Discovery of Undescribed Clerodane Diterpenoids with Antimicrobial Activity Isolated from the Roots of Solidago gigantea Ait
Abstract
1. Introduction
2. Results and Discussion
2.1. Detection and Bioassay-Guided Isolation
2.2. Structure Elucidation
2.3. Antimicrobial Assays
3. Materials and Methods
3.1. Materials and Reagents
3.2. Plant Material
3.3. TLC–UV/FLD and Derivatization with p-Anisaldehyde and Vanillin–Sulfuric Acid
3.4. TLC–DB (B. subtilis Antibacterial Assay)
3.5. Extraction and Isolation
3.6. Compound Characterization
3.7. TLC–ESI-MS
3.8. FIA–HR-HESI-MS(/MS)
3.9. Spectroscopy
3.9.1. NMR Spectroscopy
3.9.2. UV Spectroscopy
3.9.3. ATR-FTIR Spectroscopy
3.10. Polarimetry
3.11. Antibacterial and Antifungal Activity Microplate Assays
3.11.1. Cell Culture
3.11.2. Determination of Minimal Inhibitory Concentration (MIC) Values
3.11.3. Determination of Minimal Bactericidal Concentration (MBC) Values
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semple, J.C.; Beck, J.B. Revised Infrageneric Classification of Solidago (Asteraceae: Astereae). Phytoneuron 2021, 10, 1–6. [Google Scholar]
- Poljuha, D.; Sladonja, B.; Uzelac Božac, M.; Šola, I.; Damijanić, D.; Weber, T. The Invasive Alien Plant Solidago canadensis: Phytochemical Composition, Ecosystem Service Potential, and Application in Bioeconomy. Plants 2024, 13, 1745. [Google Scholar] [CrossRef] [PubMed]
- Fursenco, C.; Calalb, T.; Uncu, L.; Dinu, M.; Ancuceanu, R. Solidago virgaurea L.: A Review of Its Ethnomedicinal Uses, Phytochemistry, and Pharmacological Activities. Biomolecules 2020, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Weber, E. The Dynamics of Plant Invasions: A Case Study of Three Exotic Goldenrod Species (Solidago L.) in Europe. J. Biogeogr. 1998, 25, 147–154. [Google Scholar] [CrossRef]
- EPPO Lists of Invasive Alien Plants. Available online: https://www.eppo.int/ACTIVITIES/invasive_alien_plants/iap_lists#iap (accessed on 31 August 2025).
- Weber, E.; Jakobs, G. Biological Flora of Central Europe: Solidago gigantea Aiton. Flora Morphol. Distrib. Funct. Ecol. Plants 2005, 200, 109–118. [Google Scholar] [CrossRef]
- Pal, R.W.; Chen, S.; Nagy, D.U.; Callaway, R.M. Impacts of Solidago gigantea on Other Species at Home and Away. Biol. Invasions 2015, 17, 3317–3325. [Google Scholar] [CrossRef]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy Is Pervasive in Invasive Plants. Biol. Invasions 2021, 23, 367–371. [Google Scholar] [CrossRef]
- Goossens, E.P.; Mertens, W.; Olde Venterink, H. Solidago gigantea Invasion Homogenizes Soil Properties and Native Plant Communities. Biol. Invasions 2024, 26, 3315–3327. [Google Scholar] [CrossRef]
- Świerszcz, S.; Czarniecka-Wiera, M.; Szymura, T.H.; Szymura, M. From Invasive Species Stand to Species-Rich Grassland: Long-Term Changes in Plant Species Composition during Solidago Invaded Site Restoration. J. Environ. Manag. 2024, 353, 120216. [Google Scholar] [CrossRef]
- Kołodziej, B. Antibacterial and Antimutagenic Activity of Extracts Aboveground Parts of Three Solidago Species: Solidago virgaurea L., Solidago canadensis L. and Solidago gigantea Ait. J. Med. Plants Res. 2011, 5, 6770–6779. [Google Scholar] [CrossRef]
- Woźniak, D.; Ślusarczyk, S.; Domaradzki, K.; Dryś, A.; Matkowski, A. Comparison of Polyphenol Profile and Antimutagenic and Antioxidant Activities in Two Species Used as Source of Solidaginis herba—Goldenrod. Chem. Biodivers. 2018, 15, e1800023. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.; Marschall, H.; Bradesi, P. Constituents of the Essential Oil of Solidago gigantea Ait. (Giant Goldenrod). Flavour Fragr. J. 2001, 16, 19–26. [Google Scholar] [CrossRef]
- Leuschner, J. Anti-Inflammatory, Spasmolytic and Diuretic Effects of a Commercially Available Solidago gigantea Herb. Extract. Arzneimittelforschung 1995, 45, 165–168. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- Zekič, J.; Vovk, I.; Glavnik, V. Extraction and Analyses of Flavonoids and Phenolic Acids from Canadian Goldenrod and Giant Goldenrod. Forests 2021, 12, 40. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Nagy, D.U.; Canale, A.; Maggi, F. Evaluation of Two Invasive Plant Invaders in Europe (Solidago canadensis and Solidago gigantea) as Possible Sources of Botanical Insecticides. J. Pest Sci. 2019, 92, 805–821. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Krüzselyi, D.; Ott, P.G.; Garádi, Z.; Béni, S.; Morlock, G.E.; Bakonyi, J. Bioactive Clerodane Diterpenes of Giant Goldenrod (Solidago gigantea Ait.) Root Extract. J. Chromatogr. A 2021, 1635, 461727. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Krüzselyi, D.; Lapat, V.; Ott, P.G. Acetylcholinesterase Inhibitors in the Giant Goldenrod Root. J. Chromatogr. B 2021, 1185, 123004. [Google Scholar] [CrossRef] [PubMed]
- Reznicek, G.; Jurenitsch, J.; Michl, G.; Haslinger, E.; Hiller, K.; Kubelka, W. Structure of Two New Saponins from Solidago gigantea. Planta Med. 1989, 55, 623–624. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Jamshidi-Aidji, M.; Krüzselyi, D.; Darcsi, A.; Böszörményi, A.; Csontos, P.; Béni, S.; Ott, P.G.; Morlock, G.E. Distinction and Valorization of 30 Root Extracts of Five Goldenrod (Solidago) Species. J. Chromatogr. A 2020, 1611, 460602. [Google Scholar] [CrossRef]
- Nishidono, Y.; Tanaka, K. Comprehensive Characterization of Polyacetylenes and Diterpenes from the Underground Parts of Solidago altissima L. and Their Contribution to the Overall Allelopathic Activity. Phytochemistry 2022, 193, 112986. [Google Scholar] [CrossRef]
- Krüzselyi, D.; Bakonyi, J.; Ott, P.G.; Darcsi, A.; Csontos, P.; Morlock, G.E.; Móricz, Á.M. Goldenrod Root Compounds Active against Crop Pathogenic Fungi. J. Agric. Food Chem. 2021, 69, 12686–12694. [Google Scholar] [CrossRef]
- Baglyas, M.; Ott, P.G.; Schwarczinger, I.; Nagy, J.K.; Darcsi, A.; Bakonyi, J.; Móricz, Á.M. Antimicrobial Diterpenes from Rough Goldenrod (Solidago rugosa Mill.). Molecules 2023, 28, 3790. [Google Scholar] [CrossRef]
- Anthonsen, T.; Henderson, M.S.; Martin, A.; Murray, R.D.H.; McCrindle, R.; McMaster, D. Constituents of Solidago Species. Part IV. Solidagoic Acids A and B, Diterpenoids from Solidago gigantea var. serotina. Can. J. Chem. 1973, 51, 1332–1345. [Google Scholar] [CrossRef]
- Henderson, M.S.; McCrindle, R.; McMaster, D. Constituents of Solidago Species. Part V. Non-Acidic Diterpenoids from Solidago gigantea var. serotina. Can. J. Chem. 1973, 51, 1346–1358. [Google Scholar] [CrossRef]
- Bozsó, Z.; Lapat, V.; Ott, P.G.; Móricz, Á.M. Disparate Effects of Two Clerodane Diterpenes of Giant Goldenrod (Solidago gigantea Ait.) on Bacillus spizizenii. Int. J. Mol. Sci. 2024, 25, 1531. [Google Scholar] [CrossRef]
- Mishra, D.; Joshi, S.; Bisht, G.; Pilkhwal, S. Chemical Composition and Antimicrobial Activity of Solidago canadensis Linn. Root Essential Oil. J. Basic Clin. Pharm. 2010, 1, 187–190. [Google Scholar] [PubMed]
- Baglyas, M.; Ott, P.G.; Bozsó, Z.; Schwarczinger, I.; Bakonyi, J.; Dlauchy, D.; Darcsi, A.; Varga, S.; Móricz, Á.M. Bioassay-Guided Isolation of cis-Clerodane Diterpenoids and Monoglycerides from the Leaves of Solidago gigantea and Their Antimicrobial Activities. Plants 2025, 14, 2152. [Google Scholar] [CrossRef]
- Baglyas, M.; Ott, P.G.; Garádi, Z.; Glavnik, V.; Béni, S.; Vovk, I.; Móricz, Á.M. High-Performance Thin-Layer Chromatography—Antibacterial Assay First Reveals Bioactive Clerodane Diterpenes in Giant Goldenrod (Solidago gigantea Ait.). J. Chromatogr. A 2022, 1677, 463308. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Ott, P.G.; Krüzselyi, D.; Baglyas, M.; Morlock, G.E. High-Performance Thin-Layer Chromatography–Direct Bioautography Combined with Chemometrics for the Distinction of Goldenrod Species. JPC—J Planar Chromatogr. 2022, 35, 339–344. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Ott, P.G.; Häbe, T.T.; Darcsi, A.; Böszörményi, A.; Alberti, Á.; Krüzselyi, D.; Csontos, P.; Béni, S.; Morlock, G.E. Effect-Directed Discovery of Bioactive Compounds Followed by Highly Targeted Characterization, Isolation and Identification, Exemplarily Shown for Solidago virgaurea. Anal. Chem. 2016, 88, 8202–8209. [Google Scholar] [CrossRef]
- Nishidono, Y.; Tanaka, K. New Clerodane Diterpenoids from Solidago altissima and Stereochemical Elucidation via 13C NMR Chemical Shift Analysis. Tetrahedron 2022, 110, 132691. [Google Scholar] [CrossRef]
- Nogueira, R.T.; Shepherd, G.J.; Laverde, A., Jr.; Marsaioli, A.J.; Imamura, P.M. Clerodane-Type Diterpenes from the Seed Pods of Hymenaea courbaril var. stilbocarpa. Phytochemistry 2001, 58, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Manabe, S.; Nishino, C. Stereochemistry of cis-Clerodane Diterpenes. Tetrahedron 1986, 42, 3461–3470. [Google Scholar] [CrossRef]
- Nishidono, Y.; Tanaka, K. Structural Revision of Tinotufolins from Tinospora crispa Leaves Guided by Empirical Rules and DFT Calculations. J. Nat. Prod. 2024, 87, 774–782. [Google Scholar] [CrossRef]
- Miyata, O.; Shinada, T.; Ninomiya, I.; Naito, T. A Facile Conversion of (Z)-2-Alkenoic Esters into the (E)-Isomers with Diphenyl Disulfide. Synthesis 1990, 1990, 1123–1125. [Google Scholar] [CrossRef]
- Nishidono, Y.; Tanaka, K. Revisiting the Structure of Cacospongionolide E: An Approach Based on Empirical Rules and NMR Calculations. Magn. Reson. Chem. 2025, 63, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Morris-Natschke, S.L.; Lee, K.-H. Clerodane Diterpenes: Sources, Structures, and Biological Activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef]
- Hagiwara, H. Total Syntheses of Clerodane Diterpenoids—A Review. Nat. Prod. Commun. 2019, 14, 1934578X19843613. [Google Scholar] [CrossRef]
- Li, Z.-J.; Hu, M.; Yuan, R.-Q.; Jia, L.; Ma, Y.-X.; Liu, Z.; Shen, M.-X.; Zhu, G.-F.; Li, G.; Wu, X.-D. Clerodane Furanoditerpenoids from the Tuberous Roots of Tinospora sagittata (Oliv.) Gagnep. and Their α-Glucosidase Inhibitory Activity. Fitoterapia 2025, 185, 106680. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, S.; Li, H.; Li, Y.; Wang, Z.; Wang, W.; Zheng, B.; Bai, X.; Huang, X. Two New Clerodane Diterpenoids with Analgesic Activity from the Roots of Paratinospora sagittata (Oliv.) Wei Wang. Nat. Prod. Res. 2025, 39. in press. [Google Scholar] [CrossRef]
- Beg, M.; Shankar, K.; Varshney, S.; Rajan, S.; Singh, S.P.; Jagdale, P.; Puri, A.; Chaudhari, B.P.; Sashidhara, K.V.; Gaikwad, A.N. A Clerodane Diterpene Inhibit Adipogenesis by Cell Cycle Arrest and Ameliorate Obesity in C57BL/6 Mice. Mol. Cell. Endocrinol. 2015, 399, 373–385. [Google Scholar] [CrossRef]
- Song, Y.; Yuan, C.; Li, W.; Song, Q.; Zhu, W.; Zhang, J. New Clerodane Diterpenoids from Tinospora capillipes with Antibacterial and Anti-Inflammatory Properties. Chem. Biodivers. 2024, 21, e202401033. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, Z.; Chen, C.; Wang, H.; Liu, H.; Li, J.; Sun, C.; Lou, H.; Pan, W. New Clerodane Diterpenoids from Callicarpa pseudorubella and Their Antitumor Proliferative Activity. Fitoterapia 2024, 174, 105878. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.-C.; Li, W.-Y.; Guo, K.; Liu, Y.; Li, S.-H. Antifeedant, Cytotoxic, and Anti-Inflammatory Neo-Clerodane Diterpenoids in the Peltate Glandular Trichomes and Fresh Leaves of Ajuga forrestii. Phytochemistry 2021, 186, 112731. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.V.; Han, J.W.; Le Dang, Q.; Ryu, S.M.; Lee, D.; Kim, H.; Choi, G.J. Clerodane Diterpenoids Identified from Polyalthia Longifolia Showing Antifungal Activity against Plant Pathogens. J. Agric. Food Chem. 2021, 69, 10527–10535. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zheng, H.; Li, L.; Zhang, J.; Li, Y.; Li, S.; Zhu, R.; Zhou, J.; Zhao, S.; Jiang, Y.; et al. Terpenoids with Vasorelaxant Effects from the Chinese Liverwort Scapania carinthiaca. Bioorg. Med. Chem. 2018, 26, 4320–4328. [Google Scholar] [CrossRef]
- Novello, C.R.; Düsman, E.; Balbinot, R.B.; De Paula, J.C.; Nakamura, C.V.; De Mello, J.C.P.; Sarragiotto, M.H. Antileishmanial Activity of Neo-Clerodane Diterpenes from Croton echioides. Nat. Prod. Res. 2022, 36, 925–931. [Google Scholar] [CrossRef]
- Zhao, X.-T.; Lei, C.; You, J.-Q.; Zhao, T.; Yu, M.-H.; Shi, X.-L.; Hu, X.; Hou, A.-J. Dimeric Clerodane Diterpenoids and Antiviral Constituents of Dodonaea viscosa. Bioorg. Chem. 2021, 112, 104916. [Google Scholar] [CrossRef]
- Fan, M.; Luo, D.; Peng, L.-Y.; Li, X.-N.; Wu, X.-D.; Ji, X.; Zhao, Q.-S. Neo-Clerodane Diterpenoids from Aerial Parts of Salvia hispanica L. and Their Cardioprotective Effects. Phytochemistry 2019, 166, 112065. [Google Scholar] [CrossRef]
- Hanh, T.T.H.; Anh, D.H.; Quang, T.H.; Trung, N.Q.; Thao, D.T.; Cuong, N.T.; An, N.T.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; et al. Scutebarbatolides A-C, New Neo-Clerodane Diterpenoids from Scutellaria barbata D. Don with Cytotoxic Activity. Phytochem. Lett. 2019, 29, 65–69. [Google Scholar] [CrossRef]
- Ao, R.; Li, M.-Y.; Yang, F.-F.; Bao, J.; Zhang, J.-S.; Zhang, H. Targeted Discovery of Clerodane Diterpenoids from Tinospora sinensis as Immunomodulatory Agents. Fitoterapia 2024, 178, 106174. [Google Scholar] [CrossRef]
- Ren, X.; Yuan, X.; Jiao, S.-S.; He, X.-P.; Hu, H.; Kang, J.-J.; Luo, S.-H.; Liu, Y.; Guo, K.; Li, S.-H. Clerodane Diterpenoids from the Uygur Medicine Salvia deserta with Immunosuppressive Activity. Phytochemistry 2023, 214, 113823. [Google Scholar] [CrossRef]
- Abbaszadeh, G.; Srivastava, C.; Walia, S. Insecticidal and Antifeedant Activities of Clerodane Diterpenoids Isolated from the Indian Bhant Tree, Clerodendron infortunatum, against the Cotton Bollworm, Helicoverpa armigera. J. Insect Sci. 2014, 14, 29. [Google Scholar] [CrossRef]
- Peng, X.; Tan, Q.; Zhang, Z.; Wu, D.; Xu, J.; Zhou, H.; Gu, Q. Discovery of Neo-Clerodane Diterpenoids from Ajuga campylantha as Neuroprotective Agents against Ferroptosis and Neuroinflammation. J. Nat. Prod. 2023, 86, 2006–2021. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Wei, W.-J.; Ma, K.-L.; Zhang, J.-Y.; Li, Y.; Gao, K. Phytotoxic Neo-Clerodane Diterpenoids from the Aerial Parts of Scutellaria barbata. Phytochemistry 2020, 171, 112230. [Google Scholar] [CrossRef]
- Tokoroyama, T. Synthesis of Clerodane Diterpenoids and Related Compounds—Stereoselective Construction of the Decalin Skeleton with Multiple Contiguous Stereogenic Centers. Synthesis 2000, 2000, 611–633. [Google Scholar] [CrossRef]
- Reuk-ngam, N.; Chimnoi, N.; Khunnawutmanotham, N.; Techasakul, S. Antimicrobial Activity of Coronarin D and Its Synergistic Potential with Antibiotics. BioMed Res. Int. 2014, 2014, 581985. [Google Scholar] [CrossRef] [PubMed]
- Schwarczinger, I.; Bozsó, Z.; Szatmári, Á.; Süle, S.; Szabó, Z.; Nagy, G.; Király, L. First Report of Bacterial Leaf Spot Caused by the Quarantine Pathogen Xanthomonas arboricola pv. pruni on Peach in Hungary. Plant Dis. 2018, 102, 1654. [Google Scholar] [CrossRef]


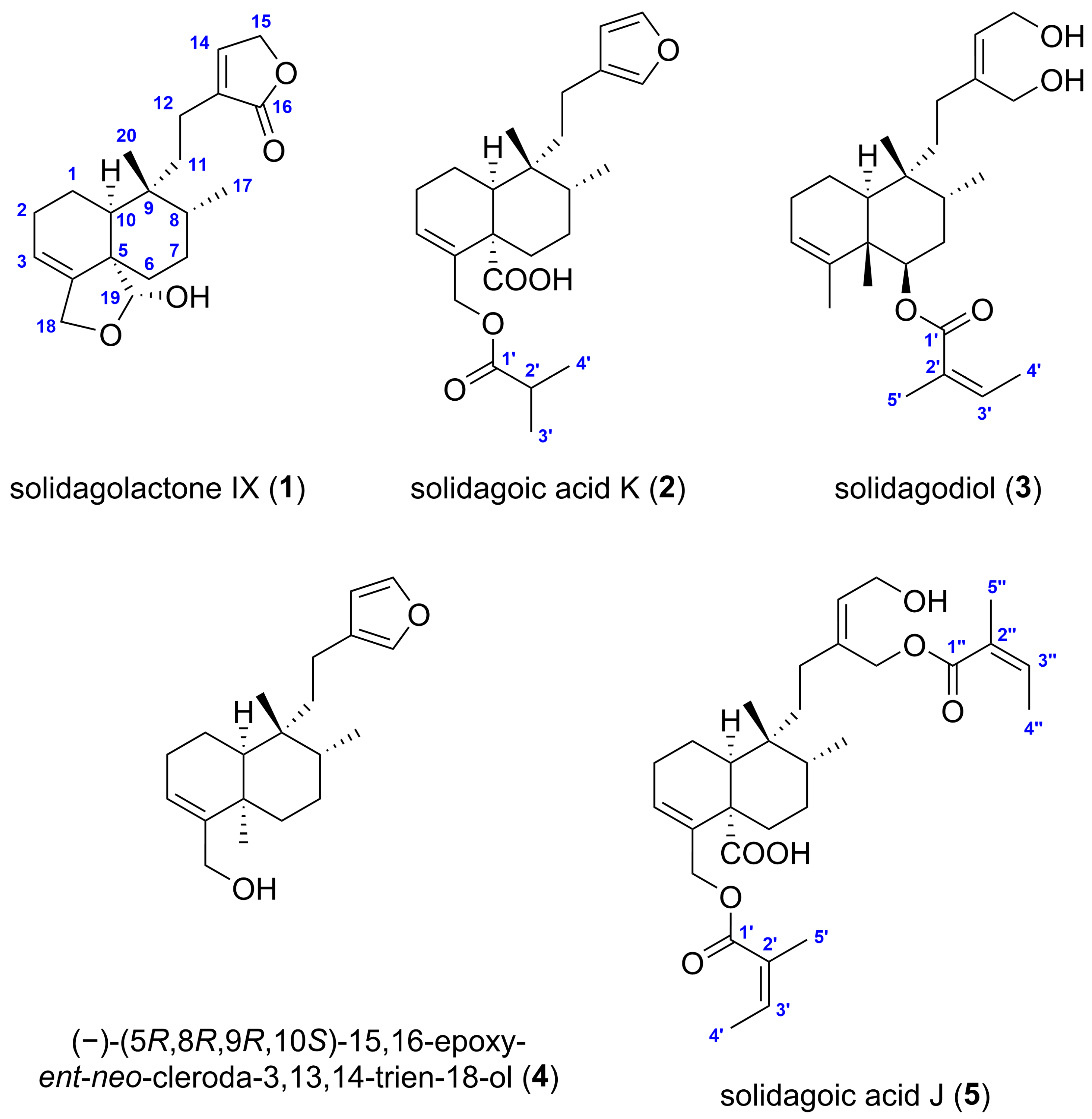
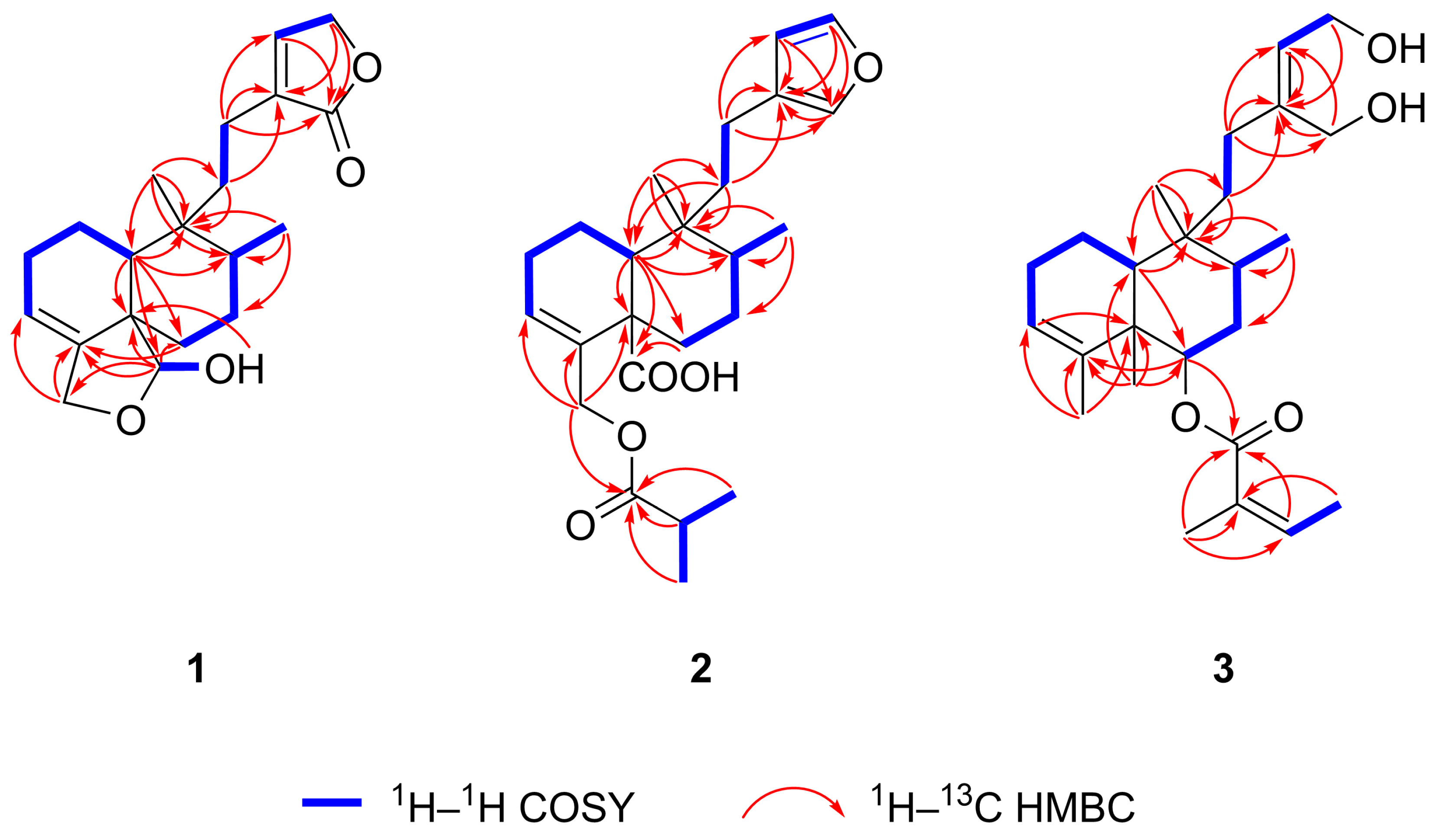
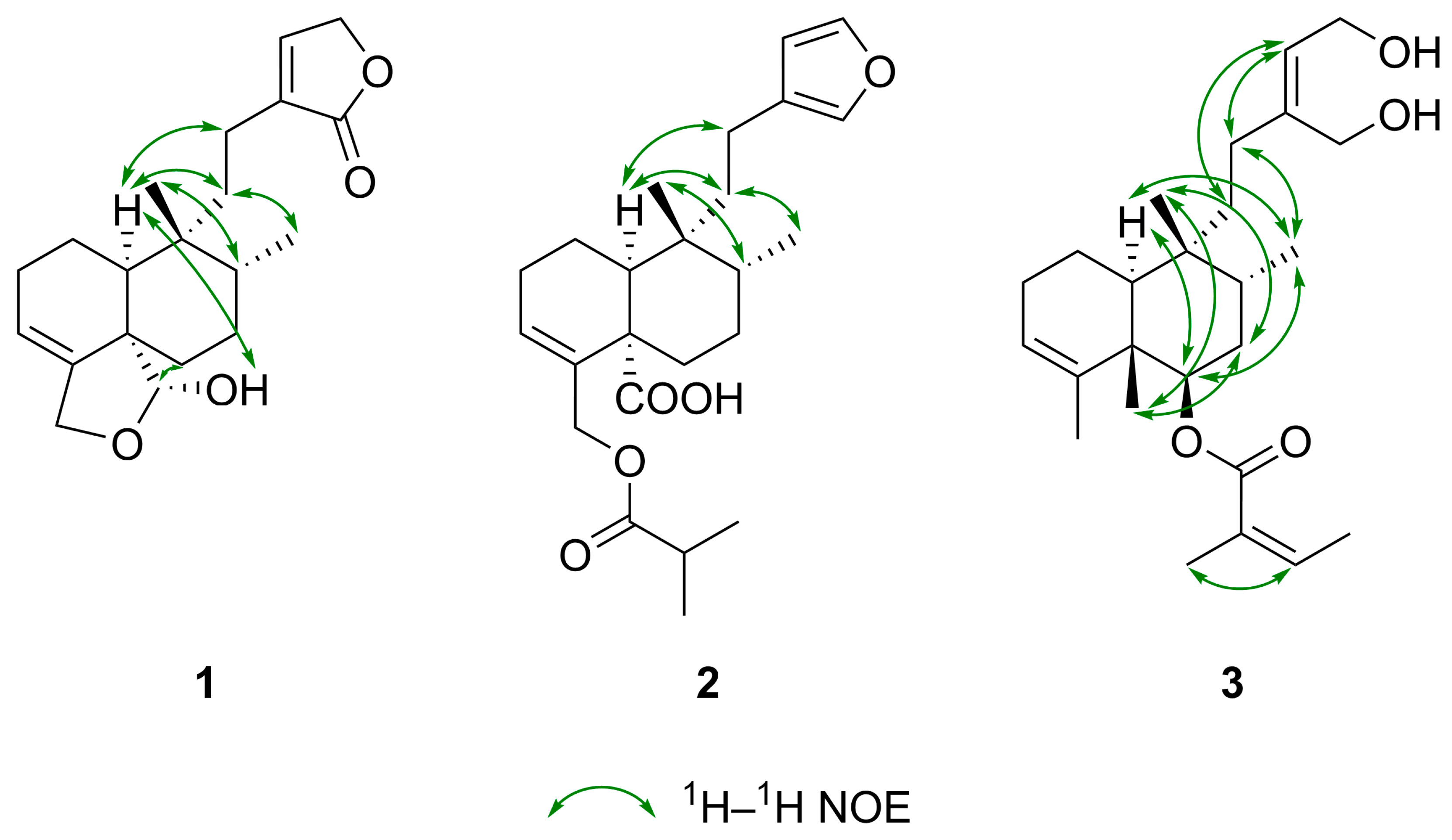
| Position | Solidagolactone IX (1) | Solidagoic Acid K (2) | Solidagodiol (3) | |||
|---|---|---|---|---|---|---|
| δH (ppm), Multiplicity, J (Hz) | δC (ppm), Type | δH (ppm), Multiplicity, J (Hz) | δC (ppm), Type | δH (ppm), Multiplicity, J (Hz) | δC (ppm), Type | |
| 1a | 1.69, ov. | 20.1, CH2 | 1.80, m | 19.6, CH2 | 1.68, ov. | 17.5, CH2 |
| 1b | 1.57, ov. | 1.56, ov. | ||||
| 2a | 2.15, m | 26.3, CH2 | 2.19, m | 26.5, CH2 | 2.04, m | 26.5, CH2 |
| 2b | 1.99, ov. | |||||
| 3 | 5.59, m | 118.3, CH | 5.91, t (4.0) | 128.5, CH | 5.17, br s | 122.3, CH |
| 4 | – | 142.1, C | – | 135.8, C | – | 143.0, C |
| 5 | – | 49.5, C | – | 50.2, C | – | 42.8, C |
| 6a | 1.69, ov. | 30.4, CH2 | 2.37, ov. | 30.1, CH2 | 5.09, dd (11.1, 4.6) | 74.2, CH |
| 6b | 1.53, m | 1.52, m | ||||
| 7a | 1.35, m | 28.1, CH2 | 1.65, ov. | 27.9, CH2 | 1.89, ov. | 32.3, CH2 |
| 7b | 1.35, m | 1.66, ov. | ||||
| 8 | 1.68, ov. | 37.2, CH | 1.66, ov. | 37.1, CH | 1.75, m | 36.1, CH |
| 9 | – | 38.2, C | – | 38.9, C | – | 37.4, C |
| 10 | 1.89, dd (12.1, 3.6) | 38.6, CH | 2.41, ov. | 42.2, CH | 1.56, ov. | 44.8, CH |
| 11a | 1.67, ov. | 32.1, CH2 | 1.59, ov. | 31.9, CH2 | 1.54, ov. | 38.0, CH2 |
| 11b | 1.17, td (13.4, 4.5) | 1.42, td (13.8, 5.1) | 1.17, m | |||
| 12a | 2.63, m | 20.6, CH2 | 2.55, ov. | 19.0, CH2 | 2.08, m | 29.2, CH2 |
| 12b | 2.18, td (13.1, 4.4) | 2.24, td (13.2, 5.1) | ||||
| 13 | – | 135.8, C | – | 126.1, C | – | 144.9, C |
| 14 | 7.17, br s | 146.0, CH | 6.25, dd (1.8, 0.9) | 111.3, CH | 5.64, t (6.9) | 126.4, CH |
| 15 | 4.79, br s | 70.6, CH2 | 7.30, t (1.7) | 142.6, CH | 4.22, dd (6.9, 2.6) | 58.8, CH2 |
| 16 | – | 176.1, C | 7.15, t (1.5) | 138.6, CH | 4.18, d (2.0) | 61.2, CH2 |
| 17 | 0.81, d (6.9) | 15.9, CH3 | 0.85, d (6.2) | 15.8, CH3 | 1.08, d (7.1) | 15.2, CH3 |
| 18 | 4.35, m | 67.2, CH2 | 4.43, m | 64.7, CH2 | 1.56, ov. | 20.76/20.81, CH3 |
| 19 | 5.52, d (5.7) | 100.8, CH | – | 179.5, C | 1.20, s | 17.1, CH3 |
| 20 | 0.97, s | 26.4, CH3 | 0.98, s | 26.9, CH3 | 0.97, s | 20.4, CH3 |
| 1′ | – | 176.9, C | – | 167.5, C | ||
| 2′ | 2.54, sept (7.0) | 34.2, CH | – | 128.7, C | ||
| 3′ | 1.15, d (7.0) | 19.10 *, CH3 | 6.04, qq (7.3, 1.3) | 137.8, CH | ||
| 4′ | 1.15, d (7.0) | 19.06 *, CH3 | 2.00, dq (7.3, 1.6) | 15.8, CH3 | ||
| 5′ | 1.89, p (1.5) | 20.76/20.81, CH3 | ||||
| 19-OH | 5.06, d (5.7) | – | ||||
| Compounds | Bs (G+) | Cff (G+) | Cm (G+) | Rf (G+) | Pstom (G−) | Xap (G−) | Bip | Fg | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MIC | |
| 1 | >402 | >402 | >402 | >402 | >402 | >402 | N/A | N/A | >402 | >402 | N/A | N/A | >502 | >502 |
| 2 | 166 | >332 | 332 | >332 | 41 | >332 | N/A | N/A | >332 | >332 | >332 | >332 | >415 | >415 b |
| 3 | 21 | >330 | 21 | >330 | 5.1 | 83 | 41 | >330 | >330 | >330 | >330 | >330 | >413 | >413 c |
| 4 | 100 | >402 | 402 | >402 | 6.3 | >402 | 201 | >402 | >402 | >402 | >402 | >402 | >502 | >502 |
| 5 [29] | 129 | >258 | 258 | >258 | 129 | >258 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Gentamicin a | 1.7 | 3.5 | 1.7 | 1.7 | 3.5 | 3.5 | 1.7 | 3.5 | 0.9 | 1.7 | 3.5 | 3.5 | ||
| Benomyl a | 3593 | 1797 | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baglyas, M.; Bozsó, Z.; Schwarczinger, I.; Ott, P.G.; Bakonyi, J.; Darcsi, A.; Móricz, Á.M. Discovery of Undescribed Clerodane Diterpenoids with Antimicrobial Activity Isolated from the Roots of Solidago gigantea Ait. Int. J. Mol. Sci. 2025, 26, 9187. https://doi.org/10.3390/ijms26189187
Baglyas M, Bozsó Z, Schwarczinger I, Ott PG, Bakonyi J, Darcsi A, Móricz ÁM. Discovery of Undescribed Clerodane Diterpenoids with Antimicrobial Activity Isolated from the Roots of Solidago gigantea Ait. International Journal of Molecular Sciences. 2025; 26(18):9187. https://doi.org/10.3390/ijms26189187
Chicago/Turabian StyleBaglyas, Márton, Zoltán Bozsó, Ildikó Schwarczinger, Péter G. Ott, József Bakonyi, András Darcsi, and Ágnes M. Móricz. 2025. "Discovery of Undescribed Clerodane Diterpenoids with Antimicrobial Activity Isolated from the Roots of Solidago gigantea Ait" International Journal of Molecular Sciences 26, no. 18: 9187. https://doi.org/10.3390/ijms26189187
APA StyleBaglyas, M., Bozsó, Z., Schwarczinger, I., Ott, P. G., Bakonyi, J., Darcsi, A., & Móricz, Á. M. (2025). Discovery of Undescribed Clerodane Diterpenoids with Antimicrobial Activity Isolated from the Roots of Solidago gigantea Ait. International Journal of Molecular Sciences, 26(18), 9187. https://doi.org/10.3390/ijms26189187






