T Cell-Specific Inactivation of the PI3K p110α Catalytic Subunit: Effect in T Cell Differentiation and Antigen-Specific Responses
Abstract
1. Introduction
2. Results
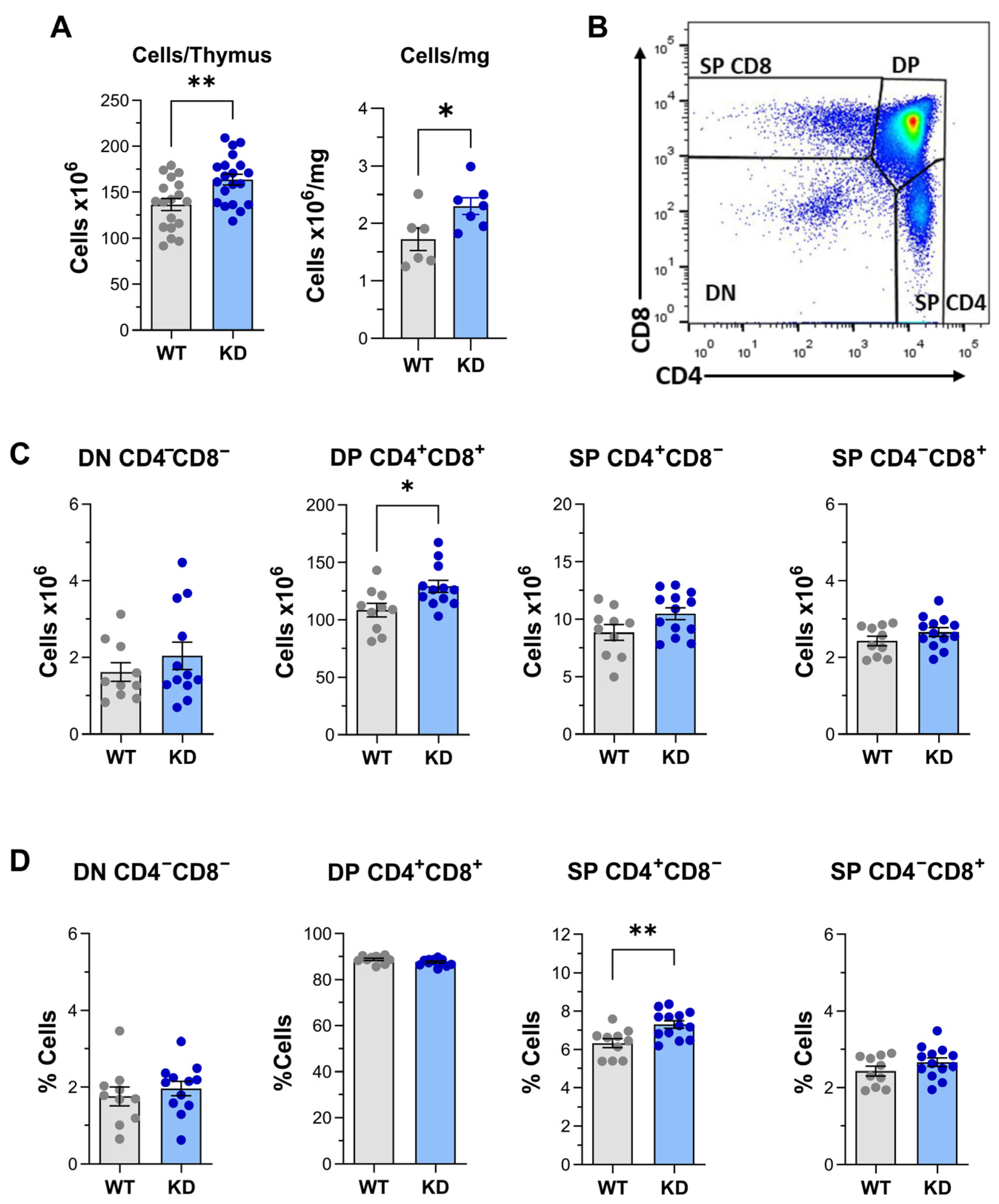
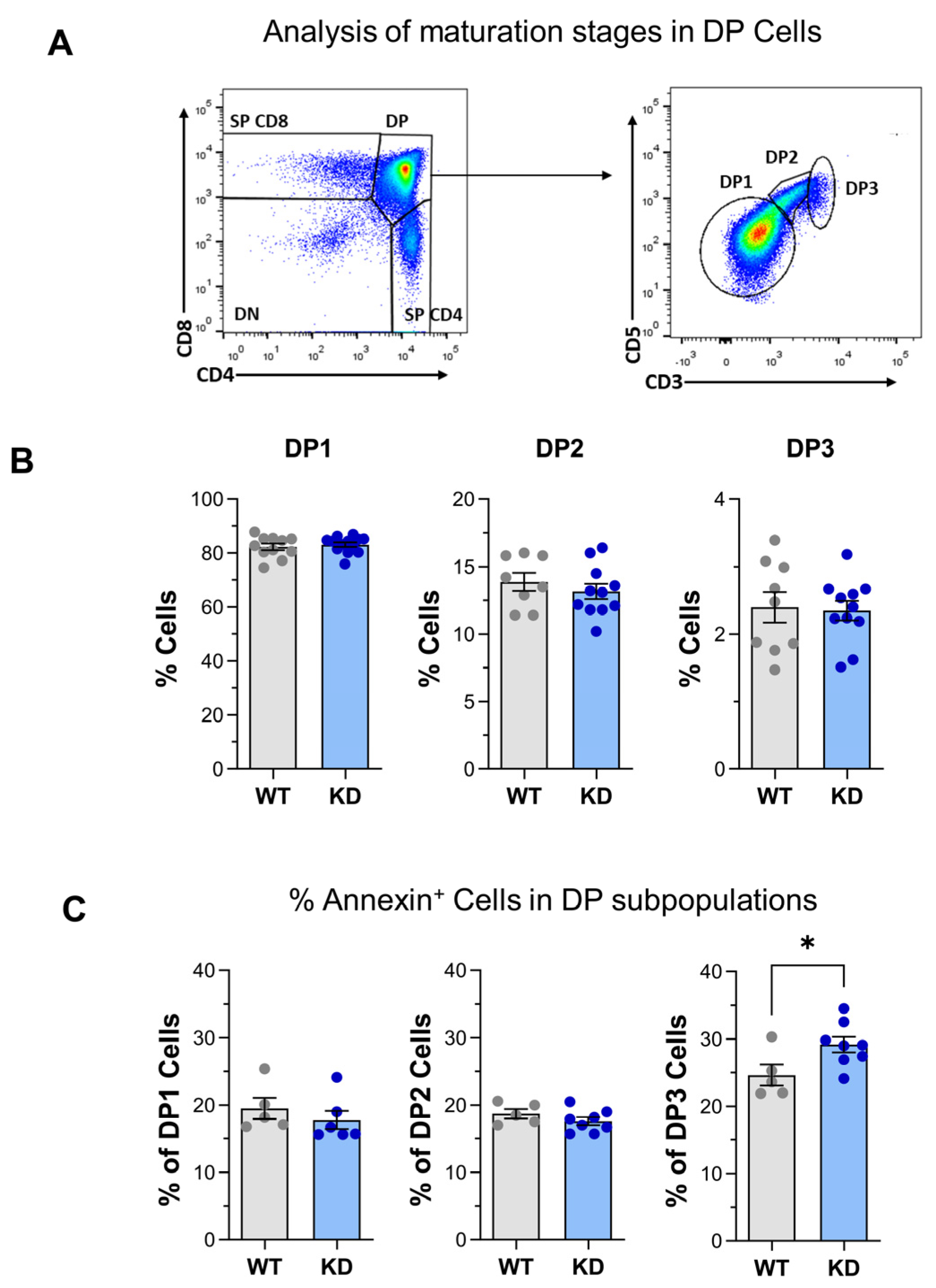
2.1. Altered Thymus Differentiation in p110αKD-T Mice
2.2. Treg Differentiation in the Thymus
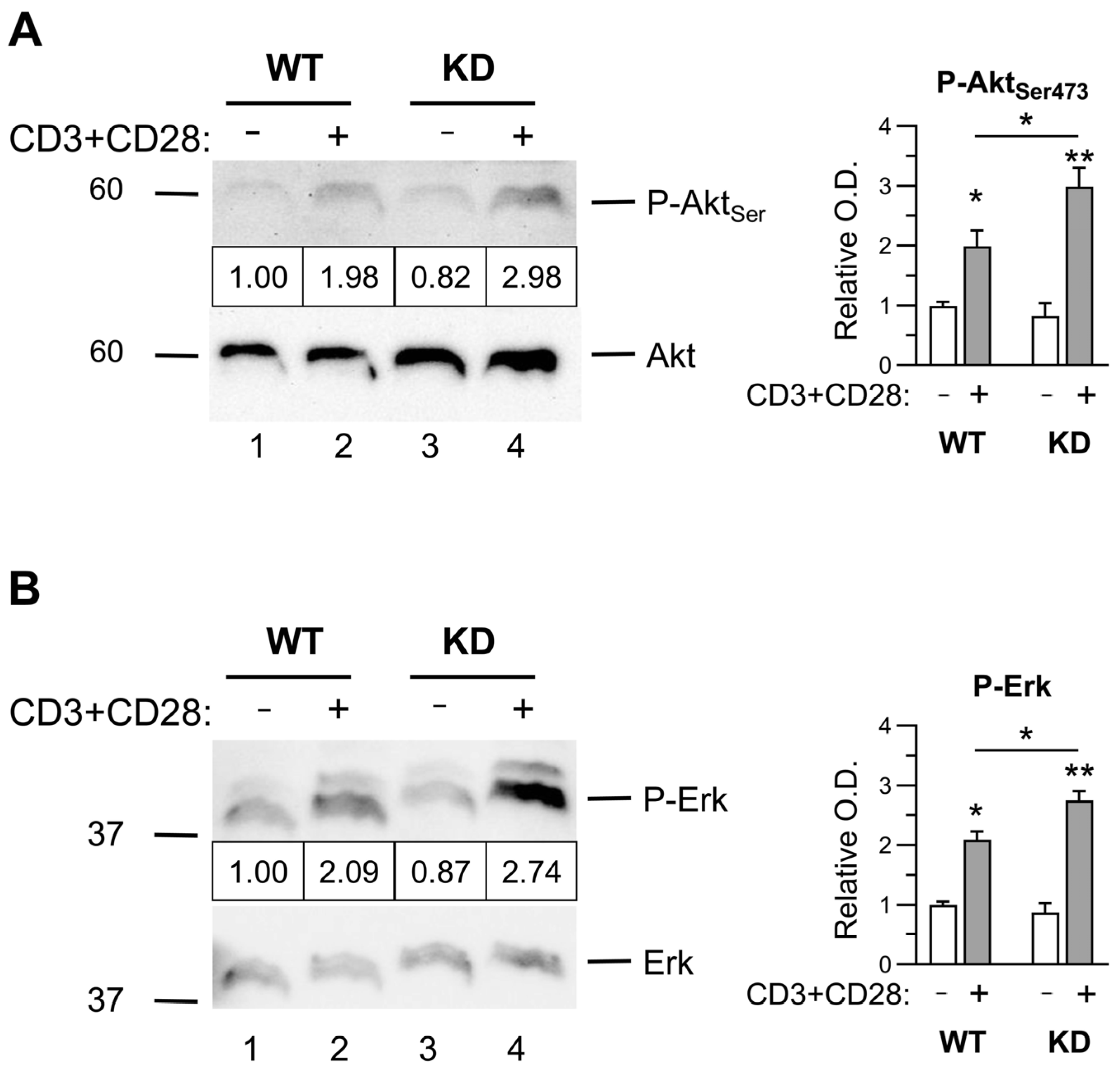
2.3. Early Activation Signals in Thymocytes
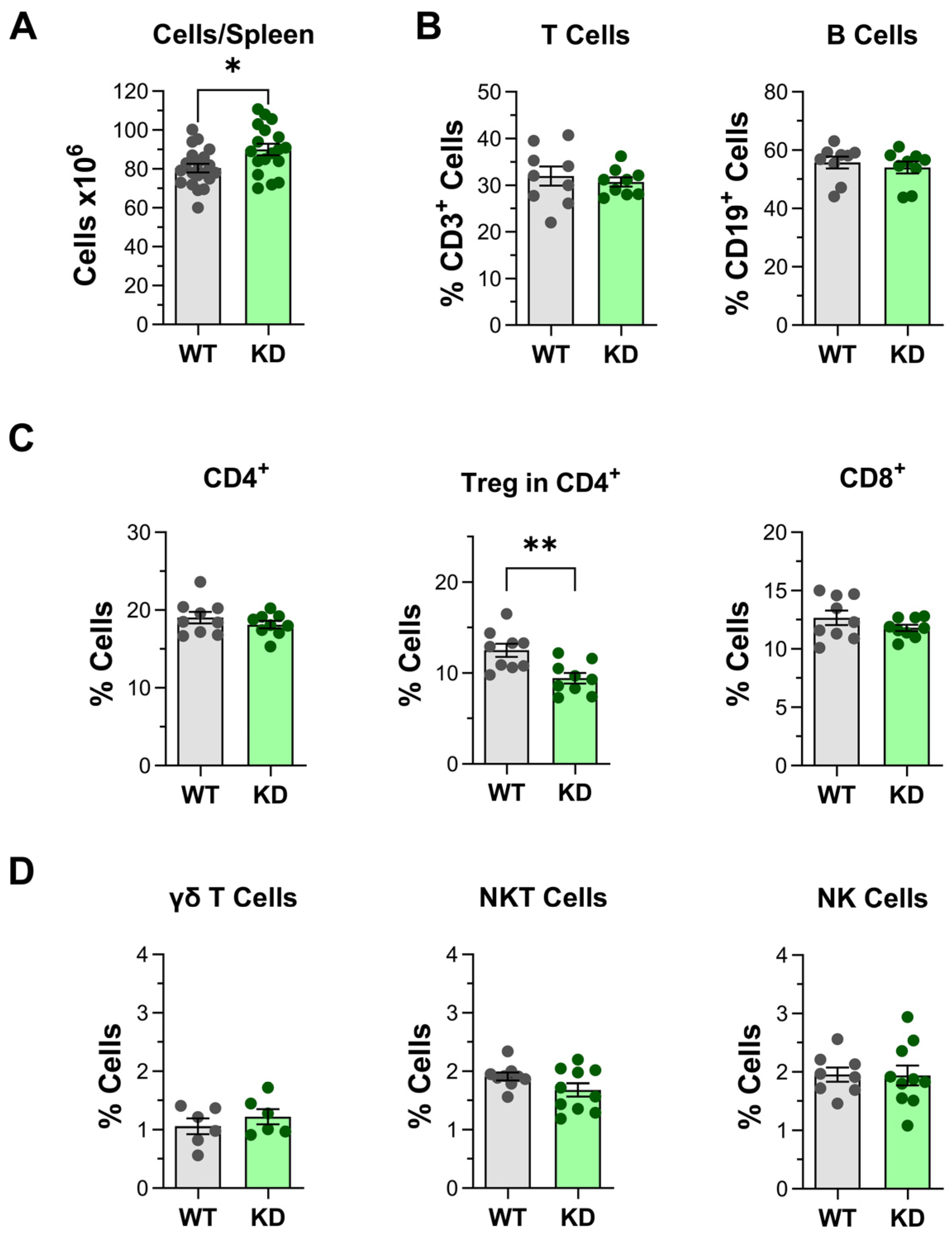
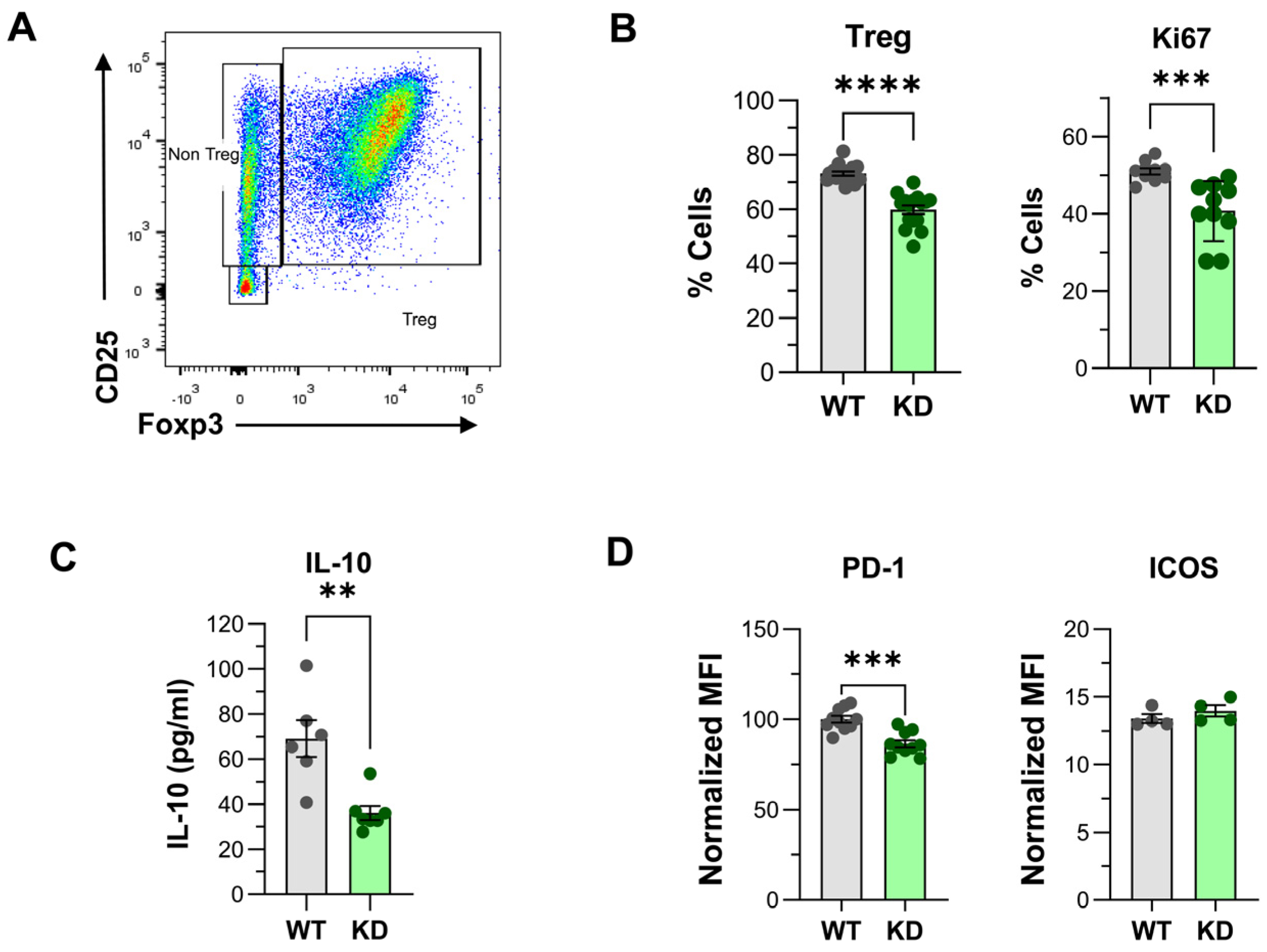
2.4. Impact of p110αKD Expression in Treg and Other Spleen Cell Populations
2.5. CD4+ Treg Differentiation “In Vitro”
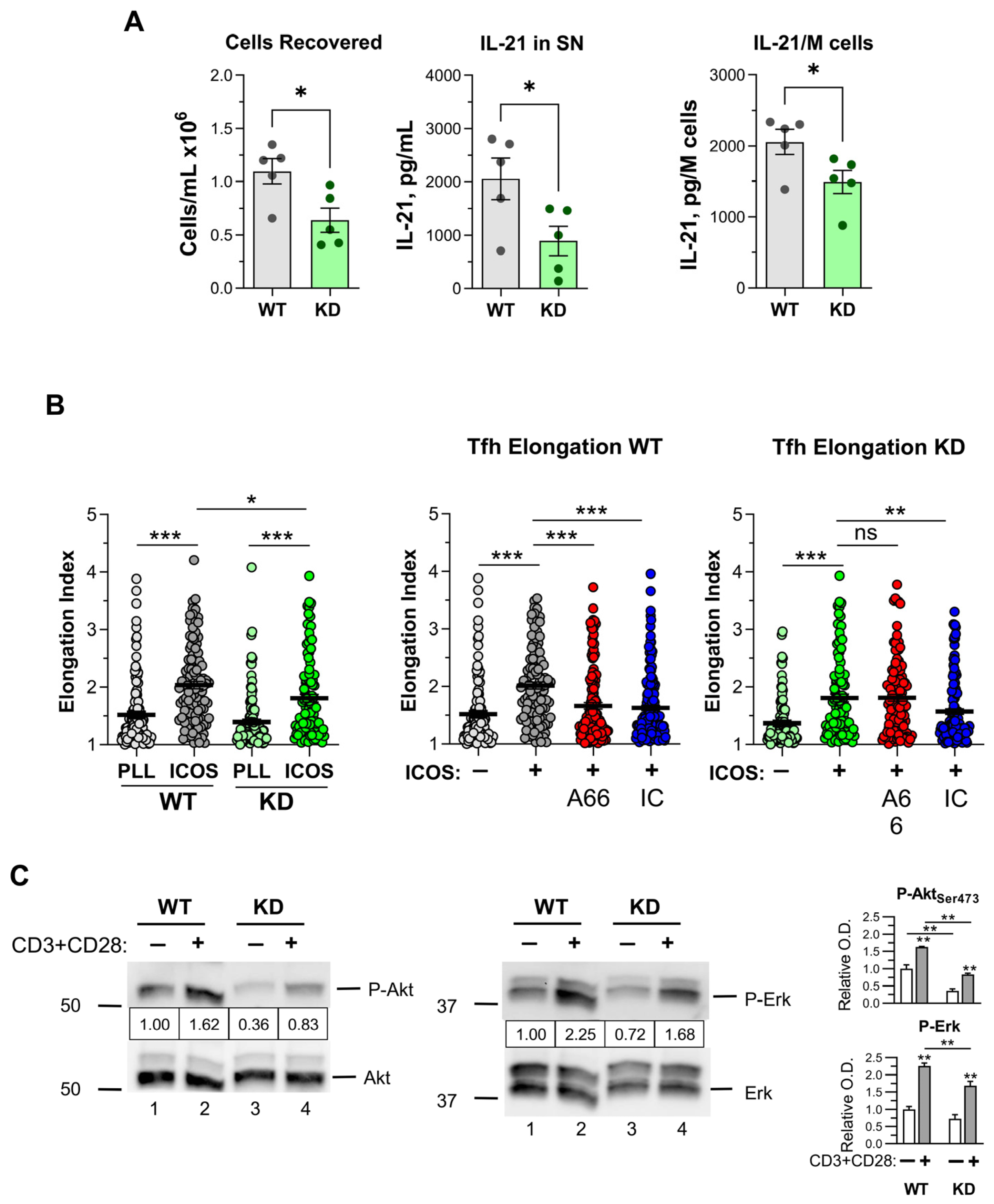
2.6. Effect of p110α Inactivation on CD4+ Th1, Th17 and Tfh Cell Differentiation In Vitro
2.7. Early Signaling in CD4+ T Cells
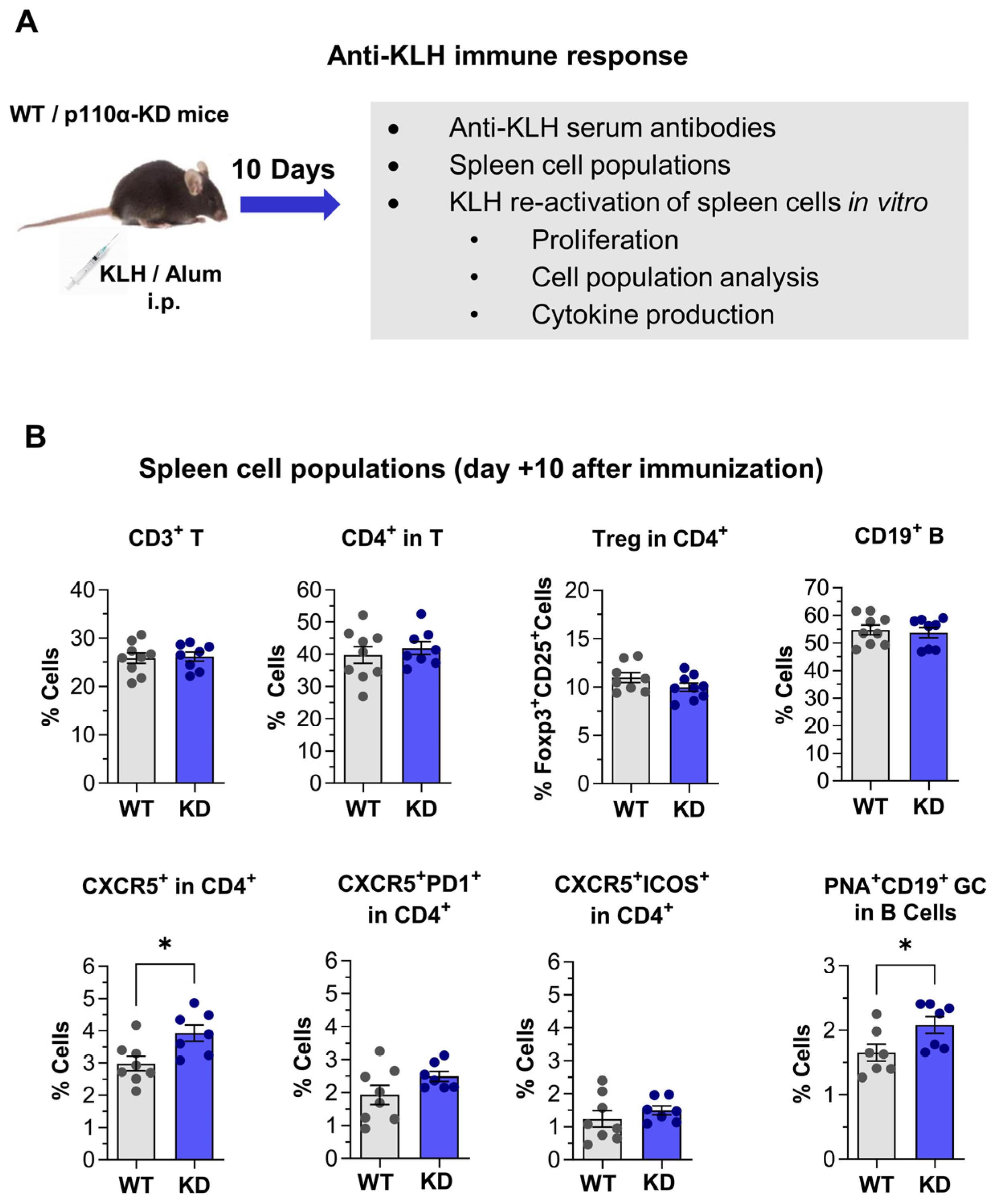
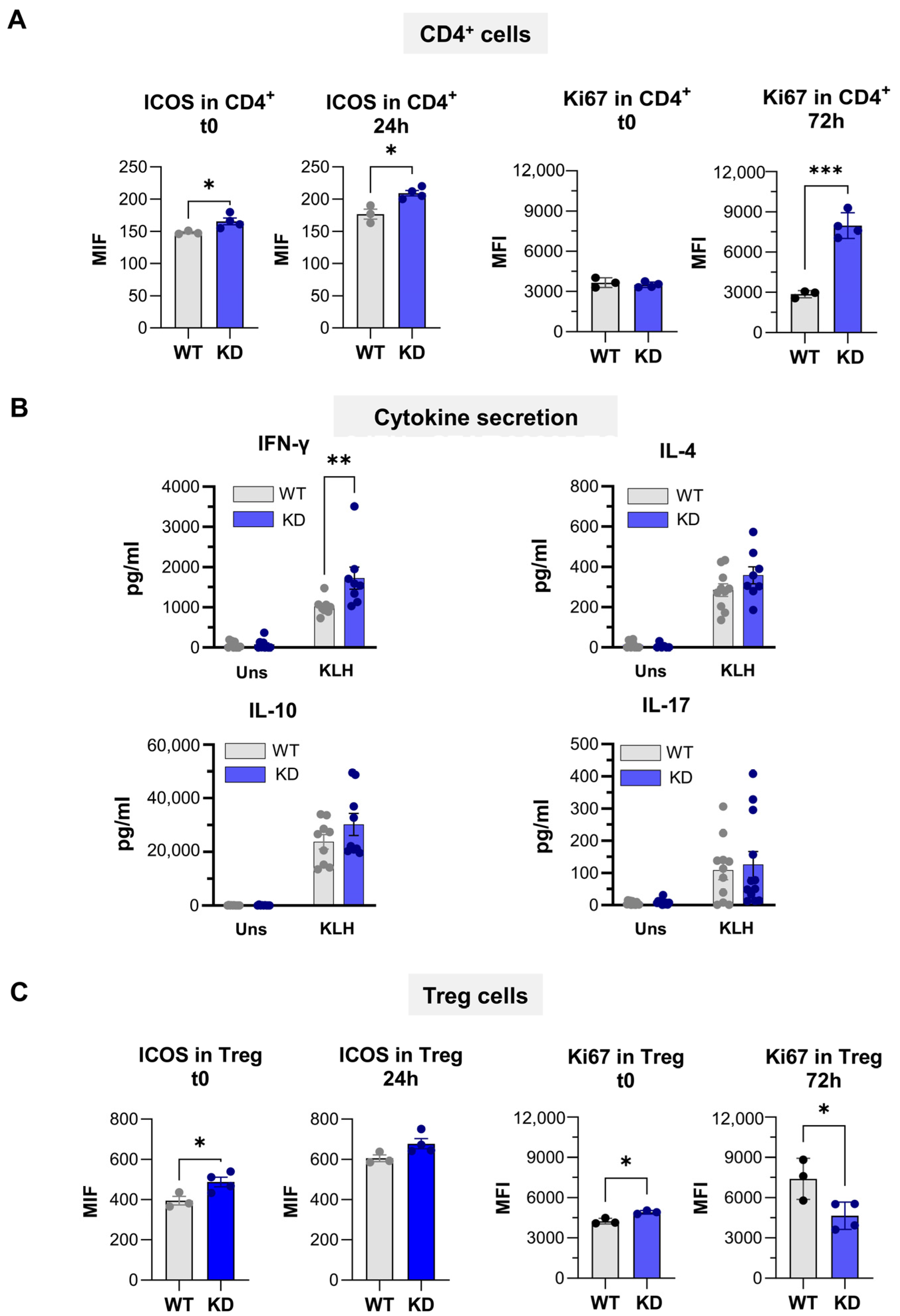
2.8. Immune Response Against KLH Protein in p110αKD-T Mice
3. Discussion
3.1. Thymus Differentiation
3.2. PI3K in Treg In Vitro and In Vivo
3.3. Tfh Generation In Vitro and In Vivo
4. Materials and Methods
4.1. Mice
4.2. Genotyping
4.3. Antibodies and Cytokines
4.4. Analysis of Surface and Intracellular Markers by Flow Cytometry
4.5. Differentiation of Helper and Regulatory CD4+ T Cells “In Vitro”
4.6. Early Activation Signals in T Cells
4.7. Elongation Assay
4.8. Cytokine ELISA
4.9. Immunization of p110αKD T Mice with KLH
4.10. Anti-KLH Serum Antibodies
4.11. KLH-Specific Responses In Vitro: Assessment of Cell Proliferation and Cytokine Secretion
4.12. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adefemi, F.; Fruman, D.A.; Marshall, A.J. A case for Phosphoinositide 3-Kinase–targeted therapy for infectious disease. J. Immunol. 2020, 205, 3237–3245. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.-K.; Sriskantharajah, S.; Hessel, E.M.; Okkenhaug, K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr. Opin. Pharmacol. 2015, 23, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Banham-Hall, E.; Clatworthy, M.R.; Okkenhaug, K. The therapeutic potential for PI3K inhibitors in autoimmune rheumatic diseases. Open Rheumatol. J. 2012, 6, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell. Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- Huang, Y.H.; Sauer, K. Lipid signaling in T-cell development and function. Cold Spring Harb. Perspect. Biol. 2010, 2, a002428. [Google Scholar] [CrossRef]
- Fruman, D.A.; Bismuth, G. Fine tuning the immune response with PI3K. Immunol. Rev. 2009, 228, 253–272. [Google Scholar] [CrossRef]
- Rommel, C.; Camps, M.; Ji, H. PI3Kδ and PI3Kγ: Partners in crime in inflammation in rheumatoid arthritis and beyond? Nat. Rev. Immunol. 2007, 7, 191–201. [Google Scholar] [CrossRef]
- Deane, J.A.; Fruman, D.A. Phosphoinositide 3-Kinase: Diverse roles in immune cell activation. Annu. Rev. Immunol. 2004, 22, 563–598. [Google Scholar] [CrossRef]
- Koyasu, S. The role of PI3K in immune cells. Nat. Immunol. 2003, 4, 313–319. [Google Scholar] [CrossRef]
- Hawkins, P.T.; Anderson, K.E.; Davidson, K.; Stephens, L.R. Signalling through Class I PI3Ks in mammalian cells. Biochem. Soc. Trans. 2006, 34, 647–662. [Google Scholar] [CrossRef]
- Okkenhaug, K.; Patton, D.T.; Bilancio, A.; Garcon, F.; Rowan, W.C.; Vanhaesebroeck, B. The p110d isoform of Phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J. Immunol. 2006, 177, 5122–5128. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Rintelen, F.; Waltzinger, C.; Bertschy Meier, D.; Bilancio, A.; Pearce, W.; Hirsch, E.; Wymann, M.P.; Ruckle, T.; Camps, M.; et al. Inactivation of PI3Kγ and PI3Kδ distorts T-cell development and causes multiple organ inflammation. Blood 2007, 110, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, T.; Marshall, A.J.; Okkenhaug, K.; Vanhaesebroeck, B.; Uzonna, J.E. The p110δ isoform of Phosphatidylinositol 3-Kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. J. Immunol. 2009, 183, 1921–1933. [Google Scholar] [CrossRef]
- Haylock-Jacobs, S.; Comerford, I.; Bunting, M.; Kara, E.; Townley, S.; Klingler-Hoffmann, M.; Vanhaesebroeck, B.; Puri, K.D.; McColl, S.R. PI3Kδ drives the pathogenesis of experimental autoimmune encephalomyelitis by inhibiting effector T cell apoptosis and promoting Th17 differentiation. J. Autoimmun. 2011, 36, 278–287. [Google Scholar] [CrossRef]
- Steinbach, E.C.; Kobayashi, T.; Russo, S.M.; Sheikh, S.Z.; Gipson, G.R.; Kennedy, S.T.; Uno, J.K.; Mishima, Y.; Borst, L.B.; Liu, B.; et al. Innate PI3K p110δ regulates Th1/Th17 development and microbiota-dependent colitis. J. Immunol. 2014, 192, 3958–3968. [Google Scholar] [CrossRef]
- Aragoneses-Fenoll, L.; Montes-Casado, M.; Ojeda, G.; Acosta, Y.Y.; Herranz, J.; Martínez, S.; Blanco-Aparicio, C.; Criado, G.; Pastor, J.; Dianzani, U.; et al. ETP-46321, a dual p110α/δ class IA phosphoinositide 3-kinase inhibitor modulates T lymphocyte activation and collagen-induced arthritis. Biochem. Pharmacol. 2016, 106, 56–69. [Google Scholar] [CrossRef]
- Aragoneses-Fenoll, L.; Ojeda, G.; Montes-Casado, M.; Acosta-Ampudia, Y.; Dianzani, U.; Portolés, P.; Rojo, J.M. T-cell specific loss of the PI-3 kinase p110α catalytic subunit results in enhanced cytokine production and anti tumor response. Front. Immunol. 2018, 9, e332. [Google Scholar] [CrossRef]
- Qiao, S.; Zheng, N.; Sun, L.; Pang, G.; Wang, S.; Jia, P.; Uzonna, J.; Bai, H.; Yang, X. The p110δ isoform of Phosphatidylinositol 3-Kinase plays an important role in host defense against Chlamydial lung infection through influencing CD4+ T cell function. Pathog. Dis. 2018, 76, fty053. [Google Scholar] [CrossRef]
- Yang, C.; Chen, X.; Wei, Z.; Xiao, J.; Chen, W.; Shang, Y.; Liu, J. Targeting the class IA PI3K isoforms p110α/δ attenuates heart allograft rejection in mice by suppressing the CD4+ T lymphocyte response. Am. J. Transl. Res. 2018, 10, 1387–1399. [Google Scholar]
- Patton, D.T.; Garden, O.A.; Pearce, W.P.; Clough, L.E.; Monk, C.R.; Leung, E.; Rowan, W.C.; Sancho, S.; Walker, L.S.K.; Vanhaesebroeck, B.; et al. Cutting edge: The phosphoinositide 3-kinase p110δ is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J. Immunol. 2006, 177, 6598–6602. [Google Scholar] [CrossRef]
- Ali, K.; Soond, D.R.; Pineiro, R.; Hagemann, T.; Pearce, W.; Lim, E.L.; Bouabe, H.; Scudamore, C.L.; Hancox, T.; Maecker, H.; et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature 2014, 510, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Cugliandolo, F.M.; Rosner, D.R.; Gyori, D.; Roychoudhuri, R.; Okkenhaug, K. Phosphoinositide 3-kinase δ inhibition promotes antitumor responses but antagonizes checkpoint inhibitors. JCI Insight 2018, 3, e120626. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.; Kushekhar, K.; Munthe, L.A.; Tjønnfjord, G.E.; Aandahl, E.M.; Okkenhaug, K.; Taskén, K. The PI3K p110δ isoform inhibitor Idelalisib preferentially inhibits human regulatory T cell function. J. Immunol. 2019, 202, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- David, J.S.; Dominik, A.; Christopher, A.L.; Krishnendu, C.; Jonathan, C.; Sumeet, P.; Karin, R.E.; Rui, C.; Athena, C.; Yuchun, D.; et al. Immunodeficiency, autoimmune thrombocytopenia and enterocolitis caused by autosomal recessive deficiency of PIK3CD-encoded phosphoinositide 3-kinase δ. Haematologica 2019, 104, e483–e486. [Google Scholar] [CrossRef]
- Stark, A.-K.; Davenport, E.C.M.; Patton, D.T.; Scudamore, C.L.; Vanhaesebroeck, B.; Veldhoen, M.; Garden, O.A.; Okkenhaug, K. Loss of phosphatidylinositol 3-kinase activity in regulatory T cells leads to neuronal inflammation. J. Immunol. 2020, 205, 78–89. [Google Scholar] [CrossRef]
- Acosta, Y.; Zafra, M.; Ojeda, G.; Bernardone, I.; Dianzani, U.; Portolés, P.; Rojo, J. Biased binding of class IA phosphatidyl inositol 3-kinase subunits to inducible costimulator (CD278). Cell. Mol. Life Sci. 2011, 68, 3065–3079. [Google Scholar] [CrossRef]
- Montes-Casado, M.; Ojeda, G.; Criado, G.; Rojo, J.M.; Portolés, P. The PI-3-kinase p110α catalytic subunit of T lymphocytes modulates Collagen-Induced Arthritis. Int. J. Mol. Sci. 2021, 22, 6405. [Google Scholar] [CrossRef]
- Rojo, J.M.; Montes-Casado, M.; Aragoneses-Fenoll, L.; Ojeda, G.; Dianzani, U.; Portolés, P. PI3-Kinase p110α deficiency modulates T cell homeostasis and function and attenuates Experimental Allergic Encephalitis in mature mice. Int. J. Mol. Sci. 2021, 22, 8698. [Google Scholar] [CrossRef]
- Acosta, Y.Y.; Montes-Casado, M.; Aragoneses-Fenoll, L.; Dianzani, U.; Portolés, P.; Rojo, J.M. Suppression of CD4+ T lymphocyte activation “in vitro” and experimental encephalomyelitis “in vivo” by the phosphatidyl inositol 3-kinase inhibitor PIK-75. Int. J. Immunopathol. Pharmacol. 2014, 27, 53–67. [Google Scholar] [CrossRef]
- Dutta, A.; Zhao, B.; Love, P.E. New insights into TCR β-selection. Trends Immunol. 2021, 42, 735–750. [Google Scholar] [CrossRef]
- Steier, Z.; Kim, E.J.Y.; Aylard, D.A.; Robey, E.A. The CD4 versus CD8 T cell fate decision: A multiomics-informed perspective. Annu. Rev. Immunol. 2024, 42, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Yablonski, D. Bridging the gap: Modulatory roles of the Grb2-family adaptor, Gads, in cellular and allergic immune responses. Front. Immunol. 2019, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Saini, M.; Sinclair, C.; Marshall, D.; Tolaini, M.; Sakaguchi, S.; Seddon, B. Regulation of Zap70 expression during thymocyte development enables temporal separation of CD4 and CD8 repertoire selection at different signaling thresholds. Sci. Signal. 2010, 3, ra23. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, C.; Bains, I.; Yates, A.J.; Seddon, B. Asymmetric thymocyte death underlies the CD4:CD8 T-cell ratio in the adaptive immune system. Proc. Natl. Acad. Sci. USA 2013, 110, E2905–E2914. [Google Scholar] [CrossRef]
- Yates, A.J. Theories and quantification of thymic selection. Front. Immunol. 2014, 5, 13. [Google Scholar] [CrossRef]
- Majumdar, S.; Deobagkar-Lele, M.; Adiga, V.; Raghavan, A.; Wadhwa, N.; Ahmed, S.M.; Rananaware, S.R.; Chakraborty, S.; Joy, O.; Nandi, D. Differential susceptibility and maturation of thymocyte subsets during Salmonella typhimurium infection: Insights on the roles of glucocorticoids and Interferon-gamma. Sci. Rep. 2017, 7, 40793. [Google Scholar] [CrossRef]
- Horkova, V.; Drobek, A.; Paprckova, D.; Niederlova, V.; Prasai, A.; Uleri, V.; Glatzova, D.; Kraller, M.; Cesnekova, M.; Janusova, S.; et al. Unique roles of co-receptor-bound LCK in helper and cytotoxic T cells. Nat. Immunol. 2023, 24, 174–185. [Google Scholar] [CrossRef]
- Fischer, A.M.; Katayama, C.D.; Pagès, G.; Pouysségur, J.; Hedrick, S.M. The role of Erk1 and Erk2 in multiple stages of T cell development. Immunity 2005, 23, 431–443. [Google Scholar] [CrossRef]
- Rincón, M. MAP-kinase signaling pathways in T cells. Curr. Opin. Immunol. 2001, 13, 339–345. [Google Scholar] [CrossRef]
- Juntilla, M.M.; Wofford, J.A.; Birnbaum, M.J.; Rathmell, J.C.; Koretzky, G.A. Akt1 and Akt2 are required for αβ thymocyte survival and differentiation. Proc. Natl. Acad. Sci. USA 2007, 104, 12105–12110. [Google Scholar] [CrossRef]
- Mao, C.; Tili, E.G.; Dose, M.; Haks, M.C.; Bear, S.E.; Maroulakou, I.; Horie, K.; Gaitanaris, G.A.; Fidanza, V.; Ludwig, T.; et al. Unequal contribution of Akt isoforms in the double-negative to double-positive thymocyte transition. J. Immunol. 2007, 178, 5443–5453. [Google Scholar] [CrossRef] [PubMed]
- Fayard, E.; Moncayo, G.; Hemmings, B.A.; Holländer, G.A. Phosphatidylinositol 3-Kinase signaling in thymocytes: The need for stringent control. Sci. Signal. 2010, 3, re5. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Irie-Sasaki, J.; Jones, R.G.; Oliveira-dos-Santos, A.J.; Stanford, W.L.; Bolon, B.; Wakeham, A.; Itie, A.; Bouchard, D.; Kozieradzki, I.; et al. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science 2000, 287, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Borlado, L.; Barber, D.F.; Hernández, C.; Rodríguez-Marcos, M.A.; Sánchez, A.; Hirsch, E.; Wymann, M.; Martínez-A, C.; Carrera, A.C. Phosphatidylinositol 3-kinase regulates the CD4/CD8 T cell differentiation ratio. J. Immunol. 2003, 170, 4475–4482. [Google Scholar] [CrossRef]
- Webb, L.M.C.; Vigorito, E.; Wymann, M.P.; Hirsch, E.; Turner, M. Cutting edge: T cell development requires the combined activities of the p110γ and p110δ catalytic isoforms of phosphatidylinositol 3-kinase. J. Immunol. 2005, 175, 2783–2787. [Google Scholar] [CrossRef]
- Swat, W.; Montgrain, V.; Doggett, T.A.; Douangpanya, J.; Puri, K.; Vermi, W.; Diacovo, T.G. Essential role of PI3Kδ and PI3Kγ in thymocyte survival. Blood 2006, 107, 2415–2422. [Google Scholar] [CrossRef]
- Janas, M.L.; Varano, G.; Gudmundsson, K.; Noda, M.; Nagasawa, T.; Turner, M. Thymic development beyond β-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J. Exp. Med. 2010, 207, 247–261. [Google Scholar] [CrossRef]
- Janas, M.L.; Turner, M. Interaction of Ras with P110γ is required for thymic β-selection in the mouse. J. Immunol. 2011, 187, 4667–4675. [Google Scholar] [CrossRef]
- Soond, D.; Slack, E.; Garden, O.; Patton, D.; Okkenhaug, K. Does the PI3K pathway promote or antagonize regulatory T cell development and function? Front. Immunol. 2012, 3, 244. [Google Scholar] [CrossRef]
- Bensinger, S.J.; Walsh, P.T.; Zhang, J.; Carroll, M.; Parsons, R.; Rathmell, J.C.; Thompson, C.B.; Burchill, M.A.; Farrar, M.A.; Turka, L.A. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J. Immunol. 2004, 172, 5287–5296. [Google Scholar] [CrossRef]
- Walsh, P.T.; Buckler, J.L.; Zhang, J.; Gelman, A.E.; Dalton, N.M.; Taylor, D.K.; Bensinger, S.J.; Hancock, W.W.; Turka, L.A. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J. Clin. Investig. 2006, 116, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Eschweiler, S.; Ramírez-Suástegui, C.; Li, Y.; King, E.; Chudley, L.; Thomas, J.; Wood, O.; von Witzleben, A.; Jeffrey, D.; McCann, K.; et al. Intermittent PI3Kδ inhibition sustains anti-tumour immunity and curbs irAEs. Nature 2022, 605, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Sauer, S.; Bruno, L.; Hertweck, A.; Finlay, D.; Leleu, M.; Spivakov, M.; Knight, Z.A.; Cobb, B.S.; Cantrell, D.; O’Connor, E.; et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. USA 2008, 105, 7797–7802. [Google Scholar] [CrossRef]
- Moser, B.; Schaerli, P.; Loetscher, P. CXCR5+ T cells: Follicular homing takes center stage in T-helper-cell responses. Trends Immunol. 2002, 23, 250–254. [Google Scholar] [CrossRef]
- Gorentla, B.K.; Wan, C.-K.; Zhong, X.-P. Negative regulation of mTOR activation by diacylglycerol kinases. Blood 2011, 117, 4022–4031. [Google Scholar] [CrossRef]
- Gamper, C.J.; Powell, J.D. All PI3Kinase signaling is not mTOR: Dissecting mTOR-dependent and independent signaling pathways in T cells. Front. Immunol. 2012, 3. [Google Scholar] [CrossRef]
- Rodriguez-Viciana, P.; Sabatier, C.; McCormick, F. Signaling specificity by Ras family GTPases is determined by the full spectrum of effectors they regulate. Mol. Cell. Biol. 2004, 24, 4943–4954. [Google Scholar] [CrossRef]
- Lee, P.P.; Fitzpatrick, D.R.; Beard, C.; Jessup, H.K.; Lehar, S.; Makar, K.W.; Pérez-Melgosa, M.; Sweetser, M.T.; Schlissel, M.S.; Nguyen, S.; et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 2001, 15, 763–774. [Google Scholar] [CrossRef]
- Graupera, M.; Guillermet-Guibert, J.; Foukas, L.C.; Phng, L.-K.; Cain, R.J.; Salpekar, A.; Pearce, W.; Meek, S.; Millan, J.; Cutillas, P.R.; et al. Angiogenesis selectively requires the p110α isoform of PI3K to control endothelial cell migration. Nature 2008, 453, 662–666. [Google Scholar] [CrossRef]
- Portoles, P.; Rojo, J.; Golby, A.; Bonneville, M.; Gromkowski, S.; Greenbaum, L.; Janeway, C.A., Jr.; Murphy, D.B.; Bottomly, K. Monoclonal antibodies to murine CD3ε define distinct epitopes, one of which may interact with CD4 during T cell activation. J. Immunol. 1989, 142, 4169–4175. [Google Scholar] [CrossRef]
- Nurieva, R.I.; Chung, Y.; Hwang, D.; Yang, X.O.; Kang, H.S.; Ma, L.; Wang, Y.-h.; Watowich, S.S.; Jetten, A.M.; Tian, Q.; et al. Generation of T Follicular Helper cells is mediated by Interleukin-21 but independent of T Helper 1, 2, or 17 cell lineages. Immunity 2008, 29, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Perry, M.W.D.; Brown, J.R.; André, F.; Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021, 20, 741–769. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briones, A.C.; del Estal, L.; Villa-Gómez, C.; Bermejo, V.; Cervera, I.; Gutiérrez-Huerta, P.; Montes-Casado, M.; Ortega, S.; Barbacid, M.; Rojo, J.M.; et al. T Cell-Specific Inactivation of the PI3K p110α Catalytic Subunit: Effect in T Cell Differentiation and Antigen-Specific Responses. Int. J. Mol. Sci. 2025, 26, 595. https://doi.org/10.3390/ijms26020595
Briones AC, del Estal L, Villa-Gómez C, Bermejo V, Cervera I, Gutiérrez-Huerta P, Montes-Casado M, Ortega S, Barbacid M, Rojo JM, et al. T Cell-Specific Inactivation of the PI3K p110α Catalytic Subunit: Effect in T Cell Differentiation and Antigen-Specific Responses. International Journal of Molecular Sciences. 2025; 26(2):595. https://doi.org/10.3390/ijms26020595
Chicago/Turabian StyleBriones, Alejandro C., Laura del Estal, Cristina Villa-Gómez, Verónica Bermejo, Isabel Cervera, Pedro Gutiérrez-Huerta, María Montes-Casado, Sagrario Ortega, Mariano Barbacid, José María Rojo, and et al. 2025. "T Cell-Specific Inactivation of the PI3K p110α Catalytic Subunit: Effect in T Cell Differentiation and Antigen-Specific Responses" International Journal of Molecular Sciences 26, no. 2: 595. https://doi.org/10.3390/ijms26020595
APA StyleBriones, A. C., del Estal, L., Villa-Gómez, C., Bermejo, V., Cervera, I., Gutiérrez-Huerta, P., Montes-Casado, M., Ortega, S., Barbacid, M., Rojo, J. M., & Portolés, P. (2025). T Cell-Specific Inactivation of the PI3K p110α Catalytic Subunit: Effect in T Cell Differentiation and Antigen-Specific Responses. International Journal of Molecular Sciences, 26(2), 595. https://doi.org/10.3390/ijms26020595





