COMP Is a Biomarker of Cartilage Destruction, Extracellular Matrix and Vascular Remodeling and Tissue Repair
Abstract
1. Introduction
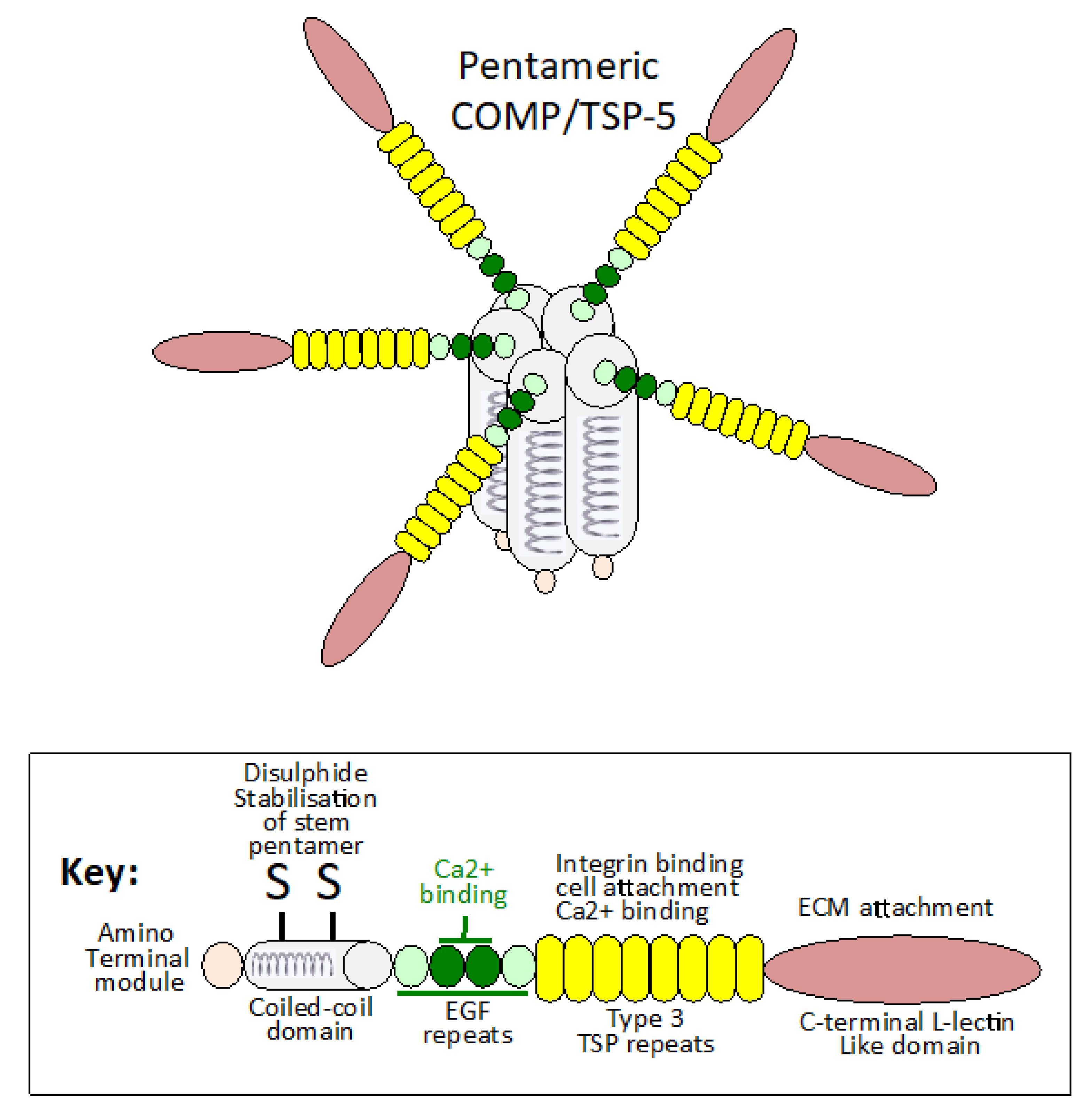
1.1. COMP and Matrix Stabilization
| Study | Features | Ref |
|---|---|---|
| Clark et al., 1999 | Serum COMP levels can distinguish OA from a normal unaffected subgroup, reflecting OA disease severity and the involvement of multiple tissues in the knee joint OA process. The focus should not just be on the articular cartilage. Knee OA is a global disease, and the synovium, meniscus, ligaments, subchondral bone and infrapatellar fat pad all have roles to play in the disease process. | [59] |
| Vilim et al., 2001 | Serum COMP is a measure of synovitis in knee OA. Elevated serum COMP levels in OA patients have been correlated with clinical joint examinations confirming synovitis and changes in other joint tissues. | [60] |
| Vilim et al., 2002 | Correlation of serum COMP levels with radiographic progression of knee OA. Serum COMP levels are a prognostic marker of progressive joint disease and have been shown to persist over a 3-year study period. | [61] |
| Wisłowska et al., 2005 | Serum COMP levels correlate with the severity of systemic lupus erythematosus and knee OA. In systemic lupus erythematosus patients (n = 30), serum COMP levels are significantly higher (p < 0.05) than serum from normal non OA affected patients (n = 30). This demonstrates the variable involvement of inflammation in disease processes in some sub-types of knee joint OA. | [62] |
| Andersson et al., 2006 | Serum COMP levels increased temporarily after physical exercise in 58 patients with knee OA. Exercise increases serum COMP levels in individuals affected with knee OA, COMP levels were decreased during rest. The increased serum COMP levels were normalized 30 min after an exercise session. This demonstrates the dynamic nature of COMP release from knee joint tissues. | [63] |
| Fernandes et al., 2007 | Correlation of serum COMP levels with clinical and radiological knee OA in a Brazilian population. Patients with symptomatic knee OA had significantly higher serum COMP levels than healthy non-OA affected controls or non-symptomatic knees that showed radiographic evidence of narrowing of the joint space. This shows the potential of COMP as a prognostic and diagnostic factor in knee joint OA. | [64] |
| Tseng et al., 2009 | Serum COMP is a marker of knee arthritis and a biomarker of ECM changes following joint trauma or cartilage degeneration. COMP is a diagnostic and prognostic marker of OA severity and can be used to assess the efficacy of anti-arthritic drugs in prospective OA treatments. | [1] |
| Hoch et al., 2011 | Elevation of serum COMP in patients with knee OA: meta-analysis. A meta-analysis of a number of studies which examined serum COMP levels in knees with radiographically diagnosed OA of variable severity showed serum COMP levels were consistently elevated in patients with knee OA and were sensitive to OA disease progression. This confirmed that COMP is a biomarker for OA development and progression. | [55] |
| Zivanović et al., 2011 | COMP, an inflammation biomarker in knee OA. Measurement of serum COMP levels in 88 OA patients examined by ultrasound to assess severity of OA disease and presence of synovitis showed serum COMP levels ranging from 52 to 66.5 ng/mL and correlated with the clinical severity of OA and the involvement of synovitis in knee OA. COMP thus was a biomarker of the severity of inflammation in knee OA. | [5] |
| Verma et al., 2013 | Serum COMP is a novel diagnostic and prognostic biomarker of knee OA. Measurement of serum COMP and inflammatory cytokine levels in OA and normal control patients by ELISA demonstrated COMP levels in OA patients were 1117.21 ng/mL (125.03–4209.75 ng/mL) compared to 338.62 ng/mL (118–589 ng/mL) in control subjects (p < 0.001). COMP levels positively correlated with the clinical severity of OA cases and demonstrated COMP was a quantitative biomarker of knee OA. | [4] |
| Lotz et al., 2013 | Current status and perspectives of OA biomarkers. OA biomarkers have been classified into burden of disease, investigative, prognostic, efficacy of intervention, diagnostic and safety categories. Serum COMP as a biomarker falls into the burden of disease, prognostic and diagnostic categories. | [65] |
| Kluzek et al., 2015 | Serum COMP in the development of radiographic, painful knee OA in a community-based cohort of middle-aged women. A study of serum COMP levels in a group of 593 middle-aged women in the development of radiographically diagnosed painful knee OA demonstrated that serum COMP levels were predictive of structural knee-joint tissue changes and the incidence of painful knee OA, independently of age and BMI. | [56] |
| Henroitin et al., 2016 | Current status of cartilage ECM OA biomarkers. Soluble cartilage biomarkers such as COMP have been proposed to be complementary drug development tools useful in the discovery of anti-arthritic drugs from preclinical stages of their development up to their evaluation in the clinic. Such biomarkers should be considered surrogate indicators of clinical and/or imaging outcomes. Use of automated assays for biomarker panels may eventually lead to personalized medicines for enhanced management of OA. | [54] |
| Ben Achour et al., 2018 | Correlation of bone and cartilage biomarkers with structural damage in RA: Cross sectional study. COMP is a biomarker of cartilage destruction that has been shown to be associated with joint erosions characteristic of RA and is predictive of radiographic damage to joint tissue in RA. | [66] |
| Georgiev et al., 2018 | Correlation of serum COMP with knee OA: a meta-analysis. The measurement of COMP is a novel knee OA diagnostic. A meta-analysis of nine knee OA studies where serum COMP levels were measured showed consistent significantly elevated serum COMP levels in knee OA patients compared to controls. Meta-analysis showed serum COMP levels could distinguish OA from non-OA patients and were discriminative enough to distinguish between different clinical grades of OA. | [67] |
| Laudon et al., 2019 | Serum COMP levels in individuals who sustained a youth sport-related intra-articular knee injury 3–10 years previously have been shown to display symptoms of knee RA-induced COMP levels and/or COMP degradation. COMP is thus a historical marker of cartilage injury and the pre-history of knee-joint loading and trauma, which both contribute to knee joint OA development. | [57] |
| Udomsinprasert et al., 2024 | Correlation of COMP protein and mRNA levels with histological evidence of damage to knee joint tissues in OA. A recent study has confirmed COMP levels were significantly elevated in serum and synovial fluid of knee OA patients, especially in advanced OA stages, and correlated with radiological severity, body composition, physical performance, knee pain, and disability. COMP mRNA expression is markedly upregulated in the inflamed synovium in knee OA, consistent with immunohistochemical localization of COMP in the inflamed lining and sub-lining layers of knee OA synovium, and positively correlated with COMP levels in OA serum and synovial fluid samples. | [58] |
1.2. COMP Is a Biomarker of Tissue Degradation
1.3. Serum COMP Levels in Variably Loaded Skeletal Tissues
Animal OA Models
2. COMP and Tissue Fibrosis
COMP Interactions with Collagen Networks in Tissue Fibrosis and Cancer
3. COMP and Regulation of the Complement System
Identification of Specific COMP Fragments in Diseased Tissues
4. COMP and TGF-β Signaling
Identification of Biomarker Proteins Including COMP in Arthritis Sub-Types
5. The Bioregulatory Roles of Matricryptins/Matrikines in Tissue Homeostasis: Emergence of COMP Fragments as New Matricryptin Members
COMP and Complement in Arthritic and Inflammatory Disorders
6. COMP Has Roles in Malignancy, Cardiovascular Diseases, and Tissue Fibrosis
7. COMP Expression in Skin and Vascular Tissues
8. COMP Activates the Complement System
9. Mutations in COMP Impact Tissue Organization and Function
10. Emerging New Areas of COMP Biology
10.1. Roles for COMP in Tumor Biology
10.2. COMP in Vascular and ECM Remodeling
10.3. COMP-Mediated TGF-β Signaling and Tissue Fibrosis
11. Roles for the Coiled-Coil COMP Domain in Tissue Organization/Stabilization
12. Therapeutic Opportunities with COMP
12.1. COMP as a Biomarker of Disease
12.2. Application of COMP Ang 1 and COMP Ang 2 Chimeric Proteins in Repair Biology
13. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAV2 | Adeno-associated viruses. |
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs. |
| AFM | Atomic force microscopy. |
| Akt | Protein kinase B. |
| BMP | Bone mineral protein. |
| COMP | Cartilage oligomeric matrix protein. |
| CTX II | C-terminal cross-linked telopeptides of type II collagen. |
| ECM | Extracellular Matrix. |
| ER | Endoplasmic reticulum. |
| GAG | Glycosaminoglycan. |
| hrAFM | High-resolution atomic force microscopy. |
| LYVE-1 | lymphatic vessel endothelial hyaluronan receptor 1. |
| MAPK | Mitogen-activated protein kinase. |
| MMP-3 | Matrix metalloprotease-3. |
| mTOR | mammalian target of rapamycin. |
| OA | Osteoarthritis. |
| PI3K | Phosphoinositide 3-kinase. |
| PSACH | Pseudoachondroplasia. |
| P-Smad | Phosphorylated Caenorhabditis elegans SMA (small worm phenotype) and MAD family (Mothers Against Decapentaplegic). |
| RA | Rheumatoid arthritis. |
| SIRT6 | Sirtuin 6 a stress responsive protein deacetylase/mono-ADP ribosyltransferase. |
| SLRP | Small leucine repeat proteoglycan. |
| TSP-5 | Thrombospondin-5. |
| TGF-β | Transforming growth factor-beta. |
| Tie2 | Endothelial receptor tyrosine kinase receptor-2. |
References
- Tseng, S.; Reddi, A.H.; Di Cesare, P.E. Cartilage Oligomeric Matrix Protein (COMP): A Biomarker of Arthritis. Biomark Insights 2009, 4, 33–44. [Google Scholar] [CrossRef]
- O’Sullivan, O.; Ladlow, P.; Steiner, K.; Hillman, C.; Stocks, J.; Bennett, A.N.; Valdes, A.M.; Kluzek, S. Current status of catabolic, anabolic and inflammatory biomarkers associated with structural and symptomatic changes in the chronic phase of post-traumatic knee osteoarthritis- a systematic review. Osteoarthr. Cart. Open 2023, 5, 100412. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, O.; Stocks, J.; Schofield, S.; Bilzon, J.; Boos, C.J.; Bull, A.M.J.; Fear, N.T.; Watt, F.E.; Bennett, A.N.; Kluzek, S.; et al. Association of serum biomarkers with radiographic knee osteoarthritis, knee pain and function in a young, male, trauma-exposed population—Findings from the ADVANCE study. Osteoarthr. Cartil. 2024, 32, 1636–1646. [Google Scholar] [CrossRef]
- Verma, P.; Dalal, K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: A novel diagnostic and prognostic biomarker. J. Orthop. Res. 2013, 31, 999–1006. [Google Scholar] [CrossRef]
- Zivanović, S.; Rackov, L.P.; Zivanović, A.; Jevtić, M.; Nikolić, S.; Kocić, S. Cartilage oligomeric matrix protein—Inflammation biomarker in knee osteoarthritis. Bosn. J. Basic Med. Sci. 2011, 11, 27–32. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Piperigkou, Z.; Tzaferi, K.; Karamanos, N.K. Trends in extracellular matrix biology. Mol. Biol. Rep. 2023, 50, 853–863. [Google Scholar] [CrossRef]
- Franchi, M.; Piperigkou, Z.; Mastronikolis, N.S.; Karamanos, N. Extracellular matrix biomechanical roles and adaptation in health and disease. FEBS J. 2024, 291, 430–440. [Google Scholar] [CrossRef]
- Theocharis, A.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Sainio, A.; Järveläinen, H. Extracellular matrix-cell interactions: Focus on therapeutic applications. Cell Signal. 2020, 66, 109487. [Google Scholar] [CrossRef]
- Theocharis, A.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Hedbom, E.; Antonsson, P.; Hjerpe, A.; Aeschlimann, D.; Paulsson, M.; Rosa-Pimentel, E.; Sommarin, Y.; Wendel, M.; Oldberg, A.; Heinegård, D. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J. Biol. Chem. 1992, 267, 6132–6136. [Google Scholar] [CrossRef] [PubMed]
- Zaia, J.; Boynton, R.E.; McIntosh, A.; Marshak, D.R.; Olsson, H.; Heinegard, D.; Barry, F.P. Post-translational modifications in cartilage oligomeric matrix protein. Characterization of the N-linked oligosaccharides by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Biol. Chem. 1997, 272, 14120–14126. [Google Scholar] [CrossRef]
- Mann, H.; Ozbek, S.; Engel, J.; Paulsson, M.; Wagener, R. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J. Biol. Chem. 2004, 279, 25294–25298. [Google Scholar] [CrossRef]
- Pihlajamaa, T.; Lankinen, H.; Ylostalo, J.; Valmu, L.; Jaalinoja, J.; Zaucke, F.; Spitznagel, L.; Gösling, S.; Puustinen, A.; Mörgelin, M.; et al. Characterization of recombinant amino-terminal NC4 domain of human collagen IX: Interaction with glycosaminoglycans and cartilage oligomeric matrix protein. J. Biol. Chem. 2004, 279, 24265–24273. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, J.; Köhler, A.; Dietmar, H.; Gompert, M.; Neundorf, I.; Zaucke, F.; Koch, M.; Baumann, U. COMP and TSP-4 interact specifically with the novel GXKGHR motif only found in fibrillar collagens. Sci. Rep. 2018, 8, 17187. [Google Scholar] [CrossRef]
- Halasz, K.; Kassner, A.; Morgelin, M.; Heinegard, D. COMP acts as a catalyst in collagen fibrillogenesis. J. Biol. Chem. 2007, 282, 31166–31173. [Google Scholar] [CrossRef]
- Sodersten, F.; Ekman, S.; Schmitz, M.; Paulsson, M.; Zaucke, F. Thrombospondin-4 and cartilage oligomeric matrix protein form heterooligomers in equine tendon. Connect. Tissue Res. 2006, 47, 85–91. [Google Scholar] [CrossRef]
- Birch, H.; Thorpe, C.T.; Rumian, A.P. Specialisation of extracellular matrix for function in tendons and ligaments. Muscles Ligaments Tendons 2013, 3, 12–22. [Google Scholar] [CrossRef]
- Müller, G.; Michel, A.; Altenburg, E. COMP (cartilage oligomeric matrix protein) is synthesized in ligament, tendon, meniscus, and articular cartilage. Connect. Tissue Res. 1998, 39, 233–244. [Google Scholar] [CrossRef]
- Smith, R.; Zunino, L.; Webbon, P.M.; Heinegard, D. The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 1997, 16, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Gerard, M.; Dowling, B.; Dart, A.J.; Birch, H.L.; Goodship, A.E. Correlation of cartilage oligomeric matrix protein (COMP) levels in equine tendon with mechanical properties: A proposed role for COMP in determining function-specific mechanical characteristics of locomotor tendons. Equine Vet. J. Suppl. 2002, 34, 241–244. [Google Scholar] [CrossRef]
- Giannoni, P.; Siegrist, M.; Hunziker, E.B.; Wong, M. The mechanosensitivity of cartilage oligomeric matrix protein (COMP). Biorheology 2003, 40, 101–109. [Google Scholar] [CrossRef]
- van Oers, R.; Rens, E.G.; LaValley, D.J.; Reinhart-King, C.A.; Merks, R.M.H. Mechanical Cell-Matrix Feedback Explains Pairwise and Collective Endothelial Cell Behavior In Vitro. PLoS Comput. Biol. 2014, 10, e1003774. [Google Scholar] [CrossRef]
- Posey, K.; Hecht, J.T. The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr. Drug Targets 2008, 9, 869–877. [Google Scholar] [CrossRef]
- Destouni, A.; Tsolis, K.C.; Economou, A.; Papathanasiou, I.; Balis, C.; Mourmoura, E.; Tsezou, A. Chondrocyte protein co-synthesis network analysis links ECM mechanosensing to metabolic adaptation in osteoarthritis. Expert Rev. Proteom. 2021, 18, 623–635. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, J. Cartilage Oligomeric Matrix Protein, Diseases, and Therapeutic Opportunities. Int. J. Mol. Sci. 2022, 23, 9253. [Google Scholar] [CrossRef]
- Farina, G.; Lemaire, R.; Korn, J.H.; Widom, R.L. Cartilage oligomeric matrix protein is overexpressed by scleroderma dermal fibroblasts. Matrix Biol. 2006, 25, 213–222. [Google Scholar] [CrossRef]
- Inui, S.; Shono, F.; Nakajima, T.; Hosokawa, K.; Itami, S. Identification and characterization of cartilage oligomeric matrix protein as a novel pathogenic factor in keloids. Am. J. Pathol. 2011, 179, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Schulz, J.N.; Blumbach, K.; Andreasson, K.; Heinegård, D.; Paulsson, M.; Mauch, C.; Eming, S.A.; Eckes, B.; Krieg, T. Enhanced deposition of cartilage oligomeric matrix protein is a common feature in fibrotic skin pathologies. Matrix Biol. 2013, 32, 325–331. [Google Scholar] [CrossRef]
- Adams, J.; Lawler, J. The thrombospondins. Int. J. Biochem. Cell Biol. 2004, 36, 961–968. [Google Scholar] [CrossRef]
- Carlson, C.; Lawler, J.; Mosher, D.F. Structures of thrombospondins. Cell. Mol. Life Sci. 2008, 65, 672–686. [Google Scholar] [CrossRef]
- Ishida, K.; Acharya, C.; Christiansen, B.A.; Yik, J.H.; DiCesare, P.E.; Haudenschild, D.R. Cartilage oligomeric matrix protein enhances osteogenesis by directly binding and activating bone morphogenetic protein-2. Bone 2013, 55, 23–35. [Google Scholar] [CrossRef]
- Caron, M.M.J.; Janssen, M.P.F.; Peeters, L.; Haudenschild, D.R.; Cremers, A.; Surtel, D.A.M.; van Rhijn, L.W.; Emans, P.J.; Welting, T.J.M. Aggrecan and COMP Improve Periosteal Chondrogenesis by Delaying Chondrocyte Hypertrophic Maturation. Front. Bioeng. Biotechnol. 2020, 8, 1036. [Google Scholar] [CrossRef]
- Tran, V.; Karsai, A.; Fong, M.C.; Cai, W.; Yik, J.H.N.; Klineberg, E.; Haudenschild, D.R.; Liu, G.-Y. Label-Free and Direct Visualization of Multivalent Binding of Bone Morphogenetic Protein-2 with Cartilage Oligomeric Matrix Protein. J. Phys. Chem. B 2019, 123, 39–46. [Google Scholar] [CrossRef]
- Tran, V.; Karsai, A.; Fong, M.C.; Cai, W.; Fraley, J.G.; Yik, J.H.; Klineberg, E.; Haudenschild, D.R.; Liu, G.-Y. Direct Visualization of the Binding of Transforming Growth Factor Beta 1 with Cartilage Oligomeric Matrix Protein via High-Resolution Atomic Force Microscopy. J. Phys. Chem. B 2020, 124, 9497–9504. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kawabata, K.; Kusaka-Kikushima, A.; Sugiyama, Y.; Mabuchi, T.; Takekoshi, S.; Miyasaka, M.; Ozawa, A.; Sakai, S. Cartilage Oligomeric Matrix Protein Increases in Photodamaged Skin. J. Investg. Dermatol. 2016, 136, 1143–1149. [Google Scholar] [CrossRef]
- Papadakos, K.; Hagerling, C.; Rydén, L.; Larsson, A.M.; Blom, A.M. High Levels of Expression of Cartilage Oligomeric Matrix Protein in Lymph Node Metastases in Breast Cancer Are Associated with Reduced Survival. Cancers 2021, 13, 5876. [Google Scholar] [CrossRef] [PubMed]
- Papadakos, K.; Lundgren, S.; Gialeli, C.; Micke, P.; Mezheyeuski, A.; Elebro, J.; Jirström, K.; Blom, A.M. Expression of cartilage oligomeric matrix protein in periampullary adenocarcinoma is associated with pancreatobiliary-type morphology, higher levels of fibrosis and immune cell exclusion. Oncoimmunology 2022, 11, 2111906. [Google Scholar] [CrossRef] [PubMed]
- Cecil, D.; Appleton, C.T.; Polewski, M.D.; Mort, J.S.; Schmidt, A.M.; Bendele, A.; Beier, F.; Terkeltaub, R. The pattern recognition receptor CD36 is a chondrocyte hypertrophy marker associated with suppression of catabolic responses and promotion of repair responses to inflammatory stimuli. J. Immunol. 2009, 182, 5024–5031. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.; Brantsing, C.; Egell, S.; Lindahl, A. Notch1, Jagged1, and HES5 are abundantly expressed in osteoarthritis. Cells Tissues Organs 2008, 188, 287–298. [Google Scholar] [CrossRef]
- Shimizu, T.; Okafuji, N.; Nakano, K.; Kurihara, S.; Kawakami, T. Jagged1 peptide appearing in mandibular condylar cartilage development. Eur. J. Med. Res. 2008, 13, 4–6. [Google Scholar]
- Oldershaw, R.; Hardingham, T.E. Notch signaling during chondrogenesis of human bone marrow stem cells. Bone 2010, 46, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Fu, S.; Xie, Y.; Zhang, C.; Wu, X. Piezo1-driven mechanotransduction as a key regulator of cartilage degradation in early osteoarthritis. Biomol. Biomed. 2025, 25, 905–913. [Google Scholar] [CrossRef]
- Kawakami, Y.; Matsuo, K.; Murata, M.; Yudoh, K.; Nakamura, H.; Shimizu, H.; Beppu, M.; Inaba, Y.; Saito, T.; Kato, T.; et al. Expression of Angiotensin II Receptor-1 in Human Articular Chondrocytes. Arthritis 2012, 2012, 648537. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, H.J.; Bae, I.H.; Lee, K.N.; Lee, K.Y.; Oh, W.M.; Kim, S.H.; Kang, I.C.; Lee, S.E.; Koh, G.Y.; et al. COMP-Ang1, a chimeric form of Angiopoietin 1, enhances BMP2-induced osteoblast differentiation and bone formation. Bone 2010, 46, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Kipnes, J.; Carlberg, A.L.; Loredo, G.A.; Lawler, J.; Tuan, R.S.; Hall, D.J. Effect of cartilage oligomeric matrix protein on mesenchymal chondrogenesis in vitro. Osteoarthr. Cartil. 2003, 11, 442–454, Erratum in Osteoarthr. Cartil. 2003, 11, 831–835. [Google Scholar] [CrossRef][Green Version]
- Riessen, R.; Fenchel, M.; Chen, H.; Axel, D.I.; Karsch, K.R.; Lawler, J. Cartilage oligomeric matrix protein (thrombospondin-5) is expressed by human vascular smooth muscle cells. Arter. Thromb. Vasc. Biol. 2001, 21, 47–54. [Google Scholar] [CrossRef]
- Fukuhara, S.; Sako, K.; Noda, K.; Zhang, J.; Minami, M.; Mochizuki, N. Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol. Histopathol. 2010, 25, 387–396. [Google Scholar] [PubMed]
- Morisada, T.; Oike, Y.; Yamada, Y.; Urano, T.; Akao, M.; Kubota, Y.; Maekawa, H.; Kimura, Y.; Ohmura, M.; Miyamoto, T.; et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood 2005, 105, 4649–4656. [Google Scholar] [CrossRef]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- Kim, H.; Jung, K.; Kim, H.M.; Cheng, Y.; Koh, G.Y. A designed angiopoietin-2 variant, pentameric COMP-Ang2, strongly activates Tie2 receptor and stimulates angiogenesis. Biochim. Biophys. Acta 2009, 1793, 772–780. [Google Scholar] [CrossRef][Green Version]
- Wallace, R.G.; Rochfort, K.D.; Barabas, P.; Curtis, T.M.; Uehara, H.; Ambati, B.K.; Cummins, P.M. COMP-Ang1: Therapeutic potential of an engineered Angiopoietin-1 variant. Vasc. Pharmacol. 2021, 141, 106919. [Google Scholar] [CrossRef]
- Vuga, L.; Milosevic, J.; Pandit, K.; Ben-Yehudah, A.; Chu, Y.; Richards, T.; Sciurba, J.; Myerburg, M.; Zhang, Y.; Parwani, A.V.; et al. Cartilage oligomeric matrix protein in idiopathic pulmonary fibrosis. PLoS ONE 2013, 8, e83120. [Google Scholar] [CrossRef]
- Henrotin, Y.; Sanchez, C.; Bay-Jensen, A.C.; Mobasheri, A. Osteoarthritis biomarkers derived from cartilage extracellular matrix: Current status and future perspectives. Ann. Phys. Rehabil. Med. 2016, 59, 145–148. [Google Scholar] [CrossRef]
- Hoch, J.; Mattacola, C.G.; Medina McKeon, J.M.; Howard, J.S.; Lattermann, C. Serum cartilage oligomeric matrix protein (sCOMP) is elevated in patients with knee osteoarthritis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2011, 19, 1396–1404. [Google Scholar] [CrossRef]
- Kluzek, S.; Bay-Jensen, A.C.; Judge, A.; Karsdal, M.A.; Shorthose, M.; Spector, T.; Hart, D.; Newton, J.L.; Arden, N.K. Serum cartilage oligomeric matrix protein and development of radiographic and painful knee osteoarthritis. A community-based cohort of middle-aged women. Biomarkers 2015, 20, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Laudon, J.; Whittaker, J.L.; Ren, G.; Jaremko, J.L.; Emery, C.A.; Krawetz, R.J. Serum cartilage oligomeric matrix protein (COMP) expression in individuals who sustained a youth sport-related intra-articular knee injury 3–10 years previously and uninjured matched controls. Osteoarthr. Cartil. 2019, 27, 286–293. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Mookkhan, N.; Tabtimnark, T.; Aramruang, T.; Ungsudechachai, T.; Saengsiwaritt, W.; Jittikoon, J.; Chaikledkaew, U.; Honsawek, S. Cartilage oligomeric matrix protein as a potential biomarker for knee osteoarthritis. Bone Jt. Res. 2024, 13, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Jordan, J.M.; Vilim, V.; Renner, J.B.; Dragomir, A.D.; Luta, G.; Kraus, V.B. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: The Johnston County Osteoarthritis Project. Arthritis Rheum. 1999, 42, 2356–2364. [Google Scholar] [CrossRef]
- Vilím, V.; Vytásek, R.; Olejárová, M.; Machácek, S.; Gatterová, J.; Procházka, B.; Kraus, V.B.; Pavelka, K. Serum cartilage oligomeric matrix protein reflects the presence of clinically diagnosed synovitis in patients with knee osteoarthritis. Osteoarthr. Cartil. 2001, 9, 612–618. [Google Scholar] [CrossRef]
- Vilím, V.; Olejárová, M.; Machácek, S.; Gatterová, J.; Kraus, V.B.; Pavelka, K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthr. Cartil. 2002, 10, 707–713. [Google Scholar] [CrossRef]
- Wisłowska, M.; Jabłońska, B. Cartilage oligomeric matrix protein in serum in systemic lupus erythematosus and knee osteoarthritis. Prelim. Commun. Rheumatol. Int. 2005, 25, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Thorstensson, C.A.; Roos, E.M.; Petersson, I.F.; Heinegård, D.; Saxne, T. Serum levels of cartilage oligomeric matrix protein (COMP) increase temporarily after physical exercise in patients with knee osteoarthritis. BMC Musculoskelet. Disord. 2006, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Pucinelli, M.L.; da Silva, N.P.; Feldman, D. Serum cartilage oligomeric matrix protein (COMP) levels in knee osteoarthritis in a Brazilian population: Clinical and radiological correlation. Scand. J. Rheumatol. 2007, 36, 211–215. [Google Scholar] [CrossRef]
- Lotz, M.; Martel-Pelletier, J.; Christiansen, C.; Brandi, M.L.; Bruyère, O.; Chapurlat, R.; Collette, J.; Cooper, C.; Giacovelli, G.; Kanis, J.A.; et al. Value of biomarkers in osteoarthritis: Current status and perspectives. Ann. Rheum. Dis. 2013, 72, 1756–1763, Erratum in Ann. Rheum. Dis. 2017, 76, e35. [Google Scholar] [CrossRef]
- Ben Achour, W.; Bouaziz, M.; Mechri, M.; Zouari, B.; Bahlous, A.; Abdelmoula, L.; Laadhar, L.; Sellami, M.; Sahli, H.; Cheour, E. A cross sectional study of bone and cartilage biomarkers: Correlation with structural damage in rheumatoid arthritis. Libyan J. Med. 2018, 13, 1512330. [Google Scholar] [CrossRef]
- Georgiev, T.; Ivanova Bi, X. Correlation of serum cartilage oligomeric matrix protein with knee osteoarthritis diagnosis: A meta-analysis. J. Orthop. Surg. Res. 2018, 13, 262. [Google Scholar] [CrossRef]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2014, 802, 31–47. [Google Scholar]
- Hultman, K.; Edsfeldt, A.; Björkbacka, H.; Dunér, P.; Sundius, L.; Nitulescu, M.; Persson, A.; Boyle, J.J.; Nilsson, J.; Hultgårdh-Nilsson, A.; et al. Cartilage Oligomeric Matrix Protein Associates With a Vulnerable Plaque Phenotype in Human Atherosclerotic Plaques. Stroke 2019, 50, 3289–3292. [Google Scholar] [CrossRef]
- Sandstedt, J.; Vargmar, K.; Björkman, K.; Ruetschi, U.; Bergström, G.; Hultén, L.M.; Skiöldebrand, E. COMP (Cartilage Oligomeric Matrix Protein) Neoepitope: A Novel Biomarker to Identify Symptomatic Carotid Stenosis. Arter. Thromb. Vasc. Biol. 2021, 41, 1218–1228. [CrossRef]
- Hecht, J.; Deere, M.; Putnam, E.; Cole, W.; Vertel, B.; Chen, H.; Lawler, J. Characterization of cartilage oligomeric matrix protein (COMP) in human normal and pseudoachondroplasia musculoskeletal tissues. Matrix Biol. 1998, 17, 269–278. [Google Scholar] [CrossRef]
- Hecht, J.; Chiu, F.; Veerisetty, A.; Hossain, M.; Posey, K.L. Health consequences of mutant cartilage oligomeric matrix protein and its relationship to abnormal growth and joint degeneration. Matrix Biol. 2023, 119, 101–111. [Google Scholar] [CrossRef]
- Merritt, T.; Alcorn, J.L.; Haynes, R.; Hecht, J.T. Expression of mutant cartilage oligomeric matrix protein in human chondrocytes induces the pseudoachondroplasia phenotype. J. Orthop. Res. 2006, 24, 700–707. [Google Scholar] [CrossRef]
- Blom, A. The role of complement inhibitors beyond controlling inflammation. J. Intern. Med. 2017, 282, 116–128. [Google Scholar] [CrossRef]
- Grevenstein, D.; Heilig, J.; Dargel, J.; Oppermann, J.; Eysel, P.; Brochhausen, C.; Niehoff, A. COMP in the Infrapatellar Fat Pad-Results of a Prospective Histological, Immunohistological, and Biochemical Case-Control Study. J. Orthop. Res. 2020, 38, 747–758. [Google Scholar] [CrossRef]
- Hauser, N.; Paulsson, M.; Heinegârd, D.; Mörgelin, M. Interaction of cartilage matrix protein with aggrecan. Increased covalent cross-linking with tissue maturation. J. Biol. Chem. 1996, 271, 32247–32252. [Google Scholar] [CrossRef][Green Version]
- Koelling, S.; Clauditz, T.S.; Kaste, M.; Miosge, N. Cartilage oligomeric matrix protein is involved in human limb development and in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2006, 8, R56. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Hashimoto, Y.; Orita, K.; Nishino, K.; Kinoshita, T.; Iida, K.; Nakamura, H. Longitudinal measurement of serum cartilage oligomeric matrix protein can detect the progression of cartilage degeneration in anterior cruciate ligament reconstruction patients. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2024, 37, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Venn, A.; Blizzard, L.; Fraser, B.J.; Jones, G.; Burgess, J.; Parameswaran, V.; March, L.; Cicuttini, F.; Ding, C.; et al. Association between osteoarthritis-related serum biochemical markers over 10–13 years and knee symptoms in middle-aged adults. Mod. Rheumatol. 2024, 35, 585–593. [Google Scholar] [CrossRef]
- de Groot, R.; Folgado, P.B.; Yamamoto, K.; Martin, D.R.; Koch, C.D.; Debruin, D.; Blagg, S.; Minns, A.F.; Bhutada, S.; Ahnström, J.; et al. Cleavage of Cartilage Oligomeric Matrix Protein (COMP) by ADAMTS4 generates a neoepitope associated with osteoarthritis and other forms of degenerative joint disease. Matrix Biol. 2025, 135, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Lisee, C.; Obudzinski, S.; Pietrosimone, B.G.; Alexander Creighton, R.; Kamath, G.; Longobardi, L.; Loeser, R.; Schwartz, T.A.; Spang, J.T. Association of Serum Biochemical Biomarker Profiles of Joint Tissue Inflammation and Cartilage Metabolism With Posttraumatic Osteoarthritis-Related Symptoms at 12 Months After ACLR. Am. J. Sports Med. 2024, 52, 2503–2511. [Google Scholar] [CrossRef]
- Liu, C.; Kong, W.; Xu, K.; Luan, Y.; Ilalov, K.; Sehgal, B.; Yu, S.; Howell, R.D.; Di Cesare, P.E. ADAMTS-12 associates with and degrades cartilage oligomeric matrix protein. J. Biol. Chem. 2006, 281, 15800–15808. [Google Scholar] [CrossRef]
- Liu, C. The role of ADAMTS-7 and ADAMTS-12 in the pathogenesis of arthritis. Nat. Clin. Pract. Rheumatol. 2009, 5, 38–45. [Google Scholar] [CrossRef]
- Luan, Y.; Kong, L.; Howell, D.R.; Ilalov, K.; Fajardo, M.; Bai, X.H.; Di Cesare, P.E.; Goldring, M.B.; Abramson, S.B.; Liu, C.J. Inhibition of ADAMTS-7 and ADAMTS-12 degradation of cartilage oligomeric matrix protein by alpha-2-macroglobulin. Osteoarthr. Cartil. 2008, 16, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Lai, Y.; Tian, Q.; Lin, E.A.; Kong, L.; Liu, C. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis Rheum. 2010, 62, 2023–2036. [Google Scholar] [CrossRef]
- Stracke, J.; Fosang, A.J.; Last, K.; Mercuri, F.A.; Pendás, A.M.; Llano, E.; Perris, R.; Di Cesare, P.E.; Murphy, G.; Knäuper, V. Matrix metalloproteinases 19 and 20 cleave aggrecan and cartilage oligomeric matrix protein (COMP). FEBS Lett. 2000, 478, 52–56. [Google Scholar] [CrossRef]
- Dickinson, S.; Vankemmelbeke, M.N.; Buttle, D.J.; Rosenberg, K.; Heinegård, D.; Hollander, A.P. Cleavage of cartilage oligomeric matrix protein (thrombospondin-5) by matrix metalloproteinases and a disintegrin and metalloproteinase with thrombospondin motifs. Matrix Biol. 2003, 22, 267–278. [Google Scholar] [CrossRef]
- Raj, A.; Wang, M.; Liu, C.; Ali, L.; Karlsson, N.G.; Claesson, P.M.; Dėdinaitė, A. Molecular synergy in biolubrication: The role of cartilage oligomeric matrix protein (COMP) in surface-structuring of lubricin. J. Colloid Interface Sci. 2017, 495, 200–206. [Google Scholar] [CrossRef]
- Valdes, A.; Meulenbelt, I.; Chassaing, E.; Arden, N.K.; Bierma-Zeinstra, S.; Hart, D.; Hofman, A.; Karsdal, M.; Kloppenburg, M.; Kroon, H.M.; et al. Large scale meta-analysis of urinary C-terminal telopeptide, serum cartilage oligomeric protein and matrix metalloprotease degraded type II collagen and their role in prevalence, incidence and progression of osteoarthritis. Osteoarthr. Cartil. 2014, 22, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Shahi, U.; Gupta, O.P.; Ray, A.; Mahdi, F.; Bajpayi, J. Role of serum levels of cartilage oligomeric matrix protein in diagnosis and assessment of severity of knee osteoarthritis. Osteoarthr. Cartil. 2022, 20, S94–S95. [Google Scholar]
- Mündermann, A.; Dyrby, C.O.; Andriacchi, T.P.; King, K.B. Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthr. Cartil. 2005, 13, 34–38. [Google Scholar] [CrossRef]
- Roberts, H.; Law, R.J.; Thom, J.M. The time course and mechanisms of change in biomarkers of joint metabolism in response to acute exercise and chronic training in physiologic and pathological conditions. Eur. J. Appl. Physiol. 2019, 119, 2401–2420. [Google Scholar] [CrossRef]
- Niehoff, A.; Müller, M.; Brüggemann, L.; Savage, T.; Zaucke, F.; Eckstein, F.; Müller-Lung, U.; Brüggemann, G.P. Deformational behaviour of knee cartilage and changes in serum cartilage oligomeric matrix protein (COMP) after running and drop landing. Osteoarthr. Cartil. 2011, 19, 1003–1010. [Google Scholar] [CrossRef]
- Jayabalan, P.; Darcy, R.; Darbhe, V.; Neuville, Z.; Tjong, V.; Hargrove, L.; Welty, L.; Schnitzer, T. A novel mechanosensitive stress test in individuals following anterior cruciate ligament reconstruction: A pilot study. Osteoarthr Cart. Open 2025, 7, 100619. [Google Scholar] [CrossRef]
- Ramos, Y.F.M.; Metrustry, S.; Arden, N.; Bay-Jensen, A.C.; Beekman, M.; de Craen, A.J.M.; Cupples, L.A.; Esko, T.; Evangelou, E.; Felson, D.T.; et al. Meta-analysis identifies loci affecting levels of the potential osteoarthritis biomarkers sCOMP and uCTX-II with genome wide significance. J. Med. Genet. 2014, 51, 596–604. [Google Scholar] [CrossRef]
- Shah, S. Editorial Commentary: Serum Cartilage Oligomeric Matrix Protein Appears to Be the Most Useful Biomarker for Tracking Early Osteoarthritis of the Knee in Anterior Cruciate Ligament Deficient Patients (But May Also Reflect Synovitis). Arthroscopy 2022, 38, 879–880. [Google Scholar] [CrossRef]
- Bai, B.; Li, Y. Combined detection of serum CTX-II and COMP concentrations in osteoarthritis model rabbits: An effective technique for early diagnosis and estimation of disease severity. J. Orthop. Surg. Res. 2016, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.; Henn, C.M.; Drewniak, E.I.; Lesieur-Brooks, A.; Machan, J.; Crisco, J.J.; Ehrlich, M.G. High dietary fat and the development of osteoarthritis in a rabbit model. Osteoarthr. Cartil. 2012, 20, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Go, E.; Kim, S.A.; Cho, M.L.; Lee, K.S.; Shetty, A.A.; Kim, S.J. A Combination of Surgical and Chemical Induction in a Rabbit Model for Osteoarthritis of the Knee. Tissue Eng. Regen. Med. 2022, 19, 1377–1388. [Google Scholar] [CrossRef]
- Yoshioka, M.; Coutts, R.D.; Amiel, D.; Hacker, S.A. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthr. Cartil. 1996, 4, 87–98. [Google Scholar] [CrossRef]
- Blaker, C.; Clarke, E.C.; Little, C.B. Using mouse models to investigate the pathophysiology, treatment, and prevention of post-traumatic osteoarthritis. J. Orthop. Res. 2017, 35, 424–439. [Google Scholar] [CrossRef]
- Barton, K.; Shekarforoush, M.; Heard, B.J.; Sevick, J.L.; Vakil, P.; Atarod, M.; Martin, R.; Achari, Y.; Hart, D.A.; Frank, C.B.; et al. Use of pre-clinical surgically induced models to understand biomechanical and biological consequences of PTOA development. J. Orthop. Res. 2017, 35, 454–465. [Google Scholar] [CrossRef]
- Fukui, D.; Nishiyama, D.; Yamanaka, M.; Tamai, H.; Nishio, N.; Kawakami, M.; Yamada, H. Development of a Novel Rat Knee Osteoarthritis Model Induced by Medial Meniscus Extrusion. Cartilage 2025, 16, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhang, Z.; Song, X.; Bai, H.; Li, Y.; Li, X.; Zhao, J.; Ma, Y.; Gao, L. Combined detection of COMP and CS846 biomarkers in experimental rat osteoarthritis: A potential approach for assessment and diagnosis of osteoarthritis. J. Orthop. Surg. Res. 2018, 13, 230. [Google Scholar] [CrossRef]
- Reece, D.; Thote, T.; Lin, A.S.P.; Willett, N.J.; Guldberg, R.E. Contrast enhanced μCT imaging of early articular changes in a pre-clinical model of osteoarthritis. Osteoarthr. Cartil. 2018, 26, 118–127. [Google Scholar] [CrossRef]
- Tessier, J.; Bowyer, J.; Brownrigg, N.J.; Peers, I.S.; Westwood, F.R.; Waterton, J.C.; Maciewicz, R.A. Characterisation of the guinea pig model of osteoarthritis by in vivo three-dimensional magnetic resonance imaging. Osteoarthr. Cartil. 2003, 11, 845–853. [Google Scholar] [CrossRef]
- Bendele, A. Animal models of osteoarthritis. J. Musculoskelet. Neuronal Interact. 2001, 1, 363–376. [Google Scholar]
- Marshall, K.; Chan, A.D. Arthroscopic anterior cruciate ligament transection induces canine osteoarthritis. J. Rheumatol. 1996, 23, 338–343. [Google Scholar] [PubMed]
- Veronesi, F.; Berni, M.; Marchiori, G.; Cassiolas, G.; Muttini, A.; Barboni, B.; Martini, L.; Fini, M.; Lopomo, N.F.; Marcacci, M.; et al. Evaluation of cartilage biomechanics and knee joint microenvironment after different cell-based treatments in a sheep model of early osteoarthritis. Int. Orthop. 2021, 45, 427–435. [Google Scholar] [CrossRef]
- Oakley, S.; Lassere, M.N.; Portek, I.; Szomor, Z.; Ghosh, P.; Kirkham, B.W.; Murrell, G.A.; Wulf, S.; Appleyard, R.C. Biomechanical, histologic and macroscopic assessment of articular cartilage in a sheep model of osteoarthritis. Osteoarthr. Cartil. 2004, 12, 667–679. [Google Scholar] [CrossRef]
- Cake, M.; Read, R.A.; Corfield, G.; Daniel, A.; Burkhardt, D.; Smith, M.M.; Little, C.B. Comparison of gait and pathology outcomes of three meniscal procedures for induction of knee osteoarthritis in sheep. Osteoarthr. Cartil. 2013, 21, 226–236. [Google Scholar] [CrossRef]
- Chabronova, A.; Walters, M.; Regårdh, S.; Jacobsen, S.; Bundgaard, L.; Anderson, J.R.; Peffers, M.J. Exploring the roles of snoRNA-induced ribosome heterogeneity in equine osteoarthritis. Front. Vet. Sci. 2025, 12, 1562508. [Google Scholar] [CrossRef] [PubMed]
- Laverty, S.; Girard, C.A.; Williams, J.M.; Hunziker, E.B.; Pritzker, K.P. The OARSI histopathology initiative–recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S53–S65. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S24–S34. [Google Scholar] [CrossRef]
- Cook, J.; Kuroki, K.; Visco, D.; Pelletier, J.P.; Schulz, L.; Lafeber, F.P. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the dog. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S66–S79. [Google Scholar] [CrossRef]
- Kraus, V.; Huebner, J.L.; DeGroot, J.; Bendele, A. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S35–S52. [Google Scholar] [CrossRef] [PubMed]
- Little, C.; Smith, M.M.; Cake, M.A.; Read, R.A.; Murphy, M.J.; Barry, F.P. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S80–S92. [Google Scholar] [CrossRef] [PubMed]
- McIlwraith, C.; Frisbie, D.D.; Kawcak, C.E.; Fuller, C.J.; Hurtig, M.; Cruz, A. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the horse. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S93–S105. [Google Scholar] [CrossRef]
- Hayashi, S.; Schulz, J.-N.; Anja Niehoff, A.; Krieg, T.; Eckes, B.; Hatamochi, A. Cartilage Oligomeric Matrix Protein (COMP) has the important role for the collagen network architecture and elasticity of the skin. J. Dermatol. Sci. 2016, 84, e27–e28. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Wang, Y.; Sun, L.; Liu, Z.; Wang, L.; Song, T.; Yao, Y.; Liu, Q.; Tu, K. HSCs-derived COMP drives hepatocellular carcinoma progression by activating MEK/ERK and PI3K/AKT signaling pathways. J. Exp. Clin. Cancer Res. 2018, 37, 231. [Google Scholar] [CrossRef]
- Nfonsam, V.; Jecius, H.C.; Janda, J.; Omesiete, P.N.; Elquza, E.; Scott, A.J.; Nfonsam, L.E.; Jandova, J. Cartilage oligomeric matrix protein (COMP) promotes cell proliferation in early-onset colon cancer tumorigenesis. Surg. Endosc. 2020, 34, 3992–3998. [Google Scholar] [CrossRef]
- Issack, P.; Liu, C.J.; Prazak, L.; Di Cesare, P.E. A silencer element in the cartilage oligomeric matrix protein gene regulates chondrocyte-specific expression. J. Orthop. Res. 2004, 22, 751–758. [Google Scholar] [CrossRef]
- Cai, X.; Li, M.; Zhong, Y.; Yang, W.; Liang, Z. COMP Improves Ang-II-Induced Atrial Fibrillation via TGF-β Signaling Pathway. Cardiovasc. Toxicol. 2023, 23, 305–316. [Google Scholar] [CrossRef]
- Magdaleno, F.; Arriazu, E.; Ruiz de Galarreta, M.; Chen, Y.; Ge, X.; Conde de la Rosa, L.; Nieto, N. Cartilage oligomeric matrix protein participates in the pathogenesis of liver fibrosis. J. Hepatol. 2016, 65, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Qiu, Q.; Tan, D.; Chen, Q.; Liu, Y.; Chen, B.; Wang, M. The Cancer-Associated Fibroblasts-Related Gene COMP Is a Novel Predictor for Prognosis and Immunotherapy Efficacy and Is Correlated with M2 Macrophage Infiltration in Colon Cancer. Biomolecules 2022, 13, 62. [Google Scholar] [CrossRef]
- Ding, J.; Zhou, H.N.; Huang, Y.F.; Peng, J.; Huang, H.Y.; Yi, H.; Zong, Z.; Ning, Z.K. TGF-β Pathways Stratify Colorectal Cancer into Two Subtypes with Distinct Cartilage Oligomeric Matrix Protein (COMP) Expression-Related Characteristics. Biomolecules 2022, 12, 1877. [Google Scholar] [CrossRef] [PubMed]
- Happonen, K.; Saxne, T.; Aspberg, A.; Mörgelin, M.; Heinegård, D.; Blom, A.M. Regulation of complement by cartilage oligomeric matrix protein allows for a novel molecular diagnostic principle in rheumatoid arthritis. Arthritis Rheum. 2010, 62, 3574–3583. [Google Scholar] [CrossRef]
- Neidhart, M.; Hauser, N.; Paulsson, M.; DiCesare, P.E.; Michel, B.A.; Häuselmann, H.J. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br. J. Rheumatol. 1997, 36, 1151–1160. [Google Scholar] [CrossRef]
- Lai, Y.; Yu, X.P.; Zhang, Y.; Tian, Q.; Song, H.; Mucignat, M.T.; Perris, R.; Samuels, J.; Krasnokutsky, S.; Attur, M.; et al. Enhanced COMP catabolism detected in serum of patients with arthritis and animal disease models through a novel capture ELISA. Osteoarthr. Cartil. 2012, 20, 854–862. [Google Scholar] [CrossRef][Green Version]
- Dakin, S.; Smith, R.K.; Heinegård, D.; Önnerfjord, P.; Khabut, A.; Dudhia, J. Proteomic analysis of tendon extracellular matrix reveals disease stage-specific fragmentation and differential cleavage of COMP (cartilage oligomeric matrix protein). J. Biol. Chem. 2014, 289, 4919–4927. [Google Scholar] [CrossRef]
- Firner, S.; Zaucke, F.; Heilig, J.; de Marées, M.; Willwacher, S.; Brüggemann, G.P.; Niehoff, A. Impact of knee joint loading on fragmentation of serum cartilage oligomeric matrix protein. J. Orthop. Res. 2020, 38, 1710–1718. [Google Scholar] [CrossRef]
- Di Cesare, P.; Carlson, C.S.; Stolerman, E.S.; Hauser, N.; Tulli, H.; Paulsson, M. Increased degradation and altered tissue distribution of cartilage oligomeric matrix protein in human rheumatoid and osteoarthritic cartilage. J. Orthop. Res. 1996, 14, 946–955. [Google Scholar] [CrossRef]
- Åhrman, E.; Lorenzo, P.; Holmgren, K.; Grodzinsky, A.J.; Dahlberg, L.E.; Saxne, T.; Heinegård, D.; Önnerfjord, P. Novel cartilage oligomeric matrix protein (COMP) neoepitopes identified in synovial fluids from patients with joint diseases using affinity chromatography and mass spectrometry. J. Biol. Chem. 2014, 289, 20908–20916. [Google Scholar] [CrossRef]
- Miller, R.; Ishihara, S.; Tran, P.B.; Golub, S.B.; Last, K.; Miller, R.J.; Fosang, A.J.; Malfait, A.M. An aggrecan fragment drives osteoarthritis pain through Toll-like receptor 2. JCI Insight 2018, 3, e95704. [Google Scholar] [CrossRef]
- Lees, S.; Golub, S.B.; Last, K.; Zeng, W.; Jackson, D.C.; Sutton, P.; Fosang, A.J. Bioactivity in an Aggrecan 32-mer Fragment Is Mediated via Toll-like Receptor 2. Arthritis Rheumatol. 2015, 67, 1240–1249. [Google Scholar] [CrossRef]
- Liu, N.; Lapcevich, R.K.; Underhill, C.B.; Han, Z.; Gao, F.; Swartz, G.; Plum, S.M.; Zhang, L.; Green, S.J. Metastatin: A hyaluronan-binding complex from cartilage that inhibits tumor growth. Cancer Res. 2001, 61, 1022–1028. [Google Scholar]
- Stanton, H.; Melrose, J.; Little, C.B.; Fosang, A.J. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim. Biophys. Acta. 2011, 1812, 1616–1629. [Google Scholar] [CrossRef]
- Calabro, A.; Hascall, V.C.; Caterson, B. Monoclonal antibodies directed against epitopes within the core protein structure of the large aggregating proteoglycan (aggrecan) from the swarm rat chondrosarcoma. Arch. Biochem. Biophys. 1992, 298, 349–360. [Google Scholar] [CrossRef]
- Hughes, C.; Caterson, B.; Fosang, A.J.; Roughley, P.J.; Mort, J.S. Monoclonal antibodies that specifically recognize neoepitope sequences generated by ‘aggrecanase’ and matrix metalloproteinase cleavage of aggrecan: Application to catabolism in situ and in vitro. Biochem. J. 1995, 305, 799–804. [Google Scholar] [CrossRef]
- Nguyen, Q.; Murphy, G.; Hughes, C.E.; Mort, J.S.; Roughley, P.J. Matrix metalloproteinases cleave at two distinct sites on human cartilage link protein. Biochem. J. 1993, 295, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Alaqeel, M.; Grant, M.P.; Epure, L.M.; Salem, O.; AlShaer, A.; Huk, O.L.; Bergeron, S.G.; Zukor, D.J.; Kc, R.; Im, H.J.; et al. Link N suppresses interleukin-1β-induced biological effects on human osteoarthritic cartilage. Eur. Cells Mater. 2020, 39, 65–76. [Google Scholar] [CrossRef]
- Antoniou, J.; Epure, L.M.; Grant, M.P.; Richard, H.; Sampalis, J.; Roughley, P.J.; Laverty, S.; Mwale, F. Short link N acts as a disease modifying osteoarthritis drug. Eur. Cells Mater. 2019, 37, 347–359. [Google Scholar] [CrossRef]
- Tendulkar, G.; Ehnert, S.; Sreekumar, V.; Chen, T.; Kaps, H.P.; Golombek, S.; Wendel, H.P.; Nüssler, A.K.; Avci-Adali, M. Exogenous Delivery of Link N mRNA into Chondrocytes and MSCs-The Potential Role in Increasing Anabolic Response. Int. J. Mol. Sci. 2019, 20, 1716. [Google Scholar] [CrossRef]
- McKenna, L.; Liu, H.; Sansom, P.A.; Dean, M.F. An N-terminal peptide from link protein stimulates proteoglycan biosynthesis in human articular cartilage in vitro. Arthritis Rheum. 1998, 41, 157–162. [Google Scholar] [CrossRef]
- Liu, H.; McKenna, L.A.; Dean, M. An N-terminal peptide from link protein stimulates synthesis of cartilage proteoglycans. Biochem. Soc. Trans. 1997, 25, 427S. [Google Scholar] [CrossRef]
- Liu, H.; McKenna, L.A.; Dean, M.F. The macromolecular characteristics of cartilage proteoglycans do not change when synthesis is up-regulated by link protein peptide. Biochim. Biophys. Acta. 1999, 1428, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Weitzmann, M.N.; Sangadala, S.; Hutton, W.C.; Yoon, S.T. Link protein N-terminal peptide binds to bone morphogenetic protein (BMP) type II receptor and drives matrix protein expression in rabbit intervertebral disc cells. J. Biol. Chem. 2013, 288, 28243–28253. [Google Scholar] [CrossRef]
- Mummert, M.; Mohamadzadeh, M.; Mummert, D.I.; Mizumoto, N.; Takashima, A. Development of a peptide inhibitor of hyaluronan-mediated leukocyte trafficking. J. Exp. Med. 2000, 192, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Morris, M.; Heitz, F.; Divita, G.; Morris, M.C. The peptide carrier Pep-1 forms biologically efficient nanoparticle complexes. Biochem. Biophys. Res. Commun. 2007, 355, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Chen, F.; Wu, Y. Recombinant PEP-1-SOD1 improves functional recovery after neural stem cell transplantation in rats with traumatic brain injury. Exp. Ther. Med. 2018, 15, 2929–2935. [Google Scholar] [CrossRef]
- Yune, T.; Lee, J.Y.; Jiang, M.H.; Kim, D.W.; Choi, S.Y.; Oh, T.H. Systemic administration of PEP-1-SOD1 fusion protein improves functional recovery by inhibition of neuronal cell death after spinal cord injury. Free Radic. Biol. Med. 2008, 45, 1190–1200. [Google Scholar] [CrossRef]
- Youn, J.; Kim, D.W.; Kim, S.T.; Park, S.Y.; Yeo, E.J.; Choi, Y.J.; Lee, H.R.; Kim, D.S.; Cho, S.W.; Han, K.H.; et al. PEP-1-HO-1 prevents MPTP-induced degeneration of dopaminergic neurons in a Parkinson’s disease mouse model. BMB Rep. 2014, 47, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Luo, S.; Li, Z.; Liang, F.; Zhu, Y.; Pei, Z.; Huang, R. Intravenous PEP-1-GDNF is protective after focal cerebral ischemia in rats. Neurosci. Lett. 2016, 617, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Kim, D.W.; Shin, M.J.; Kim, H.R.; Kim, S.M.; Woo, S.J.; Eom, S.A.; Jo, H.S.; Kim, D.S.; Cho, S.W.; et al. PEP-1-PEA-15 protects against toxin-induced neuronal damage in a mouse model of Parkinson’s disease. Biochim. Biophys. Acta. 2014, 1840, 1686–1700. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, H.Y.; Eum, W.S.; Kim, D.W.; Shin, M.J.; Ahn, E.H.; Kim, S.J.; Lee, C.H.; Yong, J.I.; Ryu, E.J.; et al. Neuroprotective effects of PEP-1-carbonyl reductase 1 against oxidative-stress-induced ischemic neuronal cell damage. Free Radic. Biol. Med. 2014, 69, 181–196. [Google Scholar] [CrossRef]
- Ahn, E.; Kim, D.W.; Shin, M.J.; Kim, Y.N.; Kim, H.R.; Woo, S.J.; Kim, S.M.; Kim, D.S.; Kim, J.; Park, J.; et al. PEP-1-ribosomal protein S3 protects dopaminergic neurons in an MPTP-induced Parkinson’s disease mouse model. Free Radic. Biol. Med. 2013, 55, 36–45. [Google Scholar] [CrossRef]
- Kim, M.; Park, M.; Kim, D.W.; Shin, M.J.; Son, O.; Jo, H.S.; Yeo, H.J.; Cho, S.B.; Park, J.H.; Lee, C.H.; et al. Transduced PEP-1-PON1 proteins regulate microglial activation and dopaminergic neuronal death in a Parkinson’s disease model. Biomaterials 2015, 64, 45–56. [Google Scholar] [CrossRef]
- Kim, D.; Sohn, E.J.; Kim, D.W.; Kim, Y.N.; Eom, S.A.; Yoon, G.H.; Cho, S.W.; Lee, S.H.; Hwang, H.S.; Cho, Y.S.; et al. PEP-1-p18 prevents neuronal cell death by inhibiting oxidative stress and Bax expression. BMB Rep. 2012, 45, 532–537. [Google Scholar] [CrossRef]
- Jeong, H.; Yoo, D.Y.; Kim, D.W.; Yeo, H.J.; Cho, S.B.; Hyeon, J.; Park, J.H.; Park, J.; Eum, W.S.; Hwang, H.S.; et al. Neuroprotective effect of PEP-1-peroxiredoxin2 on CA1 regions in the hippocampus against ischemic insult. Biochim. Biophys. Acta 2014, 1840, 2321–2330. [Google Scholar] [CrossRef]
- Cho, J.; Hwang, I.K.; Yoo, K.Y.; Kim, S.Y.; Kim, D.W.; Kwon, Y.G.; Choi, S.Y.; Won, M.H. Effective delivery of Pep-1-cargo protein into ischemic neurons and long-term neuroprotection of Pep-1-SOD1 against ischemic injury in the gerbil hippocampus. Neurochem. Int. 2008, 52, 659–668. [Google Scholar] [CrossRef]
- Wang, B.; Lv, L.; Wang, Z.; Zhao, Y.; Wu, L.; Fang, X.; Xu, Q.; Xin, H. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor α2-mediated endocytosis. Biomaterials 2014, 35, 5897–5907. [Google Scholar] [CrossRef]
- Ryu, E.; Kim, D.W.; Shin, M.J.; Jo, H.S.; Park, J.H.; Cho, S.B.; Lee, C.H.; Yeo, H.J.; Yeo, E.J.; Choi, Y.J.; et al. PEP 1 glutaredoxin 1 protects against hippocampal neuronal cell damage from oxidative stress via regulation of MAPK and apoptotic signaling pathways. Mol. Med. Rep. 2018, 18, 2216–2228. [Google Scholar] [CrossRef] [PubMed]
- Nandadasa, S.; Foulcer, S.; Apte, S.S. The multiple, complex roles of versican and its proteolytic turnover by ADAMTS proteases during embryogenesis. Matrix Biol. 2014, 35, 34–41. [Google Scholar] [CrossRef]
- Hope, C.; Emmerich, P.B.; Papadas, A.; Pagenkopf, A.; Matkowskyj, K.A.; Van De Hey, D.R.; Payne, S.N.; Clipson, L.; Callander, N.S.; Hematti, P.; et al. Versican-Derived Matrikines Regulate Batf3-Dendritic Cell Differentiation and Promote T Cell Infiltration in Colorectal Cancer. J. Immunol. 2017, 199, 1933–1941. [Google Scholar] [CrossRef]
- Zeltz, C.; Brézillon, S.; Perreau, C.; Ramont, L.; Maquart, F.X.; Wegrowski, Y. Lumcorin: A leucine-rich repeat 9-derived peptide from human lumican inhibiting melanoma cell migration. FEBS Lett. 2009, 583, 3027–3032. [Google Scholar] [CrossRef] [PubMed]
- Pietraszek, K.; Brézillon, S.; Perreau, C.; Malicka-Błaszkiewicz, M.; Maquart, F.X.; Wegrowski, Y. Lumican—Derived peptides inhibit melanoma cell growth and migration. PLoS ONE 2013, 8, e76232. [Google Scholar] [CrossRef]
- Stasiak, M.; Boncela, J.; Perreau, C.; Karamanou, K.; Chatron-Colliet, A.; Proult, I.; Przygodzka, P.; Chakravarti, S.; Maquart, F.X.; Kowalska, M.A.; et al. Lumican Inhibits SNAIL-Induced Melanoma Cell Migration Specifically by Blocking MMP-14 Activity. PLoS ONE 2016, 11, e0150226. [Google Scholar] [CrossRef]
- Brézillon, S.; Pietraszek, K.; Maquart, F.X.; Wegrowski, Y. Lumican effects in the control of tumour progression and their links with metalloproteinases and integrins. FEBS J. 2013, 280, 2369–2381. [Google Scholar] [CrossRef]
- Kao, W.; Zhang, J.; Venkatakrishnan, J.; Chang, S.H.; Yuan, Y.; Yamanaka, O.; Xia, Y.; Gesteira, T.F.; Verma, S.; Coulson-Thomas, V.J.; et al. Lumican/Lumikine Promotes Healing of Corneal Epithelium Debridement by Upregulation of EGFR Ligand Expression via Noncanonical Smad-Independent TGFβ/TBRs Signaling. Cells 2024, 13, 1599. [Google Scholar] [CrossRef]
- Gesteira, T.; Coulson-Thomas, V.J.; Yuan, Y.; Zhang, J.; Nader, H.B.; Kao, W.W. Lumican Peptides: Rational Design Targeting ALK5/TGFBRI. Sci. Rep. 2017, 7, 42057. [Google Scholar] [CrossRef]
- Parry, D.; Dixon, T.W.; Cohen, C. Analysis of the three-alpha-helix motif in the spectrin superfamily of proteins. Biophys. J. 1992, 61, 858–867. [Google Scholar] [CrossRef][Green Version]
- Adepu, S.; Ekman, S.; Leth, J.; Johansson, U.; Lindahl, A.; Skiöldebrand, E. Biglycan neo-epitope (BGN262), a novel biomarker for screening early changes in equine osteoarthritic subchondral bone. Osteoarthr. Cartil. 2022, 30, 1328–1336. [Google Scholar] [CrossRef]
- Adepu, S.; Lord, M.; Hugoh, Z.; Nyström, S.; Mattsson-Hulten, L.; Abrahamsson-Aurell, K.; Lützelschwab, C.; Skiöldebrand, E. Salivary biglycan-neo-epitope-BGN262: A novel surrogate biomarker for equine osteoarthritic sub-chondral bone sclerosis and to monitor the effect of short-term training and surface arena. Osteoarthr. Cart. Open 2023, 5, 100354. [Google Scholar] [CrossRef]
- Genovese, F.; Barascuk, N.; Larsen, L.; Larsen, M.R.; Nawrocki, A.; Li, Y.; Zheng, Q.; Wang, J.; Veidal, S.S.; Leeming, D.J.; et al. Biglycan fragmentation in pathologies associated with extracellular matrix remodeling by matrix metalloproteinases. Fibrogenesis Tissue Repair 2013, 6, 9. [Google Scholar] [CrossRef]
- Melrose, J.; Fuller, E.S.; Roughley, P.J.; Smith, M.M.; Kerr, B.; Hughes, C.E.; Caterson, B.; Little, C.B. Fragmentation of decorin, biglycan, lumican and keratocan is elevated in degenerate human meniscus, knee and hip articular cartilages compared with age-matched macroscopically normal and control tissues. Arthritis Res. Ther. 2008, 10, R79. [Google Scholar] [CrossRef]
- Brown, S.; Melrose, J.; Caterson, B.; Roughley, P.; Eisenstein, S.M.; Roberts, S. A comparative evaluation of the small leucine-rich proteoglycans of pathological human intervertebral discs. Eur. Spine J. 2012, 21 (Suppl. S2), S154–S159. [Google Scholar] [CrossRef]
- Melrose, J.; Smith, S.M.; Fuller, E.S.; Young, A.A.; Roughley, P.J.; Dart, A.; Little, C.B. Biglycan and fibromodulin fragmentation correlates with temporal and spatial annular remodelling in experimentally injured ovine intervertebral discs. Eur. Spine J. 2007, 16, 2193–2205. [Google Scholar] [CrossRef]
- Young, A.; Smith, M.M.; Smith, S.M.; Cake, M.A.; Ghosh, P.; Read, R.A.; Melrose, J.; Sonnabend, D.H.; Roughley, P.J.; Little, C.B. Regional assessment of articular cartilage gene expression and small proteoglycan metabolism in an animal model of osteoarthritis. Arthritis Res. Ther. 2005, 7, R852–R861. [Google Scholar] [CrossRef] [PubMed]
- Fuller, E.; Little, C.B.; Melrose, J. Interleukin-1α induces focal degradation of biglycan and tissue degeneration in an in-vitro ovine meniscal model. Exp. Mol. Pathol. 2016, 101, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Lee, J.W.; Moon, E.J.; Chung, Y.G.; Kim, O.S.; Kim, H.J. Anabolic effects of Peniel 2000, a peptide that regulates TGF-β1 signaling on intervertebral disc degeneration. Spine 2013, 38, E49–E58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wu, S.C.M.; Tam, W.K.; Wong, C.K.; Liao, P.; Cheah, K.S.; Chan, D.; James, A.W.; Leung, V.Y. Biglycan fragment modulates TGF-β activity in intervertebral disc via an eIF6-coupled intracellular path. Sci. Adv. 2025, 11, eadq8545. [Google Scholar] [CrossRef] [PubMed]
- Carrino, D.; Onnerfjord, P.; Sandy, J.D.; Cs-Szabo, G.; Scott, P.G.; Sorrell, J.M.; Heinegård, D.; Caplan, A.I. Age-related changes in the proteoglycans of human skin. Specific cleavage of decorin to yield a major catabolic fragment in adult skin. J. Biol. Chem. 2023, 278, 17566–17572. [Google Scholar] [CrossRef]
- Shu, C.; Flannery, C.R.; Little, C.B.; Melrose, J. Catabolism of Fibromodulin in Developmental Rudiment and Pathologic Articular Cartilage Demonstrates Novel Roles for MMP-13 and ADAMTS-4 in C-terminal Processing of SLRPs. Int. J. Mol. Sci. 2019, 20, 579. [Google Scholar] [CrossRef]
- Heathfield, T.; Onnerfjord, P.; Dahlberg, L.; Heinegård, D. Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J. Biol. Chem. 2004, 279, 6286–6295. [Google Scholar] [CrossRef]
- Mongiat, M.; Sweeney, S.M.; San Antonio, J.D.; Fu, J.; Iozzo, R.V. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J. Biol. Chem. 2003, 278, 4238–4249. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, E.; Reed, C.C.; Bix, G.; Fu, J.; Zhang, Y.; Gopalakrishnan, B.; Greenspan, D.S.; Iozzo, R.V. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J. Biol. Chem. 2005, 280, 7080–7087. [Google Scholar] [CrossRef]
- Melrose, J. Perlecan, a modular instructive proteoglycan with diverse functional properties. Int. J. Biochem. Cell Biol. 2020, 128, 105849. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Pal, N.; Concannon, M.; Paul, M.; Doran, M.; Poluzzi, C.; Sekiguchi, K.; Whitelock, J.M.; Neill, T.; Iozzo, R.V. Endorepellin, the angiostatic module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2): A dual receptor antagonism. J. Biol. Chem. 2011, 286, 25947–25962. [Google Scholar] [CrossRef]
- Douglass, S.; Goyal, A.; Iozzo, R.V. The role of perlecan and endorepellin in the control of tumor angiogenesis and endothelial cell autophagy. Connect. Tissue Res. 2015, 56, 381–391. [Google Scholar] [CrossRef]
- Felbor, U.; Dreier, L.; Bryant, R.A.; Ploegh, H.L.; Olsen, B.R.; Mothes, W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000, 19, 1187–1194. [Google Scholar] [CrossRef]
- Heljasvaara, R.; Nyberg, P.; Luostarinen, J.; Parikka, M.; Heikkilä, P.; Rehn, M.; Sorsa, T.; Salo, T.; Pihlajaniemi, T. Generation of biologically active endostatin fragments from human collagen XVIII by distinct matrix metalloproteases. Exp. Cell Res. 2005, 307, 292–304. [Google Scholar] [CrossRef]
- Zatterstrom, U.; Felbor, U.; Fukai, N.; Olsen, B.R. Collagen XVIII/endostatin structure and functional role in angiogenesis. Cell Struct. Funct. 2000, 25, 97–101. [Google Scholar] [CrossRef]
- Marneros, A.; Keene, D.R.; Hansen, U.; Fukai, N.; Moulton, K.; Goletz, P.L.; Moiseyev, G.; Pawlyk, B.S.; Halfter, W.; Dong, S.; et al. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 2004, 23, 89–99. [Google Scholar] [CrossRef]
- Deininger, M.; Meyermann, R.; Schluesener, H.J. Endostatin/collagen XVIII accumulates in patients with traumatic brain injury. J. Neurotrauma 2006, 23, 1103–1110. [Google Scholar] [CrossRef]
- Monti, E.; Sarto, F.; Sartori, R.; Zanchettin, G.; Löfler, S.; Kern, H.; Narici, M.V.; Zampieri, S. C-terminal agrin fragment as a biomarker of muscle wasting and weakness: A narrative review. J. Cachexia Sarcopenia Muscle 2023, 14, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Fatima, R.; Kim, Y.; Baek, S.; Suram, R.P.; An, S.L.; Hong, Y. C-Terminal Agrin Fragment as a Biomarker for Sarcopenia: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2025, 16, e13707. [Google Scholar] [CrossRef] [PubMed]
- Pratt, J.; De Vito, G.; Narici, M.; Segurado, R.; Pessanha, L.; Dolan, J.; Conroy, J.; Boreham, C. Plasma C-Terminal Agrin Fragment as an Early Biomarker for Sarcopenia: Results From the GenoFit Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2090–2096. [Google Scholar] [CrossRef]
- Fariello, R.; Kucsera, S.; Hettwer, S.; Dahinden, P.; Vrijbloed, J. A Neurotrypsin Resistant Agrin Fragment (NT-1654) Reverts Both Impaired Performance and Muscle Pathology in Transgenic Mice Modelling Sarcopenia (P07.195). Neurology 2012, 78, P07.195. [Google Scholar] [CrossRef]
- Xie, D.; Hui, F.; Meyers, R.; Homandberg, G.A. Cartilage chondrolysis by fibronectin fragments is associated with release of several proteinases: Stromelysin plays a major role in chondrolysis. Arch. Biochem. Biophys. 1994, 311, 205–212. [Google Scholar] [CrossRef]
- Homandberg, G.; Wen, C.; Hui, F. Cartilage damaging activities of fibronectin fragments derived from cartilage and synovial fluid. Osteoarthr. Cartil. 1998, 6, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Homandberg, G. Potential regulation of cartilage metabolism in osteoarthritis by fibronectin fragments. Front. Biosci. 1999, 4, D713–D730. [Google Scholar] [CrossRef]
- Homandberg, G.; Hui, F. High concentrations of fibronectin fragments cause short-term catabolic effects in cartilage tissue while lower concentrations cause continuous anabolic effects. Arch. Biochem. Biophys. 1994, 311, 213–218. [Google Scholar] [CrossRef]
- Hwang, H.; Park, S.J.; Cheon, E.J.; Lee, M.H.; Kim, H.A. Fibronectin fragment-induced expression of matrix metalloproteinases is mediated by MyD88-dependent TLR-2 signaling pathway in human chondrocytes. Arthritis Res. Ther. 2015, 17, 320. [Google Scholar] [CrossRef]
- He, J.; Steffen, J.H.; Thulstrup, P.W.; Pedersen, J.N.; Sauerland, M.B.; Otzen, D.E.; Hawkins, C.L.; Gourdon, P.; Davies, M.J.; Hägglund, P. Anastellin impacts on the processing of extracellular matrix fibronectin and stimulates release of cytokines from coronary artery smooth muscle cells. Sci. Rep. 2022, 12, 22051. [Google Scholar] [CrossRef] [PubMed]
- Briknarová, K.; Akerman, M.E.; Hoyt, D.W.; Ruoslahti, E.; Ely, K.R. Anastellin, an FN3 fragment with fibronectin polymerization activity, resembles amyloid fibril precursors. J. Mol. Biol. 2003, 332, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ambesi, A.; McKeown-Longo, P.J. Anastellin, the angiostatic fibronectin peptide, is a selective inhibitor of lysophospholipid signaling. Mol. Cancer Res. 2009, 7, 255–265. [Google Scholar] [CrossRef]
- Negishi, Y.; Nomizu, M. Laminin-derived peptides: Applications in drug delivery systems for targeting. Pharmacol. Ther. 2019, 202, 91–97. [Google Scholar] [CrossRef]
- Yazlovitskaya, E.; Viquez, O.M.; Tu, T.; De Arcangelis, A.; Georges-Labouesse, E.; Sonnenberg, A.; Pozzi, A.; Zent, R. The laminin binding α3 and α6 integrins cooperate to promote epithelial cell adhesion and growth. Matrix Biol. 2019, 77, 101–116. [Google Scholar] [CrossRef]
- Yeo, I.; Min, S.K.; Kang, H.K.; Kwon, T.K.; Jung, S.Y.; Min, B.M. Identification of a bioactive core sequence from human laminin and its applicability to tissue engineering. Biomaterials 2015, 73, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Horton, M.; Spragg, J.H.; Bodary, S.C.; Helfrich, M.H. Recognition of cryptic sites in human and mouse laminins by rat osteoclasts is mediated by beta 3 and beta 1 integrins. Bone 1994, 15, 639–646. [Google Scholar] [CrossRef]
- Nomizu, M.; Song, S.Y.; Kuratomi, Y.; Tanaka, M.; Kim, W.H.; Kleinman, H.K.; Yamada, Y. Active peptides from the carboxyl-terminal globular domain of laminin alpha2 and Drosophila alpha chains. FEBS Lett. 1996, 396, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.; Wu, S.; Li, L.; Ge, R.; Cheng, C.Y. Bioactive fragments of laminin and collagen chains—Lesson from the testis. Reproduction 2019, 159, R111–R123. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, A.; Zhao, S.; Paez, J.I.; Kavyanifar, A.; Salierno, M.; Cavalié, A.; Del Campo, A. In Situ, Light-Guided Axon Growth on Biomaterials via Photoactivatable Laminin Peptidomimetic IK(HANBP)VAV. ACS Appl. Mater. Interfaces 2018, 10, 41129–41137. [Google Scholar] [CrossRef]
- Ziemkiewicz, N.; Talovic, M.; Madsen, J.; Hill, L.; Scheidt, R.; Patel, A.; Haas, G.; Marcinczyk, M.; Zustiak, S.P.; Garg, K. Laminin-111 functionalized polyethylene glycol hydrogels support myogenic activity in vitro. Biomed. Mater. 2018, 13, 065007. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, S.; Feng, J.; Duan, Y.; Xing, D.; Gao, C. Micropatterned biodegradable polyesters clicked with CQAASIKVAV promote cell alignment, directional migration, and neurite outgrowth. Acta Biomater. 2018, 74, 143–155. [Google Scholar] [CrossRef]
- Hayashi, H.; Yamada, M.; Kumai, J.; Takagi, N.; Nomizu, M. Biological activities of laminin-111-derived peptide-chitosan matrices in a primary culture of rat cortical neurons. Arch. Biochem. Biophys. 2018, 648, 53–59. [Google Scholar] [CrossRef]
- Nam, K.; Maruyama, C.L.; Wang, C.S.; Trump, B.G.; Lei, P.; Andreadis, S.T.; Baker, O.J. Laminin-111-derived peptide conjugated fibrin hydrogel restores salivary gland function. PLoS ONE 2017, 12, e0187069. [Google Scholar] [CrossRef]
- Farrukh, A.; Ortega, F.; Fan, W.; Marichal, N.; Paez, J.I.; Berninger, B.; Campo, A.D.; Salierno, M.J. Bifunctional Hydrogels Containing the Laminin Motif IKVAV Promote Neurogenesis. Stem Cell Rep. 2017, 9, 1432–1440. [Google Scholar] [CrossRef]
- Nam, K.; Wang, C.S.; Maruyama, C.L.M.; Lei, P.; Andreadis, S.T.; Baker, O.J. L1 Peptide-Conjugated Fibrin Hydrogels Promote Salivary Gland Regeneration. J. Dent. Res. 2017, 96, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Jones, J.P.; Lei, P.; Andreadis, S.T.; Baker, O.J. Laminin-111 Peptides Conjugated to Fibrin Hydrogels Promote Formation of Lumen Containing Parotid Gland Cell Clusters. Biomacromolecules 2016, 17, 2293–2301. [Google Scholar] [CrossRef]
- Yeo, I.; Min, S.K.; Ki Kang, H.; Kwon, T.K.; Youn Jung, S.; Min, B.M. Adhesion and spreading of osteoblast-like cells on surfaces coated with laminin-derived bioactive core peptides. Data Brief 2015, 5, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, E.; Cristallini, C.; Guerra, G.D.; Barbani, N. Surface chemical immobilization of bioactive peptides on synthetic polymers for cardiac tissue engineering. J. Biomater. Sci. Polym. Ed. 2015, 26, 515–533. [Google Scholar] [CrossRef]
- Kikkawa, Y.; Hozumi, K.; Katagiri, F.; Nomizu, M.; Kleinman, H.K.; Koblinski, J.E. Laminin-111-derived peptides and cancer. Cell Adh. Migr. 2013, 7, 150–256. [Google Scholar] [CrossRef]
- Sugawara, Y.; Hamada, K.; Yamada, Y.; Kumai, J.; Kanagawa, M.; Kobayashi, K.; Toda, T.; Negishi, Y.; Katagiri, F.; Hozumi, K.; et al. Characterization of dystroglycan binding in adhesion of human induced pluripotent stem cells to laminin-511 E8 fragment. Sci. Rep. 2019, 9, 13037. [Google Scholar] [CrossRef]
- Motta, C.; Endres, K.J.; Wesdemiotis, C.; Willits, R.K.; Becker, M.L. Enhancing Schwann cell migration using concentration gradients of laminin-derived peptides. Biomaterials 2019, 218, 119335. [Google Scholar] [CrossRef]
- Nam, K.; Dean, S.M.; Brown, C.T.; Smith, R.J., Jr.; Lei, P.; Andreadis, S.T.; Baker, O.J. Synergistic effects of laminin-1 peptides, VEGF and FGF9 on salivary gland regeneration. Acta Biomater. 2019, 91, 186–194. [Google Scholar] [CrossRef]
- Kikkawa, Y.; Sugawara, Y.; Harashima, N.; Fujii, S.; Ikari, K.; Kumai, J.; Katagiri, F.; Hozumi, K.; Nomizu, M. Identification of laminin α5 short arm peptides active for endothelial cell attachment and tube formation. J. Pept. Sci. 2017, 23, 666–673. [Google Scholar] [CrossRef]
- Parker, F.; White, K.; Phillips, S.; Peckham, M. Promoting differentiation of cultured myoblasts using biomimetic surfaces that present alpha-laminin-2 peptides. Cytotechnology 2016, 68, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Kangwantas, K.; Pinteaux, E.; Penny, J. The extracellular matrix protein laminin-10 promotes blood-brain barrier repair after hypoxia and inflammation in vitro. J. Neuroinflam. 2016, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Guo, H.; Feng, W.; Qiu, H. LAMC2 regulated by microRNA-125a-5p accelerates the progression of ovarian cancer via activating p38 MAPK signalling. Life Sci. 2019, 232, 116648. [Google Scholar] [CrossRef]
- Caires-Dos-Santos, L.; da Silva, S.V.; Smuczek, B.; de Siqueira, A.S.; Cruz, K.S.P.; Barbuto, J.A.M.; Augusto, T.M.; Freitas, V.M.; Carvalho, H.F.; Jaeger, R.G. Laminin-derived peptide C16 regulates Tks expression and reactive oxygen species generation in human prostate cancer cells. J. Cell. Physiol. 2020, 235, 587–598. [Google Scholar] [CrossRef]
- Ishihara, J.; Ishihara, A.; Fukunaga, K.; Sasaki, K.; White, M.J.V.; Briquez, P.S.; Hubbell, J.A. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 2018, 9, 2163. [Google Scholar] [CrossRef]
- Kim, Y.; Li, H.; Song, Y.S.; Jeong, H.S.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Shin, Y.K.; Park, K.C.; Kim, D.S. Laminin peptide YIGSR enhances epidermal development of skin equivalents. J. Tissue Viability 2018, 27, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Sahab Negah, S.; Khooei, A.; Samini, F.; Gorji, A. Laminin-derived Ile-Lys-Val-ala-Val: A promising bioactive peptide in neural tissue engineering in traumatic brain injury. Cell Tissue Res. 2018, 371, 223–236, Erratum in Cell Tissue Res. 2018, 371, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, J.; Xu, D.; Jv, D.; Meng, X.; Qiao, P.; Cui, T.; Shi, B. The 37-kDa laminin receptor precursor regulates the malignancy of human glioma cells. Cell Biochem. Funct. 2016, 34, 516–521. [Google Scholar] [CrossRef]
- Siqueira, A.; Pinto, M.P.; Cruz, M.C.; Smuczek, B.; Cruz, K.S.; Barbuto, J.A.; Hoshino, D.; Weaver, A.M.; Freitas, V.M.; Jaeger, R.G. Laminin-111 peptide C16 regulates invadopodia activity of malignant cells through β1 integrin, Src and ERK 1/2. Oncotarget 2016, 7, 47904–47917. [Google Scholar] [CrossRef]
- Sweeney, T.; Kibbey, M.C.; Zain, M.; Fridman, R.; Kleinman, H.K. Basement membrane and the SIKVAV laminin-derived peptide promote tumor growth and metastases. Cancer Metastasis Rev. 1991, 10, 245–254. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Fujita, Y.; Sugioka, Y. YIGSR, a synthetic laminin peptide, inhibits the enhancement by cyclophosphamide of experimental lung metastasis of human fibrosarcoma cells. Clin. Exp. Metastasis 1992, 10, 183–189. [Google Scholar] [CrossRef]
- Gately, S.; Twardowski, P.; Stack, M.S.; Cundiff, D.L.; Grella, D.; Castellino, F.J.; Enghild, J.; Kwaan, H.C.; Lee, F.; Kramer, R.A.; et al. The mechanism of cancer-mediated conversion of plasminogen to the angiogenesis inhibitor angiostatin. Proc. Natl. Acad. Sci. USA 1997, 94, 10868–10872. [Google Scholar] [CrossRef]
- O’Reilly, M.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Moses, M.; Lane, W.S.; Cao, Y.; Sage, E.H.; Folkman, J. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994, 79, 315–328. [Google Scholar] [CrossRef]
- O’Reilly, M.; Holmgren, L.; Shing, Y.; Chen, C.; Rosenthal, R.A.; Cao, Y.; Moses, M.; Lane, W.S.; Sage, E.H.; Folkman, J. Angiostatin: A circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb. Symp. Quant. Biol. 1994, 59, 471–482. [Google Scholar] [CrossRef]
- O’Reilly, M. Angiostatin: An endogenous inhibitor of angiogenesis and of tumor growth. EXS 1997, 79, 273–294. [Google Scholar]
- Cao, Y.; Ji, R.W.; Davidson, D.; Schaller, J.; Marti, D.; Söhndel, S.; McCance, S.G.; O’Reilly, M.S.; Llinás, M.; Folkman, J. Kringle domains of human angiostatin. Characterization of the anti-proliferative activity on endothelial cells. J. Biol. Chem. 1996, 271, 29461–29467. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, A.; An, S.S.; Ji, R.W.; Davidson, D.; Llinás, M. Kringle 5 of plasminogen is a novel inhibitor of endothelial cell growth. J. Biol. Chem. 1997, 572, 22924–22928. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Smith, R.; Saxne, T.; Hickery, M.; Heinegård, D. Fibronectin fragments cause release and degradation of collagen-binding molecules from equine explant cultures. Osteoarthr. Cartil. 2004, 12, 149–159. [Google Scholar] [CrossRef]
- Clutterbuck, A.; Smith, J.R.; Allaway, D.; Harris, P.; Liddell, S.; Mobasheri, A. High throughput proteomic analysis of the secretome in an explant model of articular cartilage inflammation. J. Proteomics. 2011, 74, 704–715. [Google Scholar] [CrossRef]
- Skiöldebrand, E.; Ekman, S.; Mattsson Hultén, L.; Svala, E.; Björkman, K.; Lindahl, A.; Lundqvist, A.; Önnerfjord, P.; Sihlbom, C.; Rüetschi, U. Cartilage oligomeric matrix protein neoepitope in the synovial fluid of horses with acute lameness: A new biomarker for the early stages of osteoarthritis. Equine. Vet. J. 2017, 49, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Önnerfjord, P.; Holmgren, K.; di Grado, S.; Dudhia, J. Development of a Cartilage Oligomeric Matrix Protein Neo-Epitope Assay for the Detection of Intra-Thecal Tendon Disease. Int. J. Mol. Sci. 2020, 21, 2155. [Google Scholar] [CrossRef]
- Kaur, B.; Rana, D.; Konar, M.; Sharma, R.; Chouhan, D.K.; Saini, U.C.; Prakash, M.; Arora, A.; Dhillon, M.S.; Kaur, J.; et al. Comparative Proteomic Analysis of Osteoarthritis and Rheumatoid Arthritis: Identifying Potential Biomarkers. J. Orthop. Res. 2025, 43, 1396–1412. [Google Scholar] [CrossRef]
- Keter, D.; Thai-Paquette, V.; Miamidian, J.; Gulati, S.; Toler, K. Synovial fluid dual-biomarker algorithm accurately differentiates osteoarthritis from inflammatory arthritis. J. Orthop. Res. 2025, 43, 304–310. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Ballut, L. Matricryptins derived from collagens and proteoglycans. Front. Biosci. 2011, 16, 674–697. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Salza, R. Matricryptins and matrikines: Biologically active fragments of the extracellular matrix. Exp. Dermatol. 2014, 23, 457–463. [Google Scholar] [CrossRef]
- Su, J.; Stenbjorn, R.S.; Gorse, K.; Su, K.; Hauser, K.F.; Ricard-Blum, S.; Pihlajaniem, T.; Fox, M.A. Target-derived matricryptins organize cerebellar synapse formation through α3β1 integrins. Cell Rep. 2012, 2, 223–230. [Google Scholar] [CrossRef]
- Su, J.; Chen, J.; Lippold, K.; Monavarfeshan, A.; Carrillo, G.L.; Jenkins, R.; Fox, M.A. Collagen-derived matricryptins promote inhibitory nerve terminal formation in the developing neocortex. J. Cell Biol. 2016, 12, 721–736. [Google Scholar] [CrossRef]
- Su, J.; Cole, J.; Fox, M.A. Loss of Interneuron-Derived Collagen XIX Leads to a Reduction in Perineuronal Nets in the Mammalian Telencephalon. ASN Neuro. 2017, 9, 1759091416689020. [Google Scholar] [CrossRef]
- Pintér, P.; Alpár, A. The Role of Extracellular Matrix in Human Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 11085. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Vallet, S.D. Proteases decode the extracellular matrix cryptome. Biochimie 2016, 122, 300–313. [Google Scholar] [CrossRef]
- Groeneveld, T.; Oroszlán, M.; Owens, R.T.; Faber-Krol, M.C.; Bakker, A.C.; Arlaud, G.J.; McQuillan, D.J.; Kishore, U.; Daha, M.R.; Roos, A. Interactions of the extracellular matrix proteoglycans decorin and biglycan with C1q and collectins. J. Immunol. 2005, 175, 4715–4723. [Google Scholar] [CrossRef]
- Okroj, M.; Heinegård, D.; Holmdahl, R.; Blom, A.M. Rheumatoid arthritis and the complement system. Ann. Med. 2007, 39, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Rozelle, A.L.; Lepus, C.M.; Scanzello, C.R.; Song, J.J.; Larsen, D.M.; Crish, J.F.; Bebek, G.; Ritter, S.Y.; Lindstrom, T.M.; et al. Identification of a central role for complement in osteoarthritis. Nat. Med. 2011, 17, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Assirelli, E.; Pulsatelli, L.; Dolzani, P.; Mariani, E.; Lisignoli, G.; Addimanda, O.; Meliconi, R. Complement Expression and Activation in Osteoarthritis Joint Compartments. Front. Immunol. 2020, 11, 535010. [Google Scholar] [CrossRef] [PubMed]
- Laabei, M.; Liu, G.; Ermert, D.; Lambris, J.D.; Riesbeck, K.; Blom, A.M. Short Leucine-Rich Proteoglycans Modulate Complement Activity and Increase Killing of the Respiratory Pathogen Moraxella catarrhalis. J. Immunol. 2018, 201, 2721–2730. [Google Scholar] [CrossRef]
- Sjöberg, A.; Manderson, G.A.; Mörgelin, M.; Day, A.J.; Heinegård, D.; Blom, A.M. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol. Immunol. 2009, 46, 830–839. [Google Scholar] [CrossRef]
- Kemberi, M.; Minns, A.F.; Santamaria, S. Soluble Proteoglycans and Proteoglycan Fragments as Biomarkers of Pathological Extracellular Matrix Remodeling. Proteoglycan. Res. 2024, 2, e70011. [Google Scholar] [CrossRef]
- de Castro Brás, L.; Frangogiannis, N.G. Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol. 2020, 91–92, 176–187. [Google Scholar] [CrossRef]
- Anakha, J.; Prasad, Y.R.; Pande, A.H. Endostatin in disease modulation: From cancer to beyond. Vasc. Pharmacol. 2025, 158, 107459. [Google Scholar] [CrossRef]
- Taglietti, V.; Kefi, K.; Bronisz-Budzyńska, I.; Mirciloglu, B.; Rodrigues, M.; Cardone, N.; Coulpier, F.; Periou, B.; Gentil, C.; Goddard, M.; et al. Duchenne muscular dystrophy trajectory in R-DMDdel52 preclinical rat model identifies COMP as biomarker of fibrosis. Acta Neuropathol. Commun. 2022, 10, 60. [Google Scholar] [CrossRef]
- Udomsinprasert, W.; Angkathunyakul, N.; Jittikoon, J.; Chaikledkaew, U.; Vejchapipat, P.; Poovorawan, Y.; Honsawek, S. Cartilage oligomeric matrix protein as a marker of progressive liver fibrosis in biliary atresia. Sci. Rep. 2021, 11, 16695. [Google Scholar] [CrossRef]
- Schulz, J.; Plomann, M.; Sengle, G.; Gullberg, D.; Krieg, T.; Eckes, B. New developments on skin fibrosis—Essential signals emanating from the extracellular matrix for the control of myofibroblasts. Matrix Biol. 2018, 68–69, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Zachou, K.; Gabeta, S.; Shums, Z.; Gatselis, N.K.; Koukoulis, G.K.; Norman, G.L.; Dalekos, G.N. COMP serum levels: A new non-invasive biomarker of liver fibrosis in patients with chronic viral hepatitis. Eur. J. Intern. Med. 2017, 38, 83–88. [Google Scholar] [CrossRef]
- Wusterbarth, E.; Chen, Y.; Jecius, H.; Krall, E.; Runyan, R.B.; Pandey, R.; Nfonsam, V. Cartilage Oligomeric Matrix Protein, COMP may be a Better Prognostic Marker Than CEACAM5 and Correlates With Colon Cancer Molecular Subtypes, Tumor Aggressiveness and Overall Survival. J. Surg. Res. 2022, 270, 169–177. [Google Scholar] [CrossRef]
- Gorji-Bahri, G.; Krishna, B.M.; Hagerling, C.; Orimo, A.; Jirström, K.; Papadakos, K.S.; Blom, A.M. Stromal cartilage oligomeric matrix protein as a tumorigenic driver in ovarian cancer via Notch3 signaling and epithelial-to-mesenchymal transition. J. Transl. Med. 2024, 22, 351. [Google Scholar]
- Posey, K.; Coustry, F.; Hecht, J.T. Cartilage oligomeric matrix protein: COMPopathies and beyond. Matrix Biol. 2018, 71–72, 161–173. [Google Scholar] [CrossRef]
- Li, D.; Lyu, G. Cartilage Oligomeric Matrix Protein: A Potential Prognostic Biomarker and Therapeutic Target in Gastric Cancer. Curr. Med. Chem. 2025, 32, 8647–8663. [Google Scholar] [CrossRef]
- Network, C.G.A.R. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Razzak, M. Genetics: New molecular classification of gastric adenocarcinoma proposed by The Cancer Genome Atlas. Nat. Rev. Clin. Oncol. 2014, 11, 499. [Google Scholar] [PubMed]
- Englund, E.; Canesin, G.; Papadakos, K.S.; Vishnu, N.; Persson, E.; Reitsma, B.; Anand, A.; Jacobsson, L.; Helczynski, L.; Mulder, H.; et al. Cartilage oligomeric matrix protein promotes prostate cancer progression by enhancing invasion and disrupting intracellular calcium homeostasis. Oncotarget 2017, 8, 98298–98311. [Google Scholar] [CrossRef]
- Schuurbiers, O.; Kaanders, J.H.A.M.; Van Der Heijden, H.F.M.; Dekhuijzen, R.P.N.; Oyen, W.J.G.; Bussink, J. The PI3-K/AKT-pathway and radiation resistance mechanisms in non-small cell lung cancer. J. Thorac. Oncol. 2009, 4, 761–767. [Google Scholar] [CrossRef]
- Reno, K.; Costa-Terryll, A.; Park, S.H.; Hughes, R.T.; Farris, M.K.; Xing, F.; Willey, J.S. Cartilage Oligomeric Matrix Protein Promotes Radiation Resistance in Non-Small Cell Lung Cancer In Vitro. Int. J. Mol. Sci. 2025, 26, 2465. [Google Scholar] [CrossRef]
- Xiong, L.; Tan, B.; Lei, X.; Zhang, B.; Li, W.; Liu, D.; Xia, T. SIRT6 through PI3K/Akt/mTOR signaling pathway to enhance radiosensitivity of non-Small cell lung cancer and inhibit tumor progression. IUBMB Life 2021, 73, 1092–1102. [Google Scholar] [CrossRef]
- Tennen, R.; Berber, E.; Chua, K.F. Functional dissection of SIRT6: Identification of domains that regulate histone deacetylase activity and chromatin localization. Mech. Ageing Dev. 2010, 131, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Jin, Y.; Chen, F.; Xuan, X.; Cao, J.; Liang, Y.; Wang, Y.; Zhan, J.; Zhao, M.; Huang, C. Identification and characterization of bone/cartilage-associated signatures in common fibrotic skin diseases. Front. Genet. 2023, 14, 1121728. [Google Scholar] [CrossRef]
- Farina, G.; Lemaire, R.; Pancari, P.; Bayle, J.; Widom, R.L.; Lafyatis, R. Cartilage oligomeric matrix protein expression in systemic sclerosis reveals heterogeneity of dermal fibroblast responses to transforming growth factor beta. Ann. Rheum. Dis. 2009, 68, 435–441. [Google Scholar] [CrossRef]
- Rochfort, K.; Carroll, L.S.; Barabas, P.; Curtis, T.M.; Ambati, B.K.; Barron, N.; Cummins, P.M. COMP-Ang1 Stabilizes Hyperglycemic Disruption of Blood-Retinal Barrier Phenotype in Human Retinal Microvascular Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3547–3555. [Google Scholar] [CrossRef]
- Riessen, R.; Kearney, M.; Lawler, J.; Isner, J.M. Immunolocalization of thrombospondin-1 in human atherosclerotic and restenotic arteries. Am. Heart J. 1998, 135, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.; Lee, H.C.; Lee, S.W.; Lee, J.; Jang, H.; Lee, E.J.; Kim, H.S. COMP-Angiopoietin-1 accelerates muscle regeneration through N-cadherin activation. Sci. Rep. 2018, 8, 12323. [Google Scholar] [CrossRef]
- Chen, F.; Thomas, A.O.; Hecht, J.T.; Goldring, M.B.; Lawler, J. Cartilage oligomeric matrix protein/thrombospondin 5 supports chondrocyte attachment through interaction with integrins. J. Biol. Chem. 2005, 280, 32655–32661. [Google Scholar] [CrossRef]
- Ajona, D.; Ortiz-Espinosa, S.; Pio, R.; Lecanda, F. Complement in Metastasis: A Comp in the Camp. Front. Immunol. 2019, 10, 669. [Google Scholar] [CrossRef]
- Dunkelberger, J.; Song, W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Mastellos, D.; Hajishengallis, G.; Lambris, J.D. A guide to complement biology, pathology and therapeutic opportunity. Nat. Rev. Immunol. 2024, 24, 118–141. [Google Scholar] [CrossRef]
- Janeway, C.J.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Sarma, J.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef]
- Happonen, K.; Saxne, T.; Geborek, P.; Andersson, M.; Bengtsson, A.A.; Hesselstrand, R.; Heinegård, D.; Blom, A.M. Serum COMP-C3b complexes in rheumatic diseases and relation to anti-TNF-alpha treatment. Arthritis Res. Ther. 2012, 14, R15. [Google Scholar] [CrossRef] [PubMed]
- Melin Fürst, C.; Mörgelin, M.; Vadstrup, K.; Heinegård, D.; Aspberg, A.; Blom, A.M. The C-type lectin of the aggrecan G3 domain activates complement. PLoS ONE 2013, 8, e61407. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Mazzotti, A.; Artioli, E.; Brizola, E.; Moroni, A.; Tremosini, M.; Di Cecco, A.; Gallone, S.; Faldini, C.; Sangiorgi, L.; Gnoli, M. Multiple Osteochondritis Dissecans as Main Manifestation of Multiple Epiphyseal Dysplasia Caused by a Novel Cartilage Oligomeric Matrix Protein Pathogenic Variant: A Clinical Report. Genes 2024, 15, 1490. [Google Scholar] [CrossRef]
- Ikegawa, S.; Ohashi, H.; Nishimura, G.; Kim, K.C.; Sannohe, A.; Kimizuka, M.; Fukushima, Y.; Nagai, T.; Nakamura, Y. Novel and recurrent COMP (cartilage oligomeric matrix protein) mutations in pseudoachondroplasia and multiple epiphyseal dysplasia. Hum. Genet. 1998, 103, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, K.S.; Li, Q.W.; Koo, S.K.; Jung, S.C. Identification of cartilage oligomeric matrix protein (COMP) gene mutations in patients with pseudoachondroplasia and multiple epiphyseal dysplasia. J. Hum. Genet. 2003, 48, 222–225. [Google Scholar] [CrossRef]
- Briggs, M.; Chapman, K.L. Pseudoachondroplasia and multiple epiphyseal dysplasia: Mutation review, molecular interactions, and genotype to phenotype correlations. Hum. Mutat. 2002, 19, 465–478. [Google Scholar] [CrossRef]
- Mabuchi, A.; Haga, N.; Ikeda, T.; Manabe, N.; Ohashi, H.; Takatori, Y.; Nakamura, K.; Ikegawa, S. Novel mutation in exon 18 of the cartilage oligomeric matrix protein gene causes a severe pseudoachondroplasia. Am. J. Med. Genet. 2001, 104, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.; Jackson, G.; Ramsden, S.; Taylor, J.; Newman, W.; Wright, M.J.; Donnai, D.; Elles, R.; Briggs, M.D. COMP mutation screening as an aid for the clinical diagnosis and counselling of patients with a suspected diagnosis of pseudoachondroplasia or multiple epiphyseal dysplasia. Eur. J. Hum. Genet. 2005, 13, 547–555. [Google Scholar] [CrossRef][Green Version]
- Guo, B.; Wang, Y.; Liu, W.; Zhang, S. Cartilage oligomeric matrix protein acts as a molecular biomarker in multiple cancer types. Clin. Transl. Oncol. 2023, 25, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Rosas, S.; Kwok, A.; Moore, J.; Shi, L.; Smith, T.L.; Tallant, E.A.; Kerr, B.A.; Willey, J.S. Osteoarthritis as a Systemic Disease Promoted Prostate Cancer In Vivo and In Vitro. Int. J. Mol. Sci. 2024, 25, 6014. [Google Scholar] [CrossRef]
- Abdel-Azeez, H.; Elhady, H.A.; Fikry, A.A. Cartilage oligomeric matrix protein as a non-invasive biomarker for diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. Gastroenterol. Hepatol. Bed Bench 2022, 15, 139–145. [Google Scholar]
- Ong, K.; Hsieh, Y.Y.; Lai, H.Y.; Sun, D.P.; Chen, T.J.; Huang, S.K.; Tian, Y.F.; Chou, C.L.; Shiue, Y.L.; Wu, H.C.; et al. Cartilage oligomeric matrix protein overexpression is an independent poor prognostic indicator in patients with intrahepatic cholangiocarcinoma. Sci. Rep. 2023, 13, 17444. [Google Scholar] [CrossRef]
- Xiao, Y.; Kleeff, J.; Guo, J.; Gazdhar, A.; Liao, Q.; Di Cesare, P.E.; Büchler, M.W.; Friess, H. Cartilage oligomeric matrix protein expression in hepatocellular carcinoma and the cirrhotic liver. J. Gastroenterol. Hepatol. 2004, 19, 296–302. [Google Scholar] [CrossRef]
- Blom, A.; Gialeli, C.; Hagerling, C.; Berntsson, J.; Jirström, K.; Papadakos, K.S. Expression of Cartilage Oligomeric Matrix Protein in colorectal cancer is an adverse prognostic factor and correlates negatively with infiltrating immune cells and PD-L1 expression. Front. Immunol. 2023, 14, 1167659. [Google Scholar] [CrossRef]
- Kuo, Y.; Lai, H.Y.; Chan, T.C.; Hsing, C.H.; Huang, S.K.; Hsieh, K.L.; Chen, T.J.; Li, W.S.; Lu, J.C.; Li, C.F. Upregulation of Cartilage Oligomeric Matrix Protein Predicts Poor Prognosis in Urothelial Carcinoma. Onco Targets Ther. 2022, 15, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Guan, H.; Lin, Y.; Li, Y.; Ou, Y.; Yan, M.; Lin, L.; Zhu, X.; Shi, B.; Chen, J. Expression of cartilage oligomeric matrix protein (COMP) associates with capsular invasion in salivary gland pleomorphic adenoma. J. Oral Pathol. Med. 2022, 51, 290–300. [Google Scholar] [CrossRef]
- Cho, C.; Kim, K.E.; Byun, J.; Jang, H.S.; Kim, D.K.; Baluk, P.; Baffert, F.; Lee, G.M.; Mochizuki, N.; Kim, J.; et al. Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ. Res. 2005, 97, 86–94, Erratum in Circ. Res. 2006, 98, e69. [Google Scholar] [CrossRef]
- Jacobsen, J.; Mulvany, M.J.; Holstein-Rathlou, N.H. A mechanism for arteriolar remodeling based on maintenance of smooth muscle cell activation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1379–R1389. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Cho, C.H.; Kim, H.Z.; Baluk, P.; McDonald, D.M.; Koh, G.Y. In vivo actions of angiopoietins on quiescent and remodeling blood and lymphatic vessels in mouse airways and skin. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 564–570. [Google Scholar] [CrossRef]
- Ström, A.; Ahlqvist, E.; Franzén, A.; Heinegård, D.; Hultgårdh-Nilsson, A. Extracellular matrix components in atherosclerotic arteries of Apo E/LDL receptor deficient mice: An immunohistochemical study. Histol. Histopathol. 2004, 19, 337–347. [Google Scholar]
- Halper, J. Basic Components of Connective Tissues and Extracellular Matrix: Fibronectin, Fibrinogen, Laminin, Elastin, Fibrillins, Fibulins, Matrilins, Tenascins and Thrombospondins. Adv. Exp. Med. Biol. 2021, 1348, 105–126. [Google Scholar]
- Schulz, J.; Nüchel, J.; Niehoff, A.; Bloch, W.; Schönborn, K.; Hayashi, S.; Kamper, M.; Brinckmann, J.; Plomann, M.; Paulsson, M.; et al. COMP-assisted collagen secretion--a novel intracellular function required for fibrosis. J. Cell Sci. 2016, 129, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Andréasson, K.; Jönsson, G.; Hesselstrand, R.; Norrgren, H. Persistent elevation of fibrosis biomarker cartilage oligomeric matrix protein following hepatitis C virus eradication. World J. Hepatol. 2019, 11, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Gatselis, N.; Zachou, K.; Giannoulis, G.; Gabeta, S.; Norman, G.L.; Dalekos, G.N. Serum Cartilage Oligomeric Matrix Protein and Golgi Protein-73: New Diagnostic and Predictive Tools for Liver Fibrosis and Hepatocellular Cancer? Cancers 2021, 13, 3510. [Google Scholar] [CrossRef] [PubMed]
- Burkhard, P.; Stetefeld, J.; Strelkov, S.V. Coiled coils: A highly versatile protein folding motif. Trends Cell Biol. 2001, 11, 82–88. [Google Scholar] [CrossRef]
- Cohen, C.; Parry, D.A.D. α-Helical coiled coils—A widespread motif in proteins. Trend Biol. Sci 1986, 11, 245–248. [Google Scholar] [CrossRef]
- Mason, J.; Arndt, K.M. Coiled coil domains: Stability, specificity, and biological implications. Chembiochem 2004, 5, 170–176. [Google Scholar] [CrossRef]
- Lupas, A.; Bassler, J.; Dunin-Horkawicz, S. The Structure and Topology of α-Helical Coiled Coils. Subcell. Biochem. 2017, 82, 95–129. [Google Scholar]
- Truebestein, L.; Leonard, T.A. Coiled-coils: The long and short of it. Bioessays 2016, 38, 903–916. [Google Scholar] [CrossRef]
- Krzysztof, S.; Bukala, A.A.; Antonio Marinho da Silva Neto, A.; Jan, L.; Stanislaw, D.-H. A library of coiled-coil domains: From regular bundles to peculiar twists. Bioinformatics 2021, 36, 5368–5376. [Google Scholar]
- Nicolas, A.; Delalande, O.; Hubert, J.F.; Le Rumeur, E. The spectrin family of proteins: A unique coiled-coil fold for various molecular surface properties. J. Struct. Biol. 2014, 186, 392–401. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, C.; Zhao, Q.; Li, D. Spectrin: Structure, function and disease. Sci. China Life Sci. 2013, 56, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.; Uehara, H.; Fang, D.; Choi, S.; Zhang, X.; Singh, M.; Sandhu, Z.; Cummins, P.M.; Curtis, T.M.; Stitt, A.W.; et al. Intravitreal AAV2.COMP-Ang1 Attenuates Deep Capillary Plexus Expansion in the Aged Diabetic Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2494–2502. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, J.; Rai, R.R.; Carroll, L.S.; Uehara, H.; Zhang, X.; O’Neil, C.L.; Medina, R.J.; Das, S.K.; Muddana, S.K.; Olson, P.R.; et al. Intravitreal AAV2.COMP-Ang1 Prevents Neurovascular Degeneration in a Murine Model of Diabetic Retinopathy. Diabetes 2015, 64, 4247–4259. [Google Scholar] [CrossRef]
- Augustin, H.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin–Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Papadopoulos, N.; Aldrich, T.H.; Maisonpierre, P.C.; Huang, T.; Kovac, L.; Xu, A.; Leidich, R.; Radziejewska, E.; Rafique, A.; et al. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat. Struct. Mol. Biol. 2002, 10, 38–44, Erratum in Nat. Struct. Mol. Biol. 2003, 10, 146. [Google Scholar] [CrossRef]
- Koh, G. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol. Med. 2013, 19, 31–39. [Google Scholar] [CrossRef]
- Arita, Y.N.Y.; Matsunaga, T.; Kidoya, H.; Yamamizu, K.; Arima, Y.; Kataoka-Hashimoto, T.; Ikeoka, K.; Yasui, T.; Masaki, T.; Yamamoto, K.; et al. Myocardium-derived angiopoietin-1 is essential for coronary vein formation in the developing heart. Nat. Commun. 2014, 5, 4552. [Google Scholar] [CrossRef]
- Cho, C.; Kammerer, R.A.; Lee, H.J.; Steinmetz, M.O.; Ryu, Y.S.; Lee, S.H.; Yasunaga, K.; Kim, K.T.; Kim, I.; Choi, H.H.; et al. COMP-Ang1: A designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc. Natl. Acad. Sci. USA 2004, 101, 5547–5552. [Google Scholar] [CrossRef]
- Lim, S.; Kook, S.H.; Lee, J.C. COMP-Ang1 enhances DNA synthesis and cell cycle progression in human periodontal ligament cells via Tie2-mediated phosphorylation of PI3K/Akt and MAPKs. Mol. Cell. Biochem. 2016, 416, 157–168. [Google Scholar] [CrossRef]
- Oh, N.; Kim, K.; Kim, S.J.; Park, I.; Lee, J.E.; Seo, Y.S.; An, H.J.; Kim, H.M.; Koh, G.Y. A Designed Angiopoietin-1 Variant, Dimeric CMP-Ang1 Activates Tie2 and Stimulates Angiogenesis and Vascular Stabilization in N-glycan Dependent Manner. Sci. Rep. 2015, 5, 15291. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.; Lim, S.S.; Cho, E.S.; Lee, Y.H.; Han, S.K.; Lee, K.Y.; Kwon, J.; Hwang, J.W.; Bae, C.H.; Seo, Y.K.; et al. COMP-angiopoietin 1 increases proliferation, differentiation, and migration of stem-like cells through Tie-2-mediated activation of p38 MAPK and PI3K/Akt signal transduction pathways. Biochem. Biophys. Res. Commun. 2014, 455, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kook, S.H.; Bhattarai, G.; Cho, E.S.; Seo, Y.K.; Lee, J.C. Local delivery of COMP-angiopoietin 1 accelerates new bone formation in rat calvarial defects. J. Biomed. Mater. Res. A 2015, 103, 2942–2951. [Google Scholar] [CrossRef]
- Bhattarai, G.; Kook, S.H.; Kim, J.H.; Poudel, S.B.; Lim, S.S.; Seo, Y.K.; Lee, J.C. COMP-Ang1 prevents periodontitic damages and enhances mandible bone growth in an experimental animal model. Bone 2016, 92, 168–179. [Google Scholar] [CrossRef]
- Lee, H.; Bae, S.W.; Koh, G.Y.; Lee, Y.S. COMP-Ang1, angiopoietin-1 variant protects radiation-induced bone marrow damage in C57BL/6 mice. J. Radiat. Res. 2008, 49, 313–320. [Google Scholar] [CrossRef]
- Blumbach, K.; Bastiaansen-Jenniskens, Y.M.; DeGroot, J.; Paulsson, M.; van Osch, G.J.; Zaucke, F. Combined role of type IX collagen and cartilage oligomeric matrix protein in cartilage matrix assembly: Cartilage oligomeric matrix protein counteracts type IX collagen-induced limitation of cartilage collagen fibril growth in mouse chondrocyte cultures. Arthritis Rheum. 2009, 60, 3676–3685. [Google Scholar] [CrossRef]
- Haudenschild, D.; Hong, E.; Yik, J.H.; Chromy, B.; Mörgelin, M.; Snow, K.D.; Acharya, C.; Takada, Y.; Di Cesare, P.E. Enhanced activity of transforming growth factor β1 (TGF-β1) bound to cartilage oligomeric matrix protein. J. Biol. Chem. 2011, 286, 43250–43258. [Google Scholar] [CrossRef]
- Mak, C.; To, K.; Fekir, K.; Brooks, R.A.; Khan, W.S. Infrapatellar fat pad adipose-derived stem cells co-cultured with articular chondrocytes from osteoarthritis patients exhibit increased chondrogenic gene expression. Cell Commun. Signal. 2022, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Andrés Sastre, E.; Maly, K.; Zhu, M.; Witte-Bouma, J.; Trompet, D.; Böhm, A.M.; Brachvogel, B.; van Nieuwenhoven, C.A.; Maes, C.; van Osch, G.J.V.M.; et al. Spatiotemporal distribution of thrombospondin-4 and -5 in cartilage during endochondral bone formation and repair. Bone 2021, 150, 115999. [Google Scholar] [CrossRef] [PubMed]
- Tampoia, T.; Brescia, V.; Fallapone, P.; Zucano, F.; Scioscia, C.; Fontana, A.; Iannone, F.; Di Serio, F.; Lapadula, G. Response of Cartilage Oligomeric Matrix Protein to Monoclonal Antibody Drugs in Patients with Rheumatoid Arthritis: Impact of Biological Variability. Lab. Med. 2009, 40, 645–650. [Google Scholar] [CrossRef]
- Andersson, M.; Svensson, B.; Petersson, I.F.; Hafström, I.; Albertsson, K.; Forslind, K.; Heinegård, D.; Saxne, T. Early increase in serum-COMP is associated with joint damage progression over the first five years in patients with rheumatoid arthritis. BMC Musculoskelet. Disord. 2013, 14, 229. [Google Scholar] [CrossRef]
- Young-Min, S.; Cawston, T.; Marshall, N.; Coady, D.; Christgau, S.; Saxne, T.; Robins, S.; Griffiths, I. Biomarkers predict radiographic progression in early rheumatoid arthritis and perform well compared with traditional markers. Arthritis Rheum. 2007, 56, 3236–3247. [Google Scholar] [CrossRef]
- Nikolaisen, C.; Rekvig, O.P.; Nossent, H.C. Diagnostic impact of contemporary biomarker assays for rheumatoid arthritis. Scand. J. Rheumatol. 2007, 36, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X.; Zhang, X.; Ren, C.; Xin, J. Role of Serum cartilage oligomeric matrix protein (COMP) in the diagnosis of rheumatoid arthritis (RA): A case-control study. J. Int. Med. Res. 2016, 44, 940–949. [Google Scholar] [CrossRef]
- Lambova, S.; Batsalova, T.; Moten, D.; Dzhambazov, B. Cartilage Oligomeric Matrix Protein in Osteoarthritis and Obesity-Do New Considerations Emerge? Int. J. Mol. Sci. 2024, 25, 5263. [Google Scholar] [CrossRef]
- Lawrence, A.; Boesel, J.; Martinez Aguilar, R.; Gryczewski, D.; Moni, A.S.B. A Review and Meta-Analysis of Biomarkers in Early-Stage Osteoarthritis. Orthop. Surg. 2025, 17, 1913–1923. [Google Scholar] [CrossRef]
- Guo, Y.; Jin, Q.; Kang, Y.; Jin, W.; Liu, Y.; Chen, Q.; Liu, J.; Wang, Y.G. Integrating machine learning and neural networks for new diagnostic approaches to idiopathic pulmonary fibrosis and immune infiltration research. PLoS ONE 2025, 20, e0320242. [Google Scholar] [CrossRef]
- Huang, Y.; Bharill, S.; Karandur, D.; Peterson, S.M.; Marita, M.; Shi, X.; Kaliszewski, M.J.; Smith, A.W.; Isacoff, E.Y.; Kuriyan, J. Molecular basis for multimerization in the activation of the epidermal growth factor receptor. eLife 2016, 5, e14107. [Google Scholar] [CrossRef]
- Duke, T.; Graham, I. Equilibrium mechanisms of receptor clustering. Prog. Biophys. Mol. Biol. 2009, 100, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, C.; Dueñas, E.; Jackson, B.M.; Reusser, U.; Braus, G.H.; Hinnebusch, A.G. The transcriptional activator GCN4 contains multiple activation domains that are critically dependent on hydrophobic amino acids. Mol. Cell. Biol. 1995, 15, 1220–1233. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Jeong, B.C.; Hur, S.W.; Kim, J.W.; Lee, K.B.; Koh, J.T. The Angiopoietin-1 Variant COMP-Ang1 Enhances BMP2-Induced Bone Regeneration with Recruiting Pericytes in Critical Sized Calvarial Defects. PLoS ONE 2015, 10, e0140502. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr266. [Google Scholar] [CrossRef]
- Gurtner, G.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 543, 314–321. [Google Scholar] [CrossRef]

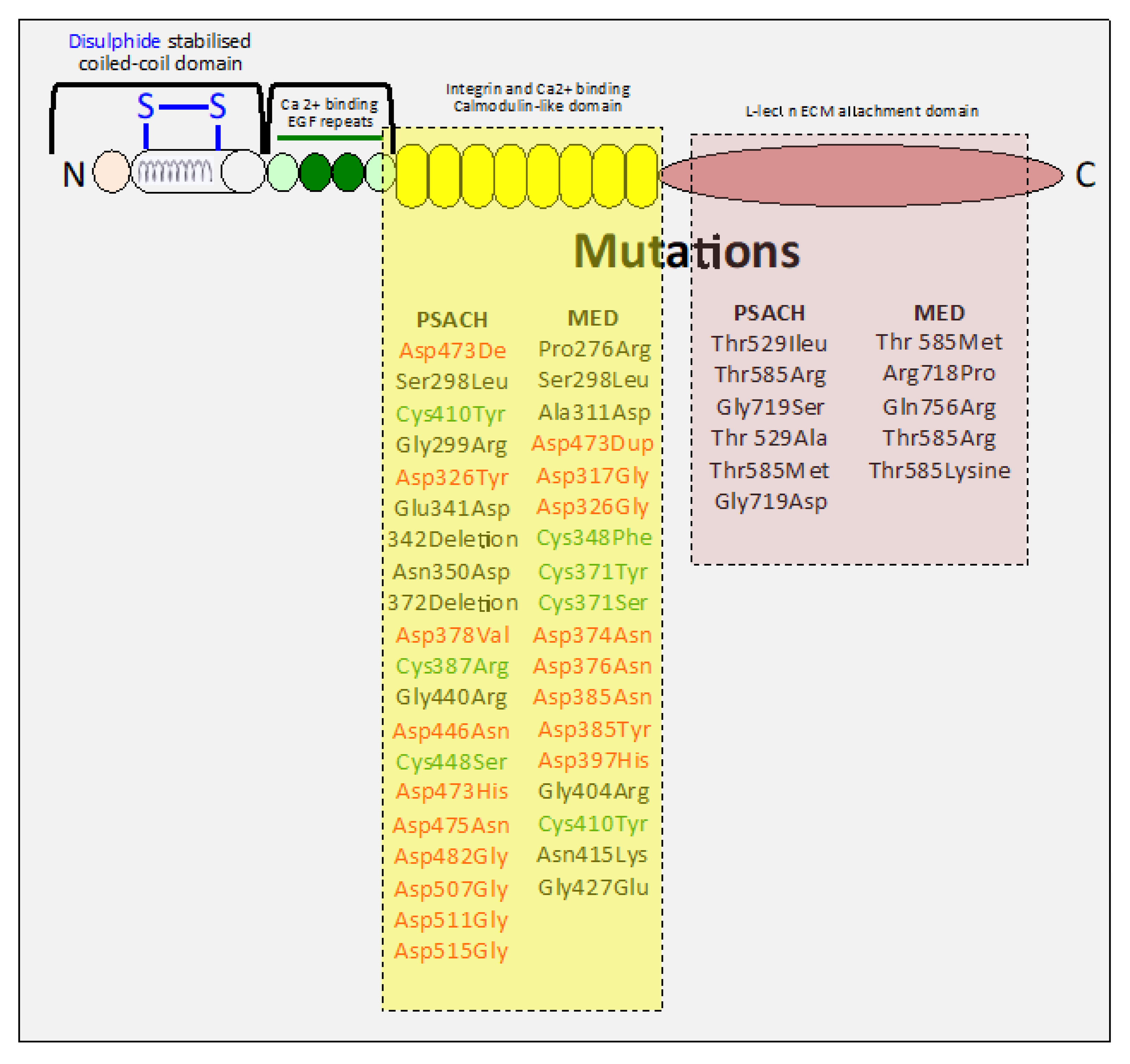
| Native Molecule | Fragment | Bioactivity of Fragment(s) |
|---|---|---|
| Aggrecan | 32 mer peptide Metstatin (BH-P) Recombinant 42 mer peptide MMP and ADAMTS generated aggrecan fragments | The 32-mer fragment is a TLR-2 ligand with the potential to accelerate cartilage destruction in vivo and drives OA-associated pain through TLR-2 [134]. Has anti-anabolic, pro-catabolic, and pro-inflammatory properties in vitro [135]. BH-P angiogenesis inhibitor peptide is of interest as a tumor growth inhibitor [136]. Cartilage aggrecan is cleaved by a wide range of MMPs and ADAMTS enzymes [137], with the IGD being a key cleavage site since this disaggregates aggregan aggregates with HA. A range of neo-epitope antibodies identify these cleavage sites, which occur in a number of pathological tissues [138,139]. |
| Link protein | Link N 16 amino acid N-terminal peptide DHLSDNYTLDHDRAIH | MMPs cleave at two sites in link protein. Stromelysins-1 and -2, gelatinases A and B and collagenase cleave between His16 and Ile17, and matrilysin, stromelysin-2 and gelatinase A cleave between Leu25 and Leu26 [140]. This N-terminal 16 amino acid peptide (Link N) displays growth factor activity, promotes collagen and aggrecan synthesis by chondrocytes, IVD cells and stem cells, and decreases inflammation and tissue fibrosis, promoting IVD and cartilage repair/regeneration [141,142,143,144,145,146,147]. |
| Brain link protein (Bral-1) HA binding peptide | PEP-1 12 mer GAHWQFNALTVR HA binding peptide | PEP-1 is an antagonist HA binding peptide that inhibits neutrophil homing to sites of inflammation [148]. PEP-1 is also a cell-penetrating peptide with the ability to translocate across biological membranes to introduce active cargo proteins and drugs into cells [149]. PEP has been used as a delivery vehicle for drugs and therapeutic proteins into a range of cells in treatment of dense tumor masses [150,151,152,153,154,155,156,157,158,159,160,161,162]. |
| Versican | Versikine | ADAMTS proteases generate a bioactive versican matricryptic fragment containing the N-terminal G1 domain, termed versikine, which promotes inflammation in transitional embryonic tissues [163]. Versikine is a novel bioactive damage-associated peptide active in immune sensing of myelomas and colon tumors [164]. The G1-DPEAAE 80 kDa fragment promotes EMT in developing and diseased tissues, cell migration, proliferation/invasion by tumor and endothelial cells, tumorigenesis and inflammation. Versikine acts in a contrasting manner to intact VCAN in tissues. The 80 kDa G1-DPEAAE fragments promote T cell infiltration and dendritic cell differentiation. |
| Lumican | Lumcorin LRR9 Chemokine LumC13(C-A) | Lumcorin is a peptide module from the 9th leucine-rich domain of lumican that is an MMP-inhibitor, is anti-angiogenic and has anti-tumor activity [165,166,167,168]. Lumikine is a synthetic peptide based on a C terminal lumican peptide with a cysteine residue replaced with alanine YEALRVANEVTLN. Lumikine binds TGF-β and the type I TGF-β receptor and displays growth factor activity [169,170]. |
| Biglycan | BGN262 peptide, Peniel 2000, Bgm1 biglycan peptide [171] YWEVQPATFR | Biglycan BGN262 [172], equine salivary biomarker for OA sub-chondral bone sclerosis [173], MMP [174], ADAMTS [137] generated and naturally occurring biglycan fragments in OA [175] and IVDD [176,177] and animal models [178,179], in silico designed Peniel 2000 binds and regulates TGF-β1 controlling IVD tissue fibrosis, inhibits IVD degeneration and stimulates IVD repair [180]. Bgm1 modulates TGF-β activity in the IVD [181]. |
| Decorin | Decorunt | Adult human skin contains a truncated form of decorin, termed decorunt, which lacks a portion of the C-terminus of decorin and has a new terminus, VRKVTF [182]. A neoepitope antibody raised to this sequence does not detect full-length decorin. Decorunt has one-hundredth the affinity for type I collage compared to full-length decorin. |
| Fibromodulin | 59, 43, 40, 27 kDa fragments | Fibromodulin is fragmented in OA in cartilage and meniscus with fragments of 27, 40, 43 and 59 kDa detected [175]. Fibromodulin is also fragmented in an ovine model of IVDD [177] and an animal OA model [178]. Fibromodulin is degraded by a range of MMPs and ADAMTSs [137,183]. MMP-13 cleaves N-terminal sulfated tyrosine residues in fibromodulin removing its HS-like interactive properties with growth factors and ECM components [184]. ADAMTS-4 and ADAMTS-5 cleave fibromodulin into 54, 45, 32 kDa fragments in vitro [183]. |
| Perlecan | Endorepellin | Endorepellin is a C-terminal 20 kDa anti-angiogenic peptide module found in domain V of perlecan [185]. Endorepellin is cleaved from the perlecan core protein by BMP-1/Tolloid-like metalloproteases [186]. The activity of endorepellin contrasts with full length perlecan, which is a pro-angiogenic multifunctional proteoglycan [187]. Endorepellin exerts a dual receptor antagonism by engaging with VEGFR2 and α2β1 integrin [188], leading to actin disassembly and blocking of endothelial cell migration required for tube formation and development of new capillaries [189]. This reduces the blood supply to tumors, providing anti-tumor properties. |
| Collagen XVIII | Endostatin, 20 kDa C-terminal peptide module of collagen XVIII | Endostain is an anti-angiogenic peptide module released from the C-terminus of collagen XVIII by cathepsin L [190] and MMPs [191], which cleave in the protease-sensitive hinge region of the C-terminal domain [192]. Endostatin is essential for vision and RPE function [193]. Reduced endostatin levels in Bruch’s membrane, RPE basal lamina, intercapillary septa, and choriocapillaris may permit choroidal vessel invasion in the retina, where neovascularization exacerbates macular degeneration, leading to impaired vision [194]. Elevated deposition of endostatin occurs in traumatic brain injuries [194]. |
| Agrin | 110 kDa C-terminal fragment NT1654, a 44 kDa C-terminal peptide of murine agrin | A C-terminal agrin fragment is a neuromuscular junction-related biomarker of muscle dysfunction and increases with aging in sarcopenia and in other muscle wasting conditions, such as diabetes, COPD, chronic heart failure, stroke, kidney disease and in pancreatic and colon cancer [195,196,197]. NT-1654 is an agrin fragment engineered to prevent cleavage by neurotrypsin. It has acetylcholine receptor clustering activity and the key functions of full-length agrin. Injection of NT-1654 in sarcopenia mouse models with impaired NMJ function results in recovery of muscle performance [198]. |
| Fibronectin | Anastellin | Fibronectin fragments (FN fs) upregulate MMP expression, significantly enhance PG degradation and temporarily suppress PG synthesis, events that are also observed in OA [199,200,201]. FN fs found in OA synovial fluid and generated within cartilage and may contribute to cartilage damage in vivo. However, this FN fs system may also be involved in normal cartilage homeostasis; low concentrations of FN fs enhance chondrocyte anabolic activities [202] and may aid in tissue repair with continuous anabolic effects; low concentrations of FN fs may promote tissue homeostasis. FN fs may thus have cartilage autocrine and paracrine regulatory properties. The MyD88-dependent TLR-2 signaling pathway may be responsible for 29-kDa fibronectin fragment-mediated cartilage catabolic responses [203]. The modulation of TLR-2 signaling activated by DAMPs is a potential regulatory mechanism over cartilage degradation in OA. Anti-metastatic inhibitory fragment of fibronectin is derived from the first FN3 domain [204,205,206]. |
| Laminin | α1 chain IKVAV, AG73 β1 chain YIGSR γ1 chain C16 | The laminins are a family of large glycoproteins with important roles in tissue development and stabilization of the basement membrane and ECM. Laminin interactive peptide modules equip it with cell adhesive and matrix stabilizing properties and roles during cell adhesion, migration and tissue development. The laminins have thus been the subject of a number of studies aimed at identifying these bioactive modules [207,208,209,210,211] to determine if synthetic versions of these can be applied in therapeutic tissue repair strategies [212,213,214,215,216,217,218,219,220,221,222]. Four peptide modules have been identified in Laminin-111 of interest in repair biology: IKVAV and AG73 in the α1 chain, YIGSR in the β1 chain and C16 in the γ1 chain [223], and these have potential in tissue repair [219,220,221,222,224,225,226,227,228,229] but in some cases can also promote tumor development [213,214,215,217,218,230,231,232,233,234,235,236,237,238]. |
| Plasminogen | Angiostatin | Human prostate carcinoma cells release enzymatic activity (urokinase) that converts plasminogen to angiostatin. Tissue-type plasminogen activator, or streptokinase, can also generate angiostatin from plasminogen [239]. Angiostatin inhibits angiogenesis in vitro and in vivo and suppresses the growth of Lewis lung carcinoma metastases [240], defining a direct mechanism for cancer-cell-mediated tumor inhibitory activity [241,242]. Angiostatin is a 38 kDa protein that inhibits endothelial cell proliferation [243]. Angiostatin is an internal fragment of plasminogen containing the first four kringle domains. Recombinant kringle 1 and kringle 3 exhibit potent inhibitory activity, while recombinant kringle 2 displays lower inhibitory activity [240]. In contrast, kringle 4 is an ineffective inhibitor of FGF-2-stimulated endothelial cell proliferation. The anti-proliferative activity of angiostatin on endothelial cells is thus shared by kringle 1, 2, and 3, but not by kringle 4 [243]. A more potent inhibitory protein is formed when kringle 4 is removed from angiostatin; Kringle 5 is more potent than angiostatin as an inhibitor of FGF-2-stimulated capillary endothelial cell proliferation [244]. |
| COMP | Fibronectin Hep I and II generated COMP fragments Serum COMP fragments in OA, RA and inflammatory arthritis. COMP fragments in surgically induced OA, collagen-induced arthritis, and TNF transgenic OA animal models. COMP is a major component of the cartilage secretome | Stimulation of equine cartilage explant cultures with N- and C-terminal heparin I and II fibronectin fragments results in chondrolysis and release of COMP fragments into synovial fluid and serum within 24 h. The Hep II fibronectin fragment was more potent than the Hep I fibronectin fragment for the generation of COMP fragments [245]. These COMP fragments are biomarkers of cartilage degradation in OA, RA and inflammatory arthritis [128] representing the early stages of cartilage degradation before disruption of collagen networks. In a study of synovial fluid from 52 OA, 85 RA and 60 patients with inflammatory arthritis, 84% of the RA, 21% of the OA and 60% of the inflammatory arthritis samples had significant amounts of low-molecular-weight COMP fragments (50–70 kDa), and 13% of SF samples taken from patients with RA or inflammatory arthritis were able to degrade COMP in vitro. A novel sandwich ELISA has been developed using an antibody that captures COMP fragments; this reproducibly measures COMP fragment levels in serum of arthritic patients and in surgically induced OA, collagen-induced arthritis, and TNF transgenic OA rodent arthritis models [129]. COMP fragment levels correlated with clinical assessment of the severity of arthritis of OA patients and the progression of surgically induced OA in murine models. This is a useful method for quantitation of serum COMP fragments and to monitor the efficacy of arthritic interventions. In a proteomics study on the secretome of cultured articular cartilage explants, 10 major cartilage peptide signatures of tryptic peptides originating from aggrecan core protein, COMP, fibronectin, fibromodulin, thrombospondin-1 (TSP-1), clusterin, cartilage intermediate layer protein-1, chondroadherin and MMP-1 and MMP-3, were detected [246]. COMP peptides are major components of the cartilage secretome under inflammatory conditions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, M.M.; Melrose, J. COMP Is a Biomarker of Cartilage Destruction, Extracellular Matrix and Vascular Remodeling and Tissue Repair. Int. J. Mol. Sci. 2025, 26, 9182. https://doi.org/10.3390/ijms26189182
Smith MM, Melrose J. COMP Is a Biomarker of Cartilage Destruction, Extracellular Matrix and Vascular Remodeling and Tissue Repair. International Journal of Molecular Sciences. 2025; 26(18):9182. https://doi.org/10.3390/ijms26189182
Chicago/Turabian StyleSmith, Margaret M., and James Melrose. 2025. "COMP Is a Biomarker of Cartilage Destruction, Extracellular Matrix and Vascular Remodeling and Tissue Repair" International Journal of Molecular Sciences 26, no. 18: 9182. https://doi.org/10.3390/ijms26189182
APA StyleSmith, M. M., & Melrose, J. (2025). COMP Is a Biomarker of Cartilage Destruction, Extracellular Matrix and Vascular Remodeling and Tissue Repair. International Journal of Molecular Sciences, 26(18), 9182. https://doi.org/10.3390/ijms26189182








