Cellular Mechanisms of Photobiomodulation in Relation to HeLa Kyoto Tumor Cells Exposed to Ionizing Radiation
Abstract
1. Introduction
2. Results
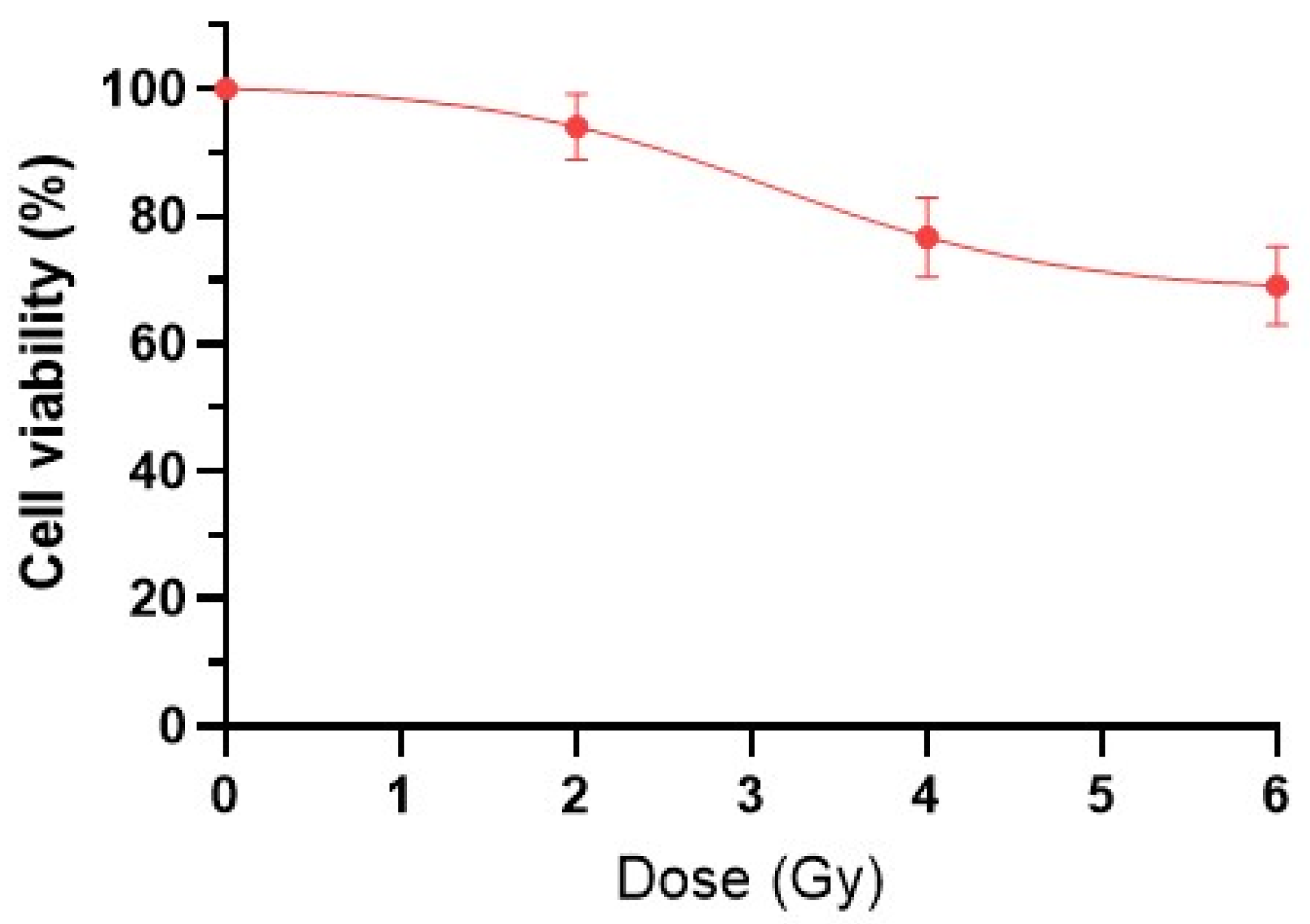
2.1. Viability of HeLa Kyoto Tumor Cells After IR
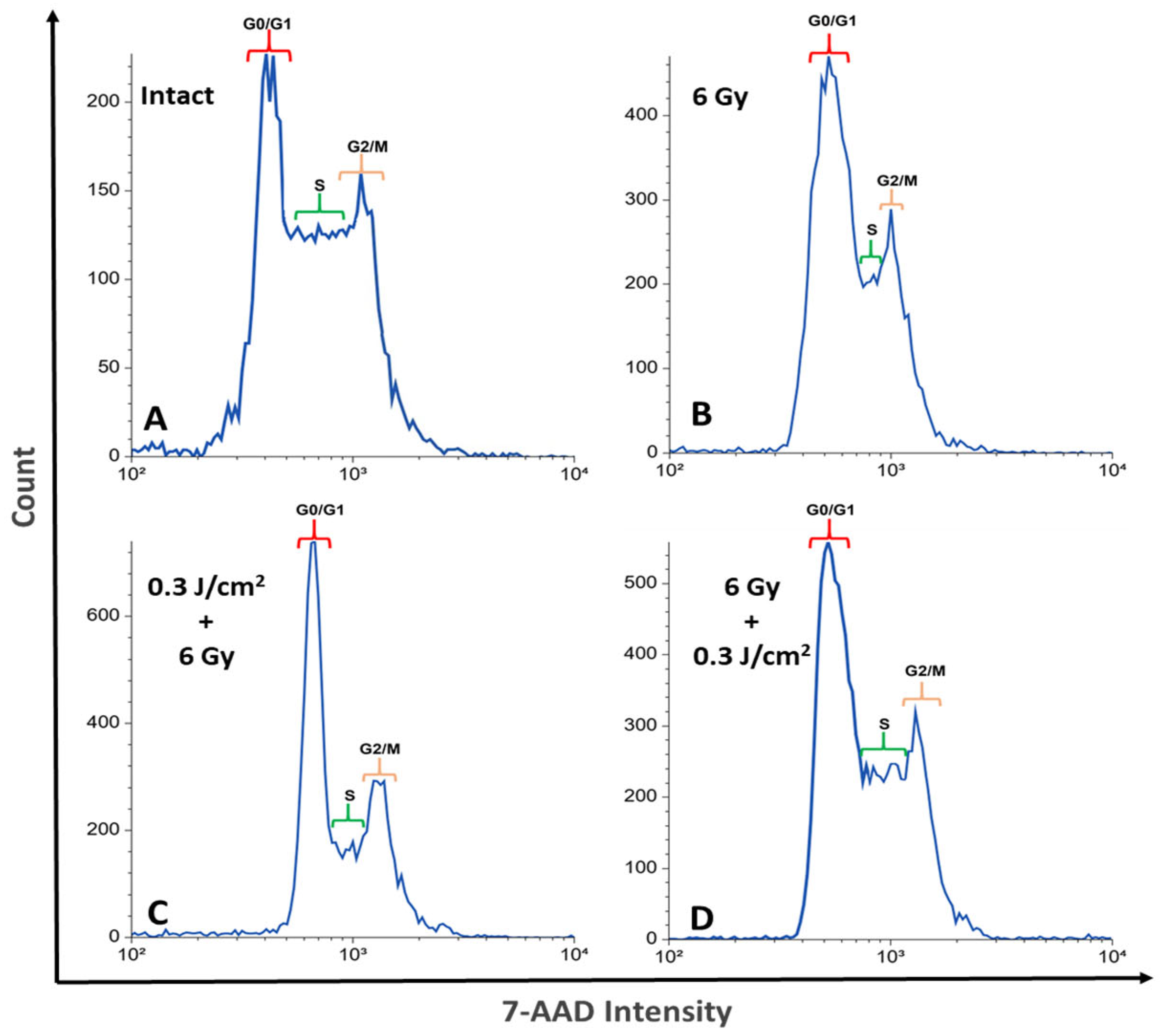
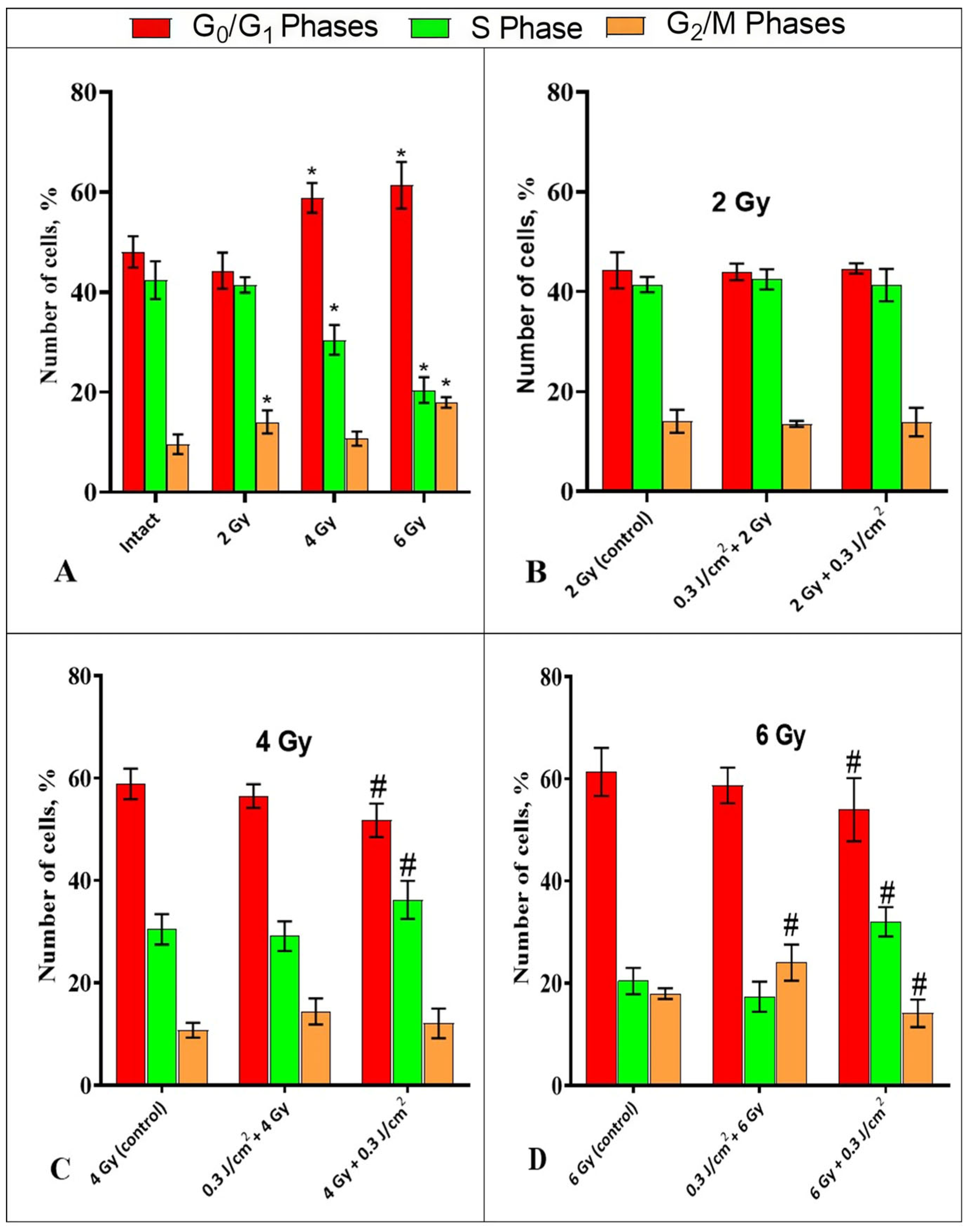
2.2. Influence of Photobiomodulation on the Cell Cycle of HeLa Kyoto Cells
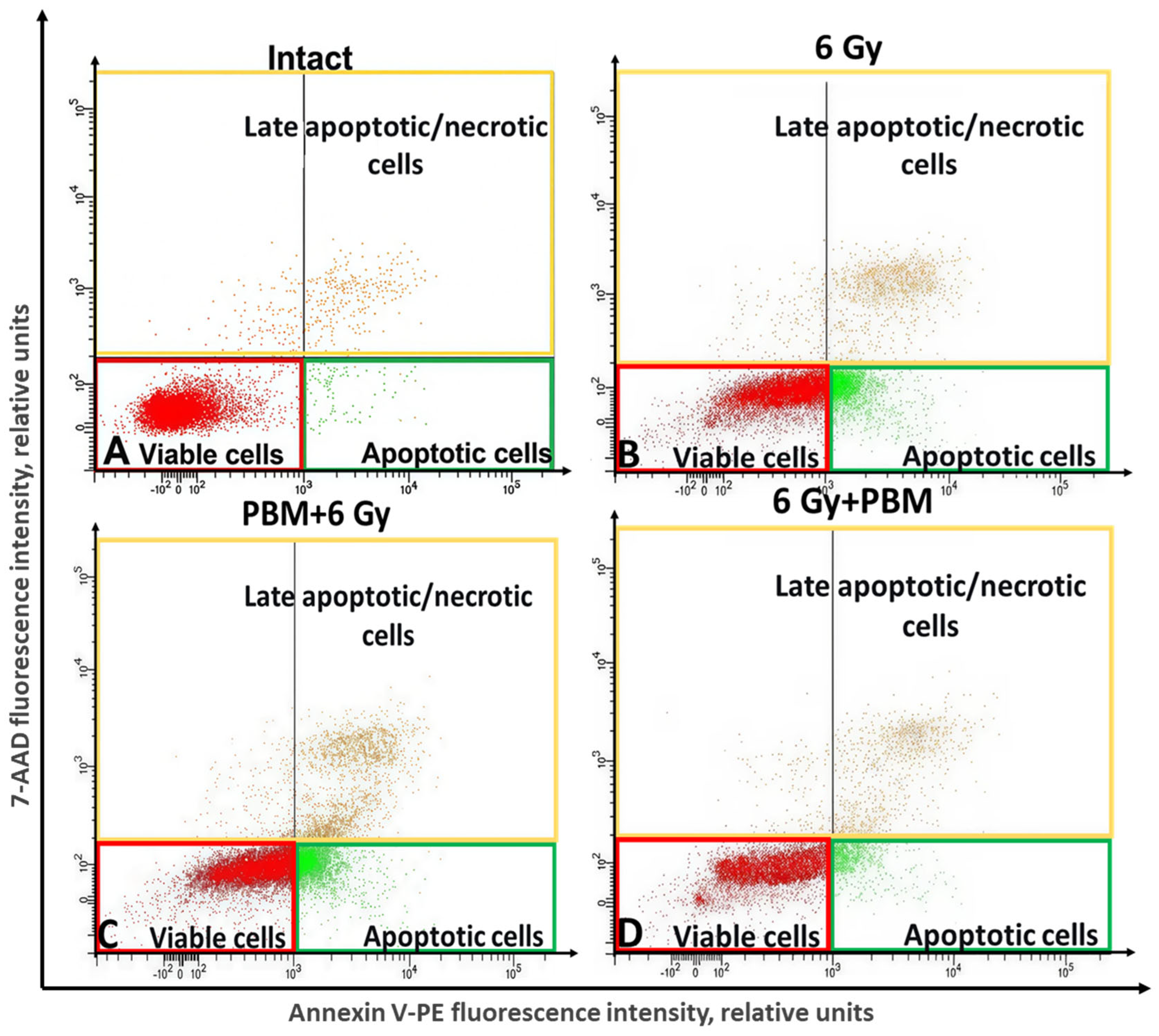
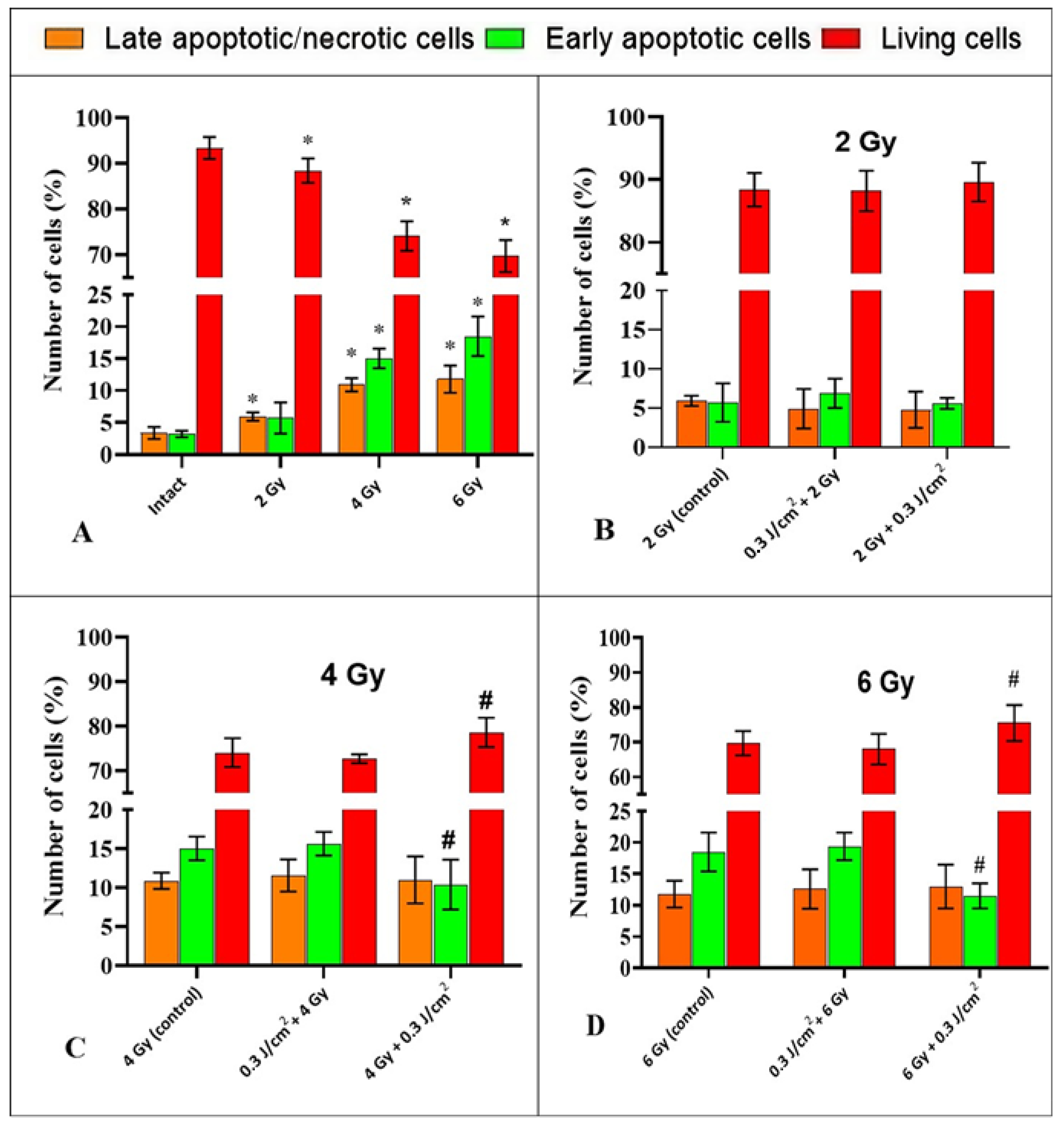
2.3. The Study of the Impact of PBM in Combination with IR on the Process of Cell Death of HeLa Cells
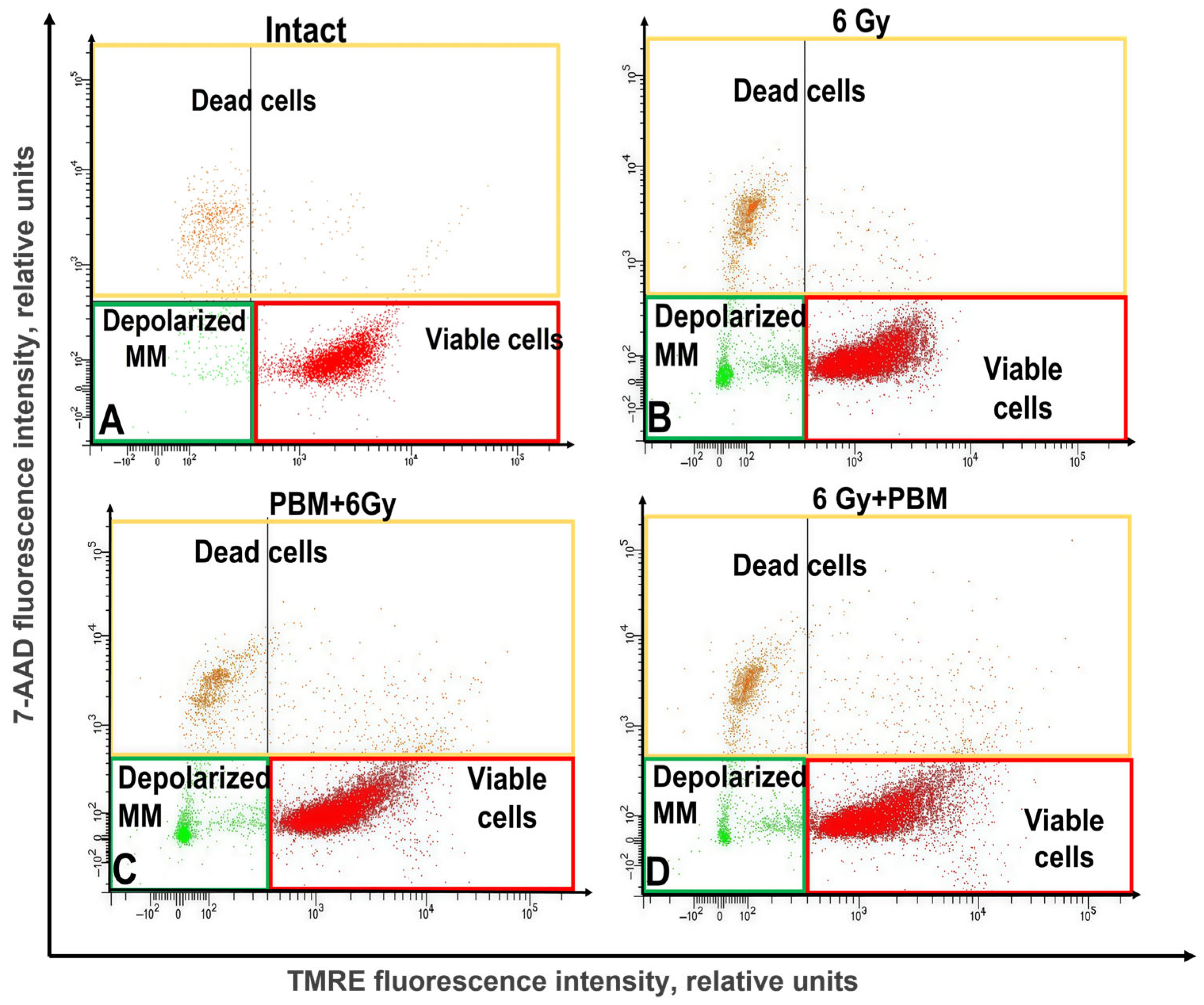
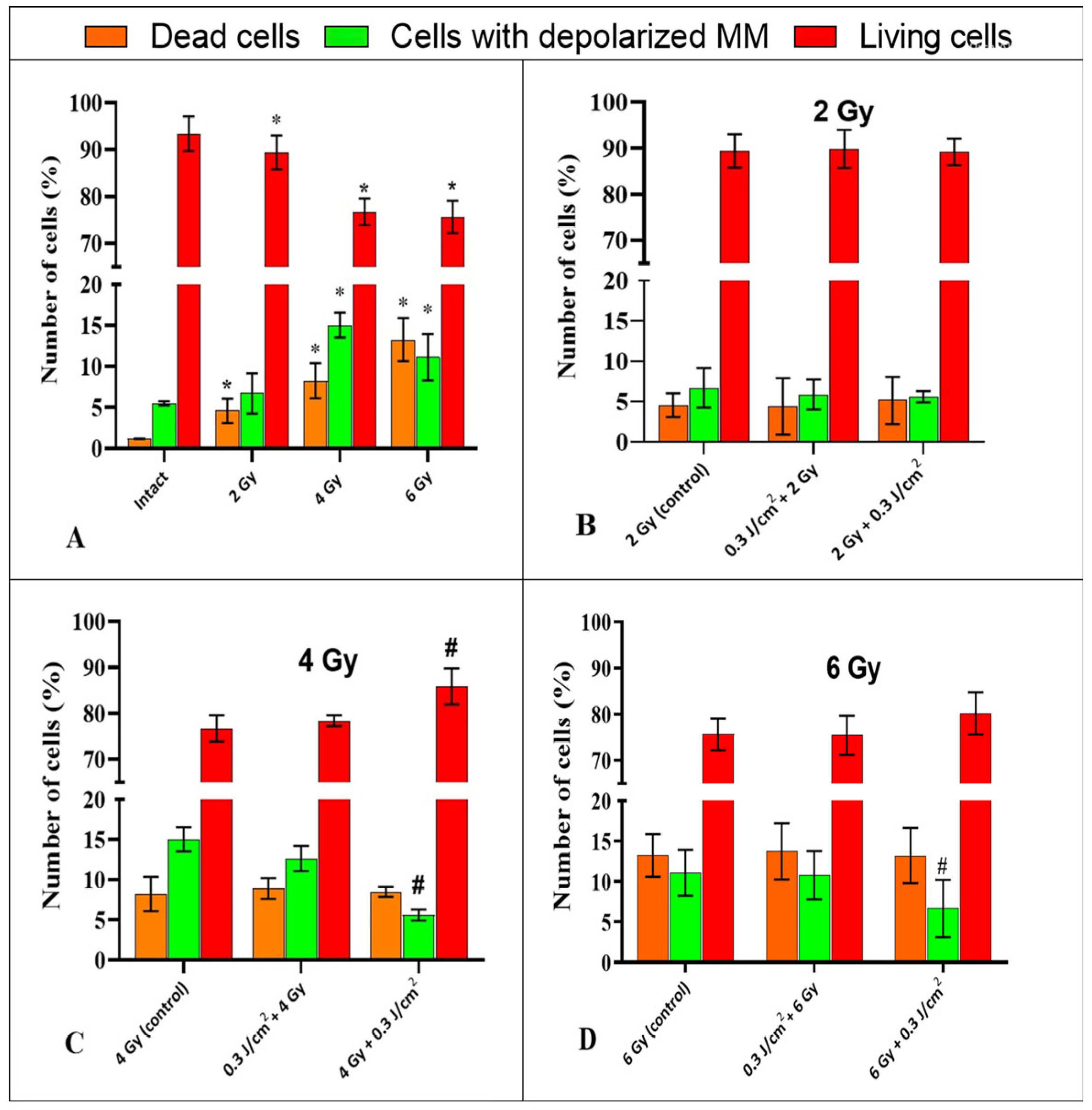
2.4. The Study of the Impact of PBM in Combination with IR on the Mitochondrial Membrane Potential of HeLa Kyoto Cells
3. Discussion
4. Materials and Methods
4.1. Cell Line
4.2. Assessment of Cell Radiosensitivity
4.3. Photobiomodulation
4.4. Ionizing Radiation
4.5. Experimental Design
4.6. Cell Cycle Definition
4.7. Transmembrane Mitochondrial Potential Study
4.8. Assessment of Cell Death Pathways
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Montay-Gruel, P.; Meziani, L.; Yakkala, C.; Vozenin, M. Expanding the therapeutic index of radiation therapy by normal tissue protection. Br. J. Radiol. 2019, 92, 20180008. [Google Scholar] [CrossRef]
- Sedova, E.S.; Yusupov, V.I.; Vorobieva, N.N.; Kanischeva, N.V.; Maslennikova, A.V. The effectiveness of low-level laser therapy for prevention and treatment of radiation-induced mucositis. Sib. Oncol. J. 2018, 17, 11–17. [Google Scholar] [CrossRef]
- Cowen, D.; Tardieu, C.; Schuber, M.; Peterson, D.; Resbeut, M.; Faucher, C.; Franquin, J. Low energy helium-neon laser in the prevention of oral mucositis in patients undergoing bone marrow transplant: Results of a double-blind randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 1997, 38, 697–703. [Google Scholar] [CrossRef]
- Tripodi, N.; Corcoran, D.; Antonello, P.; Balic, N.; Caddy, D.; Knight, A.; Meehan, C.; Sidiroglou, F.; Fraser, S.; Kiatos, D.; et al. The effects of photobiomodulation on human dermal fibroblasts in vitro: A systematic review. J. Photochem. Photobiol. B 2021, 214, 112100. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Liao, X.; Li, S.; Xie, G.; Xie, S.; Xiao, L.; Song, J.; Liu, H. Preconditioning with low-level laser irradiation enhances the therapeutic potential of human adipose-derived stem cells in a mouse model of photoaged skin. Photochem. Photobiol. 2018, 94, 780–790. [Google Scholar] [CrossRef]
- Bamps, M.; Dok, R.; Nuyts, S. Low-level laser therapy stimulates proliferation in head and neck squamous cell carcinoma cells. Front. Oncol. 2018, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Gomes Henriques, Á.C.; Ginani, F.; Oliveira, R.M.; Keesen, T.S.; Galvão Barboza, C.A.; Oliveira Rocha, H.A.; de Castro, J.F.; Della Coletta, R.; de Almeida Freitas, R. Low-level laser therapy promotes proliferation and invasion of oral squamous cell carcinoma cells. Lasers Med. Sci. 2014, 29, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Migliorati, C.; Hewson, I.; Lalla, R.V.; Antunes, H.S.; Estilo, C.L.; Hodgson, B.; Lopes, N.N.F.; Schubert, M.M.; Bowen, J.; Elad, S. Systematic review of laser and other light therapy for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.; Kim, T.; Heo, S.; Jung, W.; Kang, H. Stimulatory effects of wavelength-dependent photobiomodulation on proliferation and angiogenesis of colorectal cancer. J. Photochem. Photobiol. B 2022, 234, 112527. [Google Scholar] [CrossRef]
- Sonis, S.T.; Hashemi, S.; Epstein, J.B.; Nair, R.G.; Raber-Durlacher, J.E. Could the biological robustness of low level laser therapy (photobiomodulation) impact its use in the management of mucositis in head and neck cancer patients. Oral Oncol. 2016, 54, 7–14. [Google Scholar] [CrossRef]
- Gholamreza, E.D.; Bahram, G.; Alireza, N. Analysis of radiomodulatory effect of low-level laser irradiation by clonogenic survival assay. Photomed. Laser Surg. 2015, 33, 452–459. [Google Scholar] [CrossRef]
- Gholamreza, E.D.; Bahareh, B.; Bahram, G.; Alireza, N.; Hamblin, R. Photobiomodulation leads to enhanced radiosensitivity through induction of apoptosis and autophagy in human cervical cancer cells. J. Biophotonics 2017, 10, 1732–1742. [Google Scholar] [CrossRef]
- Barasch, A.; Raber-Durlacher, J.; Epstein, J.B.; Carroll, J. Effects of pre-radiation exposure to LLLT of normal and malignant cells. Support. Care Cancer 2015, 24, 2497–2501. [Google Scholar] [CrossRef]
- Cherkasova, E.; Babak, K.; Belotelov, A.; Labutina, J.; Yusupov, V.; Vorobieva, N.; Nerush, N.; Maslennikova, A. Effects of photobiomodulation in relation to HeLa Kyoto tumor cells exposed to ionizing radiation. J. Photochem. Photobiol. B 2020, 209, 111936. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.R.; Cabral, F.V.; de Camargo, C.F.M.; Núñez, S.C.; Yoshimura, M.T.; de Lima Luna, A.C.; Maria, D.A.; Ribeiro, M.S. Exploring the effects of low-level laser therapy on fibroblasts and tumor cells following gamma radiation exposure. J. Biophotonics 2016, 9, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.R.; de Camargo, C.F.M.; Cabral, F.V.; Ribeiro, M.S. Low-power laser irradiation did not stimulate breast cancer cells following ionizing radiation. Mech. Photobiomodulation Ther. XI. SPIE 2016, 9695, 66–72. [Google Scholar] [CrossRef]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461, Erratum in Cancer 2021, 120, 3700. [Google Scholar] [CrossRef]
- Moskvin, S.V.; DIu, K.; Antipov, E.V.; Volchkov, S.E.; Kiseleva, O.N. The influence of pulsed low-intensity laser radiation of the red (635 nm) and infrared (904 nm) spectra on the human mesenchymal stem cells in vitro. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2014, 91, 40–47. [Google Scholar]
- Lima, P.L.V.; Pereira, C.V.; Nissanka, N.; Arguello, T.; Gavini, G.; da Costa Maranduba, C.M.; Diaz, F.; Moraes, C.T. Photobiomodulation enhancement of cell proliferation at 660 nm does not require cytochrome C oxidase. J. Photochem. Photobiol. B 2019, 194, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, L. Opening the doors to cytochrome c: Changes in mitochondrial shape and apoptosis. Int. J. Biochem. Cell Biol. 2009, 41, 1875–1883. [Google Scholar] [CrossRef]
- Vucić, V.; Isenović, E.R.; Adzić, M.; Ruzdijić, S.; Radojcić, M.B. Effects of gamma-radiation on cell growth, cycle arrest, death, and superoxide dismutase expression by DU 145 human prostate cancer cells. Braz. J. Med. Biol. Res. 2006, 39, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, D.; Stigbrand, T. Radiation-induced cell death mechanisms. Tumour Biol. 2010, 31, 363–372. [Google Scholar] [CrossRef]
- Amé, J.C.; Fouquerel, E.; Gauthier, L.R.; Biard, D.; Boussin, F.D.; Dantzer, F.; de Murcia, G.; Schreiber, V. Radiation-induced mitotic catastrophe in PARG-deficient cells. J Cell Sci. 2009, 15, 1990–2002. [Google Scholar] [CrossRef] [PubMed]
- de Faria, C.; Barrera-Patiño, C.; Santana, J.P.; da Silva de Avó, L.; Bagnato, V.S. Tumor radiosensitization by photobiomodulation. J. Photochem. Photobiol. B 2021, 225, 112349. [Google Scholar] [CrossRef] [PubMed]
- Yamamori, T.; Sasagawa, T.; Ichii, O.; Hiyoshi, M.; Bo, T.; Yasui, H.; Kon, Y.; Inanami, O. Analysis of the mechanism of radiation-induced upregulation of mitochondrial abundance in mouse fibroblasts. J. Radiat. Res. 2017, 58, 292–301. [Google Scholar] [CrossRef]
- Kirkby, C.; Ghasroddashti, E. Targeting mitochondria in cancer cells using gold nanoparticle-enhanced radiotherapy: A Monte Carlo study. Med. Phys. 2015, 42, 1119–1128. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, M.; Kim, D.Y.; Kim, K.I.; Yi, J.Y. Mechanisms of energy metabolism in skeletal muscle mitochondria following radiation exposure. Cells 2019, 8, 950. [Google Scholar] [CrossRef]
- Brady, N.R.; Hamacher-Brady, A.; Westerhoff, H.V.; Gottlieb, R.A. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid. Redox Signal. 2006, 8, 1651–1665. [Google Scholar] [CrossRef]
- Juntao, Z.; Da, X.; Xuejuan, G. Low-power laser irradiation activates Src tyrosine kinase through reactive oxygen species-mediated signaling pathway. J. Cell. Physiol. 2008, 217, 518–528. [Google Scholar] [CrossRef]
- Gonçalves de Faria, C.M.; Ciol, H.; Bagnato, V.S.; Pratavieira, S. Effects of photobiomodulation on the redox state of healthy and cancer cells. Biomed. Opt. Express 2021, 12, 3902–3916. [Google Scholar] [CrossRef]
- Karu, T.I.; Kolyakov, S.F. Exact action spectra for cellular responses relevant to phototherapy. Photomed. Laser Surg. 2005, 23, 355–361. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A. Therapeutic photobiomodulation: Nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov. Med. 2011, 11, 154–159. [Google Scholar]
- Chailakhyan, R.K.; Gerasimov, Y.V.; Yusupov, V.I.; Sviridov, A.P.; Tambiev, A.K.; Vorobieva, N.N.; Grosheva, A.G.; Kuralesova, A.I.; Moskvina, I.L.; Bagratashvili, V.N. Activation of Bone Marrow Multipotent Stromal Cells by Laser and EHF Radiation and Their Combined Impacts. Mod. Technol. Med. 2017, 9, 28. [Google Scholar] [CrossRef]
- Schalch, T.D.; Fernandes, M.H.; Setúbal Destro Rodrigues, M.F. Photobiomodulation is associated with a decrease in cell viability and migration in oral squamous cell carcinoma. Lasers Med. Sci. 2019, 34, 629–636. [Google Scholar] [CrossRef]
- Levchenko, S.M.; Kuzmin, A.N.; Pliss, A.; Ohulchanskyy, T.Y.; Prasad, P.N.; Qu, J. Cellular transformations in near-infrared light-induced apoptosis in cancer cells revealed by label-free CARS imaging. J. Biophotonics 2019, 12, e201900179. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wu, S.; Xing, D. High fluence low-power laser irradiation induces apoptosis via inactivation of Akt/GSK3β signaling pathway. J. Cell. Physiol. 2011, 226, 588–601. [Google Scholar] [CrossRef]
- Zembruski, N.C.L.; Stache, V.; Haefeli, W.E.; Weiss, J. 7-Aminoactinomycin D for apoptosis staining in flow cytometry. Anal. Biochem. 2012, 429, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Reutelingsperger, C.P.; van Heerde, W.L. Annexin V, the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis. Cell. Mol. Life Sci. 1997, 53, 527–532. [Google Scholar] [CrossRef] [PubMed]
| Device | Fluence, J/cm2 | Distance from the Emitter, cm | Intensity, mW/cm2 | Time, s |
|---|---|---|---|---|
| CDM-08 | 300 ± 38 | 46 | 4.0 ± 0.5 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslennikova, A.V.; Belotelov, A.O.; Cherkasova, E.I.; Yusupov, V.I.; Kononova, U.A.; Shilyagina, N.Y.; Skamnitsky, D.V. Cellular Mechanisms of Photobiomodulation in Relation to HeLa Kyoto Tumor Cells Exposed to Ionizing Radiation. Int. J. Mol. Sci. 2025, 26, 9197. https://doi.org/10.3390/ijms26189197
Maslennikova AV, Belotelov AO, Cherkasova EI, Yusupov VI, Kononova UA, Shilyagina NY, Skamnitsky DV. Cellular Mechanisms of Photobiomodulation in Relation to HeLa Kyoto Tumor Cells Exposed to Ionizing Radiation. International Journal of Molecular Sciences. 2025; 26(18):9197. https://doi.org/10.3390/ijms26189197
Chicago/Turabian StyleMaslennikova, Anna V., Artem O. Belotelov, Elena I. Cherkasova, Vladimir I. Yusupov, Ulyana A. Kononova, Natalia Yu. Shilyagina, and Dmitry V. Skamnitsky. 2025. "Cellular Mechanisms of Photobiomodulation in Relation to HeLa Kyoto Tumor Cells Exposed to Ionizing Radiation" International Journal of Molecular Sciences 26, no. 18: 9197. https://doi.org/10.3390/ijms26189197
APA StyleMaslennikova, A. V., Belotelov, A. O., Cherkasova, E. I., Yusupov, V. I., Kononova, U. A., Shilyagina, N. Y., & Skamnitsky, D. V. (2025). Cellular Mechanisms of Photobiomodulation in Relation to HeLa Kyoto Tumor Cells Exposed to Ionizing Radiation. International Journal of Molecular Sciences, 26(18), 9197. https://doi.org/10.3390/ijms26189197











