Prevalent and Drug-Resistant Phenotypes and Genotypes of Escherichia coli Isolated from Healthy Cow’s Milk of Large-Scale Dairy Farms in China
Abstract
:1. Introduction
2. Results
2.1. Prevalence of E. coli
2.2. Antimicrobial Susceptibility Testing
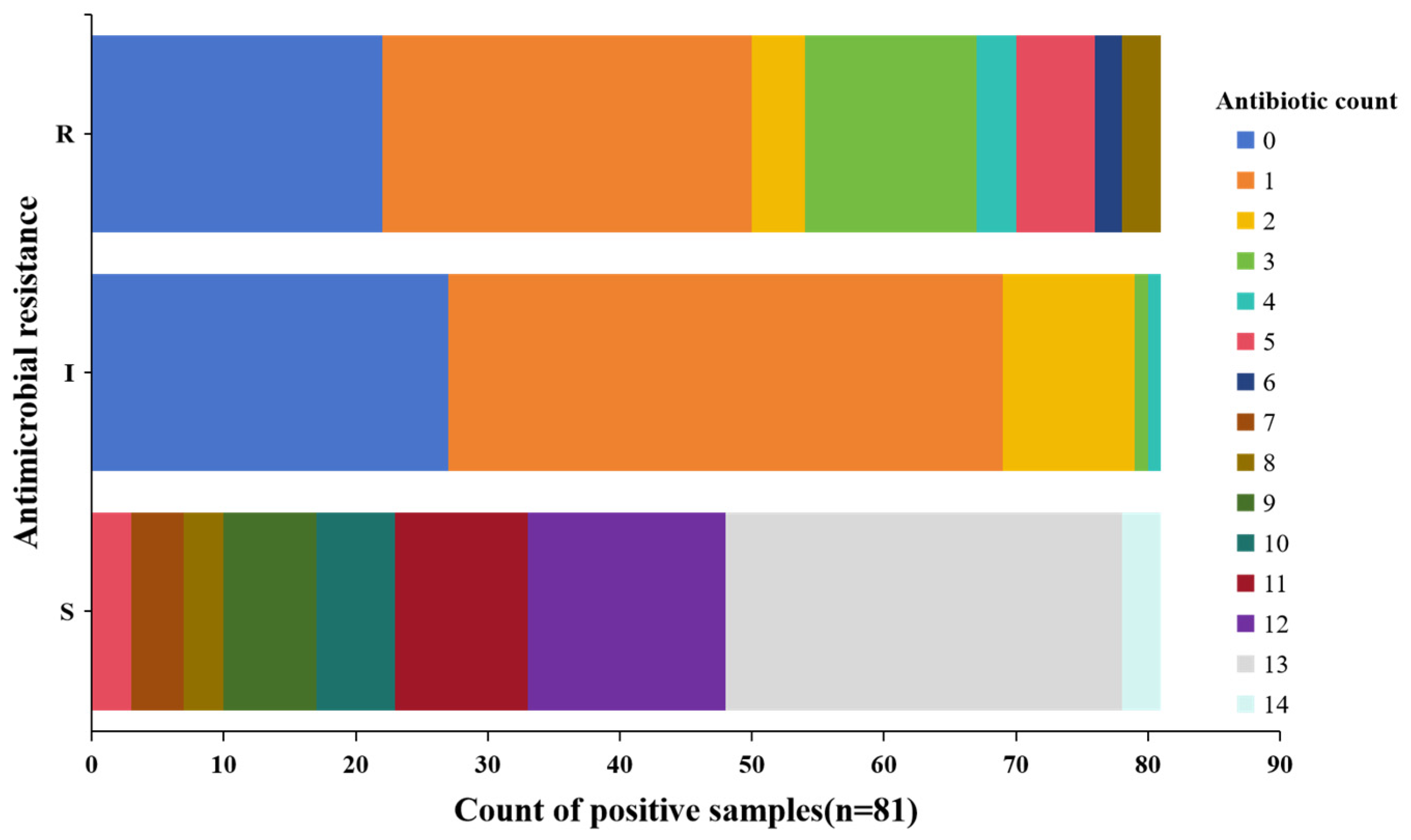
2.3. Multidrug Resistance
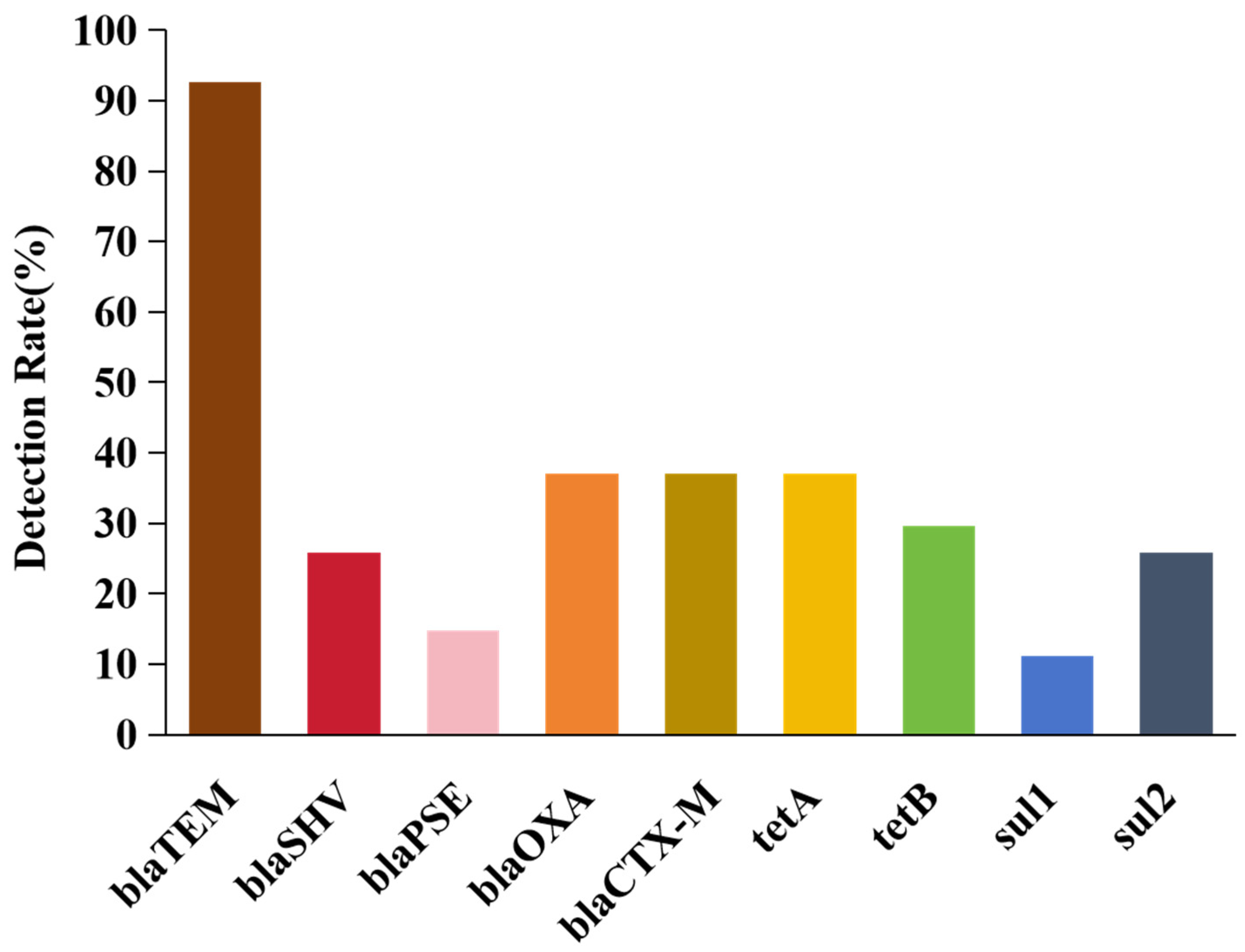
2.4. Screening Antibiotic Resistance Genes
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation and Characterization of E. coli
4.3. PCR Amplification
4.4. Antimicrobial Susceptibility Patterns
4.5. Detection of Drug Resistance Genes
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murashko, O.N.; Yeh, K.-H.; Yu, C.-H.A.; Kaberdin, V.R.; Lin-Chao, S. Sodium Fluoride Exposure Leads to ATP Depletion and Altered RNA Decay in Escherichia coli under Anaerobic Conditions. Microbiol. Spectr. 2023, 11, e0415822. [Google Scholar] [CrossRef] [PubMed]
- Gambushe, S.M.; Zishiri, O.T.; E El Zowalaty, M. Review of Escherichia coli O157:H7 Prevalence, Pathogenicity, Heavy Metal and Antimicrobial Resistance, African Perspective. Infect. Drug Resist. 2022, 15, 4645–4673. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Fox, J.G. A one health perspective for defining and deciphering Escherichia coli pathogenic potential in multiple hosts. Comp. Med. 2021, 71, 3–45. [Google Scholar] [CrossRef]
- Hwang, S.-B.; Chelliah, R.; Kang, J.E.; Rubab, M.; Banan-MwineDaliri, E.; Elahi, F.; Oh, D.-H. Role of Recent Therapeutic Applications and the Infection Strategies of Shiga Toxin-Producing Escherichia coli. Front. Cell. Infect. Microbiol. 2021, 11, 614963. [Google Scholar] [CrossRef] [PubMed]
- Ping, X. Research progress on the dairy cow mastitis. Anim. Biol. 2021, 23, 44–46. [Google Scholar] [CrossRef]
- Alves, T.d.S.; Rosa, V.S.; Leite, D.d.S.; Guerra, S.T.; Joaquim, S.F.; Guimarães, F.F.; Pantoja, J.C.d.F.; Lucheis, S.B.; Rall, V.L.M.; Hernandes, R.T.; et al. Genome-Based Characterization of Multidrug-Resistant Escherichia coli Isolated from Clinical Bovine Mastitis. Curr. Microbiol. 2023, 80, 89. [Google Scholar] [CrossRef]
- Keane, O.M. Genetic diversity, the virulence gene profile and antimicrobial resistance of clinical mastitis-associated Escherichia coli. Res. Microbiol. 2016, 167, 678–684. [Google Scholar] [CrossRef]
- Li, K.; Hou, M.; Zhang, L.; Tian, M.; Yang, M.; Jia, L.; Liang, Y.; Zou, D.; Liu, R.; Ma, Y. Analysis of antimicrobial resistance and genetic correlations of Escherichia coli in dairy cow mastitis. J. Veter. Res. 2022, 66, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Lee, Y.J. Molecular characteristics of Escherichia coli from bulk tank milk in Korea. J. Veter. Sci. 2022, 23, e9. [Google Scholar] [CrossRef]
- Burvenich, C.; Van Merris, V.; Mehrzad, J.; Diez-Fraile, A.; Duchateau, L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef]
- Sunil, D.D.; Mathew, B.; Jolly, D. Raw milk as a reservoir of antibiotic resistant enteropathogenic Escherichia coli. J. Indian Vet. Assoc. 2023, 21, 51. [Google Scholar] [CrossRef]
- Berthe, T.; Ratajczak, M.; Clermont, O.; Denamur, E.; Petit, F. Evidence for Coexistence of Distinct Escherichia coli Populations in Various Aquatic Environments and Their Survival in Estuary Water. Appl. Environ. Microbiol. 2013, 79, 4684–4693. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-Y.; Sugie, Y.; Yu, Z.; Okuno, Y.; Tanaka, H.; Ihara, M. Occurrence of E. coli and antibiotic-resistant E. coli in the southern watershed of Lake Biwa, including in wastewater treatment plant effluent and inflow rivers. Chemosphere 2022, 301, 134372. [Google Scholar] [CrossRef]
- Bengtsson, B.; Unnerstad, H.E.; Ekman, T.; Artursson, K.; Nilsson-Öst, M.; Waller, K.P. Antimicrobial susceptibility of udder pathogens from cases of acute clinical mastitis in dairy cows. Veter. Microbiol. 2009, 136, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Butaye, P.; Devriese, L.A.; Haesebrouck, F. Antimicrobial Growth Promoters Used in Animal Feed: Effects of Less Well Known Antibiotics on Gram-Positive Bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, H.; Zhou, Z.; Miao, Y.; Li, R.; Yang, B.; Cao, C.; Xiao, S.; Wang, X.; Liu, H.; et al. Characterization of Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolates That Cause Diarrhea in Sheep in Northwest China. Microbiol. Spectr. 2022, 10, e0159522. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, M.H.; Abood, I.A.; Dakheel, M.M. Antimicrobial Resistance of Tannin Extract against E. coli Isolates from Sheep. Arch. Razi Inst. 2022, 77, 697–701. [Google Scholar] [CrossRef]
- Mwasinga, W.; Shawa, M.; Katemangwe, P.; Chambaro, H.; Mpundu, P.; M’kandawire, E.; Mumba, C.; Munyeme, M. Multidrug-Resistant Escherichia coli from Raw Cow Milk in Namwala District, Zambia: Public Health Implications. Antibiotics 2023, 12, 1421. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Sarkar, N. Antibacterial sensitivity of Escherichia coli isolated from milk and milk products in Jabalpur, MP, India. Indian J. Dairy Sci. 2020, 73, 434–438. [Google Scholar] [CrossRef]
- Mendelson, M.; Sharland, M.; Mpundu, M. Antibiotic resistance: Calling time on the ‘silent pandemic’. JAC Antimicrob. Resist. 2022, 4, dlac016. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S. A parallel and silent emerging pandemic: Antimicrobial resistance (AMR) amid COVID-19 pandemic. J. Infect. Public Heal. 2023, 16, 611–617. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Zhou, X.; He, Y.; Yong, C.; Shen, M.; Szenci, O.; Han, B. Antimicrobial susceptibility, virulence genes, and randomly amplified polymorphic DNA analysis of Staphylococcus aureus recovered from bovine mastitis in Ningxia, China. J. Dairy Sci. 2016, 99, 9560–9569. [Google Scholar] [CrossRef]
- Beher, S.P.; Dayalan, S.; Jayakumar, T.; Rajendran, I. Prevalence of Escherichia coli in Raw Cow’s Milk in Cuddalore District. Int. J. Curr. Microbiol. Appl. Sci. 2022, 11, 286–294. [Google Scholar] [CrossRef]
- Ghali-Mohammed, I.; Odetokun, I.A.; Raufu, I.A.; Alhaji, N.B.; Adetunji, V.O. Prevalence of Escherichia coli O157 isolated from marketed raw cow milk in Kwara State, Nigeria. Sci. Afr. 2023, 19, e01469. [Google Scholar] [CrossRef]
- Sarba, E.J.; Wirtu, W.; Gebremedhin, E.Z.; Borena, B.M.; Marami, L.M. Occurrence and antimicrobial susceptibility patterns of Escherichia coli and Escherichia coli O157 isolated from cow milk and milk products, Ethiopia. Sci. Rep. 2023, 13, 16018. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, A.; Eid, H.; Youssif, F.; Eid, N.E.H. Escherichia Coli Isolated from Raw Milk at North Sinai Governorate. Suez Canal Veter. Med. 2017, 22, 121–131. [Google Scholar] [CrossRef]
- Imre, K.; Ban-Cucerzan, A.; Herman, V.; Sallam, K.I.; Cristina, R.T.; Abd-Elghany, S.M.; Morar, D.; Popa, S.A.; Imre, M.; Morar, A. Occurrence, Pathogenic Potential and Antimicrobial Resistance of Escherichia coli Isolated from Raw Milk Cheese Commercialized in Banat Region, Romania. Antibiotics 2022, 11, 721. [Google Scholar] [CrossRef]
- Liu, H.; Meng, L.; Dong, L.; Zhang, Y.; Wang, J.; Zheng, N. Prevalence, Antimicrobial Susceptibility, and Molecular Characterization of Escherichia coli Isolated from Raw Milk in Dairy Herds in Northern China. Front. Microbiol. 2021, 12, 730656. [Google Scholar] [CrossRef]
- Goulart, D.B.; Mellata, M. Escherichia coli Mastitis in Dairy Cattle: Etiology, Diagnosis, and Treatment Challenges. Front. Microbiol. 2022, 13, 928346. [Google Scholar] [CrossRef] [PubMed]
- Asfaw, A. Prevalence and Potential Risk Factors of Bovine Clinical Mastitis in Bonke District, Gamo Zone, Southern Ethiopia. OMO Int. J. Sci. 2023, 6, 1–11. [Google Scholar] [CrossRef]
- Aflakian, F.; Mohseni, N.; Hafiz, M.; Nikoueian, H.; Badouei, M.A.; Zomorodi, A.R. Phenotypic and genotypic investigation of antimicrobial resistance and extended-spectrum beta-lactamase production among Escherichia coli isolated from bovine mastitis. Vet. Arh. 2023, 93, 503–512. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, H.; Zhang, J.; Guo, Z.; Yan, Z.; Wang, G.; Wang, X.; Wang, L.; Li, J. Prevalence and molecular characterization of extended-spectrum β–lactamase—Producing Escherichia coli isolates from dairy cattle with endometritis in Gansu Province, China. BMC Vet. Res. 2024, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Z.; Zhang, Y.; Zhang, Z.; Lei, L.; Xia, Z. Increasing Prevalence of ESBL-Producing Multidrug Resistance Escherichia coli From Diseased Pets in Beijing, China From 2012 to 2017. Front. Microbiol. 2019, 10, 2852. [Google Scholar] [CrossRef] [PubMed]
- Tyasningsih, W.; Ramandinianto, S.C.; Ansharieta, R.; Witaningrum, A.M.; Permatasari, D.A.; Wardhana, D.K.; Effendi, M.H.; Ugbo, E.N. Prevalence and antibiotic resistance of Staphylococcus aureus and Escherichia coli isolated from raw milk in East Java, Indonesia. Vet. World 2022, 15, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Nahar, A.; Islam, A.K.M.A.; Islam, N.; Khan, M.K.; Khan, S.; Rahman, A.K.M.A.; Alam, M. Molecular characterization and antibiotic resistance profile of ESBL-producing Escherichia coli isolated from healthy cow raw milk in smallholder dairy farms in Bangladesh. Vet. World 2023, 16, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Zhao, S.; Zheng, N.; Li, S.; Zhang, Y.; Liu, H.; McKillip, J.; Wang, J. Short communication: Microbiological quality of raw cow milk and its association with herd management practices in Northern China. J. Dairy Sci. 2017, 100, 4294–4299. [Google Scholar] [CrossRef] [PubMed]
- Gelalcha, B.D.; Mohammed, R.I.; Gelgie, A.E.; Dego, O.K. Molecular epidemiology and pathogenomics of extended-spectrum beta-lactamase producing- Escherichia coli and-Klebsiella pneumoniae isolates from bulk tank milk in Tennessee, USA. Front. Microbiol. 2023, 14, 1283165. [Google Scholar] [CrossRef] [PubMed]
- Sombie, J.I.N.; Kagira, J.; Maina, N. Prevalence and Antibiogram of Escherichia coli and Staphylococcus spp. Isolated from Cattle Milk Products Sold in Juja Sub-County, Kenya. J. Trop. Med. 2022, 2022, 5251197. [Google Scholar] [CrossRef]
- Awadallah, M.; Ahmed, H.; Merwad, A.; Selim, M. Occurrence, genotyping, shiga toxin genes and associated risk factors of E. coli isolated from dairy farms, handlers and milk consumers. Vet. J. 2016, 217, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.F.; Gohar, U.F.; Tahir, S.F.; Mukhtar, H.; Pornpukdeewattana, S.; Nukthamna, P.; Ali, A.M.M.; Bavisetty, S.C.B.; Massa, S. Antimicrobial resistance: More than 70 years of war between humans and bacteria. Crit. Rev. Microbiol. 2020, 46, 578–599. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-L.; Che, R.-X.; Wu, T.; Qu, Q.-W.; Chen, M.; Zheng, S.-D.; Cai, X.-H.; Wang, G.; Li, Y.-H. New Characterization of Multi-Drug Resistance of Streptococcus suis and Biofilm Formation from Swine in Heilongjiang Province of China. Antibiotics 2023, 12, 132. [Google Scholar] [CrossRef]
- Ibrahim, D.R.; Dodd, C.E.R.; Stekel, D.J.; Meshioye, R.T.; Diggle, M.; Lister, M.; Hobman, J.L. Multidrug-Resistant ESBL-Producing E. coli in Clinical Samples from the UK. Antibiotics 2023, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Fahim, F.J.; Das, R.; Abdullah, K.S.; Islam, S.S.; Mahim, N.J.; Sultana, N.; Uddin, M.N.; Islam, M.R.; Ahmad, S.; et al. High prevalence of multidrug-resistant extended spectrum beta lactamase-producing Escherichia coli in raw milk in Bangladesh. Microbes Infect. Dis. 2024. [Google Scholar] [CrossRef]
- Saei, M.; Jamshidi, A.; Zeinali, T.; Khoramian, B. Phenotypic and genotypic determination of β-lactamase-producing Escherichia coli strains isolated from raw milk and clinical mastitis samples, Mashhad, Iran. Int. Dairy J. 2022, 133, 105406. [Google Scholar] [CrossRef]
- Xu, Z.-Q.; Flavin, M.T.; Flavin, J. Combating multidrug-resistant Gram-negative bacterial infections. Expert Opin. Investig. Drugs 2013, 23, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Liu, H.; Meng, L.; Xing, M.; Dong, L.; Gu, M.; Wang, J.; Zheng, N. Antimicrobial susceptibility, phylotypes, and virulence genes of Escherichia coli from clinical bovine mastitis in five provinces of China. Food Agric. Immunol. 2020, 31, 406–423. [Google Scholar] [CrossRef]
- Bag, M.S.; Khan, M.R.; Sami, M.H.; Begum, F.; Islam, M.; Rahman, M.; Rahman, M.; Hassan, J. Virulence determinants and antimicrobial resistance of E. coli isolated from bovine clinical mastitis in some selected dairy farms of Bangladesh. Saudi J. Biol. Sci. 2021, 28, 6317–6323. [Google Scholar] [CrossRef] [PubMed]
- Eldesoukey, I.E.; Elmonir, W.; Alouffi, A.; Beleta, E.I.M.; Kelany, M.A.; Elnahriry, S.S.; Alghonaim, M.I.; Alzeyadi, Z.A.; Elaadli, H. Multidrug-Resistant Enteropathogenic Escherichia coli Isolated from Diarrhoeic Calves, Milk, and Workers in Dairy Farms: A Potential Public Health Risk. Antibiotics 2022, 11, 999. [Google Scholar] [CrossRef]
- Pitout, J.D.; Laupland, K.B. Extended-spectrum β-lactamase-producing Enterobacteriaceae: An emerging public-health concern. Lancet Infect. Dis. 2008, 8, 159–166. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, J.; Ho, H.; Wang, Y.; Huang, S.; Han, R. Prevalence and antimicrobial-resistance phenotypes and genotypes of Escherichia coli isolated from raw milk samples from mastitis cases in four regions of China. J. Glob. Antimicrob. Resist. 2019, 22, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Meng, L.; Liu, H.; Wu, H.; Hu, H.; Zheng, N.; Wang, J.; Schroyen, M. Effect of therapeutic administration of β-lactam antibiotics on the bacterial community and antibiotic resistance patterns in milk. J. Dairy Sci. 2021, 104, 7018–7025. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Shin, M.K.; Jung, M.; Belaynehe, K.M.; Yoo, H.S. Prevalence of Antimicrobial Resistance and Transfer of Tetracycline Resistance Genes in Escherichia coli Isolates from Beef Cattle. Appl. Environ. Microbiol. 2015, 81, 5560–5566. [Google Scholar] [CrossRef]
- Allmeier, H.; Cresnar, B.; Greck, M.; Schmitt, R. Complete nucleotide sequence of Tn1721: Gene organization and a novel gene product with features of a chemotaxis protein. Gene 1992, 111, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, R.; Sewitz, S.; Lipkow, K.; Crellin, P. Complete Nucleotide Sequence of Tn10. J. Bacteriol. 2000, 182, 2970–2972. [Google Scholar] [CrossRef]
- Ombarak, R.A.; Hinenoya, A.; Elbagory, A.-R.M.; Yamasaki, S. Prevalence and Molecular Characterization of Antimicrobial Resistance in Escherichia coli Isolated from Raw Milk and Raw Milk Cheese in Egypt. J. Food Prot. 2018, 81, 226–232. [Google Scholar] [CrossRef]
- Rasheed, M.U.; Thajuddin, N.; Ahamed, P.; Teklemariam, Z.; Jamil, K. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 341–346. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 10–1128. [Google Scholar] [CrossRef]
- Jouini, A.; Ben Slama, K.; Sáenz, Y.; Klibi, N.; Costa, D.; Vinué, L.; Zarazaga, M.; Boudabous, A.; Torres, C. Detection of Multiple-Antimicrobial Resistance and Characterization of the Implicated Genes in Escherichia coli Isolates from Foods of Animal Origin in Tunis. J. Food Prot. 2009, 72, 1082–1088. [Google Scholar] [CrossRef]
- Sáenz, Y.; Briñas, L.; Domínguez, E.; Ruiz, J.; Zarazaga, M.; Vila, J.; Torres, C. Mechanisms of Resistance in Multiple-Antibiotic-Resistant Escherichia coli Strains of Human, Animal, and Food Origins. Antimicrob. Agents Chemother. 2004, 48, 3959–3967. [Google Scholar] [CrossRef] [PubMed]
| Name of Antibacterial Drugs | No. (%) of Positive Strains | ||||||
|---|---|---|---|---|---|---|---|
| Northeast China (n = 6) | North China (n = 5) | South China (n = 4) | East China (n = 14) | Northwest China (n = 50) | Southwest China (n = 2) | Total (n = 81) | |
| Ampicillin | 1 (16.7) | 2 (40.0) | 1 (25.0) | 7 (50.0) | 14 (28.0) | 0 (0.0) | 25 (30.9) |
| Amoxicillin/Clavulanic acid | 2 (33.3) | 0 (0.0) | 0 (0.0) | 4 (28.6) | 3 (6.0) | 0 (0.0) | 9 (12.4) |
| Cephalothin | 4 (75.0) | 3 (60.0) | 2 (50.0) | 10 (71.4) | 31 (62.0) | 1 (50.0) | 51 (63.0) |
| Ceftiofur | 1 (16.7) | 2 (40.0) | 1 (25.0) | 1 (7.1) | 9 (18.0) | 0 (0.0) | 14 (17.3) |
| Meropenem | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Kanamycin | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 1 (2.0) | 0 (0.0) | 2 (2.5) |
| Gentamicin | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (21.4) | 2 (4.0) | 0 (0.0) | 5 (6.2) |
| Tetracycline | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (14.3) | 9 (18.0) | 0 (0.0) | 11 (13.6) |
| Doxycycline | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 6 (12.0) | 0 (0.0) | 7 (8.7) |
| Florfenicol | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.0) | 0 (0.0) | 2 (2.5) |
| Polymyxin E | 2 (33.3) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 3 (6.0) | 0 (0.0) | 6 (7.4) |
| Ciprofloxacin | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 4 (8.0) | 0 (0.0) | 5 (6.2) |
| Sulfisoxazole | 0 (0.0) | 1 (20.0) | 1 (25.0) | 6 (42.9) | 5 (10.0) | 0 (0.0) | 13 (16.1) |
| Sulfamethoxazole | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (7.1) | 3 (6.0) | 0 (0.0) | 4 (5.0) |
| No. of Resistance Genes | Combination Patterns of Resistance Genes | No. of Resistance Gene Combination Patterns | No. of E. coli Isolates (%) (n = 27) |
|---|---|---|---|
| 1 | blaTEM | 5 | 6 (22.2) |
| blaCTX-M | 1 | ||
| 2 | blaTEM-blaOXA | 2 | 4 (14.8) |
| blaSHV-blaCTX-M | 1 | ||
| blaTEM-blaCTX-M | 1 | ||
| 3 | blaTEM-blaPSE-blaOXA | 3 | 7 (25.9) |
| blaTEM-blaSHV-blaCTX-M | 2 | ||
| blaTEM-tetA-tetB | 1 | ||
| blaTEM-blaCTX-M-tetA | 1 | ||
| 4 | blaTEM-blaSHV-blaPSE-blaOXA | 1 | 3 (11.1) |
| blaTEM-blaSHV-blaOXA-sul1 | 1 | ||
| blaTEM-tetA-tetB-sul2 | 1 | ||
| 5 | blaTEM-blaCTX-M-tetA-sul1-sul2 | 1 | 5 (18.5) |
| blaTEM-blaSHV-tetA-tetB-sul2 | 1 | ||
| blaTEM-blaOXA-tetA-tetB-sul2 | 1 | ||
| blaTEM-blaOXA-blaCTX-M-tetA-tetB | 1 | ||
| blaTEM-tetA-tetB-sul1-sul2 | 1 | ||
| 6 | blaTEM-blaOXA-blaCTX-M-tetA-tetB-sul2 | 1 | 2 (7.4) |
| blaTEM-blaSHV-blaCTX-M-tetA-tetB-sul2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Wu, Y.; Ma, X.; Xu, X.; Tuerdi, A.; Shao, W.; Zheng, N.; Zhao, Y. Prevalent and Drug-Resistant Phenotypes and Genotypes of Escherichia coli Isolated from Healthy Cow’s Milk of Large-Scale Dairy Farms in China. Int. J. Mol. Sci. 2025, 26, 454. https://doi.org/10.3390/ijms26020454
Gao J, Wu Y, Ma X, Xu X, Tuerdi A, Shao W, Zheng N, Zhao Y. Prevalent and Drug-Resistant Phenotypes and Genotypes of Escherichia coli Isolated from Healthy Cow’s Milk of Large-Scale Dairy Farms in China. International Journal of Molecular Sciences. 2025; 26(2):454. https://doi.org/10.3390/ijms26020454
Chicago/Turabian StyleGao, Jiaojiao, Yating Wu, Xianlan Ma, Xiaowei Xu, Aliya Tuerdi, Wei Shao, Nan Zheng, and Yankun Zhao. 2025. "Prevalent and Drug-Resistant Phenotypes and Genotypes of Escherichia coli Isolated from Healthy Cow’s Milk of Large-Scale Dairy Farms in China" International Journal of Molecular Sciences 26, no. 2: 454. https://doi.org/10.3390/ijms26020454
APA StyleGao, J., Wu, Y., Ma, X., Xu, X., Tuerdi, A., Shao, W., Zheng, N., & Zhao, Y. (2025). Prevalent and Drug-Resistant Phenotypes and Genotypes of Escherichia coli Isolated from Healthy Cow’s Milk of Large-Scale Dairy Farms in China. International Journal of Molecular Sciences, 26(2), 454. https://doi.org/10.3390/ijms26020454






