Curcumin Alleviates HMGB1-Mediated Inflammation Through the Signaling Pathway of TLR2-NF-κB in Bovine Ovarian Granulosa Cells
Abstract
1. Introduction
2. Results
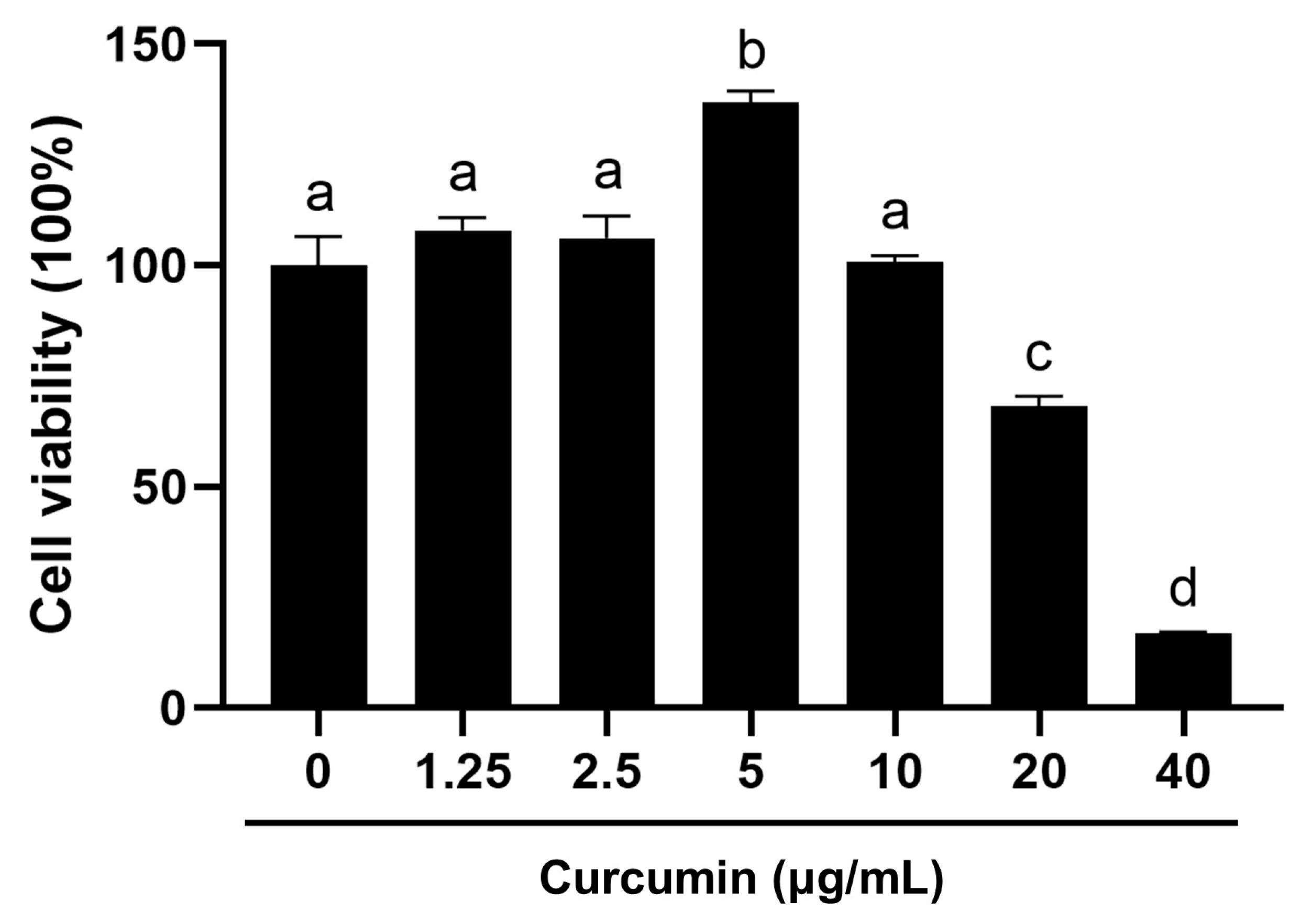
2.1. Biphasic Modulation of BOGCs Viability by Curcumin
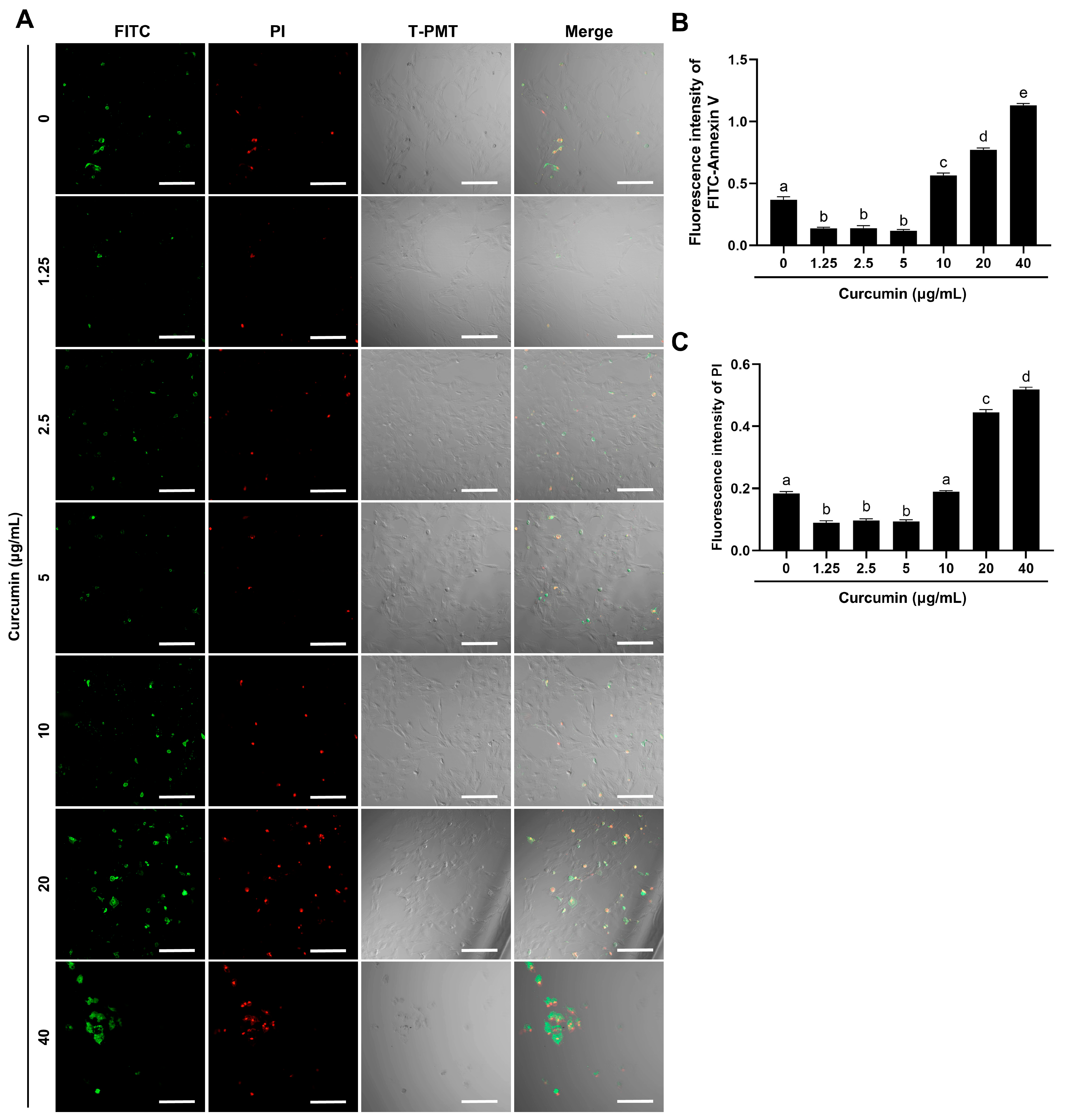
2.2. Curcumin Demonstrates Dose-Dependent Regulation of Apoptosis
2.3. Curcumin Reverses HMGB1-Driven TLR2-Associated Ovulatory Gene Upregulation
2.4. Curcumin Inhibits the HMGB1-TLR2/NF-κB Signaling Axis
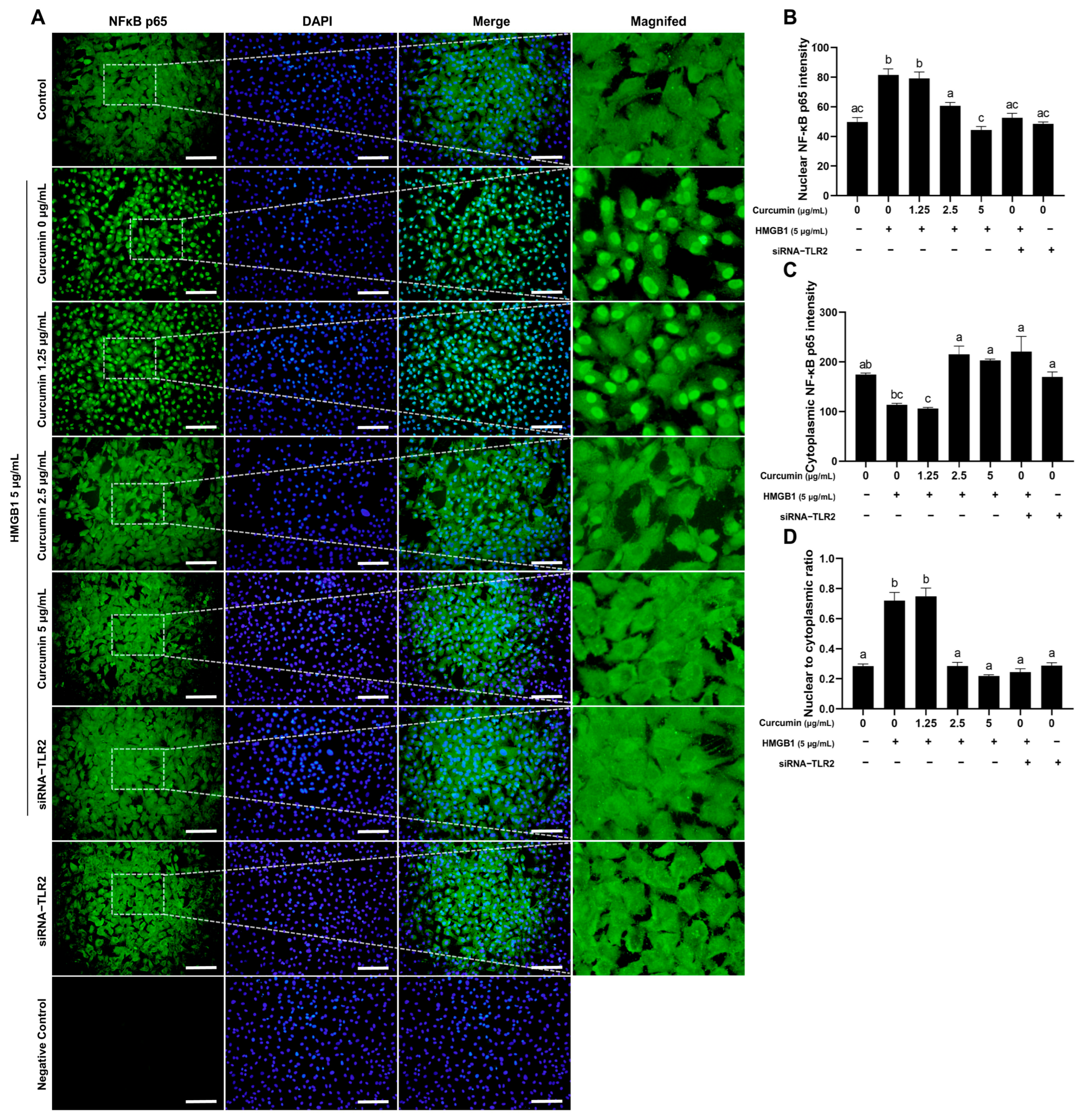
2.5. Curcumin Inhibits HMGB1-Induced NF-κB Nuclear Translocation
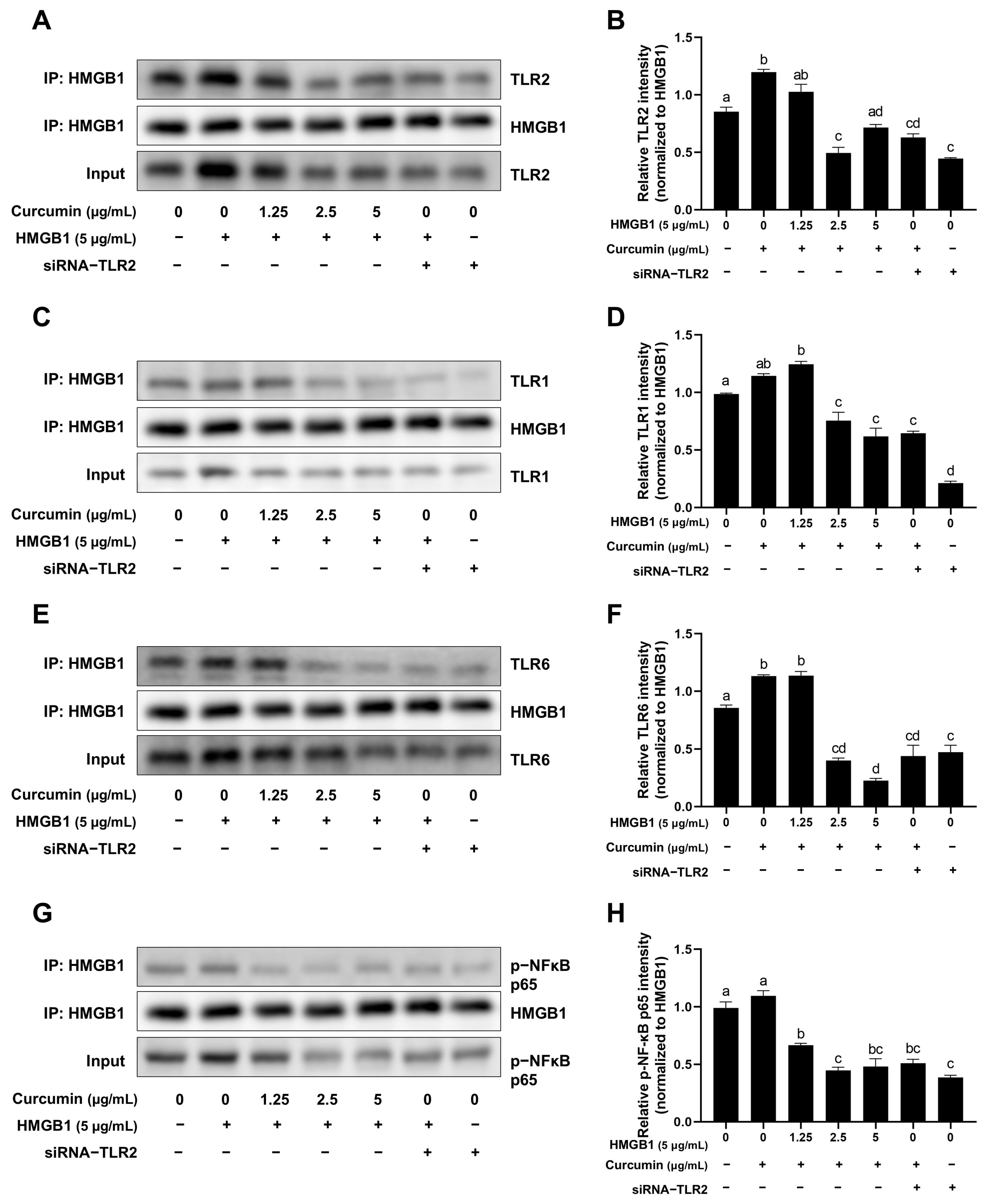
2.6. Curcumin Attenuates the Binding of HMGB1 to TLR1, TLR2, TLR6 and Phospho-NF-κB p65
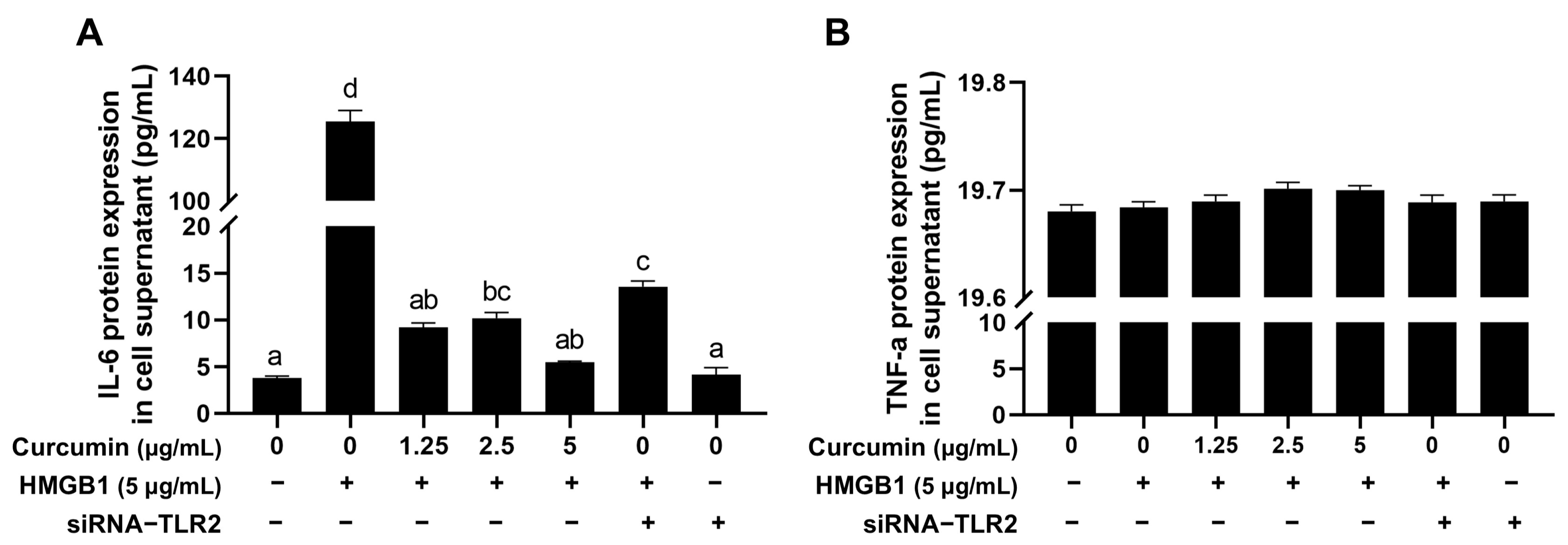
2.7. Curcumin Reduces HMGB1-Induced Increase of the Inflammatory Factor IL-6
3. Discussion
4. Materials and Methods
4.1. Primary Reagents and Consumables
4.2. Instruments and Equipment
4.3. Isolation and Culture of BOGCs
4.4. MTT Assay
4.5. Apoptosis Detection
4.6. Cell Treatment
4.7. Total RNA Extraction and cDNA Synthesis from BOGCs
4.8. Reverse Transcription-Quantitative PCR (RT-qPCR)
4.9. Western Blot (WB)
4.10. Indirect Immunofluorescence (IIF)
4.11. Co-Immunoprecipitation (CO-IP)
4.12. Enzyme-Linked Immunosorbent Assay (ELISA)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HMGB1 | High-mobility group box 1 |
| BOGCs | Bovine ovarian granulosa cells |
| TLR2 | Toll-like receptor 2 |
| DAMP | Damage-associated molecular pattern |
| RAGE | Receptor for advanced glycation end products |
| PCOS | Polycystic ovarian syndrome |
| RT-qPCR | Reverse transcription-quantitative PCR |
| WB | Western blot |
| IIF | Indirect immunofluorescence |
| CO-IP | Co-immunoprecipitation |
| ELISA | Enzyme-linked immunosorbent assay |
| IL-6 | Interleukin 6 |
| TNF-α | Tumor necrosis factor α |
References
- Dosoky, N.S.; Setzer, W.N. Chemical Composition and Biological Activities of Essential Oils of Curcuma species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Kamal, D.A.M.; Salamt, N.; Yusuf, A.N.M.; Kashim, M.; Mokhtar, M.H. Potential Health Benefits of Curcumin on Female Reproductive Disorders: A Review. Nutrients 2021, 13, 3126. [Google Scholar] [CrossRef]
- Japheth, K.P.; Kumaresan, A.; Patbandha, T.K.; Baithalu, R.K.; Selvan, A.S.; Nag, P.; Manimaran, A.; Oberoi, P.S. Supplementation of a combination of herbs improves immunity, uterine cleansing and facilitate early resumption of ovarian cyclicity: A study on post-partum dairy buffaloes. J. Ethnopharmacol. 2021, 272, 113931. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Kadasi, A.; Stochmalova, A.; Balazi, A.; Földesiová, M.; Makovicky, P.; Chrenek, P.; Harrath, A.H. Effect of turmeric on the viability, ovarian folliculogenesis, fecundity, ovarian hormones and response to luteinizing hormone of rabbits. Animal 2018, 12, 1242–1249. [Google Scholar] [CrossRef]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent Advances in the Synthesis of Diarylheptanoids. Chem. Asian J. 2024, 19, e202400380. [Google Scholar] [CrossRef]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chen, P.F.; Dai, W.C.; Zheng, Z.Q.; Wang, H.L. Curcumin Alleviates Hyperandrogenism and Promotes Follicular Proliferation in Polycystic Ovary Syndrome Rats: Insights on IRS1/PI3K/GLUT4 and PTEN Modulations. Chin. J. Integr. Med. 2022, 28, 1088–1095. [Google Scholar] [CrossRef]
- Yan, Z.; Dai, Y.; Fu, H.; Zheng, Y.; Bao, D.; Yin, Y.; Chen, Q.; Nie, X.; Hao, Q.; Hou, D.; et al. Curcumin exerts a protective effect against premature ovarian failure in mice. J. Mol. Endocrinol. 2018, 60, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Huang, H.; Hu, T.; Zou, C.; Qiao, Y.; Fang, M.; Liu, J.; Lai, S. Curcumin pretreatment attenuates myocardial ischemia/reperfusion injury by inhibiting ferroptosis, autophagy and apoptosis via HES1. Int. J. Mol. Med. 2024, 54, 110. [Google Scholar] [CrossRef]
- Dinc, D.; Seyhan, M.F.; Aktoren, O. Cytotoxicity and Alkaline Phosphatase Activity of Curcumin, Aloin and MTA on Human Dental Pulp Cells. Indian J. Dent. Res. 2024, 35, 216–220. [Google Scholar] [CrossRef]
- Ashraaf, S.; Tahir, H.M.; Raza, C.; Awad, E.M.; Ali, S.; Khan, S.Y.; Barisani-Asenbauer, T. Synergistic Effect of Silk Sericin and Curcumin to Treat an Inflammatory Condition. J. Burn Care Res. 2023, 44, 106–113. [Google Scholar] [CrossRef]
- Afshari, H.; Noori, S.; Zarghi, A. Curcumin potentiates the anti-inflammatory effects of Tehranolide by modulating the STAT3/NF-κB signaling pathway in breast and ovarian cancer cell lines. Inflammopharmacology 2023, 31, 2541–2555. [Google Scholar] [CrossRef]
- Ghorbanzadeh, V.; Pourheydar, B.; Dariushnejad, H.; Ghalibafsabbaghi, A.; Chodari, L. Curcumin improves angiogenesis in the heart of aged rats: Involvement of TSP1/NF-κB/VEGF-A signaling. Microvasc. Res. 2022, 139, 104258. [Google Scholar] [CrossRef]
- Ravindran, F.; Koroth, J.; Manjunath, M.; Narayan, S.; Choudhary, B. Curcumin derivative ST09 modulates the miR-199a-5p/DDR1 axis and regulates proliferation and migration in ovarian cancer cells. Sci. Rep. 2021, 11, 23025. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Zeh, H.J.; Lotze, M.T. The multifunctional protein HMGB1: 50 years of discovery. Nat. Rev. Immunol. 2023, 23, 824–841. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhao, L.; Sun, Y.; Wang, X.; Shi, X. HMGB1 and Toll-like receptors: Potential therapeutic targets in autoimmune diseases. Mol. Med. 2023, 29, 117. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.; Lu, Y.; Han, J.; Guo, T.; Cui, P.; Brännström, M.; Shao, L.R.; Billig, H. Regulatory mechanisms of HMGB1 and its receptors in polycystic ovary syndrome-driven gravid uterine inflammation. FEBS J. 2023, 290, 1874–1906. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, F.; Wang, J.; Liu, H.; Zhang, H.; Liu, M.; Liu, K.; Ye, Z. Molecular mechanisms regulating natural menopause in the female ovary: A study based on transcriptomic data. Front. Endocrinol. 2023, 14, 1004245. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, K.; Zhang, K.; Zhang, J.; Wang, L.; Wang, X.; Hu, X.; Liang, Z.; Li, J. Toll-like receptors and high mobility group box 1 in granulosa cells during bovine follicle maturation. J. Cell. Physiol. 2020, 235, 3447–3462. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, K.; Zhang, J.; Zhang, K.; Hu, X.; Wang, L.; Wang, X.; Li, J. High mobility group box-1 regulates expression of EGFR, VEGF, StAR and TIMP1/2 in bovine granulosa cells through a mechanism involving TLR2/NF-κB. Anim. Reprod. Sci. 2022, 247, 107152. [Google Scholar] [CrossRef]
- Duffy, D.M.; Ko, C.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels With Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef]
- Lonardo, M.S.; Cacciapuoti, N.; Guida, B.; Di Lorenzo, M.; Chiurazzi, M.; Damiano, S.; Menale, C. Hypothalamic-Ovarian axis and Adiposity Relationship in Polycystic Ovary Syndrome: Physiopathology and Therapeutic Options for the Management of Metabolic and Inflammatory Aspects. Curr. Obes. Rep. 2024, 13, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Al-Khazaleh, A.K.; Bhuyan, D.J.; Li, F.; Li, C.G. A Review of Recent Curcumin Analogues and Their Antioxidant, Anti-Inflammatory, and Anticancer Activities. Antioxidants 2024, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A.; Chaudhari, V.S.; Bose, S. Bone Healing via Carvacrol and Curcumin Nanoparticle on 3D Printed Scaffolds. Small 2024, 20, e2405642. [Google Scholar] [CrossRef]
- Zhou, H.; Zhuang, Y.; Liang, Y.; Chen, H.; Qiu, W.; Xu, H.; Zhou, H. Curcumin exerts anti-tumor activity in colorectal cancer via gut microbiota-mediated CD8+ T Cell tumor infiltration and ferroptosis. Food Funct. 2025, 16, 3671–3693. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Wang, L.; Chen, L.; Goh, B.C. Curcumin in cancer therapy: Exploring molecular mechanisms and overcoming clinical challenges. Cancer Lett. 2023, 570, 216332. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Li, J.D.; Zhang, J.G.; Zhang, K.; Zhang, A.R.; Li, P.P.; Li, Q.J.; Guo, H.Z. Mechanism of action and new developments in the study of curcumin in the treatment of osteoarthritis: A narrative review. Inflammopharmacology 2025, 33, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Rani, J.; Dhull, S.B.; Rose, P.K.; Kidwai, M.K. Drug-induced liver injury and anti-hepatotoxic effect of herbal compounds: A metabolic mechanism perspective. Phytomedicine 2024, 122, 155142. [Google Scholar] [CrossRef]
- Stassi, A.F.; Díaz, P.U.; Gasser, F.B.; Velázquez, M.M.L.; Gareis, N.C.; Salvetti, N.R.; Ortega, H.H.; Baravalle, M.E. A review on inflammation and angiogenesis as key mechanisms involved in the pathogenesis of bovine cystic ovarian disease. Theriogenology 2022, 186, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Zangui, M.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Current evidence and future perspectives for curcumin and its analogues as promising adjuncts to oxaliplatin: State-of-the-art. Pharmacol. Res. 2019, 141, 343–356. [Google Scholar] [CrossRef]
- Richards, J.S.; Liu, Z.; Shimada, M. Immune-like mechanisms in ovulation. Trends Endocrinol. Metab. 2008, 19, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Akison, L.K.; Robertson, S.A.; Gonzalez, M.B.; Richards, J.S.; Smith, C.W.; Russell, D.L.; Robker, R.L. Regulation of the ovarian inflammatory response at ovulation by nuclear progesterone receptor. Am. J. Reprod. Immunol. 2018, 79, e12835. [Google Scholar] [CrossRef]
- Wang, J.; Yin, T.; Liu, S. Dysregulation of immune response in PCOS organ system. Front. Immunol. 2023, 14, 1169232. [Google Scholar] [CrossRef]
- Tan, G.; Huang, C.; Chen, J.; Zhi, F. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J. Hematol. Oncol. 2020, 13, 149. [Google Scholar] [CrossRef]
- Feng, Z.; Meng, F.; Huo, F.; Zhu, Y.; Qin, Y.; Gui, Y.; Zhang, H.; Lin, P.; He, Q.; Li, Y.; et al. Inhibition of ferroptosis rescues M2 macrophages and alleviates arthritis by suppressing the HMGB1/TLR4/STAT3 axis in M1 macrophages. Redox Biol. 2024, 75, 103255. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Hu, S.; Bai, X. Development of a novel stirrerliquid/solid microextraction method for the separation and enrichment of trace levels of active compounds in traditional Chinese medicine. J. Sep. Sci. 2016, 39, 4290–4298. [Google Scholar] [CrossRef]
- Gao, C.; Zhou, Y.; Chen, Z.; Li, H.; Xiao, Y.; Hao, W.; Zhu, Y.; Vong, C.T.; Farag, M.A.; Wang, Y.; et al. Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics 2022, 12, 5596–5614. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Huang, Q.; Wang, Y.; Yao, Y.; Li, J.; Chen, J.; Wu, M.; Zhang, Z.; E, M.; Qi, H.; et al. Therapeutic application of natural products: NAD+ metabolism as potential target. Phytomedicine 2023, 114, 154768. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, C.; Zhou, M.; Xu, S.; Xie, Y.; Gao, S.; Yang, Y.; Deng, Z.; Zhang, L.; Shu, J.; et al. Role of curcumin in the treatment of acute kidney injury: Research challenges and opportunities. Phytomedicine 2022, 104, 154306. [Google Scholar] [CrossRef]
- Jin, M.; Fang, J.; Wang, J.J.; Shao, X.; Xu, S.W.; Liu, P.Q.; Ye, W.C.; Liu, Z.P. Regulation of toll-like receptor (TLR) signaling pathways in atherosclerosis: From mechanisms to targeted therapeutics. Acta. Pharmacol. Sin. 2023, 44, 2358–2375. [Google Scholar] [CrossRef]
- Ho, W.I.; Hu, Y.; Cheng, C.W.; Wei, R.; Yang, J.; Li, N.; Au, K.W.; Tse, Y.L.; Wang, Q.; Ng, K.M.; et al. Liposome-encapsulated curcumin attenuates HMGB1-mediated hepatic inflammation and fibrosis in a murine model of Wilson’s disease. Biomed. Pharmacother. 2022, 152, 113197. [Google Scholar] [CrossRef]
- Mirzaei, H.; Shakeri, A.; Rashidi, B.; Jalili, A.; Banikazemi, Z.; Sahebkar, A. Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed. Pharmacother. 2017, 85, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, H.; Imaizumi, A.; Sumi, Y.; Hashimoto, T.; Kanai, M.; Makino, Y.; Tsuda, T.; Takahashi, N.; Kakeya, H. Curcumin β-D-Glucuronide Plays an Important Role to Keep High Levels of Free-Form Curcumin in the Blood. Biol. Pharm. Bull. 2017, 40, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef]
- Hoehle, S.I.; Pfeiffer, E.; Metzler, M. Glucuronidation of curcuminoids by human microsomal and recombinant UDP-glucuronosyltransferases. Mol. Nutr. Food Res. 2007, 51, 932–938. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.; Zhang, L.; Wang, L.; Peng, Z.; Chen, Y. The pharmacokinetics and tissue distribution of curcumin and its metabolites in mice. Biomed. Chromatogr. 2018, 24, e4267. [Google Scholar] [CrossRef] [PubMed]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef]
- Ai, S.; Li, Y.; Zheng, H.; Zhang, M.; Tao, J.; Liu, W.; Peng, L.; Wang, Z.; Wang, Y. Collision of herbal medicine and nanotechnology: A bibliometric analysis of herbal nanoparticles from 2004 to 2023. J. Nanobiotechnol. 2024, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jia, W.; Zhang, R.; Wang, X.; Zhang, L. Improving curcumin bioavailability: Targeted delivery of curcumin and loading systems in intestinal inflammation. Food Res. Int. 2024, 196, 115079. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Primer Sequence (5′→3′) | Accession No. |
|---|---|---|
| TLR2 | Forward: 5′-GCA CTT CAA CCC TCC CTT TA-3′ Reverse: 5′-GTT CTC CGA AAG CAC AAA GAT G-3′ | NM_174197.2 |
| EGFR | Forward: 5′-CTA TGA CCC TAC CAC CTA CGA-3′ Reverse: 5′-AAA CTC ACC GAT TCC TAT TCC-3′ | XM_002696890.5 |
| VEGF | Forward: 5′-CCC ACG AAG TGG TGA AGT TCA-3′ Reverse: 5′-CCA CCA GGG TCT CGA TGG-3′ | NM_001316955.1 |
| STAR | Forward: 5′-GTG GAT TTT GCC AAT CAC CT-3′ Reverse: 5′-TTA TTG AAA ACG TGC CAC CA-3′ | NM_174189.3 |
| TIMP1 | Forward: 5′-CCT ATG CTG CTG GTT GTG AGG A-3′ Reverse: 5′-AGT GAG TGT CGC TCT GCA GTT TG-3′ | NM_174471.3 |
| TIMP2 | Forward: 5′-ACA GGT ACC AGA TGG GCT GTG AG-3′ Reverse: 5′-CGT GAC CCA GTC CAT CCA GA-3′ | NM_174472.4 |
| ACTB | Forward: 5′-ATC GGC AAT GAG CGG TTC C-3′ Reverse: 5′-GTG TTG GCG TAG AGG TCC TTG-3′ | NM_173979.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Xie, Y.; Wang, L.; Zhang, J.; Chen, X.; Feng, X.; Wang, J.; Zhang, K.; Li, J. Curcumin Alleviates HMGB1-Mediated Inflammation Through the Signaling Pathway of TLR2-NF-κB in Bovine Ovarian Granulosa Cells. Int. J. Mol. Sci. 2025, 26, 9180. https://doi.org/10.3390/ijms26189180
Liu S, Xie Y, Wang L, Zhang J, Chen X, Feng X, Wang J, Zhang K, Li J. Curcumin Alleviates HMGB1-Mediated Inflammation Through the Signaling Pathway of TLR2-NF-κB in Bovine Ovarian Granulosa Cells. International Journal of Molecular Sciences. 2025; 26(18):9180. https://doi.org/10.3390/ijms26189180
Chicago/Turabian StyleLiu, Siqi, Yingying Xie, Lei Wang, Jingyan Zhang, Xiaoliang Chen, Xiaowei Feng, Junyan Wang, Kang Zhang, and Jianxi Li. 2025. "Curcumin Alleviates HMGB1-Mediated Inflammation Through the Signaling Pathway of TLR2-NF-κB in Bovine Ovarian Granulosa Cells" International Journal of Molecular Sciences 26, no. 18: 9180. https://doi.org/10.3390/ijms26189180
APA StyleLiu, S., Xie, Y., Wang, L., Zhang, J., Chen, X., Feng, X., Wang, J., Zhang, K., & Li, J. (2025). Curcumin Alleviates HMGB1-Mediated Inflammation Through the Signaling Pathway of TLR2-NF-κB in Bovine Ovarian Granulosa Cells. International Journal of Molecular Sciences, 26(18), 9180. https://doi.org/10.3390/ijms26189180





