Rotavirus Genotype Dynamics and the Emergence of G3P[8] in Thailand Following Nationwide Vaccine Implementation
Abstract
1. Introduction
2. Results
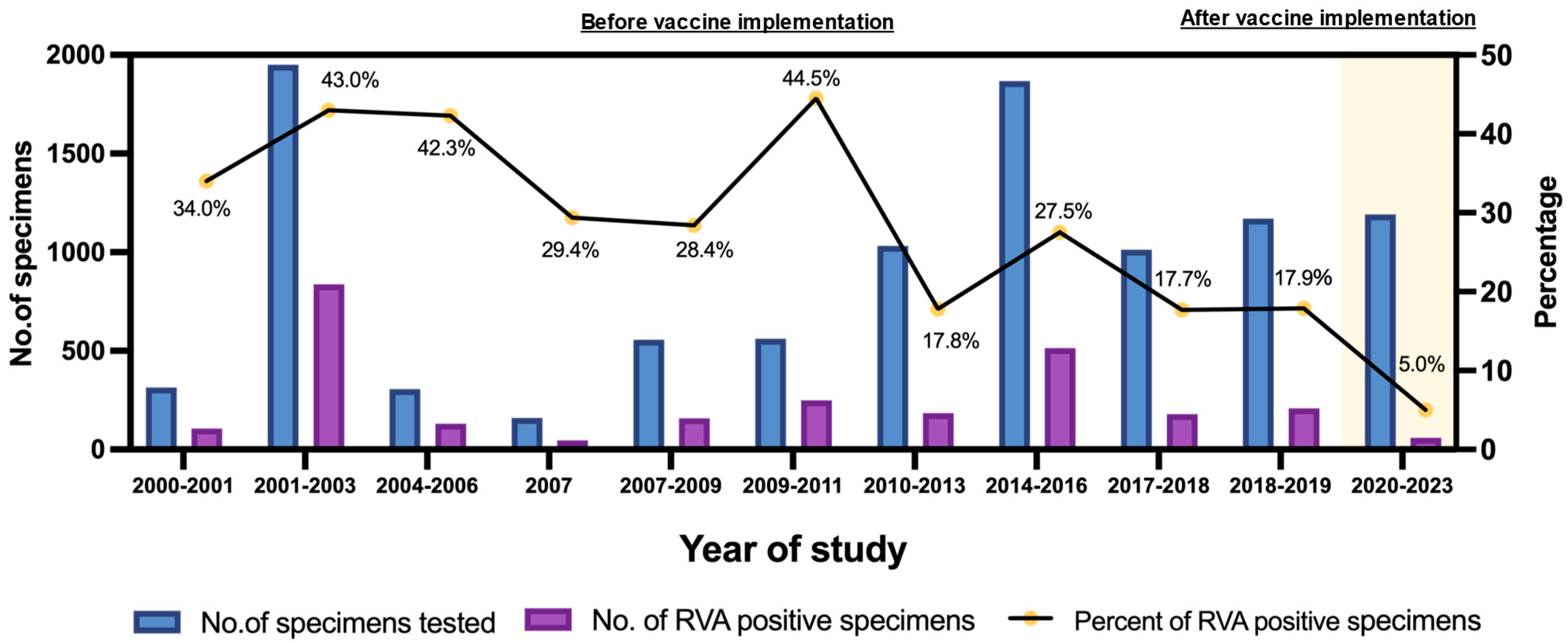
2.1. Detection Rate and Prevalence of RVA Infections
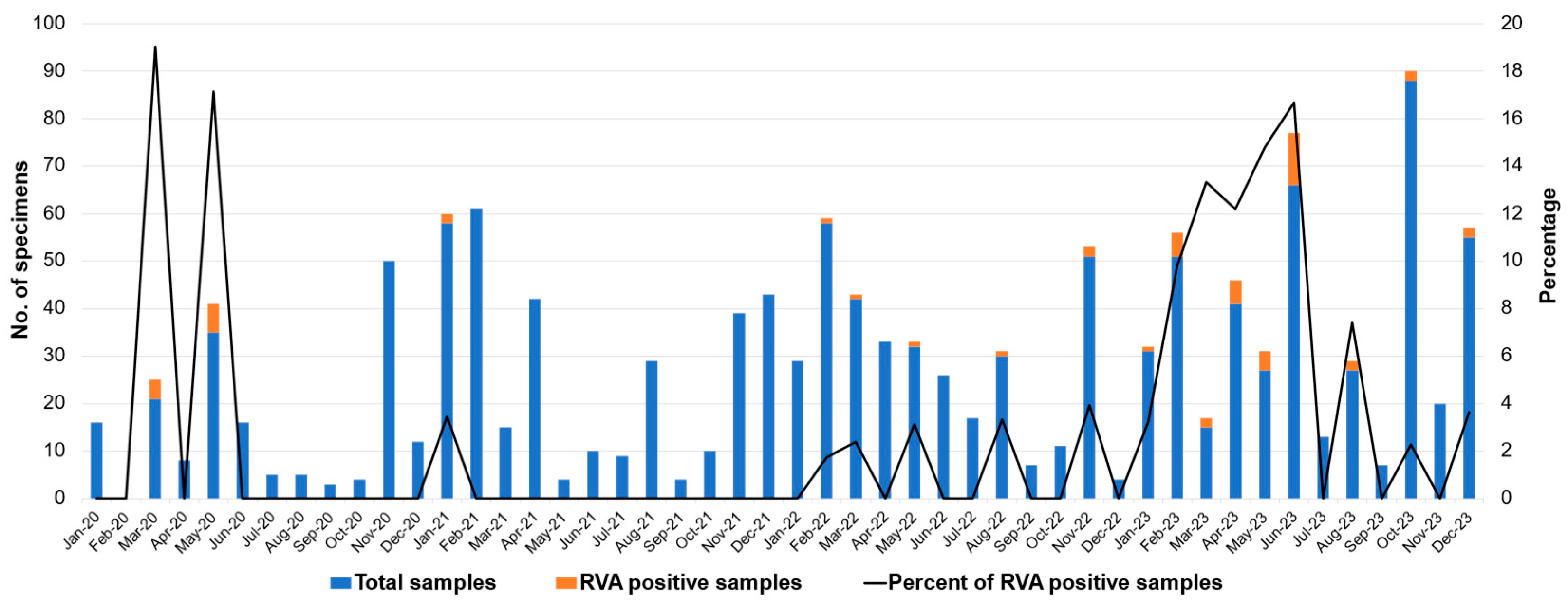
2.2. Monthly Distribution of RVA Infection
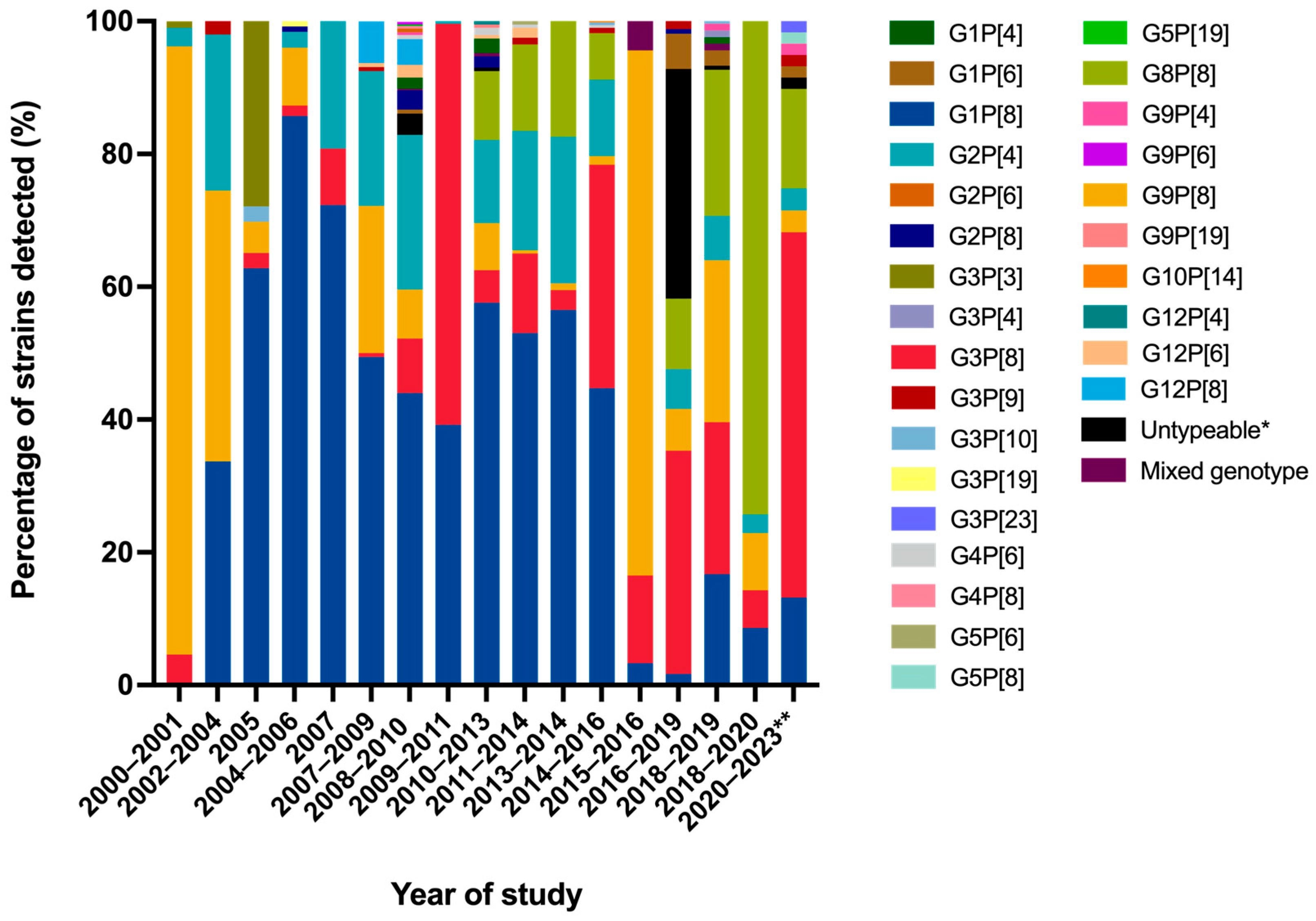
2.3. Distribution of G- and P-Genotypes
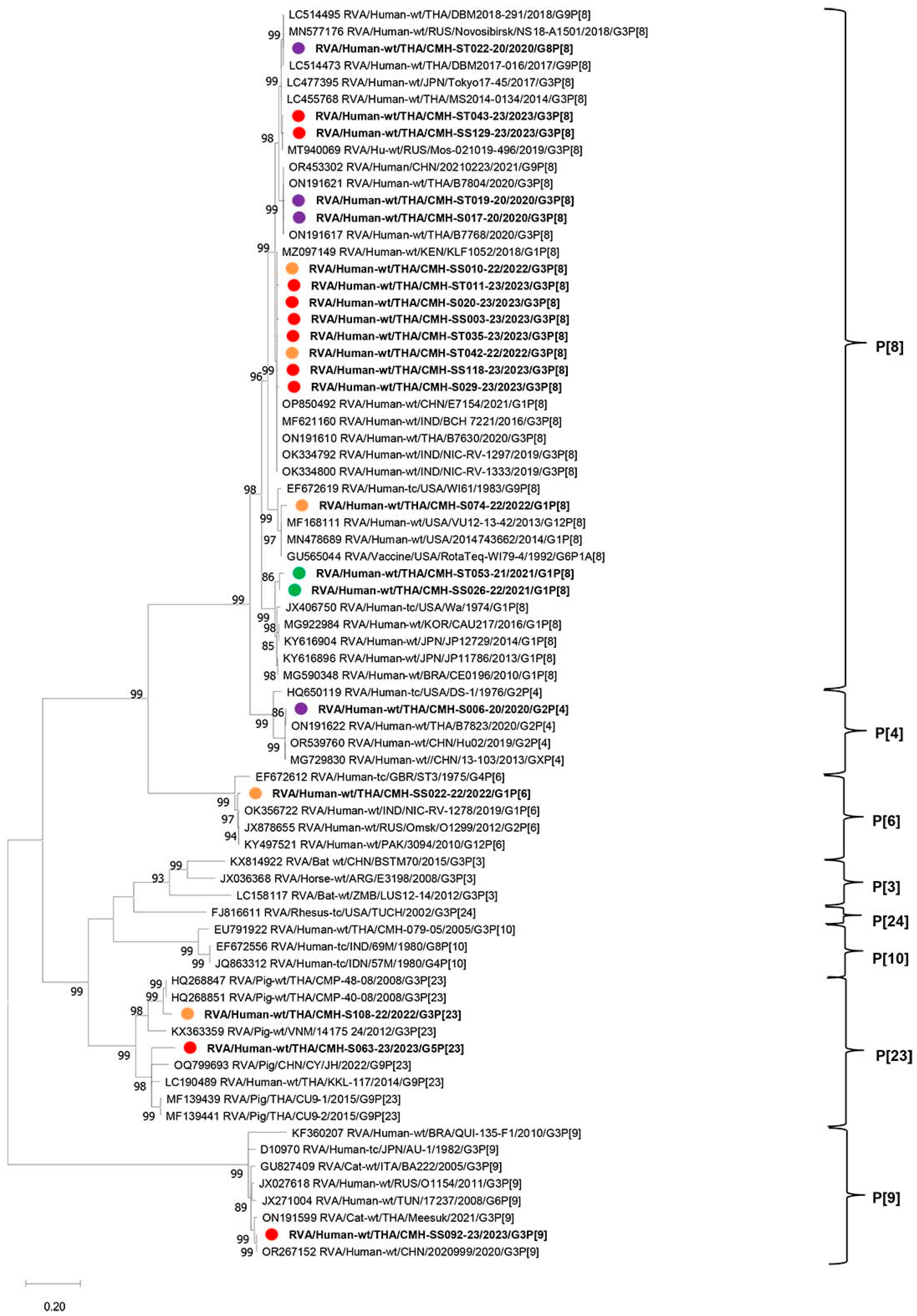
2.4. Phylogenetic Analysis of VP7 and VP4 Genes
3. Discussion
4. Materials and Methods
4.1. Study Design and Settings
4.2. Viral RNA Extraction and RVA Detection by RT-qPCR
4.3. RVA G- and P-Genotyping, Nucleotide Sequencing, and Phylogenetic Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGE | Acute gastroenteritis |
| COVID-19 | Coronavirus disease 2019 |
| DNA | Deoxyribonucleic acid |
| nm | Nanometer |
| NSP | Nonstructural protein |
| PCR | Polymerase chain reaction |
| pH | Potential of hydrogen ion |
| RT | Reverse transcription |
| RT-qPCR | Reverse transcription quantitative polymerase chain reaction |
| RNA | Ribonucleic acid |
| Rnase | Ribonuclease |
| RV | Rotavirus |
| RVs | Rotaviruses |
| RVA | Rotavirus A |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| VP | Viral protein |
References
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Parashar, U.D. Global, regional, and national estimates of rotavirus mortality in children < 5 years of age, 2000–2013. Clin. Infect. Dis. 2016, 62, 96–105. [Google Scholar]
- Jain, S.; Thakur, N.; Grover, N.; Vashistt, J.; Changotra, H. Prevalence of rotavirus, norovirus and enterovirus in diarrheal diseases in Himachal Pradesh, India. Virusdisease 2016, 27, 77–83. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.; Danthi, P.; Del Vas, M.; Dermody, T.S.; Duncan, R.; Fāng, Q.; Johne, R. ICTV virus taxonomy profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef]
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef]
- Fields, B.N. Fields’ Virology; Lippincott Williams & Wilkins: Ambler, PA, USA, 2007; Volume 1. [Google Scholar]
- Sadiq, A.; Bostan, N.; Yinda, K.C.; Naseem, S.; Sattar, S. Rotavirus: Genetics, pathogenesis and vaccine advances. Rev. Med. Virol. 2018, 28, e2003. [Google Scholar] [CrossRef]
- Asensio-Cob, D.; Rodríguez, J.M.; Luque, D. Rotavirus particle disassembly and assembly in vivo and in vitro. Viruses 2023, 15, 1750. [Google Scholar] [CrossRef]
- Estes, M.; Kapikian, A.; Knipe, D.; Howley, P. Rotaviruses. In Fields Virology; Williams and Wilkins: Philadelphia, PA, USA, 2007; pp. 1917–1974. [Google Scholar]
- Greenberg, H.B.; Estes, M.K. Rotaviruses: From pathogenesis to vaccination. Gastroenterology 2009, 136, 1939–1951. [Google Scholar] [CrossRef]
- RCWG. List of Accepted Genotypes. Available online: https://rega.kuleuven.be/cev/viralmetagenomics/virus-classification/rcwg (accessed on 25 August 2025).
- Dennehy, P.H. Transmission of rotavirus and other enteric pathogens in the home. Pediatr. Infect. Dis. J. 2000, 19, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, P.H. Rotavirus infection: A disease of the past? Infect. Dis. Clin. N. Am. 2015, 29, 617–635. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, C.; Zhang, X.; Yan, D.; Jiang, D.; Liu, X.; Yang, M.; Ding, C.; Lan, L.; Hecht, R.; et al. Global burden and trends of rotavirus infection-associated deaths from 1990 to 2019: An observational trend study. Virol. J. 2022, 19, 166. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Dias, H.G.; Gonçalves, J.L.S.; Manchego, A.; Rosadio, R.; Pezo, D.; Santos, N. Genetic diversity and zoonotic potential of rotavirus A strains in the southern Andean highlands, Peru. Transbound. Emerg. Dis. 2019, 66, 1718–1726. [Google Scholar] [CrossRef]
- Jampanil, N.; Kumthip, K.; Maneekarn, N.; Khamrin, P. Genetic diversity of rotaviruses circulating in pediatric patients and domestic animals in Thailand. Trop. Med. Infect. Dis. 2023, 8, 347. [Google Scholar] [CrossRef]
- Maneekarn, N.; Khamrin, P. Rotavirus associated gastroenteritis in Thailand. Virusdisease 2014, 25, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Maneekarn, N.; Ushijima, H. Epidemiology of rotavirus infection in Thailand. Pediatr. Int. 2000, 42, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Parashar, U.D.; Johnson, H.; Steele, A.D.; Tate, J.E. Health impact of rotavirus vaccination in developing countries: Progress and way forward. Clin. Infect. Dis. 2016, 62, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Dang, D.A.; Victor, J.C.; Shin, S.; Yunus, M.; Dallas, M.J.; Podder, G.; Vu, D.T.; Le, T.P.; Luby, S.P.; et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: A randomised, double-blind, placebo-controlled trial. Lancet 2010, 376, 615–623. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cunliffe, N.A.; Steele, D.; Witte, D.; Kirsten, M.; Louw, C.; Ngwira, B.; Victor, J.C.; Gillard, P.H.; Cheuvart, B.B.; et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N. Engl. J. Med. 2010, 362, 289–298. [Google Scholar] [CrossRef]
- Omatola, C.A.; Olaniran, A.O. Rotaviruses: From pathogenesis to disease control—A critical review. Viruses 2022, 14, 875. [Google Scholar] [CrossRef]
- Carvalho, M.F.; Gill, D. Rotavirus vaccine efficacy: Current status and areas for improvement. Hum. Vaccin. Immunother. 2019, 15, 1237–1250. [Google Scholar] [CrossRef]
- Patel, M.M.; Glass, R.; Desai, R.; Tate, J.E.; Parashar, U.D. Fulfilling the promise of rotavirus vaccines: How far have we come since licensure? Lancet Infect. Dis. 2012, 12, 561–570. [Google Scholar] [CrossRef]
- Bonura, F.; Mangiaracina, L.; Filizzolo, C.; Bonura, C.; Martella, V.; Ciarlet, M.; Giammanco, G.M.; De Grazia, S. Impact of vaccination on rotavirus genotype diversity: A nearly two-decade-long epidemiological study before and after rotavirus vaccine introduction in Sicily, Italy. Pathogens 2022, 11, 424. [Google Scholar] [CrossRef]
- Tharmaphornpilas, P.; Jiamsiri, S.; Boonchaiya, S.; Rochanathimoke, O.; Thinyounyong, W.; Tuntiwitayapun, S.; Guntapong, R.; Riewpaiboon, A.; Rasdjarmrearnsook, A.O.; Glass, R.I. Evaluating the first introduction of rotavirus vaccine in Thailand: Moving from evidence to policy. Vaccine 2017, 35, 796–801. [Google Scholar] [CrossRef]
- Charoenwat, B.; Suwannaying, K.; Paibool, W.; Laoaroon, N.; Sutra, S.; Thepsuthammarat, K. The impact of rotavirus vaccination on acute diarrhea in Thai children under 5 years of age after the first year of universal implementation of rotavirus vaccines in the national immunization program (NIP) in Thailand: A 6-year analysis. BMC Public Health 2023, 23, 2109. [Google Scholar] [CrossRef] [PubMed]
- Hungerford, D.; Allen, D.J.; Nawaz, S.; Collins, S.; Ladhani, S.; Vivancos, R.; Iturriza-Gómara, M. Impact of rotavirus vaccination on rotavirus genotype distribution and diversity in England, September 2006 to August 2016. Euro Surveill. 2019, 24, 1700774. [Google Scholar] [CrossRef]
- Roczo-Farkas, S.; Kirkwood, C.D.; Cowley, D.; Barnes, G.L.; Bishop, R.F.; Bogdanovic-Sakran, N.; Boniface, K.; Donato, C.M.; Bines, J.E. The impact of rotavirus vaccines on genotype diversity: A comprehensive analysis of 2 decades of Australian surveillance data. J. Infect. Dis. 2018, 218, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.B.; Arantes, I.; Bello, G.; Berto, L.H.; Dutra, L.H.; Kato, R.B.; Fumian, T.M. Emergence and dissemination of equine-like G3P[8] rotavirus A in Brazil between 2015 and 2021. Microbiol. Spectr. 2024, 12, e03709-23. [Google Scholar] [CrossRef]
- Vizzi, E.; Rosales, R.E.; Piñeros, O.; Fernández, R.; Inaty, D.; López, K.; Peña, L.; De Freitas-Linares, A.; Navarro, D.; Neri, S.; et al. Emergence of equine-like G3P[8] rotavirus strains infecting children in Venezuela. Viruses 2025, 17, 410. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Saokaew, S.; Prasitsuebsai, W.; Bibera, G.L.; Kengkla, K.; Zhang, X.-H.; Oh, K.-B.; Lee, C. Economic evaluation of human rotavirus vaccine in Thailand. Infect. Dis. Ther. 2019, 8, 397–415. [Google Scholar] [CrossRef]
- Chavers, T.; Cates, J.; Burnett, E.; Parashar, U.D.; Tate, J.E. Indirect protection from rotavirus vaccines: A systematic review. Expert. Rev. Vaccines 2024, 23, 789–795. [Google Scholar] [CrossRef]
- UNICEF; WHO. Rotavirus Vaccine Coverage. Available online: https://immunizationdata.who.int/global/wiise-detail-page/rotavirus-vaccination-coverage (accessed on 25 August 2025).
- Lappe, B.L.; Wikswo, M.E.; Kambhampati, A.K.; Mirza, S.A.; Tate, J.E.; Kraay, A.N.M.; Lopman, B.A. Predicting norovirus and rotavirus resurgence in the United States following the COVID-19 pandemic: A mathematical modelling study. BMC Infect. Dis. 2023, 23, 254. [Google Scholar] [CrossRef]
- Burnett, E.; Parashar, U.D.; Winn, A.; Curns, A.T.; Tate, J.E. Major changes in spatiotemporal trends of US rotavirus laboratory detections after rotavirus vaccine introduction—2009–2021. Pediatr. Infect. Dis. J. 2022, 41, 759–763. [Google Scholar] [CrossRef]
- Le Saux, N. Recommendations for the use of rotavirus vaccines in infants. Paediatr. Child Health 2017, 22, 290–294. [Google Scholar] [CrossRef][Green Version]
- Niewiesk, S. Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 2014, 5, 446. [Google Scholar] [CrossRef] [PubMed]
- Burnett, E.; Lopman, B.A.; Parashar, U.D. Potential for a booster dose of rotavirus vaccine to further reduce diarrhea mortality. Vaccine 2017, 35, 7198–7203. [Google Scholar] [CrossRef]
- Eigner, U.; Verstraeten, T.; Weil, J. Decrease in norovirus infections in Germany following COVID-19 containment measures. J. Infect. 2021, 82, 276–316. [Google Scholar] [CrossRef]
- Fukuda, Y.; Tsugawa, T.; Nagaoka, Y.; Ishii, A.; Nawa, T.; Togashi, A.; Kunizaki, J.; Hirakawa, S.; Iida, J.; Tanaka, T.; et al. Surveillance in hospitalized children with infectious diseases in Japan: Pre- and post-coronavirus disease 2019. J. Infect. Chemother. 2021, 27, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Park, J.Y.; Lim, I.S.; Chae, S.A.; Yun, S.W.; Lee, N.M.; Kim, S.Y.; Choi, B.S.; Yi, D.Y. Changes in the occurrence of gastrointestinal infections after COVID-19 in Korea. J. Korean Med. Sci. 2021, 36, e180. [Google Scholar] [CrossRef] [PubMed]
- Lazarakou, A.; Mughini-Gras, L.; Pijnacker, R. Global Impact of COVID-19 Pandemic on Gastrointestinal Infections: A Scoping Review. Foodborne Pathog. Dis. 2024. [Google Scholar] [CrossRef]
- Jampanil, N.; Kumthip, K.; Yodmeeklin, A.; Kanai, Y.; Okitsu, S.; Kobayashi, T.; Ukarapol, N.; Ushijima, H.; Maneekarn, N.; Khamrin, P. Epidemiology and genetic diversity of group A rotavirus in pediatric patients with acute gastroenteritis in Thailand, 2018–2019. Infect. Genet. Evol. 2021, 95, 104898. [Google Scholar] [CrossRef] [PubMed]
- Maneekarn, N.; Khamrin, P.; Chan-it, W.; Peerakome, S.; Sukchai, S.; Pringprao, K.; Ushijima, H. Detection of rare G3P[19] porcine rotavirus strains in Chiang Mai, Thailand, provides evidence for origin of the VP4 genes of Mc323 and Mc345 human rotaviruses. J. Clin. Microbiol. 2006, 44, 4113–4119. [Google Scholar] [CrossRef] [PubMed]
- Chan-It, W.; Chanta, C.; Ushijima, H. Predominance of DS-1-like G8P[8] rotavirus reassortant strains in children hospitalized with acute gastroenteritis in Thailand, 2018–2020. J. Med. Virol. 2023, 95, e28870. [Google Scholar] [CrossRef]
- Mwanga, M.J.; Verani, J.R.; Omore, R.; Tate, J.E.; Parashar, U.D.; Murunga, N.; Gicheru, E.; Breiman, R.F.; Nokes, D.J.; Agoti, C.N. Multiple introductions and predominance of rotavirus group A genotype G3P[8] in Kilifi, Coastal Kenya, 4 years after nationwide vaccine introduction. Pathogens 2020, 9, 981. [Google Scholar] [CrossRef]
- Manjate, F.; João, E.D.; Mwangi, P.; Chirinda, P.; Mogotsi, M.; Messa, A., Jr.; Garrine, M.; Vubil, D.; Nobela, N.; Nhampossa, T.; et al. Genomic characterization of the rotavirus G3P[8] strain in vaccinated children, reveals possible reassortment events between human and animal strains in Manhiça District, Mozambique. Front. Microbiol. 2023, 14, 1193094. [Google Scholar] [CrossRef]
- Kim, K.G.; Kee, H.Y.; Park, H.J.; Chung, J.K.; Kim, T.S.; Kim, M.J. The long-term impact of rotavirus vaccines in Korea, 2008–2020; emergence of G8P[8] strain. Vaccines 2021, 9, 406. [Google Scholar] [CrossRef]
- Kirkwood, C.D.; Boniface, K.; Barnes, G.L.; Bishop, R.F. Distribution of rotavirus genotypes after introduction of rotavirus vaccines, Rotarix® and RotaTeq®, into the national immunization program of Australia. Pediatr. Infect. Dis. J. 2011, 30, S48–S53. [Google Scholar] [CrossRef]
- Dóró, R.; László, B.; Martella, V.; Leshem, E.; Gentsch, J.; Parashar, U.; Bányai, K. Review of global rotavirus strain prevalence data from six years post vaccine licensure surveillance: Is there evidence of strain selection from vaccine pressure? Infect. Genet. Evol. 2014, 28, 446–461. [Google Scholar] [CrossRef]
- Pitzer, V.E.; Atkins, K.E.; de Blasio, B.F.; Van Effelterre, T.; Atchison, C.J.; Harris, J.P.; Shim, E.; Galvani, A.P.; Edmunds, W.J.; Viboud, C.; et al. Direct and indirect effects of rotavirus vaccination: Comparing predictions from transmission dynamic models. PLoS ONE 2012, 7, e42320. [Google Scholar] [CrossRef]
- Cowley, D.; Donato, C.M.; Roczo-Farkas, S.; Kirkwood, C.D. Emergence of a novel equine-like G3P[8] inter-genogroup reassortant rotavirus strain associated with gastroenteritis in Australian children. J. Gen. Virol. 2016, 97, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Mwanga, M.J.; Owor, B.E.; Ochieng, J.B.; Ngama, M.H.; Ogwel, B.; Onyango, C.; Juma, J.; Njeru, R.; Gicheru, E.; Otieno, G.P.; et al. Rotavirus group A genotype circulation patterns across Kenya before and after nationwide vaccine introduction, 2010–2018. BMC Infect. Dis. 2020, 20, 504. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Pitzer, V.E. Molecular epidemiology and evolution of rotaviruses. In Viral Gastroenteritis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 279–299. [Google Scholar]
- Lestari, F.B.; Chandranoi, K.; Chuchaona, W.; Vongpunsawad, S.; Poovorawan, Y. A G3P[9] rotavirus strain with an unusual genome constellation in a diarrheic cat in Thailand. Arch. Virol. 2023, 168, 24. [Google Scholar] [CrossRef]
- Chieochansin, T.; Vutithanachot, V.; Phumpholsup, T.; Posuwan, N.; Theamboonlers, A.; Poovorawan, Y. The prevalence and genotype diversity of human rotavirus A circulating in Thailand, 2011–2014. Infect. Genet. Evol. 2016, 37, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jampanil, N.; Khamrin, P.; Kumthip, K.; Longum, T.; Xie, Z.; Yodmeeklin, A.; Yamsakul, P.; Kongkaew, A.; Akari, Y.; Komoto, S.; et al. Prevalence and genetic diversity of porcine rotavirus A from diarrheic piglets in Northern Thailand. BMC Vet. Res. 2025, 21, 308. [Google Scholar] [CrossRef]
- Bassetto, M.; Van Dycke, J.; Neyts, J.; Brancale, A.; Rocha-Pereira, J. Targeting the viral polymerase of diarrhea-causing viruses as a strategy to develop a single broad-spectrum antiviral therapy. Viruses 2019, 11, 173. [Google Scholar] [CrossRef] [PubMed]
- Jothikumar, N.; Kang, G.; Hill, V.R. Broadly reactive TaqMan assay for real-time RT-PCR detection of rotavirus in clinical and environmental samples. J. Virol. Methods 2009, 155, 126–131. [Google Scholar] [CrossRef]
- Mijatovic-Rustempasic, S.; Tam, K.I.; Kerin, T.K.; Lewis, J.M.; Gautam, R.; Quaye, O.; Gentsch, J.R.; Bowen, M.D. Sensitive and specific quantitative detection of rotavirus A by one-step real-time reverse transcription-PCR assay without antecedent double-stranded-RNA denaturation. J. Clin. Microbiol. 2013, 51, 3047–3054. [Google Scholar] [CrossRef]
- Gautam, R.; Mijatovic-Rustempasic, S.; Esona, M.D.; Tam, K.I.; Quaye, O.; Bowen, M.D. One-step multiplex real-time RT-PCR assay for detecting and genotyping wild-type group A rotavirus strains and vaccine strains (Rotarix® and RotaTeq®) in stool samples. PeerJ 2016, 4, e1560. [Google Scholar] [CrossRef]
- Khamrin, P.; Okame, M.; Thongprachum, A.; Nantachit, N.; Nishimura, S.; Okitsu, S.; Maneekarn, N.; Ushijima, H. A single-tube multiplex PCR for rapid detection in feces of 10 viruses causing diarrhea. J. Virol. Methods 2011, 173, 390–393. [Google Scholar] [CrossRef]
- Gouvea, V.; Santos, N.; Timenetsky Mdo, C. Identification of bovine and porcine rotavirus G types by PCR. J. Clin. Microbiol. 1994, 32, 1338–1340. [Google Scholar] [CrossRef]
- Das, B.K.; Gentsch, J.R.; Cicirello, H.G.; Woods, P.A.; Gupta, A.; Ramachandran, M.; Kumar, R.; Bhan, M.K.; Glass, R.I. Characterization of rotavirus strains from newborns in New Delhi, India. J. Clin. Microbiol. 1994, 32, 1820–1822. [Google Scholar] [CrossRef] [PubMed]
- Gouvea, V.; Glass, R.I.; Woods, P.; Taniguchi, K.; Clark, H.F.; Forrester, B.; Fang, Z.Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 1990, 28, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Gentsch, J.R.; Glass, R.I.; Woods, P.; Gouvea, V.; Gorziglia, M.; Flores, J.; Das, B.K.; Bhan, M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]

| Year | Number of Specimen Tested | Number of RVA-Positive Specimens (%) |
|---|---|---|
| 2020 | 161 | 10 (6.2%) |
| 2021 | 303 | 10 (3.3%) |
| 2022 | 313 | 6 (1.9%) |
| 2023 | 415 | 34 (8.2%) |
| Total | 1192 | 60 (5.0%) |
| Variable | Number of Cases | Rotavirus A Positive (%) | Rotavirus A Negative (%) | |
|---|---|---|---|---|
| Gender a | Male, n (%) | 678 (56.9) | 33 (4.9) | 641 (94.5) |
| Female, n (%) | 490 (41.1) | 27 (5.5) | 467 (95.3) | |
| Total | 1168 c | 60 | 1108 c | |
| Age group (year) b | 0–1 | 457 | 9 (2.0) | 448 (98.0) |
| >1–2 | 243 | 11 (4.5) | 232 (95.5) | |
| >2–3 | 119 | 14 (11.8) | 105 (88.2) | |
| >3–4 | 97 | 8 (8.2) | 89 (91.8) | |
| >4–5 | 72 | 1 (1.4) | 71 (98.6) | |
| Total | 988 d | 43 d | 945 d | |
| Rotavirus A Genotype | Number of Rotavirus A Strains (%) | ||||
|---|---|---|---|---|---|
| 2020 (n = 161) | 2021 (n = 303) | 2022 (n = 313) | 2023 (n = 415) | Total (n = 1192) | |
| G1P[6] | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (1.7) |
| G1P[8] | 1 (10.0) | 3 (30.0) | 1 (16.7) | 3 (8.8) | 8 (13.3) |
| G2P[4] | 2 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.3) |
| G3P[8] | 4 (40.0) | 0 (0.0) | 2 (33.3) | 27 (79.5) | 33 (55.0) |
| G3P[9] | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) | 1 (1.7) |
| G3P[23] | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (1.7) |
| G5P[23] | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.9) | 1 (1.7) |
| G8P[8] | 2 (20.0) | 4 (40.0) | 1 (16.7) | 2 (5.9) | 9 (15.0) |
| G8P[X] | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| G9P[4] | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| G9P[8] | 1 (10.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 2 (3.3) |
| Total | 10 (16.7) | 10 (16.7) | 6 (10.0) | 34 (56.6) | 60 (100) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jampanil, N.; Kumthip, K.; Longum, T.; Xie, Z.; Yodmeeklin, A.; Sirilert, S.; Ukarapol, N.; Sansaard, N.; Promping, C.; Okitsu, S.; et al. Rotavirus Genotype Dynamics and the Emergence of G3P[8] in Thailand Following Nationwide Vaccine Implementation. Int. J. Mol. Sci. 2025, 26, 9249. https://doi.org/10.3390/ijms26189249
Jampanil N, Kumthip K, Longum T, Xie Z, Yodmeeklin A, Sirilert S, Ukarapol N, Sansaard N, Promping C, Okitsu S, et al. Rotavirus Genotype Dynamics and the Emergence of G3P[8] in Thailand Following Nationwide Vaccine Implementation. International Journal of Molecular Sciences. 2025; 26(18):9249. https://doi.org/10.3390/ijms26189249
Chicago/Turabian StyleJampanil, Nutthawadee, Kattareeya Kumthip, Thitapa Longum, Zhenfeng Xie, Arpaporn Yodmeeklin, Sirinart Sirilert, Nuthapong Ukarapol, Naphatrapee Sansaard, Channat Promping, Shoko Okitsu, and et al. 2025. "Rotavirus Genotype Dynamics and the Emergence of G3P[8] in Thailand Following Nationwide Vaccine Implementation" International Journal of Molecular Sciences 26, no. 18: 9249. https://doi.org/10.3390/ijms26189249
APA StyleJampanil, N., Kumthip, K., Longum, T., Xie, Z., Yodmeeklin, A., Sirilert, S., Ukarapol, N., Sansaard, N., Promping, C., Okitsu, S., Kobayashi, T., Ushijima, H., Maneekarn, N., & Khamrin, P. (2025). Rotavirus Genotype Dynamics and the Emergence of G3P[8] in Thailand Following Nationwide Vaccine Implementation. International Journal of Molecular Sciences, 26(18), 9249. https://doi.org/10.3390/ijms26189249






