XON9—A Glyco-Humanized Polyclonal Antibody Effective Against Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
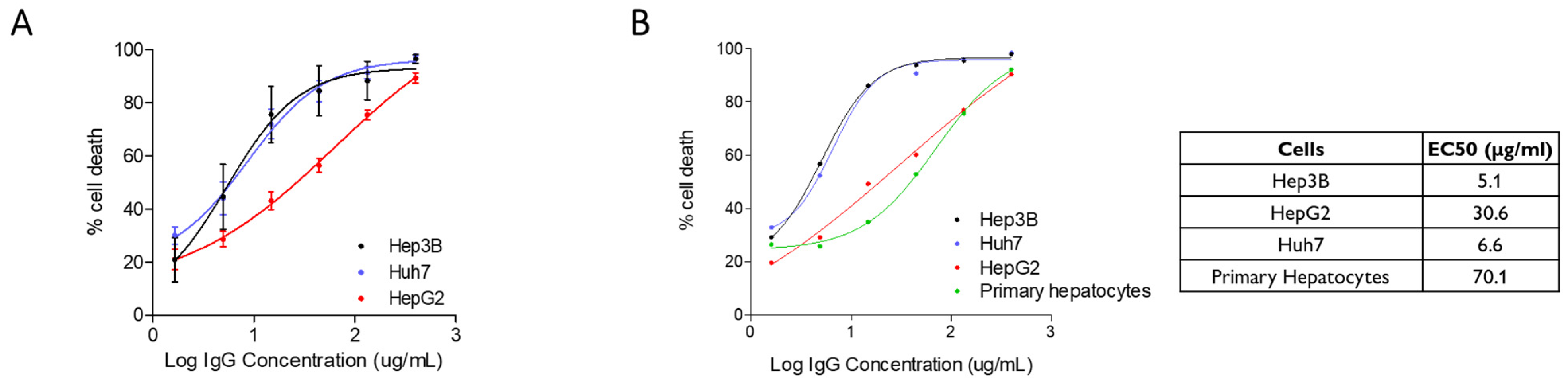
2.1. Cytotoxic Activity of XON9 Against Hepatocarcinoma Cell Lines
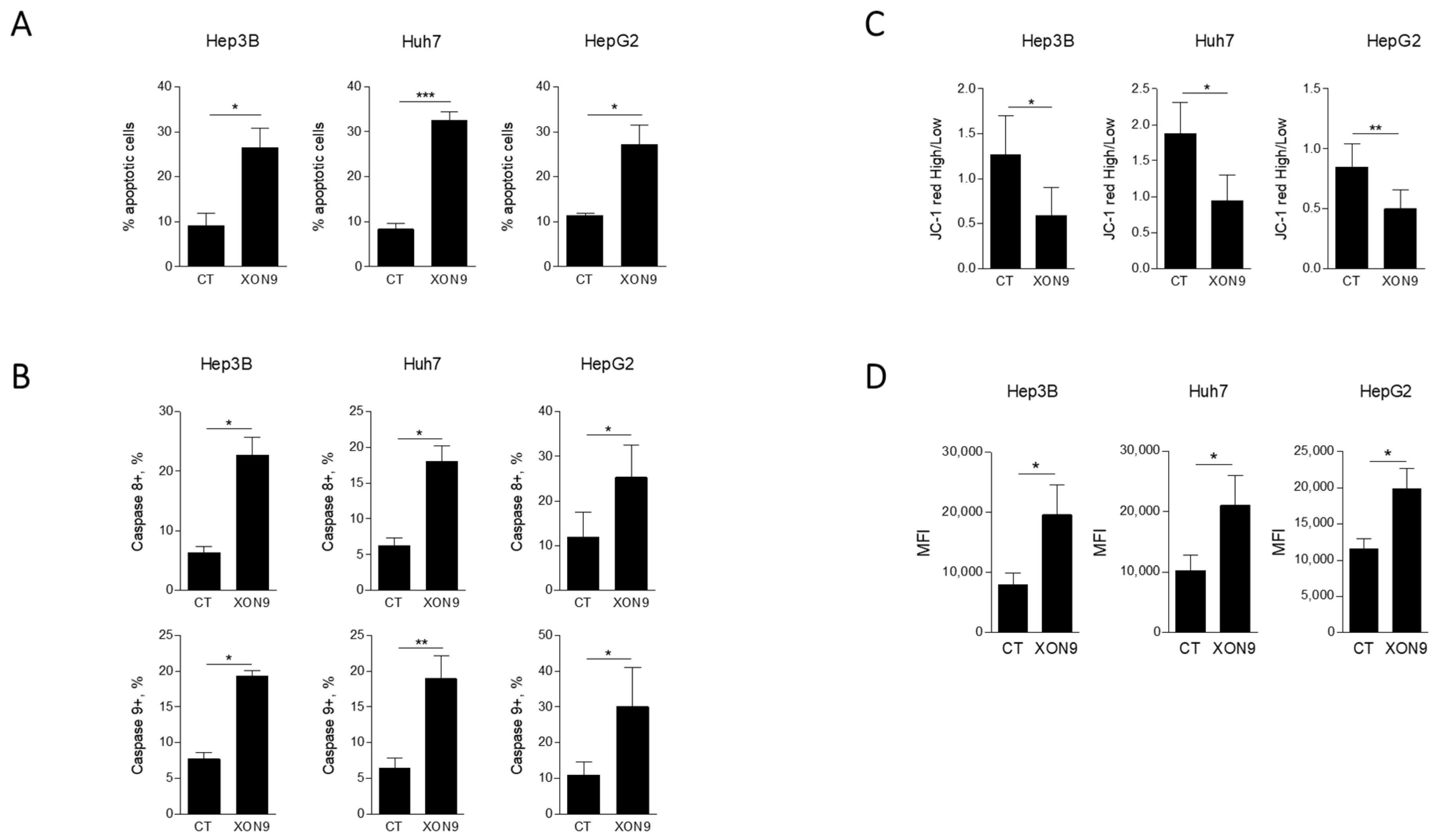
2.2. Activation of Apoptosis in HCC Cell Lines
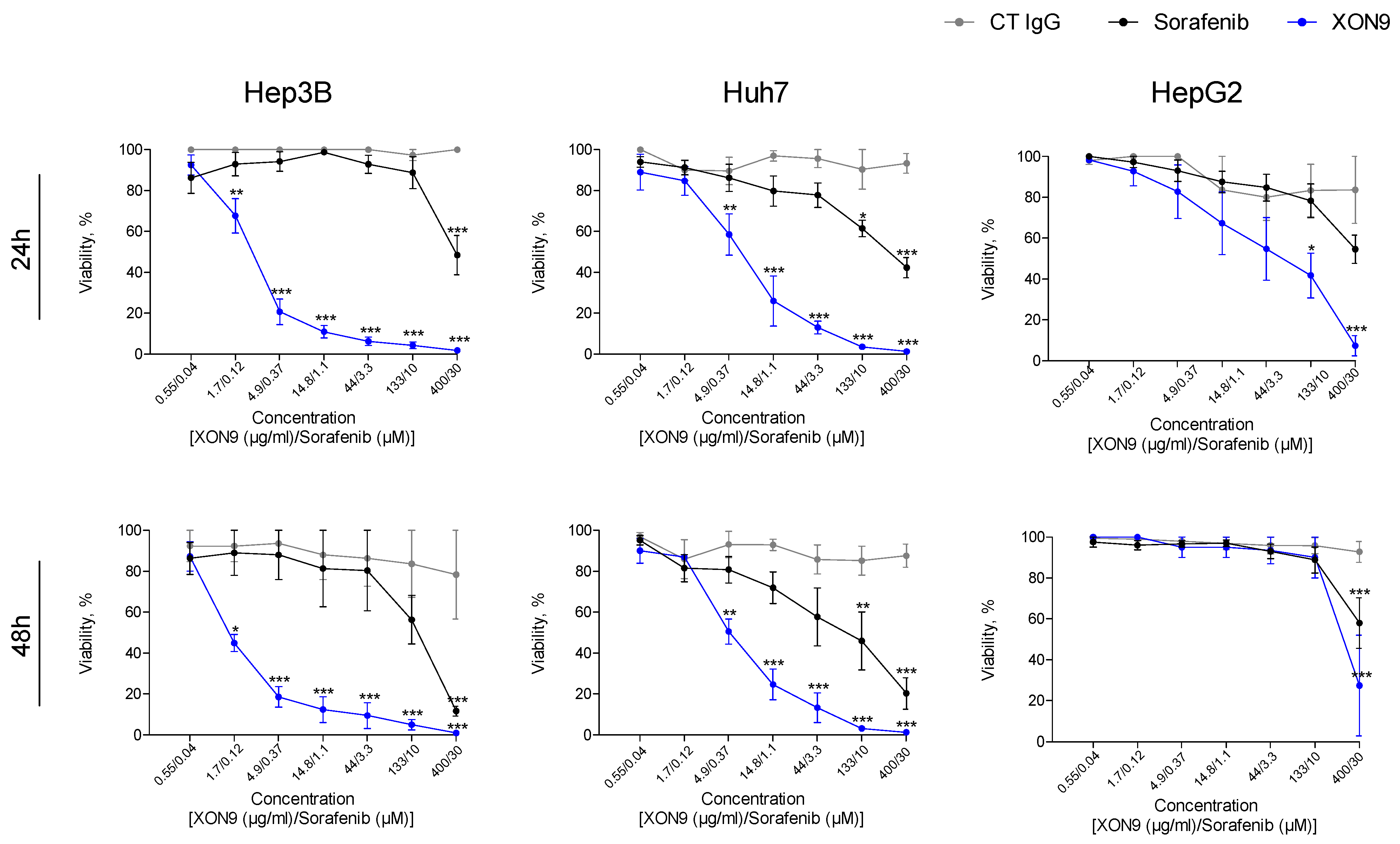
2.3. Potency Comparaison Between XON9 and Sorafenib
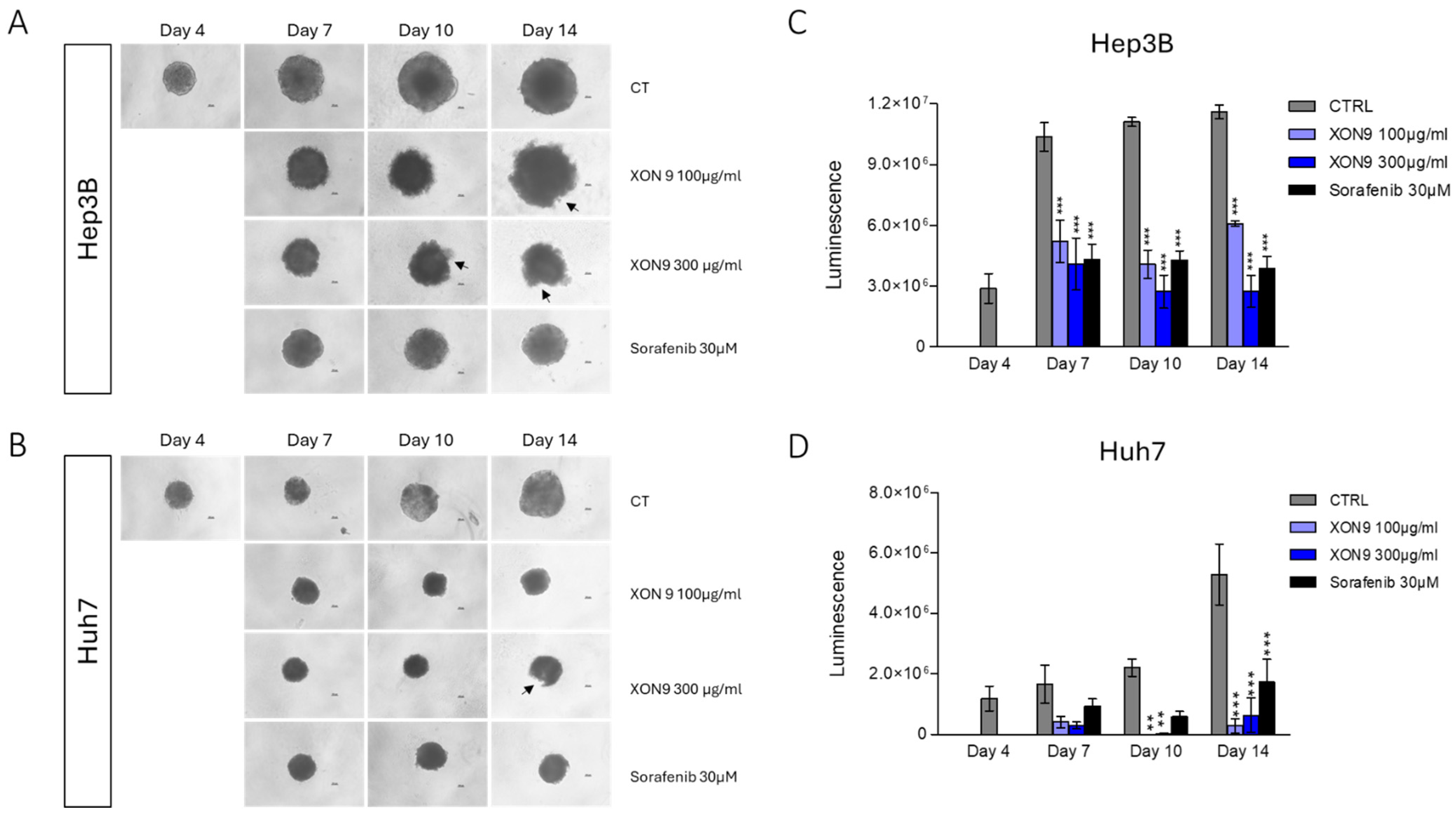
2.4. Xenograft Mice Model
2.5. Pharmacokinetic and Safety Parameters
3. Discussion
4. Material and Method
4.1. Preparation of XON9
4.2. Cells, Culture Media and Reagents
4.3. Complement Dependant Cell Cytotoxicity (CDC)
4.4. Apoptosis Assay
4.5. Mitochondrial Potential Assay
4.6. Reactive Oxygen Species Assay
4.7. Caspases 8 and 9 Assays
4.8. Cancer Cell Viability
4.9. Tumor Spheroid Assay
4.10. Tumor Xenograft Model
4.11. Pharmacokinetic and Safety Parameters in a Non-Human Primate
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Liu, Y.; Zhang, N.; Tao, F.; Yin, G. Therapeutic Advances in Hepatocellular Carcinoma: An Update from the 2024 ASCO Annual Meeting. Front. Oncol. 2024, 14, 1453412. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global Trends in Hepatocellular Carcinoma Epidemiology: Implications for Screening, Prevention and Therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Tan, D.J.H.; Tang, A.S.P.; Lim, W.H.; Ng, C.H.; Nah, B.; Fu, C.; Xiao, J.; Koh, B.; Tay, P.W.L.; Tan, E.X.; et al. Survival Trends in Sorafenib for Advanced Hepatocellular Carcinoma: A Reconstructed Individual Patient Data Meta-Analysis of Randomized Trials. Liver Cancer 2023, 12, 445–456. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, EVIDoa2100070. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.-Y.; Ren, Z.; et al. Lenvatinib plus Pembrolizumab versus Lenvatinib plus Placebo for Advanced Hepatocellular Carcinoma (LEAP-002): A Randomised, Double-Blind, Phase 3 Trial. Lancet Oncol. 2023, 24, 1399–1410, Erratum in Lancet Oncol. 2024, 25, e137. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus Atezolizumab versus Sorafenib for Advanced Hepatocellular Carcinoma (COSMIC-312): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, S.; Xu, M.; Wu, X.; Dou, W.; Li, H.; Zhang, Z.; Zhang, S. CAR-T Cell Therapy for Hepatocellular Carcinoma: Current Trends and Challenges. Front. Immunol. 2024, 15, 1489649. [Google Scholar] [CrossRef] [PubMed]
- Aggeletopoulou, I.; Kalafateli, M.; Triantos, C. Chimeric Antigen Receptor T Cell Therapy for Hepatocellular Carcinoma: Where Do We Stand? Int. J. Mol. Sci. 2024, 25, 2631. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Lahaie, Y.M.; Watier, H. Contribution of Physiologists to the Identification of the Humoral Component of Immunity in the 19th Century. mAbs 2017, 9, 774–780. [Google Scholar] [CrossRef]
- Couvrat-desvergnes, G.; Salama, A.; Berre, L.L.; Evanno, G.; Viklicky, O.; Hruba, P.; Vesely, P.; Guerif, P.; Dejoie, T.; Rousse, J.; et al. Rabbit Antithymocyte Globulin—Induced Serum Sickness Disease and Human Kidney Graft Survival. J. Clin. Investig. 2015, 125, 4655–4665. [Google Scholar] [CrossRef]
- Ciron, C.; Morice, P.; Rousse, J.; Roy, P.; Royer, P.-J.; Gauthier, O.; Brouard, S.; Duvaux, O.; Bassissi, F.; Vanhove, B. Polyclonal Antibodies, Inhibit Selectively Tumor Growth and Invasion and Synergize with Immune Checkpoint Inhibitors. JCI Insight 2023, 9, e166231. [Google Scholar] [CrossRef]
- Poulakou, G.; Royer, P.J.; Evgeniev, N.; Evanno, G.; Shneiker, F.; Marcelin, A.G.; Vanhove, B.; Duvaux, O.; Marot, S.; Calvez, V. Anti-SARS-CoV-2 Glyco-Humanized Polyclonal Antibody XAV-19: Phase II/III Randomized Placebo-Controlled Trial Shows Acceleration to Recovery for Mild to Moderate Patients with COVID-19. Front. Immunol. 2024, 15, 1330178. [Google Scholar] [CrossRef]
- Gaborit, B.; Vanhove, B.; Lacombe, K.; Guimard, T.; Hocqueloux, L.; Perrier, L.; Dubee, V.; Ferre, V.; Bressollette, C.; Josien, R.; et al. Effect of Swine Glyco-Humanized Polyclonal Neutralizing Antibody on Survival and Respiratory Failure in Patients Hospitalized with Severe COVID-19: A Randomized, Placebo-Controlled Trial. Open Forum Infect. Dis. 2023, 10, ofad525. [Google Scholar] [CrossRef]
- Viklicky, O.; Slatinska, J.; Janousek, L.; Rousse, J.; Royer, P.-J.; Toutain, P.-L.; Cozzi, E.; Galli, C.; Evanno, G.; Duvaux, O.; et al. First-in-Human Study with LIS1, a Next-Generation Porcine Low Immunogenicity Antilymphocyte Immunoglobulin in Kidney Transplantation. Transplantation 2024, 108, e139–e147. [Google Scholar] [CrossRef]
- Ivascu, A.; Kubbies, M. Rapid Generation of Single-Tumor Spheroids for High-Throughput Cell Function and Toxicity Analysis. J. Biomol. Screen. 2006, 11, 922–932. [Google Scholar] [CrossRef]
- Li, X.Y.; Huang, F.; Xu, X.; Hu, S. Polyclonal Rabbit Anti-Cancer-Associated Fibroblasts Globulins Induce Cancer Cells Apoptosis and Inhibit Tumor Growth. Int. J. Biol. Sci. 2018, 14, 1621–1629. [Google Scholar] [CrossRef]
- Schieferdecker, A.; Shoshani, O.; Westner, B.; Zipori, D.; Fehse, B.; Kröger, N.; Ayuk, F. Potent in Vitro and in Vivo Effects of Polyclonal Anti-Human-Myeloma Globulins. Oncotarget 2016, 7, 67061–67070. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ayuk, F.A.; Fang, L.; Fehse, B.; Zander, A.R.; Kröger, N. Antithymocyte Globulin Induces Complement-Dependent Cell Lysis and Caspase-Dependent Apoptosis in Myeloma Cells. Exp. Hematol. 2005, 33, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Westphal, S.; Brinkmann, H.; Kalupa, M.; Wilke, A.; Seitz-Merwald, I.; Penack, O. Anti-Tumor Effects of Anti-T-Cell Globulin. Exp. Hematol. 2014, 42, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, M.; Deng, H.; Shen, G.; Wei, Y. Polyclonal Rabbit Anti-Human Ovarian Cancer Globulins Inhibit Tumor Growth through Apoptosis Involving the Caspase Signaling. Sci. Rep. 2014, 4, 4984. [Google Scholar] [CrossRef]
- Mu, B.; Yang, J.L.; Gou, L.T.; Yao, Y.Q.; Zhou, Y.; Cheng, Z.H.; Shi, H.S.; Li, Z.Y.; Wen, Y.; Leng, F.; et al. Polyclonal Rabbit Anti-Murine Plasmacytoma Cell Globulins Induce Myeloma Cells Apoptosis and Inhibit Tumour Growth in Mice. Apoptosis 2011, 16, 370–381. [Google Scholar] [CrossRef][Green Version]
- Zand, M.S.; Vo, T.; Pellegrin, T.; Felgar, R.; Liesveld, J.L.; Ifthikharuddin, J.J.; Abboud, C.N.; Sanz, I.; Huggins, J. Apoptosis and Complement-Mediated Lysis of Myeloma Cells by Polyclonal Rabbit Antithymocyte Globulin. Blood 2006, 107, 2895–2903. [Google Scholar] [CrossRef]
- Canup, B.S.B.; Song, H.; Laroui, H. Role of CD98 in Liver Disease. Ann. Hepatol. 2020, 19, 602–607. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, J.; Sun, Y.; Liu, G.; Wei, X. ANXA5: Related Mechanisms of Osteogenesis and Additional Biological Functions. Front. Cell Dev. Biol. 2025, 13, 1553683. [Google Scholar] [CrossRef]
- Che, L.; Paliogiannis, P.; Cigliano, A.; Pilo, M.G.; Chen, X.; Calvisi, D.F. Pathogenetic, Prognostic, and Therapeutic Role of Fatty Acid Synthase in Human Hepatocellular Carcinoma. Front. Oncol. 2019, 9, 1412, Erratum in Front. Oncol. 2022, 12, 874053. [Google Scholar] [CrossRef]
- Cai, H.; Saiyin, H.; Liu, X.; Han, D.; Ji, G.; Qin, B.; Zuo, J.; Shen, S.; Yu, W.; Wu, J.; et al. Nogo-B Promotes Tumor Angiogenesis and Provides a Potential Therapeutic Target in Hepatocellular Carcinoma. Mol. Oncol. 2018, 12, 2042–2054. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, S.; Hu, X.; Jin, X.; Le, Y.; Cao, L.; Yuan, Z.; Lin, Z.; Jiang, S.; Sun, L.; et al. Knockout of the Nogo-B Gene Attenuates Tumor Growth and Metastasis in Hepatocellular Carcinoma. Neoplasia 2017, 19, 583–593. [Google Scholar] [CrossRef]
- Lim, K.; Han, S.H.; Han, S.; Lee, J.Y.; Choi, H.S.; Choi, D.; Ryu, C.J. A Monoclonal Antibody Recognizing CD98 on Human Embryonic Stem Cells Shows Anti-Tumor Activity in Hepatocellular Carcinoma Xenografts. Cancer Immunol. Immunother. 2024, 73, 231. [Google Scholar] [CrossRef]
- Pellizzari, G.; Martinez, O.; Crescioli, S.; Page, R.; Di Meo, A.; Mele, S.; Chiaruttini, G.; Hoinka, J.; Batruch, I.; Prassas, I.; et al. Immunotherapy Using IgE or CAR T Cells for Cancers Expressing the Tumor Antigen SLC3A2. J. Immunother. Cancer 2021, 9, e002140. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and Challenges for Use of Tumor Spheroids as Models to Test Drug Delivery and Efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Ménoret, S.; Ouisse, L.H.; Tesson, L.; Remy, S.; Usal, C.; Guiffes, A.; Chenouard, V.; Royer, P.J.; Evanno, G.; Vanhove, B.; et al. In Vivo Analysis of Human Immune Responses in Immunodeficient Rats. Transplantation 2020, 104, 715–723. [Google Scholar] [CrossRef]
- Gaborit, B.; Dailly, E.; Vanhove, B.; Josien, R.; Lacombe, K.; Dubee, V.; Ferre, V.; Brouard, S.; Ader, F.; Vibet, M.; et al. Pharmacokinetics and Safety of XAV-19, a Swine Glyco-Humanized Polyclonal Anti-SARS-CoV-2 Antibody, for COVID-19-Related Moderate Pneumonia: A Randomized, Double-Blind, Placebo-Controlled, Phase IIa Study. Antimicrob. Agents Chemother. 2021, 65, 10-1128. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Royer, P.-J.; Ciron, C.; Evanno, G.; Dauphouy, O.; Rousse, J.; Graur, G.; Duvaux, O.; Bassissi, F. XON9—A Glyco-Humanized Polyclonal Antibody Effective Against Hepatocellular Carcinoma. Int. J. Mol. Sci. 2025, 26, 9185. https://doi.org/10.3390/ijms26189185
Royer P-J, Ciron C, Evanno G, Dauphouy O, Rousse J, Graur G, Duvaux O, Bassissi F. XON9—A Glyco-Humanized Polyclonal Antibody Effective Against Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2025; 26(18):9185. https://doi.org/10.3390/ijms26189185
Chicago/Turabian StyleRoyer, Pierre-Joseph, Carine Ciron, Gwenaelle Evanno, Ophélie Dauphouy, Juliette Rousse, George Graur, Odile Duvaux, and Firas Bassissi. 2025. "XON9—A Glyco-Humanized Polyclonal Antibody Effective Against Hepatocellular Carcinoma" International Journal of Molecular Sciences 26, no. 18: 9185. https://doi.org/10.3390/ijms26189185
APA StyleRoyer, P.-J., Ciron, C., Evanno, G., Dauphouy, O., Rousse, J., Graur, G., Duvaux, O., & Bassissi, F. (2025). XON9—A Glyco-Humanized Polyclonal Antibody Effective Against Hepatocellular Carcinoma. International Journal of Molecular Sciences, 26(18), 9185. https://doi.org/10.3390/ijms26189185







