Utilization of AhR and GPR35 Receptor Ligands as Superfoods in Cancer Prevention for Individuals with IBD
Abstract
1. Introduction
2. IBD—Pathological and Clinical Features and Its Association with Carcinogenesis
3. The Significance of CYP1A1 and CYP1B1 Gene Expression in Inflammatory Bowel Diseases and Colorectal Cancer
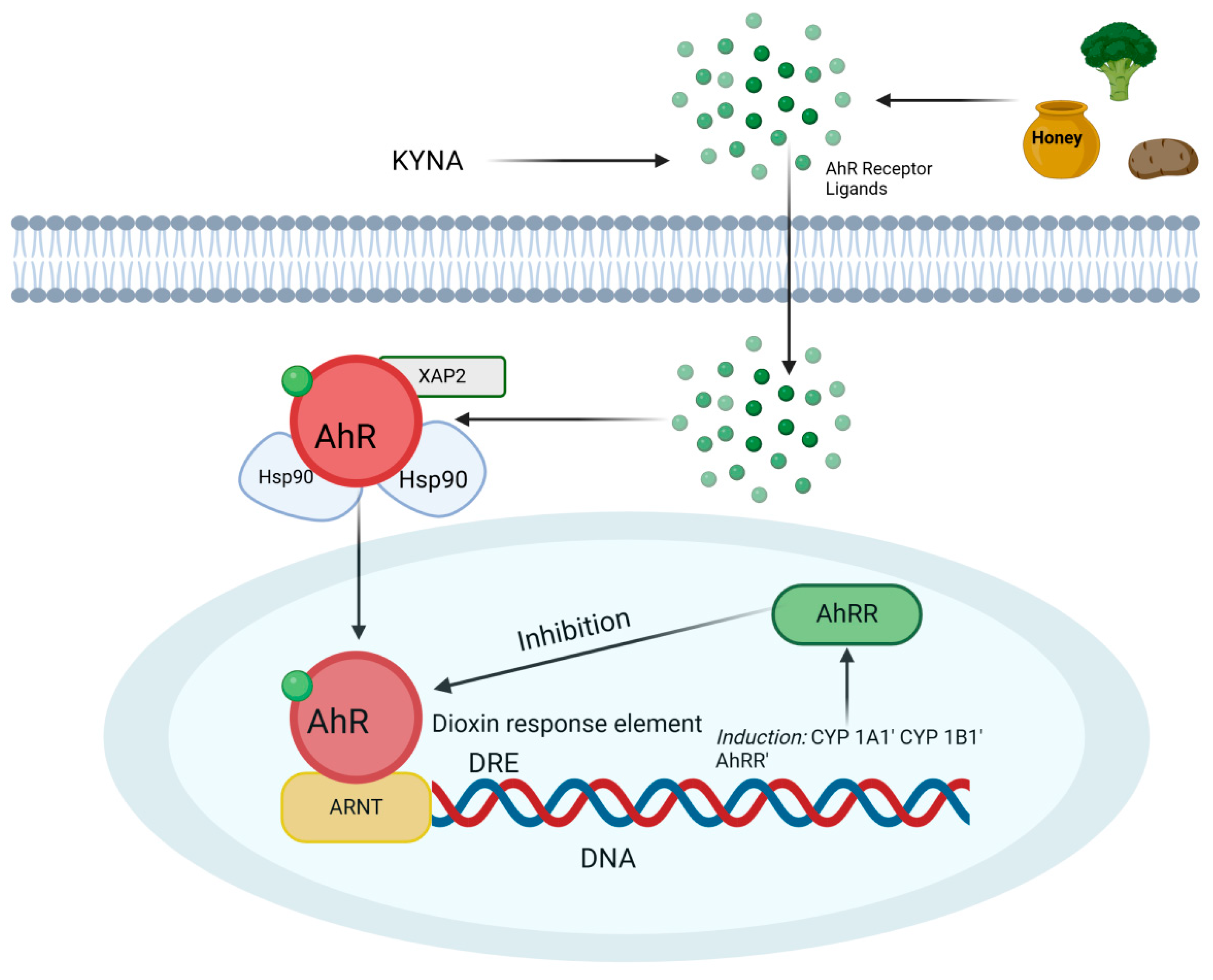
4. The Role of the AhR Receptor in Mitigating Inflammation in IBD
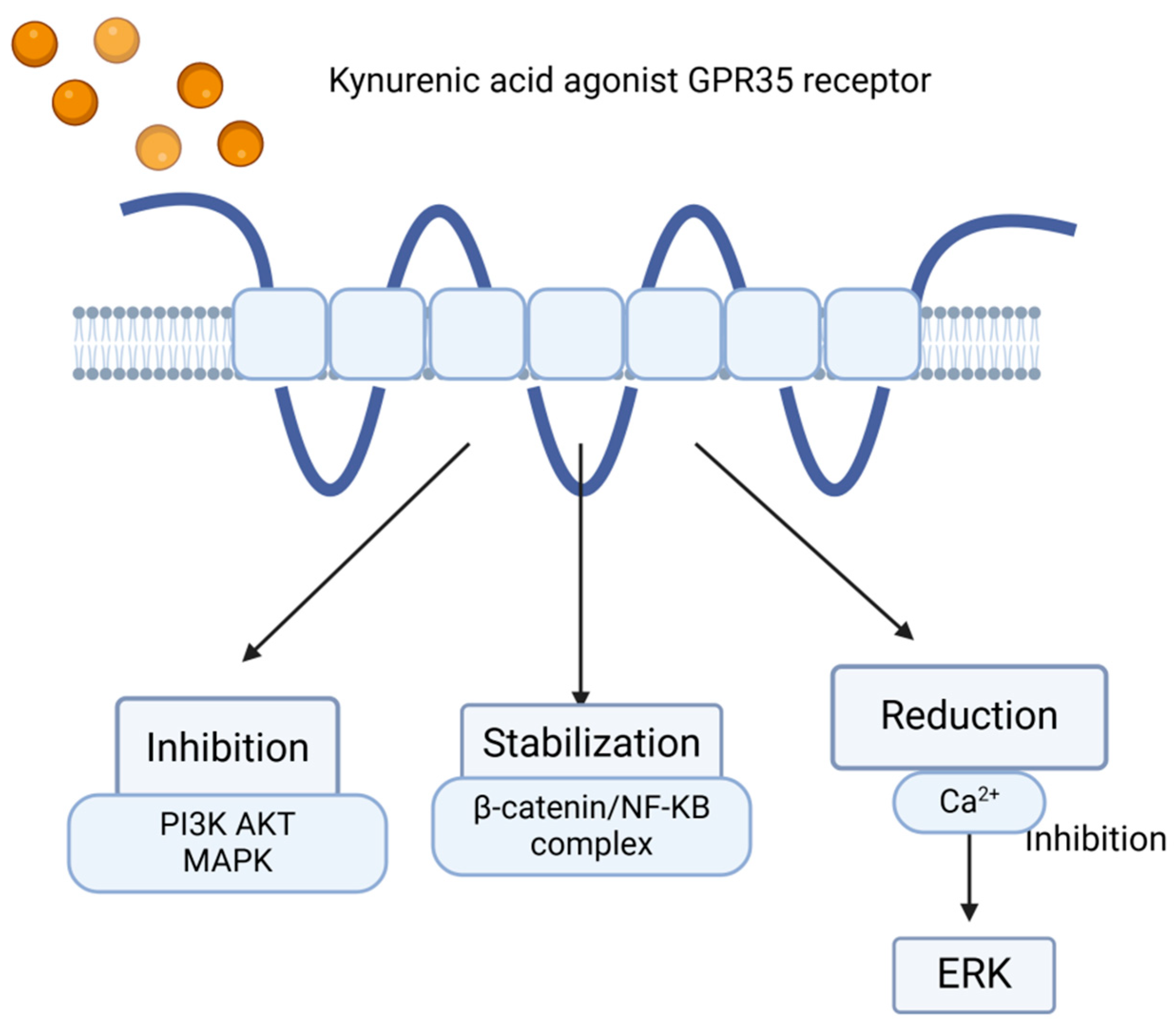
5. Role—GPR35 and KYNA in IBD
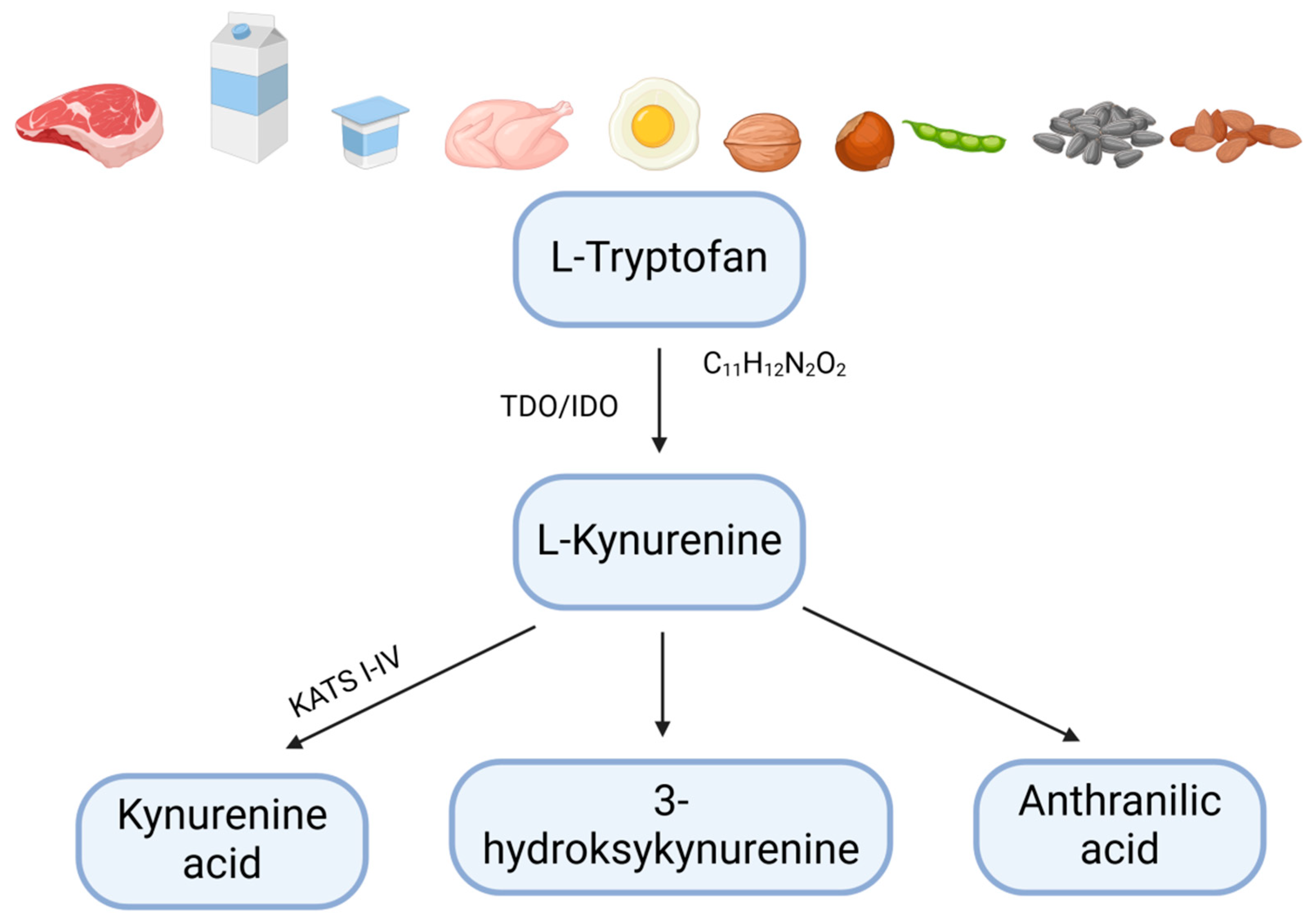
6. Kynurenic Acid as a Key Ligand of the GPR35 Receptor
7. Nutraceuticals and Functional Foods Activating Anti-Inflammatory Pathways via AhR and GPR35 Receptor Activation as Potential Therapeutic Strategies for IBD
8. Kynurenic Acid
9. Quercetin
10. Turmeric
11. Lactobacillus
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coussens, L.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Burgos-Molina, A.M.; Téllez Santana, T.; Redondo, M.; Bravo Romero, M.J. The Crucial Role of Inflammation and the Immune System in Colorectal Cancer Carcinogenesis: A Comprehensive Perspective. Int. J. Mol. Sci. 2024, 25, 6188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and Colon Cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.F.; Moore, J.B.; Kell, D.B. The Biology and Biochemistry of Kynurenic Acid, a Potential Nutraceutical with Multiple Biological Effects. Int. J. Mol. Sci. 2024, 25, 9082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esteve, E.; Ricart, W.; Fernández-Real, J.M. Dyslipidemia and inflammation: An evolutionary conserved mechanism. Clin. Nutr. 2005, 24, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.; Arlt, V.M.; Phillips, D.H. The role of cytochrome P450 enzymes in carcinogen activation and detoxication: An in vivo-in vitro paradox. Carcinogenesis 2018, 39, 851–859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meddens, C.A.; van der List, A.C.J.; Nieuwenhuis, E.E.S.; Nieuwenhuis, E.E.S.; Mokry, M. Non-coding DNA in IBD: From sequence variation in DNA regulatory elements to novel therapeutic potential. Gut 2019, 68, 928–941. [Google Scholar] [CrossRef]
- Petagna, L.; Antonelli, A.; Ganini, C.; Bellato, V.; Campanelli, M.; Divizia, A.; Efrati, C.; Franceschilli, M.; Guida, A.M.; Ingallinella, S.; et al. Pathophysiology of Crohn’s disease inflammation and recurrence. Biol. Direct 2020, 15, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Q.; Tu, H.; Li, J. Multifaceted Roles of Chemokine C-X-C Motif Ligand 7 in Inflammatory Diseases and Cancer. Front. Pharmacol. 2022, 13, 914730. [Google Scholar] [CrossRef]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, J.M. Pathophysiology of Inflammatory Bowel Disease: Innate Immune System. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lucafò, M.; Curci, D.; Franzin, M.; Decorti, G.; Stocco, G. Inflammatory Bowel Disease and Risk of Colorectal Cancer: An Overview From Pathophysiology to Pharmacological Prevention. Front. Pharmacol. 2021, 12, 772101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Ji, Y.; Chen, N.; Dai, L.; Deng, H. Colitis-associated carcinogenesis: Crosstalk between tumors, immune cells and gut microbiota. Cell Biosci. 2023, 13, 194. [Google Scholar] [CrossRef]
- Kinugasa, T.; Akagi, Y. Status of colitis-associated cancer in ulcerative colitis. World J. Gastrointest. Oncol. 2016, 8, 351–357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016, 22, 4794–4801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Pan, M.; Li, Y.; Cui, M.; Zhang, M. New targets for the treatment of ulcerative colitis: Gut microbiota and its metabolites. Comput. Struct. Biotechnol. J. 2025, 27, 1850–1863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Chen, R.; Zhang, D.; Qi, S.; Liu, Y. Metabolite interactions between host and microbiota during health and disease: Which feeds the other? Biomed. Pharmacother. 2023, 160, 114295. [Google Scholar] [CrossRef] [PubMed]
- Nebbia, M.; Yassin, N.A.; Spinelli, A. Colorectal Cancer in Inflammatory Bowel Disease. Clin. Colon. Rectal Surg. 2020, 33, 305–317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Cytochrome P450 CYP1A1: Wider roles in cancer progression and prevention. BMC Cancer 2009, 9, 187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raunio, H.; Kuusisto, M.; Juvonen, R.O.; Pentikäinen, O.T. Modeling of interactions between xenobiotics and cytochrome P450 (CYP) enzymes. Front. Pharmacol. 2015, 6, 123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Androutsopoulos, V.P.; Spyrou, I.; Ploumidis, A.; Papalampros, A.E.; Kyriakakis, M.; Delakas, D.; Spandidos, D.A.; Tsatsakis, A.M. Expression profile of CYP1A1 and CYP1B1 enzymes in colon and bladder tumors. PLoS ONE 2013, 8, e82487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sen, A.; Stark, H. Role of cytochrome P450 polymorphisms and functions in development of ulcerative colitis. World J. Gastroenterol. 2019, 25, 2846–2862. [Google Scholar] [CrossRef]
- Díaz-Díaz, C.J.; Ronnekleiv-Kelly, S.M.; Nukaya, M.; Geiger, P.G.; Balbo, S.; Dator, R.; Megna, B.W.; Carney, P.R.; Bradfield, C.A.; Kennedy, G.D. The Aryl Hydrocarbon Receptor is a Repressor of Inflammation-associated Colorectal Tumorigenesis in Mouse. Ann. Surg. 2016, 264, 429–436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neavin, D.R.; Liu, D.; Ray, B.; Weinshilboum, R.M. The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. Int. J. Mol. Sci. 2018, 19, 3851. [Google Scholar] [CrossRef]
- Goettel, J.A.; Gandhi, R.; Kenison, J.E.; Yeste, A.; Murugaiyan, G.; Sambanthamoorthy, S.; Griffith, A.E.; Patel, B.; Shouval, D.S.; Weiner, H.L.; et al. AHR Activation Is Protective against Colitis Driven by T Cells in Humanized Mice. Cell Rep. 2016, 17, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Vázquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ito, S.; Chen, C.; Satoh, J.; Yim, S.; Gonzalez, F.J. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut. J. Clin. Investig. 2007, 117, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Wei, J.; Liu, Z.Z.; Xie, J.J.; Wang, W.; Du, Y.P.; Chen, Y.; Si, H.Q.; Liu, Q.; Wu, L.X.; et al. Association between CYP1A2 and CYP1B1 polymorphisms and colorectal cancer risk: A meta-analysis. PLoS ONE 2014, 9, e100487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alhouayek, M.; Gouveia-Figueira, S.; Hammarström, M.L.; Fowler, C.J. Involvement of CYP1B1 in interferon γ-induced alterations of epithelial barrier integrity. Br. J. Pharmacol. 2018, 175, 877–890. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grishanova, A.Y.; Perepechaeva, M.L. Aryl Hydrocarbon Receptor in Oxidative Stress as a Double Agent and Its Biological and Therapeutic Significance. Int. J. Mol. Sci. 2022, 23, 6719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dudzińska, E.; Szymona, K.; Kloc, R.; Gil-Kulik, P.; Kocki, T.; Świstowska, M.; Bogucki, J.; Kocki, J.; Urbanska, E.M. Increased expression of kynurenine aminotransferases mRNA in lymphocytes of patients with inflammatory bowel disease. Ther. Adv. Gastroenterol. 2019, 12, 1756284819881304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrow, P.; McCarthy, N.; Stagg, A.; Lindsay, J. DOP017 Increased intestinal aryl hydrocarbon receptor expression and pathway sensitivity in Crohn’s disease. J. Crohn’s Colitis 2018, 12 (Suppl. S1), S041. [Google Scholar] [CrossRef][Green Version]
- Hashimoto-Hachiya, A.; Tsuji, G.; Murai, M.; Yan, X.; Furue, M. Upregulation of FLG, LOR, and IVL Expression by Rhodiola crenulata Root Extract via Aryl Hydrocarbon Receptor: Differential Involvement of OVOL1. Int. J. Mol. Sci. 2018, 19, 1654. [Google Scholar] [CrossRef]
- Champion, S.; Sauzet, C.; Bremond, P.; Benbrahim, K.; Abraldes, J.; Seree, E.; Barra, Y.; Villard, P.H. Activation of the NF κ B Pathway Enhances AhR Expression in Intestinal Caco-2 Cells. ISRN Toxicol. 2013, 2013, 792452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2018, 53, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hui, W.; Dai, Y. Therapeutic potential of aryl hydrocarbon receptor ligands derived from natural products in rheumatoid arthritis. Basic. Clin. Pharmacol. Toxicol. 2020, 126, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Busbee, P.B.; Rouse, M.; Nagarkatti, M.; Nagarkatti, P.S. Use of natural AhR ligands as potential therapeutic modalitiesagainst inflammatory disorders. Nutr. Rev. 2013, 71, 353–369. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, C.; Safe, S.H. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: Effects of structure and cell context. Environ. Health Perspect. 2003, 111, 1877–1882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vrba, J.; Kren, V.; Vacek, J.; Papouskova, B.; Ulrichova, J. Quercetin, quercetin glycosides and taxifolin differ in their ability to induce AhR activation and CYP1A1 expression in HepG2 cells. Phytother. Res. 2012, 26, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Niestroy, J.; Barbara, A.; Herbst, K.; Rode, S.; van Liempt, M.; Roos, P.H. Single and concerted effects of benzo[a]pyrene and flavonoids on the AhR and Nrf2-pathway in the human colon carcinoma cell line Caco-2. Toxicol. In Vitro 2011, 25, 671–683. [Google Scholar] [CrossRef]
- Nguyen, L.P.; Bradfield, C.A. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 2008, 21, 102–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n-3 and n-6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dudzińska, E.; Szymona, K.; Gil-Kulik, P.; Chomik, P.; Świstowska, M.; Gryzińska, M.; Kocki, J. Imbalance of Controlled Death in Peripheral Blood Lymphocytes in Crohn’s Disease and Ulcerative Colitis. Medicina 2019, 55, 231. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2018, 8, 1957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rad Pour, S.; Morikawa, H.; Kiani, N.A.; Yang, M.; Azimi, A.; Shafi, G.; Shang, M.; Baumgartner, R.; Ketelhuth, D.F.J.; Kamleh, M.A.; et al. Exhaustion of CD4+ T-cells mediated by the Kynurenine Pathway in Melanoma. Sci. Rep. 2019, 9, 12150. [Google Scholar] [CrossRef]
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci. Rep. 2018, 9, 643. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and pathogenesis of inflammatory bowel disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, R.; Cong, Y.; Liu, Z. Critical roles of G protein-coupled receptors in regulating intestinal homeostasis and inflammatory bowel disease. Mucosal Immunol. 2022, 15, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.J.; Cho, J.H.; Gevers, D.; Chu, H. Genetic Factors and the Intestinal Microbiome Guide Development of Microbe-Based Therapies for Inflammatory Bowel Diseases. Gastroenterology 2019, 156, 2174–2189. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, Y.; Jiang, Z.; Qing, H. G Protein-Coupled Receptors (GPCRs) in Alzheimer’s Disease: A Focus on BACE1 Related GPCRs. Front. Aging Neurosci. 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Melhem, H.; Kaya, B.; Ayata, C.K.; Hruz, P.; Niess, J.H. Metabolite-Sensing G Protein-Coupled Receptors Connect the Diet-Microbiota-Metabolites Axis to Inflammatory Bowel Disease. Cells 2019, 8, 450. [Google Scholar] [CrossRef]
- Law, I.K.M.; Padua, D.M.; Iliopoulos, D.; Pothoulakis, C. Role of G protein-coupled receptors-microRNA interactions in gastrointestinal pathophysiology. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G361–G372. [Google Scholar] [CrossRef]
- Dolecka, J.; Urbanik-Sypniewska, T.; Skrzydło-Radomańska, B.; Parada-Turska, J. Effect of kynurenic acid on the viability of probiotics in vitro. Pharmacol. Rep. 2011, 63, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Simonavicius, N.; Wu, X.; Swaminath, G.; Reagan, J.; Tian, H.; Ling, L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006, 281, 22021–22028. [Google Scholar] [CrossRef] [PubMed]
- Kaya, B.; Melhem, H.; Niess, J.H. GPR35 in Intestinal Diseases: From Risk Gene to Function. Front. Immunol. 2021, 12, 717392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Zuraikat, F.M.; Wood, R.A.; Barragán, R.; St-Onge, M.P. Sleep and Diet: Mounting Evidence of a Cyclical Relationship. Annu. Rev. Nutr. 2021, 41, 309–332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ala, M. Tryptophan metabolites modulate inflammatory bowel disease and colorectal cancer by affecting immune system. Int. Rev. Immunol. 2021, 41, 326–345. [Google Scholar] [CrossRef]
- Yu, F.; Du, Y.; Li, C.; Zhang, H.; Lai, W.; Li, S.; Ye, Z.; Fu, W.; Li, S.; Li, X.-G.; et al. Association between metabolites in tryptophan-kynurenine pathway and inflammatory bowel disease: A two-sample Mendelian randomization. Sci. Rep. 2024, 14, 201. [Google Scholar] [CrossRef]
- Paydaş Hataysal, E.; Körez, M.K.; Guler, E.M.; Vatansev, H.; Bozalı, K.; Basaranoglu, M.; Vatansev, H. Impaired Kynurenine Pathway in Inflammatory Bowel Disease. J. Clin. Med. 2024, 13, 6147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schneditz, G.; Elias, J.E.; Pagano, E.; Zaeem Cader, M.; Saveljeva, S.; Long, K.; Mukhopadhyay, S.; Arasteh, M.; Lawley, T.D.; Dougan, G.; et al. GPR35 promotes glycolysis, proliferation, and oncogenic signaling by engaging with the sodium potassium pump. Sci. Signal. 2019, 12, eaau9048. [Google Scholar] [CrossRef]
- Divorty, N.; Mackenzie, A.E.; Nicklin, S.A.; Milligan, G. G protein-coupled receptor 35: An emerging target in inflammatory and cardiovascular disease. Front. Pharmacol. 2015, 6, 41. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan—Kynurenine metabolism. Ann. N. Y. Acad. Sci. 2010, 1199, 1–14. [Google Scholar] [CrossRef]
- Iskandar, H.N.; Ciorba, M.A. Biomarkers in inflammatory bowel disease: Current practices and recent advances. Transl. Res. 2012, 159, 313–325. [Google Scholar] [CrossRef]
- Gupta, N.K.; Thaker, A.I.; Kanuri, N.; Riehl, T.E.; Rowley, C.W.; Stenson, W.F.; Ciorba, M.A. Serum Analysis of Tryptophan Catabolism Pathway: Correlation with Crohn’s Disease Activity. Inflamm. Bowel Dis. 2012, 18, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Małaczewska, J.; Siwicki, A.K.; Wójcik, R.M.; Turski, W.A.; Kaczorek, E. The effect of kynurenic acid on the synthesis of selected cytokines by murine splenocytes—In vitro and ex vivo studies. Cent. Eur. J. Immunol. 2016, 41, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Turski, W.A.; Rajtar, G. Kynurenic acid inhibits colon cancer proliferation in vitro: Effects on signaling pathways. Amino Acids 2014, 46, 2393–2401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bishnupuri, K.S.; Alvarado, D.M.; Khouri, A.N.; Shabsovich, M.; Chen, B.; Dieckgraefe, B.K.; Ciorba, M.A. IDO1 and Kynurenine Pathway Metabolites Activate PI3K-Akt Signaling in the Neoplastic Colon Epithelium to Promote Cancer Cell Proliferation and Inhibit Apoptosis. Cancer Res. 2019, 79, 1138–1150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walczak, K.; Wnorowski, A.; Turski, W.A.; Plech, T. Kynurenic acid and cancer: Facts and controversies. Cell. Mol. Life Sci. 2020, 77, 1531–1550. [Google Scholar] [CrossRef] [PubMed]
- Cobos, Á.; Díaz, O. ‘Superfoods’: Reliability of the Information for Consumers Available on the Web. Foods 2023, 12, 546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Verma, D.K.; Thakur, M.; Patel, A.R.; Srivastav, P.P.; Singh, S.; Gupta, A.K.; Chávez-González, M.L.; Aguilar, C.N.; Chakravorty, N.; et al. Curcumin Extraction, Isolation, Quantification and Its Application in Functional Foods: A Review With a Focus on Immune Enhancement Activities and COVID-19. Front. Nutr. 2021, 8, 747956. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turski, M.P.; Kamiński, P.; Zgrajka, W.; Turska, M.; Turski, W.A. Potato- an important source of nutritional kynurenic acid. Plant Foods Hum. Nutr. 2012, 67, 17–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turski, M.P.; Turska, M.; Paluszkiewicz, P.; Parada-Turska, J.; Oxenkrug, G.F. Kynurenic Acid in the Digestive System—New Facts, New Challenges. Int. J. Tryptophan Res. 2013, 6, 47–55. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef] [PubMed]
- Shorobi, F.M.; Nisa, F.Y.; Saha, S.; Chowdhury, M.A.H.; Srisuphanunt, M.; Hossain, K.H.; Rahman, M.A. Quercetin: A Functional Food-Flavonoid Incredibly Attenuates Emerging and Re-Emerging Viral Infections through Immunomodulatory Actions. Molecules 2023, 28, 938. [Google Scholar] [CrossRef] [PubMed]
- Najaf Najafi, N.; Armide, N.; Akbari, A.; Rahimi, V.B.; Askari, V.R. Quercetin a promising functional food additive against allergic Diseases: A comprehensive and mechanistic review. J. Funct. Foods 2024, 116, 106152. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batjargal, A.; Solek, P.; Kukula-Koch, W.; Urjin, B.; Koch, W.; Koman, D.; Dudzinska, E. Gurgem-7 toxicity assessment: Regulation of cell survival or death by traditional Mongolian prescription. Ecotoxicol. Environ. Saf. 2022, 239, 113660. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Jakubczyk, K.; Maciejewska-Markiewicz, D.; Janda, K.; Kaźmierczak-Siedlecka, K.; Kaczmarczyk, M.; Łoniewski, I.; Marlicz, W. Gut Biofactory-Neurocompetent Metabolites within the Gastrointestinal Tract. A Scoping Review. Nutrients 2020, 12, 3369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e4. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa González, A.; Guerra-Ojeda, S.; Camacho-Villa, M.A.; Valls, A.; Alegre, E.; Quintero-Bernal, R.; Martorell, P.; Chenoll, E.; Serna-García, M.; Mauricio, M.D.; et al. Effect of Probiotics on Gastrointestinal Health Through the Aryl Hydrocarbon Receptor Pathway: A Systematic Review. Foods 2024, 13, 3479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Z.; Xie, L.; Huang, L. Regulation of host immune responses by Lactobacillus through aryl hydrocarbon receptors. Med. Microecol. 2023, 16, 100081. [Google Scholar] [CrossRef]
- Takamura, T.; Harama, D.; Fukumoto, S.; Nakamura, Y.; Shimokawa, N.; Ishimaru, K.; Ikegami, S.; Makino, S.; Kitamura, M.; Nakao, A. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol. Cell Biol. 2011, 89, 817–822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poźniak, O.; Sitarz, R.; Sitarz, M.Z.; Kowalczuk, D.; Słoń, E.; Dudzińska, E. Utilization of AhR and GPR35 Receptor Ligands as Superfoods in Cancer Prevention for Individuals with IBD. Int. J. Mol. Sci. 2025, 26, 9160. https://doi.org/10.3390/ijms26189160
Poźniak O, Sitarz R, Sitarz MZ, Kowalczuk D, Słoń E, Dudzińska E. Utilization of AhR and GPR35 Receptor Ligands as Superfoods in Cancer Prevention for Individuals with IBD. International Journal of Molecular Sciences. 2025; 26(18):9160. https://doi.org/10.3390/ijms26189160
Chicago/Turabian StylePoźniak, Olga, Robert Sitarz, Monika Zofia Sitarz, Dorota Kowalczuk, Emilia Słoń, and Ewa Dudzińska. 2025. "Utilization of AhR and GPR35 Receptor Ligands as Superfoods in Cancer Prevention for Individuals with IBD" International Journal of Molecular Sciences 26, no. 18: 9160. https://doi.org/10.3390/ijms26189160
APA StylePoźniak, O., Sitarz, R., Sitarz, M. Z., Kowalczuk, D., Słoń, E., & Dudzińska, E. (2025). Utilization of AhR and GPR35 Receptor Ligands as Superfoods in Cancer Prevention for Individuals with IBD. International Journal of Molecular Sciences, 26(18), 9160. https://doi.org/10.3390/ijms26189160






