Protocadherin-7 Regulates Monocyte Migration Through Regulation of Small GTPase RhoA and Rac1
Abstract
1. Introduction
2. Results
2.1. Pcdh7 Deficiency Results in Monocytes with Impaired Motility
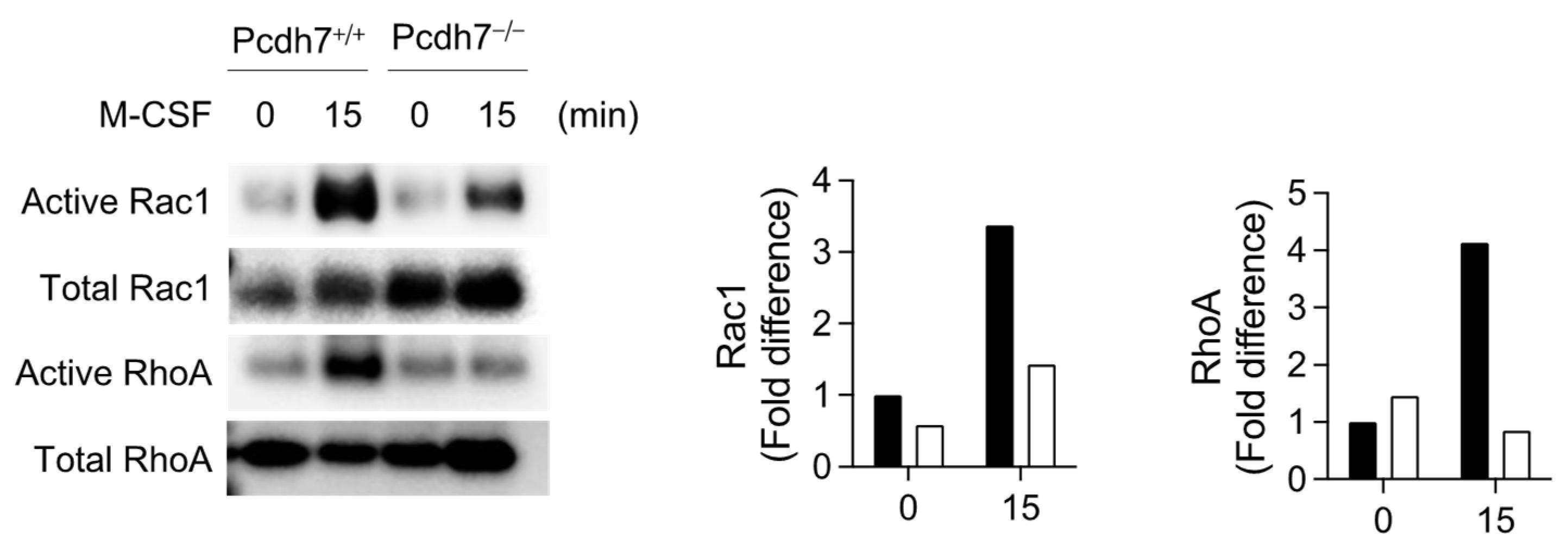
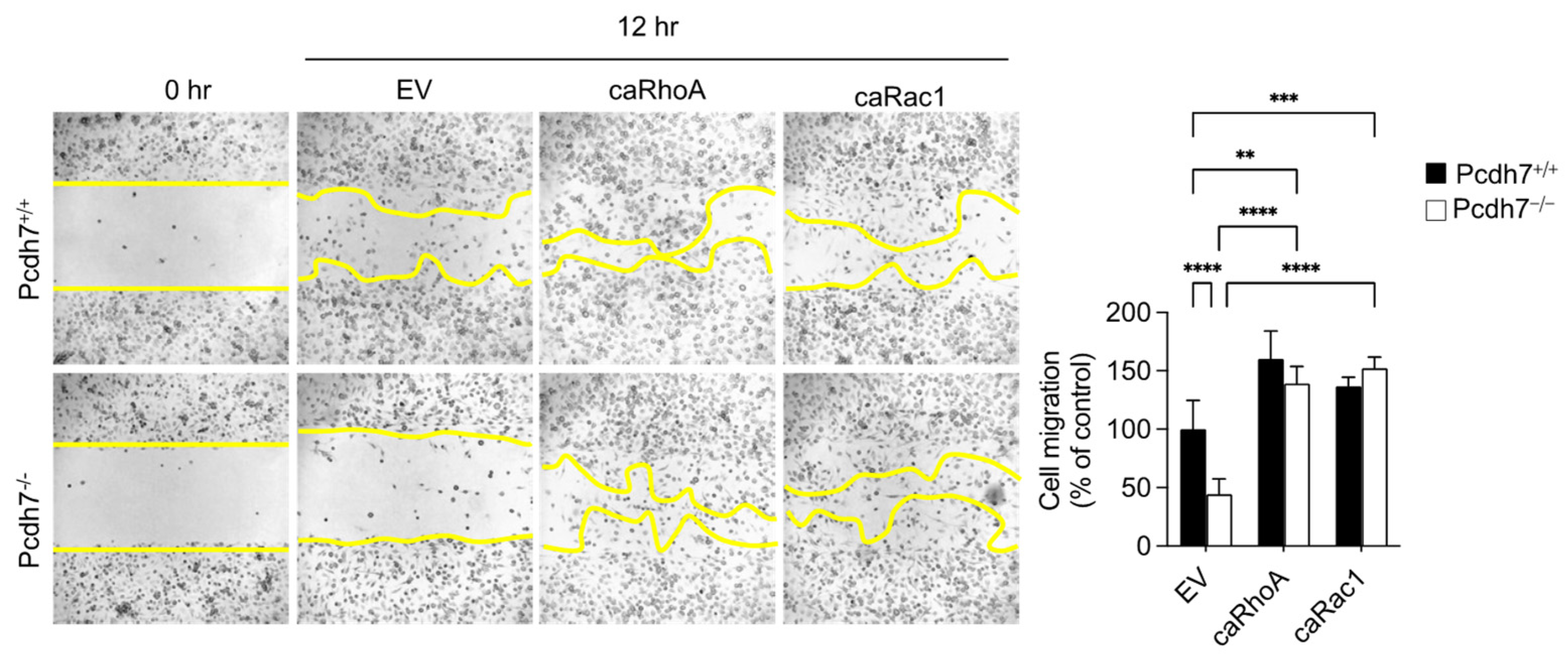
2.2. Pcdh7-Mediated Activation of RhoA and Rac1 Is Required for Monocyte Motility
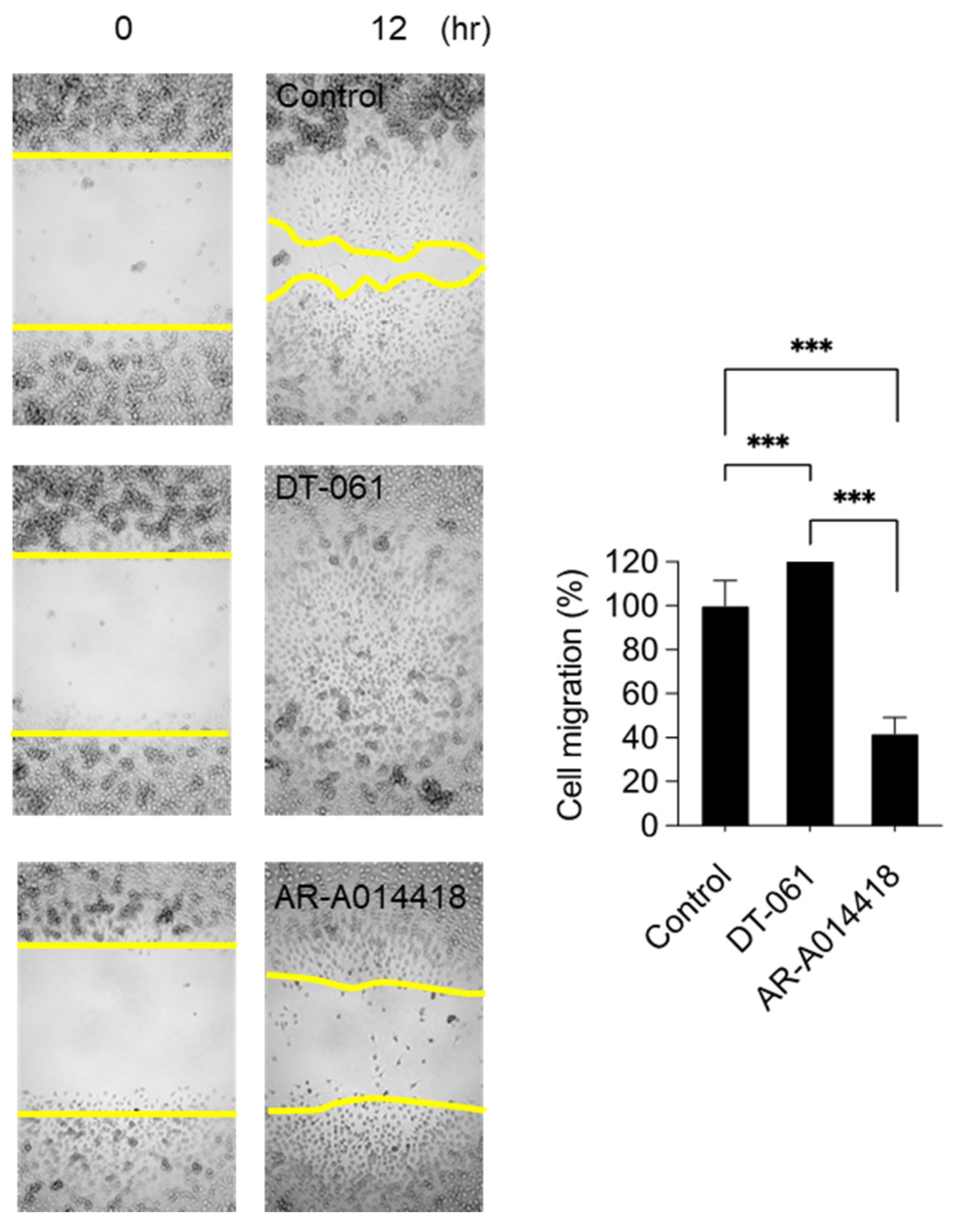
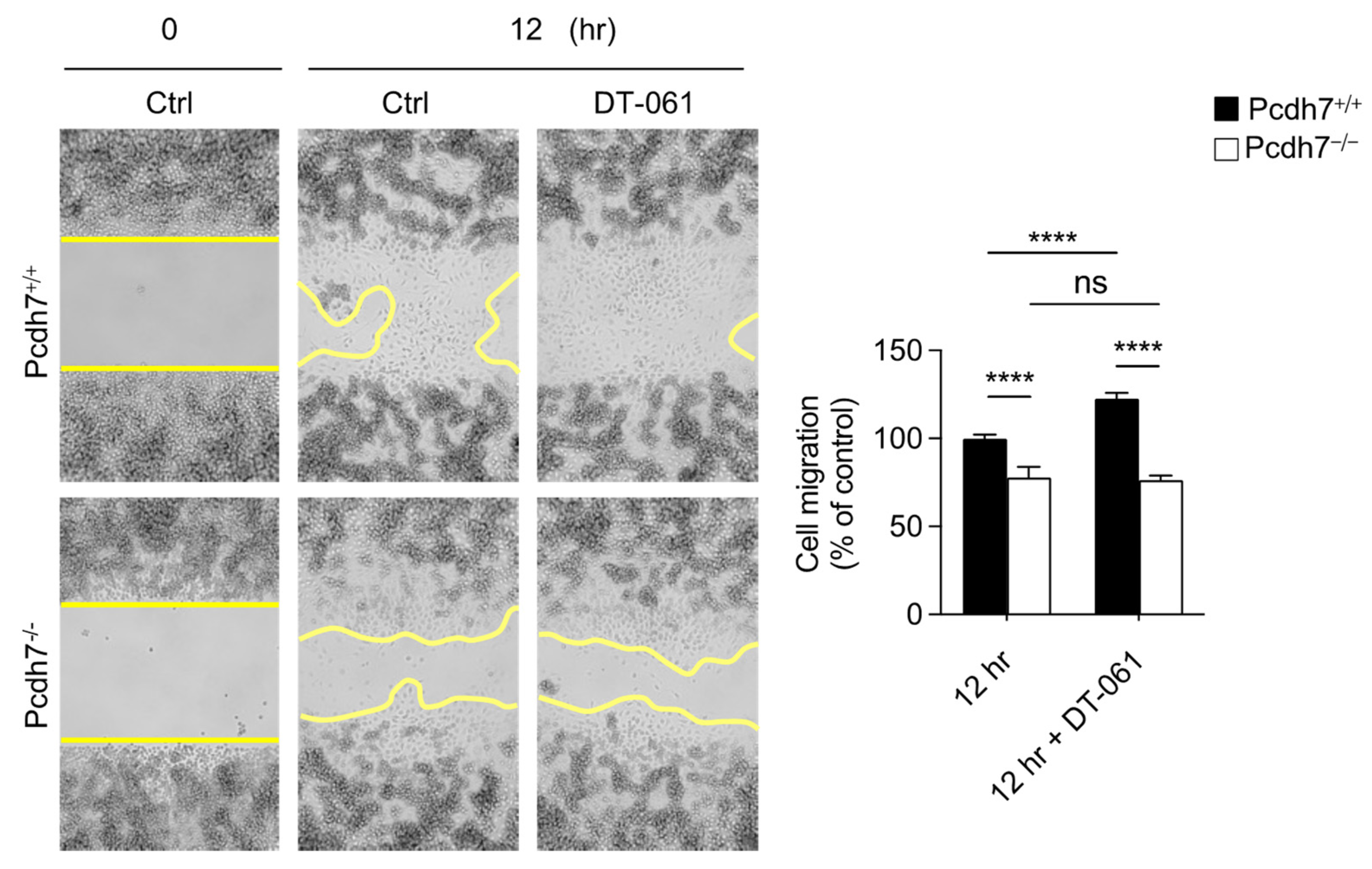
2.3. Involvement of PP2A and GSK3β in Monocyte Migration
3. Discussion
4. Materials and Methods
4.1. In Vitro Cell Culture
4.2. Retrovirus Preparation and Transduction
4.3. Migration Assay
4.4. Active GTPase and Western Blotting
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayashi, S.; Takeichi, M. Emerging roles of protocadherins: From self-avoidance to enhancement of motility. J. Cell Sci. 2015, 128, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Bassani, S.; Passafaro, M. Right Place at the Right Time: How Changes in Protocadherins Affect Synaptic Connections Contributing to the Etiology of Neurodevelopmental Disorders. Cells 2020, 9, 2711. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yoshitomo-Nakagawa, K.; Seki, N.; Sasaki, M.; Sugano, S. Cloning, expression analylsis, and chromosomal localization of BH-protocadherin (PCDH7), a novel member of the cadherin superfamily. Genomics 1998, 49, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yasuda, S.; Tanaka, H.; Yamagata, K.; Kim, H. Non-clustered protocadherin. Cell Adh. Migr. 2011, 5, 97–105. [Google Scholar] [CrossRef]
- Peek, S.L.; Mah, K.M.; Weiner, J.A. Regulation of neural circuit formation by protocadherins. Cell. Mol. Life Sci. CMLS 2017, 74, 4133–4157. [Google Scholar] [CrossRef]
- Yoshida, K. Fibroblast cell shape and adhesion in vitro is altered by overexpression of the 7a and 7b isoforms of protocadherin 7, but not the 7c isoform. Cell. Mol. Biol. Lett. 2003, 8, 735–741. [Google Scholar]
- Heggem, M.; Bradley, R. The cytoplasmic domain of Xenopus NF-protocadherin interacts with TAF1/set. Dev. Cell 2003, 4, 419–429. [Google Scholar] [CrossRef]
- Rashid, D.; Newell, K.; Shama, L.; Bradley, R. A requirement for NF-protocadherin and TAF1/set in cell adhesion and neural tube formation. Dev. Biol. 2006, 291, 170–181. [Google Scholar] [CrossRef][Green Version]
- Piper, M.; Dwivedy, A.; Leung, L.; Bradley, R.S.; Holt, C.E. NF-protocadherin and TAF1 regulate retinal axon initiation and elongation in vivo. J. Neurosci. 2008, 28, 100–105. [Google Scholar] [CrossRef]
- Leung, L.C.; Urbancic, V.; Baudet, M.L.; Dwivedy, A.; Bayley, T.G.; Lee, A.C.; Harris, W.A.; Holt, C.E. Coupling of NF-protocadherin signaling to axon guidance by cue-induced translation. Nat. Neurosci. 2013, 16, 166–173. [Google Scholar] [CrossRef]
- Zhou, X.; Padanad, M.S.; Evers, B.M.; Smith, B.; Novaresi, N.; Suresh, S.; Richardson, J.A.; Stein, E.; Zhu, J.; Hammer, R.E.; et al. Modulation of Mutant Kras(G12D) -Driven Lung Tumorigenesis In Vivo by Gain or Loss of PCDH7 Function. Mol. Cancer Res. 2019, 17, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Takegahara, N.; Walsh, M.C.; Ueda, J.; Fujihara, Y.; Ikawa, M.; Choi, Y. Protocadherin-7 contributes to maintenance of bone homeostasis through regulation of osteoclast multinucleation. Bmb Rep. 2020, 53, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Takegahara, N.; Choi, Y. Protocadherin-7 Regulates Osteoclast Differentiation through Intracellular SET-Binding Domain-Mediated RhoA and Rac1 Activation. Int. J. Mol. Sci. 2021, 22, 13117. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Takegahara, N.; Choi, Y. PP2A-Mediated GSK3β Dephosphorylation Is Required for Protocadherin-7-Dependent Regulation of Small GTPase RhoA in Osteoclasts. Cells 2023, 12, 1967. [Google Scholar] [CrossRef]
- Perrotti, D.; Neviani, P. Protein phosphatase 2A: A target for anticancer therapy. Lancet Oncol. 2013, 14, e229–e238. [Google Scholar] [CrossRef]
- Elgendy, M.; Ciro, M.; Hosseini, A.; Weiszmann, J.; Mazzarella, L.; Ferrari, E.; Cazzoli, R.; Curigliano, G.; DeCensi, A.; Bonanni, B.; et al. Combination of Hypoglycemia and Metformin Impairs Tumor Metabolic Plasticity and Growth by Modulating the PP2A-GSK3beta-MCL-1 Axis. Cancer Cell 2019, 35, 798–815 e5. [Google Scholar] [CrossRef]
- Bennecib, M.; Gong, C.X.; Grundke-Iqbal, I.; Iqbal, K. Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5, and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 2000, 485, 87–93. [Google Scholar] [CrossRef]
- Mitra, A.; Menezes, M.E.; Pannell, L.K.; Mulekar, M.S.; Honkanen, R.E.; Shevde, L.A.; Samant, R.S. DNAJB6 chaperones PP2A mediated dephosphorylation of GSK3beta to downregulate beta-catenin transcription target, osteopontin. Oncogene 2012, 31, 4472–4483. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Reviews. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Burridge, K.; Wennerberg, K. Rho and Rac Take Center Stage. Cell 2004, 116, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Parri, M.; Chiarugi, P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 2010, 8, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell Migration: Integrating Signals from Front to Back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Biro, M.; Munoz, M.A.; Weninger, W. Targeting Rho-GTPases in immune cell migration and inflammation. Br. J. Pharmacol. 2014, 171, 5491–5506. [Google Scholar] [CrossRef]
- Liu, Y.; Tejpal, N.; You, J.; Li, X.C.; Ghobrial, R.M.; Kloc, M. ROCK inhibition impedes macrophage polarity and functions. Cell Immunol. 2016, 300, 54–62. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Wu, C.; Minze, L.J.; Kubiak, J.Z.; Li, X.C.; Kloc, M.; Ghobrial, R.M. Macrophage/monocyte-specific deletion of Ras homolog gene family member A (RhoA) downregulates fractalkine receptor and inhibits chronic rejection of mouse cardiac allografts. J. Heart Lung Transpl. 2017, 36, 340–354. [Google Scholar] [CrossRef]
- Xu, J.; Wen, J.; Fu, L.; Liao, L.; Zou, Y.; Zhang, J.; Deng, J.; Zhang, H.; Liu, J.; Wang, X.; et al. Macrophage-specific RhoA knockout delays Wallerian degeneration after peripheral nerve injury in mice. J. Neuroinflammation 2021, 18, 234. [Google Scholar] [CrossRef]
- Lawson, C.D.; Burridge, K. The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases 2014, 5, e27958. [Google Scholar] [CrossRef]
- Edwin, F.; Anderson, K.; Ying, C.; Patel, T.B. Intermolecular interactions of Sprouty proteins and their implications in development and disease. Mol. Pharmacol. 2009, 76, 679–691. [Google Scholar] [CrossRef]
- Kong, M.; Bui, T.V.; Ditsworth, D.; Gruber, J.J.; Goncharov, D.; Krymskaya, V.P.; Lindsten, T.; Thompson, C.B. The PP2A-associated protein alpha4 plays a critical role in the regulation of cell spreading and migration. J. Biol. Chem. 2007, 282, 29712–29720. [Google Scholar] [CrossRef]
- Gilan, O.; Diesch, J.; Amalia, M.; Jastrzebski, K.; Chueh, A.C.; Verrills, N.M.; Pearson, R.B.; Mariadason, J.M.; Tulchinsky, E.; Hannan, R.D.; et al. PR55alpha-containing protein phosphatase 2A complexes promote cancer cell migration and invasion through regulation of AP-1 transcriptional activity. Oncogene 2015, 34, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Okamura, H.; Morimoto, H.; Teramachi, J.; Haneji, T. Protein phosphatase 2A Calpha regulates proliferation, migration, and metastasis of osteosarcoma cells. Lab. Investig. 2016, 96, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, A.; Vinci, M.; Verbinnen, I.; Treccarichi, S.; Nigliato, E.; Chiavetta, V.; Greco, D.; Vitello, G.A.; Federico, C.; Janssens, V.; et al. PPP2R5E: New gene potentially involved in specific learning disorders and myopathy. Gene 2025, 933, 148945. [Google Scholar] [CrossRef] [PubMed]
- Serguienko, A.; Hanes, R.; Grad, I.; Wang, M.Y.; Myklebost, O.; Munthe, E. PP2A Regulatory Subunit B55gamma is a Gatekeeper of Osteoblast Maturation and Lineage Maintenance. Stem Cells Dev. 2017, 26, 1375–1383. [Google Scholar] [CrossRef]
- Okamura, H.; Yoshida, K.; Morimoto, H.; Teramachi, J.; Ochiai, K.; Haneji, T.; Yamamoto, A. Role of Protein Phosphatase 2A in Osteoblast Differentiation and Function. J. Clin. Med. 2017, 6, 23. [Google Scholar] [CrossRef]
- Qureshi, M.H.; Bayraktar, H.; Cinko, M.T.; Akkaya, C.; Kamacioglu, A.; Uretmen-Kagiali, Z.C.; Bozluolcay, E.; Ozlu, N. PCDH7 Promotes Cell Migration by Regulating Myosin Activity. bioRxiv 2022. [Google Scholar] [CrossRef]
- Shishodia, G.; Koul, S.; Koul, H.K. Protocadherin 7 is overexpressed in castration resistant prostate cancer and promotes aberrant MEK and AKT signaling. Prostate 2019, 79, 1739–1751. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Guan, X.; Guan, X.; Dong, C.; Jiao, Z. Rho GTPases and related signaling complexes in cell migration and invasion. Exp. Cell Res. 2020, 388, 111824. [Google Scholar] [CrossRef]
- Dipankar, P.; Kumar, P.; Dash, S.P.; Sarangi, P.P. Functional and Therapeutic Relevance of Rho GTPases in Innate Immune Cell Migration and Function during Inflammation: An In Silico Perspective. Mediat. Inflamm. 2021, 2021, 6655412. [Google Scholar] [CrossRef]
- Prudent, J.; Popgeorgiev, N.; Gadet, R.; Deygas, M.; Rimokh, R.; Gillet, G. Mitochondrial Ca(2+) uptake controls actin cytoskeleton dynamics during cell migration. Sci. Rep. 2016, 6, 36570. [Google Scholar] [CrossRef] [PubMed]
- Romero-Garcia, S.; Prado-Garcia, H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer (Review). Int. J. Oncol. 2019, 54, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, L. Calcium ions promote migrasome formation via Synaptotagmin-1. J. Cell Biol. 2024, 223, e202402060. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.M.; Ku, R.Y.; Hashimoto-Torii, K. Prenatal Environment That Affects Neuronal Migration. Front. Cell Dev. Biol. 2019, 7, 138. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Smith, I.M.; Banerjee, S.; Moses, A.K.; Stroka, K.M. Aquaporin-mediated dysregulation of cell migration in disease states. Cell. Mol. Life Sci. CMLS 2023, 80, 48. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Takegahara, N.; Choi, Y. Protocadherin-7 Regulates Monocyte Migration Through Regulation of Small GTPase RhoA and Rac1. Int. J. Mol. Sci. 2025, 26, 572. https://doi.org/10.3390/ijms26020572
Kim H, Takegahara N, Choi Y. Protocadherin-7 Regulates Monocyte Migration Through Regulation of Small GTPase RhoA and Rac1. International Journal of Molecular Sciences. 2025; 26(2):572. https://doi.org/10.3390/ijms26020572
Chicago/Turabian StyleKim, Hyunsoo, Noriko Takegahara, and Yongwon Choi. 2025. "Protocadherin-7 Regulates Monocyte Migration Through Regulation of Small GTPase RhoA and Rac1" International Journal of Molecular Sciences 26, no. 2: 572. https://doi.org/10.3390/ijms26020572
APA StyleKim, H., Takegahara, N., & Choi, Y. (2025). Protocadherin-7 Regulates Monocyte Migration Through Regulation of Small GTPase RhoA and Rac1. International Journal of Molecular Sciences, 26(2), 572. https://doi.org/10.3390/ijms26020572






