The Mechanism of Modulation of Cardiac Force by Temperature
Abstract
1. Introduction
2. Results
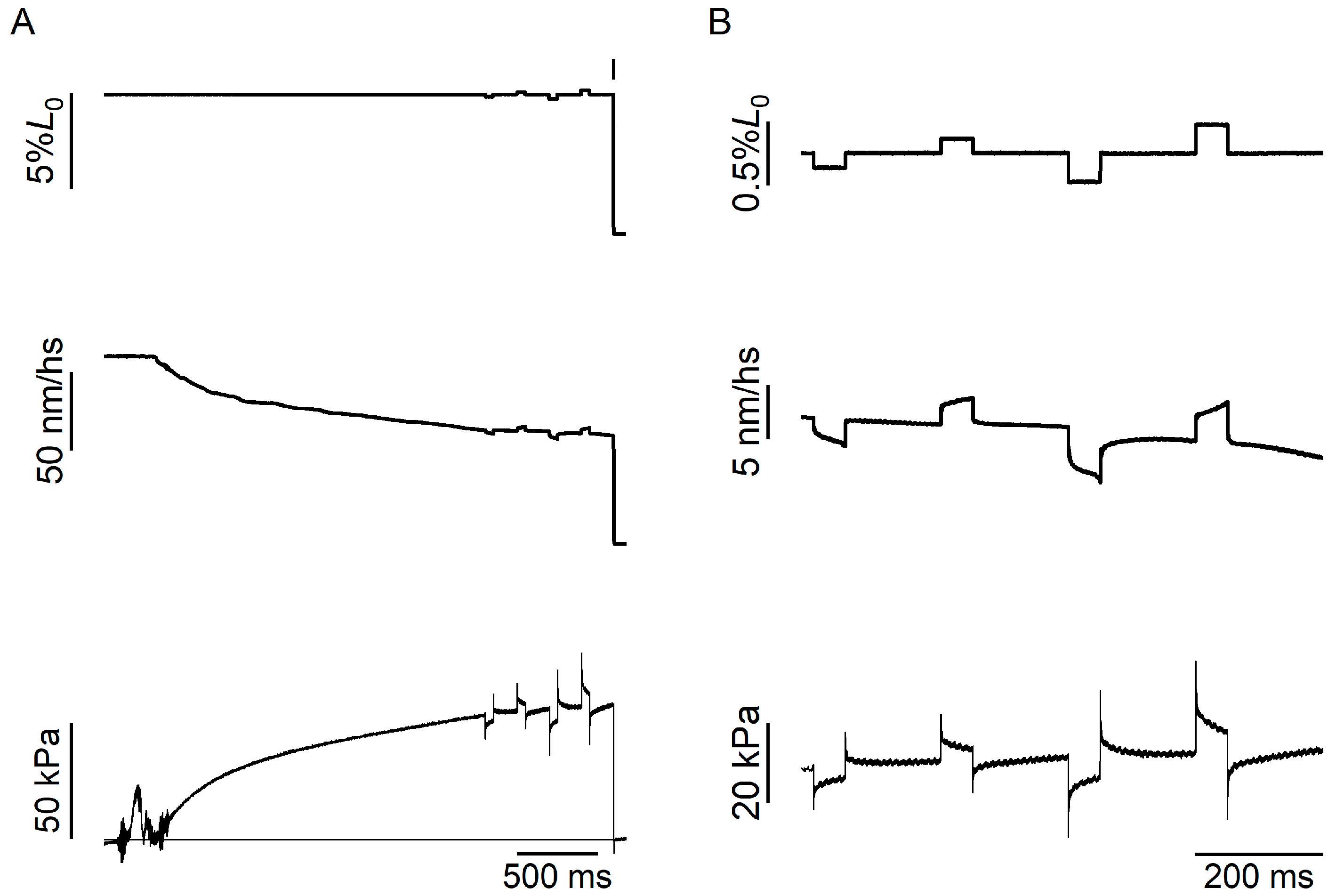
2.1. Temperature Dependence of Force and Stiffness of the Half-Sarcomere in the Maximally Activated Demembranated Trabecula
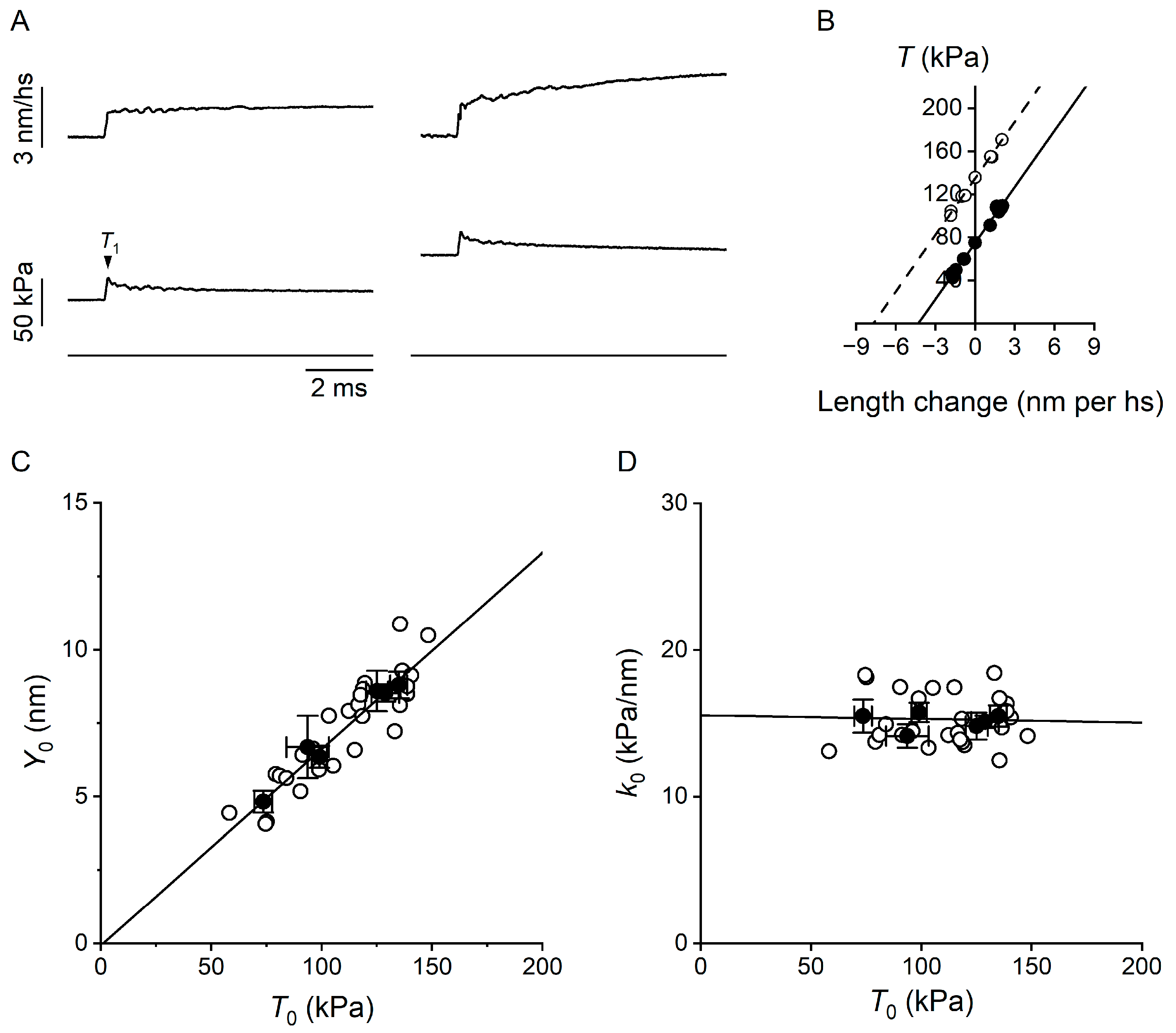
2.2. Estimate of the Temperature Dependence of the Mechanical Parameters of the Motor Array Through the Half-Sarcomere Compliance Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Ethical Approval
4.2. Sample Preparation
4.3. Experimental Protocols
4.4. Solutions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordon, A.M.; Homsher, E.; Regnier, M. Regulation of Contraction in Striated Muscle. Physiol. Rev. 2000, 80, 853–924. [Google Scholar] [CrossRef]
- Woodhead, J.L.; Zhao, F.-Q.; Craig, R.; Egelman, E.H.; Alamo, L.; Padrón, R. Atomic Model of a Myosin Filament in the Relaxed State. Nature 2005, 436, 1195–1199. [Google Scholar] [CrossRef]
- Wendt, T.; Taylor, D.; Trybus, K.M.; Taylor, K. Three-Dimensional Image Reconstruction of Dephosphorylated Smooth Muscle Heavy Meromyosin Reveals Asymmetry in the Interaction between Myosin Heads and Placement of Subfragment 2. Proc. Natl. Acad. Sci. USA 2001, 98, 4361–4366. [Google Scholar] [CrossRef] [PubMed]
- Alamo, L.; Wriggers, W.; Pinto, A.; Bártoli, F.; Salazar, L.; Zhao, F.-Q.; Craig, R.; Padrón, R. Three-Dimensional Reconstruction of Tarantula Myosin Filaments Suggests How Phosphorylation May Regulate Myosin Activity. J. Mol. Biol. 2008, 384, 780–797. [Google Scholar] [CrossRef]
- Stewart, M.A.; Franks-Skiba, K.; Chen, S.; Cooke, R. Myosin Atp Turnover Rate Is a Mechanism Involved in Thermogenesis in Resting Skeletal Muscle Fibers. Proc. Natl. Acad. Sci. USA 2010, 107, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R. The Role of the Myosin Atpase Activity in Adaptive Thermogenesis by Skeletal Muscle. Biophys. Rev. 2011, 3, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Linari, M.; Brunello, E.; Reconditi, M.; Fusi, L.; Caremani, M.; Narayanan, T.; Piazzesi, G.; Lombardi, V.; Irving, M. Force Generation by Skeletal Muscle Is Controlled by Mechanosensing in Myosin Filaments. Nature 2015, 528, 276–279. [Google Scholar] [CrossRef]
- Reconditi, M.; Caremani, M.; Pinzauti, F.; Powers, J.D.; Narayanan, T.; Stienen, G.J.; Linari, M.; Lombardi, V.; Piazzesi, G. Myosin Filament Activation in the Heart Is Tuned to the Mechanical Task. Proc. Natl. Acad. Sci. USA 2017, 114, 3240–3245. [Google Scholar] [CrossRef] [PubMed]
- Brunello, E.; Fusi, L.; Ghisleni, A.; Park-Holohan, S.J.; Ovejero, J.G.; Narayanan, T.; Irving, M. Myosin Filament-Based Regulation of the Dynamics of Contraction in Heart Muscle. Proc. Natl. Acad. Sci. USA 2020, 117, 8177–8186. [Google Scholar] [CrossRef] [PubMed]
- Morotti, I.; Caremani, M.; Marcello, M.; Pertici, I.; Squarci, C.; Bianco, P.; Narayanan, T.; Piazzesi, G.; Reconditi, M.; Lombardi, V.; et al. An Integrated Picture of the Structural Pathways Controlling the Heart Performance. Proc. Natl. Acad. Sci. USA 2024, 121, e2410893121. [Google Scholar] [CrossRef]
- Piazzesi, G.; Reconditi, M.; Koubassova, N.; Decostre, V.; Linari, M.; Lucii, L.; Lombardi, V. Temperature Dependence of the Force-Generating Process in Single Fibres from Frog Skeletal Muscle. J. Physiol. 2003, 549 Pt 1, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Decostre, V.; Bianco, P.; Lombardi, V.; Piazzesi, G. Effect of Temperature on the Working Stroke of Muscle Myosin. Proc. Natl. Acad. Sci. USA 2005, 102, 13927–13932. [Google Scholar] [CrossRef]
- Linari, M.; Brunello, E.; Reconditi, M.; Sun, Y.; Panine, P.; Narayanan, T.; Piazzesi, G.; Lombardi, V.; Irving, M. The Structural Basis of the Increase in Isometric Force Production with Temperature in Frog Skeletal Muscle. J. Physiol. 2005, 567 Pt 2, 459–469. [Google Scholar] [CrossRef]
- Caremani, M.; Brunello, E.; Linari, M.; Fusi, L.; Irving, T.C.; Gore, D.; Piazzesi, G.; Irving, M.; Lombardi, V.; Reconditi, M. Low Temperature Traps Myosin Motors of Mammalian Muscle in a Refractory State That Prevents Activation. J. Gen. Physiol. 2019, 151, 1272–1286. [Google Scholar] [CrossRef]
- Allen, D.; Kentish, J. The Cellular Basis of the Length-Tension Relation in Cardiac Muscle. J. Mol. Cell. Cardiol. 1985, 17, 821–840. [Google Scholar] [CrossRef] [PubMed]
- ter Keurs, H.E. The Interaction of Ca2+ with Sarcomeric Proteins: Role in Function and Dysfunction of the Heart. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H38–H50. [Google Scholar] [CrossRef] [PubMed]
- Ovejero, J.G.; Fusi, L.; Park-Holohan, S.-J.; Ghisleni, A.; Narayanan, T.; Irving, M.; Brunello, E. Cooling Intact and Demembranated Trabeculae from Rat Heart Releases Myosin Motors from Their Inhibited Conformation. J. Gen. Physiol. 2022, 154, e202113029. [Google Scholar] [CrossRef] [PubMed]
- Dibb, K.M.; Eisner, D.A.; Trafford, A.W. Regulation of Systolic [Ca2+]I and Cellular Ca2+ Flux Balance in Rat Ventricular Myocytes by Sr Ca2+, L-Type Ca2+ Current and Diastolic [Ca2+]I. J. Physiol. 2007, 585 Pt 2, 579–592. [Google Scholar] [CrossRef]
- Eisner, D. Calcium in the Heart: From Physiology to Disease. Exp. Physiol. 2014, 99, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- de Tombe, P.P.; Keurs, H.E.T. Force and Velocity of Sarcomere Shortening in Trabeculae from Rat Heart. Effects of Temperature. Circ. Res. 1990, 66, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.M.L.; Stull, L.B.; Marbán, E. Myofilament Properties Comprise the Rate-Limiting Step for Cardiac Relaxation at Body Temperature in the Rat. Am. J. Physiol. Circ. Physiol. 2002, 282, H499–H507. [Google Scholar] [CrossRef]
- Harrison, S.M.; Bers, D.M. Influence of Temperature on the Calcium Sensitivity of the Myofilaments of Skinned Ventricular Muscle from the Rabbit. J. Gen. Physiol. 1989, 93, 411–428. [Google Scholar] [CrossRef] [PubMed]
- de Tombe, P.P.; Stienen, G.J. Impact of Temperature on Cross-Bridge Cycling Kinetics in Rat Myocardium. J. Physiol. 2007, 584 Pt 2, 591–600. [Google Scholar] [CrossRef]
- Huxley, A. Muscle Structure and Theories of Contraction. Prog. Biophys. Biophys. Chem. 1957, 7, 255–318. [Google Scholar] [CrossRef] [PubMed]
- Fusi, L.; Brunello, E.; Reconditi, M.; Piazzesi, G.; Lombardi, V. The Non-Linear Elasticity of the Muscle Sarcomere and the Compliance of Myosin Motors. J. Physiol. 2014, 592, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Geeves, M.A.; Holmes, K.C. Structural Mechanism of Muscle Contraction. Annu. Rev. Biochem. 1999, 68, 687–728. [Google Scholar] [CrossRef] [PubMed]
- Huxley, A.F.; Simmons, R.M. Proposed Mechanism of Force Generation in Striated Muscle. Nature 1971, 233, 533–538. [Google Scholar] [CrossRef]
- Ford, L.E.; Huxley, A.F.; Simmons, R.M. Tension Responses to Sudden Length Change in Stimulated Frog Muscle Fibres near Slack Length. J. Physiol. 1977, 269, 441–515. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.E.; Huxley, A.F.; Simmons, R.M. The Relation between Stiffness and Filament Overlap in Stimulated Frog Muscle Fibres. J. Physiol. 1981, 311, 219–249. [Google Scholar] [CrossRef] [PubMed]
- Brunello, E.; Bianco, P.; Piazzesi, G.; Linari, M.; Reconditi, M.; Panine, P.; Narayanan, T.; Helsby, W.; Irving, M.; Lombardi, V. Structural Changes in the Myosin Filament and Cross-Bridges During Active Force Development in Single Intact Frog Muscle Fibres: Stiffness and X-Ray Diffraction Measurements. J. Physiol. 2006, 577 Pt 3, 971–984. [Google Scholar] [CrossRef]
- Colombini, B.; Nocella, M.; Bagni, M.A.; Griffiths, P.J.; Cecchi, G. Is the Cross-Bridge Stiffness Proportional to Tension During Muscle Fiber Activation? Biophys. J. 2010, 98, 2582–2590. [Google Scholar] [CrossRef][Green Version]
- Linari, M.; Piazzesi, G.; Pertici, I.; Dantzig, J.A.; Goldman, Y.E.; Lombardi, V. Straightening Out the Elasticity of Myosin Cross-Bridges. Biophys. J. 2020, 118, 994–1002. [Google Scholar] [CrossRef]
- Pertici, I.; Taft, M.H.; Greve, J.N.; Fedorov, R.; Caremani, M.; Manstein, D.J. Allosteric Modulation of Cardiac Myosin Mechanics and Kinetics by the Conjugated Omega-7,9 Trans-Fat Rumenic Acid. J. Physiol. 2021, 599, 3639–3661. [Google Scholar] [CrossRef] [PubMed]
- Pinzauti, F.; Pertici, I.; Reconditi, M.; Narayanan, T.; Stienen, G.J.M.; Piazzesi, G.; Lombardi, V.; Linari, M.; Caremani, M. The Force and Stiffness of Myosin Motors in the Isometric Twitch of a Cardiac Trabecula and the Effect of the Extracellular Calcium Concentration. J. Physiol. 2018, 596, 2581–2596. [Google Scholar] [CrossRef]
- Janssen, P.M.; de Tombe, P.P. Uncontrolled Sarcomere Shortening Increases Intracellular Ca2+ Transient in Rat Cardiac Trabeculae. Am. J. Physiol. Circ. Physiol. 1997, 272 Pt 2, H1892–H1897. [Google Scholar] [CrossRef] [PubMed]
- Irving, M. Functional Control of Myosin Motors in the Cardiac Cycle. Nat. Rev. Cardiol. 2024, 22, 9–19. [Google Scholar] [CrossRef]
- Goldman, Y.E.; McCray, J.A.; Ranatunga, K.W. Transient Tension Changes Initiated by Laser Temperature Jumps in Rabbit Psoas Muscle Fibres. J. Physiol. 1987, 392, 71–95. [Google Scholar] [CrossRef]
- Coupland, M.E.; Puchert, E.; Ranatunga, K.W. Temperature Dependence of Active Tension in Mammalian (Rabbit Psoas) Muscle Fibres: Effect of Inorganic Phosphate. J. Physiol. 2001, 536 Pt 3, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Bershitsky, S.Y.; Tsaturyan, A.K. The Elementary Force Generation Process Probed by Temperature and Length Perturbations in Muscle Fibres from the Rabbit. J. Physiol. 2002, 540 Pt 3, 971–988. [Google Scholar] [CrossRef]
- Linari, M.; Caremani, M.; Piperio, C.; Brandt, P.; Lombardi, V. Stiffness and Fraction of Myosin Motors Responsible for Active Force in Permeabilized Muscle Fibers from Rabbit Psoas. Biophys. J. 2007, 92, 2476–2490. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kawai, M. Kinetic and Thermodynamic Studies of the Cross-Bridge Cycle in Rabbit Psoas Muscle Fibers. Biophys. J. 1994, 67, 1655–1668. [Google Scholar] [CrossRef]
- Kawai, M. What Do We Learn by Studying the Temperature Effect on Isometric Tension and Tension Transients in Mammalian Striated Muscle Fibres? J. Muscle Res. Cell Motil. 2003, 24, 127–138. [Google Scholar] [CrossRef]
- Rayment, I.; Holden, H.; Whittaker, M.; Yohn, C.; Lorenz, M.; Holmes, K.; Milligan, R. Structure of the Actin-Myosin Complex and Its Implications for Muscle Contraction. Science 1993, 261, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.P.; Zhao, Y.; Kawai, M. Molecular Forces Involved in Force Generation During Skeletal Muscle Contraction. J. Exp. Biol. 1996, 199 Pt 12, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Reconditi, M.; Linari, M.; Lucii, L.; Stewart, A.; Sun, Y.-B.; Boesecke, P.; Narayanan, T.; Fischetti, R.F.; Irving, T.; Piazzesi, G.; et al. The Myosin Motor in Muscle Generates a Smaller and Slower Working Stroke at Higher Load. Nature 2004, 428, 578–581. [Google Scholar] [CrossRef]
- Bershitsky, S.Y.; Tsaturyan, A.K. Tension Responses to Joule Temperature Jump in Skinned Rabbit Muscle Fibres. J. Physiol. 1992, 447, 425–448. [Google Scholar] [CrossRef]
- Davis, J.; Harrington, W. A Single Order-Disorder Transition Generates Tension During the Huxley-Simmons Phase 2 in Muscle. Biophys. J. 1993, 65, 1886–1898. [Google Scholar] [CrossRef] [PubMed]
- Ranatunga, K. Endothermic Force Generation in Fast and Slow Mammalian (Rabbit) Muscle Fibers. Biophys. J. 1996, 71, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Ranatunga, K.W. Endothermic Force Generation in Skinned Cardiac Muscle from Rat. J. Muscle Res. Cell Motil. 1999, 20, 489–496. [Google Scholar] [CrossRef]
- Coupland, M.E.; Ranatunga, K.W. Force Generation Induced by Rapid Temperature Jumps in Intact Mammalian (Rat) Skeletal Muscle Fibres. J. Physiol. 2003, 548 Pt 2, 439–449. [Google Scholar] [CrossRef]
- Davis, J.S.; Epstein, N.D. Kinetic Effects of Fiber Type on the Two Subcomponents of the Huxley-Simmons Phase 2 in Muscle. Biophys. J. 2003, 85, 390–401. [Google Scholar] [CrossRef]
- Ferenczi, M.A.; Bershitsky, S.Y.; Koubassova, N.; Siththanandan, V.; Helsby, W.I.; Panine, P.; Roessle, M.; Narayanan, T.; Tsaturyan, A.K. The “Roll and Lock” Mechanism of Force Generation in Muscle. Structure 2005, 13, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Offer, G.; Ranatunga, K.W. The Endothermic Atp Hydrolysis and Crossbridge Attachment Steps Drive the Increase of Force with Temperature in Isometric and Shortening Muscle. J. Physiol. 2015, 593, 1997–2016. [Google Scholar] [CrossRef]
- Piazzesi, G.; Lucii, L.; Lombardi, V. The Size and the Speed of the Working Stroke of Muscle Myosin and Its Dependence on the Force. J. Physiol. 2002, 545 Pt 1, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Piazzesi, G.; Dolfi, M.; Brunello, E.; Fusi, L.; Reconditi, M.; Bianco, P.; Linari, M.; Lombardi, V. The Myofilament Elasticity and Its Effect on Kinetics of Force Generation by the Myosin Motor. Arch. Biochem. Biophys. 2014, 552–553, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Huxley, A.F.; Lombardi, V.; Peachey, L.D. A System for Fast Recording of Longitudinal Displacement of a Striated Muscle Fibre. J. Physiol. 1981, 317, 12P–13P. [Google Scholar]
- Brandt, P.W.; Reuben, J.P.; Grundfest, H. Regulation of Tension in the Skinned Crayfish Muscle Fiber. J. Gen. Physiol. 1972, 59, 305–317. [Google Scholar] [CrossRef]
- Goldman, Y.E.; Hibberd, M.G.; Trentham, D.R. Relaxation of Rabbit Psoas Muscle Fibres from Rigor by Photochemical Generation of Adenosine-5′-Triphosphate. J. Physiol. 1984, 354, 577–604. [Google Scholar] [CrossRef]



| 10 °C | 15 °C | 20 °C | 25 °C | 30 °C | Average Q10 (10–25 °C) | |
|---|---|---|---|---|---|---|
| T0 (kPa) | 74 ± 4 | 99 ± 3 | 125 ± 5 | 135 ± 4 | 129 ± 6 | 1.5 |
| kTR (s−1) | 5.9 ± 0.8 | 8.3 ± 0.5 | 12.9 ± 1.1 | 18.8 ± 1.7 | 29.3 ± 2.8 | 2.2 |
| k0 (kPa nm−1) | 15.5 ± 1.1 | 15.7 ± 0.7 | 14.8 ± 0.9 | 15.5 ± 0.7 | 15.1 ± 0.4 |
| A | ||||||||
|---|---|---|---|---|---|---|---|---|
| Imidazole | MgCl2 | Na2ATP | EGTA | KP | PMSF | GSH | Gly | |
| Eastwood A | 10 | 2.5 | 2.5 | 5 | 170 | 0.2 | − | − |
| Eastwood B | 20 | 5 | 5 | 10 | 340 | − | 20 | 50 |
| B | ||||||||
| TES | MgCl2 | Na2ATP | EGTA | CaEGTA | Na2CP | GSH | HDTA | |
| Pre-Activating (5 °C) | 140 | 6.93 | 5.45 | 0.1 | − | 19.49 | 10 | 24.9 |
| Relaxing (10 °C) | 112 | 8.40 | 5.44 | 25 | − | 19.11 | 10 | − |
| Activating (10 °C) | 112 | 6.76 | 5.46 | − | 25 | 19.49 | 10 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morotti, I.; Marcello, M.; Sautariello, G.; Pertici, I.; Bianco, P.; Piazzesi, G.; Linari, M.; Lombardi, V.; Reconditi, M.; Caremani, M. The Mechanism of Modulation of Cardiac Force by Temperature. Int. J. Mol. Sci. 2025, 26, 469. https://doi.org/10.3390/ijms26020469
Morotti I, Marcello M, Sautariello G, Pertici I, Bianco P, Piazzesi G, Linari M, Lombardi V, Reconditi M, Caremani M. The Mechanism of Modulation of Cardiac Force by Temperature. International Journal of Molecular Sciences. 2025; 26(2):469. https://doi.org/10.3390/ijms26020469
Chicago/Turabian StyleMorotti, Ilaria, Matteo Marcello, Giulia Sautariello, Irene Pertici, Pasquale Bianco, Gabriella Piazzesi, Marco Linari, Vincenzo Lombardi, Massimo Reconditi, and Marco Caremani. 2025. "The Mechanism of Modulation of Cardiac Force by Temperature" International Journal of Molecular Sciences 26, no. 2: 469. https://doi.org/10.3390/ijms26020469
APA StyleMorotti, I., Marcello, M., Sautariello, G., Pertici, I., Bianco, P., Piazzesi, G., Linari, M., Lombardi, V., Reconditi, M., & Caremani, M. (2025). The Mechanism of Modulation of Cardiac Force by Temperature. International Journal of Molecular Sciences, 26(2), 469. https://doi.org/10.3390/ijms26020469








