Monoterpenes in Vascular Function: A Review of Bioactivity and Mechanisms of Action
Abstract
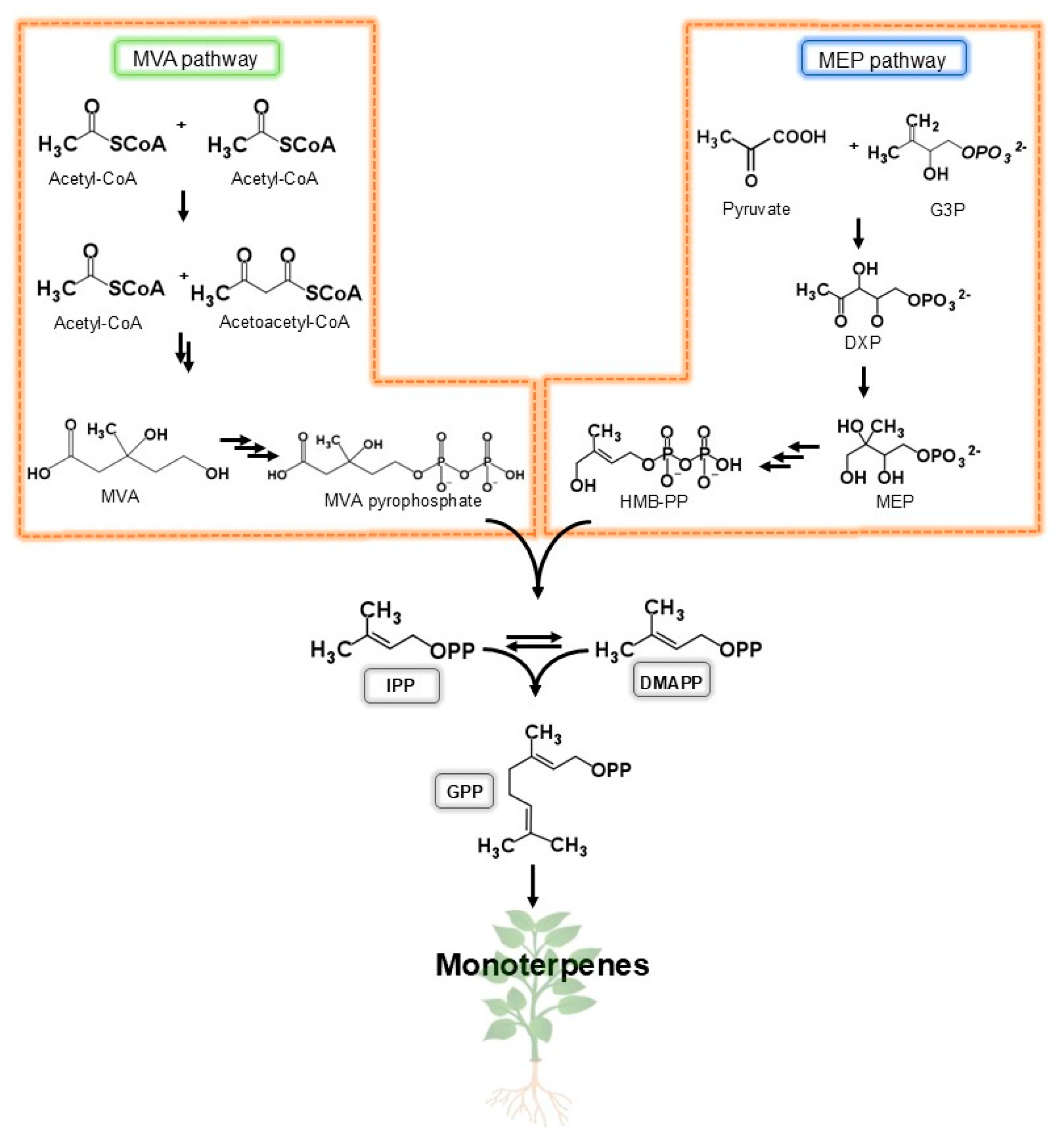
1. Introduction
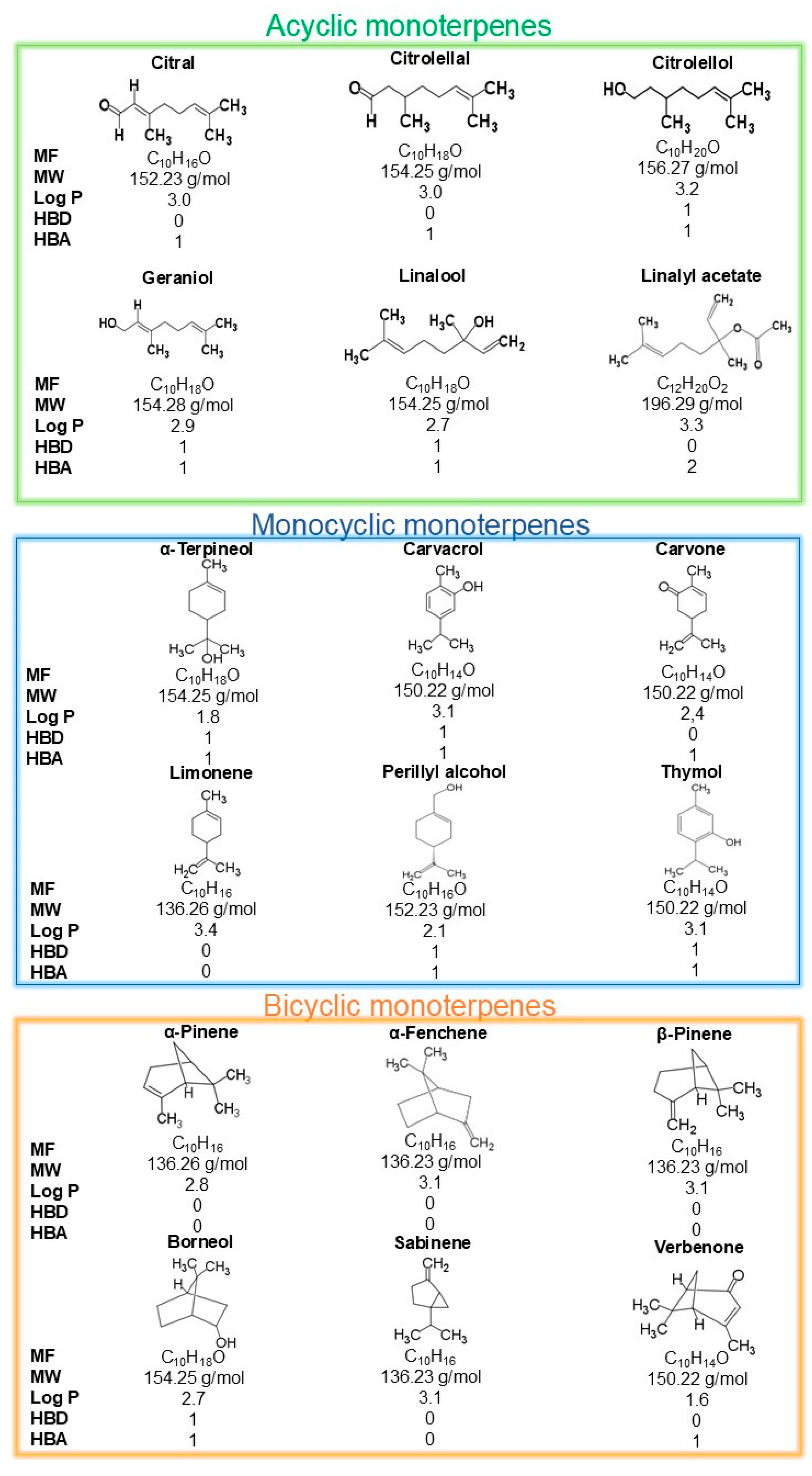
2. Monoterpenes Chemistry: An Overview
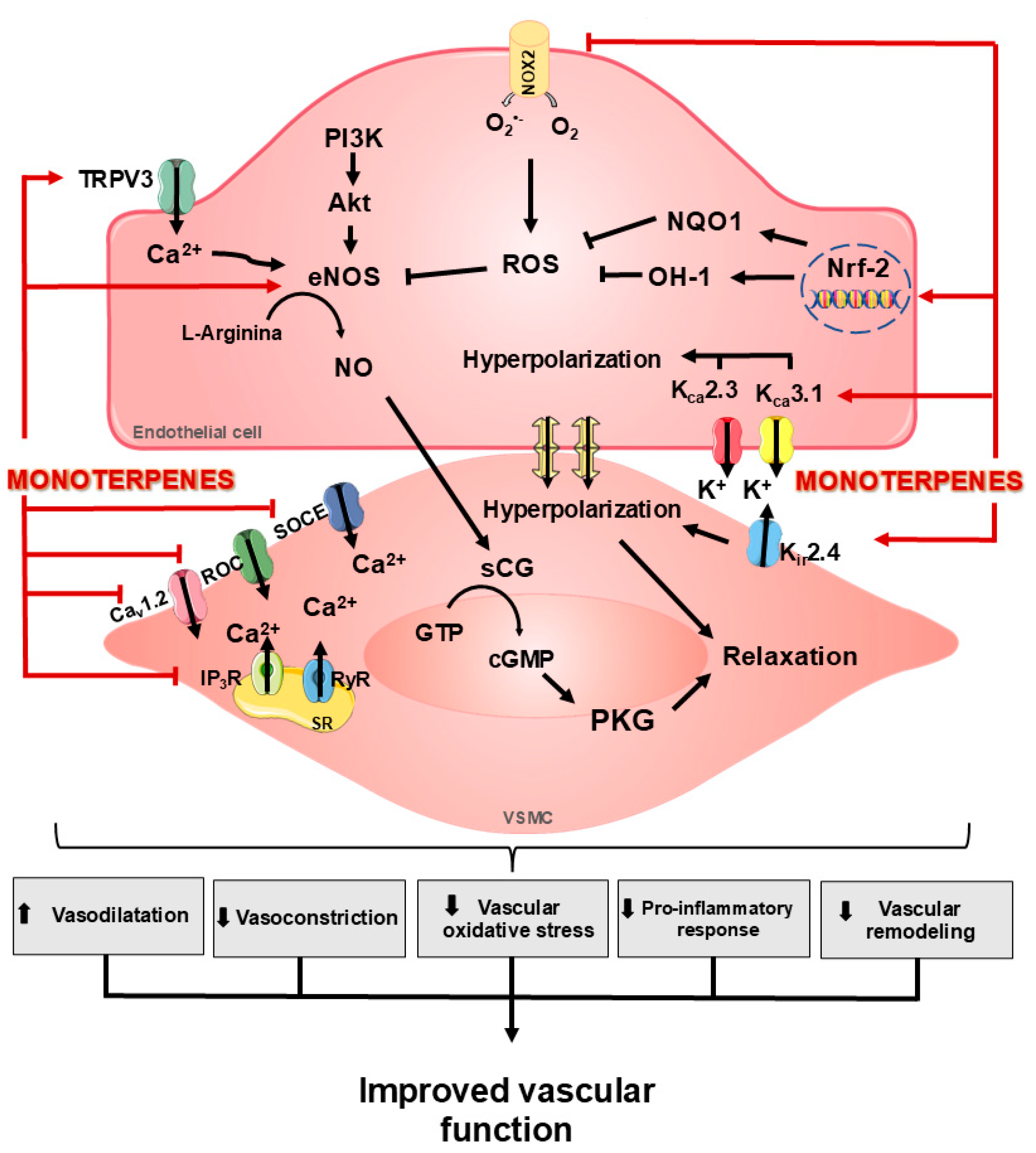
3. Role of Monoterpenes on the Vascular Function
3.1. Effects of Monoterpenes on NO Signaling and Oxidative Stress
3.2. Effects of Monoterpenes on K+ and EDHF Channels
3.3. Effects of Monoterpenes on TRP Channels and Ca2+ Signaling
4. Beneficial Effects of Monoterpenes on Vasculature
4.1. Geraniol
4.2. Carvacrol
4.3. Citronellal
4.4. Citronellol
4.5. Linalyl Acetate
4.6. Carvone
4.7. α-Terpineol
4.8. Linalool
4.9. Perillyl Alcohol
4.10. Borneol
5. Translational Challenges: Bioavailability, Clinical Evidence, and Safety
6. Study Limitations, Inconsistencies, and Gaps in Knowledge
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin converting enzyme |
| Ach | Acetylcholine |
| AMPK | AMP-activated protein kinase |
| BH4 | Tetrahydrobiopterin |
| cAMP | Cyclic Adenosine Monophosphate |
| CAT | Catalase |
| Cav | Voltage-gated calcium channels |
| cGMP | Cyclic guanosine monophosphate |
| COX | Cyclooxygenase |
| DMAPP | Dimethylallyl diphosphate |
| DRP1 | Dynamin-related protein 1 |
| DXP | 1-Deoxy-d-xylulose5-phosphate |
| EDHF | Endothelium-derived hyperpolarizing factors |
| eNOS | Endothelial nitric oxide synthase |
| EPC | Endothelial progenitor cell |
| ET-1 | Endothelin-1 |
| G3P | Glyceraldehyde-3-phosphate |
| GMP | Guanosine monophosphate |
| GPP | Geranyl diphosphate enzyme |
| GPx | Glutathione peroxidases |
| GSH | Glutathione reduced |
| GTP | Guanosine triphosphate |
| HIF-α | Hypoxia-inducible factor 1-alpha |
| HO-1 | Heme oxygenase-1 |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| IL | Interleukins |
| INDO | Indomethacin |
| iNOS | Induced nitric oxide synthase |
| IP3 | Inositol 1,4,5-trisphosphate |
| IP3R | Inositol 1,4,5-trisphosphate receptor |
| IPP | Isopentenyl diphosphate |
| KCa | Calcium-activated potassium channels |
| KCa2.3-SKCa | Potassium channels activated by small calcium conductance |
| KCa3.1-IKCa | Potassium channels activated by intermediate calcium conductance |
| Kir | ATP-sensitive potassium channels |
| Kv | Voltage-operated K+ channels |
| LDL | Low-density lipoprotein |
| L-Name | Nitro-L-arginine methyl ester (NOS inhibitors) |
| MAP | Mean arterial pressure |
| MAPK | Mitogen-activated protein kinase |
| MCT | Monocrotaline |
| MEP | 4-phosphate methylerythritol |
| MLCP | Myosin light chain phosphatase |
| MMP | Matrix metallopeptidase |
| MVA | Mevalonate |
| MW | Molecular Weigh |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Factor nuclear kappa B |
| NHE1 | Na+/H+ exchanger |
| NO | Nitric oxide |
| NQO1 | Quinone oxidoreductase-1 |
| Nrf-2 | Nuclear factor 2 related to erythroid factor 2 |
| PAH | Pulmonary hypertension |
| PDE | Phosphodiesterase |
| PI3K | Phosphatidylinositol 3-kinase |
| PKC | Protein kinase C |
| PKG | Cyclic GMP-dependent protein kinase |
| ROC | Receptor-operated calcium channels |
| ROS | Reactive oxygen species |
| RyR | Ryanodine receptor |
| S1P1 | Sphingosine-1-phosphate receptor subtype 1 |
| sCG | Soluble guanylyl cyclase |
| SCI | Spinal cord injury |
| SERCA | Sarcoendoplasmic reticulum calcium ATPase |
| SMC | Smooth muscle cells |
| SOC | Store-operated channels |
| SOCE | Store-operated calcium entry |
| SOD | Superoxide dismutase |
| SR | Sarcoplasmic reticulum |
| STZ | Streptozotocin |
| TEA | Tetraethylammonium chloride |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Protein tumor necrosis α |
| TPRM | Transient receptor potential melastatin channel |
| TPVR | Total peripheric vascular resistance |
| TRP | Transient receptor potential |
| TRPV | Transient receptor potential vanilloid |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VEGF | Vascular endothelial growth fator |
| VSMC | Vascular smooth muscle cells |
References
- Silva, E.A.P.; Santos, D.M.; de Carvalho, F.O.; Menezes, I.A.C.; Barreto, A.S.; Souza, D.S.; Quintans-Júnior, L.J.; Santos, M.R.V. Monoterpenes and their derivatives as agents for cardiovascular disease management: A systematic review and meta-analysis. Phytomedicine 2021, 88, 153451. [Google Scholar] [CrossRef]
- Silva, G.; Marques, J.N.J.; Linhares, E.P.M.; Bonora, C.M.; Costa, E.T.; Saraiva, M.F. Review of anticancer activity of monoterpenoids: Geraniol, nerol, geranial and neral. Chem. Biol. Interact. 2022, 362, 109994. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Błajet, M.; Feder-Kubis, J.J. Monoterpenes and their derivatives—Recent development in biological and medical applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Dehsheikh, A.B.; Sourestani, M.M.; Dehsheikh, P.B.; Mottaghipisheh, J.; Vitalini, S.; Iriti, M. Monoterpenes: Essential Oil Components with Valuable Features. Mini Rev. Med. Chem. 2020, 20, 958–974. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhong, Y.; Li, X.; Xiao, Y.; Wu, Y.; Xie, P. Biological evaluation of linalool on the function of blood vessels. Mol. Med. Rep. 2021, 24, 874. [Google Scholar] [CrossRef]
- de Andrade, T.U.; Brasil, G.A.; Endringer, D.C.; da Nobrega, F.R.; de Sousa, D.P. Cardiovascular Activity of the Chemical Constituents of Essential Oils. Molecules 2017, 22, 1539. [Google Scholar] [CrossRef]
- Zielińska-Blajet, M.; Pietrusiak, P.; Feder-Kubis, J. Selected Monocyclic Monoterpenes and Their Derivatives as Effective Anticancer Therapeutic Agents. Int. J. Mol. Sci. 2021, 22, 4763. [Google Scholar] [CrossRef]
- Kaspute, G.; Ivaskiene, T.; Ramanavicius, A.; Ramanavicius, S.; Prentice, U. Terpenes and Essential Oils in Pharmaceutics: Applications as Therapeutic Agents and Penetration Enhancers with Advanced Delivery Systems for Improved Stability and Bioavailability. Pharmaceutics 2025, 17, 793. [Google Scholar] [CrossRef]
- Henry, L.K.; Thomas, S.T.; Widhalm, J.R.; Lynch, J.H.; Davis, T.C.; Kessler, S.A.; Bohlmann, J.; Noel, J.P.; Dudareva, N. Contribution of isopentenyl phosphate to plant terpenoid metabolism. Nat. Plants 2018, 4, 721–729. [Google Scholar] [CrossRef]
- Ward, V.C.A.; Chatzivasileiou, A.O.; Stephanopoulos, G. Cell free biosynthesis of isoprenoids from isopentenol. Biotechnol. Bioeng. 2019, 116, 3269–3281. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, L.; Li, W.; Yang, Y.; Zhang, G.; Luo, Y. A homomeric geranyl diphosphate synthase-encoding gene from Camptotheca acuminata and its combinatorial optimization for production of geraniol in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2017, 44, 1431–1441. [Google Scholar] [CrossRef]
- Dewick, P.M. The biosynthesis of C5–C25 terpenoid compounds. Nat. Prod. Rep. 2002, 19, 181–222. [Google Scholar] [CrossRef]
- Koparal, A.T.; Zeytinoglu, M. Effects of Carvacrol on a Human Non-Small Cell Lung Cancer (NSCLC) Cell Line, A549. Cytotechnology 2003, 43, 149–154. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 611–625. [Google Scholar] [CrossRef]
- Oak, M.H.; Auger, C.; Belcastro, E.; Park, S.H.; Lee, H.H.; Schini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free. Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Vanhoutte, P.; Shimokawa, H.; Feletou, M.; Tang, E. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.J.P.O.; de Almeida Rezende, M.S.; Dantas, S.H.; de Lima Silva, S.; de Oliveira, J.C.P.L.; de Lourdes Assunção Araújo de Azevedo, F.; Alves, R.M.F.R.; de Menezes, G.M.S.; dos Santos, P.F.; Gonçalves, T.A.F.; et al. Unveiling the Role of Inflammation and Oxidative Stress on Age-Related Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2020, 8, 1954398. [Google Scholar] [CrossRef] [PubMed]
- Pourbagher-Shahri, A.M.; Farkhondeh, T.; Talebi, M.; Kopustinskiene, D.M.; Samarghandian, S.; Bernatoniene, J. An Overview of NO Signaling Pathways in Aging. Molecules 2021, 26, 4533. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular endothelial cell biology: An update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Golshiri, K.; Ataei Ataabadi, E.; Portilla Fernandez, E.C.; Jan Danser, A.; Roks, A.J. The importance of the nitric oxide-cGMP pathway in age-related cardiovascular disease: Focus on phosphodiesterase-1 and soluble guanylate cyclase. Basic Clin. Pharmacol. Toxicol. 2020, 127, 67–80. [Google Scholar] [CrossRef]
- Santos, S.E.; Ribeiro, F.; Menezes, P.M.N.; Duarte-Filho, L.A.M.; Quintans, J.S.S.; Quintans-Junior, L.J.; Silva, F.S.; Ribeiro, L.A.A. New insights on relaxant effects of (-)-borneol monoterpene in rat aortic rings. Fundam. Clin. Pharmacol. 2019, 33, 148–158. [Google Scholar] [CrossRef]
- da Silva, R.E.R.; de Morais, L.P.; Silva, A.A.; Bastos, C.M.S.; Pereira-Goncalves, A.; Kerntopf, M.R.; Menezes, I.R.A.; Leal-Cardoso, J.H.; Barbosa, R. Vasorelaxant effect of the Lippia alba essential oil and its major constituent, citral, on the contractility of isolated rat aorta. Biomed. Pharmacother. 2018, 108, 792–798. [Google Scholar] [CrossRef]
- Idris-Khodja, N.; Auger, C.; Koch, E.; Schini-Kerth, V.B. Crataegus special extract WS((R))1442 prevents aging-related endothelial dysfunction. Phytomedicine 2012, 19, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Kwon, S.; Lee, H.S.; Seol, G.H. Linalyl acetate prevents hypertension-related ischemic injury. PLoS ONE 2018, 13, e0198082. [Google Scholar] [CrossRef]
- Chen, Y.; Ba, L.; Huang, W.; Liu, Y.; Pan, H.; Mingyao, E.; Shi, P.; Wang, Y.; Li, S.; Qi, H.; et al. Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways. Eur. J. Pharmacol. 2017, 796, 90–100. [Google Scholar] [CrossRef]
- Kang, P.; Seol, G.H. Linalool elicits vasorelaxation of mouse aortae through activation of guanylyl cyclase and K+ channels. J. Pharm. Pharmacol. 2015, 67, 714–719. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Porto, D.L.; Menezes, C.P.; Antunes, A.A.; Silva, D.F.; De Sousa, D.P.; Nakao, L.S.; Braga, V.A.; Medeiros, I.A. Unravelling the cardiovascular effects induced by α-terpineol: A role for the nitric oxide–cGMP pathway. Clin. Exp. Pharmacol. Physiol. 2010, 37, 811–816. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Shabir, H.; Kundu, S.; Basir, S.F.; Khan, L.A. Modulation of Pb(II) caused aortal constriction by eugenol and carvacrol. Biol. Trace Elem. Res. 2014, 161, 116–122. [Google Scholar] [CrossRef]
- Kundu, S.; Shabir, H.; Basir, S.F.; Khan, L.A. Inhibition of As(III) and Hg(II) caused aortic hypercontraction by eugenol, linalool and carvone. J. Smooth Muscle Res. 2014, 50, 93–102. [Google Scholar] [CrossRef]
- Testai, L.; Chericoni, S.; Martelli, A.; Flamini, G.; Breschi, M.C.; Calderone, V. Voltage-operated potassium (Kv) channels contribute to endothelium-dependent vasorelaxation of carvacrol on rat aorta. J. Pharm. Pharmacol. 2016, 68, 1177–1183. [Google Scholar] [CrossRef]
- Peixoto-Neves, D.; Wang, Q.; Leal-Cardoso, J.H.; Rossoni, L.V.; Jaggar, J.H. Eugenol dilates mesenteric arteries and reduces systemic BP by activating endothelial cell TRPV4 channels. Br. J. Pharmacol. 2015, 172, 3484–3494. [Google Scholar] [CrossRef]
- Behringer, E.J.; Hakim, M.A. Functional interaction among KCa and TRP channels for cardiovascular physiology: Modern perspectives on aging and chronic disease. Int. J. Mol. Sci. 2019, 20, 1380. [Google Scholar] [CrossRef]
- Mathew John, C.; Khaddaj Mallat, R.; George, G.; Kim, T.; Mishra, R.C.; Braun, A.P. Pharmacologic targeting of endothelial Ca2+-activated K+ channels: A strategy to improve cardiovascular function. Channels 2018, 12, 126–136. [Google Scholar] [CrossRef]
- Goto, K.; Ohtsubo, T.; Kitazono, T. Endothelium-dependent hyperpolarization (EDH) in hypertension: The role of endothelial ion channels. Int. J. Mol. Sci. 2018, 19, 315. [Google Scholar] [CrossRef]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Thakore, P.; Earley, S. Transient receptor potential channels and endothelial cell calcium signaling. Compr. Physiol. 2019, 9, 1249–1277. [Google Scholar] [CrossRef]
- Earley, S.; Gonzales, A.L.; Garcia, Z.I. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol. Pharmacol. 2010, 77, 612–620. [Google Scholar] [CrossRef]
- Pires, P.W.; Sullivan, M.N.; Pritchard, H.A.; Robinson, J.J.; Earley, S. Unitary TRPV3 channel Ca2+ influx events elicit endothelium-dependent dilation of cerebral parenchymal arterioles. Am. J. Physiol.-Heart Circ. Physiol. 2015, 309, H2031–H2041. [Google Scholar] [CrossRef]
- Earley, S.; Brayden, J.E. Transient receptor potential channels in the vasculature. Physiol. Rev. 2015, 95, 645–690. [Google Scholar] [CrossRef]
- Dantas, B.P.V.; Alves, Q.L.; de Assis, K.S.; Ribeiro, T.P.; de Almeida, M.M.; de Vasconcelos, A.P.; de Araújo, D.A.M.; de Andrade Braga, V.; de Medeiros, I.A.; Alencar, J.L.; et al. Participation of the TRP channel in the cardiovascular effects induced by carvacrol in normotensive rat. Vasc. Pharmacol. 2015, 67, 48–58. [Google Scholar] [CrossRef]
- Silva, D.F.; de Almeida, M.M.; Chaves, C.G.; Braz, A.L.; Gomes, M.A.; Pinho-da-Silva, L.; Pesquero, J.L.; Andrade, V.A.; Leite Mde, F.; de Albuquerque, J.G.; et al. TRPM8 Channel Activation Induced by Monoterpenoid Rotundifolone Underlies Mesenteric Artery Relaxation. PLoS ONE 2015, 10, e0143171. [Google Scholar] [CrossRef]
- Harraz, O.F.; Jensen, L.J. Aging, calcium channel signaling and vascular tone. Mech. Ageing Dev. 2020, 191, 111336. [Google Scholar] [CrossRef]
- Ambudkar, I.S.; de Souza, L.B.; Ong, H.L. TRPC1, Orai1, and STIM1 in SOCE: Friends in tight spaces. Cell Calcium 2017, 63, 33–39. [Google Scholar] [CrossRef]
- de Siqueira, R.J.; Ribeiro-Filho, H.V.; Freire, R.S.; Cosker, F.; Freire, W.B.; Vasconcelos-Silva, A.A.; Soares, M.A.; Lahlou, S.; Magalhaes, P.J. (-)-alpha-Bisabolol inhibits preferentially electromechanical coupling on rat isolated arteries. Vasc. Pharmacol. 2014, 63, 37–45. [Google Scholar] [CrossRef]
- Cheang, W.S.; Lam, M.Y.; Wong, W.T.; Tian, X.Y.; Lau, C.W.; Zhu, Z.; Yao, X.; Huang, Y. Menthol relaxes rat aortae, mesenteric and coronary arteries by inhibiting calcium influx. Eur. J. Pharmacol. 2013, 702, 79–84. [Google Scholar] [CrossRef]
- Alvarez-Collazo, J.; Alonso-Carbajo, L.; Lopez-Medina, A.I.; Alpizar, Y.A.; Tajada, S.; Nilius, B.; Voets, T.; Lopez-Lopez, J.R.; Talavera, K.; Perez-Garcia, M.T.; et al. Cinnamaldehyde inhibits L-type calcium channels in mouse ventricular cardiomyocytes and vascular smooth muscle cells. Pflug. Arch. Eur. J. Physiol. 2014, 466, 2089–2099. [Google Scholar] [CrossRef]
- Melanaphy, D.; Johnson, C.D.; Kustov, M.V.; Watson, C.A.; Borysova, L.; Burdyga, T.V.; Zholos, A.V. Ion channel mechanisms of rat tail artery contraction-relaxation by menthol involving, respectively, TRPM8 activation and L-type Ca2+ channel inhibition. Am. J. physiology. Heart Circ. Physiol. 2016, 311, H1416–H1430. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Elberry, A.A.; Ghareib, S.A. Geraniol improves the impaired vascular reactivity in diabetes and metabolic syndrome through calcium channel blocking effect. J. Diabetes Its Complicat. 2016, 30, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Ben Ammar, R.; Mohamed, M.E.; Alfwuaires, M.; Alamer, S.A.; Bani Ismail, M.; Veeraraghavan, V.P.; Sekar, A.K.; Ksouri, R.; Rajendran, P. Anti-Inflammatory Activity of Geraniol Isolated from Lemon Grass on Ox-LDL-Stimulated Endothelial Cells by Upregulation of Heme Oxygenase-1 via PI3K/Akt and Nrf-2 Signaling Pathways. Nutrients 2022, 14, 4817, Erratum in Nutrients 2024, 16, 569. [Google Scholar] [CrossRef]
- Peixoto-Neves, D.; Silva-Alves, K.S.; Gomes, M.D.; Lima, F.C.; Lahlou, S.; Magalhaes, P.J.; Ceccatto, V.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam. Clin. Pharmacol. 2010, 24, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.C.; Mota, M.M.; Barreto, A.S.; Sousa, D.P.; Quintans-Junior, L.J.; Santos, M.R. Antihypertensive therapeutic potential of citronellal. Lat. Am. J. Pharm. 2012, 31, 767–771. [Google Scholar]
- Bastos, J.F.; Moreira, Í.J.; Ribeiro, T.P.; Medeiros, I.A.; Antoniolli, A.R.; De Sousa, D.P.; Santos, M.R. Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic Clin. Pharmacol. Toxicol. 2010, 106, 331–337. [Google Scholar] [CrossRef]
- Koto, R.; Imamura, M.; Watanabe, C.; Obayashi, S.; Shiraishi, M.; Sasaki, Y.; Azuma, H. Linalyl acetate as a major ingredient of lavender essential oil relaxes the rabbit vascular smooth muscle through dephosphorylation of myosin light chain. J. Cardiovasc. Pharmacol. 2006, 48, 850–856. [Google Scholar] [CrossRef]
- You, J.H.; Kang, P.; Min, S.S.; Seol, G.H. Bergamot essential oil differentially modulates intracellular Ca2+ levels in vascular endothelial and smooth muscle cells: A new finding seen with fura-2. J. Cardiovasc. Pharmacol. 2013, 61, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.C.; Sim, S.M.; Ismail, R. Effect of Cymbopogon citratus and Citral on Vascular Smooth Muscle of the Isolated Thoracic Rat Aorta. Evid.-Based Complement. Altern. Med. eCAM 2012, 2012, 539475. [Google Scholar] [CrossRef]
- Pereira, S.L.; Marques, A.M.; Sudo, R.T.; Kaplan, M.A.; Zapata-Sudo, G. Vasodilator activity of the essential oil from aerial parts of Pectis brevipedunculata and its main constituent citral in rat aorta. Molecules 2013, 18, 3072–3085. [Google Scholar] [CrossRef]
- Sabino, C.K.B.; Ferreira-Filho, E.S.; Mendes, M.B.; da Silva-Filho, J.C.; Ponte, M.P.T.R.; Moura, L.H.P.; Oliveira, E.C.A.; Quintans-Junior, L.J.; dos Santos, M.R.V.; de Cássia Meneses Oliveira, R. Cardiovascular effects induced by α-terpineol in hypertensive rats. Flavor Fragance J. 2013, 28, 333–339. [Google Scholar] [CrossRef]
- Anjos, P.J.; Lima, A.O.; Cunha, P.S.; De Sousa, D.P.; Onofre, A.S.; Ribeiro, T.P.; Medeiros, I.A.; Antoniolli, A.R.; Quintans-Junior, L.J.; Santosa, M.R. Cardiovascular effects induced by linalool in normotensive and hypertensive rats. Z. Fur Naturforsch. C J. Biosciences 2013, 68, 181–190. [Google Scholar] [CrossRef]
- Silva, M.; Ribeiro, F.P.; Medeiros, M.A.; Sampaio, P.A.; Silva, Y.; Silva, M.T.; Quintans, J.S.; Quintans-Júnior, L.J.; Ribeiro, L.A. The vasorelaxant effect of p-Cymene in rat aorta involves potassium channels. Sci. World J. 2015, 2015, 458080. [Google Scholar] [CrossRef]
- Cardoso-Teixeira, A.C.; Ferreira-da-Silva, F.W.; Peixoto-Neves, D.; Oliveira-Abreu, K.; Pereira-Goncalves, A.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Hydroxyl Group and Vasorelaxant Effects of Perillyl Alcohol, Carveol, Limonene on Aorta Smooth Muscle of Rats. Molecules 2018, 23, 1430. [Google Scholar] [CrossRef] [PubMed]
- Evaristo Rodrigues da Silva, R.; de Alencar Silva, A.; Pereira-de-Morais, L.; de Sousa Almeida, N.; Iriti, M.; Kerntopf, M.R.; Menezes, I.R.A.; Coutinho, H.D.M.; Barbosa, R. Relaxant Effect of Monoterpene (-)-Carveol on Isolated Human Umbilical Cord Arteries and the Involvement of Ion Channels. Molecules 2020, 25, 2681. [Google Scholar] [CrossRef] [PubMed]
- Unlu, S.; Mason, C.D.; Schachter, M.; Hughes, A.D. Perillyl alcohol, an inhibitor of geranylgeranyl transferase, induces apoptosis of immortalized human vascular smooth muscle cells in vitro. J. Cardiovasc. Pharmacol. 2000, 35, 341–344. [Google Scholar] [CrossRef]
- Moreira, I.J.; Menezes, P.P.; Serafini, M.R.; Araujo, A.A.; Quintans-Junior, L.J.; Bonjardim, L.R.; Filho, V.J.; D, B.P.J.; Santos, S.L.; Junior, W.L.; et al. Characterization and Antihypertensive Effect of the Complex of (-)-beta- pinene in beta-cyclodextrin. Curr. Pharm. Biotechnol. 2016, 17, 837–845. [Google Scholar] [CrossRef]
- Silva-Filho, J.C.; Oliveira, N.N.; Arcanjo, D.D.; Quintans-Junior, L.J.; Cavalcanti, S.C.; Santos, M.R.; Oliveira Rde, C.; Oliveira, A.P. Investigation of mechanisms involved in (-)-borneol-induced vasorelaxant response on rat thoracic aorta. Basic Clin. Pharmacol. Toxicol. 2012, 110, 171–177. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Su, M.; Sun, L.; Zhang, S.; Wang, D.; Liu, Z.; Yuan, Y.; Liu, Y.; Li, Y. Geraniol improves endothelial function by inhibiting NOX-2 derived oxidative stress in high fat diet fed mice. Biochem. Biophys. Res. Commun. 2016, 474, 182–187. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, J.; Ma, K.T.; Li, C.L.; Mai, Y.P.; Qiu, X.X.; Wei, H.; Hou, N.; Luo, J.D. Carvacrol protects against diabetes-induced hypercontractility in the aorta through activation of the PI3K/Akt pathway. Biomed. Pharmacother. 2020, 125, 109825. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, T.A.F.; Lima, V.S.; de Almeida, A.; de Arruda, A.V.; Veras, A.; Lima, T.T.; Soares, E.M.C.; Santos, A.C.D.; Vasconcelos, M.E.C.; de Almeida Feitosa, M.S.; et al. Carvacrol Improves Vascular Function in Hypertensive Animals by Modulating Endothelial Progenitor Cells. Nutrients 2023, 15, 3032. [Google Scholar] [CrossRef]
- Lu, J.X.; Guo, C.; Ou, W.S.; Jing, Y.; Niu, H.F.; Song, P.; Li, Q.Z.; Liu, Z.; Xu, J.; Li, P.; et al. Citronellal prevents endothelial dysfunction and atherosclerosis in rats. J. Cell. Biochem. 2019, 120, 3790–3800, Erratum in J. Cell. Biochem. 2025, 126, e70054. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chao, C.Y.; Jiang, L.; Zhang, J.; Niu, Q.Q.; Guo, Y.Q.; Song, Y.T.; Li, P.; Zhu, M.L.; Yin, Y.L. Citronellal alleviate macro- and micro-vascular damage in high fat diet/streptozotocin—Induced diabetic rats via a S1P/S1P1 dependent signaling pathway. Eur. J. Pharmacol. 2022, 920, 174796, Erratum in Eur. J. Pharmacol. 2025, 991, 177319. [Google Scholar] [CrossRef]
- Yin, Y.L.; Wang, H.H.; Gui, Z.C.; Mi, S.; Guo, S.; Wang, Y.; Wang, Q.Q.; Yue, R.Z.; Lin, L.B.; Fan, J.X.; et al. Citronellal Attenuates Oxidative Stress-Induced Mitochondrial Damage through TRPM2/NHE1 Pathway and Effectively Inhibits Endothelial Dysfunction in Type 2 Diabetes Mellitus. Antioxidants 2022, 11, 2241. [Google Scholar] [CrossRef]
- Dai, M.; Wu, L.; Yu, K.; Xu, R.; Wei, Y.; Chinnathambi, A.; Alahmadi, T.A.; Zhou, M. D-Carvone inhibit cerebral ischemia/reperfusion induced inflammatory response TLR4/NLRP3 signaling pathway. Biomed. Pharmacother. 2020, 132, 110870. [Google Scholar] [CrossRef]
- Camargo, S.B.; Simoes, L.O.; Medeiros, C.F.A.; de Melo Jesus, A.; Fregoneze, J.B.; Evangelista, A.; Villarreal, C.F.; Araujo, A.A.S.; Quintans-Junior, L.J.; Silva, D.F. Antihypertensive potential of linalool and linalool complexed with beta-cyclodextrin: Effects of subchronic treatment on blood pressure and vascular reactivity. Biochem. Pharmacol. 2018, 151, 38–46. [Google Scholar] [CrossRef]
- Lund, A.K.; Doyle-Eisele, M.; Lin, Y.H.; Arashiro, M.; Surratt, J.D.; Holmes, T.; Schilling, K.A.; Seinfeld, J.H.; Rohr, A.C.; Knipping, E.M.; et al. The effects of alpha-pinene versus toluene-derived secondary organic aerosol exposure on the expression of markers associated with vascular disease. Inhal. Toxicol. 2013, 25, 309–324. [Google Scholar] [CrossRef]
- AlAsmari, A.F.; Ali, N.; Alharbi, M.; Alqahtani, F.; Alasmari, F.; Almoqbel, D.; AlSwayyed, M.; Alshammari, A.; Alanazi, M.M.; Alhoshani, A.; et al. Geraniol Ameliorates Doxorubicin-Mediated Kidney Injury through Alteration of Antioxidant Status, Inflammation, and Apoptosis: Potential Roles of NF-kappaB and Nrf2/HO-1. Nutrients 2022, 14, 1620. [Google Scholar] [CrossRef]
- Lei, Y.; Fu, P.; Jun, X.; Cheng, P. Pharmacological properties of geraniol—A review. Planta Medica 2019, 85, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.H.; Guan, Y.Y.; Min, J.; He, H. Contractile responses of diabetic rat aorta to phenylephrine at different stages of diabetic duration. Acta Pharmacol. Sin. 2001, 22, 445–449. [Google Scholar] [PubMed]
- Romero-Garcia, T.; Vazquez-Jimenez, J.G.; Sanchez-Hernandez, R.; Olivares-Reyes, J.A.; Rueda, A. Insulin resistance, Ca2+ signaling alterations and vascular dysfunction in prediabetes and metabolic syndrome. Front. Physiol. 2025, 16, 1535153. [Google Scholar] [CrossRef]
- Soro-Paavonen, A.; Zhang, W.Z.; Venardos, K.; Coughlan, M.T.; Harris, E.; Tong, D.C.; Brasacchio, D.; Paavonen, K.; Chin-Dusting, J.; Cooper, M.E.; et al. Advanced glycation end-products induce vascular dysfunction via resistance to nitric oxide and suppression of endothelial nitric oxide synthase. J. Hypertens. 2010, 28, 780–788. [Google Scholar] [CrossRef]
- Mukohda, M.; Okada, M.; Hara, Y.; Yamawaki, H. Exploring mechanisms of diabetes-related macrovascular complications: Role of methylglyoxal, a metabolite of glucose on regulation of vascular contractility. J. Pharmacol. Sci. 2012, 118, 303–310. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-Garcia, O.; Dominguez-Perez, M.; Gonzalez-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Wang, J.; Su, B.; Zhu, H.; Chen, C.; Zhao, G. Protective effect of geraniol inhibits inflammatory response, oxidative stress and apoptosis in traumatic injury of the spinal cord through modulation of NF-kappaB and p38 MAPK. Exp. Ther. Med. 2016, 12, 3607–3613. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef] [PubMed]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Sabzalian, M.R. Thymol, carvacrol, and antioxidant accumulation in Thymus species in response to different light spectra emitted by light-emitting diodes. Food Chem. 2020, 307, 125521. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.Z.; Gu, C.X.; Liu, G.L.; Tian, K. Carvacrol suppresses inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes. J. Cell. Biochem. 2018, 120, 8169–8176. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, S.; Hejazian, S.H.; Jamhiri, M.; Hafizibarjin, Z.; Sadeghzadeh, S.; Safari, F. The effect of carvacrol on transcription levels of Bcl-2 family proteins in hypertrophied heart of rats. J. Physiol. Pharmacol. 2018, 22, 54–62. [Google Scholar]
- Zhao, W.; Deng, C.; Han, Q.; Xu, H.; Chen, Y. Carvacrol may alleviate vascular inflammation in diabetic db/db mice. Int. J. Mol. Med. 2020, 46, 977–988, Erratum in Int. J. Mol. Med. 2023, 52, 90. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Akpan, E.; Nyoh, A. Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (DC.) Stapf extracts. Chin. J. Nat. Med. 2015, 13, 321–337. [Google Scholar] [CrossRef]
- Melo, M.S.; Guimaraes, A.G.; Santana, M.F.; Siqueira, R.S.; De Lima Ado, C.; Dias, A.S.; Santos, M.R.; Onofre, A.S.; Quintans, J.S.; De Sousa, D.P.; et al. Anti-inflammatory and redox-protective activities of citronellal. Biol. Res. 2011, 44, 363–368. [Google Scholar] [CrossRef]
- Lu, J.X.; Qiu, Y.; Guo, L.J.; Song, P.; Xu, J.; Wan, G.R.; Wang, S.X.; Yin, Y.L.; Li, P. Potential Therapeutic Effect of Citronellal on Diabetic Cardiomyopathy in Experimental Rats. Evid.-Based Complement. Altern. Med. eCAM 2021, 2021, 9987531. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Que, H.; Chen, B.; Chao, C.; Li, S.; Guo, S.; Yin, Y.; Wang, H.; Zhu, M.; Li, P. Citronellal improves endothelial dysfunction by affecting the stability of the GCH1 protein. Acta Biochim. Et Biophys. Sin. 2024, 56, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Zhang, X.; Liu, J.; Wang, Y.; Sukhova, G.K.; Wojtkiewicz, G.R.; Liu, T.; Tang, R.; Achilefu, S.; Nahrendorf, M.; et al. Na+-H+ exchanger 1 determines atherosclerotic lesion acidification and promotes atherogenesis. Nat. Commun. 2019, 10, 3978. [Google Scholar] [CrossRef]
- Zielinska, W.; Zabrzynski, J.; Gagat, M.; Grzanka, A. The Role of TRPM2 in Endothelial Function and Dysfunction. Int. J. Mol. Sci. 2021, 22, 7635. [Google Scholar] [CrossRef] [PubMed]
- Allende, M.L.; Yamashita, T.; Proia, R.L. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood 2003, 102, 3665–3667. [Google Scholar] [CrossRef]
- Brito, R.G.; Dos Santos, P.L.; Quintans, J.S.; de Lucca Junior, W.; Araujo, A.A.; Saravanan, S.; Menezes, I.R.; Coutinho, H.D.; Quintans-Junior, L.J. Citronellol, a natural acyclic monoterpene, attenuates mechanical hyperalgesia response in mice: Evidence of the spinal cord lamina I inhibition. Chem.-Biol. Interact. 2015, 239, 111–117. [Google Scholar] [CrossRef]
- Brito, R.G.; Guimaraes, A.G.; Quintans, J.S.; Santos, M.R.; De Sousa, D.P.; Badaue-Passos, D., Jr.; de Lucca, W., Jr.; Brito, F.A.; Barreto, E.O.; Oliveira, A.P.; et al. Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activities in rodents. J. Nat. Med. 2012, 66, 637–644. [Google Scholar] [CrossRef]
- Srinivasan, S.; Muruganathan, U. Antidiabetic efficacy of citronellol, a citrus monoterpene by ameliorating the hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2016, 250, 38–46. [Google Scholar] [CrossRef]
- de Menezes, I.A.; Moreira, I.J.; de Paula, J.W.; Blank, A.F.; Antoniolli, A.R.; Quintans-Junior, L.J.; Santos, M.R. Cardiovascular effects induced by Cymbopogon winterianus essential oil in rats: Involvement of calcium channels and vagal pathway. J. Pharm. Pharmacol. 2010, 62, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, C.; Yin, Y. Anti-ischemic effect of monoterpene citronellol on experimental stroke models mediated by proinflammatroy cytokines. Comb. Chem. High Throughput Screen. 2023, 26, 1888–1899. [Google Scholar]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Lavender (Lavandula angustifolia) Essential Oil on Acute Inflammatory Response. Evid.-Based Complement. Altern. Med. eCAM 2018, 2018, 1413940. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Kang, P.; Lee, H.S.; Kim, K.Y.; Seol, G.H. Cardiovascular effects of linalyl acetate in acute nicotine exposure. Environ. Health Prev. Med. 2017, 22, 42. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Shin, Y.K.; Han, A.Y.; Kwon, S.; Seol, G.H. Linalyl acetate prevents three related factors of vascular damage in COPD-like and hypertensive rats. Life Sci. 2019, 232, 116608. [Google Scholar] [CrossRef]
- Shin, Y.K.; Hsieh, Y.S.; Kwon, S.; Lee, H.S.; Seol, G.H. Linalyl acetate restores endothelial dysfunction and hemodynamic alterations in diabetic rats exposed to chronic immobilization stress. J. Appl. Physiol. 2018, 124, 1274–1283. [Google Scholar] [CrossRef]
- Muruganathan, U.; Srinivasan, S. Beneficial effect of carvone, a dietary monoterpene ameliorates hyperglycemia by regulating the key enzymes activities of carbohydrate metabolism in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2016, 84, 1558–1567. [Google Scholar] [CrossRef]
- Kang, W.; Choi, D.; Park, S.; Park, T. Carvone Decreases Melanin Content by Inhibiting Melanoma Cell Proliferation via the Cyclic Adenosine Monophosphate (cAMP) Pathway. Molecules 2020, 25, 5191. [Google Scholar] [CrossRef]
- Ding, X.; Chen, H. Anticancer effects of Carvone in myeloma cells is mediated through the inhibition of p38 MAPK signalling pathway, apoptosis induction and inhibition of cell invasion. JBUON 2018, 23, 747–751. [Google Scholar] [PubMed]
- Asle-Rousta, M.; Amini, R.; Aghazadeh, S. Carvone suppresses oxidative stress and inflammation in the liver of immobilised rats. Arch. Physiol. Biochem. 2020, 129, 597–602. [Google Scholar] [CrossRef]
- Chen, G.; Song, Y.; Ma, F.; Ma, Y. Anti-arthritic activity of D-carvone against complete Freund’s adjuvant-induced arthritis in rats through modulation of inflammatory cytokines. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2020, 24, 453–462. [Google Scholar] [CrossRef]
- Long, J.; Sun, Y.; Liu, S.; Chen, C.; Yan, Q.; Lin, Y.; Zhang, Z.; Chu, S.; Yang, Y.; Yang, S.; et al. Ginsenoside Rg1 treats ischemic stroke by regulating CKLF1/CCR5 axis-induced neuronal cell pyroptosis. Phytomedicine 2024, 123, 155238. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Souza, D.S.; Menezes-Filho, J.E.R.; Silva-Neto, J.A.D.; Cruz, J.D.S.; Roman-Campos, D.R.; Quintans-Junior, L.J.; Vasconcelos, C.M.L. (-)-Carvone Modulates Intracellular Calcium Signaling with Antiarrhythmic Action in Rat Hearts. Arq. Bras. De Cardiol. 2022, 119, 294–304. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. J. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Sousa, G.M.; Cazarin, C.B.B.; Marostica Junior, M.R.; Lamas, C.A.; Quitete, V.; Pastore, G.M.; Bicas, J.L. The effect of alpha-terpineol enantiomers on biomarkers of rats fed a high-fat diet. Heliyon 2020, 6, e03752. [Google Scholar] [CrossRef]
- Negreiros, H.A.; de Moura, K.G.; Barreto do Nascimento, M.L.L.; do Nascimento Rodrigues, D.C.; Ferreir, P.M.P.; Braz, D.C.; de Farias, M.G.; de Sousa Corrêia, L.; Pereira, A.R.S.; Santos, L.K.B.; et al. Alpha-Terpineol as Antitumor Candidate in Pre-Clinical Studies. Anti-Cancer Agents Med. Chem. 2021, 21, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Sheikholeslami, M.A.; Ghafghazi, S.; Pouriran, R.; Parvardeh, S. Analgesic effect of α-terpineol on neuropathic pain induced by chronic constriction injury in rat sciatic nerve: Involvement of spinal microglial cells and inflammatory cytokines. Iran. J. Basic Med. Sci. 2019, 22, 1445–1451. [Google Scholar]
- Gouveia, D.N.; Costa, J.S.; Oliveira, M.A.; Rabelo, T.K.; Silva, A.; Carvalho, A.A.; Miguel-Dos-Santos, R.; Lauton-Santos, S.; Scotti, L.; Scotti, M.T.; et al. α-Terpineol reduces cancer pain via modulation of oxidative stress and inhibition of iNOS. Biomed. Pharmacother. 2018, 105, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Paulino, E.T.; Rodrigues, A.K.B.F.; Machado, M.L.D.P.; de Oliveira, K.R.V.; Bernardino, A.C.; Quintans-Júnior, L.J.; Oliveira, A.P.; Ribeiro, Ê.A.N. Alpha-terpineol prevents myocardial damage against isoproterenol-MI induced in Wistar-Kyoto rats: New possible to promote cardiovascular integrity. Life Sci. 2022, 290, 120087. [Google Scholar] [CrossRef]
- Matsumoto, T.; Taguchi, K.; Kobayashi, T. Role of TRPV4 on vascular tone regulation in pathophysiological states. Eur. J. Pharmacol. 2023, 959, 176104. [Google Scholar] [CrossRef]
- Magalhaes, P.J.; Lahlou, S.; Juca, D.M.; Coelho-de-Souza, L.N.; da Frota, P.T.; da Costa, A.M.; Leal-Cardoso, J.H. Vasorelaxation induced by the essential oil of Croton nepetaefolius and its constituents in rat aorta are partially mediated by the endothelium. Fundam. Clin. Pharmacol. 2008, 22, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yu, L.; Qiu, J.; Shen, B.; Wang, D.; Soromou, L.W.; Feng, H. Linalool attenuates lung inflammation induced by Pasteurella multocida via activating Nrf-2 signaling pathway. Int. Immunopharmacol. 2014, 21, 456–463. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, C.; Zhang, J. SIRT3-SOD2-ROS pathway is involved in linalool-induced glioma cell apoptotic death. Acta Biochim. Pol. 2017, 64, 343–350. [Google Scholar] [CrossRef]
- Sabogal-Guaqueta, A.M.; Hobbie, F.; Keerthi, A.; Oun, A.; Kortholt, A.; Boddeke, E.; Dolga, A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019, 118, 109295. [Google Scholar] [CrossRef]
- Chen, T.C.; da Fonseca, C.O.; Levin, D.; Schonthal, A.H. The Monoterpenoid Perillyl Alcohol: Anticancer Agent and Medium to Overcome Biological Barriers. Pharmaceutics 2021, 13, 2167. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, C.M.; Chen, K.S.; Miyamoto, S.; Gould, M.N. Perillyl alcohol inhibits a calcium-dependent constitutive nuclear factor-kappaB pathway. Cancer Res. 2005, 65, 8558–8566. [Google Scholar] [CrossRef]
- Tabassum, R.; Vaibhav, K.; Shrivastava, P.; Khan, A.; Ahmed, M.E.; Ashafaq, M.; Khan, M.B.; Islam, F.; Safhi, M.M.; Islam, F. Perillyl alcohol improves functional and histological outcomes against ischemia-reperfusion injury by attenuation of oxidative stress and repression of COX-2, NOS-2 and NF-kappaB in middle cerebral artery occlusion rats. Eur. J. Pharmacol. 2015, 747, 190–199. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Wang, K.S.; Mi, C.; Piao, L.X.; Xu, G.H.; Li, X.; Lee, J.J.; Jin, X. Perillyl alcohol efficiently scavenges activity of cellular ROS and inhibits the translational expression of hypoxia-inducible factor-1alpha via mTOR/4E-BP1 signaling pathways. Int. Immunopharmacol. 2016, 39, 1–9. [Google Scholar] [CrossRef]
- Khan, A.Q.; Nafees, S.; Sultana, S. Perillyl alcohol protects against ethanol induced acute liver injury in Wistar rats by inhibiting oxidative stress, NFkappa-B activation and proinflammatory cytokine production. Toxicology 2011, 279, 108–114, Correction in Toxicology 2011, 290, 362. [Google Scholar] [CrossRef]
- Beik, A.; Najafipour, H.; Joukar, S.; Rajabi, S.; Iranpour, M.; Kordestani, Z. Perillyl alcohol suppresses monocrotaline-induced pulmonary arterial hypertension in rats via anti-remodeling, anti-oxidant, and anti-inflammatory effects. Clin. Exp. Hypertens. 2021, 43, 270–280. [Google Scholar] [CrossRef]
- Kennedy, S.; Wadsworth, R.M.; Wainwright, C.L. Effect of antiproliferative agents on vascular function in normal and in vitro balloon-injured porcine coronary arteries. Eur. J. Pharmacol. 2003, 481, 101–107. [Google Scholar] [CrossRef]
- Tong, Z.; Li, G.; Su, C.; Zhou, L.; Zhang, L.; Chen, Q.; Xia, Q. L-Borneol 7-O-[beta-D-Apiofuranosyl-(1-->6)]-beta-D-Glucopyranoside Alleviates Myocardial Ischemia-Reperfusion Injury in Rats and Hypoxic/Reoxygenated Injured Myocardial Cells via Regulating the PI3K/AKT/mTOR Signaling Pathway. J. Immunol. Res. 2022, 2022, 5758303. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Xing, W.; Zhang, X.; Wang, X.; Ji, J.; Lu, J.; Yu, B.; Ruan, M. Exploring the synergic mechanism of Ligusticum striatum DC. and borneol in attenuating BMECs injury and maintaining tight junctions against cerebral ischaemia based on the HIF-1alpha/VEGF signalling pathway. J. Ethnopharmacol. 2023, 301, 115764. [Google Scholar] [CrossRef]
- Chen, Z.X.; Xu, Q.Q.; Shan, C.S.; Shi, Y.H.; Wang, Y.; Chang, R.C.; Zheng, G.Q. Borneol for Regulating the Permeability of the Blood-Brain Barrier in Experimental Ischemic Stroke: Preclinical Evidence and Possible Mechanism. Oxidative Med. Cell. Longev. 2019, 2019, 2936737. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhang, Y.; Li, W.; Zhang, J.; Wang, D.; Fu, J.; Wang, P. Comparison of Chemical Profiles, Anti-Inflammatory Activity, and UPLC-Q-TOF/MS-Based Metabolomics in Endotoxic Fever Rats between Synthetic Borneol and Natural Borneol. Molecules 2017, 22, 1446. [Google Scholar] [CrossRef]
- Kumar, M.S.; Kumar, S.; Raja, B. Antihypertensive and antioxidant potential of borneol-a natural terpene in LNAME—Induced hypertensive rats. Int. J. Pharm. Biol. Arch. 2010, 1, 271–279. [Google Scholar]
- Madhuri, K.; Naik, P.R. Ameliorative effect of borneol, a natural bicyclic monoterpene against hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic Wistar rats. Biomed. Pharmacother. 2017, 96, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Jäger, W.; Höferl, M. Metabolism of terpenoids in animal models and humans. In Handbook of Essential Oils, 3rd ed; CRC Press: Boca Raton, FL, USA, 2020; Volume I, pp. 275–301. [Google Scholar]
- Kohlert, C.; van Rensen, I.; Marz, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Medica 2000, 66, 495–505. [Google Scholar] [CrossRef]
- Khanam, S.; Mishra, P.; Faruqui, T.; Alam, P.; Albalawi, T.; Siddiqui, F.; Rafi, Z.; Khan, S. Plant-based secondary metabolites as natural remedies: A comprehensive review on terpenes and their therapeutic applications. Front. Pharmacol. 2025, 16, 1587215. [Google Scholar] [CrossRef] [PubMed]
- Selka, A.; Abidli, A.; Schiavo, L.; Jeanmart, L.; Hanquet, G.; Lubell, W.D. Recent Advances in Sustainable Total Synthesis and Chiral Pool Strategies with Emphasis on (−)-Sclareol in Natural Products Synthesis. Eur. J. Org. Chem. 2025, 28, e202400983. [Google Scholar] [CrossRef]
- Silva, J.J.M.; Campanharo, S.C.; Paschoal, J.A.R. Ethnoveterinary for food-producing animals and related food safety issues: A comprehensive overview about terpenes. Compr. Rev. Food Sci. Food Saf. 2021, 20, 48–90. [Google Scholar] [CrossRef] [PubMed]
- Potocka, W.; Assy, Z.; Bikker, F.J.; Laine, M.L. Current and Potential Applications of Monoterpenes and Their Derivatives in Oral Health Care. Molecules 2023, 28, 7178. [Google Scholar] [CrossRef]
- Milan, A.; Mioc, A.; Prodea, A.; Mioc, M.; Buzatu, R.; Ghiulai, R.; Racoviceanu, R.; Caruntu, F.; Soica, C. The Optimized Delivery of Triterpenes by Liposomal Nanoformulations: Overcoming the Challenges. Int. J. Mol. Sci. 2022, 23, 1140. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, H.; Wang, Y.; Xiao, W.; Zhang, Y.; Shi, D. Small rodent models of atherosclerosis. Biomed. Pharmacother. 2020, 129, 110426. [Google Scholar] [CrossRef]
- Boerman, E.M.; Sen, S.; Shaw, R.L.; Joshi, T.; Segal, S.S. Gene expression profiles of ion channels and receptors in mouse resistance arteries: Effects of cell type, vascular bed, and age. Microcirculation 2018, 25, e12452. [Google Scholar] [CrossRef]
- Tanner, M.R.; Beeton, C. Differences in ion channel phenotype and function between humans and animal models. Front. Biosci. (Landmark Ed.) 2018, 23, 43–64. [Google Scholar]
- de Alvarenga, J.F.R.; Genaro, B.; Costa, B.L.; Purgatto, E.; Manach, C.; Fiamoncini, J. Monoterpenes: Current knowledge on food source, metabolism, and health effects. Crit. Rev. Food Sci. Nutr. 2023, 63, 1352–1389. [Google Scholar] [CrossRef]
- de Sena Bastos, C.M.; Pereira-de-Morais, L.; de Alencar Silva, A.; de Menezes Dantas, D.; Batista, P.R.; Gomes, M.F.L.; de Araujo Delmondes, G.; de Menezes, I.R.A.; da Silva, R.E.R.; Barbosa, R. Perillyl Alcohol Promotes Relaxation in Human Umbilical Artery. Curr. Med. Chem. 2024, 31, 7072–7082. [Google Scholar] [CrossRef]
- Moreno, K.G.T.; Marques, A.A.M.; da Silva, G.P.; Bertoncelo, L.A.; Pessoal, L.B.; Goncalves, L.D.; Dos Santos, A.C.; Souza, R.I.C.; Silva, D.B.; Gasparotto Junior, A. Cardioprotective Effects of Aloysia polystachya Essential Oil on a Rat Model with Multiple Cardiovascular Risk Factors. Planta Medica 2024, 90, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Swiderski, J.; Sakkal, S.; Apostolopoulos, V.; Zulli, A.; Gadanec, L.K. Combination of Taurine and Black Pepper Extract as a Treatment for Cardiovascular and Coronary Artery Diseases. Nutrients 2023, 15, 2562. [Google Scholar] [CrossRef]
- Ju, C.; Song, S.; Hwang, S.; Kim, C.; Kim, M.; Gu, J.; Oh, Y.K.; Lee, K.; Kwon, J.; Lee, K.; et al. Discovery of novel (1S)-(-)-verbenone derivatives with anti-oxidant and anti-ischemic effects. Bioorganic Med. Chemestry Lett. 2013, 23, 5421–5425. [Google Scholar] [CrossRef]
- Abd El-Ghffar, E.A.; Eldahshan, O.A.; Barakat, A.; Efferth, T. The prophylactic effect of a Eugenia aquea extract against oxidative stress and inflammation associated with the development of arthritis in an adjuvant-induced arthritis rat model. Food Funct. 2018, 9, 6643–6651. [Google Scholar] [CrossRef]
- Kim, D.H.; Yong, H.J.; Mander, S.; Nguyen, H.T.; Nguyen, L.P.; Park, H.K.; Cha, H.K.; Kim, W.K.; Hwang, J.I. SP-8356, a (1S)-(-)-Verbenone Derivative, Inhibits the Growth and Motility of Liver Cancer Cells by Regulating NF-kappaB and ERK Signaling. Biomol. Ther. 2021, 29, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, S.; Hadjiakhoondi, A.; Yassa, N.; Khanavi, M.; Tofighi, Z. Essential oils chemical composition, antioxidant activities and total phenols of Astrodaucus persicus. Iran. J. Basic Med. Sci. 2016, 19, 159–165. [Google Scholar] [PubMed]
| Estudos In Vitro | ||||
|---|---|---|---|---|
| Monoterpene | Experimental Model | Concentration Tested | Mechanism of Action | Reference |
| Geraniol | Thoracic aorta of diabetic rats | 10–300 µmol/L | Vasorelaxation by inhibiting Cav1.2 and ROC | [51] |
| HUVEC | 25–100 µmol/L | Inhibits Ox-LDL-induced inflammation and oxidative stress by targeting PI3/AKT/NRF2 | [52] | |
| Carvacrol | Rat thoracic aorta artery | 1–1 × 103 µmol/L | Vasorelaxation by block the Ca2+ influx through the membrane | [53] |
| Posterior cerebral or cerebellar arteries from rats | 10–1 × 104 µmol/L | Relaxation induced by Ca2+ influx via TRPV3 channels in the endothelium and activation of Kca2.3 and Kca3.1 channels | [40] | |
| Rat thoracic aorta artery | 0.01–100 µmol/L | Attenuation of the vasoconstrictor action via ROS inhibition and NOS stimulation | [31] | |
| Superior mesenteric artery of rats | 0.01–300 µmol/L | Vasorelaxation by inhibition of the Ca2+ influx through Cav1.2, ROC and SOC channels. | [43] | |
| Citronellal | Superior mesenteric artery of rats | 1–1 × 105 µmol/L | Vasorelaxation by inhibiting calcium influx | [54] |
| Citronellol | Superior mesenteric artery of rats | 640–1.9 × 106 µmol/L | Vasorelaxation by inhibiting calcium influx and Cav1.2 | [55] |
| Linalyl acetate | Rabbit carotid artery | 300 µmol/L | Vasorelaxation by stimulating eNOS in vascular endothelium and stimulating MLCP in vascular smooth muscle | [56] |
| HUVEC | 509 µmol/L | Blocked the Ca2+ influx in endothelial cells | [57] | |
| Citral | Thoracic aorta of rats from hypertensive rats | 6.24–6.24 × 103 µmol/L | Vasorelaxation by NO/cyclic GMP pathway and inhibiting Cav1.2 | [58] |
| Rat thoracic aorta | 300–3 × 104 µmol/L | Vasorelaxation by NO/cyclic GMP pathway and the calcium influx through Cav1.2 | [59] | |
| Rat thoracic aorta | 6.57–6.57 × 103 µmol/L | Vasorelaxation by NO/cyclic GMP pathway and inhibiting Cav1.2 | [24] | |
| Carvone | Rat thoracic aorta | 100 µmol/L | Vasorelaxation by blocking Cav1.2 | [32] |
| α-terpineol | Superior mesenteric artery of Wistar rats | 1 × 10−6–10 µmol/L | Vasorelaxation by NO/cyclic GMP pathway | [29] |
| Superior mesenteric artery of hypertensive rats | 1.10−4–1 × 104 µmol/L | Vasorelaxation by inhibiting Cav1.2 | [60] | |
| Linalool | Rat superior mesenteric artery | 6.4–6.4 × 103 µmol/L | Vasorelaxation by inhibiting calcium influx and Cav1.2 | [61] |
| Rat thoracic aorta | 100 µmol/L | Vasorelaxation by blocking Cav1.2 and elevating NO | [32] | |
| Mouse thoracic aortas | 10–500 µmol/L | Vasorelaxation by activating sCG and K+ channels. | [28] | |
| Ang II-induced VSMCs | 50–150 µmol/L | Inhibited the proliferation and migration by inhibithing MAPK | [5] | |
| p-cymene | Rat thoracic aorta artery | 1–1 × 103 µmol/L | Vasorelaxation by activation Kir2 and Kir6 Channels | [62] |
| Thymol | Rat thoracic aorta artery | 1–1 × 103 µmol/L | Vasorelaxation by block the Ca2+ influx through the membrane | [53] |
| Carveol | Rat thoracic aorta artery | 1–5 × 103 µmol/L | Vasorelaxation by inhibiting Cav1.2 channels | [63] |
| Human umbilical artery | 1–5 × 103 µmol/L | Vasorelaxation by inhibiting Cav1.2 and partial participation of Kca1.1 channels | [64] | |
| Perillyl alcohol | Rat thoracic aorta artery | 1–5 × 103 µmol/L | Induced relaxant effect by inhibition of PKC and IP3 pathway | [63] |
| Human vascular smooth muscle cells | 100–2 × 103 µmol/L | Inhibits proliferation and also induces apoptosis | [65] | |
| β-pinene | Superior mesenteric artery of rats | 0.1–3 × 104 µmol/L | Vasorelaxant effect involve blocking Ca2+ influx through the Cav1.2 channels, associated with decreased sensitivity of contractile machinery to Ca2+ | [66] |
| Borneol | Rat thoracic aorta artery | 1 × 10−3–300 µmol/L | Vasorelaxation by calcium influx blockade through Cav1.2 channels, calcium mobilization from intracellular stores and potassium channels activation. | [67] |
| Rat thoracic aorta artery | 1 × 10−4–300 µmol/L | Vasorelaxant effect with the participation of NO and prostanoids in vascular endothelium and action on the VSMC dependent in Kir6 channels. | [23] | |
| Estudos In Vivo | ||||
| Monoterpene | Experimental Model | Dose Tested | Mechanism of Action | Reference |
| Geraniol | Mice fed with a high-fat diet | 100 mg/kg/day (intraperitoneally) | Improves endothelial function by inhibiting NOX-2 derived ROS generation | [68] |
| Carvacrol | Normotensive rats | 1–20 mg/kg (intravenous) | Induced hypotension, bradycardia, and negative inotropic and chronotropic effects | [43] |
| Diabetic rats | 10–20 mg/kg/day (intraperitoneally) | Reduced hypercontractility by activating the PI3K/Akt signaling pathway | [69] | |
| Spontaneously hypertensive rats (SHR) | 50–100 mg/kg/day (oral) | Improved reendothelialization by increasing eNOS expression and reducing senescence and oxidative stress in endothelial progenitor cells. | [70] | |
| Citronellal | Rats fed with a high-fat diet | 50–150 mg/kg/day | Improved endothelial dysfunction, increased cell migration, and suppressed oxidative stress and inflammation in vascular endothelium | [71] |
| Diabetic rats | 150 mg/kg/day | Increased expression of S1P1 and eNOS, accompanied by increased SOD levels and ROS reduction. | [72] | |
| Diabetic rats | 50–150 mg/kg/day | Suppressed the expression of NHE1 and TPRM2, alleviated oxidative stress-induced mitochondrial damage | [73] | |
| Linalyl acetate | Hypertension induced by immobilization stress and intraperitoneal injection of nicotine | 25–100 mg/kg | Suppression of phosphorylation and activation of the NADPH oxidase, decrease in ROS production and increased expression of eNOS | [26] |
| Carvone | Cerebral I/R injury in rats | 1–20 mg/kg/day (intraperitoneally) | It had antioxidative, anti-inflammatory, and anti-apoptotic effects against cerebral I/R brain injury. | [74] |
| α-terpineol | Normotensive rats | 1–30 mg/kg (oral) | Dose-dependent hypotension followed by reflex tachycardia | [29] |
| Hypertension induced by L-NAME | 25–100 mg/kg/day (intraperitoneally) | Reduce arterial pressure, decrease vascular resistance, and restore enzymatic antioxidants | [60] | |
| Linalool | Normotensive rats | 1–20 mg/kg/day (intravenous) | Hypotension and bradycardia attenuated by inhibition of muscarinic receptors | [61] |
|
Hypertensive rats
(two kidneys and a clip–2R1C) | 200 mg/kg/day (oral) | Reduced blood pressure without changing the heart rate | [61] | |
| SHR | 50–100 mg/kg/day (oral) | Reduced blood pressure, increased levels of the anti-inflammatory cytokine (IL-10) and improved vasodilator responsiveness | [75] | |
| α-Pinene | Aorta artery from ApoE/mice | Particulate matter in ratios of 10:1:1 | Increased vascular expression of HO-1, MMP-9 and ET-1 | [76] |
| β-Pinene | Hypertension induced by L-NAME | 200 mg/kg | Reduce arterial pressure | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, T.; Almeida, A.; Pontes, L.; Oliveira, J.; Feitosa, M.; Júnior, J.; Veras, R.; Medeiros, I. Monoterpenes in Vascular Function: A Review of Bioactivity and Mechanisms of Action. Int. J. Mol. Sci. 2025, 26, 9243. https://doi.org/10.3390/ijms26189243
Gonçalves T, Almeida A, Pontes L, Oliveira J, Feitosa M, Júnior J, Veras R, Medeiros I. Monoterpenes in Vascular Function: A Review of Bioactivity and Mechanisms of Action. International Journal of Molecular Sciences. 2025; 26(18):9243. https://doi.org/10.3390/ijms26189243
Chicago/Turabian StyleGonçalves, Tays, Arthur Almeida, Larisse Pontes, Julio Oliveira, Mathania Feitosa, Javanyr Júnior, Robson Veras, and Isac Medeiros. 2025. "Monoterpenes in Vascular Function: A Review of Bioactivity and Mechanisms of Action" International Journal of Molecular Sciences 26, no. 18: 9243. https://doi.org/10.3390/ijms26189243
APA StyleGonçalves, T., Almeida, A., Pontes, L., Oliveira, J., Feitosa, M., Júnior, J., Veras, R., & Medeiros, I. (2025). Monoterpenes in Vascular Function: A Review of Bioactivity and Mechanisms of Action. International Journal of Molecular Sciences, 26(18), 9243. https://doi.org/10.3390/ijms26189243







