Correlation of DJ-1, GDF15, and MFGE8 Gene Expression with Clinicopathological Findings in Gliomas and Meningiomas
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics of Glioma and Meningioma Patients
2.2. ROC Curve and AUC-Based Diagnostic Performance Analysis of DJ-1, GDF15, and MFGE8 Gene Expression in Glioma and Meningioma Tissues
3. Discussion
4. Materials and Methods
4.1. Ethical Statement Declaration
4.2. Collection of Tumour Tissue Samples
4.3. RNA Isolation and qRT-PCR
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATRX | Alpha-thalassemia X-linked intellectual disability |
| DJ-1 | Parkinsonism associated deglycase 7/PARK7 |
| GDF15 | Growth Differentiation Factor 15 |
| IDH1/2 | Isocitrate Dehydrogenase 1/2 |
| KLF4 | Kruppel-Like Factor 4 |
| MFGE8 | Milk Fat Globule-EGF Factor 8 Protein |
| NF2 | Neurofibromin 2 |
| SMO | Smoothened Frizzled Class Receptor |
| TERT | Telomerase Reverse Transcriptase |
| TP53 | Tumour Protein 53 |
| TRAF7 | TNF Receptor Associated Factor 7 |
References
- Ramkissoon, S. Surgical pathology of neoplasms of the central nervous system. In Pathobiology of Human Disease; Academic Press: San Diego, CA, USA, 2014; pp. 3592–3606. [Google Scholar]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and other primary brain malignancies in adults: A review. JAMA 2023, 329, 574–587. [Google Scholar] [CrossRef]
- Zuchero, J.B.; Barres, B.A. Glia in mammalian development and disease. Development 2015, 142, 3805–3809. [Google Scholar] [CrossRef] [PubMed]
- Huntoon, K.; Toland, A.M.S.; Dahiya, S. Meningioma: A review of clinicopathological and molecular aspects. Front. Oncol. 2020, 10, 579599. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Louis, D.N.; Holland, E.C.; Cairncross, J.G. Glioma classification: A molecular reappraisal. Am. J. Pathol. 2001, 159, 779–786. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Barthel, F.P.; Malta, T.M.; Sabedot, T.S.; Salama, S.R.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef]
- Wang, J.Z.; Landry, A.P.; Raleigh, D.R.; Sahm, F.; Walsh, K.M.; Goldbrunner, R.; Yefet, L.S.; Tonn, J.C.; Gui, C.; Ostrom, Q.T.; et al. Meningioma: International consortium on meningiomas consensus review on scientific advances and treatment paradigms for clinicians, researchers, and patients. Neuro Oncol. 2024, 26, 1742–1780. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Hsieh, A.L.; Bi, W.L.; Ramesh, V.; Brastianos, P.K.; Plotkin, S.R. Evolving concepts in meningioma management in the era of genomics. Cancer 2024, 130, 2586–2600. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid. Redox Signal. 2011, 15, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Olivo, E.; La Chimia, M.; Ceramella, J.; Catalano, A.; Chiaradonna, F.; Sinicropi, M.S.; Cuda, G.; Iacopetta, D.; Scumaci, D. Moving beyond the tip of the iceberg: DJ-1 implications in cancer metabolism. Cells 2022, 11, 1432. [Google Scholar] [CrossRef]
- Wang, C.; Fang, M.; Zhang, M.; Li, W.; Guan, H.; Sun, Y.; Xie, S.; Zhong, X. The positive correlation between DJ-1 and β-catenin expression shows prognostic value for patients with glioma. Neuropathology 2013, 33, 628–636. [Google Scholar] [CrossRef]
- Ling, T.; Zhang, J.; Ding, F.; Ma, L. Role of growth differentiation factor 15 in cancer cachexia (Review). Oncol. Lett. 2023, 26, 462. [Google Scholar] [CrossRef]
- Codó, P.; Weller, M.; Kaulich, K.; Schraivogel, D.; Silginer, M.; Reifenberger, G.; Meister, G.; Roth, P. Control of glioma cell migration and invasiveness by GDF-15. Oncotarget 2016, 7, 7732–7746. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Qi, J.; Zhou, H.; Tan, L.; Huang, J.; Huang, J.; Fang, X.; Gong, L.; Luo, J.; et al. Targeting MFGE8 secreted by cancer-associated fibroblasts blocks angiogenesis and metastasis in esophageal squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2023, 120, e2307914120. [Google Scholar] [CrossRef]
- Yamada, K.; Uchiyama, A.; Uehara, A.; Perera, B.; Ogino, S.; Yokoyama, Y.; Takeuchi, Y.; Udey, M.C.; Ishikawa, O.; Motegi, S.-I. MFG-E8 drives melanoma growth by stimulating mesenchymal stromal cell-induced angiogenesis and M2 polarization of tumor-associated macrophages. Cancer Res. 2016, 76, 4283–4292. [Google Scholar] [CrossRef] [PubMed]
- Abd El Atti, R.M.; Abou Gabal, H.H.; Osman, W.M.; Saad, A.S. Insights into the prognostic value of DJ-1 and MIB-1 in astrocytic tumors. Diagn. Pathol. 2013, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.P.; Pan, B.C.; Li, B.; Li, Y.; Tian, X.Y.; Li, Z. DJ-1 is activated in medulloblastoma and is associated with cell proliferation and differentiation. World J. Surg. Oncol. 2014, 12, 373. [Google Scholar] [CrossRef]
- Jin, W. Novel insights into PARK7 (DJ-1), a potential anti-cancer therapeutic target, and implications for cancer progression. J. Clin. Med. 2020, 9, 1256. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Y.; Hu, S.; Gao, L.; Tang, N.; Liu, R.; Qin, Y.; Ren, C.; Du, S. GDF15 expression in glioma is associated with malignant progression, immune microenvironment, and serves as a prognostic factor. CNS Neurosci. Ther. 2022, 28, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Nam, K.S.; Lee, H.J.; Kim, K.S. Ionizing radiation-induced GDF15 promotes angiogenesis in human glioblastoma models by promoting VEGFA expression through p-MAPK1/SP1 signaling. Front. Oncol. 2022, 12, 801230. [Google Scholar] [CrossRef]
- The Human Protein Atlas. MFGE8 Expression in Glioma—Pathology Section. Available online: https://www.proteinatlas.org/ENSG00000140545-MFGE8/cancer (accessed on 12 June 2025).
- Hinkle, D.A.; Mullett, S.J.; Gabris, B.E.; Hamilton, R.L. DJ-1 expression in glioblastomas shows positive correlation with p53 expression and negative correlation with epidermal growth factor receptor amplification. Neuropathology 2011, 31, 29–37. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, J.; Meng, Z.; Yin, S.; Ruan, M.; Zhang, W.; Wu, Z.; Ding, T.; Huang, F.; Wang, W. MFG-E8 promotes M2 polarization of macrophages and is associated with poor prognosis in patients with gastric cancer. Heliyon 2023, 10, e23917. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zhong, X.Y.; Du, B.; Lin, C.L.; Luo, F.; Tang, L.-J.; Chen, J. Role of DJ-1-induced PTEN down-regulation in migration and invasion of human glioma cells. Chin. J. Cancer 2010, 29, 988–994. [Google Scholar] [CrossRef]
- Malmer, B.; Feychting, M.; Lönn, S.; Ahlbom, A.; Henriksson, R. p53 genotypes and risk of glioma and meningioma. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2220–2223. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Maita, H.; Takahashi-Niki, K.; Saito, Y.; Noguchi, N.; Iguchi-Ariga, S.M.; Ariga, H. Oxidized DJ-1 inhibits p53 by sequestering p53 from promoters in a DNA-binding affinity-dependent manner. Mol. Cell. Biol. 2013, 33, 340–359. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, J.; Zhao, J.; Zhang, H.; Li, L.; Li, H.; Chen, L.; Hu, J.; Zheng, W.; Jing, Z. The U2AF2 /circRNA ARF1/miR-342–3p/ISL2 feedback loop regulates angiogenesis in glioma stem cells. J. Exp. Clin. Cancer Res. 2020, 39, 182. [Google Scholar] [CrossRef]
- Kathagen, A.; Schulte, A.; Balcke, G.; Phillips, H.S.; Martens, T.; Matschke, J.; Günther, H.S.; Soriano, R.; Modrusan, Z.; Sandmann, T.; et al. Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol. 2013, 126, 763–780. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, X.; Zhou, H.; Zhao, J.; Zhao, H.; Li, L.; Zhou, Y. Mitochondrial respiratory chain component NDUFA4: A promising therapeutic target for gastrointestinal cancer. Cancer Cell Int. 2024, 24, 97. [Google Scholar] [CrossRef]
- Guo, C.; Zhuang, Y.; Chen, Y.; Chen, S.; Peng, H.; Zhou, S. Significance of tumor protein P53 mutation in cellular process and drug selection in brain lower grade (WHO Grades II and III) glioma. Biomark. Med. 2020, 14, 1139–1150. [Google Scholar] [CrossRef]
- Mei, J.; Wang, T.; Xu, R.; Chen, D.; Zhang, Y. Clinical and molecular immune characterization of ERBB2 in glioma. Int. Immunopharmacol. 2021, 94, 107499. [Google Scholar] [CrossRef]
- Liu, P.C.; Lieu, A.S.; Lin, C.J.; Tsai, H.P.; Chai, C.Y.; Kwan, A.L. High expression of Sp1 is associated with recurrence of meningioma. World Neurosurg. 2021, 149, e1056–e1060. [Google Scholar] [CrossRef]
- Kim, J.H.; Zheng, L.T.; Lee, W.H.; Suk, K. Pro-apoptotic role of integrin β3 in glioma cells. J. Neurochem. 2011, 117, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Guo, Q.; Guan, G.F.; Cheng, W.; Cheng, P.; Wu, A.H. Integrin beta 5 is a prognostic biomarker and potential therapeutic target in glioblastoma. Front. Oncol. 2019, 9, 904. [Google Scholar] [CrossRef]
- Krenciute, G.; Prinzing, B.L.; Yi, Z.; Wu, M.F.; Liu, H.; Dotti, G.; Balyasnikova, I.V.; Gottschalk, S. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol. Res. 2017, 5, 571–581. [Google Scholar] [CrossRef]
- Guo, K.; Song, L.; Chang, J.; Cao, P.; Liu, Q. AEBP1 promotes glioblastoma progression and activates the classical NF-κB pathway. Behav. Neurol. 2020, 2020, 8890452. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhang, Z.; Yu, K.; Yang, H.; Liang, H.; Lu, T.; Ji, Y.; Chen, J.; He, W.; Chen, Z.; et al. Integrin subunit alpha V is a potent prognostic biomarker associated with immune infiltration in lower-grade glioma. Front. Neurol. 2022, 13, 964590. [Google Scholar] [CrossRef]
- Scott, J.; Tsai, Y.Y.; Chinnaiyan, P.; Yu, H.H.M. Effectiveness of radiotherapy for elderly patients with glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Karsy, M.; Burnett, B.; Di Ieva, A.; Cusimano, M.D.; Jensen, R.L. Microvascularization of Grade I meningiomas: Effect on tumor volume, blood loss, and patient outcome. J. Neurosurg. 2018, 128, 657–666. [Google Scholar] [CrossRef]
- Jovčevska, I.; Kočevar, N.; Komel, R. Glioma and glioblastoma—How much do we (not) know? Mol. Clin. Oncol. 2013, 1, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, Y.; Wang, D.; Xie, Q.; Zheng, M.; Zhou, Y.; Li, Q.; Yang, Z.; Tang, H.; Li, Y.; et al. Analysis of gene expression profiling in meningioma: Deregulated signaling pathways associated with meningioma and EGFL6 overexpression in benign meningioma tissue and serum. PLoS ONE 2012, 7, e52707. [Google Scholar] [CrossRef]
- Wong, E.; Nahar, N.; Hau, E.; Varikatt, W.; Gebski, V.; Ng, T.; Jayamohan, J.; Sundaresan, P. Cut-point for Ki-67 proliferation index as a prognostic marker for glioblastoma. Asia Pac. J. Clin. Oncol. 2019, 5, 5–9. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J.; Chen, B.; Cao, L.; Chen, C. Efficient prediction of Ki-67 proliferation index in meningiomas on MRI: From traditional radiological findings to a machine learning approach. Cancers 2022, 14, 3637. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
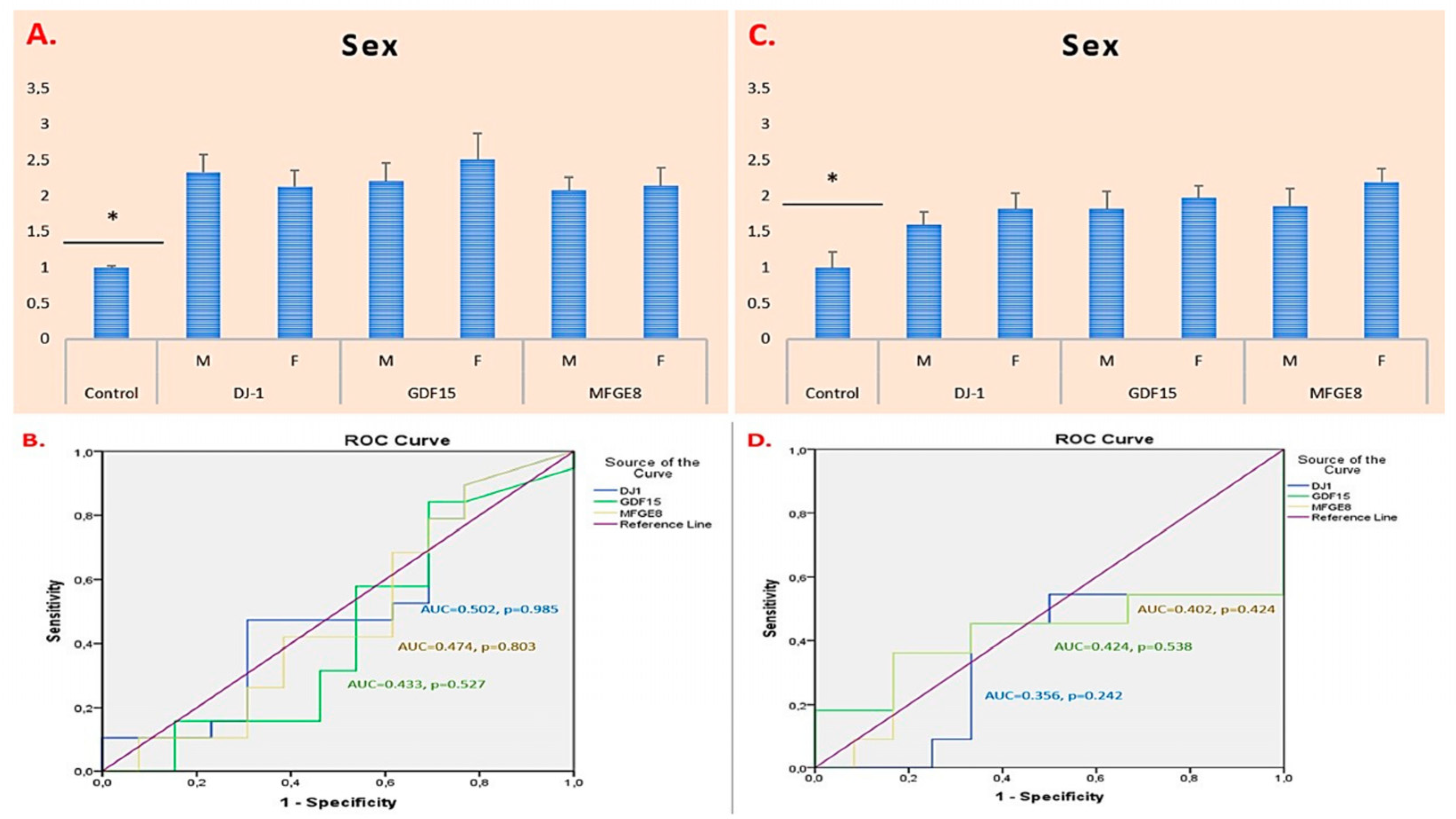
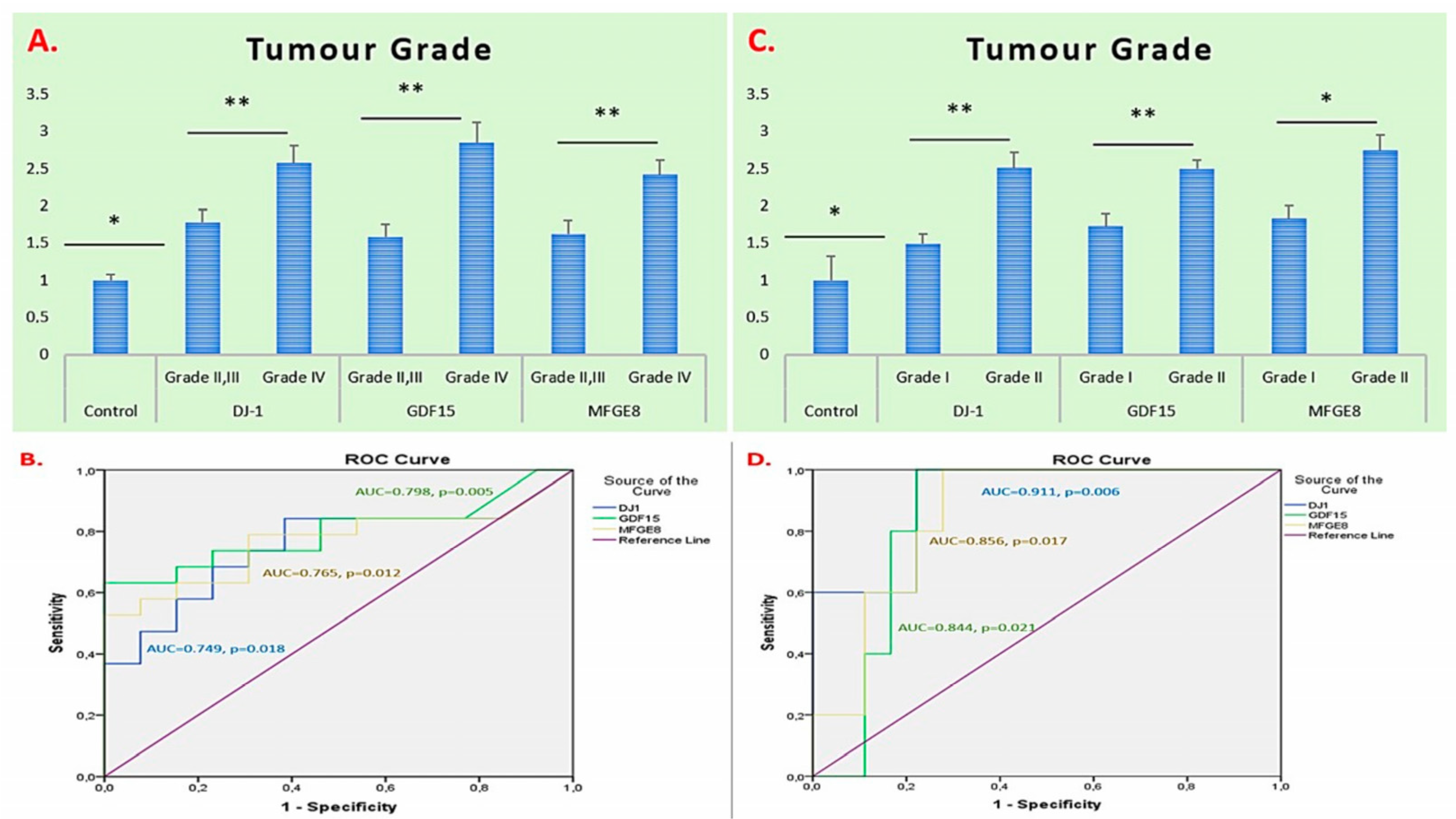
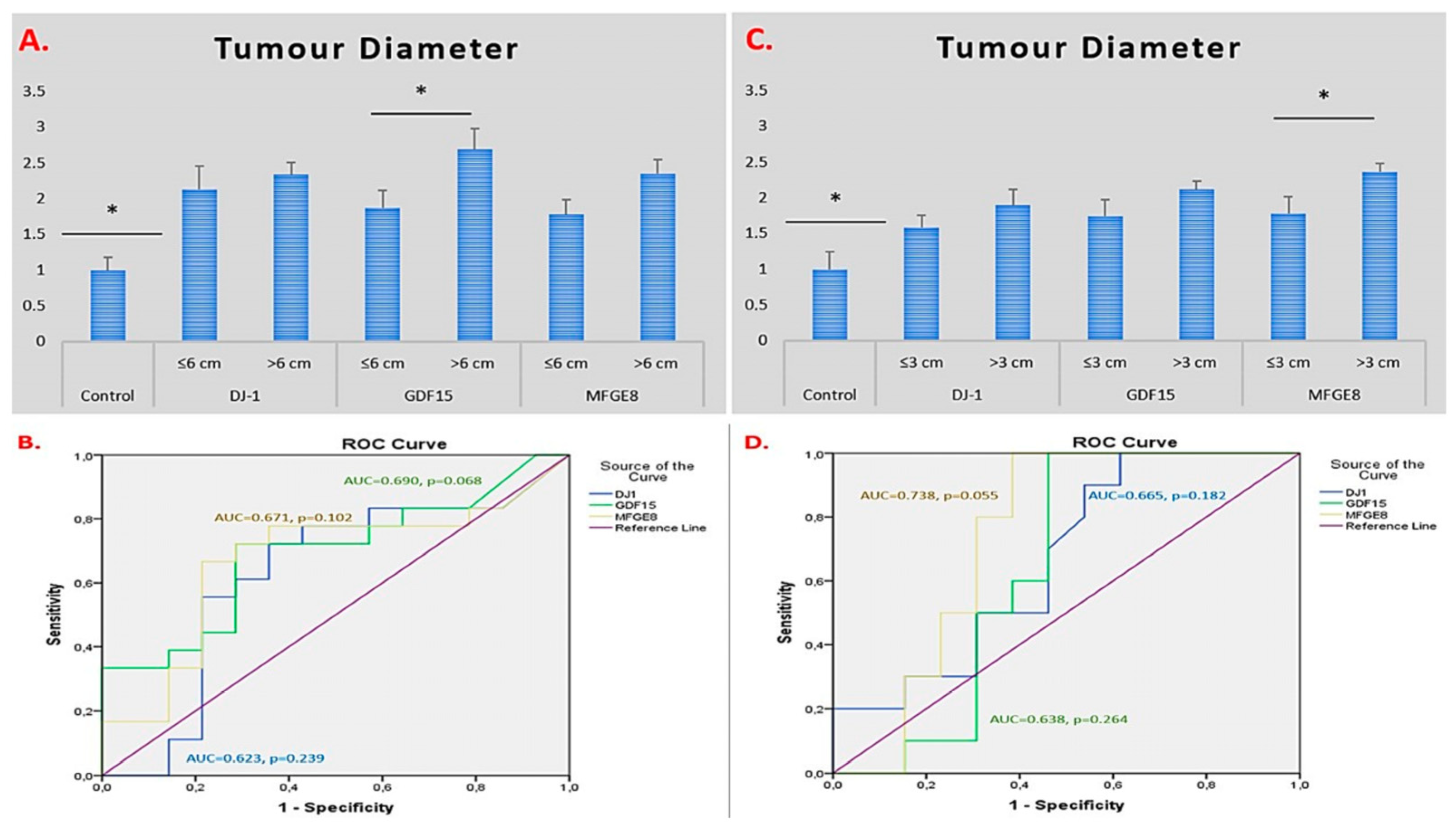
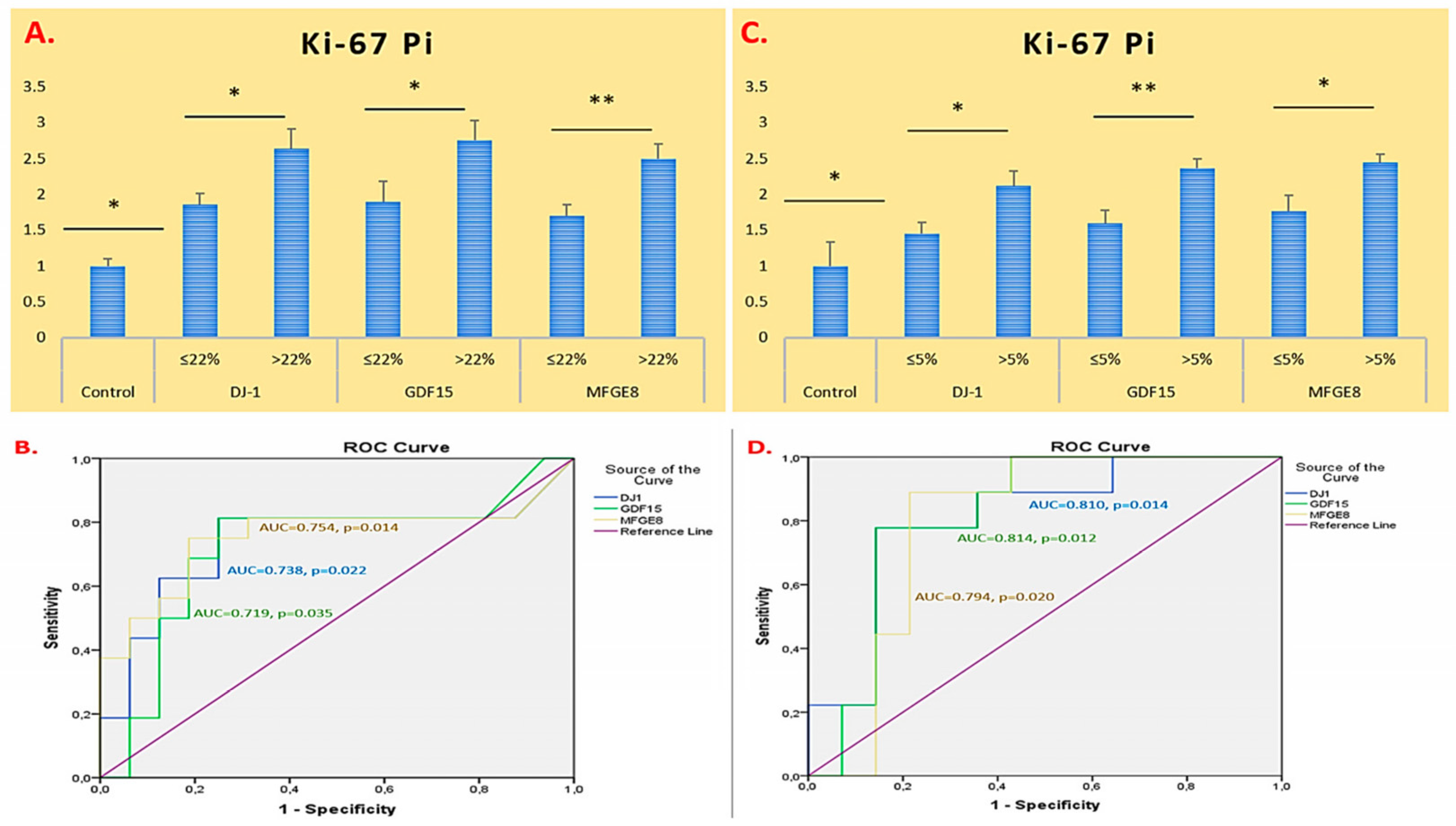

| Parameter | Glioma (n = 27) |
|---|---|
| Mean Age (year) | 50.981 ± 13.303 |
| Female: 60.442 | |
| Male: 59.521 | |
| Sex, n (%) | Female: 10 (37.037) |
| Male: 17 (62.963) | |
| Tumour Diameter, n (%) | ≤6 cm: 14 (51.852) |
| >6 cm: 13 (48.148) | |
| Ki-67 Pi, n (%) | ≤22%: 12 (44.444) |
| >22%: 15 (55.556) | |
| Grade, n (%) | II: 8 (29.630) |
| III: 3 (11.111) | |
| IV: 16 (59.259) | |
| Pathological subtype, n (%) | Astrositoma: 6 (22.222) |
| Oligodendroglioma: 5 (18.519) | |
| Glioblastoma: 16 (59.259) | |
| Localization | Frontal: 9 (33.333) |
| Occipital: 3 (11.111) | |
| Parietal: 8 (29.630) | |
| Temporal: 7 (25.926) | |
| IDH1 mutation status, (+/−) | Absent: 15 (55.556) |
| Present: 12 (44.444) |
| Parameter | Meningioma (n = 18) |
|---|---|
| Mean Age (year) | 59.873 ± 13.144 |
| Female: 59.413 | |
| Male: 60.332 | |
| Sex, n (%) | Female: 12 (66.667) |
| Male: 6 (33.333) | |
| Tumour Diameter, n (%) | ≤3 cm: 8 (44.444) |
| >3 cm: 10 (55.556) | |
| Ki-67 Pi, n (%) | ≤5%: 9 (50.000) |
| >5%: 9 (50.000) | |
| Grade, n (%) | I: 13 (72.222) |
| II: 5 (27.778) | |
| Pathological subtype, n (%) | Transitional: 5 (27.778) |
| Angiomatous: 1 (5.556) | |
| Secretory: 0 (00.00) | |
| Fibroblastic: 4 (22.222) | |
| Meningothelial: 2 (11.111) | |
| Metaplastic: 0 (00.00) | |
| Psammomatous: 1 (5.556) | |
| Clear cell: 0 (00.00) | |
| Atypical: 5 (27.778) | |
| Localization | Frontal: 8 (44.444) |
| Occipital: 2 (11.111) | |
| Parietal: 5 (27.778) | |
| Temporal: 3 (16.667) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solmaz Avcikurt, A.; Adilay, H.U.; Gunaldi, O.; Gultekin Tosun, S.; Katar, S. Correlation of DJ-1, GDF15, and MFGE8 Gene Expression with Clinicopathological Findings in Gliomas and Meningiomas. Int. J. Mol. Sci. 2025, 26, 9194. https://doi.org/10.3390/ijms26189194
Solmaz Avcikurt A, Adilay HU, Gunaldi O, Gultekin Tosun S, Katar S. Correlation of DJ-1, GDF15, and MFGE8 Gene Expression with Clinicopathological Findings in Gliomas and Meningiomas. International Journal of Molecular Sciences. 2025; 26(18):9194. https://doi.org/10.3390/ijms26189194
Chicago/Turabian StyleSolmaz Avcikurt, Ayla, Huseyin Utku Adilay, Omur Gunaldi, Sinem Gultekin Tosun, and Salim Katar. 2025. "Correlation of DJ-1, GDF15, and MFGE8 Gene Expression with Clinicopathological Findings in Gliomas and Meningiomas" International Journal of Molecular Sciences 26, no. 18: 9194. https://doi.org/10.3390/ijms26189194
APA StyleSolmaz Avcikurt, A., Adilay, H. U., Gunaldi, O., Gultekin Tosun, S., & Katar, S. (2025). Correlation of DJ-1, GDF15, and MFGE8 Gene Expression with Clinicopathological Findings in Gliomas and Meningiomas. International Journal of Molecular Sciences, 26(18), 9194. https://doi.org/10.3390/ijms26189194






