Mechanisms and Therapeutic Perspectives of Podocyte Aging in Podocytopathies
Abstract
1. Introduction
2. Podocyte Structure and Function
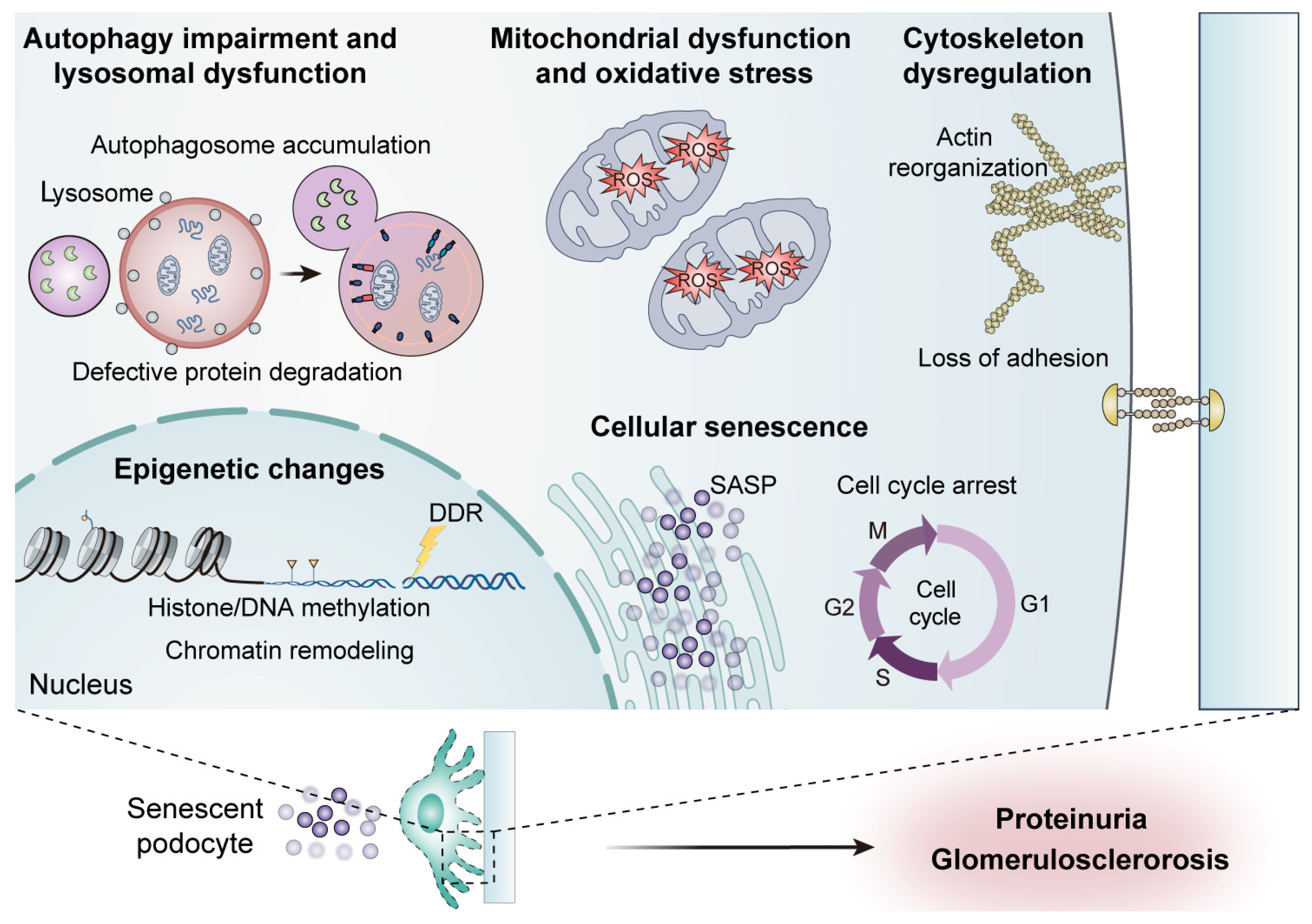
3. Cellular and Molecular Mechanisms of Podocyte Aging
3.1. Cellular Senescence
3.2. Mitochondrial Dysfunction and Oxidative Stress
3.3. Autophagy Impairment and Lysosomal Dysfunction
3.4. Epigenetic Changes
3.5. Cytoskeleton Dysregulation
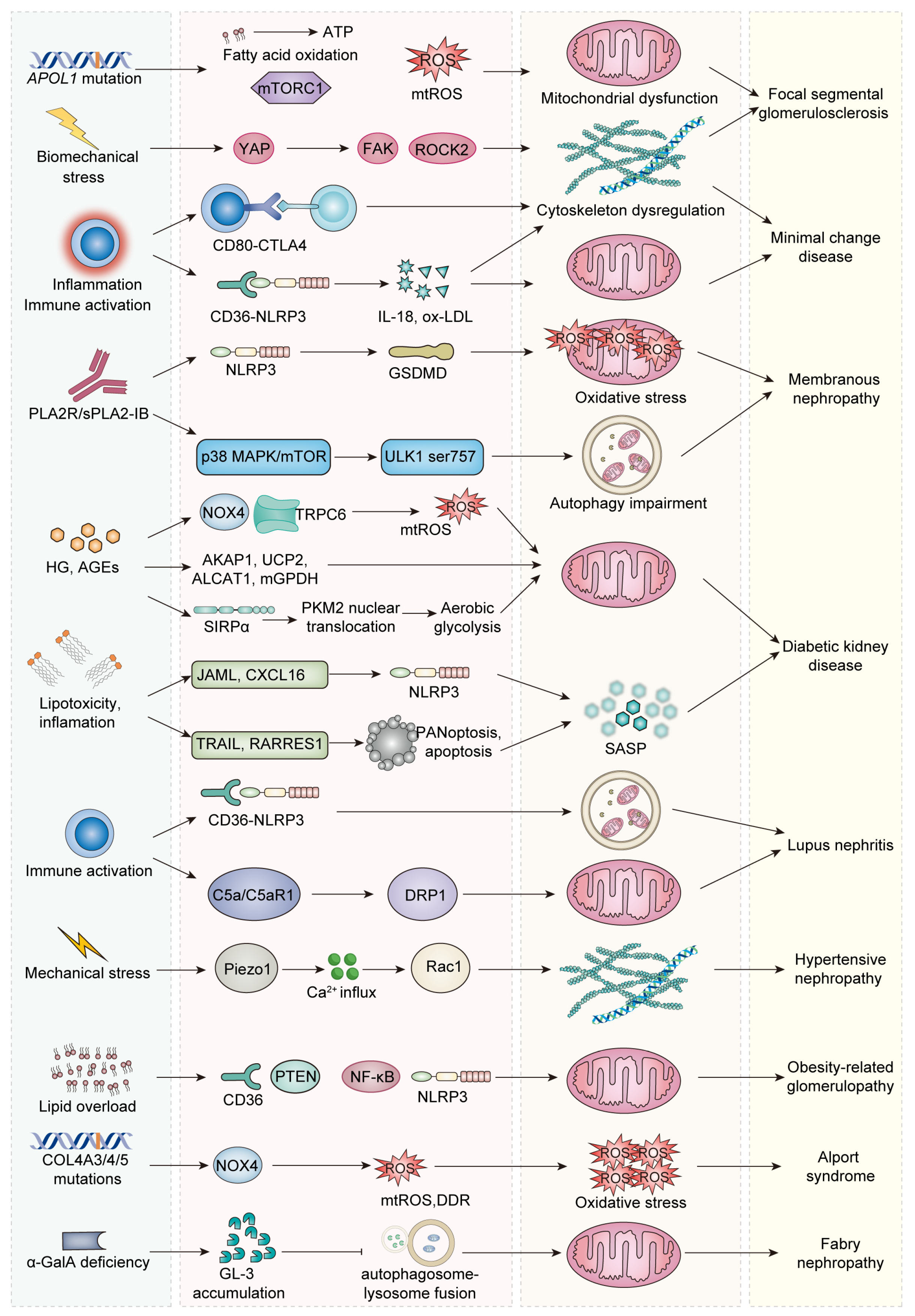
4. Podocyte Aging in Podocytopathies
4.1. Focal Segmental Glomerulosclerosis
4.2. Minimal Change Disease and Membranous Nephropathy
4.3. Diabetic Kidney Disease
4.4. Lupus Nephritis
4.5. Hypertension/Obesity-Related Glomerulopathy
4.6. Genetic Podocytopathies
5. Therapeutic Approaches Targeting Podocyte Aging
5.1. Senolytic Therapies
5.2. Podocyte Regeneration Strategies
5.2.1. Mechanisms Underlying Podocyte Regeneration
5.2.2. Drug-Based Interventions for Podocyte Regeneration
5.2.3. Genetic and Tissue Engineering Approaches
5.3. Drug Repurposing
5.3.1. Metformin and Rapamycin
5.3.2. Losartan
5.3.3. Canagliflozin
5.3.4. Curcumin
| Therapeutic Strategies | Example | Mechanisms | Diseases | Reference |
|---|---|---|---|---|
| Senolytic therapies | Dasatinib and quercetin (D + Q) | Enhancing autophagic activity through activating AMPK and suppressing mTOR signaling; activating autophagy, inhibiting Notch pathway overactivation, and improving mitochondrial integrity. | DKD | [169,170] |
| Podocyte regeneration | Retinoic acid | Enhancing podocyte differentiation by activating retinoic acid receptorα (RARα). | / | [181] |
| Angiotensin receptor blockers (ARBs) | Enhancing the differentiation of renal progenitor cells and renin–lineage cells into functional podocytes. | FSGS | [184] | |
| CRISPR/Cas9-based lineage tracing | Cellular reprogramming. | / | [185] | |
| Tissue engineering | Organoids and kidney-on-chip models. | / | [186,187] | |
| Drug repurposing | Metformin | Activating AMPK, restoring autophagic flux by promoting the ULK1-initiated autophagy complex, and enhancing mitochondrial biogenesis and function via the AMPK-PGC1α pathway. | DKD, MN | [188,189] |
| Rapamycin | Inhibiting mTORC1, enhancing autophagy, and alleviating protein synthesis burden. | DKD, MN | [190] | |
| Losartan | Upregulating Hsp70; mitigating oxidative stress and ERS, reducing NF-κB-mediated inflammatory signaling, and preserving mitochondrial function. | DKD | [133] | |
| Canagliflozin | Correcting the Th1/Th2 imbalance; restoring autophagic activity by activating the ULK1 complex and suppressing mTOR signaling. | MN | [191] | |
| Curcumin | Targeting CXCL16, reducing lipid accumulation, oxidative stress, and pro-inflammatory cytokine production; suppressing aberrant activation of the Wnt/β-catenin signaling pathway. | DKD, ORG | [109,192] |
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Assady, S.; Wanner, N.; Skorecki, K.L.; Huber, T.B. New Insights into Podocyte Biology in Glomerular Health and Disease. J. Am. Soc. Nephrol. 2017, 28, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M. Podocyte Injury and Its Consequences. Kidney Int. 2016, 89, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Shankland, S.J.; Wang, Y.; Shaw, A.S.; Vaughan, J.C.; Pippin, J.W.; Wessely, O. Podocyte Aging: Why and How Getting Old Matters. J. Am. Soc. Nephrol. 2021, 32, 2697–2713. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many Therapeutic Avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.B.; Anders, H.-J.; Susztak, K.; Podestà, M.A.; Remuzzi, G.; Hildebrandt, F.; Romagnani, P. Podocytopathies. Nat. Rev. Dis. Primers 2020, 6, 68. [Google Scholar] [CrossRef]
- Shankland, S.J.; Rule, A.D.; Kutz, J.N.; Pippin, J.W.; Wessely, O. Podocyte Senescence and Aging. Kidney360 2023, 4, 1784–1793. [Google Scholar] [CrossRef]
- Docherty, M.-H.; O’Sullivan, E.D.; Bonventre, J.V.; Ferenbach, D.A. Cellular Senescence in the Kidney. J. Am. Soc. Nephrol. 2019, 30, 726–736. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Nishinakamura, R. Podocyte Development, Disease, and Stem Cell Research. Kidney Int. 2019, 96, 1077–1082. [Google Scholar] [CrossRef]
- Lappalainen, P.; Kotila, T.; Jégou, A.; Romet-Lemonne, G. Biochemical and Mechanical Regulation of Actin Dynamics. Nat. Rev. Mol. Cell Biol. 2022, 23, 836–852. [Google Scholar] [CrossRef]
- Patrakka, J.; Tryggvason, K. Nephrin—A Unique Structural and Signaling Protein of the Kidney Filter. Trends Mol. Med. 2007, 13, 396–403. [Google Scholar] [CrossRef]
- Ning, L.; Suleiman, H.Y.; Miner, J.H. Synaptopodin Is Dispensable for Normal Podocyte Homeostasis but Is Protective in the Context of Acute Podocyte Injury. J. Am. Soc. Nephrol. 2020, 31, 2815–2832. [Google Scholar] [CrossRef]
- Shono, A.; Tsukaguchi, H.; Yaoita, E.; Nameta, M.; Kurihara, H.; Qin, X.-S.; Yamamoto, T.; Doi, T. Podocin Participates in the Assembly of Tight Junctions between Foot Processes in Nephrotic Podocytes. J. Am. Soc. Nephrol. 2007, 18, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Price, P.M. A Role for Novel Cell-Cycle Proteins in Podocyte Biology. Kidney Int. 2010, 77, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Huang, Y.; Fu, N.; Cui, C.; Peng, X.; Kang, H.; Xiao, J.; Ke, G. Podocyte Senescence: From Molecular Mechanisms to Therapeutics. Ren. Fail. 2024, 46, 2398712. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Epel, E. Stress and Telomere Shortening: Insights from Cellular Mechanisms. Ageing Res. Rev. 2022, 73, 101507. [Google Scholar] [CrossRef]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere Dysfunction in Ageing and Age-Related Diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The Senescence-Associated Secretory Phenotype and Its Physiological and Pathological Implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Hejazian, S.M.; Ardalan, M.; Hosseiniyan Khatibi, S.M.; Rahbar Saadat, Y.; Barzegari, A.; Gueguen, V.; Meddahi-Pellé, A.; Anagnostou, F.; Zununi Vahed, S.; Pavon-Djavid, G. Biofactors Regulating Mitochondrial Function and Dynamics in Podocytes and Podocytopathies. J. Cell Physiol. 2023, 238, 2206–2227. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Yu, J.; Teng, H.; Wu, S.; Wang, Y.; Zhou, H.; Li, F. Role of Mitochondria in Pathogenesis and Therapy of Renal Fibrosis. Metabolism 2024, 155, 155913. [Google Scholar] [CrossRef]
- Li, Y.; Fan, J.; Zhu, W.; Niu, Y.; Wu, M.; Zhang, A. Therapeutic Potential Targeting Podocyte Mitochondrial Dysfunction in Focal Segmental Glomerulosclerosis. Kidney Dis. 2023, 9, 254–264. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, Y.; Xue, Y.; Xing, C.; Zhang, B. Podocyte Injury in Diabetic Kidney Disease: A Focus on Mitochondrial Dysfunction. Front. Cell Dev. Biol. 2022, 10, 832887. [Google Scholar] [CrossRef]
- Chuang, P.Y.; Cai, W.; Li, X.; Fang, L.; Xu, J.; Yacoub, R.; He, J.C.; Lee, K. Reduction in Podocyte SIRT1 Accelerates Kidney Injury in Aging Mice. Am. J. Physiol. Ren. Physiol. 2017, 313, F621–F628. [Google Scholar] [CrossRef]
- Xue, H.; Li, P.; Luo, Y.; Wu, C.; Liu, Y.; Qin, X.; Huang, X.; Sun, C. Salidroside Stimulates the Sirt1/PGC-1α Axis and Ameliorates Diabetic Nephropathy in Mice. Phytomedicine 2019, 54, 240–247. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, S.; Yuan, Y.; Ding, G.; Chen, R.; Liu, B.; Yang, T.; Zhang, A. Mitochondrial Dysfunction Mediates Aldosterone-Induced Podocyte Damage: A Therapeutic Target of PPARγ. Am. J. Pathol. 2011, 178, 2020–2031. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, M.; Bai, M.; Lei, J.; Yuan, Y.; Huang, S.; Zhang, Y.; Ding, G.; Jia, Z.; Zhang, A. SIRT1 Alleviates Aldosterone-Induced Podocyte Injury by Suppressing Mitochondrial Dysfunction and NLRP3 Inflammasome Activation. Kidney Dis. 2021, 7, 293–305. [Google Scholar] [CrossRef]
- Ponticelli, C.; Moroni, G.; Reggiani, F. Autophagy and Podocytopathy. Nephrol. Dial. Transplant. 2023, 38, gfad024. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.B.; Edelstein, C.L.; Hartleben, B.; Inoki, K.; Jiang, M.; Koya, D.; Kume, S.; Lieberthal, W.; Pallet, N.; Quiroga, A.; et al. Emerging Role of Autophagy in Kidney Function, Diseases and Aging. Autophagy 2012, 8, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Bensaada, I.; Robin, B.; Perez, J.; Salemkour, Y.; Chipont, A.; Camus, M.; Lemoine, M.; Guyonnet, L.; Lazareth, H.; Letavernier, E.; et al. Calpastatin Prevents Angiotensin II-Mediated Podocyte Injury through Maintenance of Autophagy. Kidney Int. 2021, 100, 90–106. [Google Scholar] [CrossRef]
- Lenoir, O.; Jasiek, M.; Hénique, C.; Guyonnet, L.; Hartleben, B.; Bork, T.; Chipont, A.; Flosseau, K.; Bensaada, I.; Schmitt, A.; et al. Endothelial Cell and Podocyte Autophagy Synergistically Protect from Diabetes-Induced Glomerulosclerosis. Autophagy 2015, 11, 1130–1145. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Schwesinger, C. Lysosome Function in Glomerular Health and Disease. Cell Tissue Res. 2021, 385, 371–392. [Google Scholar] [CrossRef]
- Li, G.; Kidd, J.; Li, P.-L. Podocyte Lysosome Dysfunction in Chronic Glomerular Diseases. Int. J. Mol. Sci. 2020, 21, 1559. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Nonaka, K.; Koike, M.; Asanuma, K.; Takagi, M.; Oliva Trejo, J.A.; Seki, T.; Hidaka, T.; Ichimura, K.; Sakai, T.; Tada, N.; et al. Cathepsin D in Podocytes Is Important in the Pathogenesis of Proteinuria and CKD. J. Am. Soc. Nephrol. 2016, 27, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, A. (Pro)Renin Receptor and Autophagy in Podocytes. Autophagy 2012, 8, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Heintz, L.; Meyer-Schwesinger, C. The Intertwining of Autophagy and the Ubiquitin Proteasome System in Podocyte (Patho)Physiology. Cell Physiol. Biochem. 2021, 55, 68–95. [Google Scholar] [CrossRef]
- Cinà, D.P.; Onay, T.; Paltoo, A.; Li, C.; Maezawa, Y.; De Arteaga, J.; Jurisicova, A.; Quaggin, S.E. MTOR Regulates Autophagic Flux in the Glomerulus. Autophagy 2012, 8, 696–698. [Google Scholar] [CrossRef][Green Version]
- Cinà, D.P.; Onay, T.; Paltoo, A.; Li, C.; Maezawa, Y.; De Arteaga, J.; Jurisicova, A.; Quaggin, S.E. Inhibition of MTOR Disrupts Autophagic Flux in Podocytes. J. Am. Soc. Nephrol. 2012, 23, 412–420. [Google Scholar] [CrossRef]
- Feliers, D. Epigenetic Control of Podocyte Differentiation: A New Target of the Renin-Angiotensin System in Kidney Disease. Kidney Int. 2015, 88, 668–670. [Google Scholar] [CrossRef]
- Pereira, B.M.V.; Katakia, Y.T.; Majumder, S.; Thieme, K. Unraveling the Epigenetic Landscape of Glomerular Cells in Kidney Disease. J. Mol. Med. 2021, 99, 785–803. [Google Scholar] [CrossRef]
- Majumder, S.; Thieme, K.; Batchu, S.N.; Alghamdi, T.A.; Bowskill, B.B.; Kabir, M.G.; Liu, Y.; Advani, S.L.; White, K.E.; Geldenhuys, L.; et al. Shifts in Podocyte Histone H3K27me3 Regulate Mouse and Human Glomerular Disease. J. Clin. Investig. 2018, 128, 483–499. [Google Scholar] [CrossRef]
- Allison, S.J. Epigenetics: H3K27me3 in Glomerular Disease. Nat. Rev. Nephrol. 2018, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Medina Rangel, P.X.; Cross, E.; Liu, C.; Pedigo, C.E.; Tian, X.; Gutiérrez-Calabrés, E.; Nagata, S.; Priyadarshini, A.; Lerner, G.; Bunda, P.; et al. Cell Cycle and Senescence Regulation by Podocyte Histone Deacetylase 1 and 2. J. Am. Soc. Nephrol. 2023, 34, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Wang, Y.-N.; Su, W.; Zou, L.; Zhuang, S.-G.; Yu, X.-Y.; Liu, F.; Zhao, Y.-Y. Sirtuin 6 Protects against Podocyte Injury by Blocking the Renin-Angiotensin System by Inhibiting the Wnt1/β-Catenin Pathway. Acta Pharmacol. Sin. 2024, 45, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Qiao, Z.; Zhang, Y.; Zhan, P.; Yi, F. Histone Deacetylases Take Center Stage on Regulation of Podocyte Function. Kidney Dis. 2020, 6, 236–246. [Google Scholar] [CrossRef]
- Hayashi, K.; Hishikawa, A.; Itoh, H. DNA Damage Repair and DNA Methylation in the Kidney. Am. J. Nephrol. 2019, 50, 81–91. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Liu, S.; Chen, Y.; Li, R.; Lin, T.; Yu, C.; Zhang, H.; Huang, Z.; Zhao, X.; et al. DNA Methyltransferase 1 May Be a Therapy Target for Attenuating Diabetic Nephropathy and Podocyte Injury. Kidney Int. 2017, 92, 140–153. [Google Scholar] [CrossRef]
- Schell, C.; Huber, T.B. The Evolving Complexity of the Podocyte Cytoskeleton. J. Am. Soc. Nephrol. 2017, 28, 3166–3174. [Google Scholar] [CrossRef]
- Ye, Q.; Lan, B.; Liu, H.; Persson, P.B.; Lai, E.Y.; Mao, J. A Critical Role of the Podocyte Cytoskeleton in the Pathogenesis of Glomerular Proteinuria and Autoimmune Podocytopathies. Acta Physiol. 2022, 235, e13850. [Google Scholar] [CrossRef]
- Sachs, N.; Sonnenberg, A. Cell-Matrix Adhesion of Podocytes in Physiology and Disease. Nat. Rev. Nephrol. 2013, 9, 200–210. [Google Scholar] [CrossRef]
- He, F.-F.; Chen, S.; Su, H.; Meng, X.-F.; Zhang, C. Actin-Associated Proteins in the Pathogenesis of Podocyte Injury. CG 2013, 14, 477–484. [Google Scholar] [CrossRef]
- Blaine, J.; Dylewski, J. Regulation of the Actin Cytoskeleton in Podocytes. Cells 2020, 9, 1700. [Google Scholar] [CrossRef]
- Feng, D.; DuMontier, C.; Pollak, M.R. Mechanical Challenges and Cytoskeletal Impairments in Focal Segmental Glomerulosclerosis. Am. J. Physiol.-Ren. Physiol. 2018, 314, F921–F925. [Google Scholar] [CrossRef]
- Kaplan, J.M.; Kim, S.H.; North, K.N.; Rennke, H.; Correia, L.A.; Tong, H.Q.; Mathis, B.J.; Rodríguez-Pérez, J.C.; Allen, P.G.; Beggs, A.H.; et al. Mutations in ACTN4, Encoding Alpha-Actinin-4, Cause Familial Focal Segmental Glomerulosclerosis. Nat. Genet. 2000, 24, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Boyer, O.; Benoit, G.; Gribouval, O.; Nevo, F.; Tête, M.-J.; Dantal, J.; Gilbert-Dussardier, B.; Touchard, G.; Karras, A.; Presne, C.; et al. Mutations in INF2 Are a Major Cause of Autosomal Dominant Focal Segmental Glomerulosclerosis. J. Am. Soc. Nephrol. 2011, 22, 239–245. [Google Scholar] [CrossRef]
- Dessapt, C.; Baradez, M.O.; Hayward, A.; Dei Cas, A.; Thomas, S.M.; Viberti, G.; Gnudi, L. Mechanical Forces and TGFbeta1 Reduce Podocyte Adhesion through Alpha3beta1 Integrin Downregulation. Nephrol. Dial. Transplant. 2009, 24, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Fogo, A.B. Causes and Pathogenesis of Focal Segmental Glomerulosclerosis. Nat. Rev. Nephrol. 2015, 11, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Saio, M.; Picciotto, D.; Viazzi, F.; Russo, E.; Cipriani, L.; Carta, A.; Costigliolo, F.; Gaggero, G.; Salvidio, G.; et al. Cellular Senescence Is Associated with Faster Progression of Focal Segmental Glomerulosclerosis. Am. J. Nephrol. 2020, 51, 950–958. [Google Scholar] [CrossRef]
- Ge, M.; Molina, J.; Ducasa, G.M.; Mallela, S.K.; Varona Santos, J.; Mitrofanova, A.; Kim, J.-J.; Liu, X.; Sloan, A.; Mendez, A.J.; et al. APOL1 Risk Variants Affect Podocyte Lipid Homeostasis and Energy Production in Focal Segmental Glomerulosclerosis. Hum. Mol. Genet. 2021, 30, 182–197. [Google Scholar] [CrossRef]
- Li, F.; Fang, Y.; Zhuang, Q.; Cheng, M.; Moronge, D.; Jue, H.; Meyuhas, O.; Ding, X.; Zhang, Z.; Chen, J.-K.; et al. Blocking Ribosomal Protein S6 Phosphorylation Inhibits Podocyte Hypertrophy and Focal Segmental Glomerulosclerosis. Kidney Int. 2022, 102, 121–135. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, H.; Song, N.; Liang, Y.; Zhu, J.; Chen, J.; Ning, Y.; Hu, J.; Fang, Y.; Teng, J.; et al. METTL14 Aggravates Podocyte Injury and Glomerulopathy Progression through N6-Methyladenosine-Dependent Downregulating of Sirt1. Cell Death Dis. 2021, 12, 881. [Google Scholar] [CrossRef]
- Bao, D.; Su, H.; Lei, C.-T.; Tang, H.; Ye, C.; Xiong, W.; He, F.-F.; Lin, J.-H.; Hammes, H.-P.; Zhang, C. MAD2B-Mediated Cell Cycle Reentry of Podocytes Is Involved in the Pathogenesis of FSGS. Int. J. Biol. Sci. 2021, 17, 4396–4408. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.-J.; Huang, J.; Shu, Y.; Wang, M.; Ji, J.; Yang, L.; Zhao, M.-H.; Cui, Z. Complement C5a and C5a Receptor 1 Mediates Glomerular Damage in Focal Segmental Glomerulosclerosis. Clin. Immunol. 2025, 273, 110459. [Google Scholar] [CrossRef] [PubMed]
- May, C.J.; Chesor, M.; Hunter, S.E.; Hayes, B.; Barr, R.; Roberts, T.; Barrington, F.A.; Farmer, L.; Ni, L.; Jackson, M.; et al. Podocyte Protease Activated Receptor 1 Stimulation in Mice Produces Focal Segmental Glomerulosclerosis Mirroring Human Disease Signaling Events. Kidney Int. 2023, 104, 265–278. [Google Scholar] [CrossRef]
- Wilkening, A.; Krappe, J.; Mühe, A.M.; Lindenmeyer, M.T.; Eltrich, N.; Luckow, B.; Vielhauer, V. C-C Chemokine Receptor Type 2 Mediates Glomerular Injury and Interstitial Fibrosis in Focal Segmental Glomerulosclerosis. Nephrol. Dial. Transplant. 2020, 35, 227–239. [Google Scholar] [CrossRef]
- Tanoue, A.; Katayama, K.; Ito, Y.; Joh, K.; Toda, M.; Yasuma, T.; D’Alessandro-Gabazza, C.N.; Kawachi, H.; Yan, K.; Ito, M.; et al. Podocyte-Specific Crb2 Knockout Mice Develop Focal Segmental Glomerulosclerosis. Sci. Rep. 2021, 11, 20556. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Zhao, X. FAM40A Alters the Cytoskeleton of Podocytes in Familial Focal and Segmental Glomerulosclerosis by Regulating F-Actin and Nephrin. Arch. Med. Sci. 2019, 15, 165–173. [Google Scholar] [CrossRef]
- Shao, G.; Xu, J.; Hu, C.; Jia, W.; Xu, X.; Gu, Y.; Zhang, L.; Zheng, Z.; Zhong, J.; Zhu, S.; et al. Podocyte YAP Ablation Decreases Podocyte Adhesion and Exacerbates FSGS Progression through A3β1 Integrin. J. Pathol. 2025, 265, 84–98. [Google Scholar] [CrossRef]
- Vivarelli, M.; Massella, L.; Ruggiero, B.; Emma, F. Minimal Change Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 332–345. [Google Scholar] [CrossRef]
- Watts, A.J.B.; Keller, K.H.; Lerner, G.; Rosales, I.; Collins, A.B.; Sekulic, M.; Waikar, S.S.; Chandraker, A.; Riella, L.V.; Alexander, M.P.; et al. Discovery of Autoantibodies Targeting Nephrin in Minimal Change Disease Supports a Novel Autoimmune Etiology. J. Am. Soc. Nephrol. 2022, 33, 238–252. [Google Scholar] [CrossRef]
- Cara-Fuentes, G.; Wasserfall, C.H.; Wang, H.; Johnson, R.J.; Garin, E.H. Minimal Change Disease: A Dysregulation of the Podocyte CD80-CTLA-4 Axis? Pediatr. Nephrol. 2014, 29, 2333–2340. [Google Scholar] [CrossRef][Green Version]
- Liu, C.; Liu, L.; Huang, Y.; Shi, R.; Wu, Y.; Hakimah Binti Ismail, I. Contribution of IL-33/ILC2-Mediated Th2 Cytokines during the Progression of Minimal Change Disease. Int. Immunopharmacol. 2023, 114, 109493. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.E.; Thurman, J.M. The Immune System and Idiopathic Nephrotic Syndrome. Clin. J. Am. Soc. Nephrol. 2022, 17, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Kajio, Y.; Suzuki, T.; Kobayashi, K.; Kanazawa, N.; Iyoda, M.; Honda, H.; Honda, K. Activation of the Inflammasome and Pyroptosis Cascade in Podocytes of Patients with Minimal Change Disease. Clin. Kidney J. 2024, 17, sfae216. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, A.V. Membranous Nephropathy. In Contributions to Nephrology; Herrera, G.A., Ed.; S. Karger AG: Basel, Switzerland, 2011; Volume 169, pp. 107–125. ISBN 978-3-8055-9537-7. [Google Scholar]
- Kistler, A.D.; Salant, D.J. Complement Activation and Effector Pathways in Membranous Nephropathy. Kidney Int. 2024, 105, 473–483. [Google Scholar] [CrossRef]
- Van De Logt, A.-E.; Fresquet, M.; Wetzels, J.F.; Brenchley, P. The Anti-PLA2R Antibody in Membranous Nephropathy: What We Know and What Remains a Decade after Its Discovery. Kidney Int. 2019, 96, 1292–1302. [Google Scholar] [CrossRef]
- Liu, J.; Malhotra, D.; Ge, Y.; Gunning, W.; Dworkin, L.; Gong, R. THSD7A-Associated Membranous Nephropathy Involves Both Complement-Mediated and Autonomous Podocyte Injury. Front. Pharmacol. 2024, 15, 1430451. [Google Scholar] [CrossRef]
- Wang, H.; Lv, D.; Jiang, S.; Hou, Q.; Zhang, L.; Li, S.; Zhu, X.; Xu, X.; Wen, J.; Zeng, C.; et al. Complement Induces Podocyte Pyroptosis in Membranous Nephropathy by Mediating Mitochondrial Dysfunction. Cell Death Dis. 2022, 13, 281. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Lin, S.; Dai, B.; Chen, H.; Tao, X.; Li, G.; Wan, J.; Pan, Y. sPLA2-IB and PLA2R Mediate Insufficient Autophagy and Contribute to Podocyte Injury in Idiopathic Membranous Nephropathy by Activation of the p38MAPK/mTOR/ULK1ser757 Signaling Pathway. FASEB J. 2021, 35, e21170. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, Z.-H.; Chen, X.-C.; Zhao, X.-L.; Zhong, Z.; Yang, C.; Wu, H.-L.; An, N.; Li, W.-Y.; Liu, H.-F. Blockage of the Lysosome-Dependent Autophagic Pathway Contributes to Complement Membrane Attack Complex-Induced Podocyte Injury in Idiopathic Membranous Nephropathy. Sci. Rep. 2017, 7, 8643. [Google Scholar] [CrossRef]
- Verzola, D.; Gandolfo, M.T.; Gaetani, G.; Ferraris, A.; Mangerini, R.; Ferrario, F.; Villaggio, B.; Gianiorio, F.; Tosetti, F.; Weiss, U.; et al. Accelerated Senescence in the Kidneys of Patients with Type 2 Diabetic Nephropathy. Am. J. Physiol. Renal Physiol. 2008, 295, F1563–F1573. [Google Scholar] [CrossRef]
- Narasimhan, A.; Flores, R.R.; Robbins, P.D.; Niedernhofer, L.J. Role of Cellular Senescence in Type II Diabetes. Endocrinology 2021, 162, bqab136. [Google Scholar] [CrossRef] [PubMed]
- Ilatovskaya, D.V.; Blass, G.; Palygin, O.; Levchenko, V.; Pavlov, T.S.; Grzybowski, M.N.; Winsor, K.; Shuyskiy, L.S.; Geurts, A.M.; Cowley, A.W.; et al. A NOX4/TRPC6 Pathway in Podocyte Calcium Regulation and Renal Damage in Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, Z.; Ma, Y.; Yang, X.; Zhu, Z.; Zhang, Z.; Hu, J.; Liang, W.; Ding, G. AKAP1 Contributes to Impaired mtDNA Replication and Mitochondrial Dysfunction in Podocytes of Diabetic Kidney Disease. Int. J. Biol. Sci. 2022, 18, 4026–4042. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, S.; Liang, Y.; Sun, Q.; Fang, Y.; Jiang, L.; Wen, P.; Yang, J. UCP2 Deficiency Impairs Podocyte Autophagy in Diabetic Nephropathy. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166705. [Google Scholar] [CrossRef]
- Hao, Y.; Fan, Y.; Feng, J.; Zhu, Z.; Luo, Z.; Hu, H.; Li, W.; Yang, H.; Ding, G. ALCAT1-Mediated Abnormal Cardiolipin Remodelling Promotes Mitochondrial Injury in Podocytes in Diabetic Kidney Disease. Cell Commun. Signal 2024, 22, 26. [Google Scholar] [CrossRef]
- Qu, H.; Gong, X.; Liu, X.; Zhang, R.; Wang, Y.; Huang, B.; Zhang, L.; Zheng, H.; Zheng, Y. Deficiency of Mitochondrial Glycerol 3-Phosphate Dehydrogenase Exacerbates Podocyte Injury and the Progression of Diabetic Kidney Disease. Diabetes 2021, 70, 1372–1387. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, M.; Wang, Z.; Fu, Y.; Jia, M.; Wang, X.; Liu, M.; Zhang, Y.; Sun, Y.; Lu, Y.; et al. PGRN Acts as a Novel Regulator of Mitochondrial Homeostasis by Facilitating Mitophagy and Mitochondrial Biogenesis to Prevent Podocyte Injury in Diabetic Nephropathy. Cell Death Dis. 2019, 10, 524. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Jia, R.; Qian, B.; Jing, C.; Zeng, C.; Zhu, D.; Liu, Z.; Zen, K.; Li, L. Podocyte SIRPα Reduction in Diabetic Nephropathy Aggravates Podocyte Injury by Promoting Pyruvate Kinase M2 Nuclear Translocation. Redox Biol. 2024, 78, 103439. [Google Scholar] [CrossRef]
- Yang, F.; Qu, Q.; Zhao, C.; Liu, X.; Yang, P.; Li, Z.; Han, L.; Shi, X. Paecilomyces Cicadae-Fermented Radix Astragali Activates Podocyte Autophagy by Attenuating PI3K/AKT/mTOR Pathways to Protect against Diabetic Nephropathy in Mice. Biomed. Pharmacother. 2020, 129, 110479. [Google Scholar] [CrossRef]
- Salemkour, Y.; Yildiz, D.; Dionet, L.; ‘T Hart, D.C.; Verheijden, K.A.T.; Saito, R.; Mahtal, N.; Delbet, J.-D.; Letavernier, E.; Rabant, M.; et al. Podocyte Injury in Diabetic Kidney Disease in Mouse Models Involves TRPC6-Mediated Calpain Activation Impairing Autophagy. J. Am. Soc. Nephrol. 2023, 34, 1823–1842. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; He, Q.; Yang, H.-C.; Fogo, A.B.; Harris, R.C. Inhibition of Transcriptional Coactivator YAP Impairs the Expression and Function of Transcription Factor WT1 in Diabetic Podocyte Injury. Kidney Int. 2024, 105, 1200–1211. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, L.; Zeng, H.; Gao, L.; Guo, S.; Chen, C.; Liu, X.; Zhang, M.; Ma, L.; Li, Y.; et al. Circ-0000953 Deficiency Exacerbates Podocyte Injury and Autophagy Disorder by Targeting Mir665-3p-Atg4b in Diabetic Nephropathy. Autophagy 2024, 20, 1072–1097. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Huang, J.; Zhang, C.; Xie, Y.; Cao, Y.; Tao, L.; Tang, H.; Lin, J.; Hammes, H.-P.; Huang, K.; et al. Regulation of Podocyte Injury by CircHIPK3/FUS Complex in Diabetic Kidney Disease. Int. J. Biol. Sci. 2022, 18, 5624–5640. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Jing, T.; Liu, H.; Liu, Y.; Zhu, X.; Wang, J.; Xu, L. Ursolic Acid Alleviates Mitotic Catastrophe in Podocyte by Inhibiting Autophagic P62 Accumulation in Diabetic Nephropathy. Int. J. Biol. Sci. 2024, 20, 3317–3333. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, M.; Wolf, G. AGE-Induced Suppression of EZH2 Mediates Injury of Podocytes by Reducing H3K27me3. Am. J. Nephrol. 2020, 51, 676–692. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, X.; Hu, X.; Gao, L.; Zeng, H.; Wang, X.; Huang, Y.; Zhu, W.; Wang, J.; Wen, J.; et al. METTL3-Mediated m6A Modification of TIMP2 mRNA Promotes Podocyte Injury in Diabetic Nephropathy. Mol. Ther. 2022, 30, 1721–1740. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, S.; Kong, J.; Zhou, Q.; Wang, Z.; Zhang, Y.; Yan, H.; Wang, Y.; Li, T.; Xie, Y.; et al. DOT1L Protects against Podocyte Injury in Diabetic Kidney Disease through Phospholipase C-like 1. Cell Commun. Signal 2024, 22, 519. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Zhong, F.; Das, G.C.; Xie, Y.; Li, Z.; Cai, W.; Jiang, G.; Choi, J.; Sidani, M.; et al. Epigenetic Regulation of RCAN1 Expression in Kidney Disease and Its Role in Podocyte Injury. Kidney Int. 2018, 94, 1160–1176. [Google Scholar] [CrossRef]
- Liu, D.-W.; Zhang, J.-H.; Liu, F.-X.; Wang, X.-T.; Pan, S.-K.; Jiang, D.-K.; Zhao, Z.-H.; Liu, Z.-S. Silencing of Long Noncoding RNA PVT1 Inhibits Podocyte Damage and Apoptosis in Diabetic Nephropathy by Upregulating FOXA1. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Z.; He, W.; Ren, P.; He, Q.; Jin, J. LncRNA HOXB3OS Improves High Glucose-Mediated Podocyte Damage and Progression of Diabetic Kidney Disease through Enhancing SIRT1 mRNA Stability. Biomed. Pharmacother. 2025, 182, 117770. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Q.; Fan, X.; Zhen, J.; Wang, C.; Chen, H.; Liu, Y.; Zhou, P.; Zhang, T.; Huang, T.; et al. Long Noncoding RNA ENST00000436340 Promotes Podocyte Injury in Diabetic Kidney Disease by Facilitating the Association of PTBP1 with RAB3B. Cell Death Dis. 2023, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Fang, Y.; Ge, Y.; Qiu, S.; Dworkin, L.; Gong, R. The Redox-Sensitive GSK3β Is a Key Regulator of Glomerular Podocyte Injury in Type 2 Diabetic Kidney Disease. Redox Biol. 2024, 72, 103127. [Google Scholar] [CrossRef] [PubMed]
- Lizotte, F.; Rousseau, M.; Denhez, B.; Lévesque, D.; Guay, A.; Liu, H.; Moreau, J.; Higgins, S.; Sabbagh, R.; Susztak, K.; et al. Deletion of Protein Tyrosine Phosphatase SHP-1 Restores SUMOylation of Podocin and Reverses the Progression of Diabetic Kidney Disease. Kidney Int. 2023, 104, 787–802, Erratum in Kidney Int. 2023, 104, 1228. [Google Scholar] [CrossRef]
- Lu, J.; Chen, P.P.; Zhang, J.X.; Li, X.Q.; Wang, G.H.; Yuan, B.Y.; Huang, S.J.; Liu, X.Q.; Jiang, T.T.; Wang, M.Y.; et al. GPR43 Activation-Mediated Lipotoxicity Contributes to Podocyte Injury in Diabetic Nephropathy by Modulating the ERK/EGR1 Pathway. Int. J. Biol. Sci. 2022, 18, 96–111. [Google Scholar] [CrossRef]
- Lu, J.; Chen, P.P.; Zhang, J.X.; Li, X.Q.; Wang, G.H.; Yuan, B.Y.; Huang, S.J.; Liu, X.Q.; Jiang, T.T.; Wang, M.Y.; et al. GPR43 Deficiency Protects against Podocyte Insulin Resistance in Diabetic Nephropathy through the Restoration of AMPKα Activity. Theranostics 2021, 11, 4728–4742. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yuan, Q.; Tang, B.; Xie, Y.; Cao, Y.; Qiu, Y.; Zeng, J.; Wang, Z.; Su, H.; Zhang, C. CPT1A Protects Podocytes From Lipotoxicity and Apoptosis In Vitro and Alleviates Diabetic Nephropathy In Vivo. Diabetes 2024, 73, 879–895. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sun, Y.; Wang, M.; Hou, Y.; Huang, W.; Zhou, D.; Wang, Z.; Yang, S.; Tang, W.; Zhen, J.; et al. Elevation of JAML Promotes Diabetic Kidney Disease by Modulating Podocyte Lipid Metabolism. Cell Metab. 2020, 32, 1052–1062.e8. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, J.; He, Y.; Hou, X.; Fang, J.; Huang, J.; Wang, L.; Shen, J.; Zhu, B.; Wang, N.; et al. Curcumin Targets CXCL16-Mediated Podocyte Injury and Lipid Accumulation in Diabetic Kidney Disease Treatment. Arch. Pharm. Res. 2024, 47, 924–939. [Google Scholar] [CrossRef]
- Shahzad, K.; Fatima, S.; Khawaja, H.; Elwakiel, A.; Gadi, I.; Ambreen, S.; Zimmermann, S.; Mertens, P.R.; Biemann, R.; Isermann, B. Podocyte-Specific Nlrp3 Inflammasome Activation Promotes Diabetic Kidney Disease. Kidney Int. 2022, 102, 766–779. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, Z.; Fu, J.; Zhong, F.; Zhang, W.; Wei, C.; Chen, A.; Liu, B.-C.; He, J.C.; Lee, K. Podocyte-Derived Soluble RARRES1 Drives Kidney Disease Progression through Direct Podocyte and Proximal Tubular Injury. Kidney Int. 2024, 106, 50–66. [Google Scholar] [CrossRef]
- Lv, Z.; Hu, J.; Su, H.; Yu, Q.; Lang, Y.; Yang, M.; Fan, X.; Liu, Y.; Liu, B.; Zhao, Y.; et al. TRAIL Induces Podocyte PANoptosis via Death Receptor 5 in Diabetic Kidney Disease. Kidney Int. 2025, 107, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Saxena, R.; Zhao, M.-H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus Nephritis. Nat. Rev. Dis. Primers 2020, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Pippin, J.W.; Durvasula, R.; Petermann, A.; Hiromura, K.; Couser, W.G.; Shankland, S.J. DNA Damage Is a Novel Response to Sublytic Complement C5b-9-Induced Injury in Podocytes. J. Clin. Investig. 2003, 111, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Haas, M.; Glassock, R.; Zhao, M.-H. Redefining Lupus Nephritis: Clinical Implications of Pathophysiologic Subtypes. Nat. Rev. Nephrol. 2017, 13, 483–495. [Google Scholar] [CrossRef]
- Podestà, M.A.; Faravelli, I.; Ponticelli, C. Autophagy in Lupus Nephritis: A Delicate Balance between Regulation and Disease. Autoimmun. Rev. 2022, 21, 103132. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, H.; Miao, X.; Xu, J.; Yang, R.; Zhao, L.; Liu, J.; Yang, L.; Gao, F.; Zhang, W.; et al. Nestin Protects Podocyte from Injury in Lupus Nephritis by Mitophagy and Oxidative Stress. Cell Death Dis. 2020, 11, 319. [Google Scholar] [CrossRef]
- Lv, F.; He, Y.; Xu, H.; Li, Y.; Han, L.; Yan, L.; Lang, H.; Zhao, Y.; Zhao, Z.; Qi, Y. CD36 Aggravates Podocyte Injury by Activating NLRP3 Inflammasome and Inhibiting Autophagy in Lupus Nephritis. Cell Death Dis. 2022, 13, 729. [Google Scholar] [CrossRef]
- Zhou, X.-J.; Klionsky, D.J.; Zhang, H. Podocytes and Autophagy: A Potential Therapeutic Target in Lupus Nephritis. Autophagy 2019, 15, 908–912. [Google Scholar] [CrossRef]
- Carney, E.F. Lupus Nephritis: Role of NLRP3 Inflammasomes in Podocyte Injury. Nat. Rev. Nephrol. 2017, 13, 444. [Google Scholar] [CrossRef]
- Ye, B.; Chen, B.; Guo, C.; Xiong, N.; Huang, Y.; Li, M.; Lai, Y.; Li, J.; Zhou, M.; Wang, S.; et al. C5a-C5aR1 Axis Controls Mitochondrial Fission to Promote Podocyte Injury in Lupus Nephritis. Mol. Ther. 2024, 32, 1540–1560. [Google Scholar] [CrossRef]
- Lei, J.; Wen, Z. DRP1 Bridges Complement Component C5a and Podocyte Injury in Lupus Nephritis. Mol. Ther. 2024, 32, 1199–1201. [Google Scholar] [CrossRef]
- Fu, R.; Wang, W.; Huo, Y.; Li, L.; Chen, R.; Lin, Z.; Tao, Y.; Peng, X.; Huang, W.; Guo, C. The Mechanosensitive Ion Channel Piezo1 Contributes to Podocyte Cytoskeleton Remodeling and Development of Proteinuria in Lupus Nephritis. Kidney Int. 2024, 106, 625–639. [Google Scholar] [CrossRef] [PubMed]
- McKinzie, S.R.; Kaverina, N.; Schweickart, R.A.; Chaney, C.P.; Eng, D.G.; Pereira, B.M.V.; Kestenbaum, B.; Pippin, J.W.; Wessely, O.; Shankland, S.J. Podocytes from Hypertensive and Obese Mice Acquire an Inflammatory, Senescent, and Aged Phenotype. Am. J. Physiol. Renal Physiol. 2024, 326, F644–F660. [Google Scholar] [CrossRef]
- Seccia, T.M.; Caroccia, B.; Calò, L.A. Hypertensive Nephropathy. Moving from Classic to Emerging Pathogenetic Mechanisms. J. Hypertens. 2017, 35, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Ilatovskaya, D.V.; Behr, A.; Staruschenko, A.; Hall, G.; Palygin, O. Mechanistic Insights Into Redox Damage of the Podocyte in Hypertension. Hypertension 2025, 82, 14–25. [Google Scholar] [CrossRef]
- Nagase, T.; Nagase, M. Piezo Ion Channels: Long-Sought-after Mechanosensors Mediating Hypertension and Hypertensive Nephropathy. Hypertens. Res. 2024, 47, 2786–2799. [Google Scholar] [CrossRef]
- Ogino, S.; Yoshikawa, K.; Nagase, T.; Mikami, K.; Nagase, M. Roles of the Mechanosensitive Ion Channel Piezo1 in the Renal Podocyte Injury of Experimental Hypertensive Nephropathy. Hypertens. Res. 2024, 47, 747–759. [Google Scholar] [CrossRef]
- Li, S.-Y.; Chu, P.-H.; Huang, P.-H.; Hsieh, T.-H.; Susztak, K.; Tarng, D.-C. FHL2 Mediates Podocyte Rac1 Activation and Foot Process Effacement in Hypertensive Nephropathy. Sci. Rep. 2019, 9, 6693. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Z.; Liang, W.; Luo, Z.; Hu, J.; Feng, J.; Zhang, Z.; Luo, Q.; Yang, H.; Ding, G. Reduction of Anaerobic Glycolysis Contributes to Angiotensin II-Induced Podocyte Injury with Foot Process Effacement. Kidney Int. 2023, 103, 735–748. [Google Scholar] [CrossRef]
- Peng, Z.; Huang, X.; Pan, Y.; Li, W.; Hu, H.; Chen, X.; Zhang, Z.; Hu, J.; Qi, Y.; Chen, W.; et al. USP22 Promotes Angiotensin II-Induced Podocyte Injury by Deubiquitinating and Stabilizing HMGB1. Cell Signal 2025, 131, 111771. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Zou, Y.; Song, C.; Cao, K.; Wu, B.; You, S.; Lu, S.; Wang, D.; Xu, J.; et al. Deacetylation of Septin4 by SIRT2 (Silent Mating Type Information Regulation 2 Homolog-2) Mitigates Damaging of Hypertensive Nephropathy. Circ. Res. 2023, 132, 601–624. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.V.; Gil Lorenzo, A.F.; Bocanegra, V.; Vallés, P.G. Molecular Mechanisms of Hypertensive Nephropathy: Renoprotective Effect of Losartan through Hsp70. Cells 2021, 10, 3146. [Google Scholar] [CrossRef] [PubMed]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.J.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-Related Glomerulopathy: Clinical and Pathologic Characteristics and Pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Rui, H.-L.; Yang, M.; Sun, L.-J.; Dong, H.-R.; Cheng, H. CD36-Mediated Lipid Accumulation and Activation of NLRP3 Inflammasome Lead to Podocyte Injury in Obesity-Related Glomerulopathy. Mediators Inflamm. 2019, 2019, 3172647. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, C.; Zhou, X.; Li, Y.; Ma, Y.; Zhang, R.; Li, R. Downregulation of PTEN Promotes Podocyte Endocytosis of Lipids Aggravating Obesity-Related Glomerulopathy. Am. J. Physiol. Renal Physiol. 2020, 318, F589–F599. [Google Scholar] [CrossRef]
- Xu, X.; Huang, X.; Zhang, L.; Huang, X.; Qin, Z.; Hua, F. Adiponectin Protects Obesity-Related Glomerulopathy by Inhibiting ROS/NF-κB/NLRP3 Inflammation Pathway. BMC Nephrol. 2021, 22, 218. [Google Scholar] [CrossRef]
- Huang, D.; Kidd, J.M.; Zou, Y.; Wu, X.; Gehr, T.W.B.; Li, P.-L.; Li, G. Regulation of NLRP3 Inflammasome Activation and Inflammatory Exosome Release in Podocytes by Acid Sphingomyelinase During Obesity. Inflammation 2023, 46, 2037–2054. [Google Scholar] [CrossRef]
- Hou, X.-X.; Dong, H.-R.; Sun, L.-J.; Yang, M.; Cheng, H.; Chen, Y.-P. Purinergic 2X7 Receptor Is Involved in the Podocyte Damage of Obesity-Related Glomerulopathy via Activating Nucleotide-Binding and Oligomerization Domain-Like Receptor Protein 3 Inflammasome. Chin. Med. J. 2018, 131, 2713–2725. [Google Scholar] [CrossRef]
- Jakhotia, S.; Kavvuri, R.; Raviraj, S.; Baishya, S.; Pasupulati, A.K.; Reddy, G.B. Obesity-Related Glomerulopathy Is Associated with Elevated WT1 Expression in Podocytes. Int. J. Obes. 2024, 48, 1080–1091. [Google Scholar] [CrossRef]
- Haruhara, K.; Okabayashi, Y.; Sasaki, T.; Kubo, E.; D’Agati, V.D.; Bertram, J.F.; Tsuboi, N.; Yokoo, T. Podocyte Density as a Predictor of Long-Term Kidney Outcome in Obesity-Related Glomerulopathy. Kidney Int 2024, 106, 496–507. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Chen, Y.-P.; Yang, M.; Liu, B.-L.; Dong, J.; Dong, H.-R.; Rui, H.-L.; Cheng, H. Aldosterone Is Involved in the Pathogenesis of Obesity-Related Glomerulopathy through Activation of Wnt/β-Catenin Signaling in Podocytes. Mol. Med. Rep. 2018, 17, 4589–4598. [Google Scholar] [CrossRef]
- Kruegel, J.; Rubel, D.; Gross, O. Alport Syndrome--Insights from Basic and Clinical Research. Nat. Rev. Nephrol. 2013, 9, 170–178. [Google Scholar] [CrossRef]
- Najafian, B.; Silvestroni, A.; Sokolovskiy, A.; Tøndel, C.; Svarstad, E.; Obrisca, B.; Ismail, G.; Holida, M.D.; Mauer, M. A Novel Unbiased Method Reveals Progressive Podocyte Globotriaosylceramide Accumulation and Loss with Age in Females with Fabry Disease. Kidney Int. 2022, 102, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kamiyoshi, N.; Nozu, K.; Fu, X.J.; Morisada, N.; Nozu, Y.; Ye, M.J.; Imafuku, A.; Miura, K.; Yamamura, T.; Minamikawa, S.; et al. Genetic, Clinical, and Pathologic Backgrounds of Patients with Autosomal Dominant Alport Syndrome. Clin. J. Am. Soc. Nephrol. 2016, 11, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wickman, L.; Wang, S.Q.; Zhang, Y.; Wang, F.; Afshinnia, F.; Hodgin, J.; Ding, J.; Wiggins, R.C. Accelerated Podocyte Detachment and Progressive Podocyte Loss from Glomeruli with Age in Alport Syndrome. Kidney Int. 2017, 92, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.; Madison, J. Molecular and Cellular Mechanisms Underlying the Initiation and Progression of Alport Glomerular Pathology. Front. Med. 2022, 9, 846152. [Google Scholar] [CrossRef]
- Iampietro, C.; Bellucci, L.; Arcolino, F.O.; Arigoni, M.; Alessandri, L.; Gomez, Y.; Papadimitriou, E.; Calogero, R.A.; Cocchi, E.; Van Den Heuvel, L.; et al. Molecular and Functional Characterization of Urine-Derived Podocytes from Patients with Alport Syndrome. J. Pathol. 2020, 252, 88–100. [Google Scholar] [CrossRef]
- Frank, C.N.; Hou, X.; Petrosyan, A.; Villani, V.; Zhao, R.; Hansen, J.R.; Clair, G.; Salem, F.; De Filippo, R.E.; Cravedi, P.; et al. Effect of Disease Progression on the Podocyte Cell Cycle in Alport Syndrome. Kidney Int. 2022, 101, 106–118. [Google Scholar] [CrossRef]
- Tong, J.; Zheng, Q.; Gu, X.; Weng, Q.; Yu, S.; Fang, Z.; Jafar Hussain, H.M.; Xu, J.; Ren, H.; Chen, N.; et al. COL4A3 Mutation Induced Podocyte Apoptosis by Dysregulation of NADPH Oxidase 4 and MMP-2. Kidney Int. Rep. 2023, 8, 1864–1874. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Fontanella, A.M.; Molina, J.; Zhang, G.; Mallela, S.K.; Severino, L.U.; Santos J., J.V.; Tolerico, M.; Njeim, R.; Issa, W.; et al. The Enzyme SMPDL3b in Podocytes Decouples Proteinuria from Chronic Kidney Disease Progression in Experimental Alport Syndrome. Kidney Int. 2025, 108, 253–270. [Google Scholar] [CrossRef]
- Fukuda, R.; Suico, M.A.; Kai, Y.; Omachi, K.; Motomura, K.; Koga, T.; Komohara, Y.; Koyama, K.; Yokota, T.; Taura, M.; et al. Podocyte P53 Limits the Severity of Experimental Alport Syndrome. J. Am. Soc. Nephrol. 2016, 27, 144–157. [Google Scholar] [CrossRef]
- Wang, H.; Yue, Z.; Wu, J.; Liu, T.; Mo, Y.; Jiang, X.; Sun, L. The Accumulation of VEGFA in the Glomerular Basement Membrane and Its Relationship with Podocyte Injury and Proteinuria in Alport Syndrome. PLoS ONE 2015, 10, e0135648. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-J.; David, J.M.; Wilbon, S.S.; Santos, J.V.; Patel, D.M.; Ahmad, A.; Mitrofanova, A.; Liu, X.; Mallela, S.K.; Ducasa, G.M.; et al. Discoidin Domain Receptor 1 Activation Links Extracellular Matrix to Podocyte Lipotoxicity in Alport Syndrome. EBioMedicine 2021, 63, 103162. [Google Scholar] [CrossRef] [PubMed]
- Svarstad, E.; Marti, H.P. The Changing Landscape of Fabry Disease. Clin. J. Am. Soc. Nephrol. 2020, 15, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Najafian, B.; Svarstad, E.; Bostad, L.; Gubler, M.-C.; Tøndel, C.; Whitley, C.; Mauer, M. Progressive Podocyte Injury and Globotriaosylceramide (GL-3) Accumulation in Young Patients with Fabry Disease. Kidney Int. 2011, 79, 663–670. [Google Scholar] [CrossRef]
- Najafian, B.; Tøndel, C.; Svarstad, E.; Gubler, M.-C.; Oliveira, J.-P.; Mauer, M. Accumulation of Globotriaosylceramide in Podocytes in Fabry Nephropathy Is Associated with Progressive Podocyte Loss. J. Am. Soc. Nephrol. 2020, 31, 865–875. [Google Scholar] [CrossRef]
- Fall, B.; Scott, C.R.; Mauer, M.; Shankland, S.; Pippin, J.; Jefferson, J.A.; Wallace, E.; Warnock, D.; Najafian, B. Urinary Podocyte Loss Is Increased in Patients with Fabry Disease and Correlates with Clinical Severity of Fabry Nephropathy. PLoS ONE 2016, 11, e0168346. [Google Scholar] [CrossRef]
- Vujkovac, B.; Srebotnik Kirbiš, I.; Keber, T.; Cokan Vujkovac, A.; Tretjak, M.; Radoš Krnel, S. Podocyturia in Fabry Disease: A 10-Year Follow-Up. Clin. Kidney J. 2022, 15, 269–277. [Google Scholar] [CrossRef]
- Snanoudj, S.; Derambure, C.; Zhang, C.; Hai Yen, N.T.; Lesueur, C.; Coutant, S.; Abily-Donval, L.; Marret, S.; Yang, H.; Mardinoglu, A.; et al. Genome-Wide Expression Analysis in a Fabry Disease Human Podocyte Cell Line. Heliyon 2024, 10, e34357. [Google Scholar] [CrossRef]
- Pereira, E.M.; Labilloy, A.; Eshbach, M.L.; Roy, A.; Subramanya, A.R.; Monte, S.; Labilloy, G.; Weisz, O.A. Characterization and Phosphoproteomic Analysis of a Human Immortalized Podocyte Model of Fabry Disease Generated Using CRISPR/Cas9 Technology. Am. J. Physiol. Renal Physiol. 2016, 311, F1015–F1024. [Google Scholar] [CrossRef]
- Liebau, M.C.; Braun, F.; Höpker, K.; Weitbrecht, C.; Bartels, V.; Müller, R.-U.; Brodesser, S.; Saleem, M.A.; Benzing, T.; Schermer, B.; et al. Dysregulated Autophagy Contributes to Podocyte Damage in Fabry’s Disease. PLoS ONE 2013, 8, e63506. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Hong, S.-E.; Yu, S.-L.; Kang, J.; Park, C.G.; Lee, H.Y.; Lee, S.-K.; Lee, D.C.; Park, H.-W.; Hwang, W.-M.; et al. Ceria-Zirconia Nanoparticles Reduce Intracellular Globotriaosylceramide Accumulation and Attenuate Kidney Injury by Enhancing the Autophagy Flux in Cellular and Animal Models of Fabry Disease. J. Nanobiotechnol. 2022, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P. Reconceptualizing Podocyte Damage in Fabry Disease: New Findings Identify α-Synuclein as a Putative Therapeutic Target. Kidney Int. 2024, 105, 237–239. [Google Scholar] [CrossRef]
- Braun, F.; Abed, A.; Sellung, D.; Rogg, M.; Woidy, M.; Eikrem, O.; Wanner, N.; Gambardella, J.; Laufer, S.D.; Haas, F.; et al. Accumulation of α-Synuclein Mediates Podocyte Injury in Fabry Nephropathy. J. Clin. Investig. 2023, 133, e157782. [Google Scholar] [CrossRef] [PubMed]
- Wise, A.F.; Krisnadevi, I.A.; Bruell, S.; Lee, H.-C.; Bhuvan, T.; Kassianos, A.J.; Saini, S.; Wang, X.; Healy, H.G.; Qian, E.L.; et al. Fabry Disease Podocytes Reveal Ferroptosis as a Potential Regulator of Cell Pathology. Kidney Int. Rep. 2025, 10, 535–548. [Google Scholar] [CrossRef]
- Bosquetti, B.; Santana, A.A.; Gregório, P.C.; Cunha, R.S.d.; Miniskiskosky, G.; Budag, J.; Franco, C.R.C.; Ramos, E.A.d.S.; Barreto, F.C.; Stinghen, A.E.M. The Role of A3β1 Integrin Modulation on Fabry Disease Podocyte Injury and Kidney Impairment. Toxins 2023, 15, 700. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Afzal, O.; Altamimi, A.S.A.; Almalki, W.H.; Kazmi, I.; Al-Abbasi, F.A.; Alzarea, S.I.; Makeen, H.A.; Albratty, M. A Study of the Molecular Mechanism of Quercetin and Dasatinib Combination as Senolytic in Alleviating Age-Related and Kidney Diseases. J. Food Biochem. 2022, 46, e14471. [Google Scholar] [CrossRef]
- Guo, X.; Wen, S.; Wang, J.; Zeng, X.; Yu, H.; Chen, Y.; Zhu, X.; Xu, L. Senolytic Combination of Dasatinib and Quercetin Attenuates Renal Damage in Diabetic Kidney Disease. Phytomedicine 2024, 130, 155705. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, C.; Liu, L.; Xu, L.; Yao, L. Senolytic Combination of Dasatinib and Quercetin Protects against Diabetic Kidney Disease by Activating Autophagy to Alleviate Podocyte Dedifferentiation via the Notch Pathway. Int. J. Mol. Med. 2024, 53, 26. [Google Scholar] [CrossRef]
- Wang, M. Senolytics for Kidney Repair. Nat. Rev. Nephrol. 2021, 17, 512. [Google Scholar] [CrossRef]
- Mylonas, K.J.; O’Sullivan, E.D.; Humphries, D.; Baird, D.P.; Docherty, M.-H.; Neely, S.A.; Krimpenfort, P.J.; Melk, A.; Schmitt, R.; Ferreira-Gonzalez, S.; et al. Cellular Senescence Inhibits Renal Regeneration after Injury in Mice, with Senolytic Treatment Promoting Repair. Sci. Transl. Med. 2021, 13, eabb0203. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shen, Y.; Huang, L.; Liu, C.; Wang, J. Senolytic Therapy Ameliorates Renal Fibrosis Postacute Kidney Injury by Alleviating Renal Senescence. FASEB J. 2021, 35, e21229, Correction in FASEB J. 2025, 39, e70860. [Google Scholar] [CrossRef] [PubMed]
- Franzin, R.; Stasi, A.; Ranieri, E.; Netti, G.S.; Cantaluppi, V.; Gesualdo, L.; Stallone, G.; Castellano, G. Targeting Premature Renal Aging: From Molecular Mechanisms of Cellular Senescence to Senolytic Trials. Front. Pharmacol. 2021, 12, 630419. [Google Scholar] [CrossRef] [PubMed]
- Lasagni, L.; Angelotti, M.L.; Ronconi, E.; Lombardi, D.; Nardi, S.; Peired, A.; Becherucci, F.; Mazzinghi, B.; Sisti, A.; Romoli, S.; et al. Podocyte Regeneration Driven by Renal Progenitors Determines Glomerular Disease Remission and Can Be Pharmacologically Enhanced. Stem Cell Rep. 2015, 5, 248–263. [Google Scholar] [CrossRef]
- Eng, D.G.; Sunseri, M.W.; Kaverina, N.V.; Roeder, S.S.; Pippin, J.W.; Shankland, S.J. Glomerular Parietal Epithelial Cells Contribute to Adult Podocyte Regeneration in Experimental Focal Segmental Glomerulosclerosis. Kidney Int. 2015, 88, 999–1012. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Kanasaki, K.; Lovisa, S.; Alge, J.L.; Kim, J.; Chen, Y.; Teng, Y.; Gerami-Naini, B.; Sugimoto, H.; Kato, N.; et al. Genetic Reprogramming with Stem Cells Regenerates Glomerular Epithelial Podocytes in Alport Syndrome. Life Sci. Alliance 2024, 7, e202402664. [Google Scholar] [CrossRef]
- Fu, J.; Shinjo, T.; Li, Q.; St-Louis, R.; Park, K.; Yu, M.G.; Yokomizo, H.; Simao, F.; Huang, Q.; Wu, I.-H.; et al. Regeneration of Glomerular Metabolism and Function by Podocyte Pyruvate Kinase M2 in Diabetic Nephropathy. JCI Insight 2022, 7, e155260. [Google Scholar] [CrossRef]
- Romoli, S.; Angelotti, M.L.; Antonelli, G.; Kumar Vr, S.; Mulay, S.R.; Desai, J.; Anguiano Gomez, L.; Thomasova, D.; Eulberg, D.; Klussmann, S.; et al. CXCL12 Blockade Preferentially Regenerates Lost Podocytes in Cortical Nephrons by Targeting an Intrinsic Podocyte-Progenitor Feedback Mechanism. Kidney Int. 2018, 94, 1111–1126. [Google Scholar] [CrossRef]
- Moeller, M.J.; Tharaux, P.-L. Cellular Regeneration of Podocytes from Parietal Cells: The Debate Is Still Open. Kidney Int. 2019, 96, 542–544. [Google Scholar] [CrossRef]
- Vaughan, M.R.; Pippin, J.W.; Griffin, S.V.; Krofft, R.; Fleet, M.; Haseley, L.; Shankland, S.J. ATRA Induces Podocyte Differentiation and Alters Nephrin and Podocin Expression In Vitro and In Vivo. Kidney Int. 2005, 68, 133–144. [Google Scholar] [CrossRef]
- Dai, Y.; Chen, A.; Liu, R.; Gu, L.; Sharma, S.; Cai, W.; Salem, F.; Salant, D.J.; Pippin, J.W.; Shankland, S.J.; et al. Retinoic Acid Improves Nephrotoxic Serum-Induced Glomerulonephritis through Activation of Podocyte Retinoic Acid Receptor α. Kidney Int. 2017, 92, 1444–1457. [Google Scholar] [CrossRef]
- Peired, A.; Angelotti, M.L.; Ronconi, E.; la Marca, G.; Mazzinghi, B.; Sisti, A.; Lombardi, D.; Giocaliere, E.; Della Bona, M.; Villanelli, F.; et al. Proteinuria Impairs Podocyte Regeneration by Sequestering Retinoic Acid. J. Am. Soc. Nephrol. 2013, 24, 1756–1768. [Google Scholar] [CrossRef]
- Lichtnekert, J.; Kaverina, N.V.; Eng, D.G.; Gross, K.W.; Kutz, J.N.; Pippin, J.W.; Shankland, S.J. Renin-Angiotensin-Aldosterone System Inhibition Increases Podocyte Derivation from Cells of Renin Lineage. J. Am. Soc. Nephrol. 2016, 27, 3611–3627. [Google Scholar] [CrossRef]
- Muto, Y.; Humphreys, B.D. Recent Advances in Lineage Tracing for the Kidney. Kidney Int. 2021, 100, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Refaeli, I.; Brooks, C.R.; Jing, P.; Gulieva, R.E.; Hughes, M.R.; Cruz, N.M.; Liu, Y.; Churchill, A.J.; Wang, Y.; et al. Gene-Edited Human Kidney Organoids Reveal Mechanisms of Disease in Podocyte Development. Stem Cells 2017, 35, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Sateesh, J.; Guha, K.; Dutta, A.; Sengupta, P.; Yalamanchili, D.; Donepudi, N.S.; Surya Manoj, M.; Sohail, S.S. A Comprehensive Review on Advancements in Tissue Engineering and Microfluidics toward Kidney-on-Chip. Biomicrofluidics 2022, 16, 041501. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, S. Metformin Protects against Podocyte Injury in Diabetic Kidney Disease. Pharmaceuticals 2020, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, J.; Xie, C.; Yang, J.; Zhao, L.; Yang, J. Metformin Attenuates Diabetic Renal Injury via the AMPK-Autophagy Axis. Exp. Ther. Med. 2021, 21, 578. [Google Scholar] [CrossRef]
- Ma, M.; Pan, Y.; Zhang, Y.; Yang, M.; Xi, Y.; Lin, B.; Hao, W.; Liu, J.; Wu, L.; Liu, Y.; et al. Metformin Combined with Rapamycin Ameliorates Podocyte Injury in Idiopathic Membranous Nephropathy through the AMPK/mTOR Signaling Pathway. J. Cell Commun. Signal 2023, 17, 1405–1415. [Google Scholar] [CrossRef]
- Lv, X.; Wang, J.; Zhang, L.; Shao, X.; Lin, Y.; Liu, H.; Ma, G.; Li, J.; Zhou, S.; Yu, P. Canagliflozin Reverses Th1/Th2 Imbalance and Promotes Podocyte Autophagy in Rats with Membranous Nephropathy. Front. Immunol. 2022, 13, 993869. [Google Scholar] [CrossRef]
- Liu, B.-L.; Chen, Y.-P.; Cheng, H.; Wang, Y.-Y.; Rui, H.-L.; Yang, M.; Dong, H.-R.; Han, D.-N.; Dong, J. The Protective Effects of Curcumin on Obesity-Related Glomerulopathy Are Associated with Inhibition of Wnt/β-Catenin Signaling Activation in Podocytes. Evid. Based Complement. Alternat. Med. 2015, 2015, 827472. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.-J.; Zhu, Y.-T.; He, F.-F.; Zhang, C. Mechanisms and Therapeutic Perspectives of Podocyte Aging in Podocytopathies. Int. J. Mol. Sci. 2025, 26, 9159. https://doi.org/10.3390/ijms26189159
Ma S-J, Zhu Y-T, He F-F, Zhang C. Mechanisms and Therapeutic Perspectives of Podocyte Aging in Podocytopathies. International Journal of Molecular Sciences. 2025; 26(18):9159. https://doi.org/10.3390/ijms26189159
Chicago/Turabian StyleMa, Si-Jia, Yu-Ting Zhu, Fang-Fang He, and Chun Zhang. 2025. "Mechanisms and Therapeutic Perspectives of Podocyte Aging in Podocytopathies" International Journal of Molecular Sciences 26, no. 18: 9159. https://doi.org/10.3390/ijms26189159
APA StyleMa, S.-J., Zhu, Y.-T., He, F.-F., & Zhang, C. (2025). Mechanisms and Therapeutic Perspectives of Podocyte Aging in Podocytopathies. International Journal of Molecular Sciences, 26(18), 9159. https://doi.org/10.3390/ijms26189159







