Modelling Endometriosis Using In Vitro and In Vivo Systems
Abstract
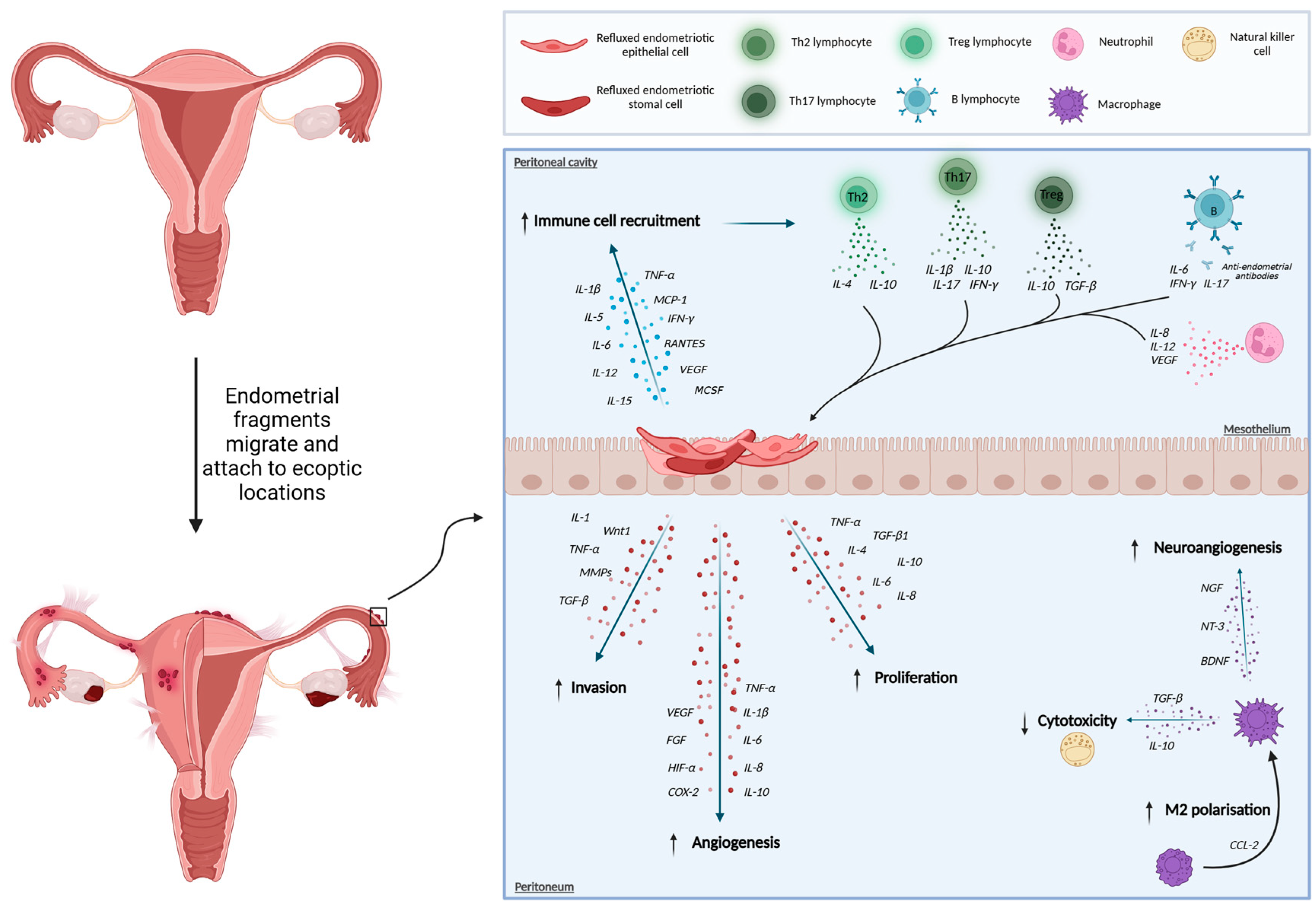
1. Introduction
2. Primary Endometrial Samples and Cell Lines
2.1. Primary Cells
2.1.1. Accessing Primary Endometrial Cells
2.1.2. Primary Cell Separation
2.1.3. Limitations of Primary Cells
2.2. Cell Lines
2.2.1. Advantages of Using Cell Lines
2.2.2. Limitations of Cell Lines
3. In Vitro Modelling
3.1. Single-Cell Studies
3.2. Two-Dimensional (2D) Cell Culture Models
3.3. Three-Dimensional Cell Culture Models
3.3.1. Three-Dimensional Matrices
3.3.2. Self-Assembling Organoids
4. In Vivo Modelling
4.1. Non-Primate Models
4.1.1. Homologous Models
4.1.2. Heterologous Models
4.2. Non-Human Primate Models
4.2.1. Spontaneous and Induced Models
4.2.2. Key Areas of Research Utilising Non-Human Primate Models
5. Cross-Cutting Models
5.1. Three-Dimensional Microfluidic Cultures
5.2. Three-Dimensional Printing
5.3. Menstrual Blood Derived Stromal Cell Models
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E.; Koninckx, P.; et al. ESHRE Guideline for the Diagnosis and Treatment of Endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C. Clinical Practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Lamceva, J.; Uljanovs, R.; Strumfa, I. The Main Theories on the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 4254. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Xanthoulea, S.; Giacomini, E.; Delvoux, B.; Alleva, E.; Vigano, P. Endometriotic Cell Culture Contamination and Authenticity: A Source of Bias in in Vitro Research? Hum. Reprod. 2020, 35, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, A.; Rahmioglu, N.; Vitonis, A.F.; Viganò, P.; Giudice, L.C.; D’Hooghe, T.M.; Hummelshoj, L.; David Adamson, G.; Becker, C.M.; Missmer, S.A.; et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: IV. Tissue Collection, Processing, and Storage in Endometriosis Research. Fertil. Steril. 2014, 102, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Guo, S.W.; Brosens, J.; Fazlebas, A.; Gargett, C.E.; Giselbrecht, S.; Gotte, M.; Griffith, L.; Taylor, H.S.; Taylor, R.N.; et al. ENDOCELL-Seud: A Delphi Protocol to Harmonise Methods in Endometrial Cell Culturing. Reprod. Fertil. 2022, 3, G1. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Hoffman, J.R.; Arora, R.; Perrone, L.A.; Gonzalez-Gomez, C.J.; Vo, K.C.; Laird, D.J.; Irwin, J.C.; Giudice, L.C. Cryopreservation and Recovery of Human Endometrial Epithelial Cells with High Viability, Purity, and Functional Fidelity. Fertil. Steril. 2015, 105, 501. [Google Scholar] [CrossRef] [PubMed]
- Dorman, B.H.; Varma, V.A.; Siegfried, J.M.; Melin, S.A.; Admaec, T.A.; Norton, C.R.; Kaufman, D.G. Morphology and Growth Potential of Stromal Cell Cultures Derived from Human Endometrium. In Vitro 1982, 18, 919–928. [Google Scholar] [CrossRef]
- Wang, Y.; Nicholes, K.; Shih, I.M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. 2020, 15, 71–95. [Google Scholar] [CrossRef]
- Weng, C.S.; Cope, A.G.; Mara, K.C.; Schoolmeester, J.K.; Khan, Z.; Burnett, T.L. Association Between Laparoscopic Appearance of Superficial Endometriosis, Positive Histology, and Systemic Hormone Use. J. Minim. Invasive Gynecol. 2022, 29, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, T.; Lin, T.; Guo, Q.; Huo, C.; Roberts, S.Z.; Yang, M.; Yang, S.; Gao, L.; Zhang, W.; et al. Novel in Vivo Endometriotic Models Associated Eutopic Endometrium by Implanting Menstrual Blood-Derived Stromal Cells from Patients with Endometriosis. Sci. Rep. 2023, 13, 8347. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Zhao, X.; Elder, K.; Jones, C.J.P.; Moffett, A.; Burton, G.J.; Turco, M.Y. Menstrual Flow as a Non-Invasive Source of Endometrial Organoids. Commun. Biol. 2021, 4, 651. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Co-Operation between the AKT and ERK Signaling Pathways May Support Growth of Deep Endometriosis in a Fibrotic Microenvironment in Vitro. Hum. Reprod. 2015, 30, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, X.; Dai, Y.; Hu, X.; Zhu, H.; Jiang, Y.; Zhang, S. Endometrial Stem Cells Repair Injured Endometrium and Induce Angiogenesis via AKT and ERK Pathways. Reproduction 2016, 152, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Satyaswaroop, P.G.; Bressler, R.S.; De La Pena, M.M.; Gurpide, E. Isolation and Culture of Human Endometrial Glands. J. Clin. Endocrinol. Metab. 1979, 48, 639–641. [Google Scholar] [CrossRef]
- Bohlouli, S.; Khazaei, M.; Rabzia, A.; Khazaei, M.R.; Sadeghi, E. Adiponectin Effect on Nitric Oxide Secretion by Normal and Endometriotic Human Endometrial Stromal Cells: In Vitro Study. Int. J. Morphol. 2015, 33, 337–341. [Google Scholar] [CrossRef]
- Sikora, J.; Mielczarek-Palacz, A.; Kondera-Anasz, Z.; Strzelczyk, J. Peripheral Blood Proinflammatory Response in Women during Menstrual Cycle and Endometriosis. Cytokine 2015, 76, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.T.; Xiao, L.; Deane, J.A.; Tan, K.S.; Cousins, F.L.; Masuda, H.; Sprung, C.N.; Rosamilia, A.; Gargett, C.E. N-Cadherin Identifies Human Endometrial Epithelial Progenitor Cells by in Vitro Stem Cell Assays. Hum. Reprod. 2017, 32, 2254–2268. [Google Scholar] [CrossRef]
- Queckbörner, S.; Syk Lundberg, E.; Gemzell-Danielsson, K.; Davies, L.C. Endometrial Stromal Cells Exhibit a Distinct Phenotypic and Immunomodulatory Profile. Stem Cell Res. Ther. 2020, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial Stem/Progenitor Cells: The First 10 Years. Hum. Reprod. Update 2015, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Takamura, M.; Koga, K.; Izumi, G.; Hirata, T.; Harada, M.; Hirota, Y.; Hiraike, O.; Fujii, T.; Osuga, Y. Simultaneous Detection and Evaluation of Four Subsets of CD4+T Lymphocyte in Lesions and Peripheral Blood in Endometriosis. Am. J. Reprod. Immunol. 2015, 74, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Januszyk, M.; Rennert, R.; Sorkin, M.; Maan, Z.; Wong, L.; Whittam, A.; Whitmore, A.; Duscher, D.; Gurtner, G. Evaluating the Effect of Cell Culture on Gene Expression in Primary Tissue Samples Using Microfluidic-Based Single Cell Transcriptional Analysis. Microarrays 2015, 4, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Riepl, B.; Knedla, A.; Lefevre, S.; Tarner, I.H.; Grifka, J.; Steinmeyer, J.; Scholmerich, J.; Gay, S.; Mueller-Ladner, U. Cell Culture and Passaging Alters Gene Expression Pattern and Proliferation Rate in Rheumatoid Arthritis Synovial Fibroblasts. Arthritis Res. Ther. 2010, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Roan, N.R. Isolation and Culture of Human Endometrial Epithelial Cells and Stromal Fibroblasts. Bio-protocol 2015, 5, e1623. [Google Scholar] [CrossRef] [PubMed]
- Jividen, K.; Movassagh, M.J.; Jazaeri, A.; Li, H. Two Methods for Establishing Primary Human Endometrial Stromal Cells from Hysterectomy Specimens. Jove-J. Vis. Exp. 2014, 10, 51513. [Google Scholar] [CrossRef]
- Klemmt, P.A.B.; Carver, J.G.; Kennedy, S.H.; Koninckx, P.R.; Mardon, H.J. Stromal Cells from Endometriotic Lesions and Endometrium from Women with Endometriosis Have Reduced Decidualization Capacity. Fertil. Steril. 2006, 85, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Katoh, N.; Nakabayashi, K.; Kato, K.; Sonoda, K.; Kitade, M.; Takeda, S.; Hata, K.; Tomikawa, J. An Improved Method for Isolation of Epithelial and Stromal Cells from the Human Endometrium. J. Reprod. Dev. 2016, 62, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kyo, S.; Nakamura, M.; Kiyono, T.; Maida, Y.; Kanaya, T.; Tanaka, M.; Yatabe, N.; Inoue, M. Successful Immortalization of Endometrial Glandular Cells with Normal Structural and Functional Characteristics. Am. J. Pathol. 2003, 163, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, L.; Hamilton, A.; Kwintkiewicz, J.; Vo, K.C.; Giudice, L.C. Steroidogenic Enzyme and Key Decidualization Marker Dysregulation in Endometrial Stromal Cells from Women with Versus Without Endometriosis. Biol. Reprod. 2009, 80, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Classen-Linke, I.; Kusche, M.; Knauthe, R.; Beier, H.M. Establishment of a Human Endometrial Cell Culture System and Characterization of Its Polarized Hormone Responsive Epithelial Cells. Cell Tissue Res. 1997, 287, 171–185. [Google Scholar] [CrossRef]
- Pierro, E.; Minici, F.; Alesiani, O.; Miceli, F.; Proto, C.; Screpanti, I.; Mancuso, S.; Lanzone, A. Stromal-Epithelial Interactions Modulate Estrogen Responsiveness in Normal Human Endometrium. Biol. Reprod. 2001, 64, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.I.; Matin, M.M.; Bahrami, A.R.; Ghasroldasht, M.M. Immortality of Cell Lines: Challenges and Advantages of Establishment. Cell Biol. Int. 2013, 37, 1038–1045. [Google Scholar] [CrossRef]
- Bouquet De Jolinière, J.; Validire, P.; Canis, M.; Doussau, M.; Levardon, M.; Gogusev, J. Human Endometriosis-Derived Permanent Cell Line (FbEM-1): Establishment and Characterization. Hum. Reprod. Update 1997, 3, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Gogusev, J.; de Jolinière, J.B.; Telvi, L.; Doussau, M.; Stojkoski, A.; Levradon, M. Cellular and Genetic Constitution of Human Endometriosis Tissues. J. Soc. Gynecol. Investig. JSGI 2000, 7, 79–87. [Google Scholar] [CrossRef]
- Gaetje, R.; Winnekendonk, D.W.; Scharl, A.; Kaufmann, M. Ovarian Cancer Antigen CA 125 Enhances the Invasiveness of the Endometriotic Cell Line EEC 145. J. Soc. Gynecol. Investig. 1999, 6, 278–281. [Google Scholar] [CrossRef]
- Zeitvogel, A.; Baumann, R.; Starzinski-Powitz, A. Identification of an Invasive, N-Cadherin-Expressing Epithelial Cell Type in Endometriosis Using a New Cell Culture Model. Am. J. Pathol. 2001, 159, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Bono, Y.; Kyo, S.; Takakura, M.; Maida, Y.; Mizumoto, Y.; Nakamura, M.; Nomura, K.; Kiyono, T.; Inoue, M. Creation of Immortalised Epithelial Cells from Ovarian Endometrioma. Br. J. Cancer 2012, 106, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yi, Y.H.; Han, H.J.; Cui, H.; Shen, D.H.; Wei, L.H.; Cheng, H.Y.; Ye, X.; Ma, R.Q.; Chang, X.H. Establishment of Human Endometriosis-Derived Immortalized Eutopic Endometrium Stromal and Epithelial Cell Lines. Int. J. Clin. Exp. Med. 2016, 9, 16450–16458. [Google Scholar]
- Banu, S.K.; Lee, J.H.; Starzinski-Powitz, A.; Arosh, J.A. Gene Expression Profiles and Functional Characterization of Human Immortalized Endometriotic Epithelial and Stromal Cells. Fertil. Steril. 2008, 90, 972–987. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Banu, S.K.; Subbarao, T.; Starzinski-Powitz, A.; Arosh, J.A. Selective Inhibition of Prostaglandin E2 Receptors EP2 and EP4 Inhibits Invasion of Human Immortalized Endometriotic Epithelial and Stromal Cells through Suppression of Metalloproteinases. Mol. Cell. Endocrinol. 2011, 332, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Dufour, J.M. Cell Lines: Valuable Tools or Useless Artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Korch, C.; Spillman, M.A.; Jackson, T.A.; Jacobsen, B.M.; Murphy, S.K.; Lessey, B.A.; Jordan, V.C.; Bradford, A.P. DNA Profiling Analysis of Endometrial and Ovarian Cell Lines Reveals Misidentification, Redundancy and Contamination. Gynecol. Oncol. 2012, 127, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Witz, C.A.; Allsup, K.T.; Montoya-Rodriguez, I.A.; Vaughn, S.L.; Centonze, V.E.; Schenken, R.S. Culture of Menstrual Endometrium with Peritoneal Explants and Mesothelial Monolayers Confirms Attachment to Intact Mesothelial Cells. Hum. Reprod. 2002, 17, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vilella, F.; Alama, P.; Moreno, I.; Mignardi, M.; Isakova, A.; Pan, W.; Simon, C.; Quake, S.R. Single-Cell Transcriptomic Atlas of the Human Endometrium during the Menstrual Cycle. Nat. Med. 2020, 26, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alonso, L.; Handfield, L.F.; Roberts, K.; Nikolakopoulou, K.; Fernando, R.C.; Gardner, L.; Woodhams, B.; Arutyunyan, A.; Polanski, K.; Hoo, R.; et al. Mapping the Temporal and Spatial Dynamics of the Human Endometrium in Vivo and in Vitro. Nat. Genet. 2021, 53, 1698–1711. [Google Scholar] [CrossRef]
- Brueggmann, D.; Templeman, C.; Starzinski-Powitz, A.; Rao, N.P.; Gayther, S.A.; Lawrenson, K. Novel Three-Dimensional in Vitro Models of Ovarian Endometriosis. J. Ovarian Res. 2014, 7, 17. [Google Scholar] [CrossRef]
- Prechapanich, J.; Kajihara, T.; Fujita, K.; Sato, K.; Uchino, S.; Tanaka, K.; Matsumoto, S.; Akita, M.; Nagashima, M.; Brosens, J.J.; et al. Effect of a Dienogest for an Experimental Three-Dimensional Endometrial Culture Model for Endometriosis. Med. Mol. Morphol. 2014, 47, 189–195. [Google Scholar] [CrossRef]
- Yamada, K.M.; Cukierman, E. Modeling Tissue Morphogenesis and Cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, B.D.; Lukowski, S.W.; Mortlock, S.; Crawford, J.; Atluri, S.; Subramaniam, S.; Johnston, R.L.; Nirgianakis, K.; Tanaka, K.; Amoako, A.; et al. Altered Differentiation of Endometrial Mesenchymal Stromal Fibroblasts Is Associated with Endometriosis Susceptibility. Commun. Biol. 2022, 5, 600. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.J.; Adelson, R.P.; Vashistha, H.; Khalili, H.; Nayyar, A.; Puran, R.; Herrera, R.; Chatterjee, P.K.; Lee, A.T.; Truskinovsky, A.M.; et al. Single-Cell Analysis of Menstrual Endometrial Tissues Defines Phenotypes Associated with Endometriosis. BMC Med. 2022, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Flynn, W.F.; Sivajothi, S.; Luo, D.; Bozal, S.B.; Davé, M.; Luciano, A.A.; Robson, P.; Luciano, D.E.; Courtois, E.T. Single-Cell Analysis of Endometriosis Reveals a Coordinated Transcriptional Programme Driving Immunotolerance and Angiogenesis across Eutopic and Ectopic Tissues. Nat. Cell Biol. 2022, 24, 1306–1318. [Google Scholar] [CrossRef]
- Schwalie, P.C.; Bafligil, C.; Russeil, J.; Zachara, M.; Biocanin, M.; Alpern, D.; Aasna, E.; Deplancke, B.; Canny, G.; Goncalves, A. Single-Cell Characterization of Menstrual Fluid at Homeostasis and in Endometriosis. eLife 2024, 13. [Google Scholar] [CrossRef]
- Schulke, L.; Berbic, M.; Manconi, F.; Tokushige, N.; Markham, R.; Fraser, I.S. Dendritic Cell Populations in the Eutopic and Ectopic Endometrium of Women with Endometriosis. Hum. Reprod. 2009, 24, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Hey-Cunningham, A.J.; Wong, C.; Hsu, J.; Fromm, P.D.; Clark, G.J.; Kupresanin, F.; Miller, E.J.; Markham, R.; McGuire, H.M. Comprehensive Analysis Utilizing Flow Cytometry and Immunohistochemistry Reveals Inflammatory Changes in Local Endometrial and Systemic Dendritic Cell Populations in Endometriosis. Hum. Reprod. 2021, 36, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Hogg, C.; Panir, K.; Dhami, P.; Rosser, M.; Mack, M.; Soong, D.; Pollard, J.W.; Jenkins, S.J.; Horne, A.W.; Greaves, E. Macrophages Inhibit and Enhance Endometriosis Depending on Their Origin. Proc. Natl. Acad. Sci. USA 2021, 118, e2013776118. [Google Scholar] [CrossRef]
- Lee, J.H.; Banu, S.K.; Rodriguez, R.; Starzinski-Powitz, A.; Arosh, J.A. Selective Blockade of Prostaglandin E2 Receptors EP2 and EP4 Signaling Inhibits Proliferation of Human Endometriotic Epithelial Cells and Stromal Cells through Distinct Cell Cycle Arrest. Fertil. Steril. 2010, 93, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.E.; Monsanto, S.P.; Ahn, S.H.; Khalaj, K.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Interleukin-33 Modulates Inflammation in Endometriosis. Sci. Rep. 2017, 7, 17903. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Ruiz, L.; Colón-Caraballo, M.; Torres-Collazo, B.J.; Monteiro, J.B.; Bayona, M.; Fazleabas, A.T.; Flores, I. Pharmacological Blockage of the CXCR4-CXCL12 Axis in Endometriosis Leads to Contrasting Effects in Proliferation, Migration, and Invasion. Biol. Reprod. 2018, 98, 4–14. [Google Scholar] [CrossRef]
- Chen, J.C.; Erikson, D.W.; Piltonen, T.T.; Meyer, M.R.; Barragan, F.; McIntire, R.H.; Tamaresis, J.S.; Vo, K.C.; Giudice, L.C.; Irwin, J.C. Coculturing Human Endometrial Epithelial Cells and Stromal Fibroblasts Alters Cell-Specific Gene Expression and Cytokine Production. Fertil. Steril. 2013, 100, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Bhadriraju, K.; Chen, C.S. Engineering Cellular Microenvironments to Improve Cell-Based Drug Testing. Drug Discov. Today 2002, 7, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Birgersdotter, A.; Sandberg, R.; Ernberg, I. Gene Expression Perturbation in Vitro--a Growing Case for Three-Dimensional (3D) Culture Systems. Semin. Cancer Biol. 2005, 15, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Burns, G.W.; Joshi, N.R.; Arora, R.; Kim, J.J.; Fazleabas, A.T. Spheroids as a Model for Endometriotic Lesions. JCI Insight 2023, 8, e160815. [Google Scholar] [CrossRef]
- Esfandiari, N.; Ai, J.; Nazemian, Z.; Javed, M.H.; Gotlieb, L.; Casper, R.F. Expression of Glycodelin and Cyclooxygenase-2 in Human Endometrial Tissue Following Three-Dimensional Culture. Am. J. Reprod. Immunol. 2007, 57, 49–54. [Google Scholar] [CrossRef]
- Esfandiari, N.; Khazaei, M.; Ai, J.; Bielecki, R.; Gotlieb, L.; Ryan, E.; Casper, R.F. Effect of a Statin on an in Vitro Model of Endometriosis. Fertil. Steril. 2007, 87, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Bedaiwy, M.A.; Falcone, T.; Mascha, E.J.; Casper, R.F. Genetic Polymorphism in the Fibrinolytic System and Endometriosis. Obstet. Gynecol. 2006, 108, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhu, Q.; Jia, X.; Yu, N.; Li, Q. In Vitro and In Vivo Effects of Tumor Suppressor Gene PTEN on Endometriosis: An Experimental Study. Med. Sci. Monit. 2016, 22, 3727. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gnecco, J.S.; Brown, A.; Buttrey, K.; Ives, C.; Goods, B.A.; Baugh, L.; Hernandez-Gordillo, V.; Loring, M.; Isaacson, K.B.; Griffith, L.G. Organoid Co-Culture Model of the Human Endometrium in a Fully Synthetic Extracellular Matrix Enables the Study of Epithelial-Stromal Crosstalk. Med 2023, 4, 554–579.e9. [Google Scholar] [CrossRef]
- Boretto, M.; Maenhoudt, N.; Luo, X.; Hennes, A.; Boeckx, B.; Bui, B.; Heremans, R.; Perneel, L.; Kobayashi, H.; Van Zundert, I.; et al. Patient-Derived Organoids from Endometrial Disease Capture Clinical Heterogeneity and Are Amenable to Drug Screening. Nat. Cell Biol. 2019, 21, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-Term, Hormone-Responsive Organoid Cultures of Human Endometrium in a Chemically Defined Medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Wiwatpanit, T.; Murphy, A.R.; Lu, Z.; Urbanek, M.; Burdette, J.E.; Woodruff, T.K.; Kim, J.J. Scaffold-Free Endometrial Organoids Respond to Excess Androgens Associated With Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 769–780. [Google Scholar] [CrossRef]
- Esfandiari, F.; Mansouri, N.; Shahhoseini, M.; Heidari Khoei, H.; Mikaeeli, G.; Vankelecom, H.; Baharvand, H. Endometriosis Organoids: Prospects and Challenges. Reprod. Biomed. Online 2022, 45, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Filby, C.E.; Wyatt, K.A.; Mortlock, S.; Cousins, F.L.; McKinnon, B.; Tyson, K.E.; Montgomery, G.W.; Gargett, C.E. Comparison of Organoids from Menstrual Fluid and Hormone-Treated Endometrium: Novel Tools for Gynecological Research. J. Pers. Med. 2021, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Dinulescu, D.M.; Ince, T.A.; Quade, B.J.; Shafer, S.A.; Crowley, D.; Jacks, T. Role of K-Ras and Pten in the Development of Mouse Models of Endometriosis and Endometrioid Ovarian Cancer. Nat. Med. 2005, 11, 63–70. [Google Scholar] [CrossRef]
- D’Hooghe, T.M. Clinical Relevance of the Baboon as a Model for the Study of Endometriosis. Fertil. Steril. 1997, 68, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Takai, E.; Taniguchi, F.; Nakamura, K.; Uegaki, T.; Iwabe, T.; Harada, T. Parthenolide Reduces Cell Proliferation and Prostaglandin E2 [Corrected] in Human Endometriotic Stromal Cells and Inhibits Development of Endometriosis in the Murine Model. Fertil. Steril. 2013, 100, 1170–1178. [Google Scholar] [CrossRef]
- Cummings, A.M.; Metcalf, J.L. Induction of Endometriosis in Mice: A New Model Sensitive to Estrogen. Reprod. Toxicol. 1995, 9, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Berkley, K.J.; Cason, A.; Jacobs, H.; Bradshaw, H.; Wood, E. Vaginal Hyperalgesia in a Rat Model of Endometriosis. Neurosci. Lett. 2001, 306, 185–188. [Google Scholar] [CrossRef]
- Berkley, K.J.; McAllister, S.L.; Accius, B.E.; Winnard, K.P. Endometriosis-Induced Vaginal Hyperalgesia in the Rat: Effect of Estropause, Ovariectomy, and Estradiol Replacement. Pain 2007, 132 (Suppl. S1), S150–S159. [Google Scholar] [CrossRef] [PubMed]
- Berkley, K.J.; Dmitrieva, N.; Curtis, K.S.; Papka, R.E. Innervation of Ectopic Endometrium in a Rat Model of Endometriosis. Proc. Natl. Acad. Sci. USA 2004, 101, 11094–11098. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, P.; Chen, X.; Hendrich, J.; Irwin, J.C.; Green, P.G.; Giudice, L.C.; Levine, J.D. Ectopic Uterine Tissue as a Chronic Pain Generator. Neuroscience 2012, 225, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, P.; Bogen, O.; Chen, X.; Giudice, L.C.; Levine, J.D. Ectopic Endometrium-Derived Leptin Produces Estrogen-Dependent Chronic Pain in a Rat Model of Endometriosis. Neuroscience 2014, 258, 111–120. [Google Scholar] [CrossRef]
- Burns, K.A.; Pearson, A.M.; Slack, J.L.; Por, E.D.; Scribner, A.N.; Eti, N.A.; Burney, R.O. Endometriosis in the Mouse: Challenges and Progress Toward a ‘Best Fit’ Murine Model. Front. Physiol. 2022, 12, 806574. [Google Scholar] [CrossRef]
- Vernon, M.W.; Wilson, E.A. Studies on the Surgical Induction of Endometriosis in the Rat. Fertil. Steril. 1985, 44, 684–694. [Google Scholar] [CrossRef]
- Yan, D.; Liu, X.; Guo, S.W. The Establishment of a Mouse Model of Deep Endometriosis. Hum. Reprod. 2019, 34, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Osuga, Y.; Yoshino, O.; Hirota, Y.; Harada, M.; Takemura, Y.; Morimoto, C.; Koga, K.; Yano, T.; Tsutsumi, O.; et al. Development of an Experimental Model of Endometriosis Using Mice That Ubiquitously Express Green Fluorescent Protein. Hum. Reprod. 2005, 20, 2092–2096. [Google Scholar] [CrossRef] [PubMed]
- Dorning, A.; Dhami, P.; Panir, K.; Hogg, C.; Park, E.; Ferguson, G.D.; Hargrove, D.; Karras, J.; Horne, A.W.; Greaves, E. Bioluminescent Imaging in Induced Mouse Models of Endometriosis Reveals Differences in Four Model Variations. Dis. Model. Mech. 2021, 14, dmm049070. [Google Scholar] [CrossRef] [PubMed]
- Bellofiore, N.; Ellery, S.; Mamrot, J.; Walker, D.; Temple-Smith, P.; Dickinson, H. First Evidence of a Menstruating Rodent: The Spiny Mouse (Acomys cahirinus). Am. J. Obstet. Gynecol. 2016, 216, 40.e1–40.e11. [Google Scholar] [CrossRef]
- Cousins, F.L.; Murray, A.; Esnal, A.; Gibson, D.A.; Critchley, H.O.; Saunders, P.T. Evidence from a Mouse Model That Epithelial Cell Migration and Mesenchymal-Epithelial Transition Contribute to Rapid Restoration of Uterine Tissue Integrity during Menstruation. PLoS ONE 2014, 9, e86378. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Collins, F.; Esnal, A.; Giakoumelou, S.; Horne, A.W.; Saunders, P.T. Estrogen Receptor (ER) Agonists Differentially Regulate Neuroangiogenesis in Peritoneal Endometriosis via the Repellent Factor SLIT3. Endocrinology 2014, 155, 4015–4026. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Cousins, F.L.; Murray, A.; Esnal-Zufiaurre, A.; Fassbender, A.; Horne, A.W.; Saunders, P.T. A Novel Mouse Model of Endometriosis Mimics Human Phenotype and Reveals Insights into the Inflammatory Contribution of Shed Endometrium. Am. J. Pathol. 2014, 184, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Horne, A.W.; Jerina, H.; Mikolajczak, M.; Hilferty, L.; Mitchell, R.; Fleetwood-Walker, S.M.; Saunders, P.T.K. EP2 Receptor Antagonism Reduces Peripheral and Central Hyperalgesia in a Preclinical Mouse Model of Endometriosis. Sci. Rep. 2017, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Temp, J.; Esnal-Zufiurre, A.; Mechsner, S.; Horne, A.W.; Saunders, P.T. Estradiol Is a Critical Mediator of Macrophage-Nerve Cross Talk in Peritoneal Endometriosis. Am. J. Pathol. 2015, 185, 2286–2297. [Google Scholar] [CrossRef]
- Tejada, M.A.; Antunez, C.; Nunez-Badinez, P.; De Leo, B.; Saunders, P.T.; Vincent, K.; Cano, A.; Nagel, J.; Gomez, R. Rodent Animal Models of Endometriosis-Associated Pain: Unmet Needs and Resources Available for Improving Translational Research in Endometriosis. Int. J. Mol. Sci. 2023, 24, 2422. [Google Scholar] [CrossRef] [PubMed]
- Arosh, J.A.; Lee, J.; Balasubbramanian, D.; Stanley, J.A.; Long, C.R.; Meagher, M.W.; Osteen, K.G.; Bruner-Tran, K.L.; Burghardt, R.C.; Starzinski-Powitz, A.; et al. Molecular and Preclinical Basis to Inhibit PGE2 Receptors EP2 and EP4 as a Novel Nonsteroidal Therapy for Endometriosis. Proc. Natl. Acad. Sci. USA 2015. [CrossRef]
- Hull, M.L.; Johan, M.Z.; Hodge, W.L.; Robertson, S.A.; Ingman, W. V Host-Derived TGFB1 Deficiency Suppresses Lesion Development in a Mouse Model of Endometriosis. Am. J. Pathol. 2012, 180, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Bruner, K.L.; Matrisian, L.M.; Rodgers, W.H.; Gorstein, F.; Osteen, K.G. Suppression of Matrix Metalloproteinases Inhibits Establishment of Ectopic Lesions by Human Endometrium in Nude Mice. J. Clin. Investig. 1997, 99, 2851–2857. [Google Scholar] [CrossRef]
- Zamah, N.M.; Dodson, M.G.; Clifton Stephens, L.; Buttram, V.C.; Besch, P.K.; Kaufman, R.H. Transplantation of Normal and Ectopic Human Endometrial Tissue into Athymic Nude Mice. Am. J. Obstet. Gynecol. 1984, 149, 591–597. [Google Scholar] [CrossRef]
- Aoki, D.; Katsuki, Y.; Shimizu, A.; Kakinuma, C.; Nozawa, S. Successful Heterotransplantation of Human Endometrium in SCID Mice. Obstet. Gynecol. 1994, 83, 220–228. [Google Scholar]
- Grümmer, R.; Schwarzer, F.; Bainczyk, K.; Hess-Stumpp, H.; Regidor, P.A.; Schindler, A.E.; Winterhager, E. Peritoneal Endometriosis: Validation of an in-Vivo Model. Hum. Reprod. 2001, 16, 1736–1743. [Google Scholar] [CrossRef]
- Pullen, N.; Birch, C.L.; Douglas, G.J.; Hussain, Q.; Pruimboom-Brees, I.; Walley, R.J. The Translational Challenge in the Development of New and Effective Therapies for Endometriosis: A Review of Confidence from Published Preclinical Efficacy Studies. Hum. Reprod. Update 2011, 17, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Gonzalez, I.; Barrientos, G.; Tariverdian, N.; Arck, P.C.; Garcia, M.G.; Klapp, B.F.; Blois, S.M. Endometriosis Research: Animal Models for the Study of a Complex Disease. J. Reprod. Immunol. 2010, 86, 141–147. [Google Scholar] [CrossRef]
- Stevens, V.C. Some Reproductive Studies in the Baboon. Hum. Reprod. Update 1997, 3, 533–540. [Google Scholar] [CrossRef]
- D’Hooghe, T.M.; Bambra, C.S.; Cornillie, F.J.; Isahakia, M.; Koninckx, P.R. Prevalence and Laparoscopic Appearance of Spontaneous Endometriosis in the Baboon (Papio Anubis, Papio Cynocephalus). Biol. Reprod. 1991, 45, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Dick, E.J., Jr.; Hubbard, G.B.; Martin, L.J.; Leland, M.M. Record Review of Baboons with Histologically Confirmed Endometriosis in a Large Established Colony. J. Med. Primatol. 2003, 32, 39–47. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, W.F.; Casey, H.W. Animal Model of Human Disease. Endometriosis. Animal Model: Endometriosis in Rhesus Monkeys. Am. J. Pathol. 1975, 80, 341–344. [Google Scholar]
- Story, L.; Kennedy, S. Animal Studies in Endometriosis: A Review. ILAR J. 2004, 45, 132–138. [Google Scholar] [CrossRef]
- Te Linde, R.W.; Scott, R.B. Experimental Endometriosis. Am. J. Obstet. Gynecol. 1950, 60, 1147–1173. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Bambra, C.S.; Koninckx, P.R. Cycle Fecundity in Baboons of Proven Fertility with Minimal Endometriosis. Gynecol. Obstet. Investig. 1994, 37, 63–65. [Google Scholar]
- Fazleabas, A.T.; Brudney, A.; Gurates, B.; Chai, D.; Bulun, S. A Modified Baboon Model for Endometriosis. Ann. N. Y Acad. Sci. 2002, 955, 308–317, 396–406, discussion 340-2. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; Agarwal, S.K.; Foster, W.G. Subchronic Exposure to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Modulates the Pathophysiology of Endometriosis in the Cynomolgus Monkey. Toxicol. Sci. 2000, 56, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, R.M.; Yudkin, P.L.; Coe, C.L.; Scheffler, J.; Uno, H.; Barlow, D.H.; Kemnitz, J.W.; Kennedy, S.H. Risk Factors for Endometriosis in the Rhesus Monkey (Macaca mulatta): A Case-Control Study. Hum. Reprod. Update 1997, 3, 109–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zondervan, K.T.; Cardon, L.R.; Kennedy, S.H. The Genetic Basis of Endometriosis. Curr. Opin. Obstet. Gynecol. 2001, 13, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Weeks, D.E.; Colman, R.; Cardon, L.R.; Hadfield, R.; Schleffler, J.; Trainor, A.G.; Coe, C.L.; Kemnitz, J.W.; Kennedy, S.H. Familial Aggregation of Endometriosis in a Large Pedigree of Rhesus Macaques. Hum. Reprod. 2004, 19, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Tapmeier, T.T.; Rahmioglu, N.; Lin, J.; de Leo, B.; Obendorf, M.; Raveendran, M.; Fischer, O.M.; Bafligil, C.; Guo, M.; Harris, R.A.; et al. Neuropeptide S Receptor 1 Is a Nonhormonal Treatment Target in Endometriosis. Sci. Transl. Med. 2021, 13, eabd6469. [Google Scholar] [CrossRef] [PubMed]
- DeVito, M.J.; Birnbaum, L.S.; Farland, W.H.; Gasiewicz, T.A. Comparisons of Estimated Human Body Burdens of Dioxinlike Chemicals and TCDD Body Burdens in Experimentally Exposed Animals. Environ. Health Perspect. 1995, 103, 820–831. [Google Scholar] [CrossRef]
- Rier, S.E.; Martin, D.C.; Bowman, R.E.; Dmowski, W.P.; Becker, J.L. Endometriosis in Rhesus Monkeys (Macaca mulatta) Following Chronic Exposure to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Fundam. Appl. Toxicol. 1993, 21, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Vanden Heuvel, J.P.; Clark, G.C.; Tritscher, A.; Lucier, G.W. Accumulation of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans in Liver of Control Laboratory Rats. Fundam. Appl. Toxicol. 1994, 23, 465–469. [Google Scholar] [CrossRef] [PubMed]
- D’Hooghe, T.M.; Nugent, N.P.; Cuneo, S.; Chai, D.C.; Deer, F.; Debrock, S.; Kyama, C.M.; Mihalyi, A.; Mwenda, J.M. Recombinant Human TNFRSF1A (r-HTBP1) Inhibits the Development of Endometriosis in Baboons: A Prospective, Randomized, Placebo- and Drug-Controlled Study. Biol. Reprod. 2006, 74, 131–136. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Craessaerts, M.; Timmerman, D.; Cornillie, F.; Kennedy, S. Anti-TNF-Alpha Treatment for Deep Endometriosis-Associated Pain: A Randomized Placebo-Controlled Trial. Hum. Reprod. 2008, 23, 2017–2023. [Google Scholar] [CrossRef]
- Xiao, S.; Coppeta, J.R.; Rogers, H.B.; Isenberg, B.C.; Zhu, J.; Olalekan, S.A.; McKinnon, K.E.; Dokic, D.; Rashedi, A.S.; Haisenleder, D.J.; et al. A Microfluidic Culture Model of the Human Reproductive Tract and 28-Day Menstrual Cycle. Nat. Commun. 2017, 8, 14584. [Google Scholar] [CrossRef]
- Izumi, G.; Koga, K.; Takamura, M.; Makabe, T.; Satake, E.; Takeuchi, A.; Taguchi, A.; Urata, Y.; Fujii, T.; Osuga, Y. Involvement of Immune Cells in the Pathogenesis of Endometriosis. J. Obstet. Gynaecol. Res. 2018, 44, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, C.J.; Kim, D.-J.; Kang, J. Immune Cells in the Female Reproductive Tract. Immune Netw. 2015, 15, 16. [Google Scholar] [CrossRef]
- Ahn, J.; Yoon, M.J.; Hong, S.H.; Cha, H.; Lee, D.; Koo, H.S.; Ko, J.E.; Lee, J.; Oh, S.; Jeon, N.L.; et al. Three-Dimensional Microengineered Vascularised Endometrium-on-a-Chip. Hum. Reprod. 2021, 36, 2720. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Hill, C.J.; Paterson, K.; Mellin, R.; Zagnoni, M.; Hapangama, D.K.; Sandison, M.E. Functional, Patient-Derived 3D Tri-Culture Models of the Uterine Wall in a Microfluidic Array. Hum. Reprod. 2024, 39, 2537–2550. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of Tissues and Organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Laronda, M.M.; Rutz, A.L.; Xiao, S.; Whelan, K.A.; Duncan, F.E.; Roth, E.W.; Woodruff, T.K.; Shah, R.N. A Bioprosthetic Ovary Created Using 3D Printed Microporous Scaffolds Restores Ovarian Function in Sterilized Mice. Nat. Commun. 2017, 8, 15261. [Google Scholar] [CrossRef]
- Rogers, H.B.; Zhou, L.T.; Kusuhara, A.; Zaniker, E.; Shafaie, S.; Owen, B.C.; Duncan, F.E.; Woodruff, T.K. Dental Resins Used in 3D Printing Technologies Release Ovo-Toxic Leachates. Chemosphere 2021, 270, 129003. [Google Scholar] [CrossRef]
- Liu, H.; Lang, J.H. Is Abnormal Eutopic Endometrium the Cause of Endometriosis? The Role of Eutopic Endometrium in Pathogenesis of Endometriosis. Med. Sci. Monit. 2011, 17, RA92–RA99. [Google Scholar] [CrossRef] [PubMed]
- Vallvé-Juanico, J.; Santamaria, X.; Vo, K.C.; Houshdaran, S.; Giudice, L.C. Macrophages Display Proinflammatory Phenotypes in the Eutopic Endometrium of Women with Endometriosis with Relevance to an Infectious Etiology of the Disease. Fertil. Steril. 2019, 112, 1118–1128. [Google Scholar] [CrossRef]
- Adamczyk, M.; Wender-Ozegowska, E.; Kedzia, M. Epigenetic Factors in Eutopic Endometrium in Women with Endometriosis and Infertility. Int. J. Mol. Sci. 2022, 23, 3804. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, S.; Ebtekar, M.; Jeddi-Tehrani, M.; Shervin, A.; Bozorgmehr, M.; Vafaei, S.; Kazemnejad, S.; Zarnani, A.H. Menstrual Blood-Derived Stromal Stem Cells from Women with and without Endometriosis Reveal Different Phenotypic and Functional Characteristics. Mol. Hum. Reprod. 2014, 20, 905–918. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | Cell Line ID | Tissue of Origin | Primary Research Area | Reference |
|---|---|---|---|---|
| Endometriotic epithelial cells | FbEM-1 | Peritoneal lesion | Genomic studies | [33,34] |
| EEC 145T | Peritoneal lesion | Invasion studies | [35] | |
| 10B, 10Z, 11Z, 11E, 12Z, 33Z, 39Z, 42B, 45Z, 49Z, 50Z, 108Z | Peritoneal lesion | Invasion, proliferation, apoptosis angiogenesis and inflammation studies | [36] | |
| EMosis-CC/TERT1, EMosis-CC/TERT2, EMosis-E6/E7/TERT1, EMosis-E6/E7/TERT2 | Ovarian lesion | Transcriptomic studies, NOTCH signalling, neoplastic transformation | [37] | |
| EEC16-TERT | Ovarian lesion | Transcriptome analysis | [37] | |
| hEM5B2 | Ovarian lesion | Cell line establishment | [38] | |
| Endometriotic stromal cells | 3, 4, 9-4Z, 9-8Z, 17B, 18B, 20B, 22B, 25Z, 40Z, 55Z, 57Z-T1, 57Z-T2 | Peritoneal lesion | Proliferation, apoptosis, inflammation, angiogenesis and invasion studies | [36] |
| hEM15A | Endometrium | Cell line establishment | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Black, V.; Bafligil, C.; Greaves, E.; Zondervan, K.T.; Becker, C.M.; Hellner, K. Modelling Endometriosis Using In Vitro and In Vivo Systems. Int. J. Mol. Sci. 2025, 26, 580. https://doi.org/10.3390/ijms26020580
Black V, Bafligil C, Greaves E, Zondervan KT, Becker CM, Hellner K. Modelling Endometriosis Using In Vitro and In Vivo Systems. International Journal of Molecular Sciences. 2025; 26(2):580. https://doi.org/10.3390/ijms26020580
Chicago/Turabian StyleBlack, Verity, Cemsel Bafligil, Erin Greaves, Krina T. Zondervan, Christian M. Becker, and Karin Hellner. 2025. "Modelling Endometriosis Using In Vitro and In Vivo Systems" International Journal of Molecular Sciences 26, no. 2: 580. https://doi.org/10.3390/ijms26020580
APA StyleBlack, V., Bafligil, C., Greaves, E., Zondervan, K. T., Becker, C. M., & Hellner, K. (2025). Modelling Endometriosis Using In Vitro and In Vivo Systems. International Journal of Molecular Sciences, 26(2), 580. https://doi.org/10.3390/ijms26020580






