Ginkgo Seed as Medicine–Food Homology for Migraine: Network Pharmacology and Molecular Docking Insights
Abstract
1. Introduction
2. Results
2.1. Screening of Active Compounds and Targets of Ginkgo Seed
2.2. Intersection Targets of Ginkgo Seed and Migraine
2.3. Construction of “Compound–Target” Network
2.4. PPI Network Analysis
2.5. GO and KEGG Pathways Enrichment Analysis
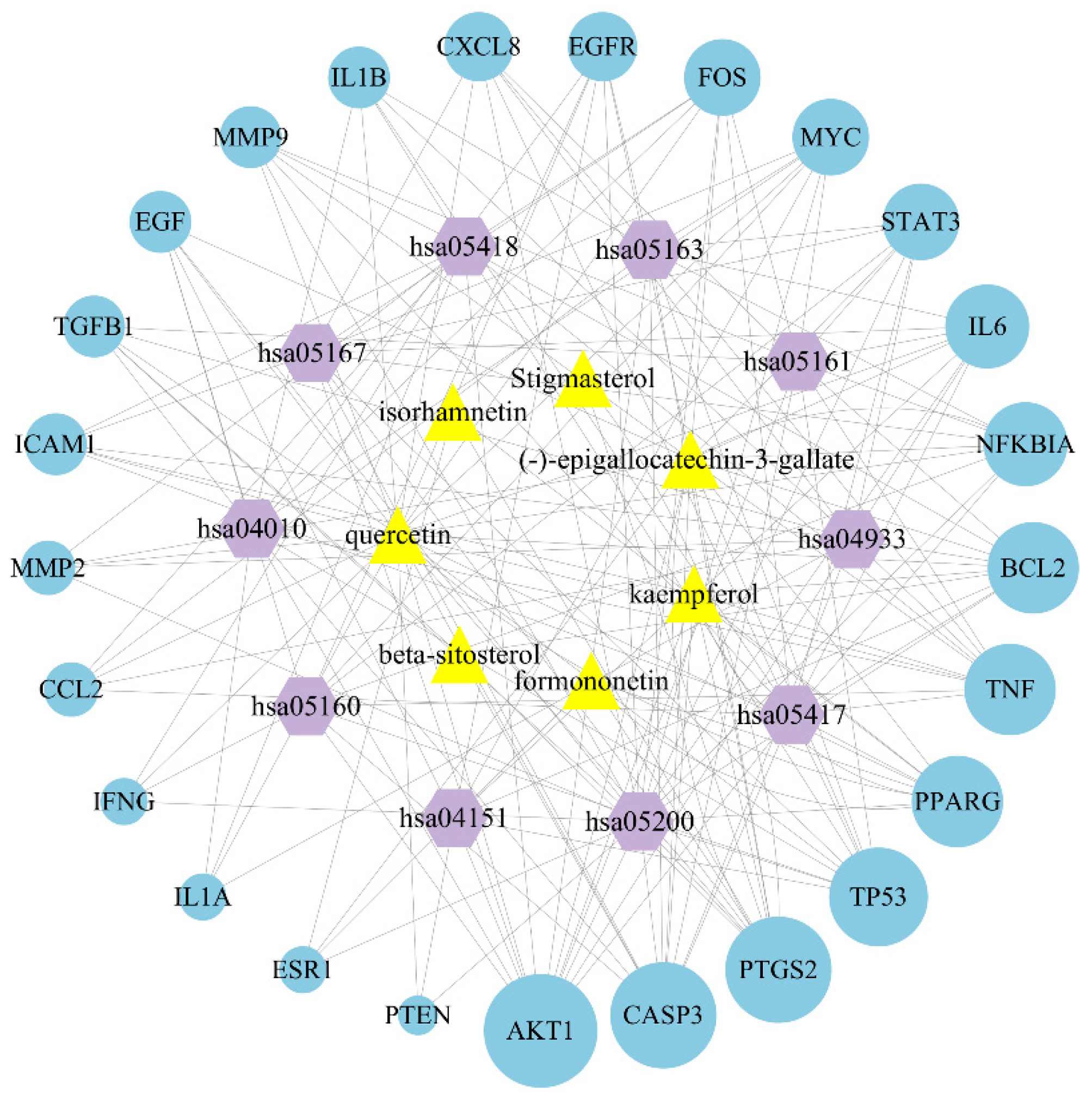
2.6. Construction of Bioactive Compound-Target-Pathway Network
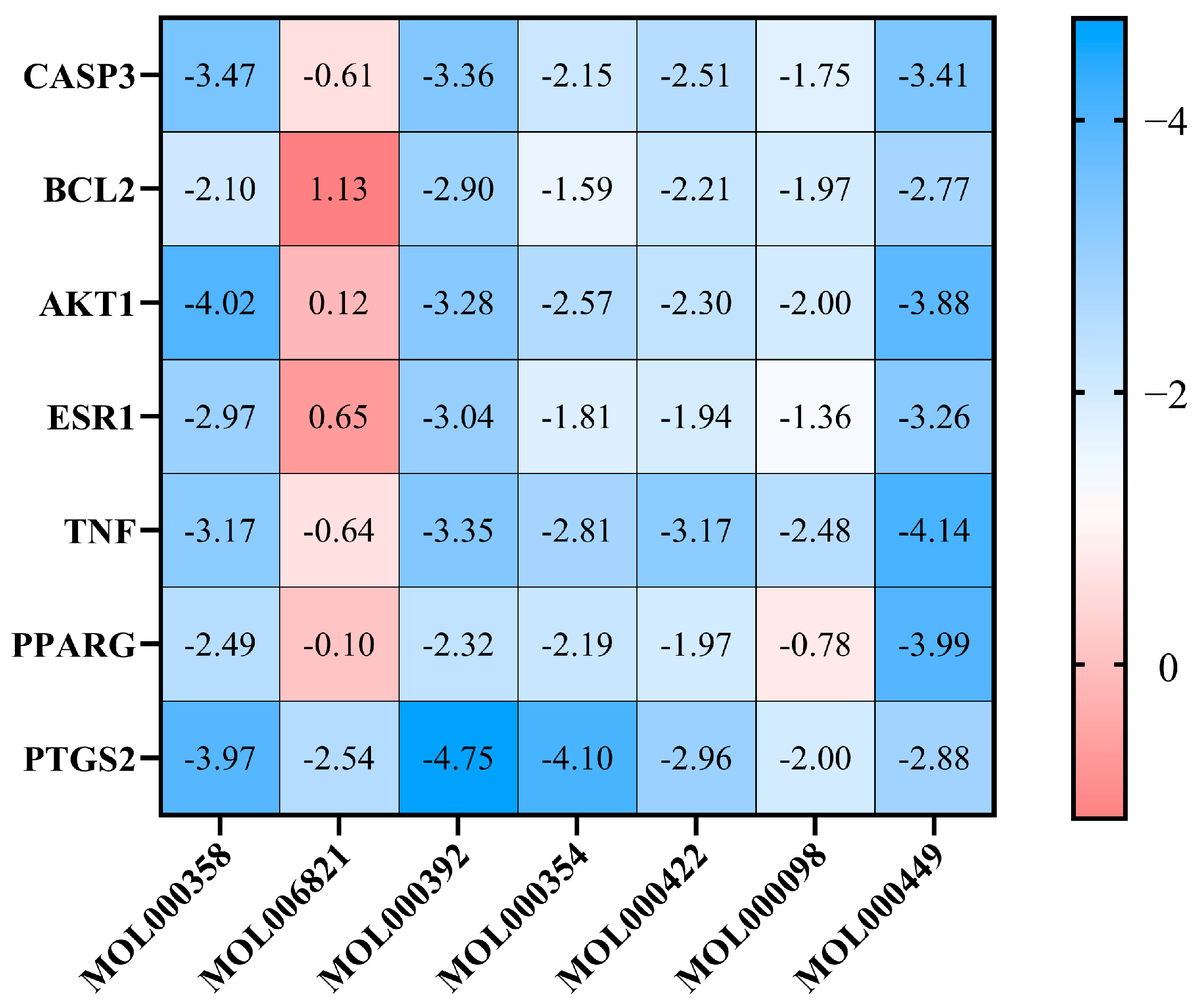
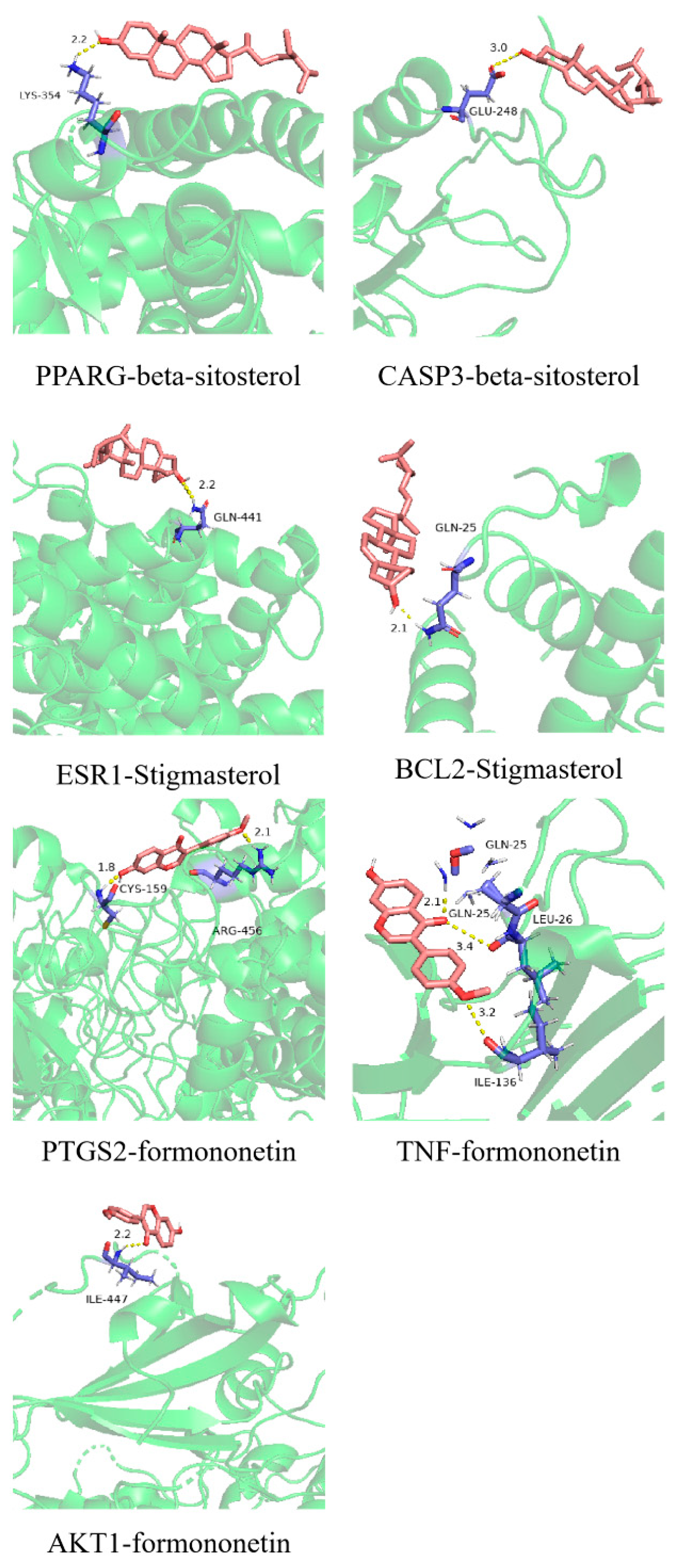
2.7. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Screening Strategy for Active Components and Targets in Ginkgo Seeds
4.2. Prediction of Related Targets of Ginkgo Seed and Migraine
4.3. Draw “Compound-Target” Network
4.4. Protein–Protein Interaction (PPI) Network
4.5. GO and KEGG Enrichment Analysis
4.6. Constructing Ginkgo Seed–Target–Pathway Network
4.7. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dodick, D.W. Migraine. Lancet 2018, 391, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Goel, K.; Chhetri, A.; Ludhiadch, A.; Munshi, A. Current Update on Categorization of Migraine Subtypes on the Basis of Genetic Variation: A Systematic Review. Mol. Neurobiol. 2024, 61, 4804–4833. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.; Gold, M.; Vazquez, A. The Impact of Stress and Sex on Sympathetic Regulation of Dural Vessel Tone. J. Pain 2019, 20, S23. [Google Scholar] [CrossRef]
- Ferrari, M.D.; Goadsby, P.J.; Burstein, R.; Kurth, T.; Ayata, C.; Charles, A.; Ashina, M.; van den Maagdenberg, A.; Dodick, D.W. Migraine. Nat. Rev. Dis. Primers 2022, 8, 2. [Google Scholar] [CrossRef]
- Kowalska, M.; Prendecki, M.; Piekut, T.; Kozubski, W.; Dorszewska, J. Migraine: Calcium Channels and Glia. Int. J. Mol. Sci. 2021, 22, 2688. [Google Scholar] [CrossRef]
- Lange, K.; Waliszewska-Prosół, M.; Onan, D.; Marschollek, K.; Wiels, W.; Mikulenka, P.; Farham, F.; Gollion, C.; Ducros, A. Genetics of migraine: Where are we now? J. Headache Pain 2023, 24, 12. [Google Scholar] [CrossRef]
- Crawford, J.; Liu, S.; Tao, F. Gut Microbiota and Migraine. Neurobiol. Pain. 2022, 11, 100090. [Google Scholar] [CrossRef]
- Cho, S.J.; Song, T.J.; Chu, M. Treatment Update of Chronic Migraine. Curr. Pain Headache Rep. 2017, 21, 26. [Google Scholar] [CrossRef]
- Geng, L.M.; Jiang, J.G. The neuroprotective effects of formononetin: Signaling pathways and molecular targets. J. Funct. Foods 2022, 88, 104911. [Google Scholar] [CrossRef]
- D’Onofrio, F.; Raimo, S.; Spitaleri, D.; Casucci, G.; Bussone, G. Usefulness of nutraceuticals in migraine prophylaxis. Neurol. Sci. 2017, 38, 117–120. [Google Scholar] [CrossRef]
- Ferroni, P.; Barbanti, P.; Della-Morte, D.; Palmirotta, R.; Jirillo, E.; Guadagni, F. Redox Mechanisms in Migraine: Novel Therapeutics and Dietary Interventions. Antioxid Redox Sign. 2018, 28, 1144–1183. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A.; Russo, A. Is targeting CGRP the right pathway to prevent migraine? Lancet 2019, 394, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Ackley, S.; Rist, P.; Brenowitz, W.; Graff, R.; Glymour, M. The association between migraine genetic risk and dementia: A Mendelian randomization study: Genetics/genetic factors of Alzheimer’s disease. Alzheimers Dement 2020, 16, e045405. [Google Scholar] [CrossRef]
- Zhao, Y.; Martins-Oliveira, M.; Akerman, S.; Goadsby, P. Comparative effects of traditional Chinese and Western migraine medicines in an animal model of nociceptive trigeminovascular activation. Cephalalgia 2017, 38, 1215–1224, Erratum in Cephalalgia 2018, 38, NP1. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, Y.Q. The main active constituents and detoxification process of Ginkgo biloba seeds and their potential use in functional health foods. J. Food Compos. Anal. 2019, 83, 103247. [Google Scholar] [CrossRef]
- Kang, H. Hypocholesterolemic effect of Ginkgo biloba seeds extract from high fat diet mice. Biomed. Sci. Lett. 2017, 23, 138–143. [Google Scholar] [CrossRef]
- Yu, Y.Y. Toxic and Active Compositions and the Intervention Effect on Alzheimer’s Disease of Ginkgo Seeds. Master’s Thesis, Jiangsu University, Zhenjiang, China, 2017. [Google Scholar]
- Shen, N.; Zeng, W.; Leng, F.; Lu, J.; Lu, Z.; Cui, J.; Wang, L.; Jin, B. Ginkgo seed extract promotes longevity and stress resistance of Caenorhabditis elegans. Food Funct. 2021, 12, 12395. [Google Scholar] [CrossRef]
- Liu, Z.H.; Sun, X.B. Network pharmacology: New opportunity for the modernization of traditional Chinese medicine. Acta Pharm. Sin. 2012, 47, 696–703. [Google Scholar]
- Yu, S.; Fan, C.; Li, Y.; Pei, H.; Tian, Y.; Zuo, Z.; Wang, Z.; Liu, C.; Zhao, X.; Wang, Z. Network pharmacology and experimental verification to explore the anti-migraine mechanism of Yufeng Ningxin Tablet. J. Ethnopharmacol. 2023, 310, 116384. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; Li, N.; Chen, J.; Xu, H.; Wang, Y.; Liang, Q. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J. Ethnopharmacol. 2023, 309, 116306. [Google Scholar] [CrossRef]
- Zhao, Y.; Ling, X.; Zhang, H.; Sun, P.; Sun, Y.; Yin, W.; Fan, K.; Yang, H.; Zhong, J.; Zhang, Z.; et al. Network pharmacology and experimental validation to reveal the target of matrine against PRRSV. iScience 2023, 26, 106371. [Google Scholar] [CrossRef]
- Sariyer, E.; Yakarsonmez Kocer, S.; Danış, Ö.; Turgut-Balik, D. In vitro inhibition studies of coumarin derivatives on bos taurus enolase and elucidating their interaction by molecular docking, molecular dynamics simulations and mmgb(pb)sa binding energy calculation. Bioorg. Chem. 2021, 110, 104796. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef]
- Arijit, M.; Tapan, K.M.; Anupam, B. Analgesic and anti-inflammatory activities of quercetin-3-methoxy-4′-glucosyl-7-glucoside isolated from indian medicinal plant melothria heterophylla. Medicines 2019, 6, 59. [Google Scholar]
- Xu, A.J.; Zhou, Y.Q.; Liu, C.; Liu, D.Q.; Tian, Y.K.; Mei, W.; Tian, X.B. The emerging role of quercetin in the treatment of chronic pain. Curr. Neuropharmacol. 2022, 20, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Foudah, A.; Devi, S.; Alqarni, M.; Salkini, M.; Kumar, M.; Almalki, H. Quercetin Attenuates Nitroglycerin-Induced Migraine Headaches by Inhibiting Oxidative Stress and Inflammatory Mediators. Nutrients 2022, 14, 4871. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.S.; Lee, E.G.; Jeon, H.S.; Chae, H.J.; Park, S.J.; Lee, Y.; Yoo, W.H. Quercetin Inhibits IL-1β-Induced Proliferation and Production of MMPs, COX-2, and PGE2 by Rheumatoid Synovial Fibroblast. Inflammation 2012, 35, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Harder, A.; Onderwater, G.; Dongen, R.; Heijink, M.; Zwet, E.; Giera, M.; Maagdenberg, A.; Terwindt, G. Prostaglandin-E2 levels over the course of glyceryl trinitrate provoked migraine attacks. Neurobiol. Pain 2022, 13, 100112. [Google Scholar] [CrossRef]
- Khan, A.; Ali, T.; Rehman, S.; Khan, M.; Alam, S.; Ikram, M.; Muhammad, T.; Saeed, K.; Badshah, H.; Kim, M. Neuroprotective Effect of Quercetin Against the Detrimental Effects of LPS in the Adult Mouse Brain. Front. Pharmacol. 2018, 9, 1383. [Google Scholar] [CrossRef]
- Khalatbary, A.; Khademi, E. The green tea polyphenolic catechin epigallocatechin gallate and neuroprotection. Nutr. Neurosci. 2018, 23, 281–294. [Google Scholar] [CrossRef]
- Bulboaca, A.; Porfire, A.; Barbalata, C.; Bolboaca, S.; Nicula, C.; Boarescu, P.-M.; Stanescu, I.; Dogaru, G. The effect of liposomal epigallocatechin gallate and metoclopramide hydrochloride co-administration. Farmacia 2019, 67, 905–911. [Google Scholar] [CrossRef]
- Wang, T.; Ma, C.; Hu, Y.; Guo, S.; Bai, G.; Yang, R.; Yang, G. Effects of food formulation on bioavailability of phytosterols: Phytosterols structure, delivery carriers, and food matrix. Food Funct. 2023, 14, 5465–5477. [Google Scholar] [CrossRef]
- An, T.; Hirst, J.; Cady, R.; Durham, P. β-Sitosterol Isolated from Cocoa Powder Functions to Increase Expression of Anti-Inflammatory Proteins in Trigeminal Neurons: Implications for Treatment of Migraine and TMJ Disorders. Planta Med. 2011, 77, 133. [Google Scholar] [CrossRef]
- Vafaei, A.; Vafaeian, A.; Iranmehr, A.; Nassireslami, E.; Hasannezhad, B.; Hosseini, Y. Effects of β-sitosterol on anxiety in migraine-induced rats: The role of oxidative/nitrosative stress and mitochondrial function. CNS Neurosci. Ther. 2024, 30, e14892. [Google Scholar] [CrossRef]
- Ayaz, M.; Wadood, A.; Sadiq, A.; Ullah, F.; Anichkina, O.; Ghufran, M. In-silico evaluations of the isolated phytosterols from polygonum hydropiper L against BACE1 and MAO drug targets. J. Biomol. Struct. Dyn. 2022, 40, 10230–10238. [Google Scholar] [CrossRef]
- Tian, J.; Wang, X.Q.; Tian, Z. Focusing on Formononetin: Recent Perspectives for its Neuroprotective Potentials. Front. Pharmacol. 2022, 13, 905898. [Google Scholar] [CrossRef] [PubMed]
- El-Bakoush, A.; Olajide, O.A. Formononetin inhibits neuroinflammation and increases estrogen receptor beta (ERβ) protein expression in BV2 microglia. Int. Immunopharmacol. 2018, 61, 325–337. [Google Scholar] [CrossRef]
- Esposito, M.; Carotenuto, M. Ginkgolide B complex efficacy for brief prophylaxis of migraine in school-aged children: An open-label study. Neurol. Sci. 2011, 32, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, M.; Antinolfi, L.; Faraldo, M.; Dona, A.D.; Esposito, M. Nutraceutical preparations in childhood migraine prophylaxis. J. Headache Pain 2013, 14 (Suppl. S1), 15. [Google Scholar] [CrossRef]
- Shahbaz, M.; Imran, M.; Alsagaby, S.A.; Naeem, H.; Al Abdulmonem, W.; Hussain, M.; Abdelgawad, M.A.; El-Ghorab, A.H.; Ghoneim, M.M.; El-Sherbiny, M.; et al. Anticancer, antioxidant, ameliorative and therapeutic properties of kaempferol. Int. J. Food Prop. 2023, 26, 1140–1166. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Huang, J.H.; Wang, N.; Tan, H.Y.; Cheung, F.; Chen, F.Y.; Feng, Y.B. Integrating Network Pharmacology and Pharmacological Evaluation for Deciphering the Action Mechanism of Herbal Formula Zuojin Pill in Suppressing Hepatocellular Carcinoma. Front. Pharmacol. 2019, 10, 1185. [Google Scholar] [CrossRef]
- Vardi, Y.; Rabey, I.M.; Streifler, M.; Schwartz, A.; Lindner, H.R.; Zor, U. Migraine attacks. Alleviation by an inhibitor of prostaglandin synthesis and action. Neurology 1976, 26, 447–450. [Google Scholar] [CrossRef]
- Nuvoli, B.; Galati, R. Cyclooxygenase-2, Epidermal Growth Factor Receptor, and Aromatase Signaling in Inflammation and Mesothelioma. Mol. Cancer Ther. 2013, 12, 844–852. [Google Scholar] [CrossRef]
- Liu, M.L.; Fan, G.H.; Zhang, D.P.; Zhu, M.J.; Zhang, H.L. Study on Mechanism of Jiawei Chaiqin Wendan Decoction in Treatment of Vestibular Migraine Based on Network Pharmacology and Molecular Docking Technology. Evid.-Based Complement. Altern. Med. 2021, 2021, 5528403. [Google Scholar] [CrossRef]
- Strafella, C.; Caputo, V.; Termine, A.; Fabrizio, C.; Calvino, G.; Megalizzi, D.; Ruffo, P.; Toppi, E.; Banaj, N.; Bassi, A.; et al. Identification of Genetic Networks Reveals Complex Associations and Risk Trajectory Linking Mild Cognitive Impairment to Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 821789. [Google Scholar] [CrossRef]
- Chen, J.H.; Shi, Z.H.; Zhang, C.L.; Xiong, K.; Zhao, W.; Wang, Y.H. Oroxin A alleviates early brain injury after subarachnoid hemorrhage by regulating ferroptosis and neuroinflammation. J. Neuroinflamm. 2024, 21, 116. [Google Scholar] [CrossRef]
- Othman, M.S.; Obeidat, S.T.; Aleid, G.M.; Abdel-Daim, M.M.; Habotta, O.A.; Schwartz, L.; Al-Bagawi, A.H.; Hussein, M.M.; Bakkar, A. Pergularia tomentosa coupled with selenium nanoparticles salvaged lead acetate-induced redox imbalance, inflammation, apoptosis, and disruption of neurotransmission in rats’ brain. Open Chem. 2022, 20, 1313–1326. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Chu, C.Y.; Liu, S. Astaxanthin protects retinal ganglion cells from acute glaucoma via the Nrf2/HO-1 pathway. J. Chem. Neuroanat. 2020, 110, 101876. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, F.; Zoia, C.P.; Bazzini, C.; Bolognini, A.; Saresella, M.; Conti, E.; Ferrarese, C.; Piancone, F.; Marventano, I.; Galimberti, D.; et al. Modulation of MAPK- and PI3/AKT-Dependent Autophagy Signaling by Stavudine (D4T) in PBMC of Alzheimer’s Disease Patients. Cells 2022, 11, 2180. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, E.; Negri, G.L.; Tan, S.S.N.; Macaldaz, M.E.; Ding, S.S.; Long, J.S.; Nielsen, K.; Spencer, S.E.; Morin, G.B.; Eaves, C.J. Dependence of human cell survival and proliferation on the CASP3 prodomain. Cell Death Discov. 2024, 10, 63. [Google Scholar] [CrossRef]
- Kursun, O.; Yemisci, M.; van den Maagdenberg, A.; Karatas, H. Migraine and neuroinflammation: The inflammasome perspective. J. Headache Pain 2021, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Kraig, R.P.; Mitchell, H.M.; Christie-Pope, B.; Kunkler, P.E.; White, D.M.; Tang, Y.P.; Langan, G. TNF-α and microglial hormetic involvement in neurological health & migraine. Dose-Response 2010, 8, 389–413. [Google Scholar]
- Sudershan, A.; Sudershan, S.; Sharma, I.; Kumar, H.; Panjaliya, R.; Kumar, P. Role of TNF-α in the Pathogenesis of Migraine. Pain Res. Manag. 2024, 2024, 1377143. [Google Scholar] [CrossRef] [PubMed]
- Duggal, S.; Jailkhani, N.; Midha, M.K.; Agrawal, N.; Rao, K.V.S.; Kumar, A. Defining the Akt1 interactome and its role in regulating the cell cycle. Sci. Rep. 2018, 8, 1303. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Zhu, L.D.; Shi, H.J.; Ye, S.; Li, Q.; Yin, X.F.; Xie, Q.D.; Xu, Q.Z.; Wei, J.X.; Mei, F.; et al. Puerarin prevents sepsis-associated encephalopathy by regulating the AKT1 pathway in microglia. Phytomedicine 2023, 121, 155119. [Google Scholar] [CrossRef]
- Berköz, M.; Krosniak, M.; Özkan-Yilmaz, F.; Özlüer-Hunt, A. Prophylactic effect of Biochanin A in lipopolysaccharide-stimulated BV2 microglial cells. Immunopharm. Immunot. 2020, 42, 330–339. [Google Scholar] [CrossRef]
- Pavlovic, J.M.; Allshouse, A.A.; Santoro, N.F.; Crawford, S.L.; Thurston, R.C.; Neal-Perry, G.S.; Lipton, R.B.; Derby, C.A. Sex hormones in women with and without migraine: Evidence of migraine-specific hormone profiles. Neurology 2016, 87, 49–56. [Google Scholar] [CrossRef]
- Gunes Tatar, I.; Ergun, O.; Celtikci, P.; Kurt, A.; Yavasoglu, N.; Birgi, E.; Tatar, T.; Hekimoglu, B. Evaluation of subclinical atherosclerosis in migraine patients by ultrasound radiofrequency data technology: Preliminary results. Agri 2016, 28, 121–126. [Google Scholar]
- Liu, Y.Y.; Jiao, Z.Y.; Li, W.; Tian, Q. PI3K/AKT signaling pathway activation in a rat model of migraine. Mol. Med. Rep. 2017, 16, 4849–4854. [Google Scholar] [CrossRef]
- Xiao, H.X.; Song, B.; Li, Q.; Shao, Y.M.; Zhang, Y.B.; Chang, X.L.; Zhou, Z.J. Paraquat mediates BV-2 microglia activation by raising intracellular ROS and inhibiting Akt1 phosphorylation. Toxicol. Lett. 2022, 355, 116–126, Erratum in Toxicol. Lett. 2023, 383, 213–214. [Google Scholar] [CrossRef]
- Du, W.Q.; Liang, X.; Wang, S.Z.; Lee, P.; Zhang, Y.L. The underlying mechanism of Paeonia lactiflora pall. in parkinson’s disease based on a network pharmacology approach. Front. Pharmacol. 2020, 11, 581984. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, Y.X.; Li, M.M.; Ge, X. Dyrk2 mediated the release of proinflammatory cytokines in LPS-induced BV2 cells. Int. J. Biol. Macromol. 2018, 109, 1115–1124. [Google Scholar] [CrossRef]
- Sun, S.T.; Fan, Z.Z.; Liu, X.J.; Wang, L.D.; Ge, Z.M. Microglia TREM1-mediated neuroinfammation contributes to central sensitization via the NF-κB pathway in a chronic migraine model. J. Headache Pain 2024, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.P.; Cui, Y.S.; Du, H.; Wu, J.H.; Zhou, M.F.; Ouyang, H.; Feng, Y.L.; Yang, S.L. San Pian decoction can treat nitroglycerin-induced migraine in rats by inhibiting the PI3K/AKT and MAPK signaling pathways. J. Ethnopharmacol. 2022, 296, 115470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Wang, Y.; Chen, Z.T.; Liu, B.; Wang, W.J.; Li, Y.L. Study on the mechanism of Shugan Lidan Xiaoshi granule in preventing acute pancreatitis based on network pharmacology and molecular docking. Heliyon 2024, 10, e27365. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Martin, M.J.; O’Donovan, C.; Magrane, M.; Apweiler, R.; Alpi, E.; Antunes, R.; Ar-Ganiska, J.; Bely, B.; Bingley, M.; et al. UniProt: A hub for protein information. Nucleic Acids Res. 2015, 43, D204–D212. [Google Scholar]
- Rebhan, M.; Chalifa-Caspi, V.; Prilusky, J.; Lancet, D. GeneCards: Integrating information about genes, proteins and diseases. Trends Genet. TIG 1997, 13, 163. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.T.; Fu, Y.H.; Li, Z.R.; Zou, Y.J.; Dai, Y.L. Network pharmacology and untargeted metabolomic-based investigation of anti-osteoporotic effects of viscozyme-assisted polysaccharide from Portulaca oleracea L. J. Pharm. Biomed. Anal. 2024, 243, 116104. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Sanner, M.F. AutoDock CrankPep: Combining folding and docking to predict protein-peptide complexes. Bioinformatics 2019, 35, 5121–5127. [Google Scholar] [CrossRef]
- Yuan, S.; Chan, H.C.S.; Hu, Z.Q. Using PyMOL as a platform for computational drug design. Wires Comput. Mol. Sci. 2017, 7, e1298. [Google Scholar] [CrossRef]


| MOL ID | Molecule Name | MW | OB (%) | DL |
|---|---|---|---|---|
| MOL011072 | Quinicine | 324.46 | 75.44 | 0.33 |
| MOL011074 | Scillaren A_qt | 384.56 | 57.67 | 0.78 |
| MOL011075 | Shikodonin | 362.46 | 78.16 | 0.56 |
| MOL001771 | poriferast-5-en-3beta-ol | 414.79 | 36.91 | 0.75 |
| MOL002773 | beta-carotene | 536.96 | 37.18 | 0.58 |
| MOL000449 | Stigmasterol | 412.77 | 43.83 | 0.76 |
| MOL000354 | isorhamnetin | 316.28 | 49.6 | 0.31 |
| MOL000358 | beta-sitosterol | 414.79 | 36.91 | 0.75 |
| MOL000392 | formononetin | 268.28 | 69.67 | 0.21 |
| MOL000422 | kaempferol | 286.25 | 41.88 | 0.24 |
| MOL004350 | Ruvoside_qt | 390.57 | 36.12 | 0.76 |
| MOL000492 | (+)-catechin | 290.29 | 54.83 | 0.24 |
| MOL005236 | gibberellin | 346.41 | 81.59 | 0.53 |
| MOL006821 | (-)-epigallocatechin-3-gallate | 458.4 | 55.09 | 0.77 |
| MOL000098 | quercetin | 302.25 | 46.43 | 0.28 |
| MOL ID | Molecule Name | Degree | Betweenness | Closeness |
|---|---|---|---|---|
| MOL000098 | quercetin | 99 | 17,269.80 | 0.53 |
| MOL006821 | (-)-epigallocatechin-3-gallate | 73 | 11,579.29 | 0.45 |
| MOL000422 | kaempferol | 39 | 3007.39 | 0.40 |
| MOL000358 | beta-sitosterol | 27 | 3520.40 | 0.38 |
| MOL000354 | isorhamnetin | 26 | 1176.60 | 0.36 |
| MOL000392 | formononetin | 23 | 2206.78 | 0.37 |
| MOL000449 | Stigmasterol | 23 | 2619.81 | 0.36 |
| MOL002773 | beta-carotene | 19 | 1344.66 | 0.36 |
| MOL011072 | Quinicine | 14 | 1086.41 | 0.35 |
| MOL000492 | (+)-catechin | 4 | 365.78 | 0.34 |
| MOL011074 | Scillaren A_qt | 3 | 363.09 | 0.24 |
| MOL004350 | Ruvoside_qt | 1 | 0.00 | 0.21 |
| MOL001771 | poriferast-5-en-3beta-ol | 1 | 0.00 | 0.24 |
| MOL011075 | Shikodonin | 1 | 0.00 | 0.21 |
| Gene | Target Name | Degree | Betweenness | Closeness |
|---|---|---|---|---|
| PTGS2 | Prostaglandin G/H synthase 2 | 12 | 3313.14 | 0.50 |
| PTGS1 | Prostaglandin G/H synthase 1 | 9 | 1057.28 | 0.41 |
| PPARG | Peroxisome proliferator activated receptor gamma | 8 | 1018.63 | 0.44 |
| ADRB2 | Beta-2 adrenergic receptor | 6 | 611.36 | 0.40 |
| GABRA1 | Gamma-aminobutyric acid receptor subunit alpha-1 | 6 | 386.94 | 0.39 |
| NOS3 | Nitric-oxide synthase, endothelial | 6 | 291.27 | 0.38 |
| BCL2 | Apoptosis regulator Bcl-2 | 5 | 518.97 | 0.45 |
| CASP3 | Caspase-3 | 5 | 518.97 | 0.45 |
| CASP8 | Caspase-8 | 5 | 675.86 | 0.44 |
| AKR1B1 | Aldose reductase | 5 | 314.05 | 0.37 |
| PRSS1 | Trypsin-1 | 5 | 162.54 | 0.38 |
| F2 | Thrombin | 5 | 162.54 | 0.38 |
| AKT1 | RAC-alpha serine/threonine-protein kinase | 4 | 249.45 | 0.42 |
| CASP9 | Caspase-9 | 4 | 416.57 | 0.44 |
| MMP1 | Matrix metalloproteinase-9 | 4 | 249.45 | 0.42 |
| PLAU | Urokinase-type plasminogen activator | 4 | 575.35 | 0.42 |
| NOS2 | Nitric oxide synthase, inducible | 4 | 238.92 | 0.36 |
| AR | Androgen receptor | 4 | 129.72 | 0.38 |
| DPP4 | Dipeptidyl peptidase IV | 4 | 129.72 | 0.38 |
| BAX | Apoptosis regulator BAX | 4 | 406.73 | 0.44 |
| KCNH2 | Potassium voltage-gated channel subfamily H member 2 | 3 | 191.49 | 0.37 |
| SCN5A | Sodium channel protein type 5 subunit alpha | 3 | 191.49 | 0.37 |
| VEGFA | Vascular endothelial growth factor A | 3 | 167.23 | 0.40 |
| MMP2 | 72 kDa type IV collagenase | 3 | 167.23 | 0.40 |
| CAV1 | Caveolin-1 | 3 | 167.23 | 0.40 |
| RELA | Transcription factor p65 | 3 | 168.00 | 0.41 |
| TNF | Tumor necrosis factor | 3 | 168.00 | 0.41 |
| STAT1 | Signal transducer and activator of transcription 1-alpha/beta | 3 | 168.00 | 0.41 |
| NFKBIA | NF-kappa-B inhibitor alpha | 3 | 199.72 | 0.40 |
| ODC1 | Ornithine decarboxylase | 3 | 199.72 | 0.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yu, S.; Chen, B.; Su, E.; Cao, F. Ginkgo Seed as Medicine–Food Homology for Migraine: Network Pharmacology and Molecular Docking Insights. Int. J. Mol. Sci. 2025, 26, 9225. https://doi.org/10.3390/ijms26189225
Li Z, Yu S, Chen B, Su E, Cao F. Ginkgo Seed as Medicine–Food Homology for Migraine: Network Pharmacology and Molecular Docking Insights. International Journal of Molecular Sciences. 2025; 26(18):9225. https://doi.org/10.3390/ijms26189225
Chicago/Turabian StyleLi, Zhifan, Shuangyuan Yu, Bolin Chen, Erzheng Su, and Fuliang Cao. 2025. "Ginkgo Seed as Medicine–Food Homology for Migraine: Network Pharmacology and Molecular Docking Insights" International Journal of Molecular Sciences 26, no. 18: 9225. https://doi.org/10.3390/ijms26189225
APA StyleLi, Z., Yu, S., Chen, B., Su, E., & Cao, F. (2025). Ginkgo Seed as Medicine–Food Homology for Migraine: Network Pharmacology and Molecular Docking Insights. International Journal of Molecular Sciences, 26(18), 9225. https://doi.org/10.3390/ijms26189225






