Nanomedicine-Driven Modulation of the Gut–Brain Axis: Innovative Approaches to Managing Chronic Inflammation in Alzheimer’s and Parkinson’s Disease
Abstract
1. Introduction
1.1. Key Facts and Players in Chronic Neuroinflammation
1.2. Molecular Mediators of Chronic Neuroinflammation
2. Gut–Brain Interactions
2.1. Artificial Intelligence in Mapping Gut–Brain Interactions
2.2. Analytical Tools and Technologies for AI in Mapping Gut–Brain Interactions
3. Alzheimer’s Disease (AD)
AI Mapping of Gut–Brain Interactions in Alzheimer’s Disease
4. Parkinson’s Disease
Robot-Assisted Gait Training and AI Applications in Parkinson’s Disease
5. Imaging in AI
6. Types of AI Algorithms Used in Microbiome Research
6.1. Machine Learning in Microbiome Research
6.1.1. Transfer Learning in Alzheimer’s Disease Detection
6.1.2. Tau and Tubulin
6.2. Random Forest (RF)
6.3. K-Means Clustering
6.4. Support Vector Machines (SVMs)
6.5. Naive Bayes (NB)
6.6. Deep Learning (DL) and Pattern Recognition
7. Biochips as a New Trend
7.1. Optically Transparent Microfluidic Culture-Chips
7.2. Biochips for Neurostimulation
7.3. Utility of Biochips
7.4. BIOCARD Data
8. Organ-on-a-Chip
8.1. Gut-on-a-Chip
8.2. Brain-on-a-Chip
8.3. Gut–Brain-on-a-Chip
9. Exposome
9.1. Future Directions of Exposome and AI
9.2. Artificial Intelligence = New Exposome Opportunities
10. Future Research Directions: Bridging Microbiome and AI
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | Artificial Bee Colony |
| ABM | Agent-Based Modeling |
| ABMHAP | Attention-Based Multi-Head Adaptive Pooling |
| AD | Alzheimer’s Disease |
| ADASYN | Adaptive Synthetic Sampling Approach for Imbalanced Learning |
| ADD | Alzheimer’s Disease Detection |
| ADNI | Alzheimer’s Disease Neuroimaging Initiative |
| ADRD | Alzheimer’s Disease and Related Dementias |
| AI | Artificial Intelligence |
| ALOX | Arachidonate Lipoxygenase |
| ALX | Lipoxin A4 Receptor |
| ANN | Artificial Neural Network |
| AP | Activator Protein |
| ATN | Amyloid/Tau/Neurodegeneration |
| ATP | Adenosine Triphosphate |
| AUC | Area Under the Curve |
| BAT | Bat Algorithm |
| BBB | Blood–Brain Barrier |
| BCI | Brain–Computer Interface |
| BIOCARD | Biomarkers of Cognitive Decline Among Normal Individuals |
| BPA | Bisphenol A |
| CART | Classification and Regression Tree |
| CDRSUM | Clinical Dementia Rating Sum of Boxes |
| CNN | Convolutional Neural Network |
| CNS | Central Nervous System |
| COX | Cyclooxygenase |
| CRP | C-Reactive Protein |
| CSF | Cerebrospinal Fluid |
| CWT | Continuous Wavelet Transform |
| DAMPs | Damage-Associated Molecular Patterns |
| DBS | Deep Brain Stimulation |
| DCCA | Detrended Cross-Correlation Analysis |
| DL | Deep Learning |
| DNA | Deoxyribonucleic Acid |
| DNN | Deep Neural Network |
| DT | Decision Tree |
| DYRK | Dual-specificity Tyrosine-Regulated Kinase |
| ECM | Extracellular Matrix |
| EEG | Electroencephalography |
| ERC | Extracellular Receptor Kinase (likely context-dependent, check text) |
| ERK | Extracellular Signal-Regulated Kinase |
| EXPANSE | Environmental Policy for the Ageing Population (project acronym) |
| FDG | Fluorodeoxyglucose |
| FFT | Fast Fourier Transform |
| FTLD | Frontotemporal Lobar Degeneration |
| GA | Genetic Algorithm |
| GBA | Gut–Brain Axis |
| GBM | Gradient Boosting Machine |
| GI | Gastrointestinal |
| GIS | Geographic Information System |
| GOC | Gut-on-a-Chip |
| HC | Healthy Control |
| HEALS | Health and Environment-wide Associations based on Large population Surveys (project acronym) |
| IBD | Inflammatory Bowel Disease |
| IEB | Intestinal Epithelial Barrier |
| IFN | Interferon |
| IL | Interleukin |
| IRT | Item Response Theory (context-dependent, check text) |
| KNN | K-Nearest Neighbors |
| LBP | Lipopolysaccharide-Binding Protein |
| LDA | Linear Discriminant Analysis |
| LOA | Lion Optimization Algorithm |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-Activated Protein Kinase |
| MCI | Mild Cognitive Impairment |
| MD | Mean Diffusivity |
| MGBA | Microbiota–Gut–Brain Axis |
| MINERVA | Mechanism-based Integrated Systems for Neurodegeneration and Disease Variation (project acronym) |
| ML | Machine Learning |
| MLP | Multi-Layer Perceptron |
| MMSE | Mini-Mental State Examination |
| MPS | Microphysiological Systems |
| MRI | Magnetic Resonance Imaging |
| MTAP | Multi-Task Attention Pooling |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NB | Naïve Bayes |
| NDD | Neurodegenerative Disease |
| NDDS | Novel Drug-Delivery Systems |
| NF | Nuclear Factor |
| NLP | Natural Language Processing |
| NN | Neural Network |
| NSD | Neuronal alpha-Synuclein Disease |
| OASIS | Open Access Series of Imaging Studies |
| OOC | Organ-on-a-Chip |
| PD | Parkinson’s Disease |
| PDMS | Polydimethylsiloxane |
| PET | Positron Emission Tomography |
| PFAS | Per- and Polyfluoroalkyl Substances |
| PSO | Particle Swarm Optimization |
| QDA | Quadratic Discriminant Analysis |
| RAGE | Receptor for Advanced Glycation End Products |
| RAGT | Robot-Assisted Gait Training |
| RF | Random Forest |
| RGB | Red, Green, Blue (color channels) |
| RNA | Ribonucleic Acid |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SCFA | Short-Chain Fatty Acids |
| SD | Standard Deviation |
| SPM | Specialized Pro-resolving Mediators |
| SSL | Semi-Supervised Learning |
| STHAM | Smart Technologies for Healthy Ageing with Multimorbidity (project acronym) |
| SVM | Support Vector Machine |
| TNF | Tumor Necrosis Factor |
| TRIF | TIR-domain-containing Adapter-Inducing Interferon-β |
| WGCNA | Weighted Gene Co-expression Network Analysis |
References
- Bekbolatova, M.; Mayer, J.; Ong, C.W.; Toma, M. Transformative Potential of AI in Healthcare: Definitions, Applications, and Navigating the Ethical Landscape and Public Perspectives. Healthcare 2024, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, N. Artificial intelligence and neuroscience: An update on fascinating relationships. Process Biochem. 2022, 125, 113–120. [Google Scholar] [CrossRef]
- Mathur, S.; Bhattacharjee, S.; Sehgal, S.; Shekhar, R. Role of artificial intelligence and Internet of Things in neurodegenerative diseases. In Studies in Computational Intelligence; Springer: Cham, Switzerland, 2024; pp. 35–62. [Google Scholar] [CrossRef]
- Krsek, A.; Baticic, L. Nanotechnology-Driven Therapeutic Innovations in Neurodegenerative Disorders: A Focus on Alzheimer’s and Parkinson’s Disease. Future Pharmacol. 2024, 4, 352–379. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 1486. [Google Scholar] [CrossRef]
- Tamatta, R.; Pai, V.; Jaiswal, C.; Singh, I.; Singh, A.K. Neuroinflammaging and the immune landscape: The role of autophagy and senescence in the aging brain. Biogerontology 2025, 26, 52. [Google Scholar] [CrossRef]
- Madore, C.; Yin, Z.; Leibowitz, J.; Butovsky, O. Microglia, lifestyle stress, and neurodegeneration. Immunity 2020, 52, 222–240. [Google Scholar] [CrossRef]
- Skaria, A.P. The Economic and Societal Burden of Alzheimer Disease: Managed Care Considerations. Am. J. Manag. Care 2022, 28, S188–S196. [Google Scholar]
- Dong, T.S.; Mayer, E. Advances in Brain-Gut-Microbiome Interactions: A Comprehensive Update on Signaling Mechanisms, Disorders, and Therapeutic Implications. Cell. Mol. Gastroenterol. Hepatol. 2024, 18, 1–13. [Google Scholar] [CrossRef]
- Page, M.J.; Kell, D.B.; Pretorius, E. The role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Pålsson-McDermott, E.M.; O’Neill, L.A. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004, 113, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Lopez, T. Peripheral inflammation and insulin resistance: Their impact on blood–brain barrier integrity and glia activation in Alzheimer’s disease. Int. J. Mol. Sci. 2025, 26, 4209. [Google Scholar] [CrossRef]
- Wang, X.; Wen, X.; Yuan, S.; Zhang, J. Gut-brain axis in the pathogenesis of sepsis-associated encephalopathy. Neurobiol. Dis. 2024, 195, 106499. [Google Scholar] [CrossRef]
- Lei, L.; Deng, D.; Xu, W.; Yue, M.; Wu, D.; Fu, K.; Shi, Z. Increased intestinal permeability and lipopolysaccharide contribute to swainsonine-induced systemic inflammation. Ecotoxicol. Environ. Saf. 2024, 284, 116912. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- D’Urso, F.; Broccolo, F. Applications of Artificial Intelligence in Microbiome Analysis and Probiotic Interventions—An Overview and Perspective Based on the Current State of the Art. Appl. Sci. 2024, 14, 8627. [Google Scholar] [CrossRef]
- Boland, M.; Alam, F.; Bronlund, J. Modern Technologies for Personalized Nutrition. In Advances in Personalized Nutrition; Elsevier: Cambridge, MA, USA, 2019; pp. 195–222. [Google Scholar] [CrossRef]
- Fakorede, S.; Lateef, O.M.; Garuba, W.A.; Akosile, P.O.; Okon, D.A.; Aborode, A.T. Dual impact of neuroinflammation on cognitive and motor impairments in Alzheimer’s disease. J. Alzheimer’s Dis. Rep. 2025, 9, 25424823251341870. [Google Scholar] [CrossRef]
- Rodríguez-Nuevo, A.; Zorzano, A. The sensing of mitochondrial DAMPs by non-immune cells. Cell Stress 2019, 3, 195–207. [Google Scholar] [CrossRef]
- Cieri, M.B.; Ramos, A.J. Astrocytes, reactive astrogliosis, and glial scar formation in traumatic brain injury. Neural Regen. Res. 2025, 20, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Li, X.; Zhou, S.; Jiang, P.; Liu, X.; Ouyang, M.; Nie, Y.; Chen, X.; Zhang, L.; Liu, Y.; et al. Interplay between RAGE and TLR4 regulates HMGB1-induced inflammation by promoting cell surface expression of RAGE and TLR4. J. Immunol. 2020, 205, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Venereau, E.; Casalgrandi, M.; Schiraldi, M.; Antoine, D.J.; Cattaneo, A.; De Marchis, F.; Liu, J.; Antonelli, A.; Preti, A.; Raeli, L.; et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 2012, 209, 1519–1528. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Zahoor, I.; Giri, S. Specialized pro-resolving lipid mediators: Emerging therapeutic candidates for multiple sclerosis. Clin. Rev. Allergy Immunol. 2021, 60, 147–163. [Google Scholar] [CrossRef]
- Han, T.; Xu, Y.; Sun, L.; Hashimoto, M.; Wei, J. Microglial response to aging and neuroinflammation in the development of neurodegenerative diseases. Neural Regen. Res. 2024, 19, 1241–1248. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, Y.; Deng, F.; Deng, Z. Oxidative stress: Signaling pathways, biological functions, and disease. MedComm 2025, 6, e70268. [Google Scholar] [CrossRef]
- Lecroq, T.; Soualmia, L.F. Managing large-scale genomic datasets and translation into clinical practice. Yearb. Med. Inform. 2014, 9, 212–214. [Google Scholar] [CrossRef][Green Version]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Zhou, W.; Sailani, M.R.; Contrepois, K.; Zhou, Y.; Ahadi, S.; Leopold, S.R.; Zhang, M.J.; Rao, V.; Avina, M.; Mishra, T.; et al. Longitudinal multi-omics of host–microbe dynamics in prediabetes. Nature 2019, 569, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Sharma, V.; Saxena, A.R.; Patel, A. Harnessing AI and gut microbiome research for precision health. J. Artif. Intell. Gut Sci. 2024, 3, 74–88. [Google Scholar] [CrossRef]
- Abavisani, M.; Khoshrou, A.; Foroushan, S.K.; Ebadpour, N.; Sahebkar, A. Deciphering the gut microbiome: The revolution of artificial intelligence in microbiota analysis and intervention. Curr. Res. Biotechnol. 2024, 7, 100211. [Google Scholar] [CrossRef]
- Devika, N.T.; Raman, K. Deciphering the metabolic capabilities of Bifidobacteria using genome-scale metabolic models. Sci. Rep. 2019, 9, 18222. [Google Scholar] [CrossRef] [PubMed]
- Shima, H.; Masuda, S.; Date, Y.; Shino, A.; Tsuboi, Y.; Kajikawa, M.; Inoue, Y.; Kanamoto, T.; Kikuchi, J. Exploring the Impact of Food on the Gut Ecosystem Based on the Combination of Machine Learning and Network Visualization. Nutrients 2017, 9, 1307. [Google Scholar] [CrossRef]
- Pirrone, D.; Weitschek, E.; Di Paolo, P.; De Salvo, S.; De Cola, M.C. EEG signal processing and supervised machine learning to early diagnose Alzheimer’s disease. Appl. Sci. 2022, 12, 5413. [Google Scholar] [CrossRef]
- Thulasimani, V.; Shanmugavadivel, K.; Cho, J.; Veerappampalayam Easwaramoorthy, S. A Review of Datasets, Optimization Strategies, and Learning Algorithms for Analyzing Alzheimer’s Dementia Detection. Neuropsychiatr. Dis. Treat. 2024, 20, 2203–2225. [Google Scholar] [CrossRef]
- Safi, M.S.; Safi, S.M.M. Early detection of Alzheimer’s disease from EEG signals using Hjorth parameters. Biomed. Signal Process. Control 2021, 65, 102338. [Google Scholar] [CrossRef]
- Hazarika, R.A.; Kumar Maji, A.; Kandar, D.; Jasinska, E.; Krejci, P.; Leonowicz, Z.; Jasinski, M. An approach for classification of Alzheimer’s disease using deep neural network and brain magnetic resonance imaging (MRI). Electronics 2023, 12, 676. [Google Scholar] [CrossRef]
- AlSaeed, D.; Omar, S.F. Brain MRI analysis for Alzheimer’s disease diagnosis using CNN-based feature extraction and machine learning. Sensors 2022, 22, 2911. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Kršek, A.; Batičić, L. Neutrophils in the Focus: Impact on Neuro-Immune Dynamics and the Gut–Brain Axis. Gastrointest. Disord. 2024, 6, 557–606. [Google Scholar] [CrossRef]
- Chaudhry, T.S.; Senapati, S.G.; Gadam, S.; Mannam, H.P.S.S.; Voruganti, H.V.; Abbasi, Z.; Abhinav, T.; Challa, A.B.; Pallipamu, N.; Bheemisetty, N.; et al. The impact of microbiota on the gut–brain axis: Examining the complex interplay and implications. J. Clin. Med. 2023, 12, 5231. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of early Alzheimer’s disease: Clinical practice in 2021. J. Prev. Alzheimer’s Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Juganavar, A.; Joshi, A.; Shegekar, T. Navigating early Alzheimer’s diagnosis: A comprehensive review of diagnostic innovations. Cureus 2023, 15, e44937. [Google Scholar] [CrossRef]
- Surianarayanan, C.; Lawrence, J.J.; Chelliah, P.R.; Prakash, E.; Hewage, C. Convergence of artificial intelligence and neuroscience towards the diagnosis of neurological disorders—A scoping review. Sensors 2023, 23, 3062. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Bloem, B.R. The Parkinson pandemic—A call to action. JAMA Neurol. 2018, 75, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]
- McFarthing, K.; Buff, S.; Rafaloff, G.; Oleskey, L.; Bonner, L.; McKenzie, G.; Croucher, L.; Shill, H.A.; Morris, M.; Stott, S.R.W.; et al. Parkinson’s disease drug therapies in the clinical-trial pipeline: 2023 update. J. Park. Dis. 2023, 13, 427–439. [Google Scholar] [CrossRef]
- Janssen Daalen, J.M.; Schootemeijer, S.; Richard, E.; Darweesh, S.K.L.; Bloem, B.R. Lifestyle interventions for the prevention of Parkinson disease: A recipe for action. Neurology 2022, 99, 42–51. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Adams, J.; Antoniades, C.; Bloem, B.R.; Carroll, C.; Cedarbaum, J.; Cosman, J.; Dexter, D.T.; Dockendorf, M.F.; Edgerton, J.; et al. Accelerating Parkinson’s disease drug development with federated learning approaches. npj Park. Dis. 2024, 10, 225. [Google Scholar] [CrossRef] [PubMed]
- Höglinger, G.U.; Adler, C.H.; Berg, D.; Klein, C.; Outeiro, T.F.; Poewe, W.; Postuma, R.; Stoessl, A.J.; Lang, A.E. A biological classification of Parkinson’s disease: The SynNeurGe research diagnostic criteria. Lancet Neurol. 2024, 23, 191–204, Erratum in Lancet Neurol. 2024, 23, e7. [Google Scholar] [CrossRef] [PubMed]
- Simuni, T.; Chahine, L.M.; Poston, K.; Brumm, M.; Buracchio, T.; Campbell, M.; Chowdhury, S.; Coffey, C.; Concha-Marambio, L.; Dam, T.; et al. A biological definition of neuronal α-synuclein disease: Towards an integrated staging system for research. Lancet Neurol. 2024, 23, 178–190. [Google Scholar] [CrossRef]
- Cardoso, F.; Goetz, C.G.; Mestre, T.A.; Sampaio, C.; Adler, C.H.; Berg, D.; Bloem, B.R.; Burn, D.J.; Fitts, M.S.; Gasser, T.; et al. A statement of the MDS on biological definition, staging, and classification of Parkinson’s disease. Mov. Disord. 2024, 39, 259–266. [Google Scholar] [CrossRef]
- Stephenson, D.; Belfiore-Oshan, R.; Karten, Y.; Keavney, J.; Kwok, D.K.; Martinez, T.; Montminy, J.; Müller, M.L.T.M.; Romero, K.; Sivakumaran, S. Transforming drug development for neurological disorders: Proceedings from a multidisease-area workshop. Neurotherapeutics 2023, 20, 1682–1691. [Google Scholar] [CrossRef]
- Liu, Q.; Joshi, A.; Standing, J.F.; van der Graaf, P.H. Artificial intelligence/machine learning: The new frontier of clinical pharmacology and precision medicine. Clin. Pharmacol. Ther. 2024, 115, 637–642. [Google Scholar] [CrossRef]
- Podichetty, J.T.; Sardar, S.; Henscheid, N.; Lee, G.V.; Abrams, J.R.; Anderson, W.; Ma, S.C.; Romero, K. Accelerating healthcare innovation: The role of artificial intelligence and digital-health technologies in Critical Path Institute’s public–private partnerships. Clin. Transl. Sci. 2024, 17, e13851. [Google Scholar] [CrossRef]
- Araújo, B.; Caridade-Silva, R.; Soares-Guedes, C.; Martins-Macedo, J.; Gomes, E.D.; Monteiro, S.; Teixeira, F.G. Neuroinflammation and Parkinson’s disease—From neurodegeneration to therapeutic opportunities. Cells 2022, 11, 2908. [Google Scholar] [CrossRef]
- Eo, H.; Kim, S.; Jung, U.J.; Kim, S.R. Alpha-synuclein and microglia in Parkinson’s disease: From pathogenesis to therapeutic prospects. J. Clin. Med. 2024, 13, 7243. [Google Scholar] [CrossRef]
- Galiano-Landeira, J.; Torra, A.; Vila, M.; Bové, J. CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson’s disease. Brain 2020, 143, 3717–3733. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Pinto, M.F.; Empadinhas, N.; Cardoso, S.M. The neuromicrobiology of Parkinson’s disease: A unifying theory. Ageing Res. Rev. 2021, 70, 101396. [Google Scholar] [CrossRef] [PubMed]
- Wunderle, C.; Martin, E.; Wittig, A.; Tribolet, P.; Lutz, T.A.; Köster-Hegmann, C.; Stanga, Z.; Mueller, B.; Schuetz, P. Comparison of the inflammatory biomarkers IL-6, TNF-α, and CRP to predict the effect of nutritional therapy on mortality in medical patients at risk of malnutrition: A secondary analysis of the randomized clinical trial EFFORT. J. Inflamm. 2025, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Barnig, C.; Lutzweiler, G.; Giannini, M.; Lejay, A.; Charles, A.L.; Meyer, A.; Geny, B. Resolution of inflammation after skeletal muscle ischemia-reperfusion injury: A focus on the lipid mediators lipoxins, resolvins, protectins and maresins. Antioxidants 2022, 11, 1213. [Google Scholar] [CrossRef] [PubMed]
- Folasole, N.A. Deep learning for biomarker discovery in heterogeneous data from autoimmune and inflammatory conditions. World J. Adv. Res. Rev. 2024, 24, 3407–3424. [Google Scholar] [CrossRef]
- Carmignano, S.M.; Fundarò, C.; Bonaiuti, D.; Calabrò, R.S.; Cassio, A.; Mazzoli, D.; Bizzarini, E.; Campanini, I.; Cerulli, S.; Chisari, C.; et al. Robot-assisted gait training in patients with Parkinson’s disease: Implications for clinical practice—A systematic review. NeuroRehabilitation 2022, 51, 649–663. [Google Scholar] [CrossRef]
- Lo, A.C.; Chang, V.C.; Gianfrancesco, M.A.; Friedman, J.H.; Patterson, T.S.; Benedicto, D.F. Reduction of freezing of gait in Parkinson’s disease by repetitive robot-assisted treadmill training: A pilot study. J. Neuroeng. Rehabil. 2010, 7, 51. [Google Scholar] [CrossRef]
- Nardo, A.; Anasetti, F.; Servello, D.; Porta, M. Quantitative gait analysis in patients with Parkinson treated with deep-brain stimulation: The effects of a robotic gait training. NeuroRehabilitation 2014, 35, 779–788. [Google Scholar] [CrossRef]
- Picelli, A.; Melotti, C.; Origano, F.; Waldner, A.; Fiaschi, A.; Santilli, V.; Smania, N. Robot-assisted gait training in patients with Parkinson disease: A randomized controlled trial. Neurorehabil. Neural Repair 2012, 26, 353–361. [Google Scholar] [CrossRef]
- Fundarò, C.; Maestri, R.; Ferriero, G.; Chimento, P.; Taveggia, G.; Casale, R. Self-selected-speed gait training in Parkinson’s disease: Robot-assisted gait training with virtual reality versus gait training on the ground. Eur. J. Phys. Rehabil. Med. 2019, 55, 456–462. [Google Scholar] [CrossRef]
- Galli, M.; Cimolin, V.; De Pandis, M.F.; Le Pera, D.; Sova, I.; Albertini, G.; Stocchi, F.; Franceschini, M. Robot-assisted gait training versus treadmill training in patients with Parkinson’s disease: A kinematic evaluation with gait-profile score. Funct. Neurol. 2016, 31, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.G.; Yun, S.J.; Shin, H.I.; Kim, E.; Lee, H.H.; Oh, B.M.; Seo, H.G. Effects of robot-assisted gait training in patients with Parkinson’s disease: Study protocol for a randomized controlled trial. Trials 2019, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Capecci, M.; Pournajaf, S.; Galafate, D.; Sale, P.; Le Pera, D.; Goffredo, M.; De Pandis, M.F.; Andrenelli, E.; Pennacchioni, M.; Ceravolo, M.G.; et al. Clinical effects of robot-assisted gait training and treadmill training for Parkinson’s disease: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2019, 62, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Arami, A.; Poulakakis-Daktylidis, A.; Tai, Y.F.; Burdet, E. Prediction of gait freezing in Parkinsonian patients: A binary classification augmented with time-series prediction. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1909–1919. [Google Scholar] [CrossRef]
- Shalin, G.; Pardoel, S.; Nantel, J.; Lemaire, E.D.; Kofman, J. Prediction of freezing of gait in Parkinson’s disease from foot plantar-pressure arrays using a convolutional neural network. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC 2020), Montréal, QC, Canada, 20–24 July 2020; pp. 244–247. [Google Scholar] [CrossRef]
- Bevilacqua, R.; Maranesi, E.; Di Rosa, M.; Luzi, R.; Casoni, E.; Rinaldi, N.; Baldoni, R.; Lattanzio, F.; Di Donna, V.; Pelliccioni, G.; et al. Rehabilitation of older people with Parkinson’s disease: An innovative protocol (RCT) to evaluate the potential of robotic-based technologies. BMC Neurol. 2020, 20, 186. [Google Scholar] [CrossRef]
- Perju-Dumbrava, L.; Barsan, M.; Leucuta, D.; Popa, L.C.; Pop, C.; Tohanean, N.; Popa, S.L. Artificial-intelligence applications and robotic systems in Parkinson’s disease—A review. Exp. Ther. Med. 2022, 23, 116. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, M.; Stiles, W.R.; Choi, H.S. Neuroimaging modalities in Alzheimer’s disease: Diagnosis and clinical features. Int. J. Mol. Sci. 2022, 23, 6079. [Google Scholar] [CrossRef]
- Chitradevi, D.; Prabha, S.; Daniel Prabhu, A. Diagnosis of Alzheimer disease in MR brain images using optimization techniques. Neural Comput. Appl. 2021, 33, 223–237. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, R. Recognition, analysis and classification of Alzheimer ailment using hybrid genetic and particle-swarm with deep-learning technique. Int. J. Comput. Appl. Inf. Technol. 2022, 13, 428–438. [Google Scholar]
- Hernández-Medina, R.; Kutuzova, S.; Nor Nielsen, K.; Johansen, J.; Hestbjerg Hansen, L.; Nielsen, M.; Rasmussen, S. Machine-learning and deep-learning applications in microbiome research. ISME Commun. 2022, 2, 98. [Google Scholar] [CrossRef]
- Othmani, A.; Brahem, B.; Haddou, Y. Machine-learning-based approaches for post-traumatic-stress-disorder diagnosis using video and EEG sensors: A review. IEEE Sens. J. 2023, in press. [CrossRef]
- Loosen, A.M.; Kato, A.; Gu, X. Revisiting the role of computational neuroimaging in the era of integrative neuroscience. Neuropsychopharmacology 2024, 50, 103–113. [Google Scholar] [CrossRef]
- Pellegrini, E.; Ballerini, L.; Hernandez, M.D.C.V.; Chappell, F.M.; González-Castro, V.; Anblagan, D.; Danso, S.; Muñoz-Maniega, S.; Job, D.; Pernet, C.; et al. Machine learning of neuroimaging for assisted diagnosis of cognitive impairment and dementia: A systematic review. Alzheimer’s Dement. (Amst.) 2018, 10, 519–535. [Google Scholar] [CrossRef]
- Bratić, B.; Kurbalija, V.; Ivanović, M.; Oder, I.; Bosnić, Z. Machine learning for predicting cognitive diseases: Methods, data sources and risk factors. J. Med. Syst. 2018, 42, 243. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.; Igel, C.; Hansen, N.L.; Osler, M.; Lauritzen, M.; Rostrup, E.; Nielsen, M.; Alzheimer’s Disease Neuroimaging Initiative; Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing. Early detection of Alzheimer’s disease using MRI hippocampal texture. Hum. Brain Mapp. 2016, 37, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Karahanoğlu, F.I.; Caballero-Gaudes, C.; Lazeyras, F.; Van de Ville, D. Total activation: fMRI deconvolution through spatio-temporal regularization. NeuroImage. 2013, 73, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Sadybekov, A.V.; Katritch, V. Computational approaches streamlining drug discovery. Nature 2023, 616, 673–685. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Chen, J.; Chen, J. RFtest: A robust and flexible community-level test for microbiome data powerfully detects phylogenetically-clustered signals. Front. Genet. 2022, 12, 749573. [Google Scholar] [CrossRef]
- Ieracitano, C.; Mammone, N.; Hussain, A.; Morabito, F.C. A convolutional-neural-network self-learning approach for classifying neurodegenerative states from EEG signals in dementia. In Proceedings of the 2020 International Joint Conference on Neural Networks (IJCNN), Glasgow, UK, 19–24 July 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–8. [Google Scholar]
- Ruiz-Gómez, S.J.; Gómez, C.; Poza, J.; García, M.; Gutiérrez, R.; Tola-Arribas, M.A.; Hornero, R. Automated multiclass classification of spontaneous EEG activity in Alzheimer’s disease and mild cognitive impairment. Entropy 2018, 20, 35. [Google Scholar] [CrossRef]
- Kulkarni, N. Use of complexity-based features in diagnosis of mild Alzheimer disease using EEG signals. Int. J. Inf. Technol. 2018, 10, 59–64. [Google Scholar] [CrossRef]
- Liu, M.; Fan, L.; Yan, H.; Wang, K.; Ma, Y.; Shen, L.; Alzheimer’s Disease Neuroimaging Initiative. A multi-model deep-convolutional neural network for automatic hippocampus segmentation and classification in Alzheimer’s disease. Neuroimage 2020, 208, 116459. [Google Scholar] [CrossRef] [PubMed]
- Ismail, W.N.; Fathimathul Rajeena, P.P.; Ali, M.A.S. A meta-heuristic multi-objective optimisation method for Alzheimer’s-disease detection based on multi-modal data. Mathematics 2023, 11, 957. [Google Scholar] [CrossRef]
- Ozdemir, C.; Dogan, Y. Advancing early diagnosis of Alzheimer’s disease with next-generation deep-learning methods. Biomed. Signal Process. Control 2024, 96, 106614. [Google Scholar] [CrossRef]
- Ozdemir, C. Adapting transfer-learning models to datasets through pruning and Avg-TopK pooling. Neural Comput. Appl. 2024, 36, 6257–6270. [Google Scholar] [CrossRef]
- Gunes, S.; Aizawa, Y.; Sugashi, T.; Sugimoto, M.; Rodrigues, P.P. Biomarkers for Alzheimer’s disease in the current state: A narrative review. Int. J. Mol. Sci. 2022, 23, 4962. [Google Scholar] [CrossRef]
- Azizi, T. Impact of mental arithmetic task on the electrical activity of the human brain. Neurosci. Inform. 2024, 4, 100162. [Google Scholar] [CrossRef]
- Rathore, S.; Habes, M.; Iftikhar, M.A.; Shacklett, A.; Davatzikos, C. A review on neuroimaging-based classification studies and associated feature-extraction methods for Alzheimer’s disease and its prodromal stages. Neuroimage 2017, 155, 530–548. [Google Scholar] [CrossRef]
- Beheshti, I.; Mishra, S.; Sone, D.; Khanna, P.; Matsuda, H. T1-weighted MRI-driven brain-age estimation in Alzheimer’s disease and Parkinson’s disease. Aging Dis. 2020, 11, 618–628. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M.; Liu, M.; Zhang, D. A survey on deep learning for neuroimaging-based brain-disorder analysis. arXiv 2020, arXiv:2005.04573. [Google Scholar] [CrossRef]
- De Ninno, G.; Giuffrè, G.M.; Urbani, A.; Baroni, S. Current perspectives on Alzheimer’s-disease fluid biomarkers and future challenges: A narrative review. J. Lab. Precis. Med. 2024, 9, 25. [Google Scholar] [CrossRef]
- Albala, B.; Appelmans, E.; Burress, R.; De Santi, S.; Devins, T.; Klein, G.; Logovinsky, V.; Novak, G.P.; Ribeiro, K.; Schmidt, M.E.; et al. The Alzheimer’s Disease Neuroimaging Initiative and the role and contributions of the Private Partners Scientific Board (PPSB). Alzheimer’s Dement. 2023, 20, 695–708. [Google Scholar] [CrossRef]
- Sorour, S.E.; El-Mageed, A.A.A.; Albarrak, K.M.; Alnaim, A.K.; Wafa, A.A.; El-Shafeiy, E. Classification of Alzheimer’s disease using MRI data based on deep-learning techniques. J. King Saud Univ. Comput. Inf. Sci. 2024, 36, 101940. [Google Scholar] [CrossRef]
- Raju, M.; Sudila, T.V.; Varun, P. Classification of mild cognitive impairment and Alzheimer’s disease from magnetic-resonance images using deep learning. In Proceedings of the 2020 International Conference on Recent Trends on Electronics, Information, Communication and Technology (RTEICT), Bengaluru, India, 12–13 November 2020; pp. 52–57. [Google Scholar]
- Hilton, C.B.; Milinovich, A.; Felix, C.; Vakharia, N.; Crone, T.; Donovan, C.; Proctor, A.; Nazha, A. Personalized predictions of patient outcomes during and after hospitalization using artificial intelligence. npj Digit. Med. 2020, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Márquez, F.; Yassa, M.A. Neuroimaging biomarkers for Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Moon, S.W.; Lee, B.; Choi, Y.C. Changes in hippocampal volume and shape in early-onset mild cognitive impairment. Psychiatry Investig. 2018, 15, 531–537. [Google Scholar] [CrossRef]
- Georgakas, J.E.; Howe, M.D.; Thompson, L.I.; Riera, N.M.; Riddle, M.C. Biomarkers of Alzheimer’s disease: Past, present and future clinical use. Biomark. Neuropsychiatry 2023, 8, 100063. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Pearlson, G.D.; Sui, J. Data-driven approaches to neuroimaging biomarkers for neurological and psychiatric disorders: Emerging approaches and examples. Curr. Opin. Neurol. 2021, 34, 469–479. [Google Scholar] [CrossRef]
- Wen, G.; Shim, V.; Holdsworth, S.J.; Fernandez, J.; Qiao, M.; Kasabov, N.; Wang, A. Machine learning for brain-MRI data harmonisation: A systematic review. Bioengineering 2023, 10, 397. [Google Scholar] [CrossRef]
- Abbasi, S.; Lan, H.; Choupan, J.; Sheikh-Bahaei, N.; Pandey, G.; Varghese, B. Deep learning for the harmonisation of structural MRI scans: A survey. Biomed. Eng. Online 2024, 23, 90. [Google Scholar] [CrossRef]
- Wang, R.; Erus, G.; Chaudhari, P.; Davatzikos, C. Adapting machine-learning diagnostic models to new populations using a small amount of data: Results from clinical neuroscience. arXiv 2024, arXiv:2308.03175v2. [Google Scholar]
- Sato, R.; Iwamoto, Y.; Cho, K.; Kang, D.-Y.; Chen, Y.-W. Comparison of CNN models with different plane images and their combinations for classification of Alzheimer’s disease using PET images. In Innovation in Medicine and Healthcare Systems and Multimedia; Lecture Notes in Networks and Systems; Springer: Singapore, 2019; pp. 169–177. [Google Scholar]
- Deepanshi, I.B.; Garg, D. Alzheimer’s disease classification using transfer learning. In Proceedings of the International Advanced Computing Conference (IACC 2021), Msida, Malta, 18–19 December 2021; Springer: Cham, Switzerland, 2021; pp. 73–81. [Google Scholar]
- Afzal, S.; Maqsood, M.; Nazir, F.; Khan, U.; Aadil, F.; Awan, K.M. A data-augmentation framework to handle class-imbalance problem for Alzheimer’s-stage detection. IEEE Access 2019, 7, 115528–115539. [Google Scholar] [CrossRef]
- Islam, J.; Zhang, Y. Brain-MRI analysis for Alzheimer’s-disease diagnosis using an ensemble system of deep convolutional neural networks. Brain Inform. 2018, 5, 2. [Google Scholar] [CrossRef]
- Ramzan, F.; Khan, M.U.G.; Rehmat, A.; Iqbal, S.; Saba, T.; Rehman, A.; Mehmood, Z. A deep-learning approach for automated diagnosis and multi-class classification of Alzheimer’s-disease stages using resting-state fMRI and residual neural networks. J. Med. Syst. 2020, 44, 37. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sohn, J.H.; Kawczynski, M.G.; Trivedi, H.; Harnish, R.; Jenkins, N.W.; Lituiev, D.; Copeland, T.P.; Aboian, M.S.; Aparici, C.M.; et al. A deep-learning model to predict diagnosis of Alzheimer disease using 18F-FDG PET of the brain. Radiology 2019, 290, 456–464. [Google Scholar] [CrossRef]
- Cui, Z.; Gao, Z.; Leng, J.; Zhang, T.; Quan, P.; Zhao, W. Alzheimer’s-disease diagnosis using an enhanced Inception network based on brain MRI. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), San Diego, CA, USA, 18–21 November 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 2324–2330. [Google Scholar]
- Hazarika, R.A.; Kandar, D.; Kumar Maji, A. An experimental analysis of deep-learning models for Alzheimer’s-disease classification using brain MRI. J. King Saud Univ. Comput. Inf. Sci. 2022, 34, 8576–8598. [Google Scholar] [CrossRef]
- Sistaninejhad, B.; Rasi, H.; Nayeri, P. A review article on deep learning for medical-image analysis. Comput. Math. Methods Med. 2023, 2023, 7091301. [Google Scholar] [CrossRef]
- Wang, H.; Shen, Y.; Wang, S.; Xiao, T.; Deng, L.; Wang, X.; Zhao, X. Ensemble of 3-D densely connected convolutional networks for diagnosis of mild cognitive impairment and Alzheimer’s disease. Neurocomputing 2019, 333, 145–156. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Adeli, E.; Shen, D. Joint classification and regression via deep multi-task multi-channel learning for Alzheimer’s-disease diagnosis. IEEE Trans. Biomed. Eng. 2019, 66, 1195–1206. [Google Scholar] [CrossRef]
- Cui, R.; Liu, M. Hippocampus analysis by combining 3-D DenseNet and shape features for Alzheimer’s-disease diagnosis. IEEE J. Biomed. Health Inform. 2019, 23, 2099–2107. [Google Scholar] [CrossRef]
- Bae, J.; Stocks, J.; Heywood, A.; Jung, Y.; Jenkins, L.; Hill, V.; Katsaggelos, A.; Popuri, K.; Rosen, H.; Beg, M.F.; et al. Transfer learning to predict conversion from mild cognitive impairment to Alzheimer-type dementia using 3-D CNN. Neurobiol. Aging 2021, 99, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Spasov, S.E.; Passamonti, L.; Duggento, A.; Lio, P.; Toschi, N. A multi-modal CNN framework for prediction of Alzheimer’s disease. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 1271–1274. [Google Scholar]
- Ajagbe, S.A.; Amuda, K.A.; Oladipupo, M.A.; Oluwaseyi, F.; Okesola, K.I. Multi-classification of Alzheimer disease on MRI using deep-CNN approaches. Int. J. Adv. Comput. Res. 2021, 11, 51. [Google Scholar] [CrossRef]
- Arijit, D.; Chowdhury, A.S. DTI-based Alzheimer’s-disease classification with rank-modulated fusion of CNNs and random forest. Expert Syst. Appl. 2021, 169, 114338. [Google Scholar] [CrossRef]
- Li, F.; Liu, M.; Alzheimer’s Disease Neuroimaging Initiative. A hybrid convolutional and recurrent neural network for hippocampus analysis in Alzheimer’s disease. J. Neurosci. Methods 2019, 323, 108–118. [Google Scholar] [CrossRef]
- Maqsood, M.; Nazir, F.; Khan, U.; Aadil, F.; Jamal, H.; Mehmood, I.; Song, O.-Y. Transfer-learning-assisted classification and detection of Alzheimer’s-disease stages using 3-D MRI scans. Sensors 2019, 19, 2645. [Google Scholar] [CrossRef]
- Yiğit, A.; Baştanlar, Y.; Işık, Z. Dementia diagnosis by ensemble deep neural networks using FDG-PET scans. Signal Image Video Process. 2022, 16, 2203–2210. [Google Scholar] [CrossRef]
- Yiğit, A.; Işık, Z.; Baştanlar, Y. Dementia detection with deep networks using multi-modal image data. In Diagnosis of Neurological Disorders Based on Deep Learning Techniques; CRC Press: Boca Raton, FL, USA, 2023; pp. 185–203. [Google Scholar]
- Ning, A.; Ding, H.; Yang, J.; Rhoda, A.; Ang, T.F.A. Deep ensemble learning for Alzheimer’s-disease classification. J. Biomed. Inform. 2020, 105, 103411. [Google Scholar]
- Khan, M.S.; Sworna, N.S.; Islam, A.K.M.M.; Alzheimer’s Disease Neuroimaging Initiative. A slice-selection-guided deep integrated pipeline for Alzheimer’s prediction from structural brain MRI. Biomed. Signal Process. Control 2024, 89, 105773. [Google Scholar] [CrossRef]
- Ozdemir, C.; Dogan, Y.; Kaya, Y. Advancing brain-tumour classification through MTAP model: An innovative approach in medical diagnostics. Med. Biol. Eng. Comput. 2024, in press.
- Ozdemir, C.; Dogan, Y.; Kaya, Y. RGB-Angle-Wheel: A new data-augmentation method for deep-learning models. Knowl. Based Syst. 2024, 291, 111615. [Google Scholar] [CrossRef]
- Chen, J.H.; Tu, H.J.; Lin, T.E.; Peng, Z.X.; Wu, Y.W.; Yen, S.C.; Sung, T.Y.; Hsieh, J.H.; Lee, H.Y.; Pan, S.L.; et al. Discovery of dual-specificity tyrosine-phosphorylation-regulated kinase 1A inhibitors using an artificial-intelligence model and their effects on tau and tubulin dynamics. Biomed. Pharmacother. 2024, 181, 117688. [Google Scholar] [CrossRef] [PubMed]
- Jarhad, D.B.; Mashelkar, K.K.; Kim, H.R.; Noh, M.; Jeong, L.S. Dual-specificity tyrosine-phosphorylation-regulated kinase 1A inhibitors as potential therapeutics. J. Med. Chem. 2018, 61, 9791–9810. [Google Scholar] [CrossRef] [PubMed]
- Arbones, M.L.; Thomazeau, A.; Nakano-Kobayashi, A.; Hagiwara, M.; Delabar, J.M. DYRK1A and cognition: A lifelong relationship. Pharmacol. Ther. 2019, 194, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Deboever, E.; Fistrovich, A.; Hulme, C.; Dunckley, T. The omnipresence of DYRK1A in human diseases. Int. J. Mol. Sci. 2022, 23, 9355. [Google Scholar] [CrossRef]
- Hervy, J.; Bicout, D.J. Dynamical decoration of stabilised microtubules by Tau proteins. Sci. Rep. 2019, 9, 12473. [Google Scholar] [CrossRef]
- Branca, C.; Shaw, D.M.; Belfiore, R.; Gokhale, V.; Shaw, A.Y.; Foley, C.; Smith, B.; Hulme, C.; Dunckley, T.; Meechoovet, B.; et al. Dyrk1 inhibition improves Alzheimer’s disease-like pathology. Aging Cell 2017, 16, 1146–1154. [Google Scholar] [CrossRef]
- Lee, H.J.; Woo, H.; Lee, H.E.; Jeon, H.; Ryu, K.Y.; Nam, J.H.; Jeon, S.G.; Park, H.; Lee, J.S.; Han, K.-M.; et al. The novel DYRK1A inhibitor KVN93 regulates cognitive function, amyloid-β pathology and neuroinflammation. Free Radic. Biol. Med. 2020, 160, 575–595. [Google Scholar] [CrossRef]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine-intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef]
- Sarica, A.; Cerasa, A.; Quattrone, A. Random-forest algorithm for classification of neuroimaging data in Alzheimer’s disease: A systematic review. Front. Aging Neurosci. 2017, 9, 329. [Google Scholar] [CrossRef]
- Dauwan, M.; van der Zande, J.J.; van Dellen, E.; Sommer, I.E.C.; Scheltens, P.; Lemstra, A.W.; Stam, C.J. Random forest to differentiate dementia with Lewy bodies from Alzheimer’s disease. Alzheimer’s Dement. 2016, 4, 99–106. [Google Scholar] [CrossRef]
- Menze, B.H.; Kelm, B.M.; Masuch, R.; Himmelreich, U.; Bachert, P.; Petrich, W.; Hamprecht, F.A. A comparison of random forest and its Gini importance with standard chemometric methods for feature selection and classification of spectral data. BMC Bioinform. 2009, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Jiang, D.; Zhao, Q.; Wang, L.; Yin, K. Comparison of random forest, artificial neural networks and support-vector machine for intelligent diagnosis of rotating machinery. Trans. Inst. Meas. Control 2018, 40, 2681–2693. [Google Scholar] [CrossRef]
- Dimitriadis, S.I.; Liparas, D.; Tsolaki, M.N.; Alzheimer’s Disease Neuroimaging Initiative. Random-forest feature selection, fusion and ensemble strategy: Combining multiple morphological MRI measures to discriminate among healthy elderly, MCI, cMCI and Alzheimer’s-disease patients. J. Neurosci. Methods 2018, 302, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, A.K.; Westman, E.; Borza, T.; Beyer, M.K.; Engedal, K.; Aarsland, D.; Selbæk, G.; Håberg, A.K. MRI-based classification models to predict mild cognitive impairment and dementia in late-life depression. Front. Aging Neurosci. 2017, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.; Taylor, J.P.; Hamilton, C.A.; Firbank, M.; Cromarty, R.A.; Donaghy, P.C.; Roberts, G.; Allan, L.; Lloyd, J.; Durcan, R.; et al. Quantitative EEG as a biomarker in mild cognitive impairment with Lewy bodies. Alzheimer’s Res. Ther. 2020, 12, 82. [Google Scholar] [CrossRef]
- Kim, J.; Lee, M.; Lee, M.K.; Wang, S.M.; Kim, N.Y.; Kang, D.W.; Um, Y.H.; Na, H.R.; Woo, Y.S.; Lee, C.U.; et al. Development of a random-forest-based prediction model of Alzheimer’s disease using neurodegeneration patterns. Psychiatry Investig. 2021, 18, 69–79. [Google Scholar] [CrossRef]
- Alatrany, A.S.; Khan, W.; Hussain, A.; Al-Taee, A.; Khan, S.; Hussain, I.; Mohammed, S.; Ali, F.; Rehman, A.; Shah, M.B.; et al. An explainable machine-learning approach for Alzheimer’s-disease classification. Sci. Rep. 2024, 14, 2637. [Google Scholar] [CrossRef]
- Song, M.; Jung, H.; Lee, S.; Kim, D.; Ahn, M. Diagnostic classification and biomarker identification of Alzheimer’s disease with random-forest algorithm. Brain Sci. 2021, 11, 453. [Google Scholar] [CrossRef]
- Liao, B.; Chen, Y.; Wang, Z.; Smith, C.D.; Liu, J. A comparative study on 1.5 T–3 T MRI conversion through deep-neural-network models. arXiv 2022, arXiv:2210.06362. [Google Scholar] [CrossRef]
- Henschel, L.; Conjeti, S.; Estrada, S.; Diers, K.; Fischl, B.; Reuter, M. FastSurfer—A fast and accurate deep-learning-based neuroimaging pipeline. Neuroimage 2020, 219, 117012. [Google Scholar] [CrossRef]
- Querbes, O.; Aubry, F.; Pariente, J.; Lotterie, J.A.; Démonet, J.F.; Duret, V.; Puel, M.; Berry, I.; Fort, J.C.; Celsis, P.; et al. Early diagnosis of Alzheimer’s disease using cortical thickness: Impact of cognitive reserve. Brain 2009, 132, 2036–2047. [Google Scholar] [CrossRef]
- Choi, J.B.; Cho, K.J.; Kim, J.C.; Kim, C.H.; Chung, Y.-A.; Jeong, H.S.; Shim, Y.S.; Koh, J.S. Effect of daily low-dose tadalafil on cerebral perfusion and cognition in patients with erectile dysfunction and mild cognitive impairment. Clin. Psychopharmacol. Neurosci. 2019, 17, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Hartigan, J.A.; Wong, M.A. Algorithm AS 136: A k-means clustering algorithm. J. R. Stat. Soc. C Appl. Stat. 1979, 28, 100–108. [Google Scholar] [CrossRef]
- Bellezza, M.; Di Palma, A.; Frosini, A. Predicting conversion from mild cognitive impairment to Alzheimer’s disease using k-means clustering on MRI data. Information 2024, 15, 96. [Google Scholar] [CrossRef]
- Naeem, S.; Ali, A.; Anam, S.; Ahmed, M.M. Unsupervised machine-learning algorithms: Comprehensive review. Int. J. Comput. Digit. Syst. 2023, 13, 911–921. [Google Scholar] [CrossRef]
- Yang, D.; Xu, W. Clustering on human-microbiome sequencing data: A distance-based unsupervised-learning model. Microorganisms 2020, 8, 1612. [Google Scholar] [CrossRef]
- Li, P.; Luo, H.; Ji, B. Machine learning for data integration in human gut microbiome. Microb. Cell Fact. 2022, 21, 241. [Google Scholar] [CrossRef]
- Hayes, C.L.; Peters, B.J.; Foster, J.A. Microbes and mental health: Can the microbiome help explain clinical heterogeneity in psychiatry? Front. Neuroendocrinol. 2020, 58, 100849. [Google Scholar] [CrossRef]
- Topçuoğlu, B.D.; Lesniak, N.A.; Rufin, M.T.; Wiens, J.; Schloss, P.D. A framework for effective application of machine learning to microbiome-based classification problems. mBio 2020, 11, e00434-20. [Google Scholar] [CrossRef]
- Sánchez-DelaCruz, E.; Loeza-Mejía, C.; Primero-Huerta, C.; Fuentes-Ramos, M. Automatic selection model to identify neurodegenerative diseases. Digit. Health 2024, 10, 20552076241284376. [Google Scholar] [CrossRef]
- Jiao, B.; Li, R.; Zhou, H.; Qing, K.; Liu, H.; Pan, H.; Lei, Y.; Fu, W.; Wang, X.; Xiao, X.; et al. Neural biomarker diagnosis and prediction for mild cognitive impairment and Alzheimer’s disease using EEG technology. Alzheimer’s Res. Ther. 2023, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Miltiadous, A.; Tzimourta, K.D.; Giannakeas, N.; Tsipouras, M.G.; Afrantou, T.; Ioannidis, P.; Tzallas, A.T. Alzheimer’s disease and frontotemporal dementia: A robust classification method of EEG signals and a comparison of validation methods. Diagnostics 2021, 11, 1437. [Google Scholar] [CrossRef] [PubMed]
- Durongbhan, P.; Zhao, Y.; Chen, L.; Wang, Z.; Liu, Y.; Li, K. A dementia-classification framework using frequency and time-frequency features based on EEG signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Tzimourta, K.D.; Giannakeas, N.; Tzallas, A.T.; Astrakas, L.G.; Afrantou, T.; Ioannidis, P.; Grigoriadis, N.; Angelidis, P.; Tsalikakis, D.G.; Tsipouras, M.G. EEG window-length evaluation for the detection of Alzheimer’s disease over different brain regions. Brain Sci. 2019, 9, 81. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Alù, F.; Menna, M.; Judica, E.; Cotelli, M.; Rossini, P.M. Classification of Alzheimer’s disease versus physiological ageing with innovative EEG biomarkers in a machine-learning implementation. J. Alzheimer’s Dis. 2020, 75, 1253–1261. [Google Scholar] [CrossRef]
- Nobukawa, S.; Yamanishi, T.; Kasakawa, S.; Nishimura, H.; Kikuchi, M.; Takahashi, T. Classification methods based on complexity and synchronization of electroencephalography signals in Alzheimer’s disease. Front. Psychiatry 2020, 11, 255. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, L.; Wang, R.; Song, Z.; Deng, B.; Wang, J.; Yu, H. DCCA cross-correlation coefficients reveal changes in synchronization and oscillation in EEG of Alzheimer’s-disease patients. Phys. A Stat. Mech. Appl. 2018, 490, 171–184. [Google Scholar] [CrossRef]
- Cicalese, P.A.; Li, R.; Ahmadi, M.B.; Wang, C.; Francis, J.T.; Selvaraj, S.; Schulz, P.E.; Zhang, Y. An EEG–fNIRS hybridisation technique in four-class classification of Alzheimer’s disease. J. Neurosci. Methods 2020, 336, 108618. [Google Scholar] [CrossRef]
- Song, Z.; Deng, B.; Wang, J.; Wang, R. Biomarkers for Alzheimer’s disease defined by a novel brain functional-network measure. IEEE Trans. Biomed. Eng. 2019, 66, 41–49. [Google Scholar] [CrossRef]
- Lee, E.; Choi, J.-S.; Kim, M.; Suk, H.-I.; Alzheimer’s Disease Neuroimaging Initiative. Toward an interpretable Alzheimer’s-disease diagnostic model with regional abnormality representation via deep learning. Neuroimage 2019, 202, 116113. [Google Scholar] [CrossRef]
- Albright, J.; Alzheimer’s Disease Neuroimaging Initiative. Forecasting the progression of Alzheimer’s disease using neural networks and a novel preprocessing algorithm. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Kotwal, S.; Manhas, J.; Sharma, V.; Sharma, S. Machine-learning- and deep-learning-based computational approaches in automatic microorganism image recognition: Methodologies, challenges and developments. Arch. Comput. Methods Eng. 2022, 29, 1801–1837. [Google Scholar] [CrossRef] [PubMed]
- Fung, D.L.X.; Li, X.; Leung, C.K.; Hu, P. A self-knowledge-distillation-driven CNN–LSTM model for predicting disease outcomes using longitudinal microbiome data. Bioinform. Adv. 2023, 3, vbad059. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Mohsen Pedram, M.; Moradi, A.; Ouchani, M. Diagnosis of Alzheimer’s disease by time-dependent power-spectrum descriptors and convolutional neural network using EEG signal. Comput. Math. Methods Med. 2021, 2021, 5511922. [Google Scholar] [CrossRef]
- Suriya, M.; Chandran Venkatesan, M.G.S.; Manoharan, S. DEMNET: A deep-learning model for early diagnosis of Alzheimer disease and dementia from MR images. IEEE Access 2021, 9, 90319–90329. [Google Scholar] [CrossRef]
- Ayub, N.; Zubair Ahmad Shah, S.; Assad, A.; Mohi Ud Din, N. Deep 3D-CNN using magnetic-resonance imaging for diagnosing Alzheimer’s. In Proceedings of the 3rd International Conference on Artificial Intelligence and Signal Processing (AISP 2023), Hyderabad, India, 18–20 March 2023; pp. 1–5. [Google Scholar]
- Cui, R.; Liu, M.; Alzheimer’s Disease Neuroimaging Initiative. RNN-based longitudinal analysis for diagnosis of Alzheimer’s disease. Comput. Med. Imaging Graph. 2019, 73, 1–10. [Google Scholar] [CrossRef]
- Hsu, T.; Epting, W.K.; Kim, H.; Abernathy, H.W.; Hackett, G.A.; Rollett, A.D.; Salvador, P.A.; Holm, E.A. Microstructure generation via generative adversarial network for heterogeneous, topologically complex 3-D materials. JOM 2021, 73, 90–102. [Google Scholar] [CrossRef]
- Furusawa, C.; Tanabe, K.; Ishii, C.; Kagata, N.; Tomita, M.; Fukuda, S. Decoding gut microbiota by imaging analysis of fecal samples. iScience 2021, 24, 103481. [Google Scholar] [CrossRef]
- Solovchuk, D.R. Advances in AI-assisted biochip technology for biomedicine. Biomed. Pharmacother. 2024, 177, 116997. [Google Scholar] [CrossRef]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in biosensors for continuous glucose monitoring toward wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing biosensors with machine learning. ACS Sens. 2020, 5, 3346–3364. [Google Scholar] [CrossRef]
- Rodoplu, D.; Chang, C.S.; Kao, C.Y.; Hsu, C.H. A simple magnetic-assisted microfluidic method for rapid detection and phenotypic characterisation of ultralow concentrations of bacteria. Talanta 2021, 230, 122291. [Google Scholar] [CrossRef]
- Rodoplu, D.; Chang, C.; Kao, C.; Hsu, C. A micro-pupil device for point-of-care testing of viable Escherichia coli in tap water. Microchem. J. 2022, 178, 107390. [Google Scholar] [CrossRef]
- Carrara, S.; Ghoreishizadeh, S.; Olivo, J.; Taurino, I.; Baj-Rossi, C.; Cavallini, A.; de Beeck, M.O.; Dehollain, C.; Burleson, W.; Moussy, F.G.; et al. Fully integrated biochip platforms for advanced healthcare. Sensors 2012, 12, 11013–11060. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.; Javani, A.; Park, J.; Velasco, V.; Xu, B.; Razorenova, O.; Esfandyarpour, R. A machine-learning-assisted nanoparticle-printed biochip for real-time single-cancer-cell analysis. Adv. Biosyst. 2020, 4, 2000160. [Google Scholar] [CrossRef] [PubMed]
- Hochstetter, A. Lab-on-a-chip technologies for the single-cell level: Separation, analysis and diagnostics. Micromachines 2020, 11, 468. [Google Scholar] [CrossRef]
- Yeh, C.F.; Juang, D.S.; Chen, Y.W.; Rodoplu, D.; Hsu, C.H. A portable controllable compressive-stress device to monitor human breast-cancer cell protrusions at single-cell resolution. Front. Bioeng. Biotechnol. 2022, 10, 852318. [Google Scholar] [CrossRef]
- Rodoplu, D.; Matahum, J.S.; Hsu, C.H. A microfluidic hanging-drop-based spheroid co-culture platform for probing tumour angiogenesis. Lab Chip 2022, 22, 1275–1285. [Google Scholar] [CrossRef]
- Holloway, P.M.; Willaime-Morawek, S.; Siow, R.; Barber, M.; Owens, R.M.; Sharma, A.D.; Rowan, W.; Hill, E.; Zagnoni, M. Advances in microfluidic in vitro systems for neurological disease modelling. J. Neurosci. Res. 2021, 99, 1276–1307. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y. Advances in organ-on-a-chip for the treatment of cardiovascular diseases. MedComm Biomater. Appl. 2023, 2, 63. [Google Scholar] [CrossRef]
- Wu, X.; Shi, W.; Liu, X.; Gu, Z. Recent advances in 3-D-printing-based organ-on-a-chip. Eng. Med. 2024, 1, 100003. [Google Scholar] [CrossRef]
- Hu, M.; Ling, Z.; Ren, X. Extracellular-matrix dynamics: Tracking in biological systems and their implications. J. Biol. Eng. 2022, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; De Haan, P.; Peterson, B.W.; De Jong, E.D.; Verpoorte, E.; Van Der Mei, H.C.; Busscher, H.J. Visualisation of bacterial colonisation and cellular layers in a gut-on-a-chip system using optical coherence tomography. Microsc. Microanal. 2020, 26, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, C.; Cao, J.; Cui, Z.; Du, J.; Fu, Z.; Yang, H.; Chen, P. Organ-on-a-chip meets artificial intelligence in drug evaluation. Theranostics 2023, 13, 4526–4558. [Google Scholar] [CrossRef]
- Mercier, M.R.; Dubarry, A.S.; Tadel, F.; Avanzini, P.; Axmacher, N.; Cellier, D.; Vecchio, M.D.; Hamilton, L.S.; Hermes, D.; Kahana, M.J.; et al. Advances in human intracranial electroencephalography research: Guidelines and good practices. Neuroimage 2022, 260, 119438. [Google Scholar] [CrossRef]
- Yogev, D.; Goldberg, T.; Arami, A.; Tejman-Yarden, S.; Winkler, T.E.; Maoz, B.M. Current state of the art and future directions for implantable sensors in medical technology: Clinical needs and engineering challenges. APL Bioeng. 2023, 7, 031506. [Google Scholar] [CrossRef]
- Cuschieri, A.; Borg, N.; Zammit, C. Closed-loop deep-brain stimulation: A systematic scoping review. Clin. Neurol. Neurosurg. 2022, 223, 107516. [Google Scholar] [CrossRef]
- Altyar, A.E.; El-Sayed, A.; Abdeen, A.; Piscopo, M.; Mousa, S.A.; Najda, A.; Abdel-Daim, M.M. Future regenerative-medicine developments and their therapeutic applications. Biomed. Pharmacother. 2023, 158, 114131. [Google Scholar] [CrossRef]
- Jafari, M.; Marquez, G.; Dechiraju, H.; Gomez, M.; Rolandi, M. Merging machine learning and bioelectronics for closed-loop control of biological systems and homeostasis. Cell Rep. Phys. Sci. 2023, 4, 101535. [Google Scholar] [CrossRef]
- Gutiérrez-Martínez, J.; Toledo-Peral, C.; Mercado-Gutiérrez, J.; Vera-Hernández, A.; Leija-Salas, L. Neuroprosthesis devices based on micro- and nanosensors: A systematic review. J. Sens. 2020, 2020, 8865889. [Google Scholar] [CrossRef]
- Ma, J.; Zhong, J.; Sun, F.; Liu, B.; Peng, Z.; Lian, J.; Wu, X.; Li, L.; Hao, M.; Zhang, T. Hydrogel sensors for biomedical electronics. Chem. Eng. J. 2023, 481, 148317. [Google Scholar] [CrossRef]
- Wei, W.; Hao, M.; Zhou, K.; Wang, Y.; Lu, Q.; Zhang, H.; Wu, Y.; Zhang, T.; Liu, Y. In situ multimodal transparent electrophysiological hydrogel for in vivo miniature two-photon neuroimaging and electrocorticogram analysis. Acta Biomater. 2022, 152, 86–99. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Liu, W.; Liu, X.; Song, Z.; Yu, D.; Li, G.; Ge, S.; Wang, H. Establishing a corrugated carbon network with a crack structure in a hydrogel for improving sensing performance. ACS Appl. Mater. Interfaces 2023, 15, 48462–48474. [Google Scholar] [CrossRef] [PubMed]
- Kondaveeti, S.; Choi, G.; Veerla, S.C.; Kim, S.; Kim, J.; Lee, H.J.; Kuzhiumparambil, U.; Ralph, P.J.; Yeo, J.; Jeong, H.E. Mussel-inspired resilient hydrogels with strong skin adhesion and high sensitivity for wearable devices. Nano Converg. 2024, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.; Rao, Z.; Zou, Z.; Ershad, F.; Lei, J.; Thukral, A.; Chen, J.; Huang, Q.A.; Xiao, J.; Yu, C. Metal-oxide-semiconductor nanomembrane-based soft unnoticeable multifunctional electronics for wearable human–machine interfaces. Sci. Adv. 2019, 5, eaav9653. [Google Scholar] [CrossRef] [PubMed]
- Afanasenkau, D.; Kalinina, D.; Lyakhovetskii, V.; Tondera, C.; Gorsky, O.; Moosavi, S.; Pavlova, N.; Merkulyeva, N.; Kalueff, A.V.; Minev, I.R.; et al. Rapid prototyping of soft bioelectronic implants for use as neuromuscular interfaces. Nat. Biomed. Eng. 2020, 4, 1010–1022. [Google Scholar] [CrossRef]
- Deng, J.; Yuk, H.; Wu, J.; Varela, C.E.; Chen, X.; Roche, E.T.; Guo, C.F.; Zhao, X. Electrical bioadhesive interface for bioelectronics. Nat. Mater. 2021, 20, 229–236. [Google Scholar] [CrossRef]
- Summerer, L.; Izzo, D.; Rossini, L. Brain-machine interfaces for space applications-research, technological development, and opportunities. Int. Rev. Neurobiol. 2009, 86, 213–223. [Google Scholar] [CrossRef]
- Ortiz, M.; Ferrero, L.; Iáñez, E.; Azorín, J.M.; Contreras-Vidal, J.L. Sensory integration in human movement: A new brain–machine interface based on gamma band and attention level for controlling a lower-limb exoskeleton. Front. Bioeng. Biotechnol. 2020, 8, 735. [Google Scholar] [CrossRef]
- Sun, J.; Ren, Y.; Ji, J.; Guo, Y.; Sun, X. A novel concentration-gradient microfluidic chip for high-throughput antibiotic susceptibility testing of bacteria. Anal. Bioanal. Chem. 2021, 413, 1127–1136. [Google Scholar] [CrossRef]
- Li, C.; McCrone, S.; Warrick, J.W.; Andes, D.R.; Hite, Z.; Volk, C.F.; Rose, W.E.; Beebe, D.J. Under-oil open microfluidic systems for rapid phenotypic antimicrobial susceptibility testing. Lab Chip 2023, 23, 2005–2015. [Google Scholar] [CrossRef]
- Jin, L.; Tang, Y.; Coole, J.B.; Tan, M.T.; Zhao, X.; Badaoui, H.; Robinson, J.T.; Williams, M.D.; Vigneswaran, N.; Gillenwater, A.M.; et al. DeepDOF-SE: An affordable deep-learning microscopy platform for slide-free histology. Nat. Commun. 2024, 15, 2935. [Google Scholar] [CrossRef] [PubMed]
- Yousefirizi, F.; Decazes, P.; Amyar, A.; Ruan, S.; Saboury, B.; Rahmim, A. AI-based detection, classification and prediction/prognosis in medical imaging: Toward radiophenomics. PET Clin. 2022, 17, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Eloranta, S.; Boman, M. Predictive models for clinical decision making: Deep dives in practical machine learning. J. Intern. Med. 2022, 292, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Rudrapati, R. Using Industry 4.0 technologies to combat the COVID-19 pandemic. Ann. Med. Surg. 2022, 78, 103811. [Google Scholar] [CrossRef]
- Przybyszewski, A.W.; Bojakowska, K.; Nowacki, J.P.; Drabik, A. Rough set rules predominantly based on cognitive tests can predict Alzheimer-related dementia. In Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2022; pp. 129–141. [Google Scholar] [CrossRef]
- Przybyszewski, A.W. AI classifications applied to neuropsychological trials in normal individuals that predict progression to cognitive decline. In Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2022; pp. 150–156. [Google Scholar] [CrossRef]
- Przybyszewski, A.W. Multi-granular computing can predict prodromal Alzheimer’s-disease indications in normal subjects. In Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2023; pp. 278–285. [Google Scholar] [CrossRef]
- Chudzik, A.; Śledzianowski, A.; Przybyszewski, A.W. Machine learning and digital biomarkers can detect early stages of neurodegenerative diseases. Sensors 2024, 24, 1572. [Google Scholar] [CrossRef]
- Shin, W.; Kim, H.J. 3-D in vitro morphogenesis of human intestinal epithelium in a gut-on-a-chip or a hybrid chip with a cell-culture insert. Nat. Protoc. 2022, 17, 910–939. [Google Scholar] [CrossRef]
- Mittal, E.; Cupp, G.; Kang, Y.A. Simulating the effect of gut microbiome on cancer-cell growth using a microfluidic device. Sensors 2023, 23, 1265. [Google Scholar] [CrossRef]
- Milani, N.; Parrott, N.; Ortiz Franyuti, D.; Godoy, P.; Galetin, A.; Gertz, M.; Fowler, S. Application of a gut-liver-on-a-chip device and mechanistic modelling to the quantitative in vitro pharmacokinetic study of mycophenolate mofetil. Lab Chip 2022, 22, 2853–2868. [Google Scholar] [CrossRef]
- Liu, J.; Lu, R.; Zheng, X.; Hou, W.; Wu, X.; Zhao, H.; Wang, G.; Tian, T. Establishment of a gut-on-a-chip device with controllable oxygen gradients to study the contribution of Bifidobacterium bifidum to inflammatory bowel disease. Biomater. Sci. 2023, 11, 2504–2517. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, S.; Zhuang, J.; Liang, L. Advances in gut–brain organ chips. Cell Prolif. 2024, 57, e13724. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, V.; Sgambato, C.; Urciuolo, F.; Vecchione, R.; Netti, P.A.; Imparato, G. Immunoresponsive microbiota-gut-on-chip reproduces barrier dysfunction, stromal reshaping and probiotics translocation under inflammation. Biomaterials 2022, 286, 121573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, G.; Wu, J.; Liu, X.; Fan, Y.; Chen, J.; Wallace, G.; Gu, Q. Microphysiological constructs and systems: Biofabrication tactics, biomimetic evaluation approaches and biomedical applications. Small Methods 2024, 8, e2300685. [Google Scholar] [CrossRef]
- Pediaditakis, I.; Kodella, K.R.; Manatakis, D.V.; Le, C.Y.; Hinojosa, C.D.; Tien-Street, W.; Manolakos, E.S.; Vekrellis, K.; Hamilton, G.A.; Ewart, L.; et al. Modeling alpha-synuclein pathology in a human brain-chip to assess blood–brain barrier disruption. Nat. Commun. 2021, 12, 5907. [Google Scholar] [CrossRef]
- Lyu, Z.; Park, J.; Kim, K.M.; Jin, H.J.; Wu, H.; Rajadas, J.; Kim, D.H.; Steinberg, G.K.; Lee, W. A neurovascular-unit-on-a-chip for evaluating the restorative potential of stem-cell therapies for ischaemic stroke. Nat. Biomed. Eng. 2021, 5, 847–863. [Google Scholar] [CrossRef]
- Rust, R.; Nih, L.R.; Liberale, L.; Yin, H.; Amki, M.E.; Ong, L.K.; Zlokovic, B.V. Brain-repair mechanisms after cell therapy for stroke. Brain 2024, 147, 3286–3305. [Google Scholar] [CrossRef]
- Maoz, B.M. Brain-on-a-chip: Characterising the next generation of advanced in vitro platforms for modelling the central nervous system. APL Bioeng. 2021, 5, 030902. [Google Scholar] [CrossRef]
- Osaki, T.; Sivathanu, V.; Kamm, R.D. Engineered 3-D vascular and neuronal networks in a microfluidic platform. Sci. Rep. 2018, 8, 5168. [Google Scholar] [CrossRef]
- Cho, A.N.; Jin, Y.; An, Y.; Kim, J.; Choi, Y.S.; Lee, J.S.; Kim, J.; Choi, W.Y.; Koo, D.J.; Yu, W.; et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat. Commun. 2021, 12, 4730. [Google Scholar] [CrossRef]
- Rao, Z.; Lin, T.; Qiu, S.; Zhou, J.; Liu, S.; Chen, S.; Wang, T.; Liu, X.; Zhu, Q.; Bai, Y.; et al. Decellularised nerve-matrix hydrogel scaffolds with longitudinally oriented and size-tunable microchannels for peripheral-nerve regeneration. Mater. Sci. Eng. C 2021, 120, 111791. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.; Zhang, M.; Liu, S.; Zhou, L.; Yang, C.; Liu, C. The role of the gastrointestinal system in neuroinvasion by SARS-CoV-2. Front. Neurosci. 2021, 15, 694446. [Google Scholar] [CrossRef] [PubMed]
- Cadena, M.; Ning, L.; King, A.; Hwang, B.; Jin, L.; Serpooshan, V.; Sloan, S.A. 3-D bioprinting of neural tissues. Adv. Healthc. Mater. 2021, 10, e2001600. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, M.; Chen, Z.; Zhang, T.; Huang, J.; Dai, J.; Zhang, Z. 3-D bioprinted neural-tissue constructs for spinal-cord-injury repair. Biomaterials 2021, 272, 120771. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.E.; Lee, J.; Kim, J.R.; Kawakami, C.; Kim, C.Y.; Qazi, R.; Jang, K.I.; Jeong, J.W.; McCall, J.G. Customisable, wireless and implantable neural-probe design and fabrication via 3-D printing. Nat. Protoc. 2023, 18, 3–21. [Google Scholar] [CrossRef]
- Sullivan, M.A.; Lane, S.; Volkerling, A.; Engel, M.; Werry, E.L.; Kassiou, M. Three-dimensional bioprinting of stem-cell-derived CNS cells enables astrocyte growth, vasculogenesis and enhances neural differentiation/function. Biotechnol. Bioeng. 2023, 120, 3079–3091. [Google Scholar] [CrossRef]
- Tang, M.; Rich, J.N.; Chen, S. Biomaterials and 3-D bioprinting strategies to model glioblastoma and the blood–brain barrier. Adv. Mater. 2021, 33, e2004776. [Google Scholar] [CrossRef]
- Mao, S.; Fonder, C.; Rubby, M.F.; Phillips, G.J.; Sakaguchi, D.S.; Que, L. An integrated microfluidic chip for studying the effects of neurotransmitters on neurospheroids. Lab Chip 2023, 23, 1649–1663. [Google Scholar] [CrossRef]
- Tunesi, M.; Izzo, L.; Raimondi, I.; Albani, D.; Giordano, C. A miniaturised hydrogel-based in vitro model for dynamic culturing of human cells over-expressing β-amyloid precursor protein. J. Tissue Eng. 2020, 11, 2041731420945633. [Google Scholar] [CrossRef]
- Chen, Y.P.; Ding, Z.Y.; Yu, Y.S.; He, P.; Zhou, Y.; Liu, Y.; Feng, X. Recent advances in investigating odour–taste interactions: Psychophysics, neuroscience and microfluidic techniques. Trends Food Sci. Technol. 2023, 138, 500–510. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, D.; Sung, J.H. A gut–brain axis-on-a-chip for studying transport across epithelial and endothelial barriers. J. Ind. Eng. Chem. 2021, 101, 126–134. [Google Scholar] [CrossRef]
- Raimondi, M.T.; Albani, D.; Giordano, C. An organ-on-a-chip engineered platform to study the microbiota–gut–brain axis in neurodegeneration. Trends Mol. Med. 2019, 25, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Kim, M.H.; van Noort, D.; Sung, J.H.; Park, S. Organ-on-a-chip for studying gut–brain interaction mediated by extracellular vesicles in the gut microenvironment. Int. J. Mol. Sci. 2021, 22, 13513. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Lee, H.Y.; Choi, Y.Y.; Mo, S.J.; Jeon, S.; Ha, J.H.; Park, S.D.; Shim, J.J.; Lee, J.; Chung, B.G. Effect of gut-microbiota-derived metabolites and extracellular vesicles on neurodegenerative disease in a gut–brain-axis chip. Nano Converg. 2024, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Chapin, A.A.; Rajasekaran, P.R.; Quan, D.N.; Hu, L.; Herberholz, J.; Bentley, W.E.; Ghodssi, R. Electrochemical measurement of serotonin by Au-CNT electrodes fabricated on microporous cell-culture membranes. Microsyst. Nanoeng. 2020, 6, 90. [Google Scholar] [CrossRef]
- Trapecar, M.; Wogram, E.; Svoboda, D.; Communal, C.; Omer, A.; Lungjangwa, T.; Sphabmixay, P.; Velazquez, J.; Schneider, K.; Wright, C.W.; et al. Human physiomimetic model integrating microphysiological systems of the gut, liver and brain for studies of neurodegenerative diseases. Sci. Adv. 2021, 7, eabd1707. [Google Scholar] [CrossRef]
- Picollet-D’hahan, N.; Zuchowska, A.; Lemeunier, I.; Le Gac, S. Multiorgan-on-a-chip: A systemic approach to model and decipher inter-organ communication. Trends Biotechnol. 2021, 39, 788–810. [Google Scholar] [CrossRef]
- Park, D.; Lee, J.; Chung, J.J.; Jung, Y.; Kim, S.H. Integrating organs-on-chips: Multiplexing, scaling, vascularisation and innervation. Trends Biotechnol. 2020, 38, 99–112. [Google Scholar] [CrossRef]
- Wagner, K.T.; Nash, T.R.; Liu, B.; Vunjak-Novakovic, G.; Radisic, M. Extracellular vesicles in cardiac regeneration: Potential applications for tissues-on-a-chip. Trends Biotechnol. 2021, 39, 755–773. [Google Scholar] [CrossRef]
- Monti, C.; Pangallo, M.; De Francisci Morales, G.; Bonchi, F. On learning agent-based models from data. Sci. Rep. 2023, 13, 9268. [Google Scholar] [CrossRef]
- Wilensky, U.; Rand, W. An Introduction to Agent-Based Modeling: Modeling Natural, Social, and Engineered Complex Systems with NetLogo; MIT Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Railsback, S.F.; Grimm, V. Agent-Based and Individual-Based Modeling: A Practical Introduction; Princeton University Press: Princeton, NJ, USA, 2019. [Google Scholar]
- Stephan, S.; Galland, S.; Labbani Narsis, O.; Shoji, K.; Vachenc, S.; Gerart, S.; Nicolle, C. Agent-based approaches for biological modelling in oncology: A literature review. Artif. Intell. Med. 2024, 152, 102884. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Weber, E.S.; Manz, K.E.; McCarthy, K.J.; Chen, Y.; Schüffler, P.J.; Zhu, C.W.; Tracy, M. Assessing the impact and cost-effectiveness of exposome interventions on Alzheimer’s disease: A review of agent-based modelling and other data-science methods for causal inference. Genes 2024, 15, 1457. [Google Scholar] [CrossRef] [PubMed]
- Luke, D.A.; Stamatakis, K.A. Systems-science methods in public health: Dynamics, networks and agents. Annu. Rev. Public Health 2012, 33, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Tracy, M.; Gordis, E.; Strully, K.; Marshall, B.D.L.; Cerdá, M. Applications of agent-based modelling in trauma research. Psychol. Trauma 2023, 15, 939–950. [Google Scholar] [CrossRef]
- Squires, H.; Kelly, M.P.; Gilbert, N.; Sniehotta, F.; Purshouse, R.C. The long-term effectiveness and cost-effectiveness of public-health interventions: How can we model behaviour? Health Econ. 2023, 32, 2836–2854. [Google Scholar] [CrossRef]
- Taucare, G.; Chan, G.; Nilsson, S.; Toms, L.L.; Zhang, X.; Mueller, J.F.; Jolliet, O. Temporal trends of per- and polyfluoroalkyl substances concentrations: Insights from Australian human biomonitoring 2002–2021 and the U.S. NHANES programmes 2003–2018. Environ. Res. 2024, 262, 119777. [Google Scholar] [CrossRef]
- Lund, A.M.; Gouripeddi, R.; Facelli, J.C. STHAM: An agent-based model for simulating human exposure across high-resolution spatiotemporal domains. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 459–468. [Google Scholar] [CrossRef]
- Brandon, N.; Price, P.S. Calibrating an agent-based model of longitudinal human-activity patterns using the Consolidated Human Activity Database. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 194–204. [Google Scholar] [CrossRef]
- Brandon, N.; Dionisio, K.L.; Isaacs, K.; Tornero-Velez, R.; Kapraun, D.; Setzer, R.W.; Price, P.S. Simulating exposure-related behaviours using agent-based models embedded with needs-based artificial intelligence. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 184–193. [Google Scholar] [CrossRef]
- Chapizanis, D.; Karakitsios, S.; Gotti, A.; Sarigiannis, D.A. Assessing personal exposure using agent-based modelling informed by sensor technology. Environ. Res. 2021, 192, 110141. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Shu, Y.; Sun, L.; Jin, Z.; Ma, Z.; Liu, M.; Bi, J.; Kinney, P.L. A stochastic exposure model integrating random forest and agent-based approaches: Evaluation for PM2.5 in Jiangsu, China. J. Hazard. Mater. 2022, 431, 128639. [Google Scholar] [CrossRef]
- Sundar, S.; Battistoni, C.; McNulty, R.; Morales, F.; Gorky, J.; Foley, H.; Dhurjati, P. An agent-based model to investigate microbial initiation of Alzheimer’s via the olfactory system. Theor. Biol. Med. Model. 2020, 17, 5. [Google Scholar] [CrossRef]
- Hoffman, T.E.; Hanneman, W.H.; Moreno, J.A. Network simulations reveal molecular signatures of vulnerability to age-dependent stress and tau accumulation. Front. Mol. Biosci. 2020, 7, 590045. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Copeland, L.L.; Sussman, J.B.; Hayward, R.A.; Gross, A.L.; Briceño, E.M.; Whitney, R.; Giordani, B.J.; Elkind, M.S.V.; Manly, J.J.; et al. Development and validation of the Michigan Chronic Disease Simulation Model (MICROSIM). PLoS ONE 2024, 19, e0300005. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, T.; Scheider, S.; de Wit, G.A.; Tonne, C.C.; Vermeulen, R. Agent-based modelling of urban exposome interventions: Prospects, model architectures and methodological challenges. Exposome 2022, 2, osac009. [Google Scholar] [CrossRef] [PubMed]
- Tracy, M.; Cerdá, M.; Keyes, K.M. Agent-based modelling in public health: Current applications and future directions. Annu. Rev. Public Health 2018, 39, 77–94. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, M.; Jung, D. Policy evaluation of economic–environmental trade-offs in regulating industrial water use: An agent-based model. J. Environ. Manag. 2023, 346, 118988. [Google Scholar] [CrossRef]
- Shi, H.; Wang, S.; Li, J.; Zhang, L. Modelling the impacts of policy measures on residents’ PM2.5-reduction behaviour: An agent-based simulation analysis. Environ. Geochem. Health 2020, 42, 895–913. [Google Scholar] [CrossRef]
- Wilson, A.M.; Verhougstraete, M.P.; Donskey, C.J.; Reynolds, K.A. An agent-based modelling approach to estimate pathogen-exposure risks from wheelchairs. Am. J. Infect. Control 2021, 49, 206–214. [Google Scholar] [CrossRef]
- Tracy, M. Systems approaches to understanding how the environment influences population health and population-health interventions. In Systems Science and Population Health; Oxford University Press: Oxford, UK, 2017; pp. 151–165. [Google Scholar]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Siroux, V.; Agier, L.; Slama, R. The exposome concept: A challenge and a potential driver for environmental-health research. Eur. Respir. Rev. 2016, 25, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Liu, X.; Zheng, Y.; He, X.; Hart, J.; James, P.; Laden, F.; Chen, Y.; Bian, J. Methodological challenges in spatial and contextual exposome-health studies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 827–846. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chen, Y.; Kuiper, J.R.; Ho, E.; Buckley, J.P.; Feuerstahler, L. Applying latent-variable models to estimate cumulative-exposure burden to chemical mixtures and identify latent exposure subgroups: A critical review and future directions. Stat. Biosci. 2024, 16, 482–502. [Google Scholar] [CrossRef] [PubMed]
- Sarigiannis, D. Unravelling the exposome: A practical view. In The HEALS Project; Springer: Cham, Switzerland, 2019; pp. 405–422. [Google Scholar]
- Vlaanderen, J.; de Hoogh, K.; Hoek, G.; Peters, A.; Probst-Hensch, N.; Scalbert, A.; Melén, E.; Tonne, C.; de Wit, G.A.; Chadeau-Hyam, M.; et al. Developing the building blocks to elucidate the impact of the urban exposome on cardiometabolic-pulmonary disease: The EU EXPANSE project. Environ. Epidemiol. 2021, 5, e162. [Google Scholar] [CrossRef]
- Kind, A.J.H.; Buckingham, W.R. Making neighborhood-disadvantage metrics accessible—The Neighborhood Atlas. N. Engl. J. Med. 2018, 378, 2456–2458. [Google Scholar] [CrossRef]
- Liu, S.H.; Feuerstahler, L.; Chen, Y.; Braun, J.M.; Buckley, J.P. Developing an exposure-burden score for chemical mixtures using item-response theory, with applications to PFAS mixtures. Environ. Health Perspect. 2022, 130, 117001. [Google Scholar] [CrossRef]
- Liu, S.H.; Feuerstahler, L.; Chen, Y.; Braun, J.M.; Buckley, J.P. Toward advancing precision environmental health: Developing a customised exposure-burden score to PFAS mixtures to enable equitable comparisons across population subgroups, using mixture item-response theory. Environ. Sci. Technol. 2023, 57, 18104–18115. [Google Scholar] [CrossRef]
- Liu, S.H.; Chen, Y.; Feuerstahler, L.; Chen, A.; Starling, A.; Dabelea, D.; Wang, X.; Cecil, K.; Lanphear, B.; Yolton, K.; et al. The U.S. PFAS exposure-burden calculator for 2017–2018: Application to the HOME Study, with comparison of epidemiological findings from NHANES. Neurotoxicol. Teratol. 2024, 102, 107321. [Google Scholar] [CrossRef]
- Chen, Y.; Feuerstahler, L.; Martinez Steele, E.; Buckley, J.P.; Liu, S.H. Phthalate mixtures and insulin resistance: An item-response-theory approach to quantify exposure burden. J. Expo. Sci. Environ. Epidemiol. 2023, 34, 581–590. [Google Scholar] [CrossRef]
- Balestriero, R.; Ibrahim, M.; Sobal, V.; Morcos, A.S.; Shekhar, S.; Goldstein, T.; Bordes, F.; Bardes, A.; Mialon, G.; Tian, Y.; et al. A cookbook of self-supervised learning. arXiv 2023, arXiv:2304.12210. [Google Scholar] [CrossRef]
- Krishnan, R.; Rajpurkar, P.; Topol, E.J. Self-supervised learning in medicine and healthcare. Nat. Biomed. Eng. 2022, 6, 1346–1352. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, H.; Gu, Y. Dive into the details of self-supervised learning for medical-image analysis. Med. Image Anal. 2023, 89, 102879. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Teng, Z.; Li, Z.; Chen, J. CVGAE: A self-supervised generative method for gene-regulatory-network inference using single-cell RNA sequencing data. Interdiscip. Sci. Comput. Life Sci. 2024, 16, 990–1004. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Islam, M.T.; Zhou, Y.; Xing, L. Self-supervised deep learning of gene–gene interactions for improved gene-expression recovery. Brief. Bioinform. 2024, 25, bba1031. [Google Scholar] [CrossRef] [PubMed]
- Kostas, D.; Aroca-Ouellette, S.; Rudzicz, F. BENDR: Using transformers and a contrastive self-supervised learning task to learn from massive amounts of EEG data. Front. Hum. Neurosci. 2021, 15, 653659. [Google Scholar] [CrossRef]
- Mehta, N.P. Microbiome and mind: Current insights and future directions in gut–brain research. World J. Biol. Pharm. Health Sci. 2024, 19, 86–94. [Google Scholar] [CrossRef]
- Krothapalli, M.; Buddendorff, L.; Yadav, H.; Schilaty, N.D.; Jain, S. From gut microbiota to brain waves: The potential of the microbiome and EEG as biomarkers for cognitive impairment. Int. J. Mol. Sci. 2024, 25, 6678. [Google Scholar] [CrossRef]
- Nakhal, M.M.; Yassin, L.K.; Alyaqoubi, R.; Saeed, S.; Alderei, A.; Alhammadi, A.; Alshehhi, M.; Almehairbi, A.; Al Houqani, S.; BaniYas, S.; et al. The microbiota–gut–brain axis and neurological disorders: A comprehensive review. Life 2024, 14, 1234. [Google Scholar] [CrossRef]
- Meystre, S.; van Stiphout, R.; Goris, A.; Gaitan, S. AI-based gut–brain-axis digital twins. Stud. Health Technol. Inform. 2023, 302, 1007–1008. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, J.; Huang, D.; Liu, Y.; Li, D.D. Machine-learning advances in microbiology: A review of methods and applications. Front. Microbiol. 2022, 13, 925454. [Google Scholar] [CrossRef]
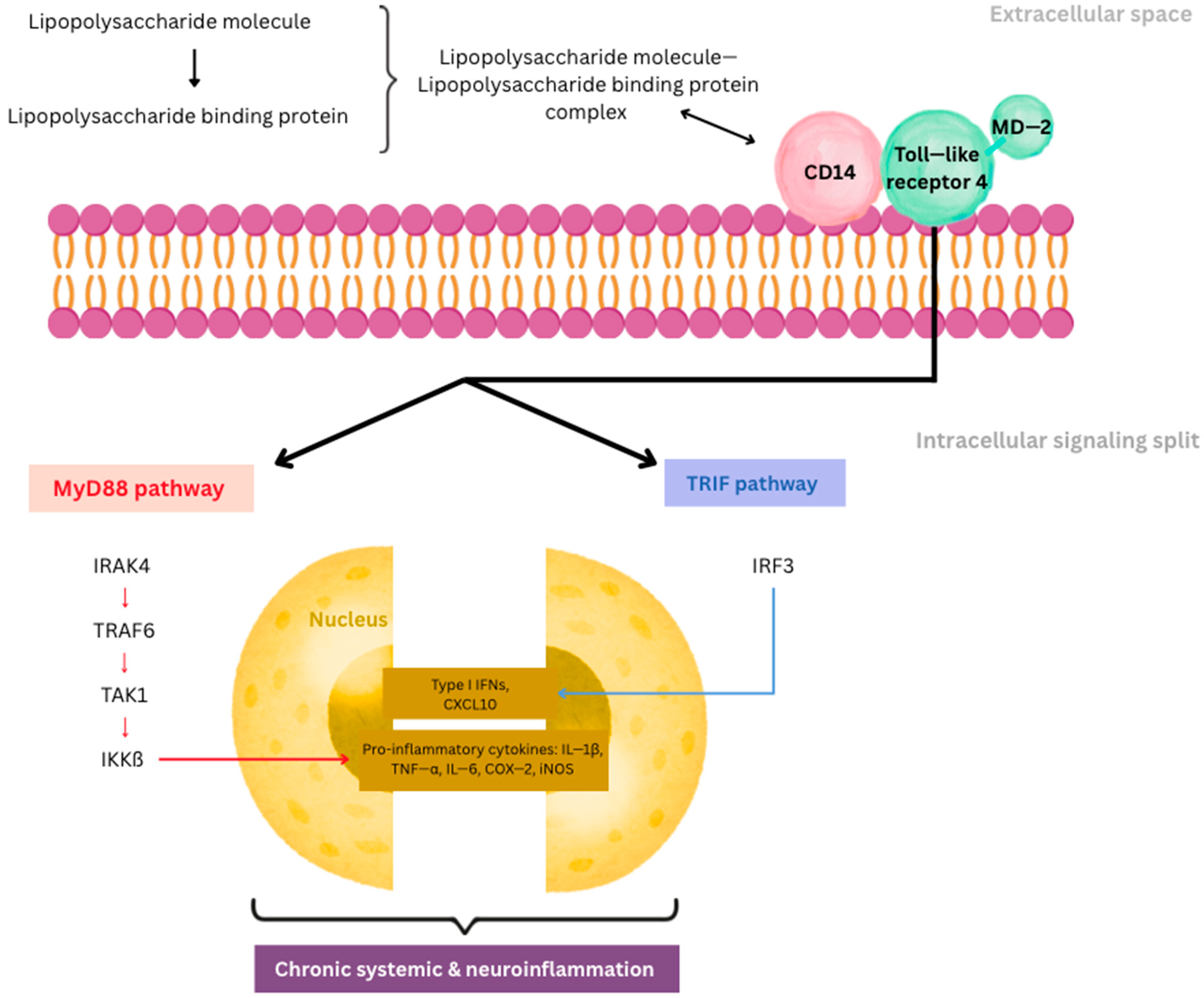

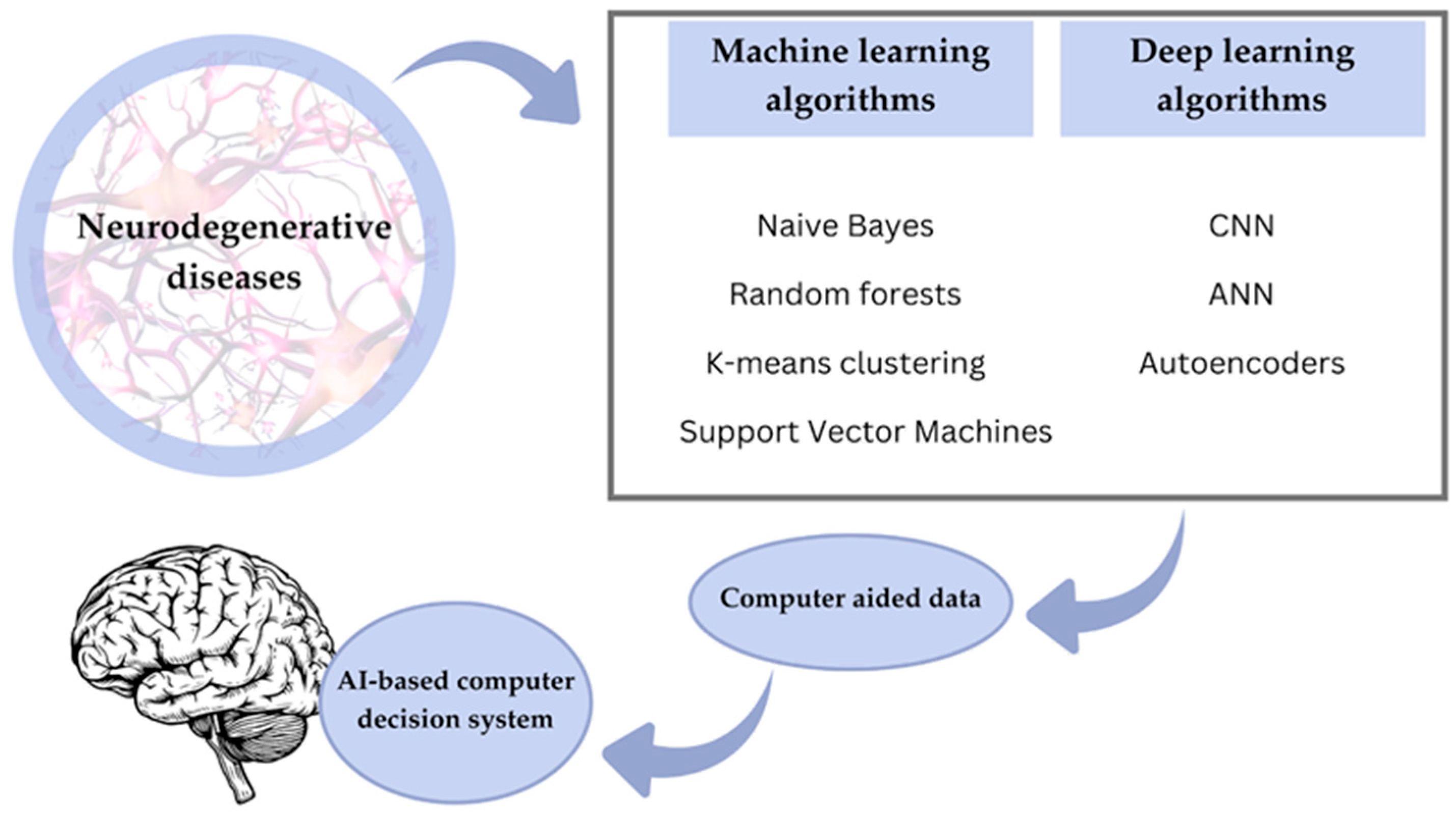
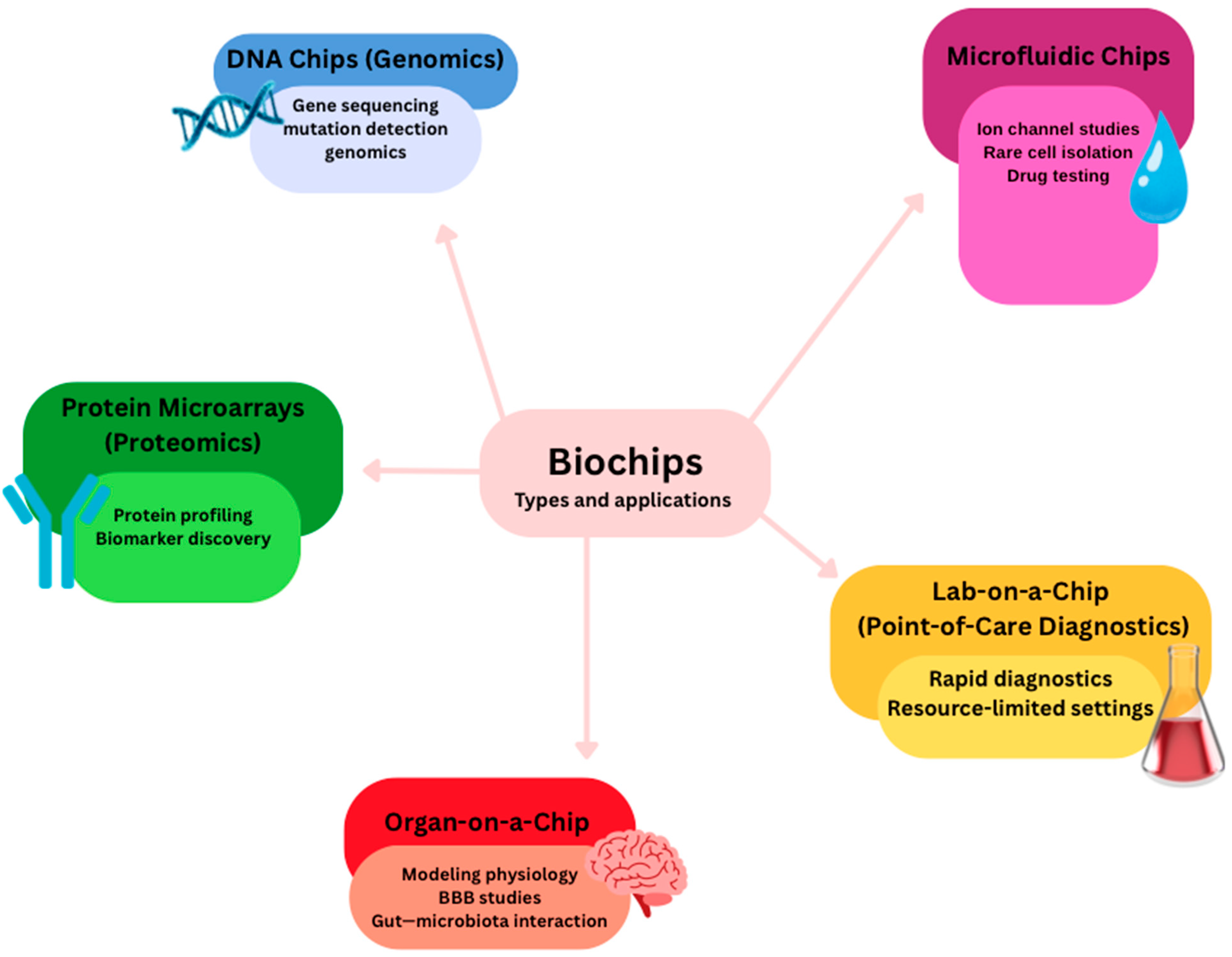
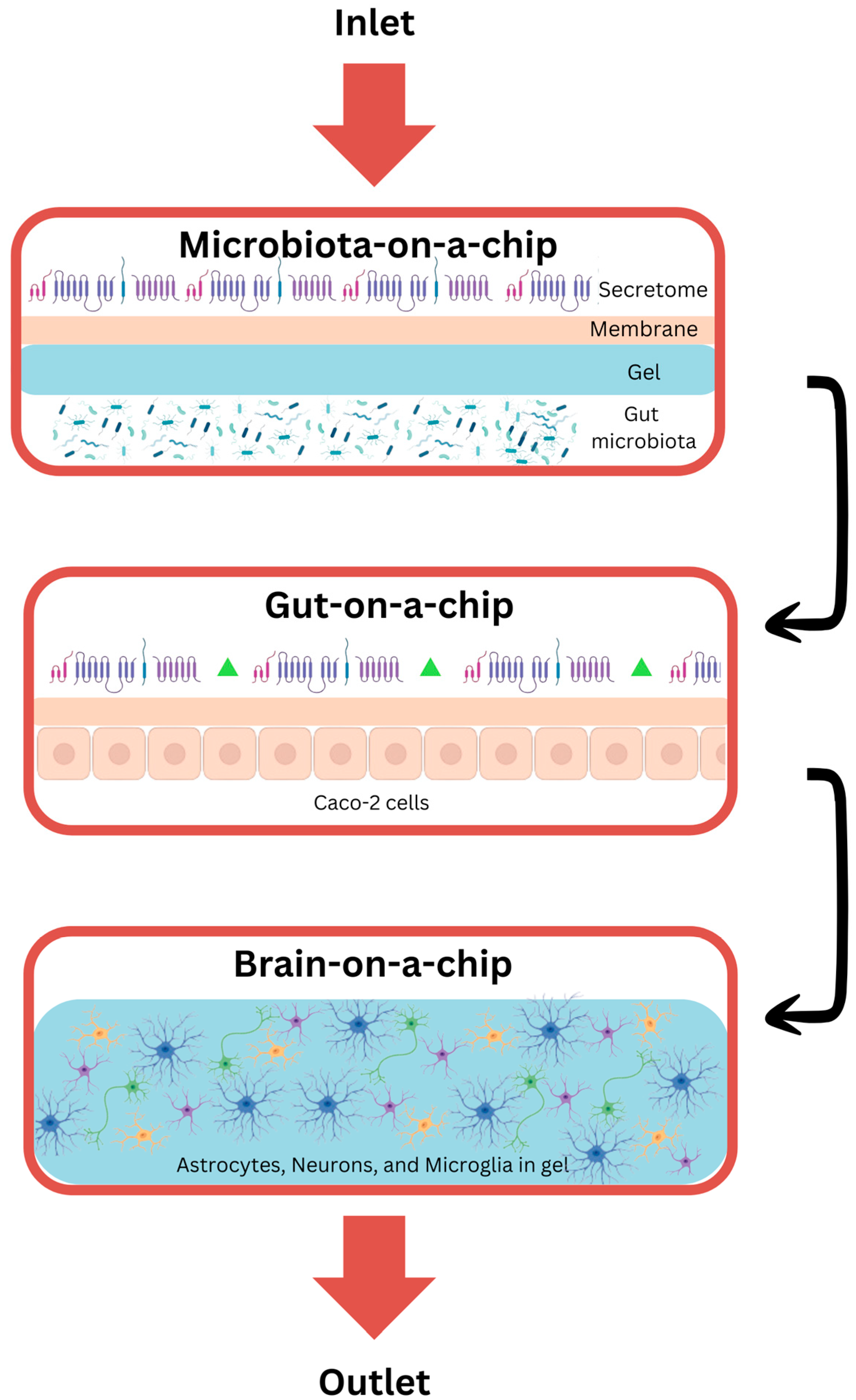
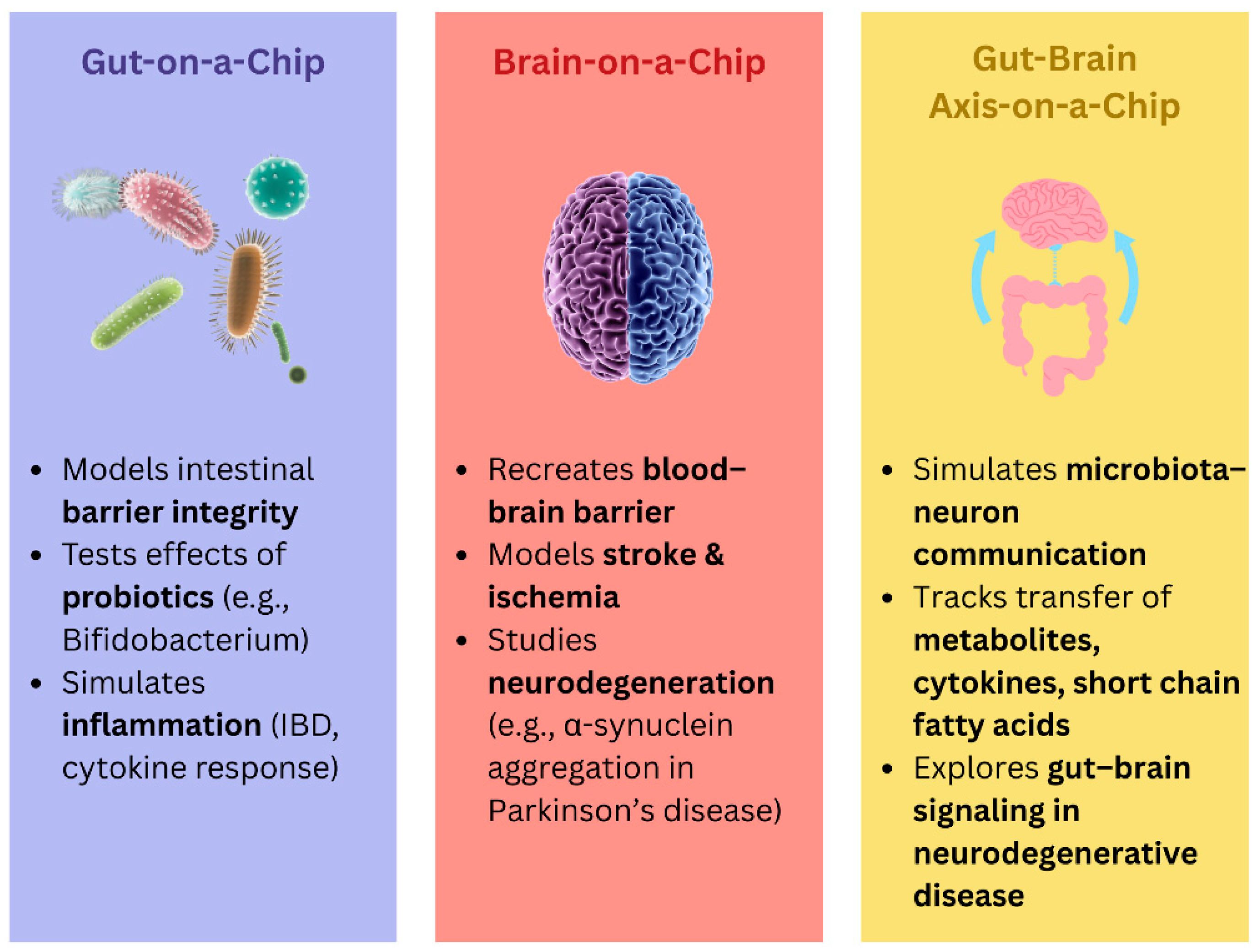

| Mechanism/Pathway | Key Mediators and Processes | Impact on Neurodegeneration | Source |
|---|---|---|---|
| Glial activation and cytokine release | Microglial and astrocytic activation; IL-1β, TNF-α, IL-6 secretion | Creates a toxic environment, synaptic dysfunction, neuronal death | [1,2,3,4,5,6] |
| Protein aggregates and DAMPs | Amyloid-β, α-synuclein, mitochondrial DNA, oxidized lipids | Engage PRRs (TLRs, NLRs); activate NLRP3 inflammasome; propagate inflammation | [6,21,22] |
| Inflammasome and autophagy dysfunction | NLRP3 inflammasome, cathepsin B, impaired lysosomal clearance | Self-sustaining inflammasome activation, reduced clearance of protein aggregates | [6,24] |
| HMGB1–RAGE/TLR4 signaling | HMGB1 redox states, ERK, MAPK, NF-κB pathways | Sustains pro-inflammatory phenotype, epigenetic remodeling of glia | [25,26] |
| Glutamate excitotoxicity | Reduced astrocytic EAAT1/2, excess extracellular glutamate | Postsynaptic calcium overload, neuronal apoptosis (hippocampus, substantia nigra) | [23] |
| Metabolic reprogramming | NF-κB-driven shift to glycolysis, lactate accumulation | Impaired mitochondrial biogenesis, extracellular acidification, myelin damage | [14,27] |
| Impaired resolution of inflammation | Reduced pro-resolving mediators (resolvins, protectins, maresins, lipoxins) | Failure to terminate inflammation, persistent microglial activation | [28,29] |
| Gut–brain axis and systemic inflammation | Gut dysbiosis, LPS translocation, TLR4–MD2–CD14 signaling, immunometabolism | BBB disruption, peripheral priming of microglia, chronic low-grade CNS inflammation | [9,10,11,12,13,14,15,16,17,18] |
| LPS-driven neurotoxicity | NF-κB, AP-1, IRF3, COX-2, iNOS activation; ROS production | Enhances tau/α-synuclein pathology, synaptic loss, mitochondrial dysfunction | [12,13,14,15,16,17] |
| Therapeutic innovations | Nanomedicine (BBB-penetrating nanoparticles, microbiota-targeting nanosystems); AI for data integration | Targeted modulation of inflammation, gut–brain homeostasis restoration, personalized therapy | [19,20] |
| Computational Approach | Main Use | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Brain imaging with computer learning (MRI, PET, EEG) | Diagnosis (early detection, staging) | Captures structural, functional, and molecular brain changes; combines multimodal data; large datasets exist. | Accuracy varies across scanners and hospitals; requires diverse data. | [63,64,65] |
| Deep learning and transfer learning (neural networks on MRI/PET/EEG) | Diagnosis (detecting Alzheimer’s, predicting progression) | Learns complex features; pre-trained models improve accuracy; ensemble methods show high performance. | Needs large, annotated datasets; limited clinical translation so far. | [77,78,79,80] |
| Random forest models (on MRI and cognitive data) | Diagnosis (differentiating Alzheimer’s, mild cognitive impairment, healthy controls) | High accuracy (~90–93%); identifies key brain regions; robust to noise. | Less accurate for mild cognitive impairment; heavy preprocessing required. | [81,82,83,84] |
| Classic machine learning (support vector machines, gradient boosting, etc.) | Diagnosis (using MRI, EEG) | Works well with small/medium datasets; effective on EEG/imaging. | Dataset-dependent; single models may not generalize. | [53,85,86] |
| Unsupervised clustering (e.g., k-means) | Research and patient subgrouping | Simple; finds hidden patterns in MRI and microbiome data. | Exploratory only; requires validation. | [87,88,89] |
| Artificial intelligence applied to gut microbiome | Diagnosis and treatment (biomarkers, diet, probiotics) | Identifies microbial biomarkers; supports personalized therapies. | High variability; still early for clinical translation. | [90,91] |
| Artificial intelligence for drug discovery (in silico modeling, docking, machine learning) | Treatment (finding new medicines) | Accelerates screening; finds promising inhibitors. | Many targets not yet in clinical trials. | [92,93,94] |
| Computational Approach | Main Use | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Classic machine learning on wearable sensors (support vector machines, gradient boosting) | Diagnosis (movement monitoring, detection) | Achieves high accuracy (>90%) using movement sensors; wearable devices enable real-world use. | Dependent on dataset quality; clinical robustness still limited. | [95] |
| Artificial intelligence applied to gait training (robot-assisted rehab, deep learning on pressure data) | Treatment and monitoring | Robot-assisted gait training improves walking and endurance; deep learning detects freezing-of-gait in real time. | Optimal training programs unclear; long-term benefits uncertain. | [96,97,98] |
| Brain imaging with computer learning (MRI, PET, EEG) | Diagnosis (early detection, tracking disease progression) | Captures structural and functional brain changes; allows early detection. | Needs harmonization across centers; performance may drop on unseen datasets. | [53,95] |
| Artificial intelligence applied to gut microbiome | Diagnosis and treatment (biomarker discovery; guiding therapies) | Reveals associations between gut bacteria and Parkinson’s; supports personalized therapeutic strategies. | Data variability across studies; translation into clinical use still limited. | [90,91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krsek, A.; Schleicher, L.M.S.; Jagodic, A.; Baticic, L. Nanomedicine-Driven Modulation of the Gut–Brain Axis: Innovative Approaches to Managing Chronic Inflammation in Alzheimer’s and Parkinson’s Disease. Int. J. Mol. Sci. 2025, 26, 9178. https://doi.org/10.3390/ijms26189178
Krsek A, Schleicher LMS, Jagodic A, Baticic L. Nanomedicine-Driven Modulation of the Gut–Brain Axis: Innovative Approaches to Managing Chronic Inflammation in Alzheimer’s and Parkinson’s Disease. International Journal of Molecular Sciences. 2025; 26(18):9178. https://doi.org/10.3390/ijms26189178
Chicago/Turabian StyleKrsek, Antea, Lou Marie Salomé Schleicher, Ana Jagodic, and Lara Baticic. 2025. "Nanomedicine-Driven Modulation of the Gut–Brain Axis: Innovative Approaches to Managing Chronic Inflammation in Alzheimer’s and Parkinson’s Disease" International Journal of Molecular Sciences 26, no. 18: 9178. https://doi.org/10.3390/ijms26189178
APA StyleKrsek, A., Schleicher, L. M. S., Jagodic, A., & Baticic, L. (2025). Nanomedicine-Driven Modulation of the Gut–Brain Axis: Innovative Approaches to Managing Chronic Inflammation in Alzheimer’s and Parkinson’s Disease. International Journal of Molecular Sciences, 26(18), 9178. https://doi.org/10.3390/ijms26189178








