Mesoporous Silicas of Well-Organized Structure: Synthesis, Characterization, and Investigation of Physical Processes Occurring in Confined Pore Spaces
Abstract
1. Introduction
2. Results and Discussion
2.1. Textural Characteristics of Silicas and Their Relationship to Synthesis Conditions
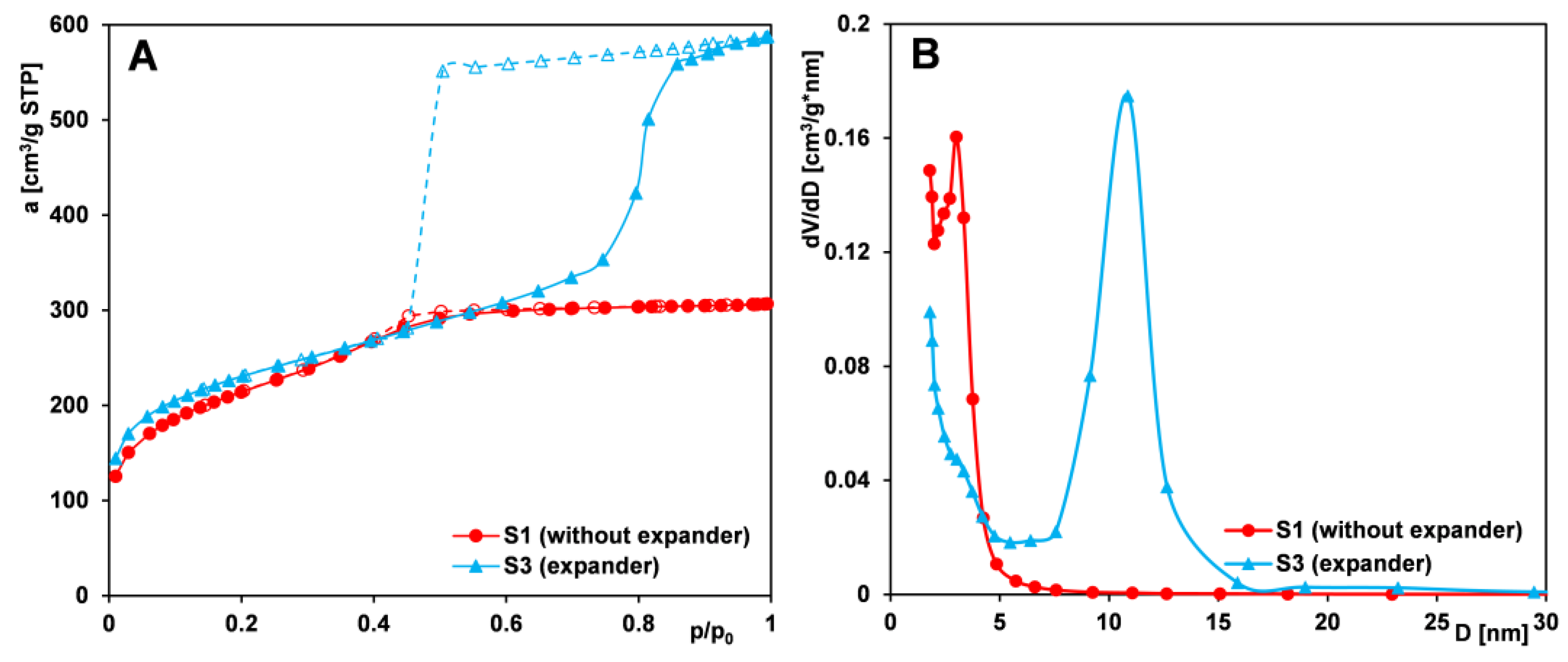
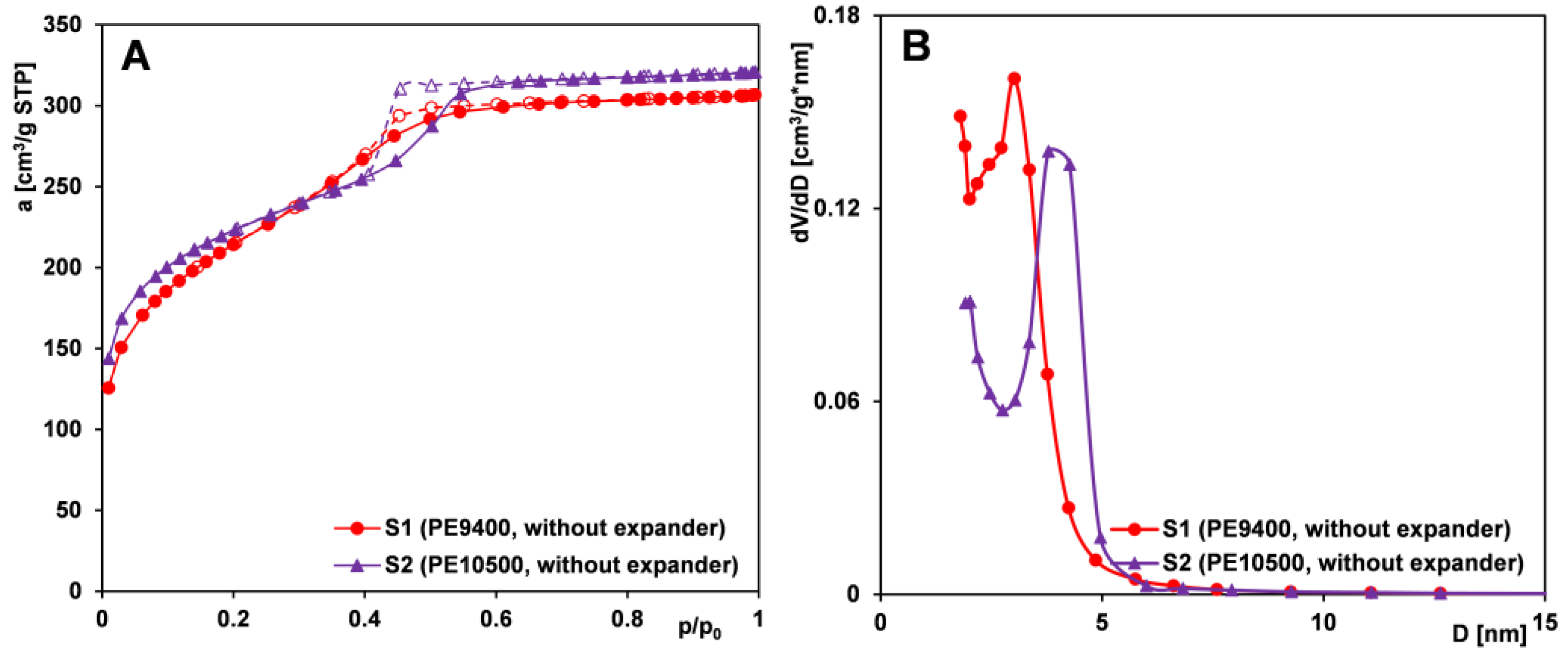
2.1.1. Effect of the Expander Addition
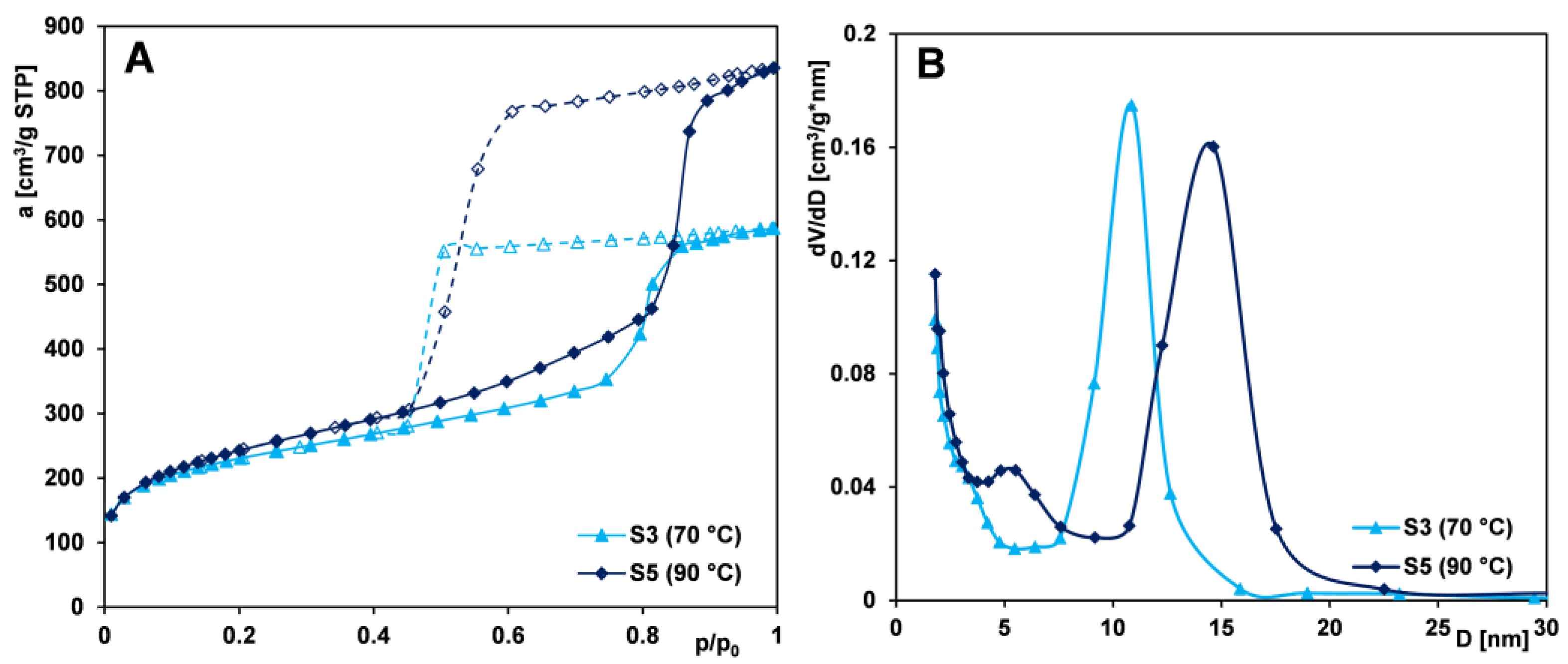
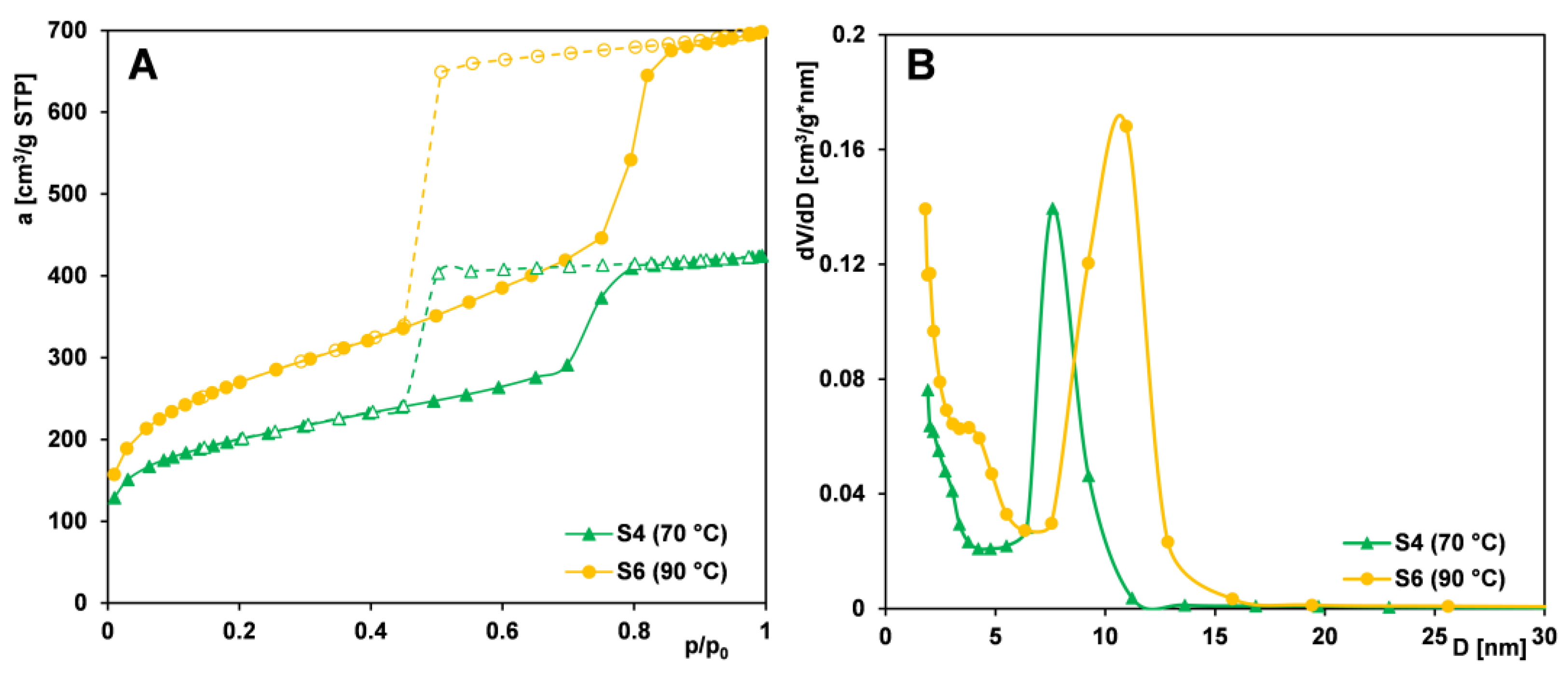
2.1.2. Effect of Aging Temperature
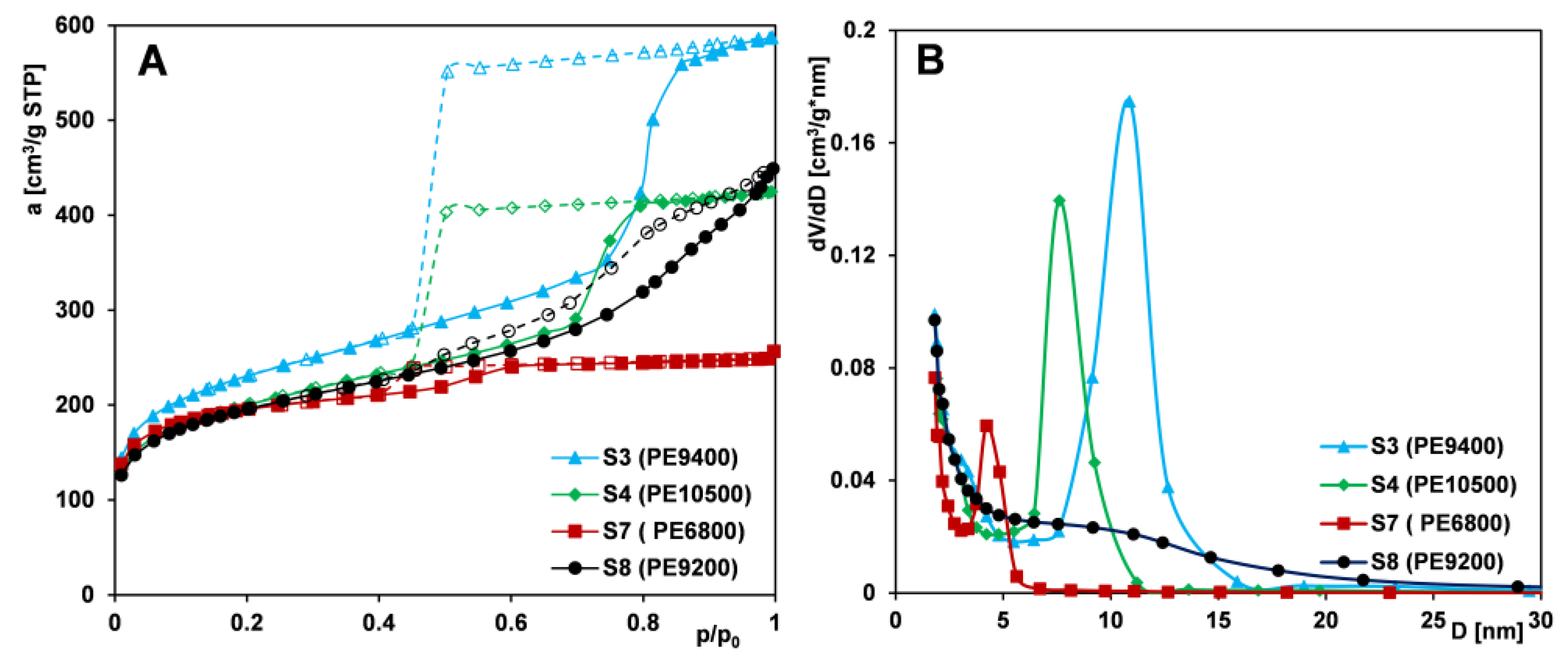
2.1.3. Effect of Copolymer Type
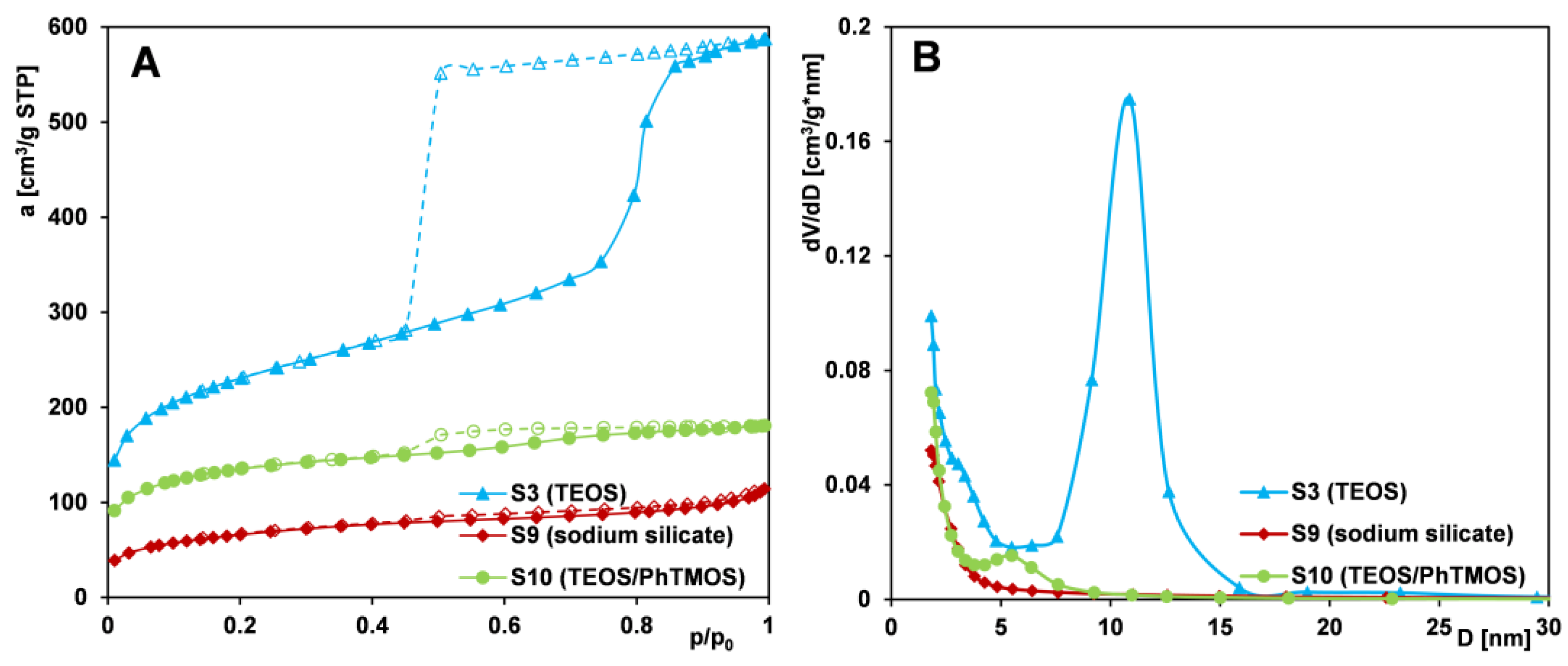
2.1.4. Effect of Silica Source
2.2. Mechanism of Formation of MCF-Type Mesoporous Materials
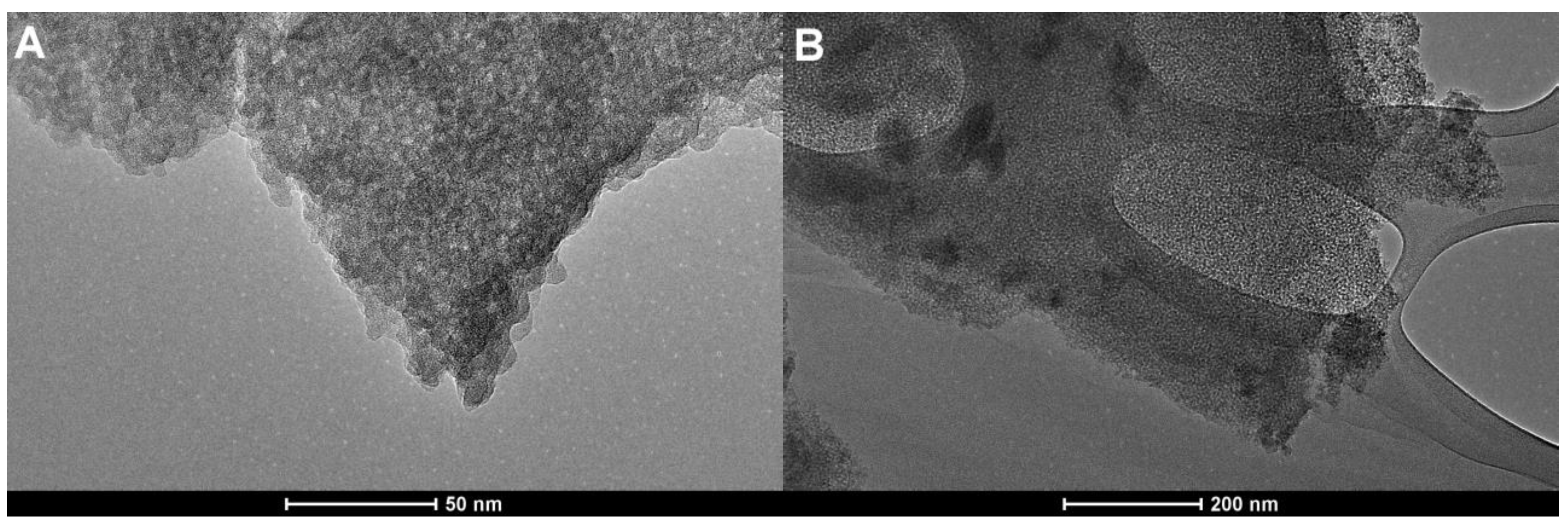
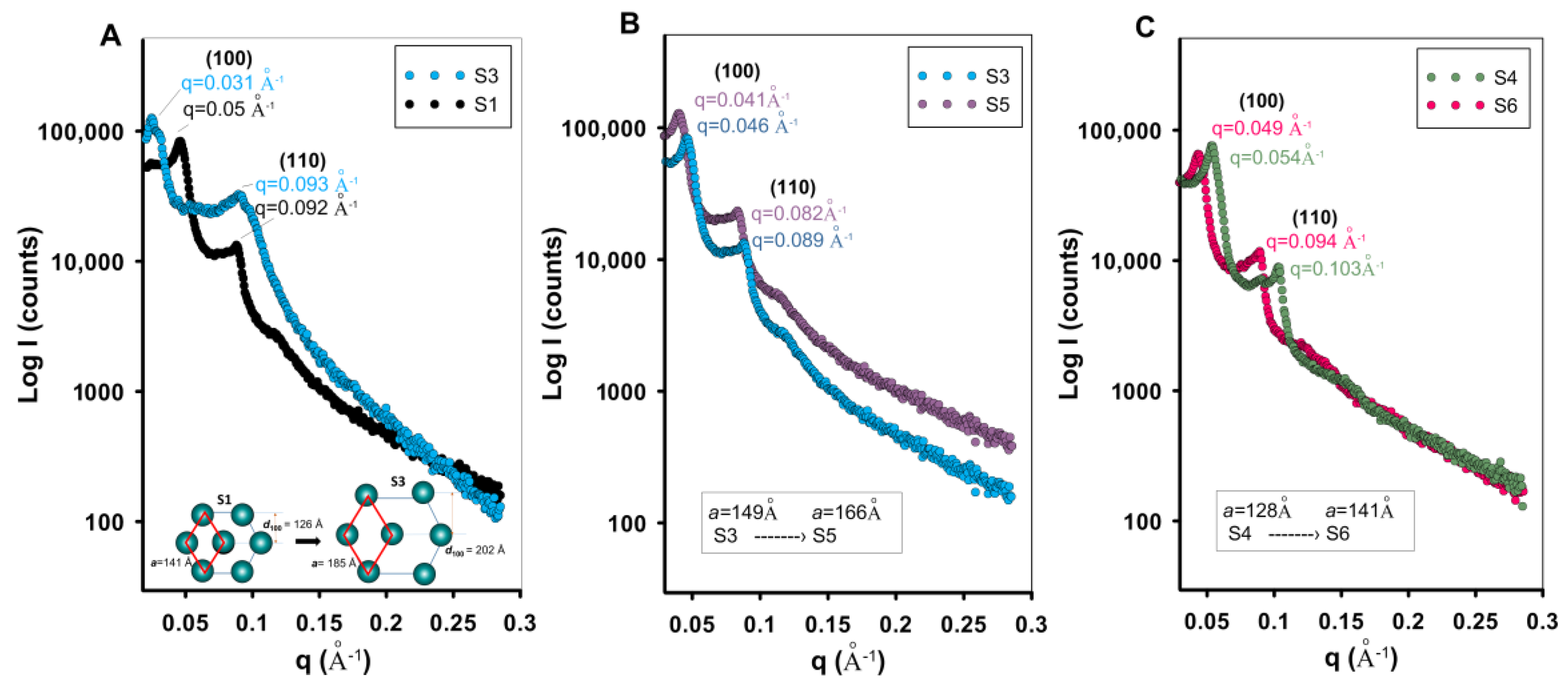
2.3. Structural and Morphological Characteristics
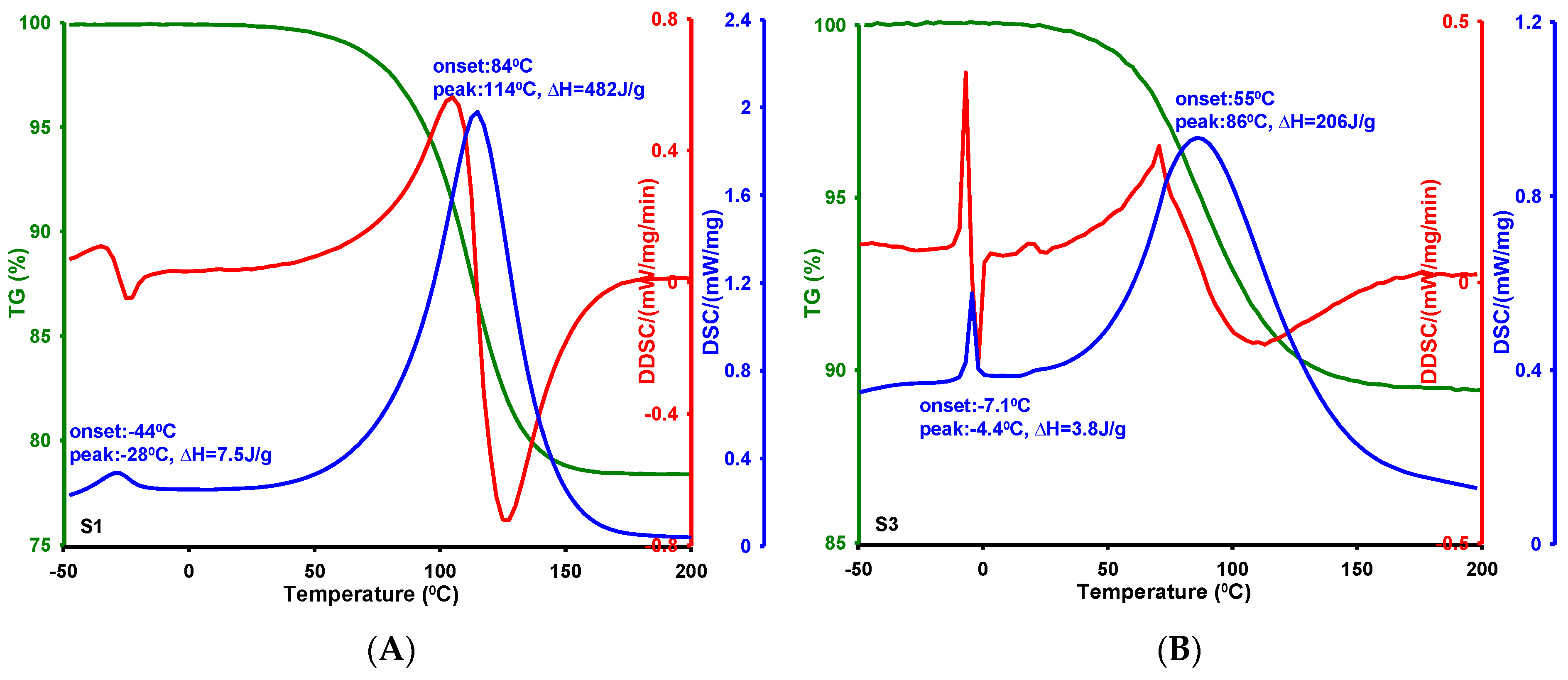
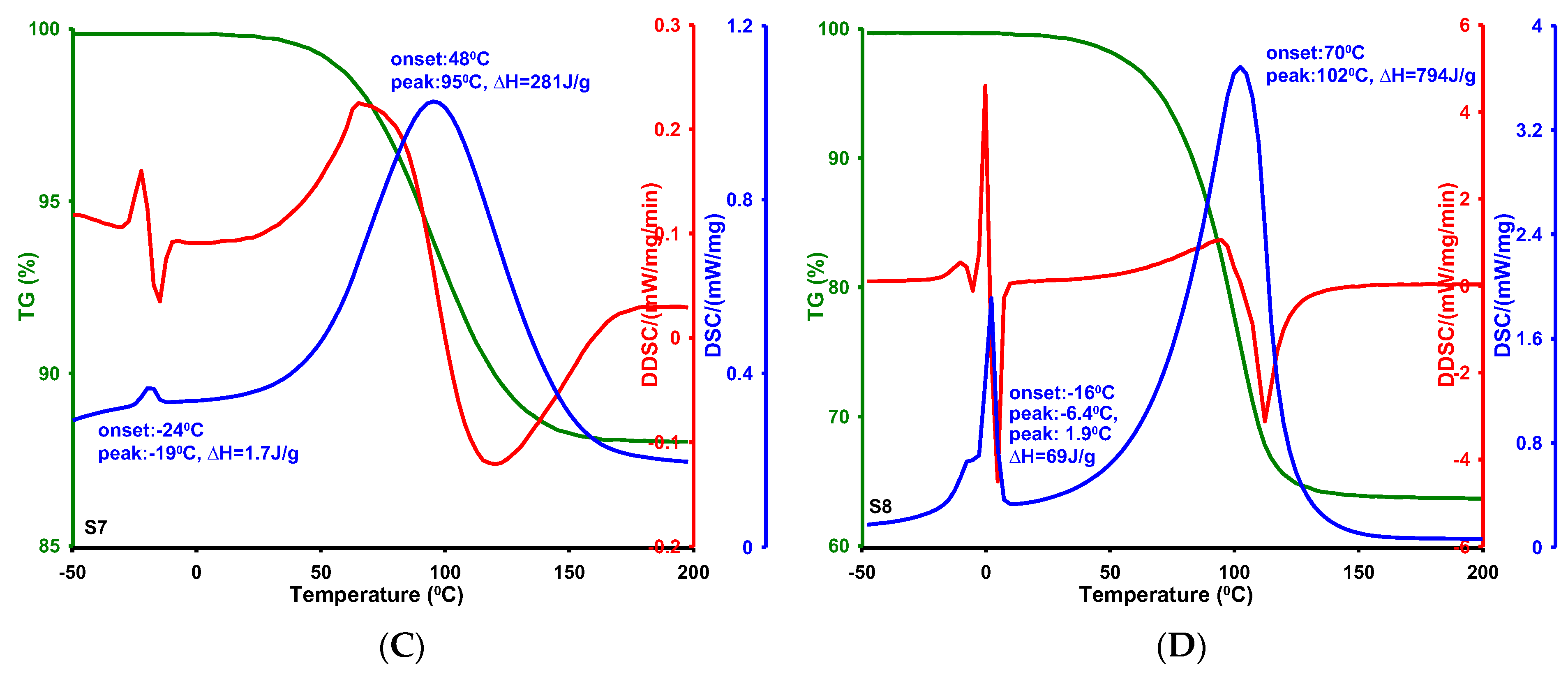
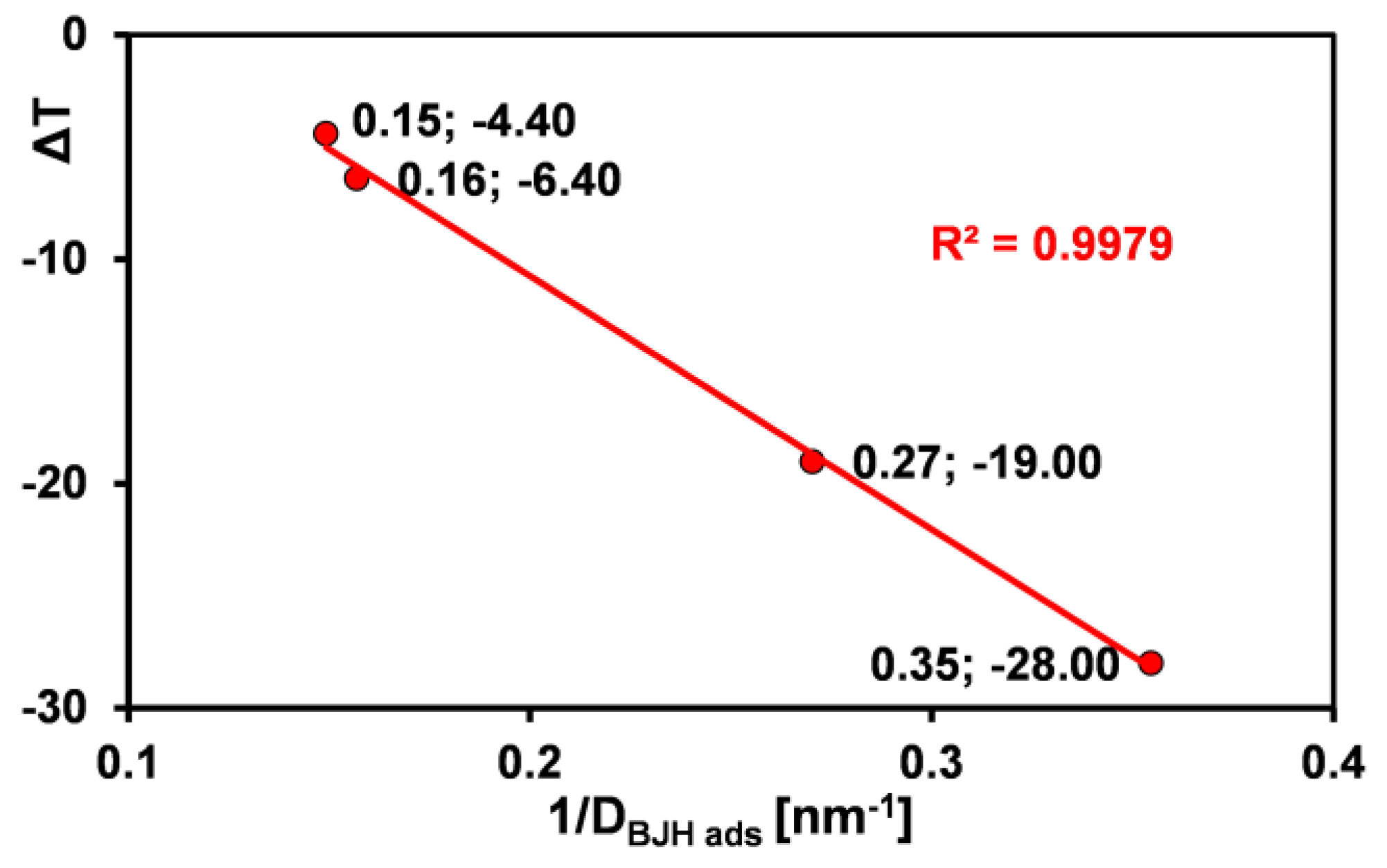
2.4. Phase Transformations in Confined Pore Spaces of Silicas
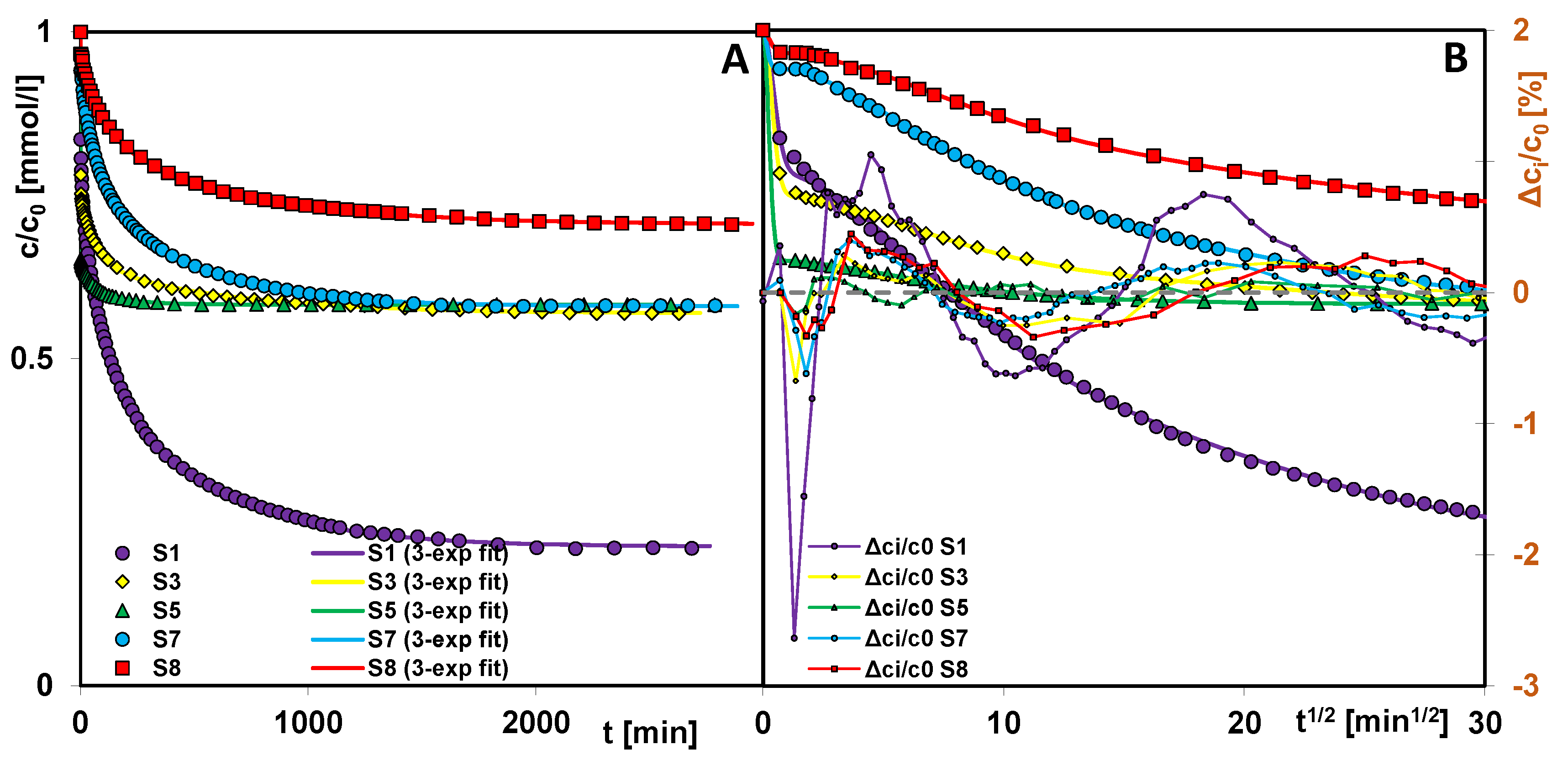
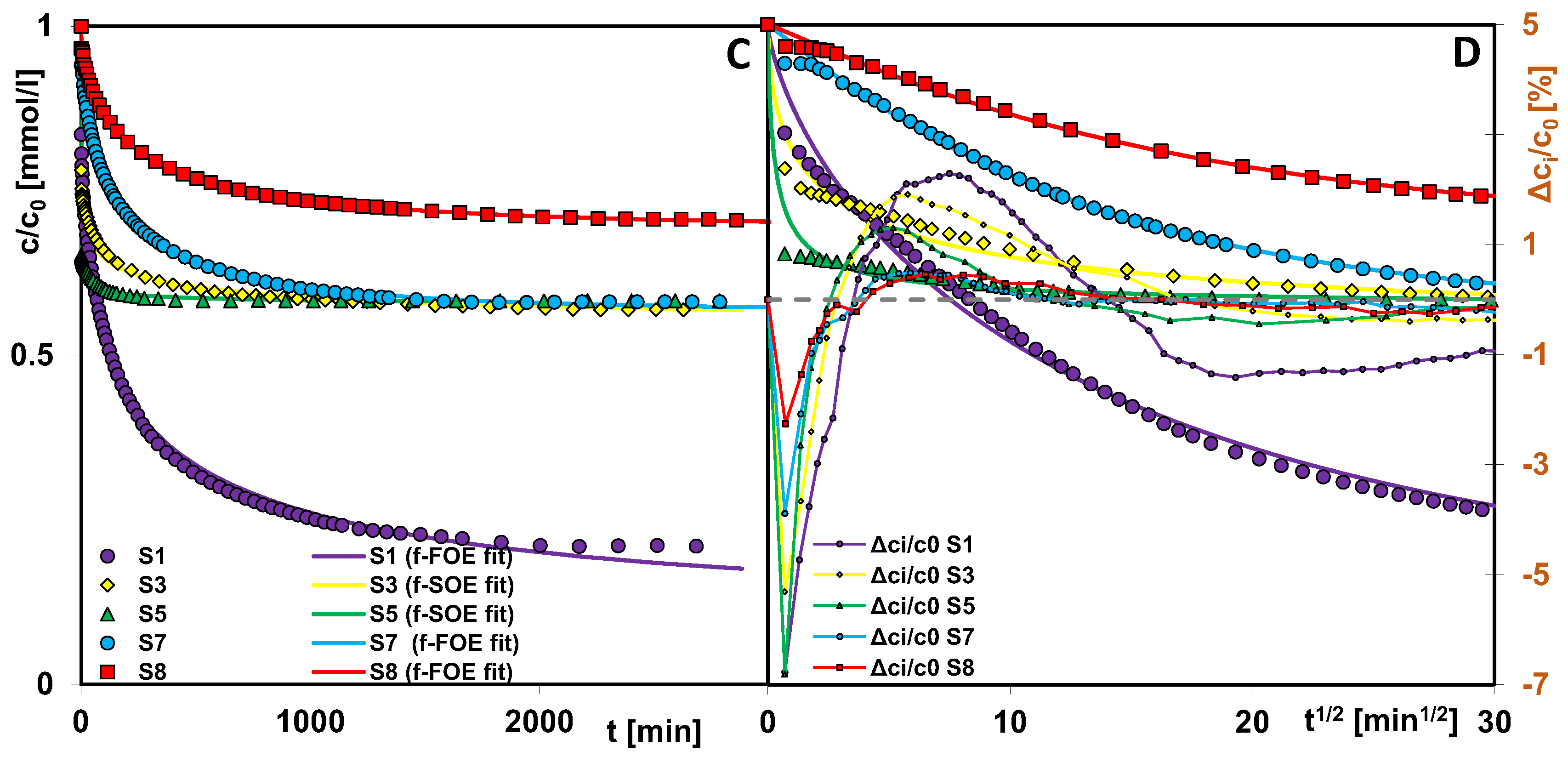
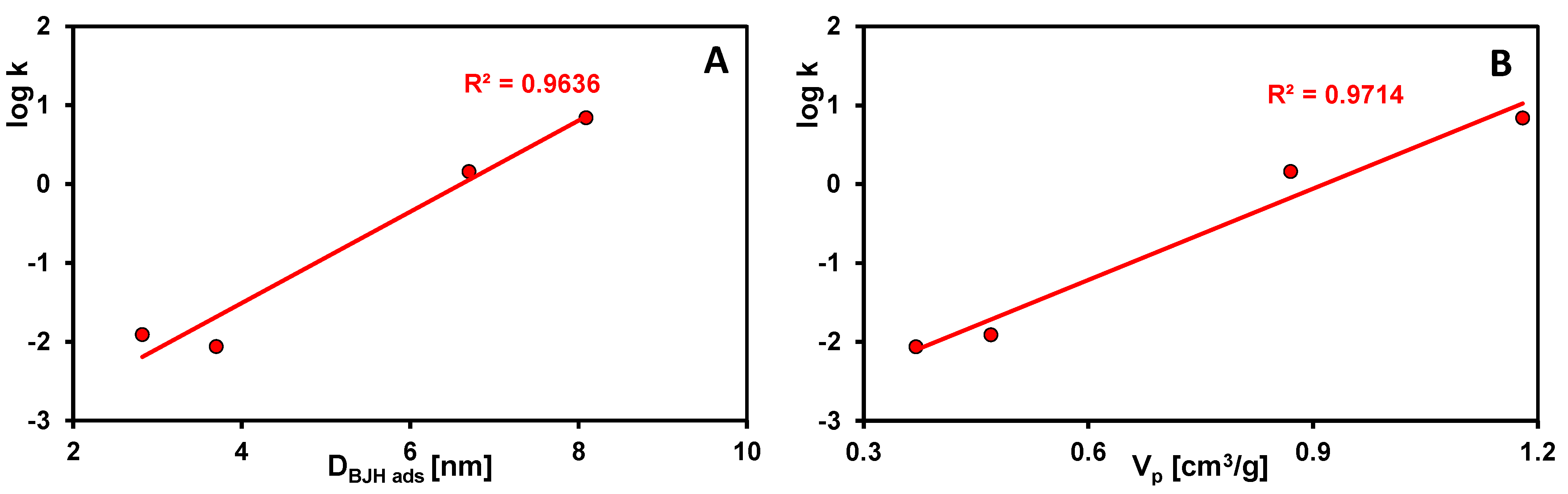
2.5. Adsorption of Methylene Blue on Selected Silicas
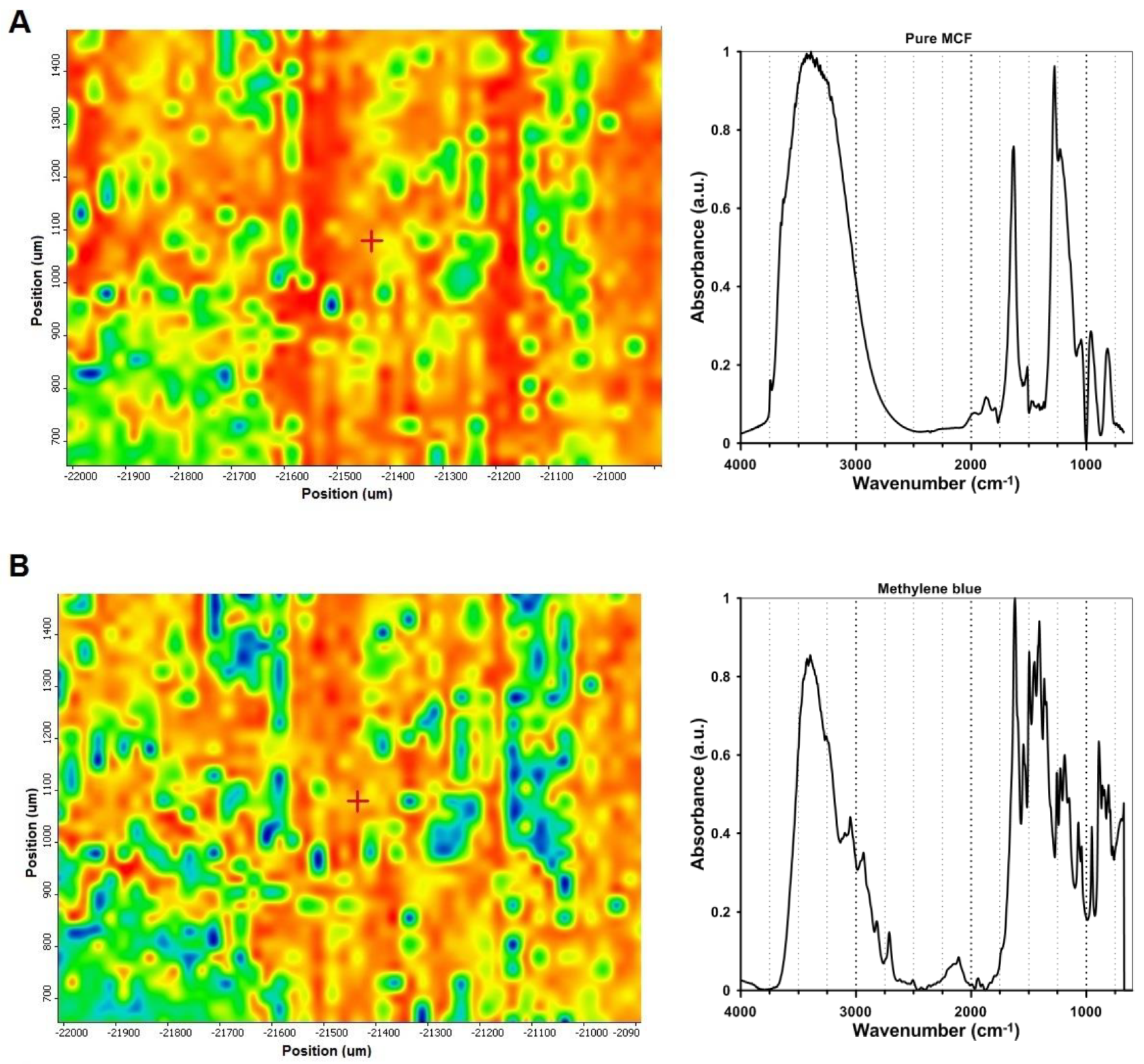
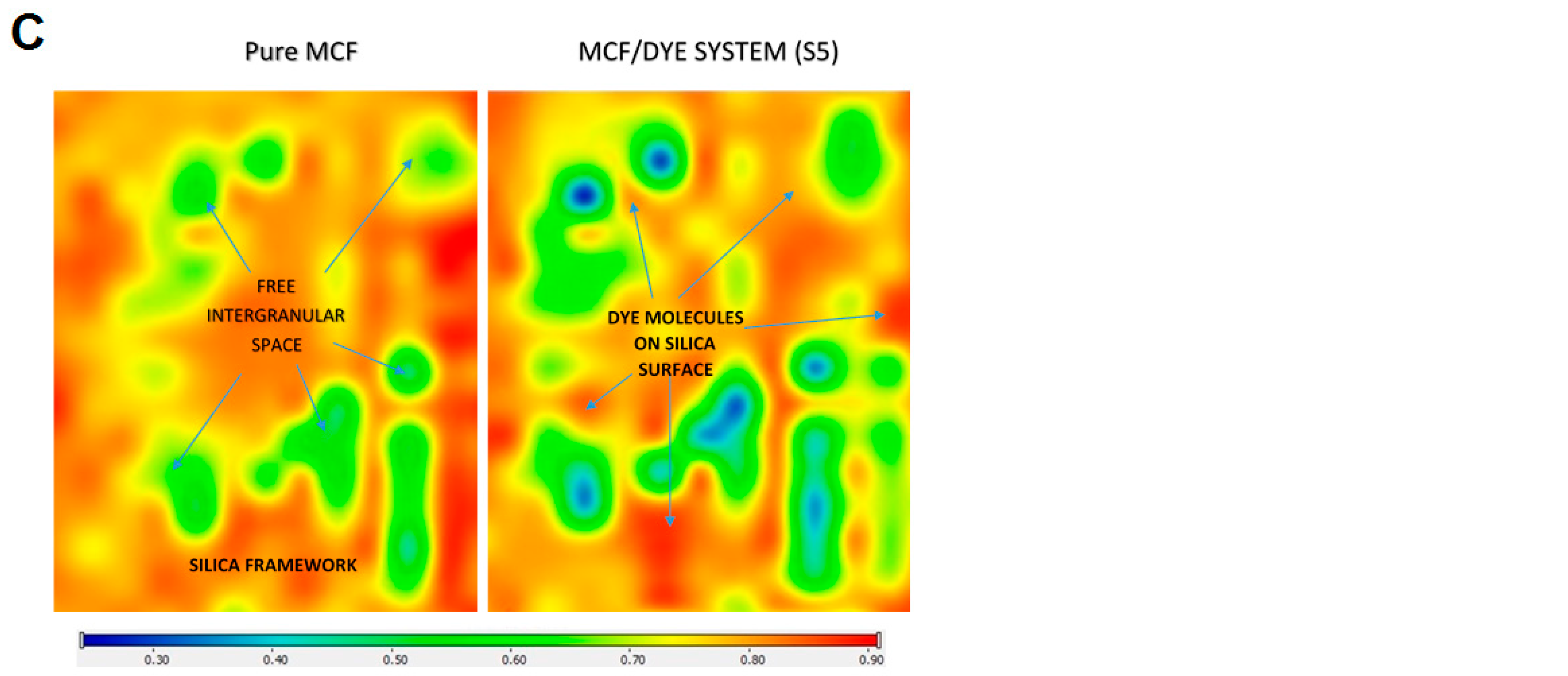
2.6. Visualization of Dye Adsorption on Silica Grains
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Synthesis of MCF Materials
3.3. Experimental Techniques
3.3.1. Material Characterization
3.3.2. Measurements of Liquid Phase Transitions
3.3.3. Dye Adsorption Kinetics Measurements
4. Conclusions
- All copolymers (PE6800, PE9200, PE9400, PE10500) allow the production of mesoporous materials with lower and medium mesopore sizes. The material synthesized using the PE9400 copolymer exhibits the most favorable structural parameters and high uniformity in pore size. The PE9200 and PE6800 copolymers were the least effective. The type of matrix plays a significant role in the formation of the porous structure, which is related to their properties and structure and the number of central poly(isopropylene oxide) (PO) chains and poly(ethylene oxide) (EO) side segments. The copolymers with a high content of the PO block enable optimal utilization of the pore-enlarging agent, TMB. In the absence of an expander, pore sizes are determined by the dimensions of the copolymer macromolecules.
- Raising the aging temperature improves the structural parameters of the tested materials. The sample obtained using the PE9400 copolymer and aged at 90 °C is characterized by the largest pore volume and average pore size (1.29 cm3/g and 8.09 nm, respectively), resulting from partial dissolution of the silica framework and generation of additional mesopores. The sample aged at 90 °C, but synthesized using the PE10500 copolymer, has a more rigid silica framework (thermally stable), so its porous structure is characterized by the greatest specific surface area (BET method—944 m2/g) of all the samples tested. Pore enlargement as a temperature effect is accompanied by the maintenance of uniformity in the size distribution.
- The addition of an expander (TMB) to the reaction mixture enables the production of materials with bottle-shaped mesopores (narrow openings and wide interiors), larger pore volumes, and larger pore sizes. Samples obtained without the use of TMB have pores with cylindrical geometry.
- Comparing silicas obtained from three different silica sources (TEOS, sodium silicate, TEOS/PhTMOS mixture), one can identify the sample obtained from TEOS as the one with the most favorable structural parameters.
- The wide-angle XRD patterns revealed amorphous features of the synthesized silicas, indicative of the lack of long-range order. The results demonstrated that the use of a swelling agent effectively increased the lattice constant, thereby enlarging the mesopore size. Similarly, raising the synthesis temperature led to an increase in the lattice constant, confirming that both TMB addition and thermal conditions play a crucial role in tailoring the pore architecture. Importantly, the mesostructural order was preserved across most samples despite these modifications. The study shows the effective modulation of mesostructural features in silica materials through synthesis parameters such as swelling agent content and temperature, while emphasizing the complementary nature of XRD, SAXS, and nitrogen sorption in evaluating porous materials.
- The textural properties of MCF-type silica materials significantly influenced the thermal behavior of water confined within their porous structures. Differential scanning calorimetry (DSC) revealed that melting and evaporation processes are sensitive to variations in pore size and shape, with the onset and maximum temperatures of melting showing a clear correlation with average pore diameter. Specifically, smaller pores result in lower melting points due to enhanced water–surface interactions and restricted crystallization, consistent with the Gibbs–Thomson effect. These findings underscore the importance of pore architecture in governing phase transition behaviors within confined systems.
- Pore size, shape, and structural order of silicas influenced the adsorption behavior of methylene blue. Kinetic studies showed that materials with larger mesopores and open-pore architecture exhibited faster adsorption rates due to improved molecular diffusion and easier access to adsorption sites. However, the highest adsorption was observed for silica S1, attributed to the optimal ratio of pore size to adsorbate dimensions and a significant presence of small mesopores, which enhance packing efficiency and molecular interactions. Despite its lower surface area and total pore volume, its structure allows for more effective retention of methylene blue molecules. These findings offer practical guidance for tailoring silica adsorbents for dye removal and other environmental purification applications, with specific materials better suited for targeting molecules of different sizes or diffusion characteristics.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohamed Isa, E.D.; Mahmud, I.S.; Ahmad, H.; Abdul Rahman, M.B. Dependence of mesoporous silica properties on its template. Ceram. Int. 2019, 45, 12149–12153. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Kim, S.-G.; Iskandar, F.; Okuyama, K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous Mesoporous Mater. 2009, 120, 447–453. [Google Scholar] [CrossRef]
- Nuntang, S.; Yousatit, S.; Yokoi, T.; Ngamcharussrivichai, C. Tunable mesoporosity and hydrophobicity of natural rubber/hexagonal mesoporous silica nanocomposites. Microporous Mesoporous Mater. 2019, 275, 235–243. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Mesoporous silica and organosilica materials—Review of their synthesis and organic functionalization. Can. J. Chem. 2012, 90, 1015–1031. [Google Scholar] [CrossRef]
- Gottuso, A.; Armetta, F.; Cataldo, A.; Nardo, V.M.; Parrino, F.; Saladino, M.L. Functionalization of mesoporous silica nanoparticles through one-pot co-condensation in w/o emulsion. Microporous Mesoporous Mater. 2022, 335, 111833. [Google Scholar] [CrossRef]
- Grisolia, A.; De Santo, M.; Curcio, M.; Cavallaro, P.A.; Morelli, C.; Leggio, A.; Pasqua, L. Engineered Mesoporous Silica-Based Nanoparticles: Characterization of Surface Properties. Materials 2024, 17, 3352. [Google Scholar] [CrossRef]
- Chircov, C.; Spoială, A.; Păun, C.; Crăciun, L.; Ficai, D.; Ficai, A.; Andronescu, E.; Turculeƫ, Ș.C. Mesoporous Silica Platforms with Potential Applications in Release and Adsorption of Active Agents. Molecules 2020, 25, 3814. [Google Scholar] [CrossRef]
- Karandikar, P.; Patil, K.R.; Mitra, A.; Kakade, B.; Chandwadkar, A.J. Synthesis and characterization of mesoporous carbon through inexpensive mesoporous silica as template. Microporous Mesoporous Mater. 2007, 98, 189–199. [Google Scholar] [CrossRef]
- Pang, J.; Luan, Y.; Yang, X.; Jiang, Y.; Zhao, L.; Zong, Y.; Li, Z. Functionalized Mesoporous Silica Particles for Application in Drug Delivery System. Mini-Rev. Med. Chem. 2012, 12, 775–788. [Google Scholar] [CrossRef]
- Palaniappan, A.; Su, X.; Tay, F.E.H. Functionalized mesoporous silica films for gas sensing applications. J. Electroceramics 2006, 16, 503–505. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y. Brief History, Preparation Method, and Biological Application of Mesoporous Silica Molecular Sieves: A Narrative Review. Molecules 2023, 28, 2013. [Google Scholar] [CrossRef]
- Singh, B.; Na, J.; Konarova, M.; Wakihara, T.; Yamauchi, Y.; Salomon, C.; Gawande, M.B. Functional Mesoporous Silica Nanomaterials for Catalysis and Environmental Applications. Bull. Chem. Soc. Jpn. 2020, 93, 1459–1496. [Google Scholar] [CrossRef]
- Yun, H.-S.; Kim, S.-E.; Hyun, Y.-T. Preparation of 3D cubic ordered mesoporous bioactive glasses. Solid State Sci. 2008, 10, 1083–1092. [Google Scholar] [CrossRef]
- Yan, M.; Dourdain, S.; Gibaud, A. Analysis of water condensation in P123 templated 2D hexagonal mesoporous silica films by X-ray reflectivity. Thin Solid Film. 2008, 516, 7955–7961. [Google Scholar] [CrossRef]
- Sujandi; Prasetyanto, E.A.; Lee, S.-C.; Park, S.-E. Microwave synthesis of large pored chloropropyl functionalized mesoporous silica with p6mm, Ia-3d, and Im3m structures. Microporous Mesoporous Mater. 2009, 118, 134–142. [Google Scholar] [CrossRef]
- Lin, C.-F.; Lin, H.-P.; Mou, C.-Y.; Liu, S.-T. Periodic mesoporous silicas via templating of new triblock amphiphilic copolymers. Microporous Mesoporous Mater. 2006, 91, 151–155. [Google Scholar] [CrossRef]
- Huang, J.; Tian, G.; Wang, H.; Xu, L.; Kan, Q. Large-pore cubic Ia-3d mesoporous silicas: Synthesis, modification and catalytic applications. J. Mol. Catal. A Chem. 2007, 271, 200–208. [Google Scholar] [CrossRef]
- Florent, M.; Goldfarb, D. The interaction between the surfactant and the co-structure directing agent in anionic surfactant-templated mesoporous silicas. Microporous Mesoporous Mater. 2012, 163, 291–299. [Google Scholar] [CrossRef]
- Gao, C.; Che, S. Organically Functionalized Mesoporous Silica by Co-structure-Directing Route. Adv. Funct. Mater. 2010, 20, 2750–2768. [Google Scholar] [CrossRef]
- Laurinavičius, G.; Poškus, V. Mesoporous Silica Synthesis: Different Precursors, Catalysts and Structure Directing Agents. Silicon 2025, 17, 2381–2391. [Google Scholar] [CrossRef]
- Yan, X.; Han, S.; Hou, W.; Yu, X.; Zeng, C.; Zhao, X.; Che, H. Synthesis of highly ordered mesoporous silica using cationic trimeric surfactant as structure-directing agent. Colloids Surf. A Physicochem. Eng. Asp. 2007, 303, 219–225. [Google Scholar] [CrossRef]
- Jaramillo, L.Y.; Arango-Benítez, K.; Henao, W.; Vargas, E.; Recio-Sánchez, G.; Romero-Sáez, M. Synthesis of ordered mesoporous silicas from rice husk with tunable textural properties. Mater. Lett. 2019, 257, 126749. [Google Scholar] [CrossRef]
- Trofymluk, O.; Levchenko, A.A.; Navrotsky, A. Mesoporous silica synthesis: Energetics of interaction between framework and structure directing agent. Microporous Mesoporous Mater. 2012, 149, 119–125. [Google Scholar] [CrossRef]
- Liu, F.; Yan, X.; Fan, F.T.; Zhao, C.C.; Liu, R.T.; Gao, Y.; Wang, Y.Q. Application of micro-meso hierarchical porous carbon for toluene adsorption treatment. Micro Nano Lett. 2016, 11, 372–377. [Google Scholar] [CrossRef]
- Wang, Y.; Song, H.; Zhang, H.; Liao, L.; Liu, N.; Chen, X. Direct synthesis of flat cake-type ordered mesoporous carbon in a double surfactant system of P123/CTAB. J. Mater. Chem. 2011, 21, 5576–5579. [Google Scholar] [CrossRef]
- Yang, H.; Xu, R.; Xue, X.; Li, F.; Li, G. Hybrid surfactant-templated mesoporous silica formed in ethanol and its application for heavy metal removal. J. Hazard. Mater. 2008, 152, 690–698. [Google Scholar] [CrossRef]
- Schmidt-Winkel, P.; Lukens, W.W.; Yang, P.; Margolese, D.I.; Lettow, J.S.; Ying, J.Y.; Stucky, G.D. Microemulsion Templating of Siliceous Mesostructured Cellular Foams with Well-Defined Ultralarge Mesopores. Chem. Mater. 2000, 12, 686–696. [Google Scholar] [CrossRef]
- Hermida, L.; Agustian, J.; Abdullah, A.Z.; Mohamed, A.R. Review of large-pore mesostructured cellular foam (MCF) silica and its applications. Open Chem. 2019, 17, 1000–1016. [Google Scholar] [CrossRef]
- Schmidt-Winkel, P.; Lukens, W.W.; Zhao, D.; Yang, P.; Chmelka, B.F.; Stucky, G.D. Mesocellular Siliceous Foams with Uniformly Sized Cells and Windows. J. Am. Chem. Soc. 1999, 121, 254–255. [Google Scholar] [CrossRef]
- Schmidt-Winkel, P.; Glinka, C.J.; Stucky, G.D. Microemulsion Templates for Mesoporous Silica. Langmuir 2000, 16, 356–361. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Z.; Feng, D.; Tu, B.; Zhao, D. Hydrothermal Stability of Mesostructured Cellular Silica Foams. J. Phys. Chem. C 2010, 114, 5012–5019. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Steel, A.; Carr, S.W.; Anderson, M.W. 14N NMR study of surfactant mesophases in the synthesis of mesoporous silicates. J. Chem. Soc. Chem. Commun. 1994, 1571–1572. [Google Scholar] [CrossRef]
- Monnier, A.; Schüth, F.; Huo, Q.; Kumar, D.; Margolese, D.; Maxwell, R.S.; Stucky, G.D.; Krishnamurty, M.; Petroff, P.; Firouzi, A.; et al. Cooperative formation of inorganic-organic interfaces in the synthesis of silicate mesostructures. Science 1993, 261, 1299–1303. [Google Scholar] [CrossRef]
- Glazneva, T.S.; Rebrov, E.V.; Schouten, J.C.; Paukshtis, E.A.; Ismagilov, Z.R. Synthesis and characterization of mesoporous silica thin films as a catalyst support on a titanium substrate. Thin Solid Film. 2007, 515, 6391–6394. [Google Scholar] [CrossRef]
- Longloilert, R.; Chaisuwan, T.; Luengnaruemitchai, A.; Wongkasemjit, S. Synthesis of MCM-48 from silatrane via sol–gel process. J. Sol-Gel Sci. Technol. 2011, 58, 427–435. [Google Scholar] [CrossRef]
- Yuan, Q.; Chi, Y.; Yu, N.; Zhao, Y.; Yan, W.; Li, X.; Dong, B. Amino-functionalized magnetic mesoporous microspheres with good adsorption properties. Mater. Res. Bull. 2014, 49, 279–284. [Google Scholar] [CrossRef]
- Chen, D.; Li, Z.; Yu, C.; Shi, Y.; Zhang, Z.; Tu, B.; Zhao, D. Nonionic Block Copolymer and Anionic Mixed Surfactants Directed Synthesis of Highly Ordered Mesoporous Silica with Bicontinuous Cubic Structure. Chem. Mater. 2005, 17, 3228–3234. [Google Scholar] [CrossRef]
- Hanrahan, J.P.; Donovan, A.; Morris, M.A.; Holmes, J.D. Synthesis and swelling of large pore diameter mesoporous silica spheres. J. Mater. Chem. 2007, 17, 3881–3887. [Google Scholar] [CrossRef]
- Kim, S.S.; Karkamkar, A.; Pinnavaia, T.J.; Kruk, M.; Jaroniec, M. Synthesis and Characterization of Ordered, Very Large Pore MSU-H Silicas Assembled from Water-Soluble Silicates. J. Phys. Chem. B 2001, 105, 7663–7670. [Google Scholar] [CrossRef]
- López, T.; Basaldella, E.I.; Ojeda, M.L.; Manjarrez, J.; Alexander-Katz, R. Encapsulation of valproic acid and sodic phenytoin in ordered mesoporous SiO2 solids for the treatment of temporal lobe epilepsy. Opt. Mater. 2006, 29, 75–81. [Google Scholar] [CrossRef]
- Do, X.-D.; Hoang, V.-T.; Kaliaguine, S. MIL-53(Al) mesostructured metal-organic frameworks. Microporous Mesoporous Mater. 2011, 141, 135–139. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Izquierdo-Barba, I.; Arcos, D.; Vallet-Regí, M. Tailoring the biological response of mesoporous bioactive materials. J. Mater. Chem. B 2015, 3, 3810–3819. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Ryoo, R.; Kruk, M.; Gierszal, K.P.; Jaroniec, M.; Kamiya, S.; Terasaki, O. Tailoring the Pore Structure of SBA-16 Silica Molecular Sieve through the Use of Copolymer Blends and Control of Synthesis Temperature and Time. J. Phys. Chem. B 2004, 108, 11480–11489. [Google Scholar] [CrossRef]
- Esteban Vázquez, J.; Birnbach, J.; Schmiedel, P.; Hesami, M.; Gettinger, M.; Hellweg, T. Influence of additives on a Pluronic-based cubic phase. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131491. [Google Scholar] [CrossRef]
- Georgiev, R.; Christova, D.; Todorova, L.; Georgieva, B.; Vasileva, M.; Babeva, T. Generating porosity in metal oxides thin films through introduction of polymer micelles. Opt. Quantum Electron. 2018, 50, 156. [Google Scholar] [CrossRef]
- Kiss, É.; Erdélyi, K.; Szendrö, I.; Vargha-Butler, E.I. Adsorption and wetting properties of pluronic block copolymers on hydrophobic surfaces studied by optical waveguide lightmode spectroscopy and dynamic tensiometric method. J. Adhes. 2004, 80, 815–829. [Google Scholar] [CrossRef]
- Kócs, L.; Tegze, B.; Albert, E.; Major, C.; Szalai, A.; Fodor, B.; Basa, P.; Sáfrán, G.; Hórvölgyi, Z. Ammonia-vapour-induced two-layer transformation of mesoporous silica coatings on various substrates. Vacuum 2021, 192, 110415. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Marczewski, A.W.; Skrzypek, I.; Pikus, S.; Kozak, M. Effect of addition of pore expanding agent on changes of structure characteristics of ordered mesoporous silicas. Appl. Surf. Sci. 2008, 255, 2851–2858. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Derylo-Marczewska, A.; Wasilewska, M. Mesocellular Silica Foams (MCFs) with Tunable Pore Size as a Support for Lysozyme Immobilization: Adsorption Equilibrium and Kinetics, Biocomposite Properties. Int. J. Mol. Sci. 2020, 21, 5479. [Google Scholar] [CrossRef]
- Szymańska, K.; Bryjak, J.; Mrowiec-Białoń, J.; Jarzębski, A.B. Application and properties of siliceous mesostructured cellular foams as enzymes carriers to obtain efficient biocatalysts. Microporous Mesoporous Mater. 2007, 99, 167–175. [Google Scholar] [CrossRef]
- Skendrović, D.; Primožič, M.; Rezić, T.; Vrsalović Presečki, A. Mesocellular Silica Foam as Immobilization Carrier for Production of Statin Precursors. Int. J. Mol. Sci. 2024, 25, 1971. [Google Scholar] [CrossRef] [PubMed]
- Deryło-Marczewska, A.; Marczewski, A.W.; Skrzypek, I.; Pikus, S.; Kozak, M. The effect of aging temperature on structure characteristics of ordered mesoporous silicas. Appl. Surf. Sci. 2005, 252, 625–632. [Google Scholar] [CrossRef]
- Kipkemboi, P.; Fogden, A.; Alfredsson, V.; Flodström, K. Triblock Copolymers as Templates in Mesoporous Silica Formation: Structural Dependence on Polymer Chain Length and Synthesis Temperature. Langmuir 2001, 17, 5398–5402. [Google Scholar] [CrossRef]
- Hanrahan, J.P.; Copley, M.P.; Ziegler, K.J.; Spalding, T.R.; Morris, M.A.; Steytler, D.C.; Heenan, R.K.; Schweins, R.; Holmes, J.D. Pore Size Engineering in Mesoporous Silicas Using Supercritical CO2. Langmuir 2005, 21, 4163–4167. [Google Scholar] [CrossRef]
- Yu, C.; Fan, J.; Tian, B.; Stucky, G.D.; Zhao, D. Synthesis of Mesoporous Silica from Commercial Poly(ethylene oxide)/Poly(butylene oxide) Copolymers: Toward the Rational Design of Ordered Mesoporous Materials. J. Phys. Chem. B 2003, 107, 13368–13375. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Q.; Zhao, X.S.; Zhang, L. Pore size control of mesoporous silicas from mixtures of sodium silicate and TEOS. Microporous Mesoporous Mater. 2007, 106, 62–67. [Google Scholar] [CrossRef]
- Yu, N.; Gong, Y.; Wu, D.; Sun, Y.; Luo, Q.; Liu, W.; Deng, F. One-pot synthesis of mesoporous organosilicas using sodium silicate as a substitute for tetraalkoxysilane. Microporous Mesoporous Mater. 2004, 72, 25–32. [Google Scholar] [CrossRef]
- AlMohaimadi, K.M.; Albishri, H.M.; Thumayri, K.A.; AlSuhaimi, A.O.; Mehdar, Y.T.H.; Hussein, B.H.M. Facile Hydrothermal Assisted Basic Catalyzed Sol Gel Synthesis for Mesoporous Silica Nanoparticle from Alkali Silicate Solutions Using Dual Structural Templates. Gels 2024, 10, 839. [Google Scholar] [CrossRef]
- Schreiber, A.; Ketelsen, I.; Findenegg, G.H. Melting and freezing of water in ordered mesoporous silica materials. Phys. Chem. Chem. Phys. 2001, 3, 1185–1195. [Google Scholar] [CrossRef]
- Kittaka, S.; Ueda, Y.; Fujisaki, F.; Iiyama, T.; Yamaguchi, T. Mechanism of freezing of water in contact with mesoporous silicas MCM-41, SBA-15 and SBA-16: Role of boundary water of pore outlets in freezing. Phys. Chem. Chem. Phys. 2011, 13, 17222–17233. [Google Scholar] [CrossRef]
- Sliwinska-Bartkowiak, M.; Jazdzewska, M.; Huang, L.L.; Gubbins, K.E. Melting behavior of water in cylindrical pores: Carbon nanotubes and silica glasses. Phys. Chem. Chem. Phys. 2008, 10, 4909–4919. [Google Scholar] [CrossRef]
- Iza, M.; Woerly, S.; Danumah, C.; Kaliaguine, S.; Bousmina, M. Determination of pore size distribution for mesoporous materials and polymeric gels by means of DSC measurements: Thermoporometry. Polymer 2000, 41, 5885–5893. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, R.; Kim, J.M.; Shin, C.H.; Lee, J.Y. Synthesis and hydrothermal stability of a disordered mesoporous molecular sieve. In Studies in Surface Science and Catalysis; Chon, H., Ihm, S.-K., Uh, Y.S., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 105, pp. 45–52. [Google Scholar]
- Jaroniec, M.; Kruk, M.; Olivier, J.P. Standard Nitrogen Adsorption Data for Characterization of Nanoporous Silicas. Langmuir 1999, 15, 5410–5413. [Google Scholar] [CrossRef]
- Haul, R.S.J.; Gregg, K.S.W. Sing: Adsorption, Surface Area and Porosity. 2. Auflage, Academic Press, London 1982. 303 Seiten, Preis: $ 49.50. Berichte Bunsenges. Phys. Chem. 1982, 86, 957. [Google Scholar] [CrossRef]
- Blachnio, M.; Zienkiewicz-Strzalka, M.; Derylo-Marczewska, A.; Nosach, L.V.; Voronin, E.F. Chitosan– silica composites for adsorption application in the treatment of water and wastewater from anionic dyes. Int. J. Mol. Sci. 2023, 24, 11818. [Google Scholar] [CrossRef]
- Blachnio, M.; Zienkiewicz-Strzalka, M.; Kutkowska, J.; Derylo-Marczewska, A. Nanosilver–Biopolymer–Silica Composites: Preparation, and Structural and Adsorption Analysis with Evaluation of Antimicrobial Properties. Int. J. Mol. Sci. 2024, 25, 13548. [Google Scholar] [CrossRef]
- Haerifar, M.; Azizian, S. Fractal-Like Adsorption Kinetics at the Solid/Solution Interface. J. Phys. Chem. C 2012, 116, 13111–13119. [Google Scholar] [CrossRef]
- Marczewski, A.W. Analysis of Kinetic Langmuir Model. Part I: Integrated Kinetic Langmuir Equation (IKL): A New Complete Analytical Solution of the Langmuir Rate Equation. Langmuir 2010, 26, 15229–15238. [Google Scholar] [CrossRef]

| Silica | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Type of copolymer | PE9400 | PE10500 | PE9400 | PE10500 | PE9400 | PE10500 | PE6800 | PE9200 | PE9400 | PE9400 |
| Type of pore expander | - | - | TMB | TMB | TMB | TMB | TMB | TMB | TMB | TMB |
| Silica source | TEOS | TEOS | TEOS | TEOS | TEOS | TEOS | TEOS | TEOS | sodium silicate | TEOS/PhTMOS |
| Taging 1 [°C] | 70 | 70 | 70 | 70 | 90 | 90 | 70 | 70 | 70 | 70 |
| SBET 2 [m2/g] | 754 | 759 | 792 | 689 | 852 | 944 | 651 | 669 | 232 | 457 |
| Sext 3 [m2/g] | 4 | 4.5 | 19 | 14 | 55 | 25 | 9 | 80 | 27 | 4 |
| Vt 4 [cm3/g] | 0.47 | 0.50 | 0.91 | 0.66 | 1.29 | 1.08 | 0.40 | 0.69 | 0.17 | 0.28 |
| Vp 5 [cm3/g] | 0.47 | 0.49 | 0.87 | 0.63 | 1.18 | 1.03 | 0.37 | 0.54 | 0.12 | 0.27 |
| Vp/Vt 6 | 0.99 | 0.98 | 0.96 | 0.95 | 0.91 | 0.96 | 0.93 | 0.78 | 0.69 | 0.97 |
| DBJH ads 7 [nm] | 2.82 | 3.25 | 6.70 | 5.39 | 8.09 | 6.01 | 3.70 | 6.37 | 4.28 | 3.56 |
| Silica | T1 on 1 [°C] | T1 max 2 [°C] | ΔH1 3 [J/g] | T2 on 4 [°C] | T2 max 5 [°C] | ΔH2 6 [J/g] | DBJH ads 7 [nm] |
|---|---|---|---|---|---|---|---|
| S1 | −44 | −28 | 7.5 | 84 | 114 | 482 | 2.82 |
| S3 | −7.1 | −4.4 | 3.8 | 55 | 86 | 206 | 6.70 |
| S7 | −24 | −19 | 1.7 | 48 | 95 | 281 | 3.70 |
| S8 | −16 | −6.4/1.9 | 69 | 70 | 102 | 794 | 6.37 |
| Silica | Fit | f2/p | log k 1 | t0.5 [min] | ueq | a [mmol/g] | SD(c/c0) [%] | 1-R2 |
|---|---|---|---|---|---|---|---|---|
| S1 | 3-exp | - | −1.91 | 57 | 0.79 | 0.037 | 0.61 | 7.89 × 10−4 |
| f-FOE | 0/0.36 | −2.31 | 74 | 0.89 | - | 1.78 | 7.30 × 10−2 | |
| S3 | 3-exp | - | 0.16 | 0.50 | 0.43 | 0.020 | 0.19 | 4.38 × 10−4 |
| f-SOE | 1/0.30 | 0.70 | 5 | 0.50 | - | 1.31 | 2.42 × 10−2 | |
| S5 | 3-exp | - | 0.84 | 0.10 | 0.42 | 0.020 | 0.07 | 8.63 × 10−5 |
| f-SOE | 1/0.39 | 1.00 | 0.10 | 0.43 | - | 1.40 | 4.01 × 10−2 | |
| S7 | 3-exp | - | −2.06 | 80 | 0.42 | 0.020 | 0.20 | 2.32 × 10−4 |
| f-FOE | 0/0.52 | −2.24 | 86 | 0.43 | - | 0.63 | 2.50 × 10−3 | |
| S8 | 3-exp | - | −2.25 | 123 | 0.30 | 0.014 | 0.19 | 3.67 × 10−4 |
| f-FOE | 0/0.54 | −2.43 | 135 | 0.30 | - | 0.40 | 1.74 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blachnio, M.; Zienkiewicz-Strzalka, M.; Derylo-Marczewska, A. Mesoporous Silicas of Well-Organized Structure: Synthesis, Characterization, and Investigation of Physical Processes Occurring in Confined Pore Spaces. Int. J. Mol. Sci. 2025, 26, 9255. https://doi.org/10.3390/ijms26189255
Blachnio M, Zienkiewicz-Strzalka M, Derylo-Marczewska A. Mesoporous Silicas of Well-Organized Structure: Synthesis, Characterization, and Investigation of Physical Processes Occurring in Confined Pore Spaces. International Journal of Molecular Sciences. 2025; 26(18):9255. https://doi.org/10.3390/ijms26189255
Chicago/Turabian StyleBlachnio, Magdalena, Malgorzata Zienkiewicz-Strzalka, and Anna Derylo-Marczewska. 2025. "Mesoporous Silicas of Well-Organized Structure: Synthesis, Characterization, and Investigation of Physical Processes Occurring in Confined Pore Spaces" International Journal of Molecular Sciences 26, no. 18: 9255. https://doi.org/10.3390/ijms26189255
APA StyleBlachnio, M., Zienkiewicz-Strzalka, M., & Derylo-Marczewska, A. (2025). Mesoporous Silicas of Well-Organized Structure: Synthesis, Characterization, and Investigation of Physical Processes Occurring in Confined Pore Spaces. International Journal of Molecular Sciences, 26(18), 9255. https://doi.org/10.3390/ijms26189255








