Progressive Ocular Axial Elongation and Retinal Ganglion Cell Degeneration in Mice with Elastic Fiber Disorder
Abstract
1. Introduction
2. Results
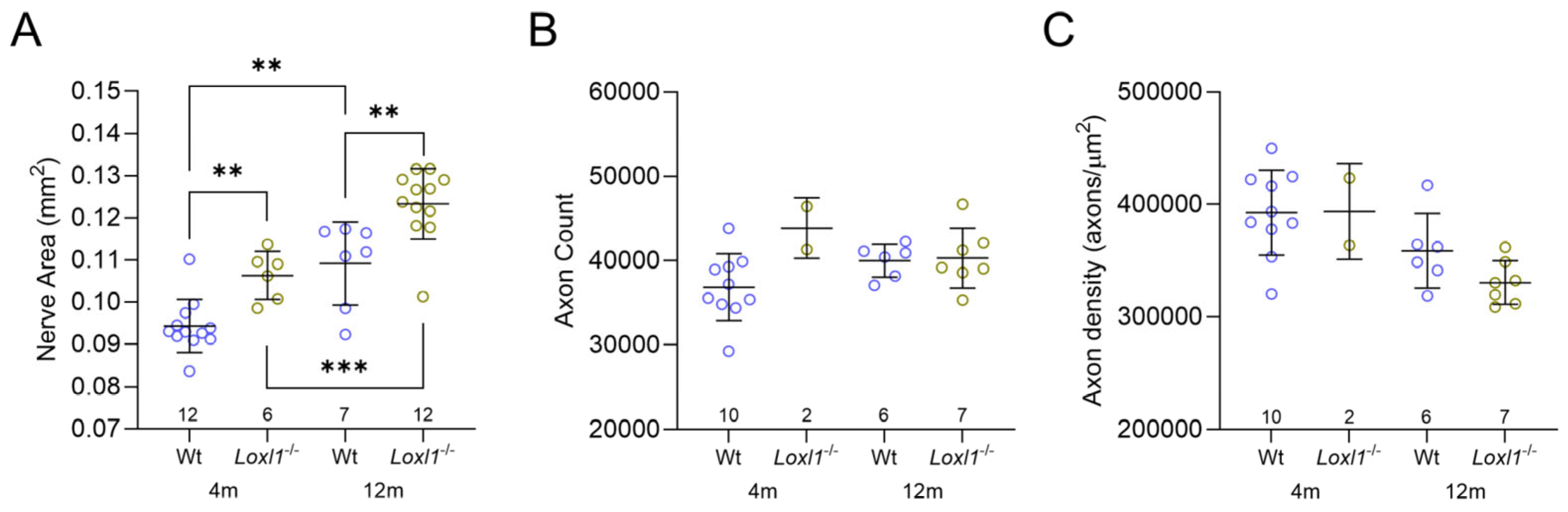
2.1. Loxl1−/− Mice Have Expanded Optic Nerves
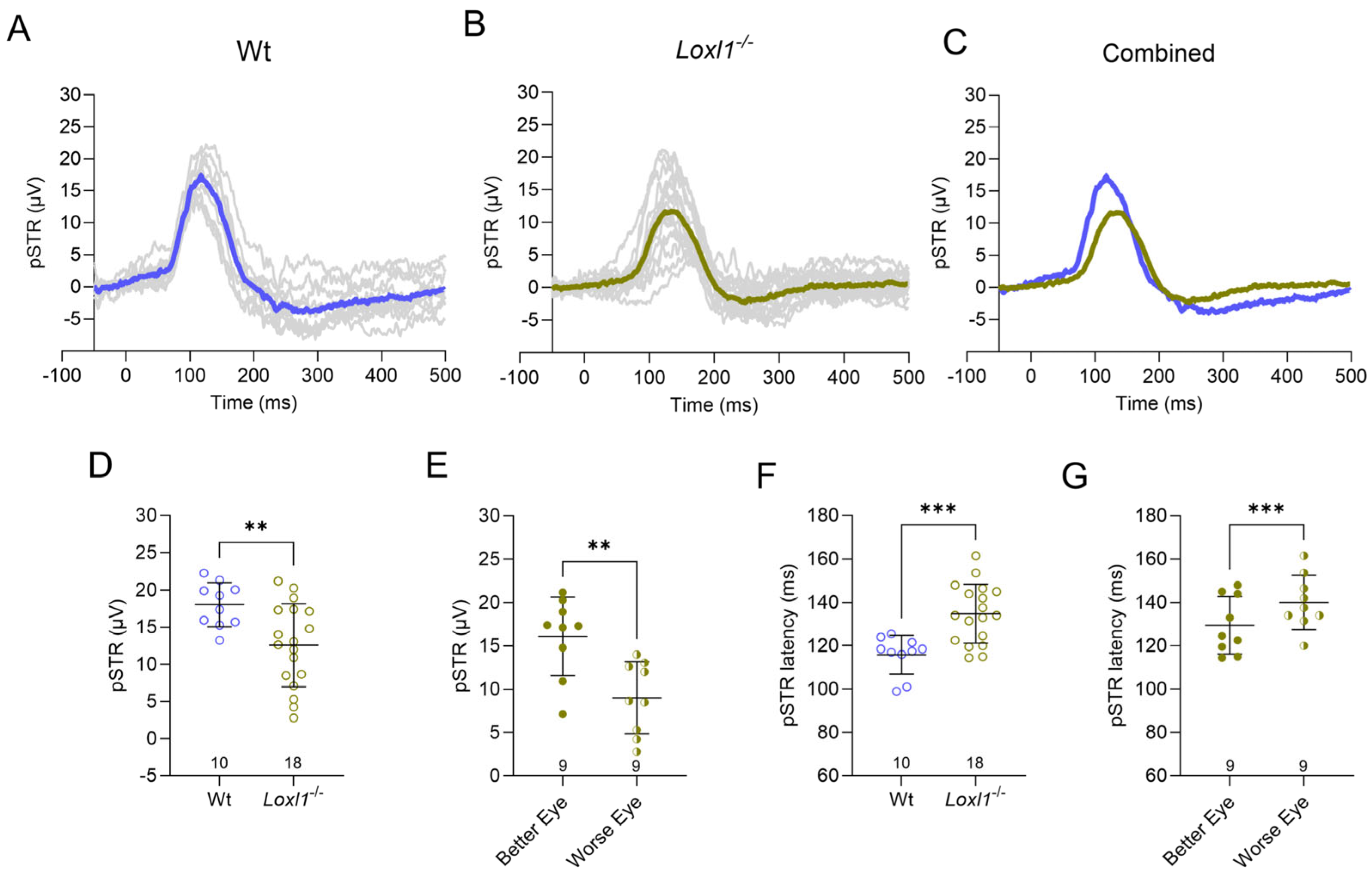
2.2. Loxl1−/− Mice Have Reduced RGC Function
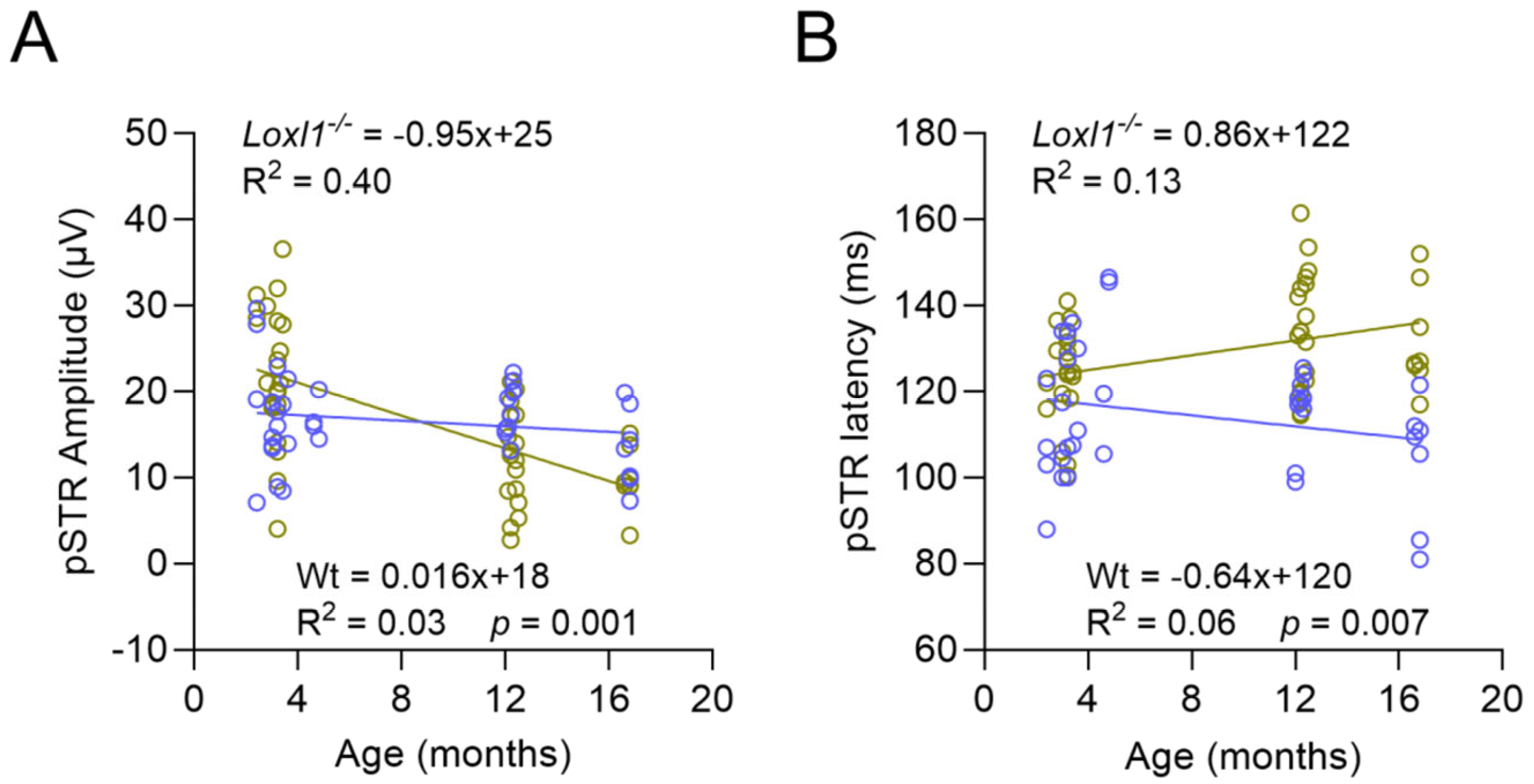
2.3. Loxl1−/− Mice Have Accelerated Age-Related Decline of RGC Function
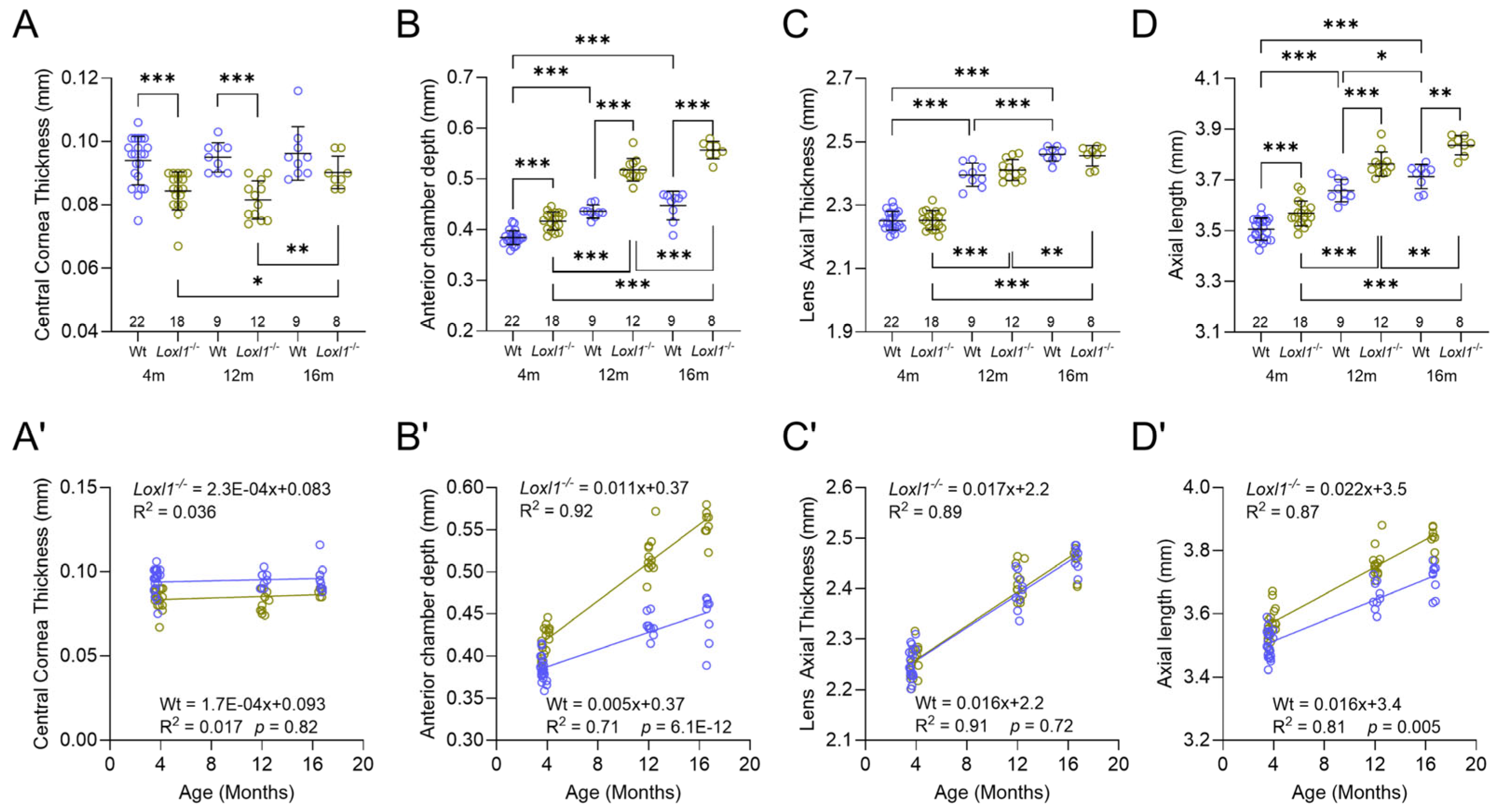
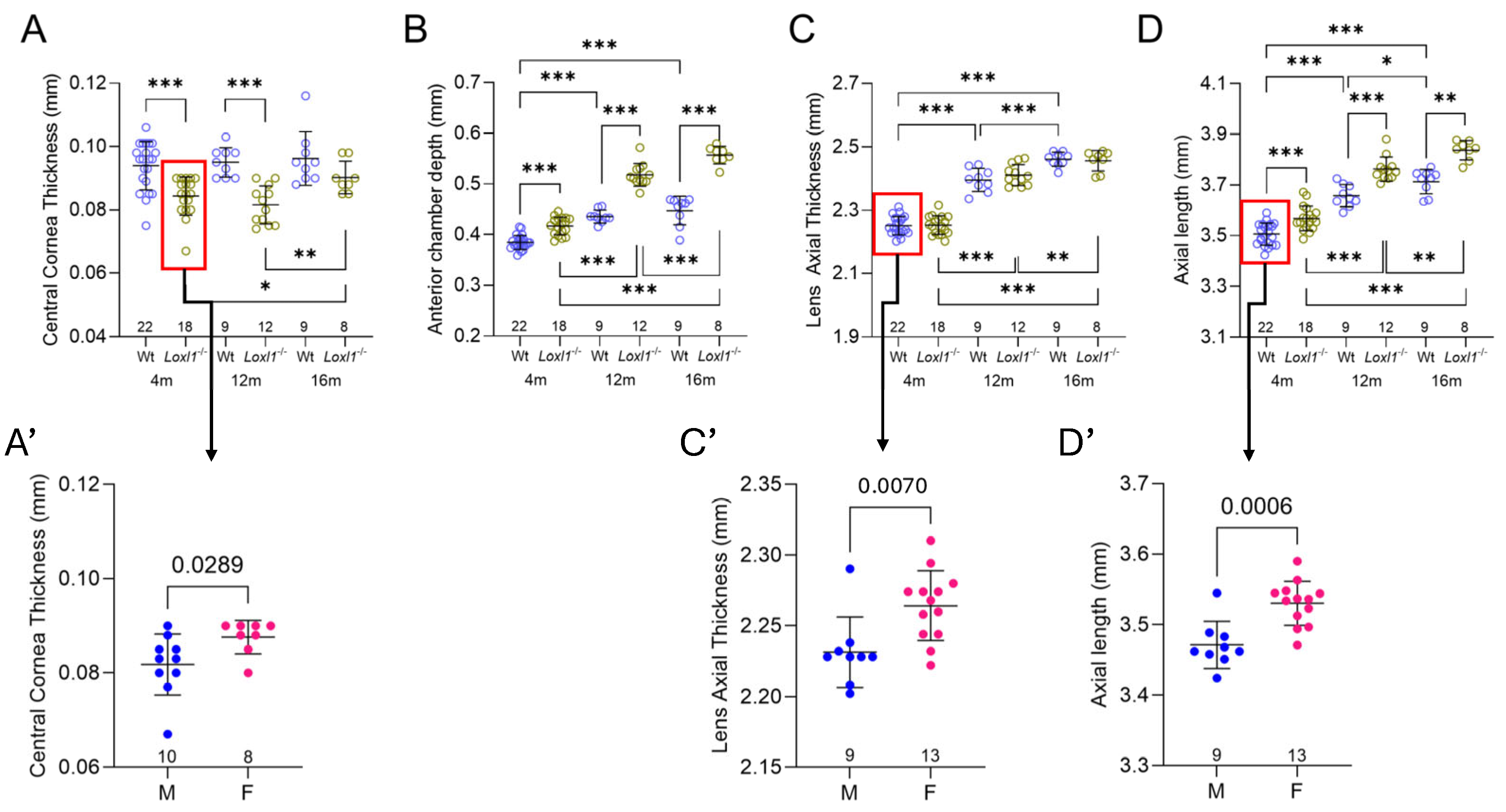
2.4. Loxl1−/− Mice Have Significant Anterior Segment Biometric Changes
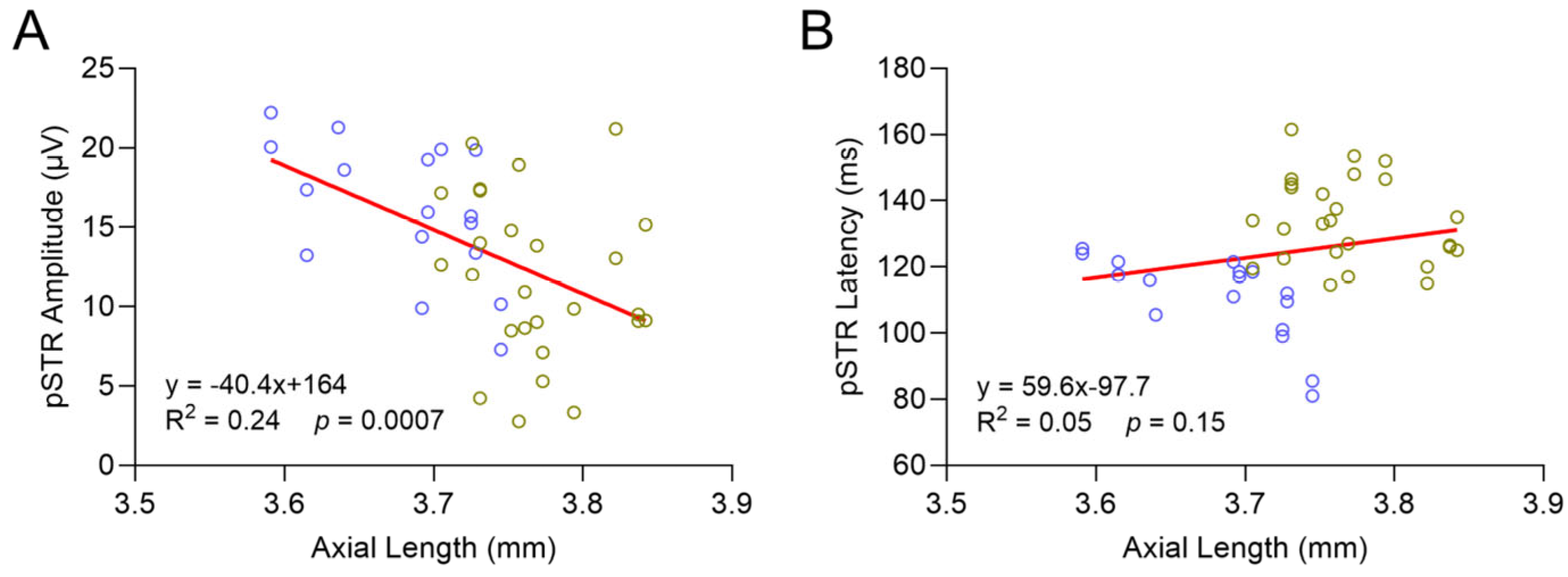
2.5. Loxl1−/− Mice Have Globe Elongation, Which Is Correlated with Decreased RGC Function
2.6. No Changes in IOP or Visual Acuity (VA) in Loxl1−/−Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Intraocular Pressure (IOP) Measurements
4.3. Visual Acuity via Optomotor Reflex
4.4. Spectral Domain Ocular Coherence Tomography (SD-OCT)
4.5. Scotopic Flash Electroretinograms (ERGs)
4.6. Optic Nerve Histology and Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burgoyne, C.F.; Crawford Downs, J.; Bellezza, A.J.; Francis Suh, J.-K.; Hart, R.T. The Optic Nerve Head as a Biomechanical Structure: A New Paradigm for Understanding the Role of IOP-Related Stress and Strain in the Pathophysiology of Glaucomatous Optic Nerve Head Damage. Prog. Retin. Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef]
- Coudrillier, B.; Pijanka, J.K.; Jefferys, J.L.; Goel, A.; Quigley, H.A.; Boote, C.; Nguyen, T.D. Glaucoma-Related Changes in the Mechanical Properties and Collagen Micro-Architecture of the Human Sclera. PLoS ONE 2015, 10, e0131396. [Google Scholar] [CrossRef]
- Marcus, M.W.; de Vries, M.M.; Montolio, F.G.J.; Jansonius, N.M. Myopia as a Risk Factor for Open-Angle Glaucoma: A Systematic Review and Meta-Analysis. Ophthalmology 2011, 118, 1989–1994.e2. [Google Scholar] [CrossRef]
- Grytz, R.; Yang, H.; Hua, Y.; Samuels, B.C.; Sigal, I.A. Connective Tissue Remodeling in Myopia and Its Potential Role in Increasing Risk of Glaucoma. Curr. Opin. Biomed. Eng. 2020, 15, 40–50. [Google Scholar] [CrossRef]
- Elhawy, E.; Kamthan, G.; Dong, C.Q.; Danias, J. Pseudoexfoliation Syndrome, a Systemic Disorder with Ocular Manifestations. Hum. Genom. 2012, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Thorleifsson, G.; Magnusson, K.P.; Sulem, P.; Walters, G.B.; Gudbjartsson, D.F.; Stefansson, H.; Jonsson, T.; Jonasdottir, A.; Jonasdottir, A.; Stefansdottir, G.; et al. Common Sequence Variants in the LOXL1 Gene Confer Susceptibility to Exfoliation Glaucoma. Science 2007, 317, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, K. Lysyl Oxidases: A Novel Multifunctional Amine Oxidase Family. Prog. Nucleic Acid. Res. Mol. Biol. 2001, 70, 1–32. [Google Scholar] [CrossRef]
- Vallet, S.D.; Ricard-Blum, S. Lysyl Oxidases: From Enzyme Activity to Extracellular Matrix Cross-Links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Y.; Gao, J.; Pawlyk, B.; Starcher, B.; Spencer, J.A.; Yanagisawa, H.; Zuo, J.; Li, T. Elastic Fiber Homeostasis Requires Lysyl Oxidase–like 1 Protein. Nat. Genet. 2004, 36, 178–182. [Google Scholar] [CrossRef]
- Wareham, L.K.; Kuchtey, J.; Wu, H.-J.; Krystofiak, E.; Wu, Y.; Reinhart-King, C.A.; Kuchtey, R.W. Lysyl Oxidase-like 1 Deficiency Alters Ultrastructural and Biomechanical Properties of the Peripapillary Sclera in Mice. Matrix Biol. Plus 2022, 16, 100120. [Google Scholar] [CrossRef]
- Pazos, M.; Yang, H.; Gardiner, S.K.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C.; Burgoyne, C.F. Expansions of the Neurovascular Scleral Canal and Contained Optic Nerve Occur Early in the Hypertonic Saline Rat Experimental Glaucoma Model. Exp. Eye Res. 2016, 145, 173–186. [Google Scholar] [CrossRef]
- Cooper, M.L.; Crish, S.D.; Inman, D.M.; Horner, P.J.; Calkins, D.J. Early Astrocyte Redistribution in the Optic Nerve Precedes Axonopathy in the DBA/2J Mouse Model of Glaucoma. Exp. Eye Res. 2016, 150, 22–33. [Google Scholar] [CrossRef]
- Wu, H.-J.; Hazlewood, R.J.; Kuchtey, J.; Kuchtey, R.W. Enlarged Optic Nerve Axons and Reduced Visual Function in Mice with Defective Microfibrils. eNeuro 2018, 5, ENEURO.0260-18.2018. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-J.; Krystofiak, E.; Kuchtey, J.; Kuchtey, R.W. Enhanced Optic Nerve Expansion and Altered Ultrastructure of Elastic Fibers Induced by Lysyl Oxidase Inhibition in a Mouse Model of Marfan Syndrome. Am. J. Pathol. 2024, 194, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, J.; de Leon, J.M.; Azarbod, P.; Chen, A.; Morales, E.; Nouri-Mahdavi, K.; Coleman, A.; Yu, F.; Afifi, A. Trabeculectomy Can Improve Long-Term Visual Function in Glaucoma. Ophthalmology 2016, 123, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Saszik, S.M.; Robson, J.G.; Frishman, L.J. The Scotopic Threshold Response of the Dark-Adapted Electroretinogram of the Mouse. J. Physiol. 2002, 543, 899–916. [Google Scholar] [CrossRef]
- Levine, R.A.; Demirel, S.; Fan, J.; Keltner, J.L.; Johnson, C.A.; Kass, M.A.; the Ocular Hypertension Treatment Study Group. Asymmetries and Visual Field Summaries as Predictors of Glaucoma in the Ocular Hypertension Treatment Study. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3896–3903. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Leung, C.K.S.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary Open-Angle Glaucoma. Nat. Rev. Dis. Primer 2016, 2, 1–19. [Google Scholar] [CrossRef]
- Kuchtey, R.W.; Insignares, S.; Yang, T.S.; Kuchtey, J. In Search of Mouse Models for Exfoliation Syndrome. Am. J. Ophthalmol. 2024, 267, 271–285. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, J.; Yang, L.; Zhang, X.; Shi, W.; Yang, H.; Zhang, G.; She, J.; Li, H. Sex Differences in Ocular Biometric Measurements: A Twin Study. Front. Med. 2022, 9, 936738. [Google Scholar] [CrossRef]
- Miao, A.; Tang, Y.; Zhu, X.; Qian, D.; Zheng, T.; Lu, Y. Associations between Anterior Segment Biometry and High Axial Myopia in 3438 Cataractous Eyes in the Chinese Population. BMC Ophthalmol. 2022, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, J.L.; Pawlyk, B.; Connolly, E.; Adamian, M.; Miller, J.W.; Pasquale, L.R.; Haddadin, R.I.; Grosskreutz, C.L.; Rhee, D.J.; Li, T. Disruption of the Blood–Aqueous Barrier and Lens Abnormalities in Mice Lacking Lysyl Oxidase-Like 1 (LOXL1). Investig. Ophthalmol. Vis. Sci. 2014, 55, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.F.; Schmitt, H.M.; Kuhn, M.S.; Watkins, T.; Hake, K.M.; Weisz, T.; Flynn, E.J., III.; Elliott, M.H.; Hauser, M.A.; Stamer, W.D. Genetic Background Determines Severity of Loxl1-Mediated Systemic and Ocular Elastosis in Mice. Dis. Model. Mech. 2023, 16, dmm050392. [Google Scholar] [CrossRef] [PubMed]
- Bui, B.V.; Fortune, B. Ganglion Cell Contributions to the Rat Full-Field Electroretinogram. J. Physiol. 2004, 555, 153–173. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Armaly, M.F. Cup/disc Ratio in Early Open-Angle Glaucoma. Doc. Ophthalmol. 1969, 26, 526–533. [Google Scholar] [CrossRef]
- Sullivan-Mee, M.; Ruegg, C.C.; Pensyl, D.; Halverson, K.; Qualls, C. Diagnostic Precision of Retinal Nerve Fiber Layer and Macular Thickness Asymmetry Parameters for Identifying Early Primary Open-Angle Glaucoma. Am. J. Ophthalmol. 2013, 156, 567–577.e1. [Google Scholar] [CrossRef]
- Jonas, J.B.; Fernández, M.C.; Naumann, G.O.H. Correlation of the Optic Disc Size to Glaucoma Susceptibility. Ophthalmology 1991, 98, 675–680. [Google Scholar] [CrossRef]
- Jonas, J.B.; Schmidt, A.M.; Müller-Bergh, J.A.; Schlötzer-Schrehardt, U.M.; Naumann, G.O. Human Optic Nerve Fiber Count and Optic Disc Size. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2012–2018. [Google Scholar]
- Weinreb, R.N.; Zangwill, L.M.; Jain, S.; Becerra, L.M.; Dirkes, K.; Piltz-Seymour, J.R.; Cioffi, G.A.; Trick, G.L.; Coleman, A.L.; Brandt, J.D.; et al. Predicting the Onset of Glaucoma. Ophthalmology 2010, 117, 1674–1683. [Google Scholar] [CrossRef]
- Nishida, T.; Pham, V.Q.; Moghimi, S.; Girkin, C.A.; Fazio, M.A.; Liebmann, J.M.; Zangwill, L.M.; Weinreb, R.N. Optic Disc Size and Circumpapillary Retinal Nerve Fiber Layer Thinning in Glaucoma. Ophthalmol. Glaucoma 2025, 8, 343–350. [Google Scholar] [CrossRef]
- Sultan, G.; Baudouin, C.; Auzerie, O.; De Saint Jean, M.; Goldschild, M.; the Marfan Study Group; Pisella, P.-J. Cornea in Marfan Disease: Orbscan and In Vivo Confocal Microscopy Analysis. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1757–1764. [Google Scholar]
- Correia Barão, R.; Santos, M.; Marques, R.E.; Quintas, A.M.; Guerra, P. Keratoconus Tomographic Indices in Osteogenesis Imperfecta. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 2585–2592. [Google Scholar] [CrossRef]
- Guo, D.; Liu, L.; Yang, F.; Young, C.A.; Zheng, D.; Jin, G. Characteristics and Genotype–Phenotype Correlations in ADAMTS17 Mutation–Related Weill–Marchesani Syndrome. Exp. Eye Res. 2023, 234, 109606. [Google Scholar] [CrossRef]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; Backer, J.D.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The Revised Ghent Nosology for the Marfan Syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef]
- De Maria, A.; Wilmarth, P.A.; David, L.L.; Bassnett, S. Proteomic Analysis of the Bovine and Human Ciliary Zonule. Investig. Ophthalmol. Vis. Sci. 2017, 58, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Ritch, R.; Schlötzer-Schrehardt, U. Exfoliation Syndrome. Surv. Ophthalmol. 2001, 45, 265–315. [Google Scholar] [CrossRef]
- Tang, T.; Ren, C.; Cai, Y.; Li, Y.; Wang, K.; Zhao, M. Lifelong Changes in the Choroidal Thickness, Refractive Status, and Ocular Dimensions in C57BL/6J Mouse. Investig. Ophthalmol. Vis. Sci. 2024, 65, 26. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.V.; Quigley, H.A. Risk Factors and Open-Angle Glaucoma: Classification and Application. J. Glaucoma 2007, 16, 406. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Bikbov, M.M.; Kazakbaeva, G.M.; Wang, Y.X.; Xu, J.; Nangia, V.; Nangia, P.V.; Panda-Jonas, S. Positive and Negative Associations of Myopia with Ocular Diseases in Population-Based Studies. Ophthalmology 2024, 131, 1427–1435. [Google Scholar] [CrossRef]
- Huh, M.G.; Jeong, Y.; Shin, Y.I.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Assessing Glaucoma Severity and Progression in Individuals with Asymmetric Axial Length: An Intrapatient Comparative Study. Ophthalmology 2025, 132, 39–51. [Google Scholar] [CrossRef]
- Ha, A.; Kim, C.Y.; Shim, S.R.; Chang, I.B.; Kim, Y.K. Degree of Myopia and Glaucoma Risk: A Dose-Response Meta-Analysis. Am. J. Ophthalmol. 2022, 236, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Wang, P.; Tian, N. Effect of General Anesthetics on IOP in Elevated IOP Mouse Model. Exp. Eye Res. 2011, 92, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Dalvin, L.A.; Fautsch, M.P. Analysis of Circadian Rhythm Gene Expression With Reference to Diurnal Pattern of Intraocular Pressure in Mice. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2657–2663. [Google Scholar] [CrossRef]
- Wu, H.-J.; Mortlock, D.P.; Kuchtey, R.W.; Kuchtey, J. Altered Ocular Fibrillin Microfibril Composition in Mice With a Glaucoma-Causing Mutation of Adamts10. Investig. Ophthalmol. Vis. Sci. 2021, 62, 26. [Google Scholar] [CrossRef]
- Bermudez, M.A.; Vicente, A.F.; Romero, M.C.; Arcos, M.D.; Abalo, J.M.; Gonzalez, F. Time Course of Cold Cataract Development in Anesthetized Mice. Curr. Eye Res. 2011, 36, 278–284. [Google Scholar] [CrossRef]
- Koehn, D.; Meyer, K.J.; Syed, N.A.; Anderson, M.G. Ketamine/Xylazine-Induced Corneal Damage in Mice. PLoS ONE 2015, 10, e0132804. [Google Scholar] [CrossRef] [PubMed]
- Sadun, A.A.; Smith, L.E.; Kenyon, K.R. Paraphenylenediamine: A New Method for Tracing Human Visual Pathways. J. Neuropathol. Exp. Neurol. 1983, 42, 200–206. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Inman, D.M.; Sappington, R.M.; Horner, P.J.; Calkins, D.J. Quantitative Correlation of Optic Nerve Pathology with Ocular Pressure and Corneal Thickness in the DBA/2 Mouse Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 986–996. [Google Scholar] [CrossRef][Green Version]
- Goyal, V.; Read, A.T.; Ritch, M.D.; Hannon, B.G.; Rodriguez, G.S.; Brown, D.M.; Feola, A.J.; Hedberg-Buenz, A.; Cull, G.A.; Reynaud, J.; et al. AxoNet 2.0: A Deep Learning-Based Tool for Morphometric Analysis of Retinal Ganglion Cell Axons. Transl. Vis. Sci. Technol. 2023, 12, 9. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Insignares, S.; Kuchtey, J.; Kuchtey, R.W. Progressive Ocular Axial Elongation and Retinal Ganglion Cell Degeneration in Mice with Elastic Fiber Disorder. Int. J. Mol. Sci. 2025, 26, 9221. https://doi.org/10.3390/ijms26189221
Insignares S, Kuchtey J, Kuchtey RW. Progressive Ocular Axial Elongation and Retinal Ganglion Cell Degeneration in Mice with Elastic Fiber Disorder. International Journal of Molecular Sciences. 2025; 26(18):9221. https://doi.org/10.3390/ijms26189221
Chicago/Turabian StyleInsignares, Samuel, John Kuchtey, and Rachel W. Kuchtey. 2025. "Progressive Ocular Axial Elongation and Retinal Ganglion Cell Degeneration in Mice with Elastic Fiber Disorder" International Journal of Molecular Sciences 26, no. 18: 9221. https://doi.org/10.3390/ijms26189221
APA StyleInsignares, S., Kuchtey, J., & Kuchtey, R. W. (2025). Progressive Ocular Axial Elongation and Retinal Ganglion Cell Degeneration in Mice with Elastic Fiber Disorder. International Journal of Molecular Sciences, 26(18), 9221. https://doi.org/10.3390/ijms26189221





