Characteristics and Functions of PmHDS, a Terpenoid Synthesis-Related Gene in Pinus massoniana Lamb.
Abstract
1. Introduction
2. Results
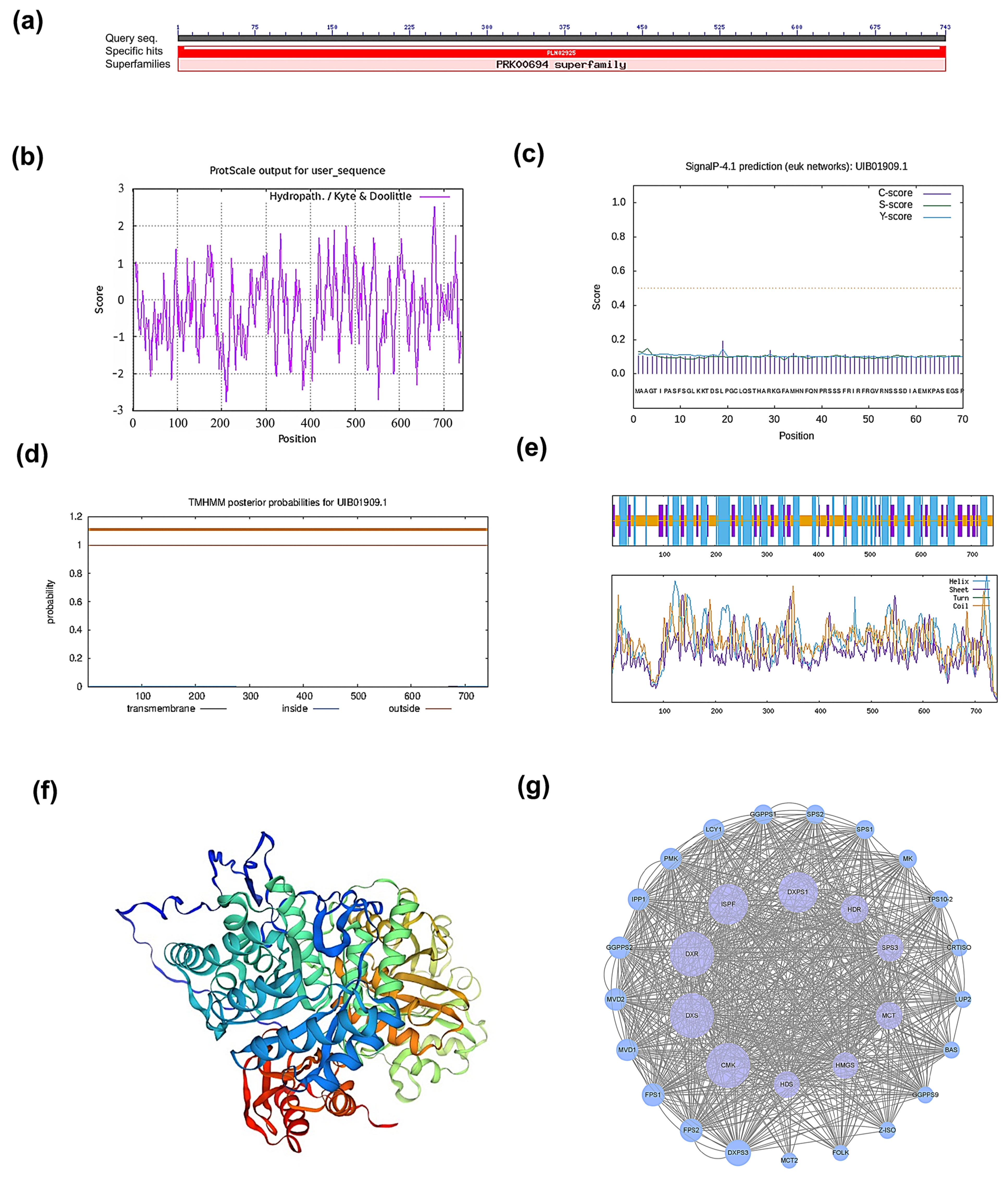
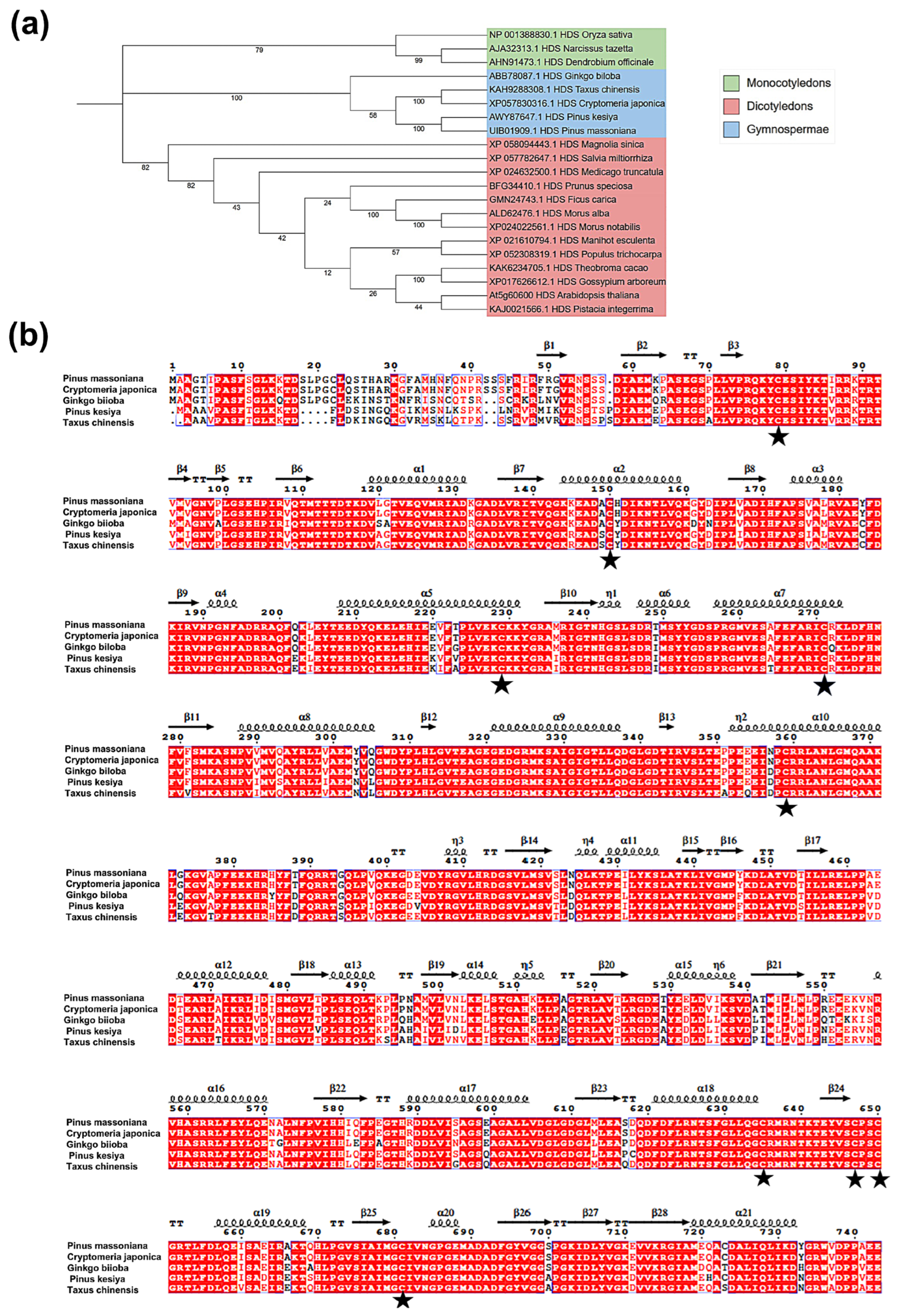
2.1. Cloning of PmHDS and Bioinformatics Analysis
2.2. Analysis of Tissue-Specific Expression Pattern of PmHDS
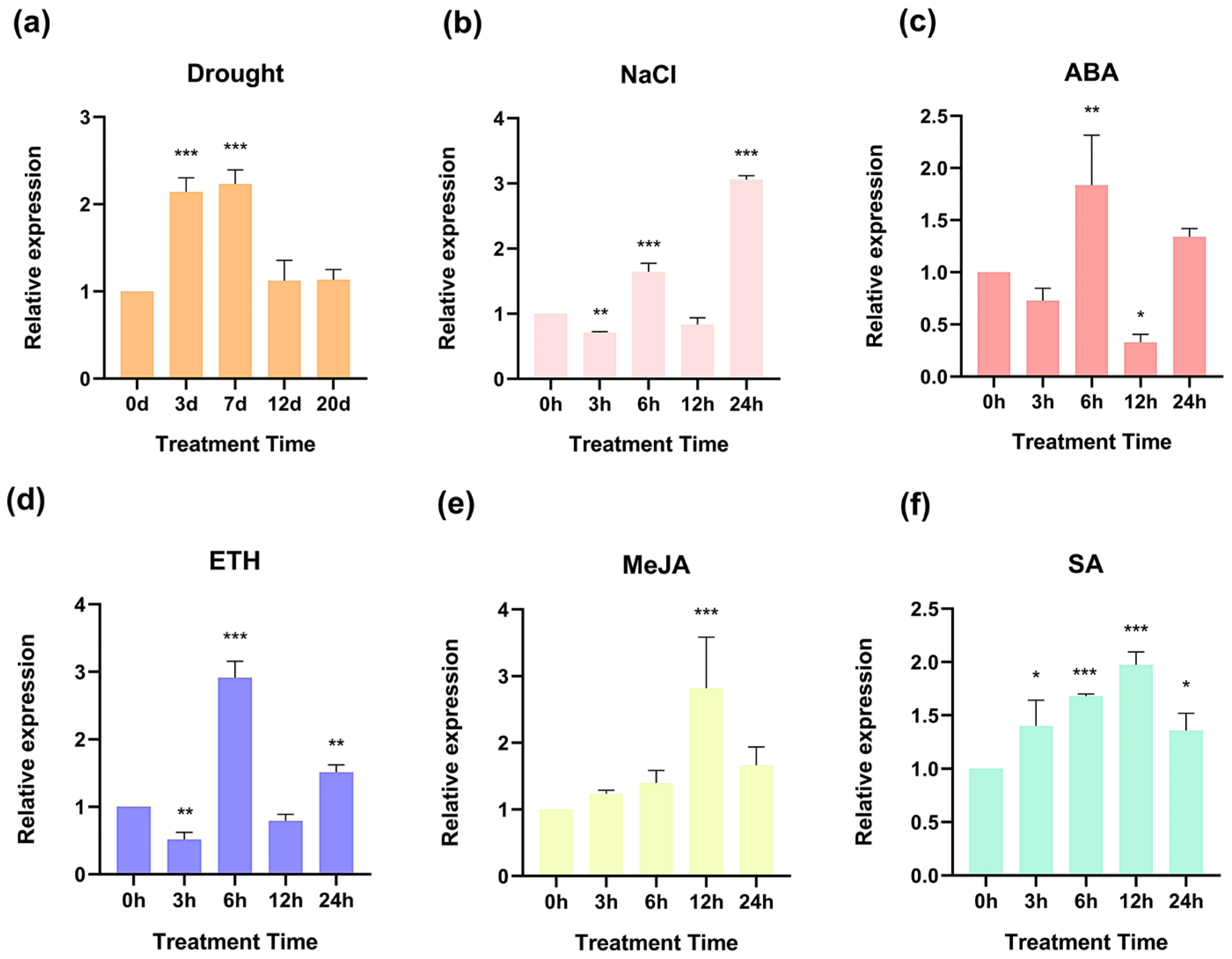
2.3. The Expression Levels of PmHDS in Response to Various Treatments
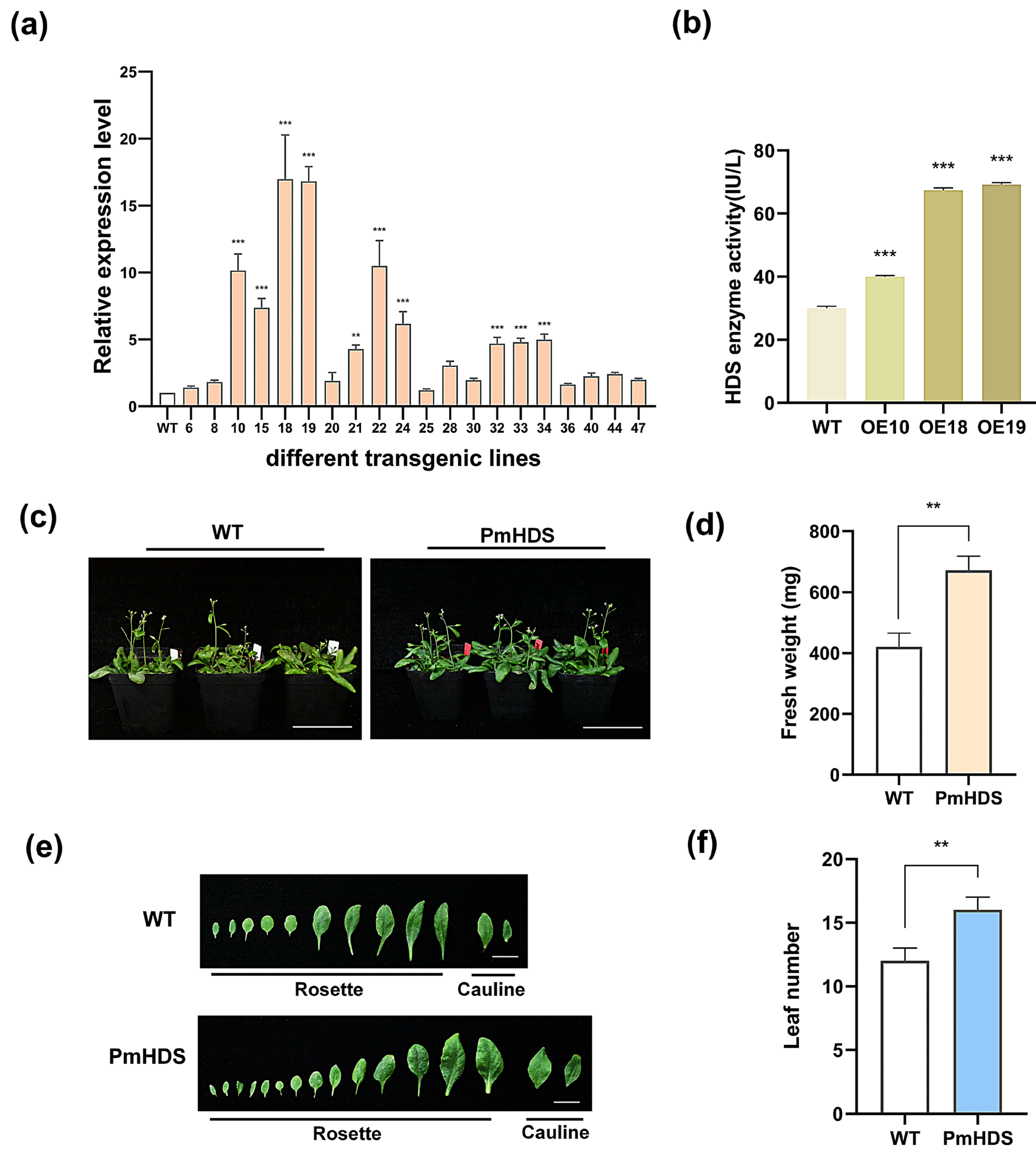
2.4. Identification, Expression Level Assessment, and Phenotypic Characterization of Transgenic Plants
2.5. Overexpression of PmHDS in Transgenic A. thaliana Enhances Growth Under Hormonal and Abiotic Stress Compared to Wild-Type Plants
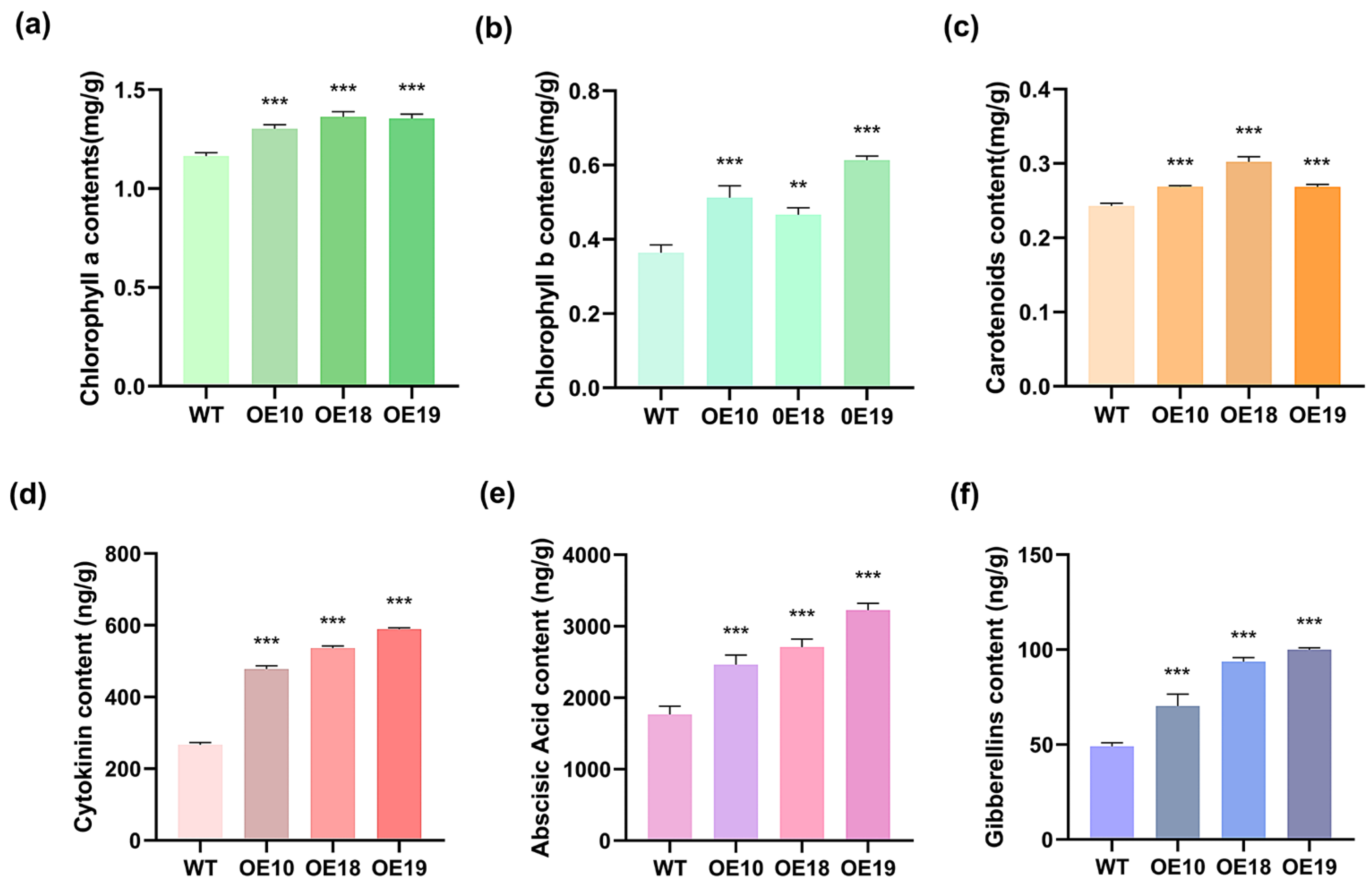
2.6. Overexpression of PmHDS Enhances Photosynthetic Pigment Content and Increases the Levels of Hormones Associated with Terpenoids’ Synthesis
2.7. Overexpression of PmHDS in A. thaliana Affects the Expression of Genes Related to Terpenoid Synthesis Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Cloning of PmHDS and Its Promoter
4.3. Bioinformatics Analysis of the PmHDS Protein Sequence
4.4. Non-Biological Stress and Hormone Treatment Experimental Materials
4.5. Real-Time Quantitative PCR Analysis
4.6. Determination of Photosynthetic Pigment Content and Terpenoid Synthesis Hormone Content
4.7. GUS Staining
4.8. Relative Expression Levels of Genes Related to Terpenoid Synthesis Pathways
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014, 79, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.M.; Wang, Q.; Webster, F.X.; Kiemle, D.; Hong, Y.J.; Tantillo, D.J.; Coates, R.M.; Wray, A.T.; Askew, W.; O’Donnell, C.; et al. Formation of the unusual semivolatile diterpene rhizathalene by the Arabidopsis class I terpene synthase TPS08 in the root stele is involved in defense against belowground herbivory. Plant Cell 2013, 25, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.E.; Williams, T.C.; Peng, B.; Cherry, J. Recent advances in synthetic biology for engineering isoprenoid production in yeast. Curr. Opin. Chem. Biol. 2017, 40, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, S.; Long, R.M.; Williams, R.M.; Croteau, R. Cytochrome p450 taxadiene 5alpha-hydroxylase, a mechanistically unusual monooxygenase catalyzing the first oxygenation step of taxol biosynthesis. Chem. Biol. 2004, 11, 379–387. [Google Scholar] [CrossRef]
- Teoh, K.H.; Polichuk, D.R.; Reed, D.W.; Nowak, G.; Covello, P.S. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 2006, 580, 1411–1416. [Google Scholar] [CrossRef]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krekling, T.; Christiansen, E. Application of methyl jasmonate on Picea abies (Pinaceae) stems induces defense-related responses in phloem and xylem. Am. J. Bot. 2002, 89, 578–586. [Google Scholar] [CrossRef]
- Sapir-Mir, M.; Mett, A.; Belausov, E.; Tal-Meshulam, S.; Frydman, A.; Gidoni, D.; Eyal, Y. Peroxisomal localization of Arabidopsis isopentenyl diphosphate isomerases suggests that part of the plant isoprenoid mevalonic acid pathway is compartmentalized to peroxisomes. Plant Physiol. 2008, 148, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.D.; Du, H.Y.; Du, L.Y.; Wu, Y.T.N.; Huang, H.Y. Cloning and sequence characterization of the full length cDNA for 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate synthase gene from Eucommia ulmoides Oliv. Bull. Bot. Res. 2012, 32, 444–451. [Google Scholar] [CrossRef]
- Ahn, C.S.; Pai, H.S. Physiological function of IspE, a plastid MEP pathway gene for isoprenoid biosynthesis, in organelle biogenesis and cell morphogenesis in Nicotiana benthamiana. Plant Mol. Biol. 2008, 66, 503–517. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Jiang, C.; Yuan, Y.; Chen, P. Cloning and bioinformatic analysis of IspE and IspH genes in Lonicera japonic and its substitutes. China J. Chin. Mater. Med. 2013, 38, 32–36. [Google Scholar]
- Liu, S.N. Comparison of HDS Genes and Functions in MEP Pathway among Different Plants. Bot. Res. 2020, 09, 387–391. [Google Scholar] [CrossRef]
- Liu, L.H.; Li, C.H.; Yang, J.L.; Xia, D.A. Bioinformatics analysis of the gene encoding 1-hydroxy-2-methyl-2-(E)-butenyl-4- diphosphate synthase (HDS) in Populus trichocarpa. J. Agric. Sci. Technol. 2012, 13, 24–28. [Google Scholar] [CrossRef]
- Li, A. Cloning, Characterization, and Expression Analysis of the HDS Gene from Huperzia serrata. Master’s Thesis, Liaoning Normal University, Dalian, China, 2016. [Google Scholar]
- Zheng, Y. Cloning of MCT, HDS, and SGD Genes from Rauvolfia verticillata (Lour.) Baill and Development of a Genetic Transformation System. Master’s Thesis, Southwest University, Chongqing, China, 2011. [Google Scholar]
- Jiang, Z.Z. Comprehensive Cloning, Functional Analysis, and Expression Profiling of HDS and HDR Genes in the MEP Pathway of Tea Tree. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2013. [Google Scholar]
- Hu, Z.H.; Yan, W.X.; Zhang, X.; Liu, S.J.; Leng, P.S. Cloning and expression analysis of the ‘Siberia’ lily LiHDS gene. Plant Physiol. J. 2023, 59, 362–372. [Google Scholar]
- Wang, X.; Wu, L.S.; Wu, Q.J.; Lin, Y.; Cai, Y.P.; Fan, H.H. Cloning and preliminary expression analysis of HDS gene in Dendrobium officinale Kimura & Migo. Chin. Herb. Med. 2016, 47, 803–809. [Google Scholar]
- Hemmerlin, A.; Harwood, J.L.; Bach, T.J. A raison d’être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lipid. Res. 2012, 51, 95–148. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.S.; Xu, L.A.; Wang, D.B.; Ni, Z.X.; Wang, Z.R. Progress and achievement of genetic improvement on Masson pine (Pinus massoniana Lamb.) in China. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2022, 46, 10–22. [Google Scholar]
- Zerbe, P.; Chiang, A.; Yuen, M.; Hamberger, B.; Hamberger, B.; Draper, J.A.; Britton, R.; Bohlmann, J. Bifunctional cis-Abienol Synthase from Abies balsamea Discovered by Transcriptome Sequencing and Its Implications for Diterpenoid Fragrance Production. J. Biol. Chem. 2012, 287, 12121–12131. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; He, X.; Chen, J.; Gu, T.; Liu, P.; Xu, T.; Teale, S.A.; Hao, D. Traumatic Resin Duct Development, Terpenoid Formation, and Related Synthase Gene Expression in Pinus massoniana under Feeding Pressure of Monochamus alternatus. J. Plant Growth Regul. 2018, 38, 897–908. [Google Scholar] [CrossRef]
- Martin, D.M.; Bohlmann, J. Chapter Two-Molecular Biochemistry and Genomics of Terpenoid Defenses in Conifers. Recent Adv. Phytochem. 2005, 39, 29–56. [Google Scholar]
- Mao, J.; He, Z.; Hao, J.; Liu, T.; Chen, J.; Huang, S. Identification, expression, and phylogenetic analyses of terpenoid biosynthesis-related genes in secondary xylem of loblolly pine (Pinus taeda L.) based on transcriptome analyses. PeerJ 2019, 7, e6124. [Google Scholar] [CrossRef]
- Mei, L.N. Identification and Functional Analysis of Genes Related to Pinus massoniana Resin Production. Doctoral Thesis, Guizhou University, Guizhou, China, 2021. [Google Scholar]
- Fellermeier, M.; Raschke, M.; Sagner, S.; Wungsintaweekul, J.; Schuhr, C.A.; Hecht, S.; Kis, K.; Radykewicz, T.; Adam, P.; Rohdich, F.; et al. Studies on the nonmevalonate pathway of terpene biosynthesis. The role of 2C-methyl-D-erythritol 2,4-cyclodiphosphate in plants. Eur. J. Biochem. 2001, 268, 6302–6310. [Google Scholar] [CrossRef] [PubMed]
- Rekittke, I.; Nonaka, T.; Wiesner, J.; Demmer, U.; Warkentin, E.; Jomaa, H.; Ermler, U. Structure of the E-1-hydroxy-2-methyl-but-2-enyl-4-diphosphate synthase (GcpE) from Thermus thermophilus. FEBS Lett. 2011, 585, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Honzatko, R.B.; Peters, R.J. Terpenoid synthase structures: A so far incomplete view of complex catalysis. Nat. Prod. Rep. 2012, 29, 1153–1175. [Google Scholar] [CrossRef]
- Moses, T.; Pollier, J.; Thevelein, J.M.; Goossens, A. Bioengineering of plant (tri)terpenoids: From metabolic engineering of plants to synthetic biology in vivo and in vitro. New Phytol. 2013, 200, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Pateraki, I.; Kanellis, A.K. Stress and developmental responses of terpenoid biosynthetic genes in Cistus creticus subsp. creticus. Plant Cell Rep. 2010, 29, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Zhan, W.Y.; Zhao, F.; Xie, K.; Zhao, Q. Cloning and expression analysis of the HDS gene in Fritillaria cirrhosa D. Don. Mol. Plant Breed. 2022, 20, 4984–4989. [Google Scholar] [CrossRef]
- Lorenzo, O.; Solano, R. Molecular players regulating the jasmonate signalling network. Curr. Opin. Plant Biol. 2005, 8, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, F.; Lunn, J.E. The Role of Trehalose 6-Phosphate (Tre6P) in Plant Metabolism and Development. Annu. Rev. Plant Biol. 2021, 72, 737–760. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Xie, Y.; Wei, Y. Two terpene synthases in resistant Pinus massoniana contribute to defence against Bursaphelenchus xylophilus. Plant Cell Environ. 2021, 44, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Bent, A. Arabidopsis thaliana floral dip transformation method. Methods Mol. Biol. 2006, 343, 87–103. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, P.; Zhu, L.; Wu, F.; Chen, Y.; Zhu, P.; Ji, K. Characterization and Function of the 1-Deoxy-D-xylose-5-Phosphate Synthase (DXS) Gene Related to Terpenoid Synthesis in Pinus massoniana. Int. J. Mol. Sci. 2021, 22, 848. [Google Scholar] [CrossRef] [PubMed]
- Dalton, C.C.; Iqbal, K.N.; Turner, D.A. Iron phosphate precipitation in Murashige and Skoog media. Physiol. Plant. 1983, 57, 472–476. [Google Scholar] [CrossRef]
- Gan, X.Y.; Gong, P.; Zhang, L.; Chen, Y.C.; Nie, F.J.; Song, Y.X. Cloning and expression analysis of Solanum tuberosum L. GATA12 gene. Mol. Plant Breed. 2021, 19, 2123–2128. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Zhao, Y.; Yu, W.; Zhang, J.; Huang, Z.; Zhang, M.; Yu, Q.; Ji, K. Characteristics and Functions of PmHDS, a Terpenoid Synthesis-Related Gene in Pinus massoniana Lamb. Int. J. Mol. Sci. 2025, 26, 457. https://doi.org/10.3390/ijms26020457
Ren X, Zhao Y, Yu W, Zhang J, Huang Z, Zhang M, Yu Q, Ji K. Characteristics and Functions of PmHDS, a Terpenoid Synthesis-Related Gene in Pinus massoniana Lamb. International Journal of Molecular Sciences. 2025; 26(2):457. https://doi.org/10.3390/ijms26020457
Chicago/Turabian StyleRen, Xingyue, Yulu Zhao, Wenya Yu, Jingjing Zhang, Zichen Huang, Mengyang Zhang, Qiong Yu, and Kongshu Ji. 2025. "Characteristics and Functions of PmHDS, a Terpenoid Synthesis-Related Gene in Pinus massoniana Lamb." International Journal of Molecular Sciences 26, no. 2: 457. https://doi.org/10.3390/ijms26020457
APA StyleRen, X., Zhao, Y., Yu, W., Zhang, J., Huang, Z., Zhang, M., Yu, Q., & Ji, K. (2025). Characteristics and Functions of PmHDS, a Terpenoid Synthesis-Related Gene in Pinus massoniana Lamb. International Journal of Molecular Sciences, 26(2), 457. https://doi.org/10.3390/ijms26020457






