Alginate/PVA Hydrogel Incorporating HA-Liposomes and Aronia-Derived Silver Nanoparticles for Advanced Wound Management
Abstract
1. Introduction
2. Results
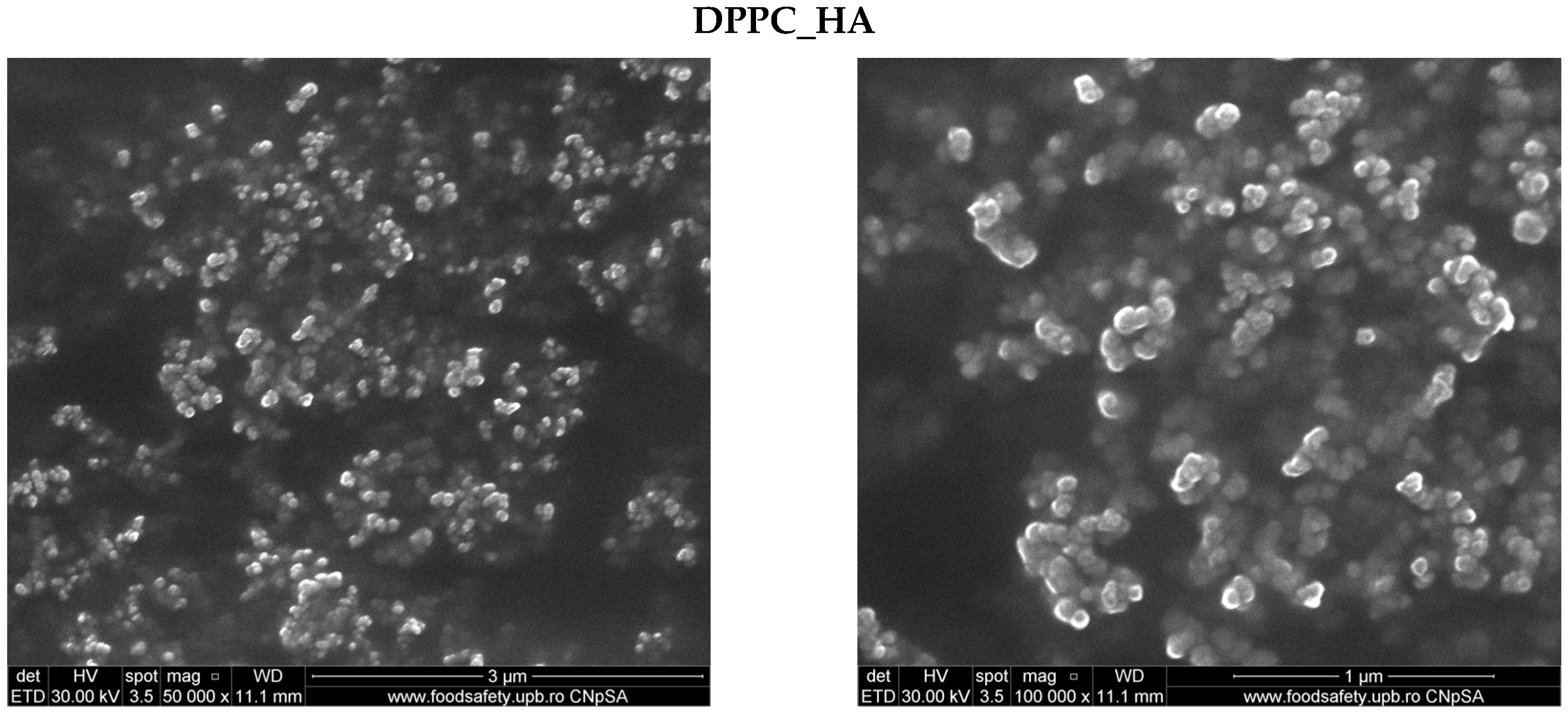
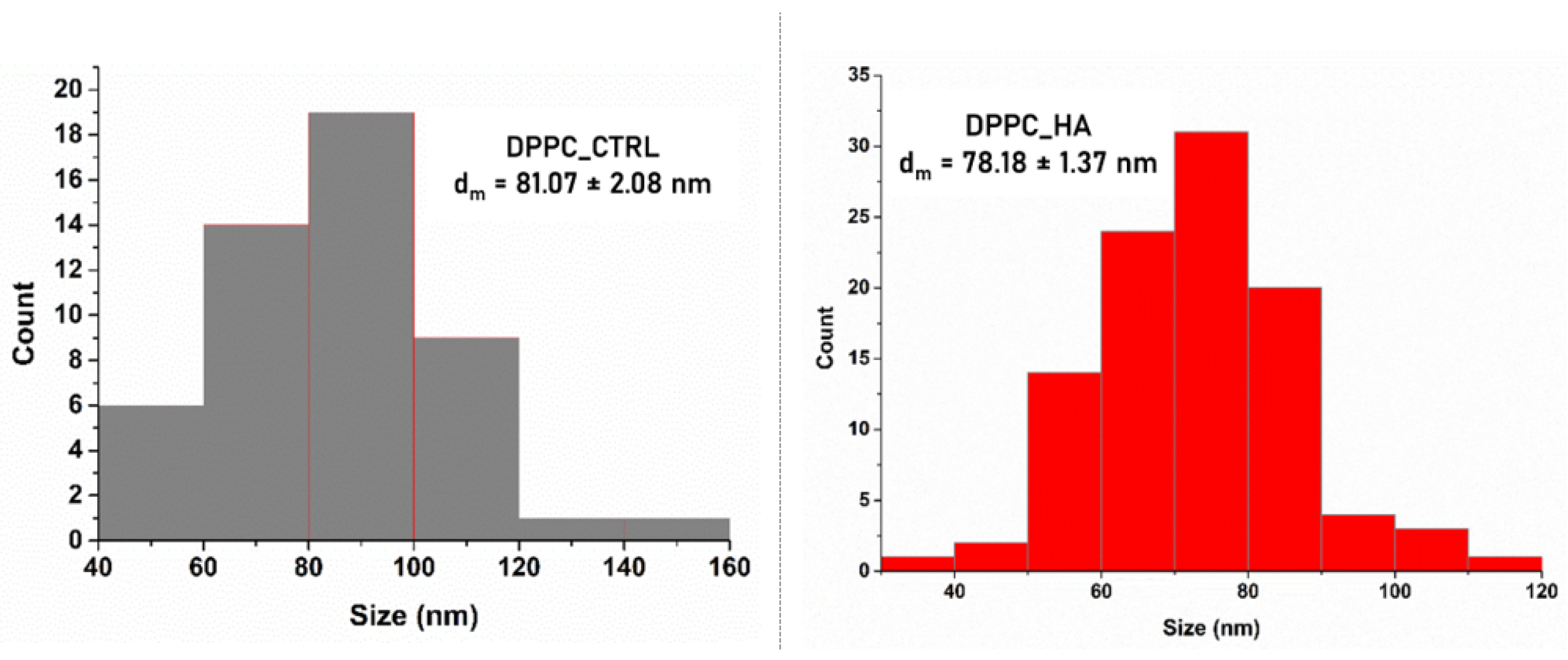
2.1. Characterization of DPPC-Based Liposomes
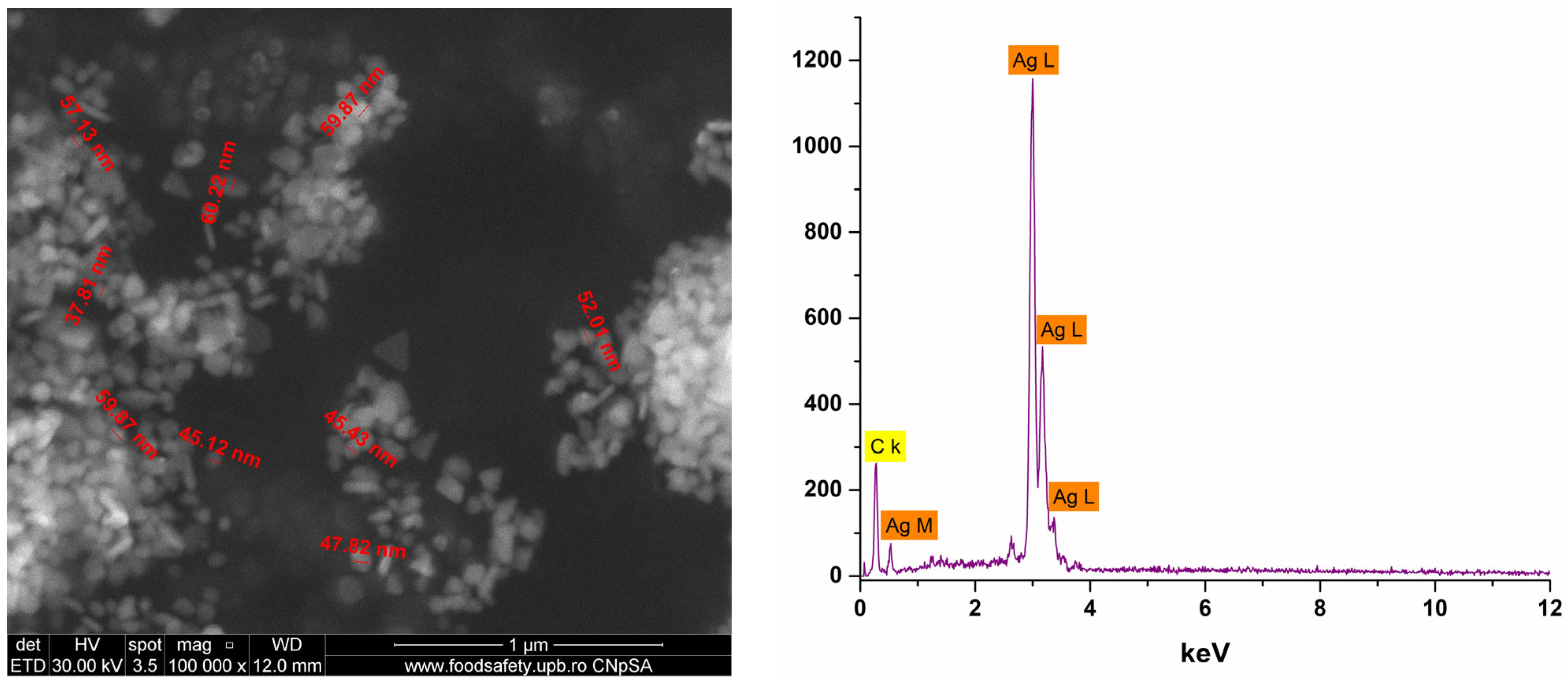
2.2. Characterization of Ag_Aro Silver Nanoparticles
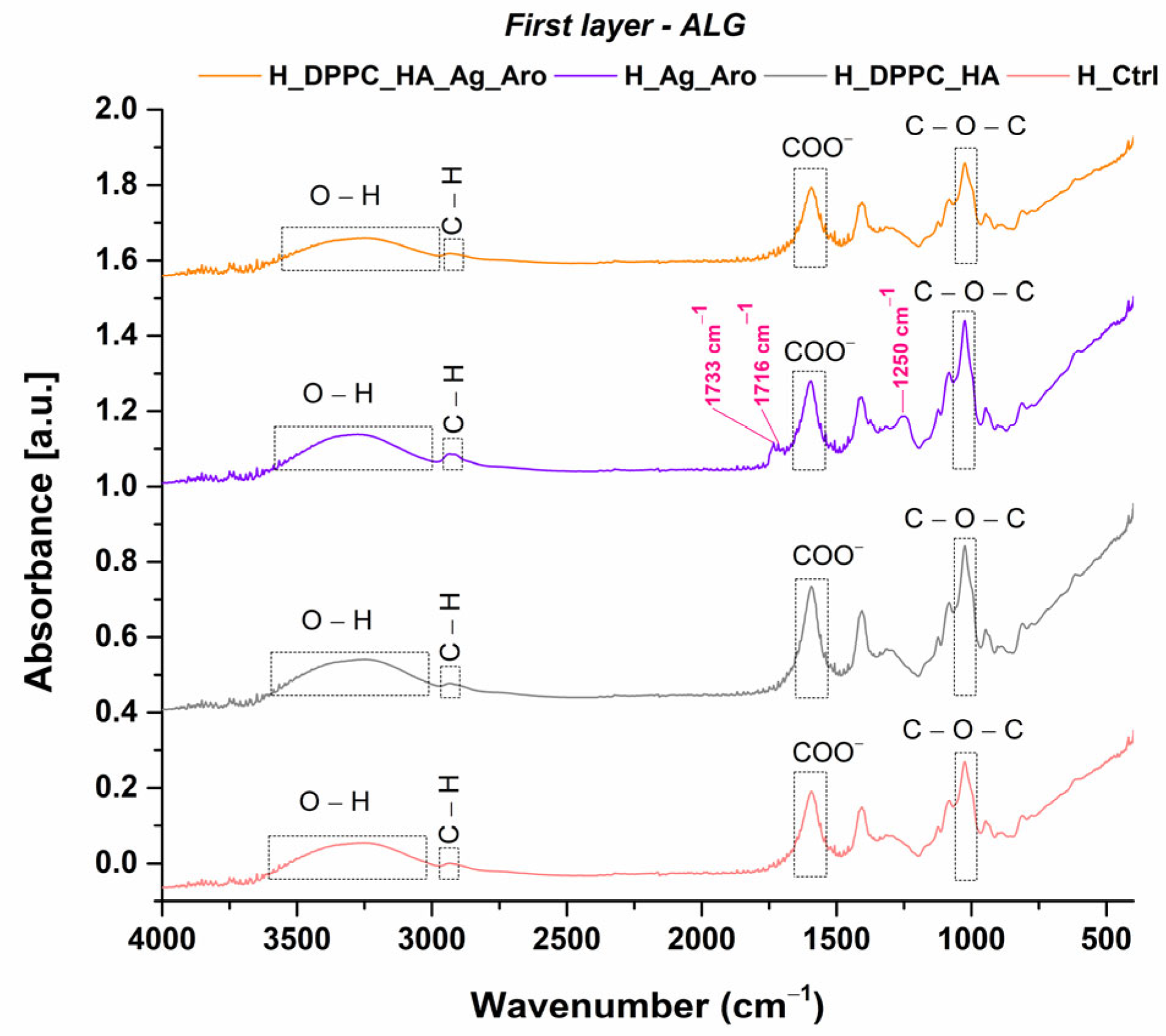
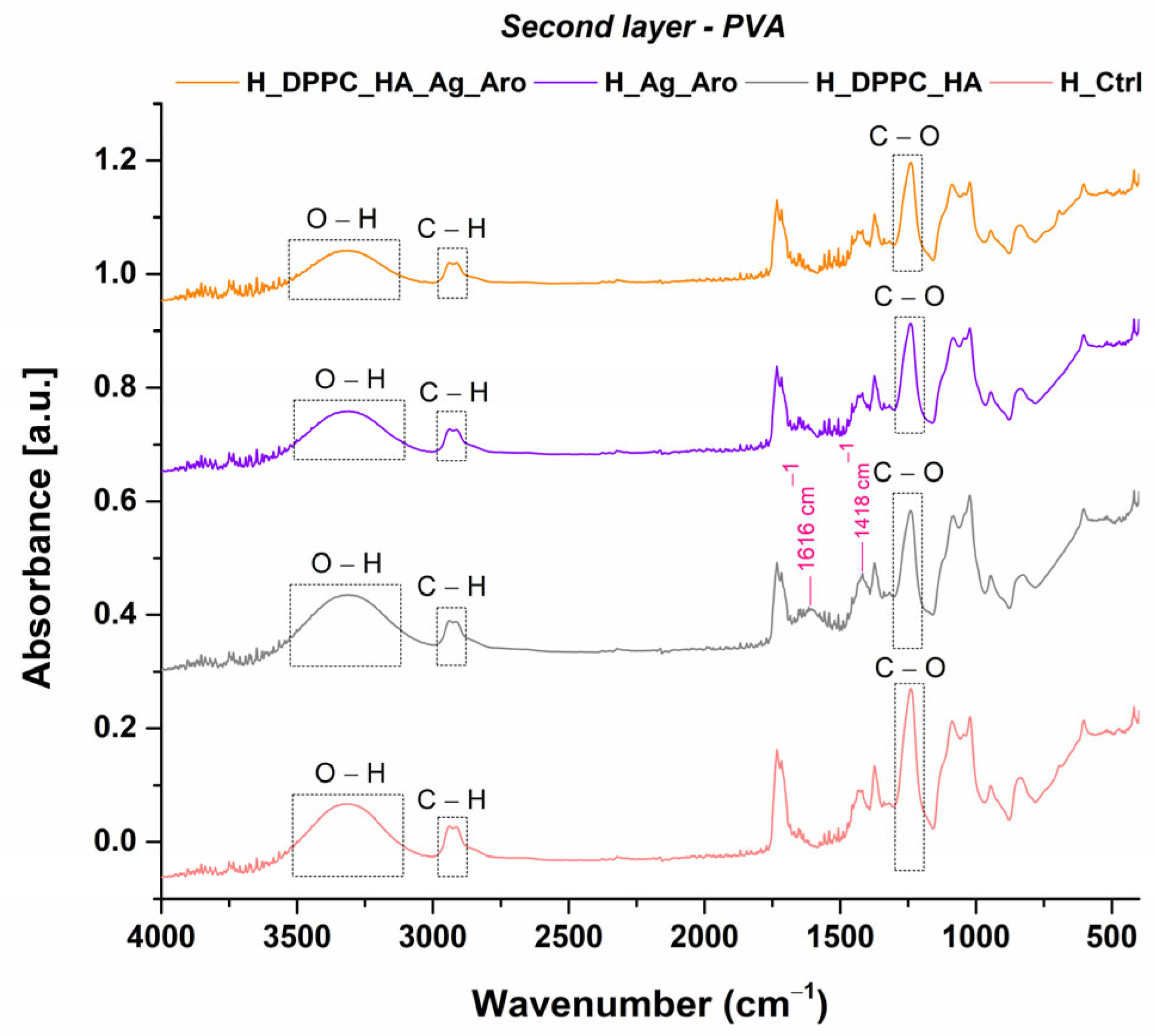
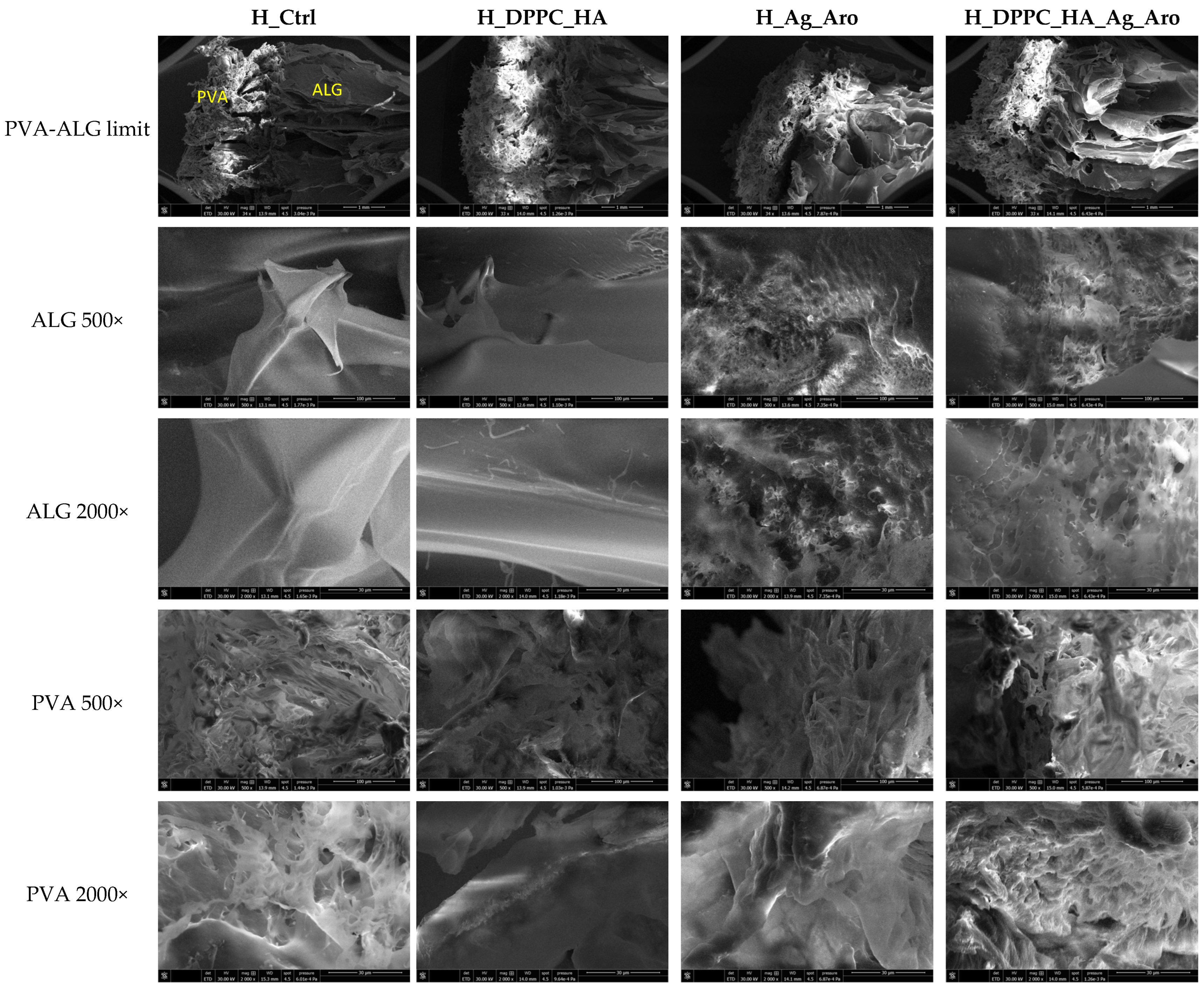
2.3. Characterization of Hydrogels
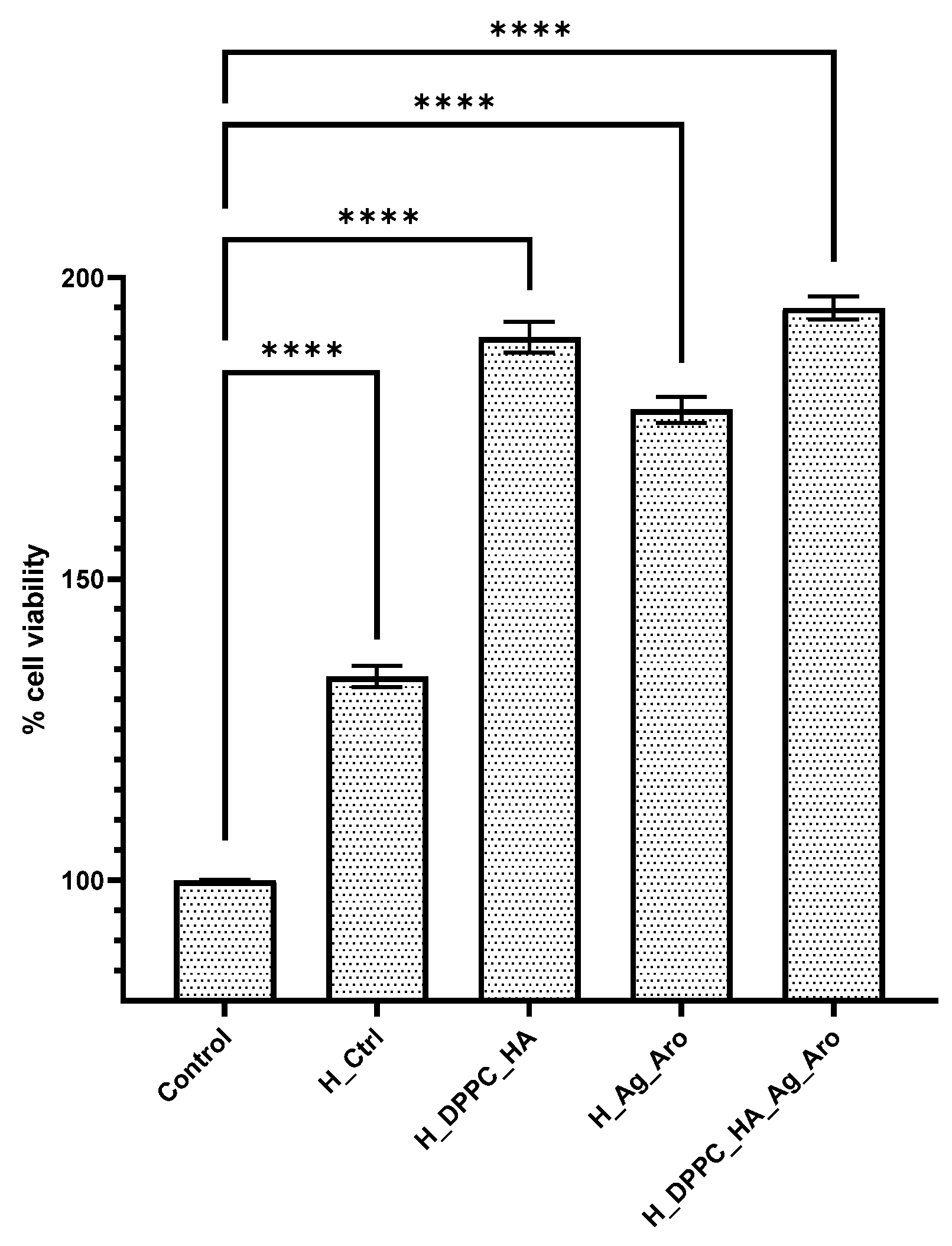
2.4. Biological Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of DPPC-Based Lipid Vesicles Loaded with Hyaluronic Acid
4.3. Green Synthesis of Silver Nanoparticles Using Aronia Powder
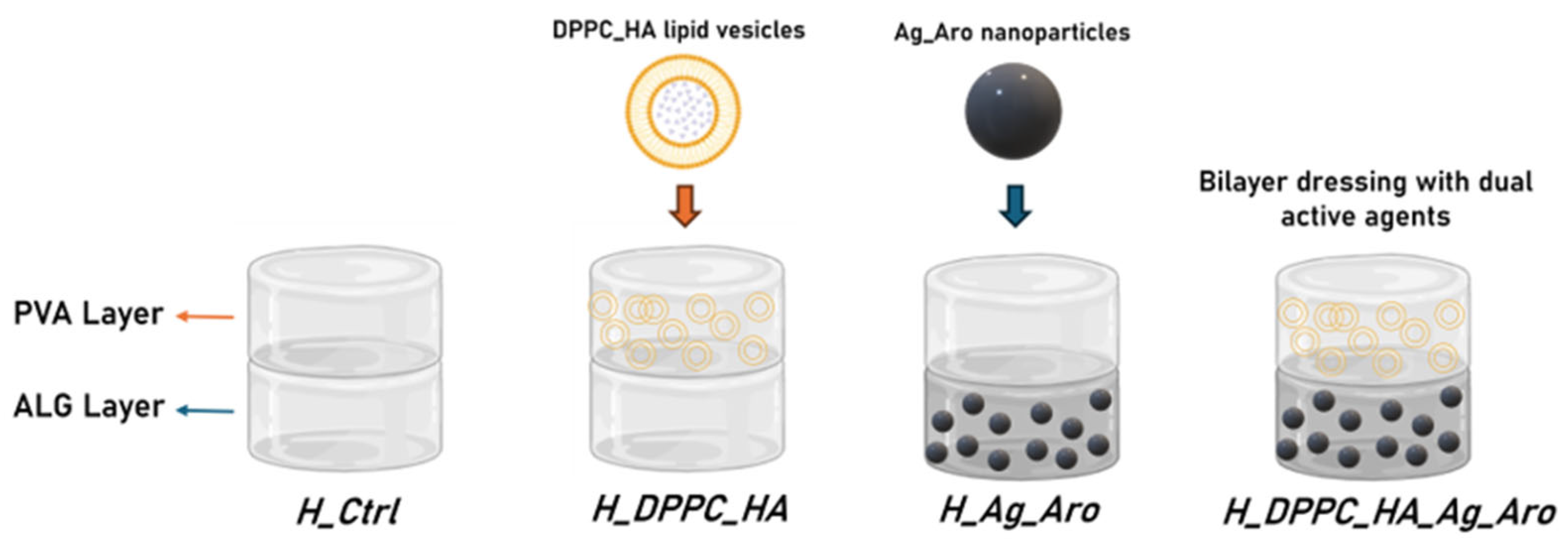
4.4. Fabrication and Layering of Hydrogel-Based Wound Dressings
4.5. Characterization Methods
4.5.1. X-Ray Diffraction (XRD)
4.5.2. Scanning Electron Microscopy (SEM)
4.5.3. Dynamic Light Scattering (DLS)
4.5.4. Fourier Transform Infrared Spectroscopy (FTIR)
4.5.5. Swelling Rate
4.5.6. Degradation Rate
4.5.7. Biological Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.d.A.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Gushiken, L.F.; Beserra, F.P.; Bastos, J.K.; Jackson, C.J.; Pellizzon, C.H. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels 2025, 11, 271. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Gould, L.J. Opportunities and Challenges of the Management of Chronic Wounds: A Multidisciplinary Viewpoint. Chronic Wound Care Manag. Res. 2020, 7, 27–36. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Bîrcă, A.C.; Mogoşanu, G.D.; Rădulescu, M.; Holban, A.M.; Manuc, D.; Alberts, A.; Grumezescu, A.M.; Mogoantă, L. Zinc Alginate Hydrogel-Coated Wound Dressings: Fabrication, Characterization, and Evaluation of Anti-Infective and In Vivo Performance. Gels 2025, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. J. Compos. Sci. 2025, 9, 133. [Google Scholar] [CrossRef]
- Pastar, I.; Balukoff, N.C.; Marjanovic, J.; Chen, V.Y.; Stone, R.C.; Tomic-Canic, M. Molecular pathophysiology of chronic wounds: Current state and future directions. Cold Spring Harb. Perspect. Biol. 2023, 15, a041243. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. New Perspectives of Hydrogels in Chronic Wound Management. Molecules 2025, 30, 686. [Google Scholar] [CrossRef] [PubMed]
- Fauzian, F.; Garmana, A.N.; Mauludin, R. Applications of nanotechnology-based drug delivery system for delivering natural products into acute and chronic wounds: A review. Biointerface Res. Appl. Chem 2023, 13, 426. [Google Scholar]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Kapp, S.; Santamaria, N. The financial and quality-of-life cost to patients living with a chronic wound in the community. Int. Wound J. 2017, 14, 1108–1119. [Google Scholar] [CrossRef]
- Britto, E.J.; Nezwek, T.A.; Popowicz, P.; Robins, M. Wound Dressings. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470199/ (accessed on 23 July 2025).
- Ferraz, M.P. Wound Dressing Materials: Bridging Material Science and Clinical Practice. Appl. Sci. 2025, 15, 1725. [Google Scholar] [CrossRef]
- Özcan Bülbül, E.; Okur, M.E.; Üstündağ Okur, N.; Siafaka, P.I. Chapter 2-Traditional and advanced wound dressings: Physical characterization and desirable properties for wound healing. In Natural Polymers in Wound Healing and Repair; Sah, M.K., Kasoju, N., Mano, J.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 19–50. [Google Scholar]
- Al-Mamari, A.; Shahitha, F.; Al-Sibani, M.; Al Saadi, A.; Al Harrasi, A.; Ahmad, A. Novel antibacterial wound healing hydrogels based on HEC/SA/HA using gree n chemistry approach. Lett. Appl. NanoBioSci 2023, 12, 69. [Google Scholar]
- Nasra, S.; Patel, M.; Shukla, H.; Bhatt, M.; Kumar, A. Functional hydrogel-based wound dressings: A review on biocompatibility and therapeutic efficacy. Life Sci. 2023, 334, 122232. [Google Scholar] [CrossRef]
- Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of Poly(vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Luneva, O.; Olekhnovich, R.; Uspenskaya, M. Bilayer Hydrogels for Wound Dressing and Tissue Engineering. Polymers 2022, 14, 3135. [Google Scholar] [CrossRef]
- Rigo, D.; da Silva, L.M.; Fischer, B.; Colet, R.; Dallago, R.M.; Zeni, J. Hyaluronic Acid—From Production to Application: A Review. Biointerface Res. Appl. Chem 2023, 13, 211. [Google Scholar]
- Huang, P.; Wang, L.; Heng, B.C.; Haririan, I.; Cai, Q.; Ge, Z. Property-Tailoring Chemical Modifications of Hyaluronic Acid for Regenerative Medicine Applications. Acta Biomater. 2025, 201, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Park, S.; Santosh, S.S.; Ganeshalingam, A.; Thiripuranathar, G.; Sathiyaseelan, A.; Vijayasarathy, S.; Swaminathan, A.; Priya, V.V.; Wang, M.-H. Application of hyaluronic acid in tissue engineering, regenerative medicine, and nanomedicine: A review. Int. J. Biol. Macromol. 2022, 222, 2744–2760. [Google Scholar] [CrossRef]
- He, X.; Hu, Y.; Wu, Y.; Luo, Y.; Feng, H.; Wu, Q.; Liu, H.; Gao, L.; Yang, H.; Long, Y.; et al. Hyaluronic acid modified chuanxiong oil liposomes as a novel therapeutic agent for photoaging prevention. Sci. Rep. 2025, 15, 12237. [Google Scholar] [CrossRef]
- Najm, A.; Bîrcă, A.C.; Niculescu, A.-G.; Alberts, A.; Grumezescu, A.M.; Gălățeanu, B.; Vasile, B.Ș.; Beuran, M.; Gaspar, B.S.; Hudiță, A. Dipalmitoylphosphatidylcholine Lipid Vesicles for Delivering HMB, NMN, and L-Leucine in Sarcopenia Therapy. Molecules 2025, 30, 1437. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- da Silva Gomes, A.; Reis, F.M.P.; Ceravolo, I.P.; Dias-Souza, M.V. Effectiveness of Free and Liposome-Entrapped Antitumoral Drugs against Hepatocellular Carcinoma: A Comparative In vitro Study. Biointerface Res. Appl. Chem. 2023, 13, 122. [Google Scholar]
- Aparecida Nunes Ribeiro, T.; Crístian Ferreira Soares, D.; Aparecida dos Santos, G.; Lino de Souza, M.C.; Xavier Gonçalves, B.; Fernanda da Silva Valentim, P.; Oliveira de Paula, M.; Sachs, D. Liposomes applied in healing bacterially infected wounds: A systematic review. J. Liposome Res. 2025, 1–17. [Google Scholar] [CrossRef]
- Dominguez-Arca, V.; Sabín, J.; Garcia-Rio, L.; Bastos, M.; Taboada, P.; Barbosa, S.; Prieto, G. On the structure and stability of novel cationic DPPC liposomes doped with gemini surfactants. J. Mol. Liq. 2022, 366, 120230. [Google Scholar] [CrossRef]
- Nicolae, C.-L.; Pîrvulescu, D.-C.; Antohi, A.M.; Niculescu, A.G.; Grumezescu, A.M.; Croitoru, G.-A. Silica nanoparticles in medicine: Overcoming pathologies through advanced drug delivery, diagnostics, and therapeutic strategies. Rom. J. Morphol. Embryol. 2024, 65, 173. [Google Scholar] [CrossRef]
- Pirușcă, I.A.; Balaure, P.C.; Grumezescu, V.; Irimiciuc, S.-A.; Oprea, O.-C.; Bîrcă, A.C.; Vasile, B.; Holban, A.M.; Voinea, I.C.; Stan, M.S.; et al. New Fe3O4-Based Coatings with Enhanced Anti-Biofilm Activity for Medical Devices. Antibiotics 2024, 13, 631. [Google Scholar] [CrossRef]
- Solomon, M.; Holban, A.M.; Bălăceanu-Gurău, B.; Dițu, L.M.; Alberts, A.; Grumezescu, A.M.; Manolescu, L.S.; Mihai, M.M. Silver Nanoparticles Functionalized with Polymeric Substances to Reduce the Growth of Planktonic and Biofilm Opportunistic Pathogens. Int. J. Mol. Sci. 2025, 26, 3930. [Google Scholar] [CrossRef]
- Rybka, M.; Mazurek, Ł.; Konop, M. Beneficial effect of wound dressings containing silver and silver nanoparticles in wound healing—From experimental studies to clinical practice. Life 2022, 13, 69. [Google Scholar] [CrossRef]
- Vaniushenkova, A.A.; Poberezhniy, D.Y.; Panyukova, N.S.; Morozov, A.N.; Kalenov, S.V.; Belov, A.A. The Co-Use of Various Forms of Silver and Proteases in the Development of New Dressing Biomaterials for Wound Healing. Biointerface Res. Appl. Chem 2024, 14, 119. [Google Scholar]
- Nguyen, T.D.; Pham, N.M.; Thai, N.T.-T.; Nguyen, H.T.-T.; Hoang, Y.H.; Ha, A.C. Characterization and applications of silver nanoparticles photosynthesized from Durio Zibethinus peel extract. Biointerface Res. Appl. Chem 2024, 14, 116. [Google Scholar]
- Wan Shamsudin, W.N.A.Y.; Che Ku Yahya, C.K.M.F.; Shaarani, S.H.N. Antifungal Activity of Biosynthesized Silver Nanoparticles Mediated by Neem Leaf Extract against Aspergillus sp. Biointerface Res. Appl. Chem 2023, 13, 583. [Google Scholar] [CrossRef]
- Santana, J.; Antunes, S.; Bonelli, R.; Backx, B. Development of antimicrobial dressings by the action of silver nanoparticles based on green nanotechnology. Lett. Appl. NanoBioScience 2023, 12, 35. [Google Scholar]
- Matar, G.; Akyuz, G.; Kaymazlar, E.; Andaç, M. An investigation of green synthesis of silver nanoparticles using Turkish honey against pathogenic bacterial strains. Biointerface Res. Appl. Chem. 2023, 13, 195. [Google Scholar]
- Sellami, H.; Khan, S.A.; Ahmad, I.; Alarfaj, A.A.; Hirad, A.H.; Al-Sabri, A.E. Green Synthesis of Silver Nanoparticles Using Olea europaea Leaf Extract for Their Enhanced Antibacterial, Antioxidant, Cytotoxic and Biocompatibility Applications. Int. J. Mol. Sci. 2021, 22, 12562. [Google Scholar] [CrossRef]
- Arshad, F.; Naikoo, G.A.; Hassan, I.U.; Chava, S.R.; El-Tanani, M.; Aljabali, A.A.; Tambuwala, M.M. Bioinspired and green synthesis of silver nanoparticles for medical applications: A green perspective. Appl. Biochem. Biotechnol. 2024, 196, 3636–3669. [Google Scholar] [CrossRef]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar]
- Hayward, S.L.; Wilson, C.L.; Kidambi, S. Hyaluronic acid-conjugated liposome nanoparticles for targeted delivery to CD44 overexpressing glioblastoma cells. Oncotarget 2016, 7, 34158–34171. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Ravar, F.; Saadat, E.; Gholami, M.; Dehghankelishadi, P.; Mahdavi, M.; Azami, S.; Dorkoosh, F.A. Hyaluronic acid-coated liposomes for targeted delivery of paclitaxel, in-vitro characterization and in-vivo evaluation. J. Control. Release Off. J. Control. Release Soc. 2016, 229, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular uptake and retention of nanoparticles: Insights on particle properties and interaction with cellular components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Tseu, G.Y.W.; Kamaruzaman, K.A. A review of different types of liposomes and their advancements as a form of gene therapy treatment for breast cancer. Molecules 2023, 28, 1498. [Google Scholar] [CrossRef]
- Ogiso, T.; Yamaguchi, T.; Iwaki, M.; Tanino, T.; Miyake, Y. Effect of Positively and Negatively Charged Liposomes on Skin Permeation of Drugs. J. Drug Target. 2001, 9, 49–59. [Google Scholar] [CrossRef]
- Gillet, A.; Compère, P.; Lecomte, F.; Hubert, P.; Ducat, E.; Evrard, B.; Piel, G. Liposome surface charge influence on skin penetration behaviour. Int. J. Pharm. 2011, 411, 223–231. [Google Scholar] [CrossRef]
- Cauzzo, J.; Jayakumar, N.; Ahluwalia, B.S.; Ahmad, A.; Škalko-Basnet, N. Characterization of Liposomes Using Quantitative Phase Microscopy (QPM). Pharmaceutics 2021, 13, 590. [Google Scholar] [CrossRef]
- Filippov, S.K.; Khusnutdinov, R.; Murmiliuk, A.; Inam, W.; Zakharova, L.Y.; Zhang, H.; Khutoryanskiy, V.V. Dynamic light scattering and transmission electron microscopy in drug delivery: A roadmap for correct characterization of nanoparticles and interpretation of results. Mater. Horiz. 2023, 10, 5354–5370. [Google Scholar] [CrossRef]
- Chen, Y.; Rong, D.; He, S.; Rong, Q. Molecularly engineered hyaluronic acid-derived hygroscopic hydrogel electrolytes with anti-freezing and moisturizing capabilities realizing environmental adaptive semi-open flexible zinc-air batteries. J. Power Sources 2025, 640, 236804. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.-L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liu, J.; Li, N.; Garamus, V.M.; Zou, A. Hyaluronic acid-coated liposome for active targeting on CD44 expressing tumors. J. Nanoparticle Res. 2018, 20, 235. [Google Scholar] [CrossRef]
- Parvathalu, K.; Chinmayee, S.; Preethi, B.; Swetha, A.; Maruthi, G.; Pritam, M.; Sreenivas, B.; Naidu, S.R.; Merlinsheeba, G.L.; Murali, B.; et al. Green Synthesis of Silver Nanoparticles Using Argyreia nervosa Leaf Extract and Their Antimicrobial Activity. Plasmonics 2023, 18, 1075–1081. [Google Scholar] [CrossRef]
- Mehta, B.K.; Chhajlani, M.; Shrivastava, B.D. Green synthesis of silver nanoparticles and their characterization by XRD. J. Phys. Conf. Ser. 2017, 836, 012050. [Google Scholar] [CrossRef]
- Kora, A.J.; Beedu, S.R.; Jayaraman, A. Size-controlled green synthesis of silver nanoparticles mediated by gum ghatti (Anogeissus latifolia) and its biological activity. Org. Med. Chem. Lett. 2012, 2, 17. [Google Scholar] [CrossRef]
- Pochapski, D.J.; Carvalho dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef]
- Corciovă, A.; Mircea, C.; Fifere, A.; Turin-Moleavin, I.-A.; Roşca, I.; Macovei, I.; Ivănescu, B.; Vlase, A.-M.; Hăncianu, M.; Burlec, A.F. Biogenic Synthesis of Silver Nanoparticles Mediated by Aronia melanocarpa and Their Biological Evaluation. Life 2024, 14, 1211. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef]
- Estrada-Villegas, G.M.; Morselli, G.; Oliveira, M.J.A.; González-Pérez, G.; Lugão, A.B. PVGA/Alginate-AgNPs hydrogel as absorbent biomaterial and its soil biodegradation behavior. Polym. Bull. 2020, 77, 4147–4166. [Google Scholar] [CrossRef]
- Janković, B.; Marinović-Cincović, M.; Janković, M. Application of the Kinetic Triplets and Geometrical Characteristics of Thermal Analysis Curves in Identifying the Main Bioactive Compounds (BC) that Govern the Thermal and Thermo-Oxidative Degradation Mechanism of Aronia melanocarpa (Black Chokeberry). Food Biophys. 2016, 11, 128–141. [Google Scholar] [CrossRef]
- Tirla, A.; Timar, A.V.; Becze, A.; Memete, A.R.; Vicas, S.I.; Popoviciu, M.S.; Cavalu, S. Designing New Sport Supplements Based on Aronia melanocarpa and Bee Pollen to Enhance Antioxidant Capacity and Nutritional Value. Molecules 2023, 28, 6944. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Hao, J.; Jiang, M.; Zhao, C.; Fan, Z. Antioxidant Activity and Structural Characterization of Anthocyanin–Polysaccharide Complexes from Aronia melanocarpa. Int. J. Mol. Sci. 2024, 25, 13347. [Google Scholar] [CrossRef]
- Kharazmi, A.; Faraji, N.; Hussin, R.M.; Saion, E.; Yunus, W.M.M.; Behzad, K. Structural, optical, opto-thermal and thermal properties of ZnS–PVA nanofluids synthesized through a radiolytic approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef]
- Xu, L.; Shang, Z.; Liu, D.; Zhao, C.; Zhao, C.; Pei, X.; Zhang, Z. Preparation, characterization and efficacy of hyaluronic acid-modified two targeted liposomes for ectoin delivery to the skin. Colloids Surf. A Physicochem. Eng. Asp. 2025, 719, 137013. [Google Scholar] [CrossRef]
- Sohail, M.; Ahmad, M.; Minhas, M.U.; Ali, L.; Munir, A.; Khalid, I. Synthesis and characterization of graft PVA composites for controlled delivery of valsartan. Lat. Am. J. Pharm. 2014, 33, 1237–1244. [Google Scholar]
- Smith, B. The infrared spectra of polymers, VI: Polymers with CO bonds. Spectroscopy 2022, 37, 15–19,27. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, F.; Zhao, R.; Wang, C.; Hu, K.; Sun, Y.; Politis, C.; Shavandi, A.; Nie, L. Polyvinyl Alcohol/Sodium Alginate Hydrogels Incorporated with Silver Nanoclusters via Green Tea Extract for Antibacterial Applications. Des. Monomers Polym. 2020, 23, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Pang, Y.; Liu, J. Swelling-strengthening hydrogels by embedding with deformable nanobarriers. Nat. Commun. 2020, 11, 4502. [Google Scholar] [CrossRef] [PubMed]
- Malik, K.; Kazmi, A.; Sultana, T.; Raja, N.I.; Bibi, Y.; Abbas, M.; Badruddin, I.A.; Ali, M.M.; Bashir, M.N. A mechanistic overview on green assisted formulation of nanocomposites and their multifunctional role in biomedical applications. Heliyon 2025, 11, e41654. [Google Scholar] [CrossRef] [PubMed]
- Skóra, B.; Piechowiak, T.; Szychowski, K.A.; Gmiński, J. Entrapment of silver nanoparticles in L-α-phosphatidylcholine/cholesterol-based liposomes mitigates the oxidative stress in human keratinocyte (HaCaT) cells. Eur. J. Pharm. Biopharm. 2021, 166, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.M.; Campelo, M.d.S.; Câmara Neto, J.F.; Lima, A.B.N.; Silva, G.d.A.; Dias, A.T.d.F.F.; Ricardo, N.M.P.S.; Kaplan, D.L.; Ribeiro, M.E.N.P. Alginate/polyvinyl alcohol films for wound healing: Advantages and challenges. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 220–233. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef]
- Barba, B.J.D.; Oyama, T.G.; Taguchi, M. Simple fabrication of gelatin–polyvinyl alcohol bilayer hydrogel with wound dressing and nonadhesive duality. Polym. Adv. Technol. 2021, 32, 4406–4414. [Google Scholar] [CrossRef]
- Kamali, A.; Shamloo, A. Fabrication and evaluation of a bilayer hydrogel-electrospinning scaffold prepared by the freeze-gelation method. J. Biomech. 2020, 98, 109466. [Google Scholar] [CrossRef]
- Malektaj, H.; Drozdov, A.D.; Fini, E.; Christiansen, J.C. The Effect of pH on the Viscoelastic Response of Alginate-Montmorillonite Nanocomposite Hydrogels. Molecules 2024, 29, 244. [Google Scholar] [CrossRef]
- Souza, A.; Parnell, M.; Rodriguez, B.J.; Reynaud, E.G. Role of pH and Cross-linking Ions on Cell Viability and Metabolic Activity in Alginate–Gelatin 3D Prints. Gels 2023, 9, 853. [Google Scholar] [CrossRef]
- FitzSimons, T.M.; Anslyn, E.V.; Rosales, A.M. Effect of pH on the Properties of Hydrogels Cross-Linked via Dynamic Thia-Michael Addition Bonds. ACS Polym. Au 2022, 2, 129–136. [Google Scholar] [CrossRef]
- Corciova, A.; Mircea, C.; Fifere, A.; Turin Moleavin, I.-A.; Burlec, A.F.; Ivanescu, B.; Vlase, A.-M.; Hancianu, M.; Macovei, I. A Green Integrated Approach to Multifunctional Silver Nanoparticles Derived from Aronia melanocarpa. Pharmaceutics 2025, 17, 669. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green synthesis of silver nanoparticles: A comprehensive review of methods, influencing factors, and applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Eker, F.; Akdaşçi, E.; Duman, H.; Bechelany, M.; Karav, S. Green Synthesis of Silver Nanoparticles Using Plant Extracts: A Comprehensive Review of Physicochemical Properties and Multifunctional Applications. Int. J. Mol. Sci. 2025, 26, 6222. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Casey, A. Liposomal encapsulation of silver nanoparticles (AgNP) improved nanoparticle uptake and induced redox imbalance to activate caspase-dependent apoptosis. Apoptosis Int. J. Program. Cell Death 2020, 25, 120–134. [Google Scholar] [CrossRef]
- Ni, C.; Zhang, Z.; Wang, Y.; Zhang, Z.; Guo, X.; Lv, H. Hyaluronic acid and HA-modified cationic liposomes for promoting skin penetration and retention. J. Control. Release 2023, 357, 432–443. [Google Scholar] [CrossRef]
- Qhattal, H.S.; Liu, X. Characterization of CD44-mediated cancer cell uptake and intracellular distribution of hyaluronan-grafted liposomes. Mol. Pharm. 2011, 8, 1233–1246. [Google Scholar] [CrossRef]
- Fang, Y.; Nie, T.; Li, G.; Wang, L.; Du, J.; Wu, J. Multifunctional antibiotic hydrogel doped with antioxidative lycopene-based liposome for accelerative diabetic wound healing. Chem. Eng. J. 2024, 480, 147930. [Google Scholar] [CrossRef]
- Forsey, R.W.; Fisher, J.; Thompson, J.; Stone, M.H.; Bell, C.; Ingham, E. The effect of hyaluronic acid and phospholipid based lubricants on friction within a human cartilage damage model. Biomaterials 2006, 27, 4581–4590. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țăin, A.-E.; Bîrcă, A.C.; Naulea, A.M.I.; Niculescu, A.-G.; Grumezescu, A.M.; Croitoru, G.-A. Alginate/PVA Hydrogel Incorporating HA-Liposomes and Aronia-Derived Silver Nanoparticles for Advanced Wound Management. Int. J. Mol. Sci. 2025, 26, 9203. https://doi.org/10.3390/ijms26189203
Țăin A-E, Bîrcă AC, Naulea AMI, Niculescu A-G, Grumezescu AM, Croitoru G-A. Alginate/PVA Hydrogel Incorporating HA-Liposomes and Aronia-Derived Silver Nanoparticles for Advanced Wound Management. International Journal of Molecular Sciences. 2025; 26(18):9203. https://doi.org/10.3390/ijms26189203
Chicago/Turabian StyleȚăin (Anastasiu), Anca-Elena, Alexandra Cătălina Bîrcă, Ana Maria Isabela Naulea, Adelina-Gabriela Niculescu, Alexandru Mihai Grumezescu, and George-Alexandru Croitoru. 2025. "Alginate/PVA Hydrogel Incorporating HA-Liposomes and Aronia-Derived Silver Nanoparticles for Advanced Wound Management" International Journal of Molecular Sciences 26, no. 18: 9203. https://doi.org/10.3390/ijms26189203
APA StyleȚăin, A.-E., Bîrcă, A. C., Naulea, A. M. I., Niculescu, A.-G., Grumezescu, A. M., & Croitoru, G.-A. (2025). Alginate/PVA Hydrogel Incorporating HA-Liposomes and Aronia-Derived Silver Nanoparticles for Advanced Wound Management. International Journal of Molecular Sciences, 26(18), 9203. https://doi.org/10.3390/ijms26189203







