Bioelectrocatalyst for O2 Reduction Based on a Novel Recombinant Two-Domain Laccase from Streptomyces ochraceisleroticus Immobilized on Naphthyl-Modified MWCNTs
Abstract
1. Introduction
2. Results
2.1. Expression and Purification of the SoSL Enzyme
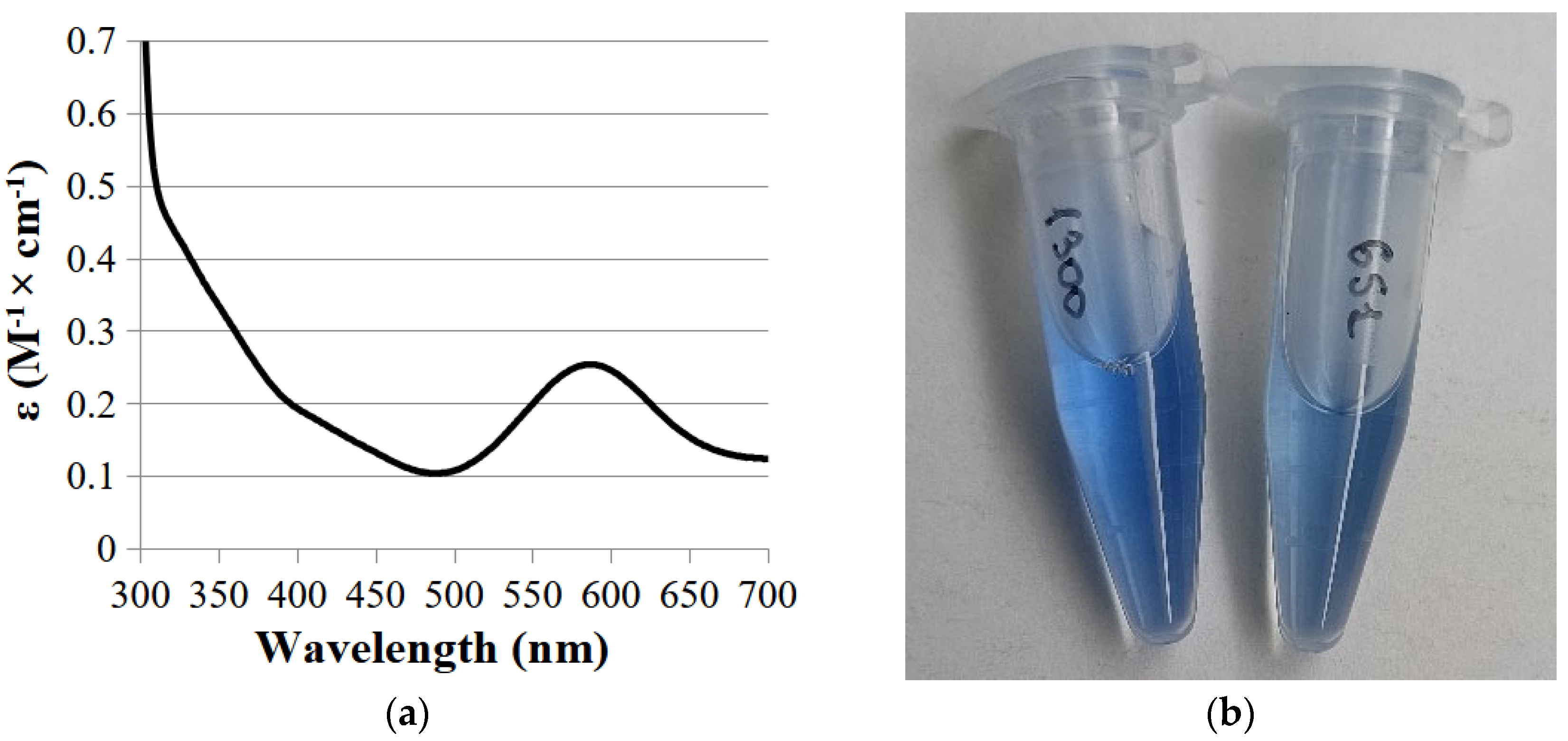
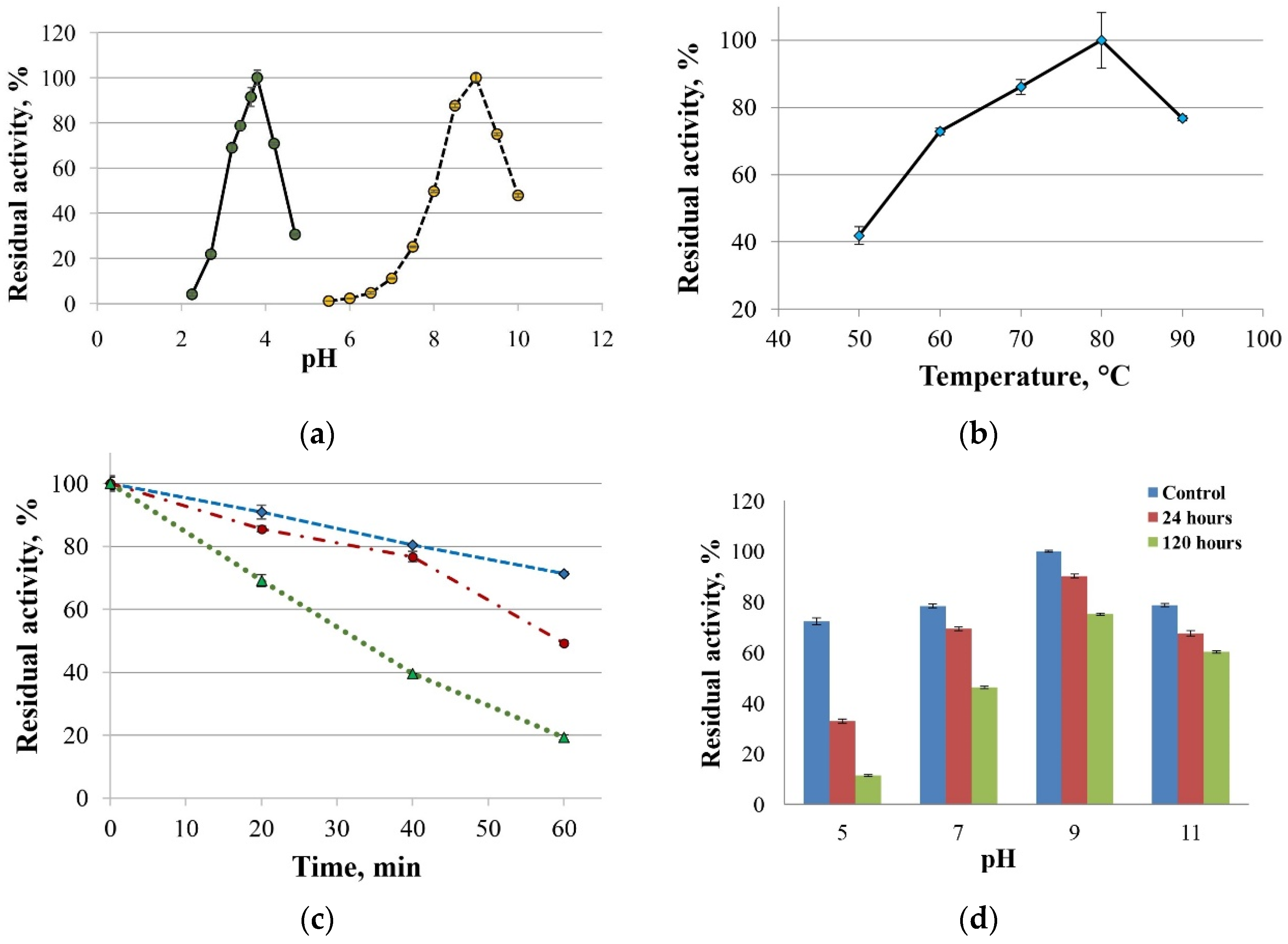
2.2. Biochemical and Computational Characterization of SoSL
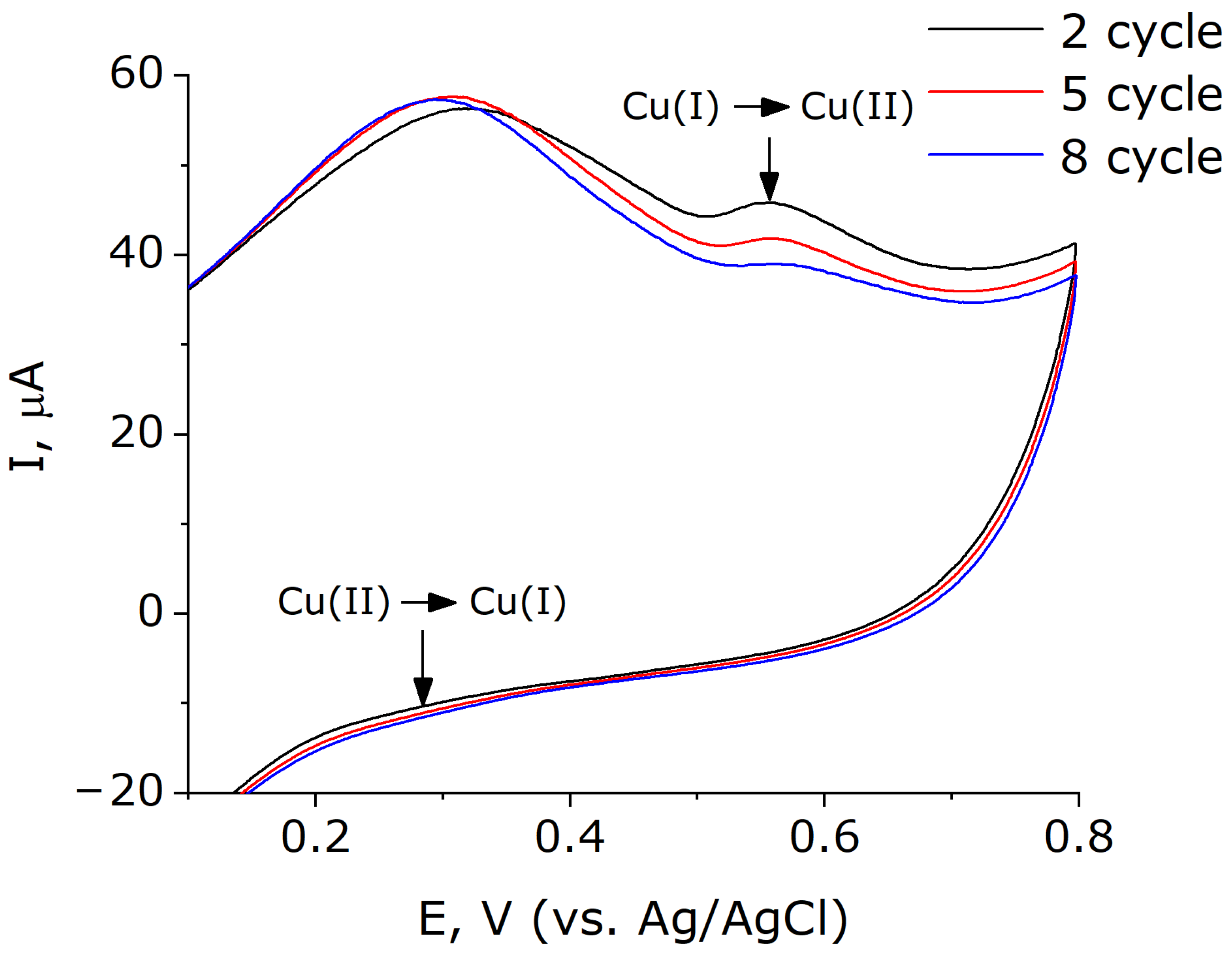
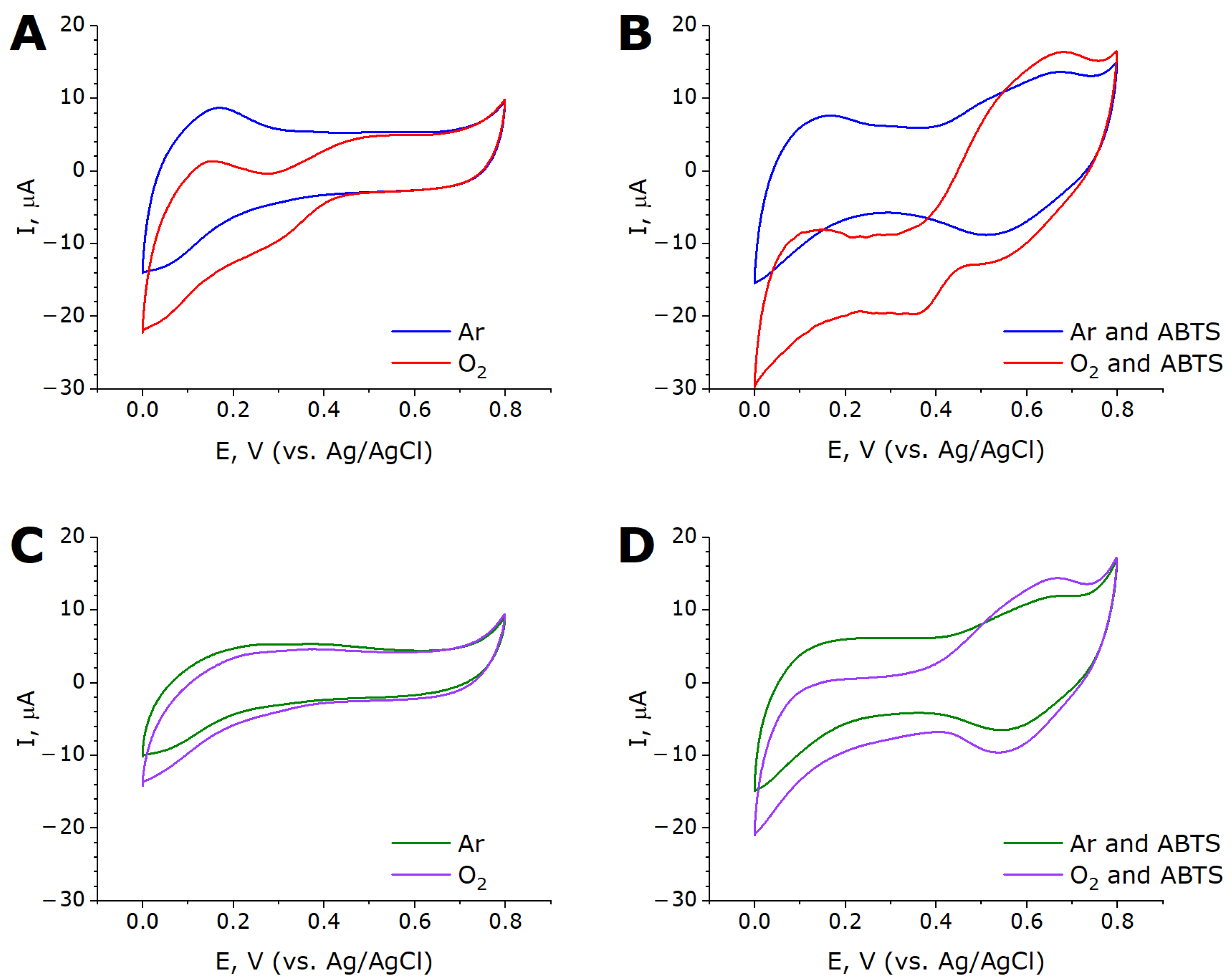
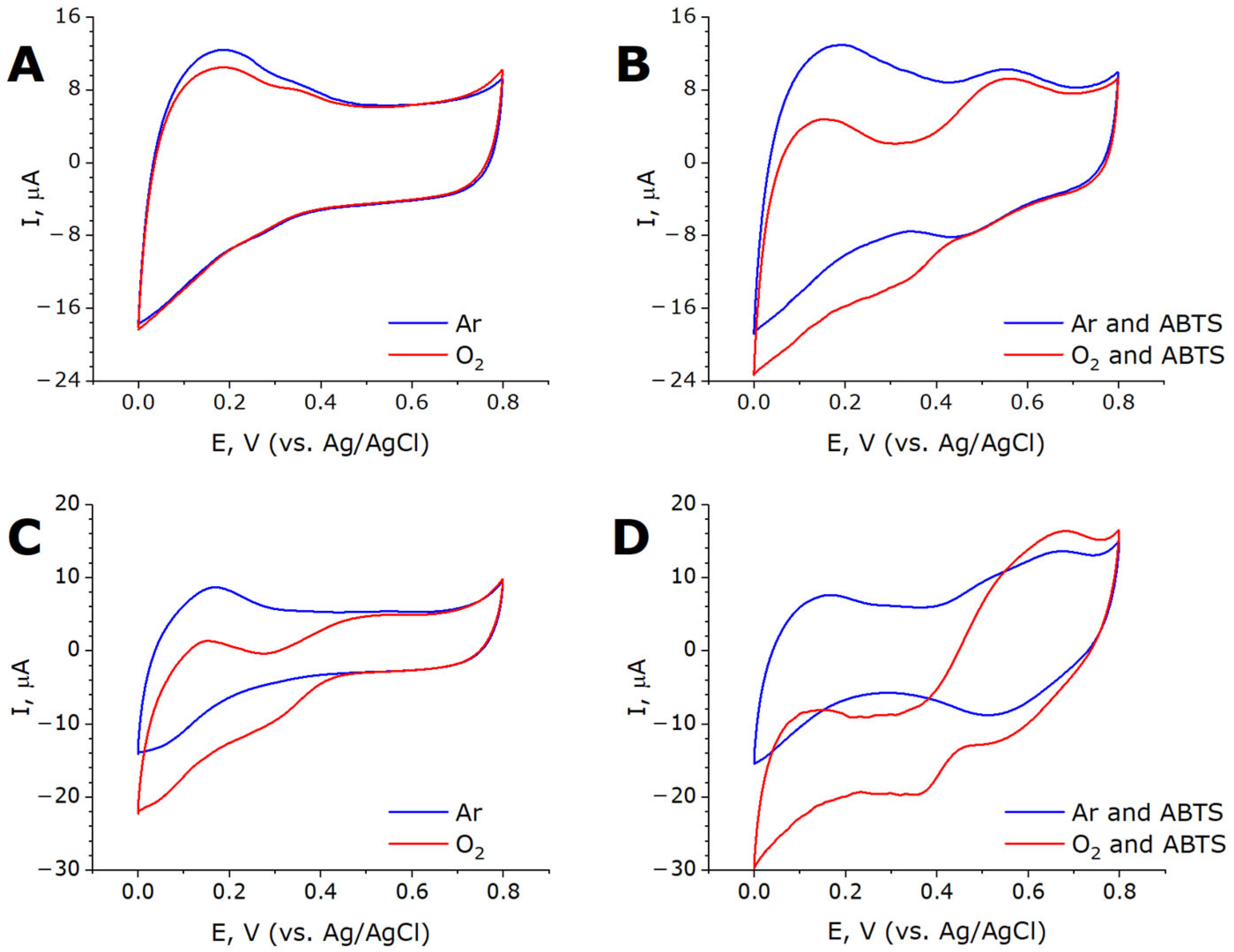
2.3. Bioelectrocatalysis
3. Discussion
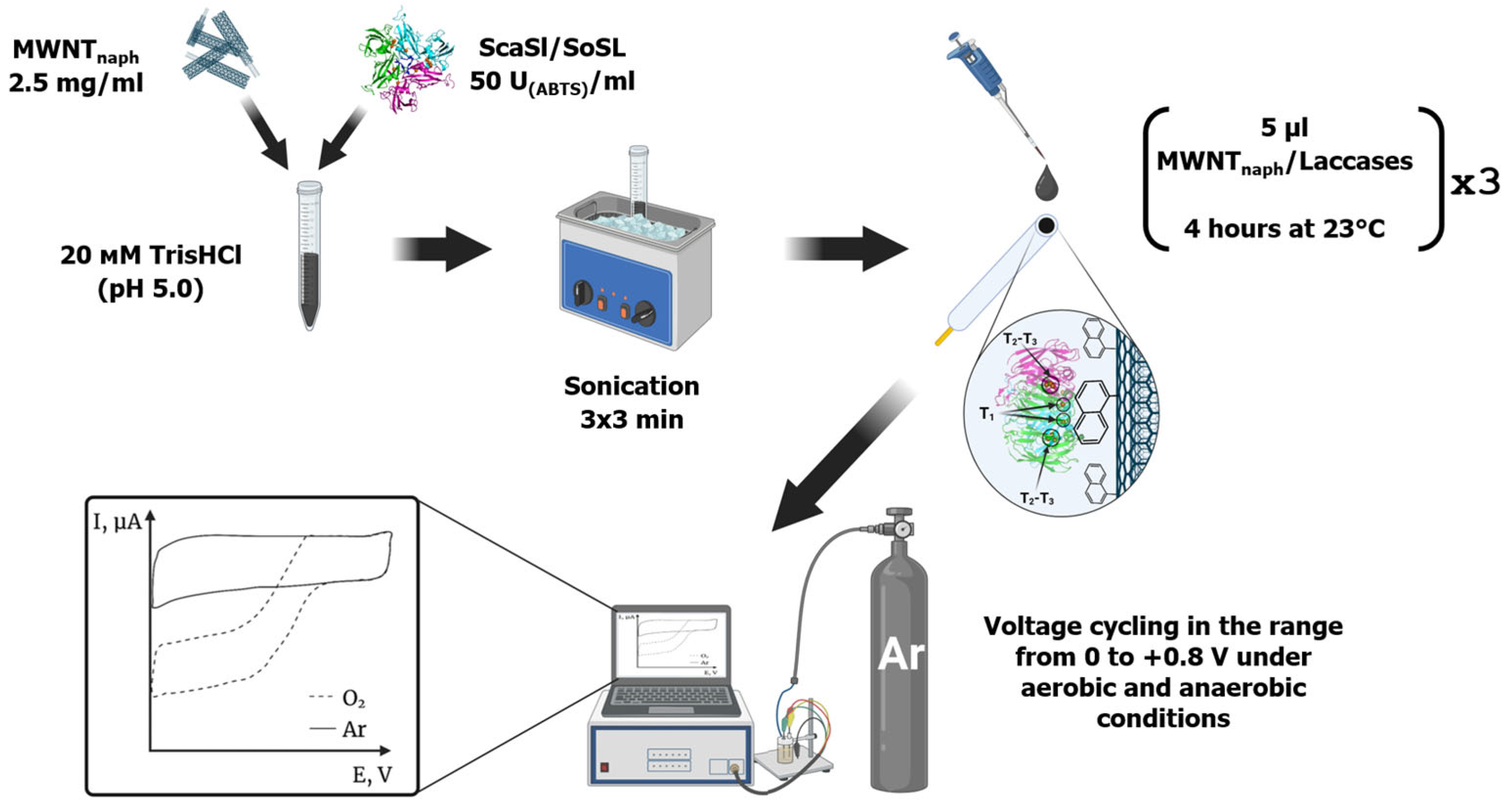
4. Materials and Methods
4.1. Microorganism Cultivation, Gene Cloning, Protein Purification
4.2. Characterization of SoSL
4.3. Homology Modeling
4.4. Molecular Docking
4.5. Graphical Protein–Ligand Complex Representation
4.6. Multiwall Carbon Nanotubes Processing and Laccase Modification
4.7. Preparation of Bioelectrodes
4.8. Electrochemical Measurements
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,6-DMP | 2,6-dimethoxyphenol |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| DET | Direct electron transfer |
| MET | Mediated electron transfer |
| MWCNT | Multiwalled carbon nanotubes |
| NHE | Normal hydrogen electrode |
| PAGE | Polyacrylamide gel electrophoresis |
| SDS | Sodium dodecylsulfate |
| SLAC | Small laccase |
| SoSL | Streptomyces ochraceiscleroticus small laccase |
| TAT | Twin-arginine translocation pathway |
Appendix A

References
- Kaur, R.; Salwan, R.; Sharma, V. Structural Properties, Genomic Distribution of Laccases from Streptomyces and Their Potential Applications. Process Biochem. 2022, 118, 133–144. [Google Scholar] [CrossRef]
- Lisov, A.V.; Trubitsina, L.I.; Lisova, Z.A.; Trubitsin, I.V.; Zavarzina, A.G.; Leontievsky, A.A. Transformation of Humic Acids by Two-Domain Laccase from Streptomyces anulatus. Process Biochem. 2019, 76, 128–135. [Google Scholar] [CrossRef]
- Trubitsina, L.I.; Lisov, A.V.; Belova, O.V.; Trubitsin, I.V.; Demin, V.V.; Konstantinov, A.I.; Zavarzina, A.G.; Leontievsky, A.A. Transformation of Low Molecular Compounds and Soil Humic Acid by Two Domain Laccase of Streptomyces puniceus in the Presence of Ferulic and Caffeic Acids. PLoS ONE 2020, 15, e0239005. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, D.; Sun, Y.; Zhi, Y.; Mao, L.; Luo, Y.; Xu, L.; Wang, L.; Zhou, P. Expression and Characterization of a Recombinant Laccase with Alkalistable and Thermostable Properties from Streptomyces griseorubens JSD-1. Appl. Biochem. Biotechnol. 2015, 176, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Lukk, T.; Solbiati, J.O.; Bauer, S.; Nair, S.K.; Cronan, J.E.; Gerlt, J.A. Roles of Small Laccases from Streptomyces in Lignin Degradation. Biochemistry 2014, 53, 4047–4058. [Google Scholar] [CrossRef]
- Lu, L.; Zeng, G.; Fan, C.; Zhang, J.; Chen, A.; Chen, M.; Jiang, M.; Yuan, Y.; Wu, H.; Lai, M.; et al. Diversity of Two-Domain Laccase-Like Multicopper Oxidase Genes in Streptomyces Spp.: Identification of Genes Potentially Involved in Extracellular Activities and Lignocellulose Degradation during Composting of Agricultural Waste. Appl. Environ. Microbiol. 2014, 80, 3305–3314. [Google Scholar] [CrossRef] [PubMed]
- Endo, K.; Hosono, K.; Beppu, T.; Ueda, K. A Novel Extracytoplasmic Phenol Oxidase of Streptomyces: Its Possible Involvement in the Onset of Morphogenesis The DDBJ Accession Number for the Sequence Reported in This Paper Is AB056583. Microbiology 2002, 148, 1767–1776. [Google Scholar] [CrossRef]
- Mot, A.C.; Silaghi-Dumitrescu, R. Laccases: Complex Architectures for One-Electron Oxidations. Biochem. Mosc. 2012, 77, 1395–1407. [Google Scholar] [CrossRef]
- Trubitsina, L.I.; Trubitsin, I.V.; Lisov, A.V.; Gabdulkhakov, A.G.; Zavarzina, A.G.; Belova, O.V.; Larionova, A.P.; Tishchenko, S.V.; Leontievsky, A.A. A Novel Two-Domain Laccase with Middle Redox Potential: Physicochemical and Structural Properties. Biochem. Mosc. 2023, 88, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Abdullatypov, A.; Oskin, P.; Fedina, V.; Trubitsina, L.; Yakimovich, S.; Shuvalova, E.; Verma, P.; Dyachkova, T.; Ponamoreva, O.; Alferov, S. Functionalization of MWCNTs for Bioelectrocatalysis by Bacterial Two-Domain Laccase from Catenuloplanes japonicus. Nanomaterials 2023, 13, 3019. [Google Scholar] [CrossRef]
- Trubitsina, L.I.; Abdullatypov, A.V.; Larionova, A.P.; Trubitsin, I.V.; Alferov, S.V.; Ponamoreva, O.N.; Leontievsky, A.A. Expression of Thermophilic Two-Domain Laccase from Catenuloplanes japonicus in Escherichia coli and Its Activity against Triarylmethane and Azo Dyes. PeerJ 2021, 9, e11646. [Google Scholar] [CrossRef]
- Berezin, I.V.; Bogdanovskaya, V.A.; Varfolomeev, S.D.; Tarasevich, M.R.; Yaropolov, A.I. Bioelectrocatalysis-Equilibrium Oxygen Potential in Presence of Laccase. Dokl. Akad. Nauk SSSR 1978, 240, 610–618. [Google Scholar]
- Tarasevich, M.R.; Yaropolov, A.I.; Bogdanovskaya, V.A.; Varfolomeev, S.D. 293-Electrocatalysis of a Cathodic Oxygen Reduction by Laccase. Bioelectrochem. Bioenerg. 1979, 6, 393–403. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, Z.; Zhou, J.; Xin, F.; Ma, J.; Wu, H.; Fang, Y.; Jiang, M.; Dong, W. Application of Eukaryotic and Prokaryotic Laccases in Biosensor and Biofuel Cells: Recent Advances and Electrochemical Aspects. Appl. Microbiol. Biotechnol. 2018, 102, 10409–10423. [Google Scholar] [CrossRef]
- Aleksejeva, O.; Mateljak, I.; Ludwig, R.; Alcalde, M.; Shleev, S. Electrochemistry of a High Redox Potential Laccase Obtained by Computer-Guided Mutagenesis Combined with Directed Evolution. Electrochem. Commun. 2019, 106, 106511. [Google Scholar] [CrossRef]
- Dey, B.; Dutta, T. Laccases: Thriving the Domain of Bio-Electrocatalysis. Bioelectrochemistry 2022, 146, 108144. [Google Scholar] [CrossRef]
- Skálová, T.; Dohnálek, J.; Østergaard, L.H.; Østergaard, P.R.; Kolenko, P.; Dušková, J.; Štěpánková, A.; Hašek, J. The Structure of the Small Laccase from Streptomyces coelicolor Reveals a Link between Laccases and Nitrite Reductases. J. Mol. Biol. 2009, 385, 1165–1178. [Google Scholar] [CrossRef]
- Gallaway, J.; Wheeldon, I.; Rincon, R.; Atanassov, P.; Banta, S.; Barton, S.C. Oxygen-Reducing Enzyme Cathodes Produced from SLAC, a Small Laccase from Streptomyces coelicolor. Biosens. Bioelectron. 2008, 23, 1229–1235. [Google Scholar] [CrossRef]
- Lörcher, S.; Lopes, P.; Kartashov, A.; Ferapontova, E.E. Direct Bio-electrocatalysis of O2 Reduction by Streptomyces coelicolor Laccase Orientated at Promoter-Modified Graphite Electrodes. ChemPhysChem 2013, 14, 2112–2124. [Google Scholar] [CrossRef]
- Galai, S.; Korri-Youssoufi, H.; Marzouki, M.N. Characterization of Yellow Bacterial Laccase SmLac /Role of Redox Mediators in Azo Dye Decolorization. J. Chem. Tech. Biotech. 2014, 89, 1741–1750. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, L.; Yu, P.; Chen, J.; Wu, F.; Mao, L. Comparative Investigation of Small Laccase Immobilized on Carbon Nanomaterials for Direct Bioelectrocatalysis of Oxygen Reduction. Electrochem. Commun. 2019, 101, 82–87. [Google Scholar] [CrossRef]
- Ivnitski, D.; Atanassov, P. Electrochemical Studies of Intramolecular Electron Transfer in Laccase from Trametes versicolor. Electroanalysis 2007, 19, 2307–2313. [Google Scholar] [CrossRef]
- González Arzola, K.; Gimeno, Y.; Arévalo, M.C.; Falcón, M.A.; Hernández Creus, A. Electrochemical and AFM Characterization on Gold and Carbon Electrodes of a High Redox Potential Laccase from Fusarium Proliferatum. Bioelectrochemistry 2010, 79, 17–24. [Google Scholar] [CrossRef]
- Shleev, S.; Jarosz-Wilkolazka, A.; Khalunina, A.; Morozova, O.; Yaropolov, A.; Ruzgas, T.; Gorton, L. Direct Electron Transfer Reactions of Laccases from Different Origins on Carbon Electrodes. Bioelectrochemistry 2005, 67, 115–124. [Google Scholar] [CrossRef]
- Pita, M.; Shleev, S.; Ruzgas, T.; Fernandez, V.; Yaropolov, A.; Gorton, L. Direct Heterogeneous Electron Transfer Reactions of Fungal Laccases at Bare and Thiol-Modified Gold Electrodes. Electrochem. Commun. 2006, 8, 747–753. [Google Scholar] [CrossRef]
- Klis, M.; Maicka, E.; Michota, A.; Bukowska, J.; Sek, S.; Rogalski, J.; Bilewicz, R. Electroreduction of Laccase Covalently Bound to Organothiol Monolayers on Gold Electrodes. Electrochim. Acta 2007, 52, 5591–5598. [Google Scholar] [CrossRef]
- Bucchieri, D.; Mangiagalli, M.; Martani, F.; Butti, P.; Lotti, M.; Serra, I.; Branduardi, P. A Novel Laccase from Trametes Polyzona with High Performance in the Decolorization of Textile Dyes. AMB Expr. 2024, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Vacalie, E.; Preda, D.; Oancea, P.; Leonties, A.R.; Aricov, L.; Raducan, A. Effective Degradation of Azo Dyes by ABTS (2,2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulfonic Acid)) Mediated Laccase. Kinetic Studies. Process Biochem. 2024, 145, 311–319. [Google Scholar] [CrossRef]
- Karmacharya, J.; Shrestha, P.; Han, S.-R.; Lee, J.H.; Oh, T.-J. Exploiting CotA Laccase from Antarctic Bacillus Sp. PAMC28748 for Efficient Mediator-Assisted Dye Decolorization and ABTS Regeneration. Chemosphere 2025, 372, 144137. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Pandya, M.D.; Baraiya, M.G. In Vitro Chrysene Degradation by Purified Cell Free Laccase (P-CFL) from Cochliobolus Lunatus Strain CHR4D in the Presence of Various Redox Mediator Systems (RMSs) and Computational Evaluation of Their Laccase-Ligand Interactions. Environ. Sci. Pollut. Res. 2025, 32, 9735–9746. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Wang, X.; Luo, H.; Wang, Y.; Yao, B.; Huang, H.; Tian, J.; Guan, F. Influence of Mutations at Different Distances from the Active Center on the Activity and Stability of Laccase 13B22. Bioresour. Bioprocess. 2025, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Sanyal, D.; Dey, P. The Optimization of Enzymatic Oxidation of Levofloxacin, a Fluoroquinolone Antibiotic for Wastetwater Treatment. Biodegradation 2021, 32, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Xu, W.; Zhang, W.; Guang, C.; Mu, W. Efficient Removal of Sulfonamides and Tetracyclines Residues by the Laccase-Mediator System Employing a Novel Laccase from Lysinibacillus Fusiformis. J. Environ. Chem. Eng. 2022, 10, 108809. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, Y.; Meng, D.; Zhai, L.; Shen, Y.; You, C.; Guan, Z.; Liao, X. Simultaneous Removal of Tetracycline and Sulfamethoxazole by Laccase-Mediated Oxidation and Ferrate(VI) Oxidation: The Impact of Mediators and Metal Ions. Environ. Sci. Pollut. Res. 2022, 30, 15708–15721. [Google Scholar] [CrossRef]
- Jia, M.; Yu, X.; Xu, K.; Gu, X.; Harmer, N.J.; Zhao, Y.; Xiang, Y.; Sheng, X.; Li, C.; Du, X.-D.; et al. The High-Efficiency Degradation of Multiple Mycotoxins by Lac-W Laccase in the Presence of Mediators. Toxins 2024, 16, 477. [Google Scholar] [CrossRef]
- Qin, X.; Xin, Y.; Zou, J.; Su, X.; Wang, X.; Wang, Y.; Zhang, J.; Tu, T.; Yao, B.; Luo, H.; et al. Efficient Degradation of Aflatoxin B1 and Zearalenone by Laccase-like Multicopper Oxidase from Streptomyces thermocarboxydus in the Presence of Mediators. Toxins 2021, 13, 754. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Guo, Y.; Qiao, Y.; Ma, Q.; Ji, C.; Zhao, L. Degradation of Zearalenone and Aflatoxin B1 by Lac2 from Pleurotus Pulmonarius in the Presence of Mediators. Toxicon 2021, 201, 1–8. [Google Scholar] [CrossRef]
- Martin, E.; Audonnet, F.; Yaacoub, D.; Dubessay, P.; Michaud, P. Nernst-Michaelis-Menten Framework Unlocks Electrochemical Kinetics for Laccases. Bioelectrochemistry 2025, 165, 109003. [Google Scholar] [CrossRef]
- Song, H.-K.; Palmore, G.T.R. Conductive Polypyrrole via Enzyme Catalysis. J. Phys. Chem. B 2005, 109, 19278–19287. [Google Scholar] [CrossRef]
- Gellett, W.; Schumacher, J.; Kesmez, M.; Le, D.; Minteer, S.D. High Current Density Air-Breathing Laccase Biocathode. J. Electrochem. Soc. 2010, 157, B557. [Google Scholar] [CrossRef]
- Johnson, D.L.; Thompson, J.L.; Brinkmann, S.M.; Schuller, K.A.; Martin, L.L. Electrochemical Characterization of Purified Rhus Vernicifera Laccase: Voltammetric Evidence for a Sequential Four-Electron Transfer. Biochemistry 2003, 42, 10229–10237. [Google Scholar] [CrossRef]
- Thuesen, M.H.; Farver, O.; Reinhammar, B.; Ulstrup, J.; Guirgis, G.A.; Rosendahl, C.N.; Søtofte, I.; Långström, B. Cyclic Voltammetry and Electrocatalysis of the Blue Copper Oxidase Polyporus Versicolor Laccase. Acta Chem. Scand. 1998, 52, 555–562. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-Scale Protein Function Classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating Signal Peptides from Transmembrane Regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 6.0 Predicts All Five Types of Signal Peptides Using Protein Language Models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Wariishi, H.; Valli, K.; Gold, M.H. Manganese(II) Oxidation by Manganese Peroxidase from the Basidiomycete Phanerochaete Chrysosporium. Kinetic Mechanism and Role of Chelators. J. Biol. Chem. 1992, 267, 23688–23695. [Google Scholar] [CrossRef]
- Heinfling, A.; Martínez, M.J.; Martínez, A.T.; Bergbauer, M.; Szewzyk, U. Purification and Characterization of Peroxidases from the Dye-Decolorizing Fungus Bjerkandera adusta. FEMS Microbiol. Lett. 1998, 165, 43–50. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Robinson, R.A. CXCVIII.—Universal Buffer Solutions and the Dissociation Constant of Veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]
- Šali, A.; Blundell, T.L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New Data Content and Improved Web Interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Krieger, E.; Dunbrack, R.L.; Hooft, R.W.W.; Krieger, B. Assignment of Protonation States in Proteins and Ligands: Combining pKa Prediction with Hydrogen Bonding Network Optimization. Methods Mol. Biol. 2012, 819, 405–421. [Google Scholar] [CrossRef]
- Land, H.; Humble, M.S. YASARA: A Tool to Obtain Structural Guidance in Biocatalytic Investigations. In Protein Engineering; Bornscheuer, U.T., Höhne, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1685, pp. 43–67. ISBN 978-1-4939-7364-4. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dyachkova, T.P.; Khan, Y.A.; Orlova, N.V.; Kondrashov, S.V. Oxidation of Multiwalled Carbon Nanotubes by Hydrogene Peroxide Vapor: Laws and Effects. Vestnik 2016, 22, 323–333. [Google Scholar] [CrossRef]


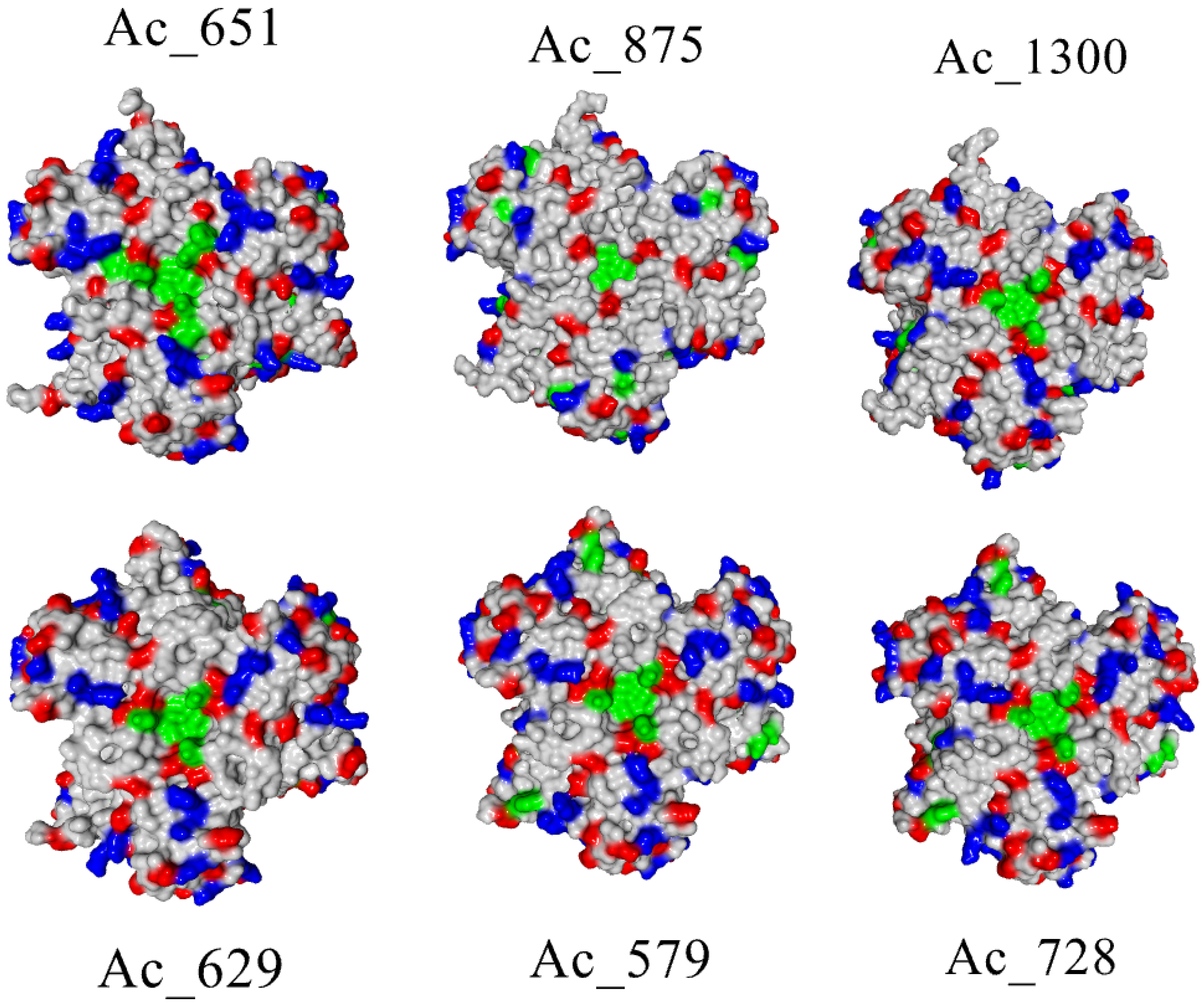
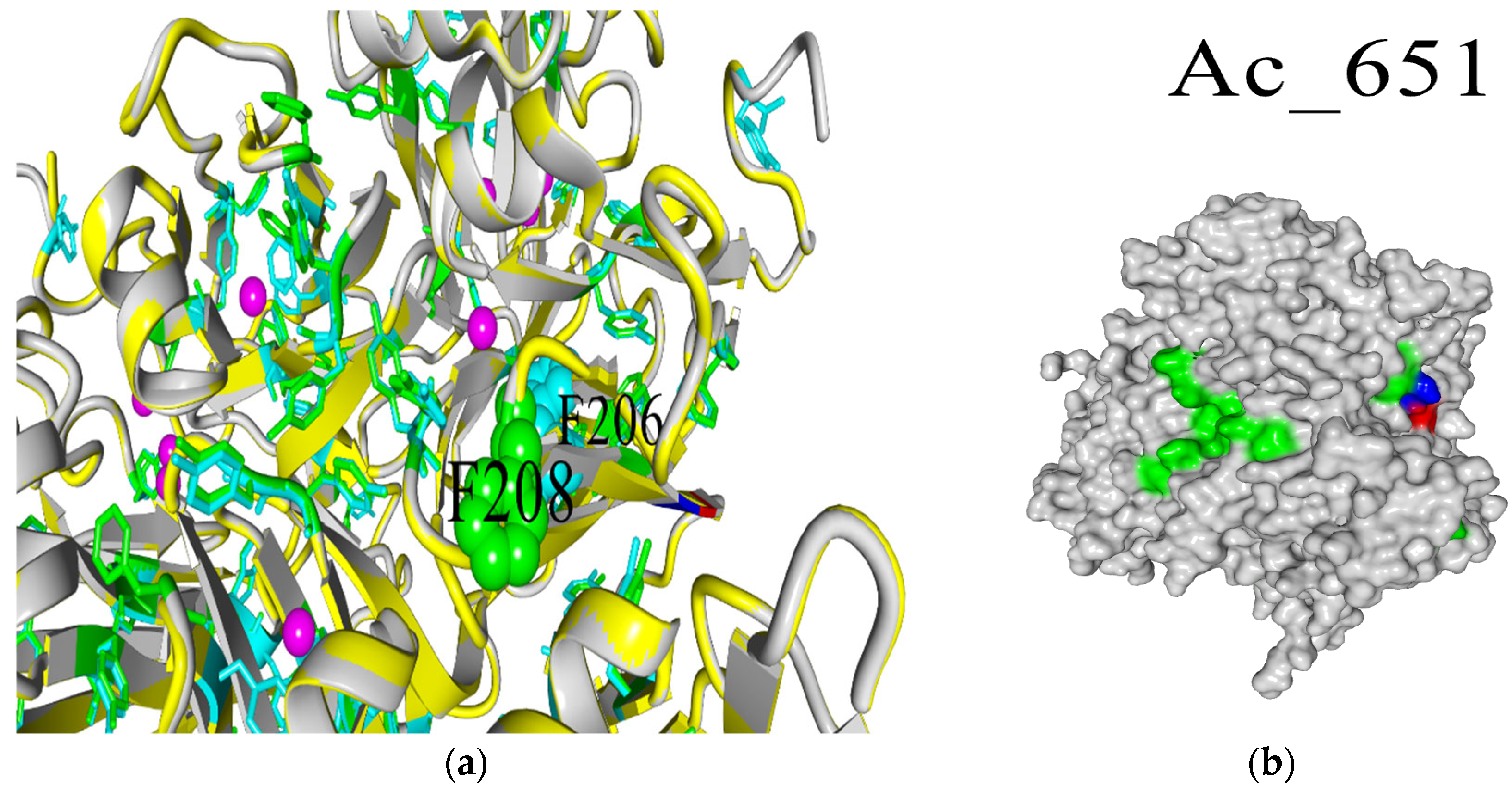
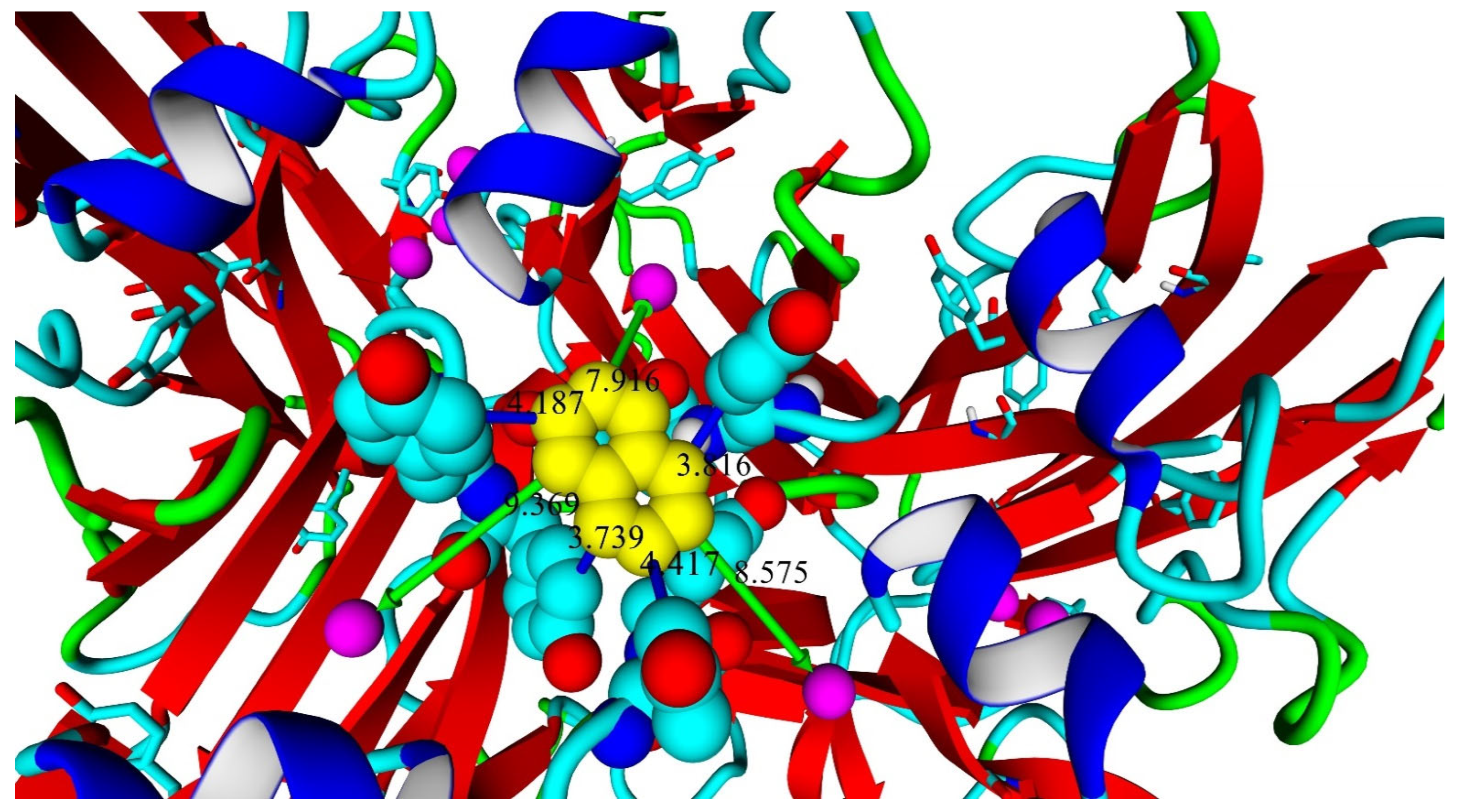
| Substrates | SoSL | SpSL | ScaSL | CjSL |
|---|---|---|---|---|
| Caffeic acid | + | − | + | + |
| Coumaric acid | − | − | − | + |
| Ferulic acid | + | − | + | + |
| Gallic acid | − | − | + | + |
| Gentisic acid | + | − | + | + |
| Syringic acid | − | − | + | + |
| Tannic acid | − | − | + | + |
| Vanillic acid | − | − | − | + |
| 2-thiobarbituric acid | − | − | − | − |
| 3,4-dihydroxybenzoic acid | − | − | − | + |
| 4-hydroxybenzoic acid | − | − | − | − |
| 2,6-dimethoxyphenol | + | + | + | + |
| 3,5-dimethoxyphenol | − | − | − | − |
| 3,4,5-trimethoxyphenol | − | − | + | + |
| 2-aminophenol | + | + | + | + |
| 4-chloro-1-naphthol | + | + | + | + |
| Catechol | + | + | + | + |
| Guaiacol | + | − | + | + |
| Hydroquinone | + | + | + | + |
| Pyrogallol | + | + | + | + |
| Methylhydroquinone | + | + | + | + |
| Syringaldehyde | − | − | + | + |
| Syringaldazine | + | − | + | + |
| Vanillin | − | − | − | − |
| Tyrosine | − | − | − | − |
| Laccase | Km ABTS, μM | pH Optimum for ABTS | Docking-Derived Binding Constant, μM | Docking-Derived Binding Constant (After Energy Minimization and pH Adjustment of the Receptor), μM |
|---|---|---|---|---|
| SoSL (Ac-651) | 50 | 3.5 | 24 | 5 |
| CjSL (Ac-875) | 390 | 3.6 | 65 | 0.06 |
| ScSL (Ac-1300) | 100 | 4.7 | 9 | 3 |
| SvSL (Ac-629) | 300 | 4 | 3 | 2 |
| SpSL (Ac-579) | 370 | 3.5 | 2 | 2 |
| SaSL (Ac-728) | 170 | 3 | 4 | 2 |
| pH | Control | 1 mM | 10 mM | 100 mM |
|---|---|---|---|---|
| 3.7 | 100 ± 0.6 | 84.1 ± 1.2 | 67.8 ± 0.6 | 49.4 ± 3 * |
| 8.7 | 100 ± 0.8 | 100 ± 2.3 | 99 ± 1.3 | 100 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trubitsina, L.; Egorov, K.; Abdullatypov, A.; Petrakova, M.; Trubitsin, I.; Alferov, S.; Leontievsky, A.; Ponamoreva, O. Bioelectrocatalyst for O2 Reduction Based on a Novel Recombinant Two-Domain Laccase from Streptomyces ochraceisleroticus Immobilized on Naphthyl-Modified MWCNTs. Int. J. Mol. Sci. 2025, 26, 9143. https://doi.org/10.3390/ijms26189143
Trubitsina L, Egorov K, Abdullatypov A, Petrakova M, Trubitsin I, Alferov S, Leontievsky A, Ponamoreva O. Bioelectrocatalyst for O2 Reduction Based on a Novel Recombinant Two-Domain Laccase from Streptomyces ochraceisleroticus Immobilized on Naphthyl-Modified MWCNTs. International Journal of Molecular Sciences. 2025; 26(18):9143. https://doi.org/10.3390/ijms26189143
Chicago/Turabian StyleTrubitsina, Liubov, Konstantin Egorov, Azat Abdullatypov, Marina Petrakova, Ivan Trubitsin, Sergey Alferov, Alexey Leontievsky, and Olga Ponamoreva. 2025. "Bioelectrocatalyst for O2 Reduction Based on a Novel Recombinant Two-Domain Laccase from Streptomyces ochraceisleroticus Immobilized on Naphthyl-Modified MWCNTs" International Journal of Molecular Sciences 26, no. 18: 9143. https://doi.org/10.3390/ijms26189143
APA StyleTrubitsina, L., Egorov, K., Abdullatypov, A., Petrakova, M., Trubitsin, I., Alferov, S., Leontievsky, A., & Ponamoreva, O. (2025). Bioelectrocatalyst for O2 Reduction Based on a Novel Recombinant Two-Domain Laccase from Streptomyces ochraceisleroticus Immobilized on Naphthyl-Modified MWCNTs. International Journal of Molecular Sciences, 26(18), 9143. https://doi.org/10.3390/ijms26189143






