The Metabolic Screening in Wounded Scales of Hippeastrum × hybridum Hort. Bulbs
Abstract
1. Introduction
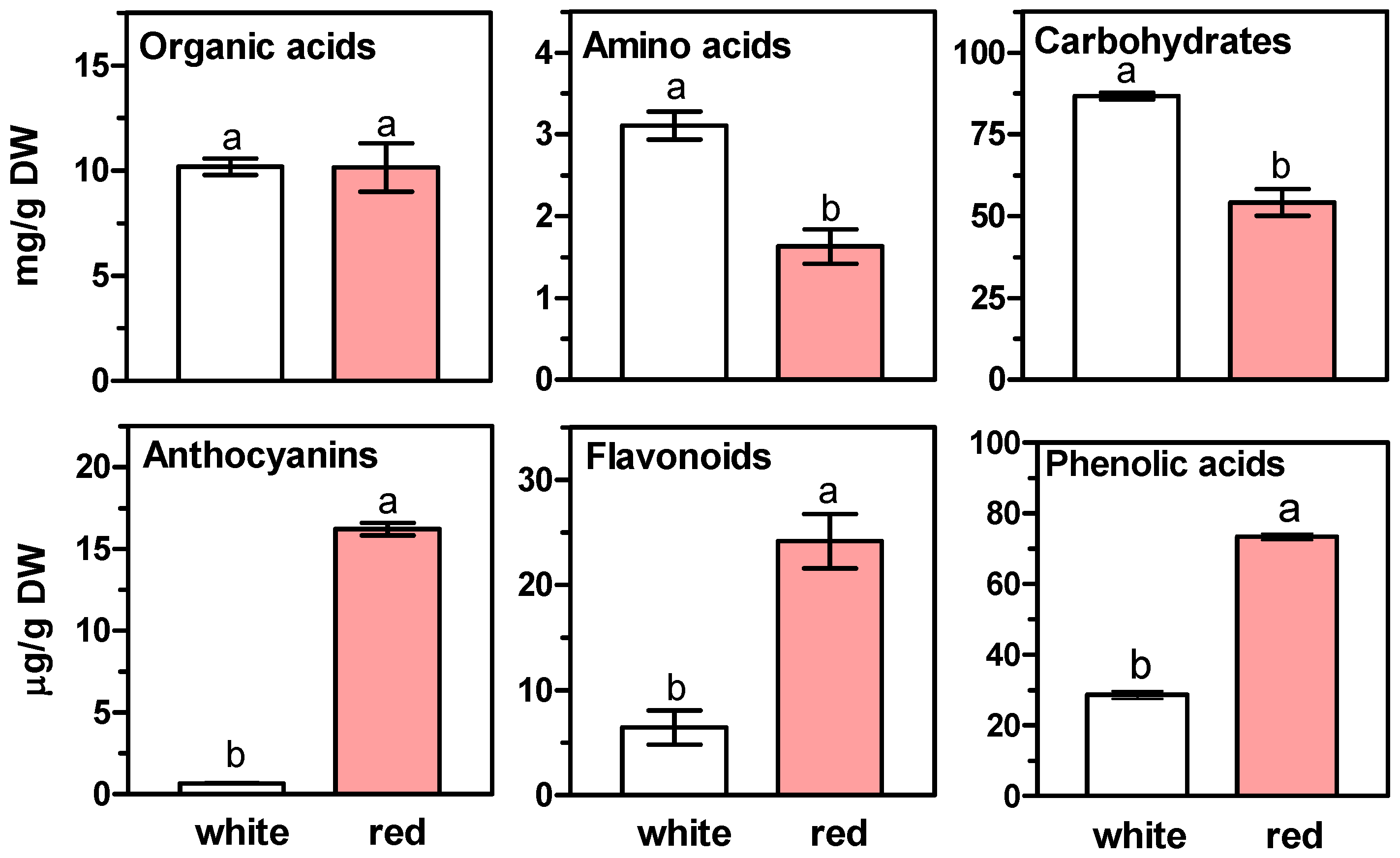
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Determination of Organic Acids and Amino Acids
4.3. Determination of Soluble Carbohydrates
4.4. Determination of Flavonoids and Phenolic Acids
4.5. Determination of Anthocyanins
4.6. Statistical Evaluation of Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| JA-Me | Jasmonic acid methyl ester; methyl jasmonate |
| l-phe | l-phenylalanine |
| SD | Standard deviation |
| DW | Dry weight |
| Tr | Traces |
| GABA | γ-Amino butyric acid |
References
- León, J.; Rojo, E.; Sánchez-Serrano, J.J. Wound signalling in plants. J. Exp. Bot. 2001, 52, 1–9. [Google Scholar] [CrossRef]
- Hoermayer, L.; Friml, J. Feeling the danger: Local wound signaling in plants. Cell Res. 2024, 34, 761–762. [Google Scholar] [CrossRef]
- Ryan, C. The systemin signaling pathway: Differential activation of plant defensive genes. Biochim. Biophys. Acta 2000, 1477, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Lapointe, G.; Rutledge, R.G.; Seguin, A. Induction of chalcone synthase expression in white spruce by wounding and jasmonate. Plant Cell Physiol. 2000, 41, 982–987. [Google Scholar] [CrossRef]
- Grayer, R.J.; Kokubun, T. Plant-fungal interactions: The search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 2001, 56, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Mitsuhara, I.; Seo, S.; Imai, T.; Koga, J.; Okada, K.; Yamane, H.; Ohashi, Y. Phytoalexin accumulation in the interaction between rice and the blast fungus. Mol. Plant Microbe Interact. 2010, 23, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Ron Mittler, R. Reactive oxygen species-dependent wound responses in animals and plants. Free Radic. Biol. Med. 2012, 53, 2269–2276. [Google Scholar] [CrossRef]
- Ka, S.; Koirala, M.; Merindol, N.; Desgagne-Penix, I. Biosynthesis and Biological Activities of Newly Discovered Amaryllidaceae Alkaloids. Molecules 2020, 25, 4901. [Google Scholar] [CrossRef]
- Rodríguez-Escobar, M.L.; Lara, R.F.; Atahuachi, M.; Fuentes, A.F.; Maldonado, C.; Bastida, J.; Tallini, R.R.; Torras-Claveria, L. Alkaloid Profile Characterisation and Bioactivity Evaluation of Bolivian Hippeastrum Species (Amaryllidaceae) as Cholinesterase Inhibitors. Life 2025, 15, 719. [Google Scholar] [CrossRef]
- Khalifa, M.F.; Fahim, J.R.; Allam, A.E.; Shoman, M.E.; El Zawily, A.; Kamel, M.S.; Shimizu, K.; Attia, E.Z. Studies on the nonalkaloidal secondary metabolites of Hippeastrum vittatum (L’Her.) Herb. Bulbs. ACS Omega 2023, 8, 26749–26761. [Google Scholar] [CrossRef]
- Ibrakaw, A.S.; Akinfenwa, A.O.; Hussein, A.A. A comprehensive review of non-alkaloidal metabolites from the subfamily Amaryllidoideae (Amaryllidaceae). Open Chem. 2023, 21, 20220252. [Google Scholar] [CrossRef]
- Saniewska, A.; Prus-Głowacki, W. Mycelial growth, pathogenicity and electrophoretic characteristics of some enzymes among isolates of Phoma narcissi (Aderh.) Boerema, de Gruyter et Noordel., comb. nov. from Hippeastrum, Narcissus and Hymenocallis. Phytopathol. Pol. 1998, 15, 5–13. [Google Scholar]
- Saniewska, A.; Budzianowski, J. The nature of red pigment formed in wounded and infected Hippeastrum bulb scale response to wounding tissues by Stagonospora curtissi (Berk.) Sacc. (Phoma narcissi). Acta Hortic. 1997, 430, 843–848. [Google Scholar] [CrossRef]
- Wink, M.; Lehmann, P. Wounding-and Elicitor-induced Formation of Coloured Chalcones and Flavans (as Phytoalexins) in Hippeastrum × hortorum. Bot. Acta 1996, 109, 412–421. [Google Scholar] [CrossRef]
- Saniewska, A.; Saniewski, M. Studies in the factor responsible for reddish coloration of mechanically injured tissue and tissue infected by fungus, Stagonospora curtisii (Berk.) Sacc. in Hippeastrum × hybr. hort. Folia Hortic. 1992, 4, 3–9. [Google Scholar]
- Saniewski, M.; Urbanek, H.; Puchalski, J. Wound-induced phenolic metabolism in scales of Hippeastrum × hybr. hort. Acta Hortic. 1992, 325, 303–306. [Google Scholar] [CrossRef]
- Saniewska, A.; Horbowicz, M.; Saniewski, M. The effect of salicylic acid and acetylsalicylic acid on red pigment formation in mechanically wounded scales of Hippeastrum × hybr. hort. and on the growth and development of Phoma narcissi. Acta Agrobot. 2005, 58, 81–90. [Google Scholar] [CrossRef][Green Version]
- Saniewski, M.; Horbowicz, M.; Saniewska, A. The effect of ruthenium red, a Ca++ channel blocker, on a red pigment formation in mechanically wounded scales of Hippeastrum × hybr. hort., and on the growth and development of Phoma narcissi. J. Fruit Ornam. Plant Res. 2006, 14, 211–222. [Google Scholar][Green Version]
- Wilmowicz, E.; Frankowski, K.; Grzegorzewska, W.; Kęsy, J.; Kućko, A.; Banach, M.; Szmidt-Jaworska, A.; Saniewski, M. The role of jasmonates in the formation of a compound of chalcones and flavans with phytoalexin-like properties in mechanically wounded scales of Hippeastrum × hybr. bulbs. Acta Biol. Cracov. Bot. 2014, 56, 54–58. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Lea, A.G.H. Flavor, Color, and Stability in Fruit Products: The Effect of Polyphenols. In Plant Polyphenols: Synthesis, Properties, Significance; Hemingway, R.W., Laks, P.E., Eds.; Basic Life Sciences; Springer: Boston, MA, USA, 1992; Volume 59, pp. 827–847. [Google Scholar] [CrossRef]
- Rahim, M.A.; Zhang, X.; Busatto, N. Phenylpropanoid biosynthesis in plants. Front. Plant Sci. 2023, 14, 1230664. [Google Scholar] [CrossRef]
- Hahlbrock, K.; Scheel, D. Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Ray, A.; Kundu, S.; Mohapatra, S.S.; Sinha, S.; Khoshru, B.; Keswani, C.; Mitra, D. An Insight into the Role of Phenolics in Abiotic Stress Tolerance in Plants: Current Perspective for Sustainable Environment. J. Pure Appl. Microbiol. 2024, 18, 64–79. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Bernards, M.A. Metabolite profiling of potato (Solanum tuberosum L.) tubers during wound-induced suberization. Metabolomics 2007, 3, 147–159. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.-J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Mattern, L.; Kneusel, R.F. Phenolic compounds in plant disease resistance. Phytoparasitica 1988, 16, 9–17. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Zhang, Y.; Zhang, Z.; Wang, Q.; Xu, Y.; Wei, J. Transcriptomics and Metabolomics Analyses Reveal Defensive Responses and Flavonoid Biosynthesis of Dracaena cochinchinensis (Lour.) Under Wound Stress in Natural Conditions. Molecules 2022, 27, 4514. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Luo, W.; Tanaka, G.; Konishi, Y.; Matsuura, H.; Takahashi, K. Wounding stress induces phenylalanine ammonia lyases, leading to the accumulation of phenylpropanoids in the model liverwort Marchantia polymorpha. Phytochemistry 2018, 155, 30–36. [Google Scholar] [CrossRef] [PubMed]
- AbouZid, S.F.; Elsherbeiny, G.M. Increase in flavonoids content in red onion peel by mechanical shredding. J. Med. Plants Res. 2008, 2, 258–260. [Google Scholar]
- Chen, Q.M.; Fu, M.R.; Yue, F.L.; Cheng, Y.Y. Effect of superfine grinding on physicochemical properties, antioxidant activity and phenolic content of red rice (Oryza sativa L.). Food Nutr. Sci. 2015, 6, 1277–1284. [Google Scholar] [CrossRef]
- Ogawa, T.; Ara, T.; Aoki, K.; Suzuki, H.; Shibata, D. Transient increase in salicylic acid and its glucose conjugates after wounding in Arabidopsis leaves. Plant Biotechnol. 2010, 27, 205–209. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, X.; Guo, X.; Liu, J.; Liu, Y.; Guo, Q.; Tang, Z. The specific responses to mechanical wound in leaves and roots of Catharanthus roseus seedlings by metabolomics. J. Plant Interact. 2018, 13, 450–460. [Google Scholar] [CrossRef]
- An, F.; Cui, M.; Chen, T.; Cheng, C.; Liu, Z.; Luo, X.; Xue, J.; Tang, Y.; Cai, J.; Chen, S. Flavonoid accumulation modulates the responses of cassava tuberous roots to postharvest physiological deterioration. Postharvest Biol. Technol. 2023, 198, 112254. [Google Scholar] [CrossRef]
- Heredia, J.B.; Cisneros-Zevallos, L. The effect of exogenous ethylene and methyl jasmonate on pal activity, phenolic profiles and antioxidant capacity of carrots (Daucus carota) under different wounding intensities. Postharvest Biol. Technol. 2009, 51, 242–249. [Google Scholar] [CrossRef]
- Gomes-Copeland, K.K.P.; Meireles, C.G.; Gomes, J.V.D.; Torres, A.G.; Sinoti, S.B.P.; Fonseca-Bazzo, Y.M.; Magalhães, P.d.O.; Fagg, C.W.; Simeoni, L.A.; Silveira, D. Hippeastrum stapfianum (kraenzl.) R.S.Oliveira & Dutilh (Amaryllidaceae) ethanol extract activity on acetylcholinesterase and PPAR-alpha/gamma receptors. Plants 2022, 11, 3179. [Google Scholar] [CrossRef]
- Estrada, O.; Alves, R.; Togawa, G.; Fagg, C.W.; Almeida, G.; Fonseca-Bazzo, Y.; Magalhães, P.d.O.; Silveira, D.A. A new chemical study of Hippeastrum stapfianum (kraenzl.) RS Oliveira & dutilh (amaryllidaceae). Biochem. Syst. Ecol. 2025, 121, 105015. [Google Scholar] [CrossRef]
- Mancinelli, A.L. Interaction between light quality and light quantity in the photoregulation of anthocyanin production. Plant Physiol. 1990, 92, 1191–1195. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, Y.; Xuan, Y.; Kari, A.; Yang, C.; Wang, C.; Zhang, C.; Gu, W.; Wang, H.; Hu, Y.; et al. Integrative transcriptome and metabolome analysis reveals the mechanisms of light-induced pigmentation in purple waxy maize. Front. Plant Sci. 2023, 14, 1203284. [Google Scholar] [CrossRef]
- Matsushita, K.; Sakayori, A.; Ikeda, T. The effect of high air temperature on anthocyanin concentration and the expressions of its biosynthetic genes in strawberry Sachinoka. Environ. Control Biol. 2016, 54, 101–107. [Google Scholar] [CrossRef]
- Yang, J.F.; Li, B.; Shi, W.; Gong, Z.; Chen, L.; Hou, Z. Transcriptional activation of anthocyanin biosynthesis in developing fruit of blueberries (Vaccinium corymbosum L.) by preharvest and postharvest UV irradiation. J. Agric. Food Chem. 2018, 66, 10931–10942. [Google Scholar] [CrossRef]
- Fan, W.; Bai, P.; Chen, R.; Guo, T.; Tian, Y.; Tian, H.; Ren, H. Postharvest Light Irradiation Induces Anthocyanin Accumulation in Fresh-Cut Lily Bulb (Lilium davidii var. unicolor) Scales. J. Food Process. Pres. 2024, 2024, 7984106. [Google Scholar] [CrossRef]
- Gude, H.; Dijkema, M.H.G.E. The effects of light quality and cold treatment on the propagation of hyacinth bulbs. Acta Hortic. 1992, 325, 157–164. [Google Scholar] [CrossRef]
- Wallace, W.; Secor, J.; Schrader, L.E. Rapid accumulation of 4-aminobutyric acid and alanine in soybean leaves in response to an abrupt transfer to lower temperature, darkness, or mechanical manipulation. Plant Physiol. 1984, 75, 170–175. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Yang, Y.; Sui, C.; Xu, Y.; Wei, J. Interxylary phloem and xylem rays are the structural foundation of agarwood resin formation in the stems of Aquilaria sinensis. Trees 2019, 33, 533–542. [Google Scholar] [CrossRef]
- Whetten, R.W.; MacKay, J.J.; Sederoff, R.R. Recent advances in understanding lignin biosynthesis. Annu. Rev. Plant Phys. 1998, 49, 585–609. [Google Scholar] [CrossRef]
- Zhong, R.; Ye, Z.-H. Transcriptional regulation of lignin biosynthesis. Plant Signal Behav. 2009, 4, 1028–1034. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Szablińska-Piernik, J.; Lahuta, L.B. Changes in Polar Metabolites during Seed Germination and Early Seedling Development of Pea, Cucumber, and Wheat. Agriculture 2023, 13, 2278. [Google Scholar] [CrossRef]
- Szablińska-Piernik, J.; Lahuta, L.B. Metabolite profiling of semi-leafless pea (Pisum sativum L.) under progressive soil drought and subsequent re-watering. J. Plant Physiol. 2021, 256, 153314. [Google Scholar] [CrossRef]
- Lahuta, L.B. Biosynthesis of raffinose family oligosaccharides and galactosyl pinitols in developing and maturing seeds of winter vetch (Vicia villosa Roth.). Acta Soc. Bot. Pol. 2006, 75, 219–227. [Google Scholar] [CrossRef][Green Version]
- Dębski, H.; Wiczkowski, W.; Horbowicz, M. Effect of elicitation with iron chelate and sodium metasilicate on phenolic compounds in legume sprouts. Molecules 2021, 26, 1345. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Szawara-Nowak, D.; Romaszko, J. The impact of red cabbage fermentation on bioavailability of anthocyanins and antioxidant capacity of human plasma. Food Chem. 2016, 190, 730–740. [Google Scholar] [CrossRef] [PubMed]

| White | Red | |||||
|---|---|---|---|---|---|---|
| Ferulic acid | ||||||
| Total | 22.70 ± 0.55 b | 50.73 ± 1.16 a | ||||
| A/E/G | 0.58 ± 0.09 c | 22.03 ± 0.43 b | 0.09 ± 0.03 d | 1.27 ± 0.48 c | 49.08 ± 0.61 a | 0.38 ± 0.08 c |
| 4-Coumaric acid | ||||||
| Total | 0.70 ± 0.03 b | 3.77 ± 0.07 a | ||||
| A/E/G | 0.03 ± 0.01 d | 0.66 ± 0.03 c | Tr | 1.10 ± 0.01 b | 2.63 ± 0.05 a | 0.04 ± 0.01 d |
| Protocatechuic acid | ||||||
| Total | 0.10 ± 0.02 b | 0.37 ± 0.05 a | ||||
| A/E/G | Tr | Tr | 0.07 ± 0.01 c | 0.06 ± 0.02 c | 0.15 ± 0.01 a | 0.16 ± 0.02 a |
| Caffeic acid | ||||||
| Total | 2.31 ± 0.10 b | 11.88 ± 0.36 a | ||||
| A/E/G | 0.04 ± 0.01 e | 2.14 ± 0.08 b | 0.12 ± 0.02 d | 0.63 ± 0.01 c | 11.06 ± 0.35 a | 0.19 ± 0.01 d |
| Caftaric acid | ||||||
| Total | 0.03 ± 0.01 a | 0.09 ± 0.03 a | ||||
| A/E/G | Tr | 0.02 ± 0.01 a | Tr | 0.07 ± 0.02 a | 0.02 ± 0.02 a | Tr |
| Ellagic acid | ||||||
| Total | 1.77 ± 0.19 b | 4.36 ± 0.21 a | ||||
| A/E/G | 0.77 ± 0.01 b | 0.33 ± 0.11 cd | 0.67 ± 0.07 bc | 3.46 ± 0.20 a | 0.29 ± 0.01 d | 0.61 ± 0.01 c |
| Synapic acid | ||||||
| Total | 1.06 ± 0.24 b | 2.25 ± 0.02 a | ||||
| A/E/G | Tr | 1.06 ± 0.24 b | Tr | Tr | 2.25 ± 0.02 a | Tr |
| White | Red | |||||
|---|---|---|---|---|---|---|
| Quercetin | ||||||
| Total | 0.02 ± 0.01 b | 0.36 ± 0.02 a | ||||
| A/E/G | Tr | Tr | 0.02 ± 0.01 b | 0.33 ± 0.01 a | Tr | 0.03 ± 0.01 b |
| Apigenin | ||||||
| Total | Tr | 0.05 ± 0.01 | ||||
| A/E/G | Tr | Tr | Tr | 0.05 ± 0.01 | Tr | Tr |
| Kaempferol | ||||||
| Total | 0.02 ± 0.01 b | 0.78 ± 0.09 a | ||||
| A/E/G | Tr | Tr | 0.02 ± 0.01 b | 0.70 ± 0.07 a | 0.07 ± 0.02 b | Tr |
| Luteolin | ||||||
| Total | 0.07 ± 0.03 b | 13.05 ± 1.64 a | ||||
| A/E/G | 0.03 ± 0.01 c | Tr | 0.04 ± 0.01 c | 12.43 ±1.62 a | 0.56 ± 0.03 b | 0.06 ± 0.01 c |
| Catechin | ||||||
| Total | 6.33 ± 1.54 a | 4.12 ± 0.55 a | ||||
| A/E/G | 0.23 ± 0.21 bc | 0.08 ± 0.02 c | 6.01 ± 1.31 a | 0.71 ± 0.39 bc | 0.27 ± 0.07 c | 3.14 ± 0.09 a |
| Naringenin | ||||||
| Total | 0.02 ± 0.01 b | 5.83 ± 0.28 a | ||||
| A/E/G | 0.02 ± 0.01 c | Tr | Tr | 5.70 ± 0.27 a | 0.12 ± 0.01 b | Tr |
| Determined Anthocyanin | White | Red |
|---|---|---|
| Cyanidin | Tr | 5.75 ± 0.11 |
| Pelargonidin | 0.02 ± 0.01 b | 2.66 ± 0.07 a |
| Cyanidin 3-glucoside | 0.31 ± 0.04 b | 1.14 ± 0.03 a |
| Delphinidin 3-glucoside | Tr | 0.42 ± 0.05 |
| Pelargonidin 3-glucoside | Tr | 1.20 ± 0.01 |
| Cyanidin diglucoside | 0.02 ± 0.01 b | 2.29 ± 0.01 a |
| Peonidin diglucoside | Tr | 1.54 ± 0.07 |
| Delphinidin diglucoside | 0.29 ± 0.01 b | 1.20 ± 0.05 a |
| Determined Metabolite | White | Red |
|---|---|---|
| Organic acids | ||
| Acetic acid | 0.17 ± 0.02 a | 0.07 ± 0.01 b |
| Succinic acid | 0.08 ± 0.01 a | 0.09 ± 0.01 a |
| Malic acid | 3.27 ± 0.09 a | 4.57 ± 0.53 a |
| Citric acid | 6.68 ± 0.32 a | 5.42 ± 0.61 a |
| Amino acids | ||
| Valine | 0.18 ± 0.02 a | 0.10 ± 0.01 b |
| Alanine | 0.08 ± 0.01 a | 0.08 ± 0.01 a |
| Serine | 0.45 ± 0.01 a | 0.19 ± 0.03 b |
| Leucine | 0.14 ± 0.01 a | 0.06 ± 0.01 b |
| Isoleucine | 0.23 ± 0.01 a | 0.09 ± 0.02 b |
| Proline | 0.12 ± 0.01 a | 0.06 ± 0.01 b |
| Hydroxyproline | 0.49 ± 0.12 a | 0.33 ± 0.07 a |
| l-Phenylalanine | 0.13 ± 0.03 a | 0.01 ± 0.01 b |
| Aspargic acid | 0.70 ± 0.03 a | 0.42 ± 0.06 b |
| Glutamic acid | 0.14 ± 0.09 a | 0.04 ± 0.02 a |
| Asparagine | 0.06 ± 0.01 a | 0.12 ± 0.02 a |
| γ-Amino butyric acid (GABA) | 0.38 ± 0.07 a | 0.13 ± 0.04 b |
| Carbohydrates | ||
| Fructose | 17.48 ± 0.15 a | 16.28 ± 1.87 a |
| Galactose | 3.26 ± 0.29 a | 1.81 ± 0.22 b |
| Glucose | 17.69 ± 0.66 a | 7.81 ± 0.71 b |
| myo-Inositol | 0.20 ± 0.02 a | 0.22 ± 0.04 a |
| Sucrose | 27.66 ± 1.35 a | 13.69 ± 1.62 b |
| 1-Kestose | 20.40 ± 0.35 a | 14.38 ± 0.36 b |
| Others | ||
| Glycerol | 0.22 ± 0.04 a | 0.26 ± 0.06 a |
| Phosphoric acid | 3.42 ± 0.09 a | 5.06 ± 0.67 a |
| Total polar compounds | 103.63 ± 1.62 a | 71.29 ± 6.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiczkowski, W.; Lahuta, L.B.; Szawara-Nowak, D.; Stałanowska, K.; Saniewski, M.; Marasek-Ciołakowska, A.; Góraj-Koniarska, J.; Horbowicz, M. The Metabolic Screening in Wounded Scales of Hippeastrum × hybridum Hort. Bulbs. Int. J. Mol. Sci. 2025, 26, 9179. https://doi.org/10.3390/ijms26189179
Wiczkowski W, Lahuta LB, Szawara-Nowak D, Stałanowska K, Saniewski M, Marasek-Ciołakowska A, Góraj-Koniarska J, Horbowicz M. The Metabolic Screening in Wounded Scales of Hippeastrum × hybridum Hort. Bulbs. International Journal of Molecular Sciences. 2025; 26(18):9179. https://doi.org/10.3390/ijms26189179
Chicago/Turabian StyleWiczkowski, Wiesław, Lesław B. Lahuta, Dorota Szawara-Nowak, Karolina Stałanowska, Marian Saniewski, Agnieszka Marasek-Ciołakowska, Justyna Góraj-Koniarska, and Marcin Horbowicz. 2025. "The Metabolic Screening in Wounded Scales of Hippeastrum × hybridum Hort. Bulbs" International Journal of Molecular Sciences 26, no. 18: 9179. https://doi.org/10.3390/ijms26189179
APA StyleWiczkowski, W., Lahuta, L. B., Szawara-Nowak, D., Stałanowska, K., Saniewski, M., Marasek-Ciołakowska, A., Góraj-Koniarska, J., & Horbowicz, M. (2025). The Metabolic Screening in Wounded Scales of Hippeastrum × hybridum Hort. Bulbs. International Journal of Molecular Sciences, 26(18), 9179. https://doi.org/10.3390/ijms26189179








