Selective Senolysis of 5FU-Induced CRC Senescent Cells by Piceatannol Through Mitochondrial Depolarization and AIF-Dependent Apoptosis
Abstract
1. Introduction
2. Results
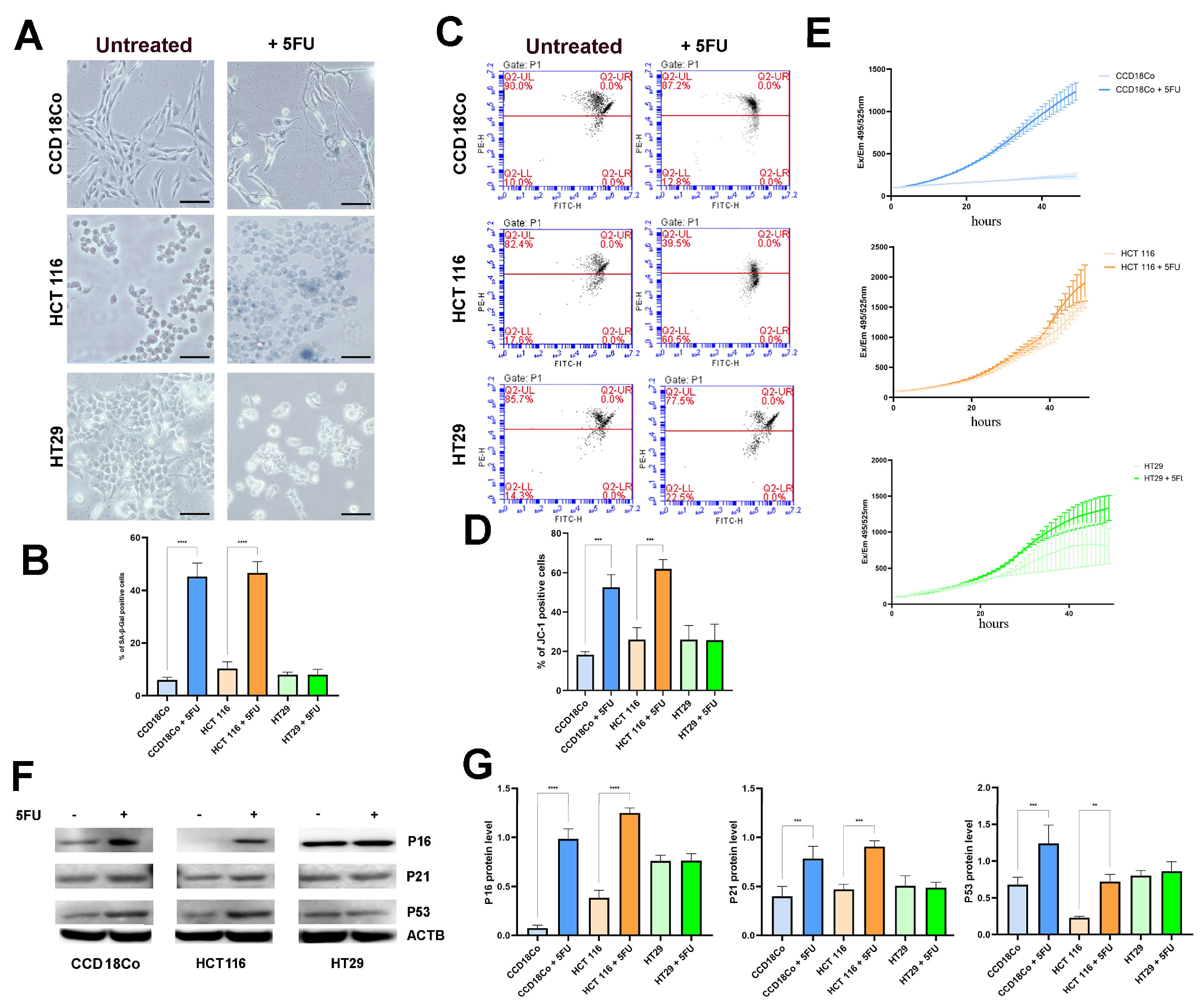
2.1. 5FU Treatment Induces a Senescent Phenotype in Normal and Tumor Cells
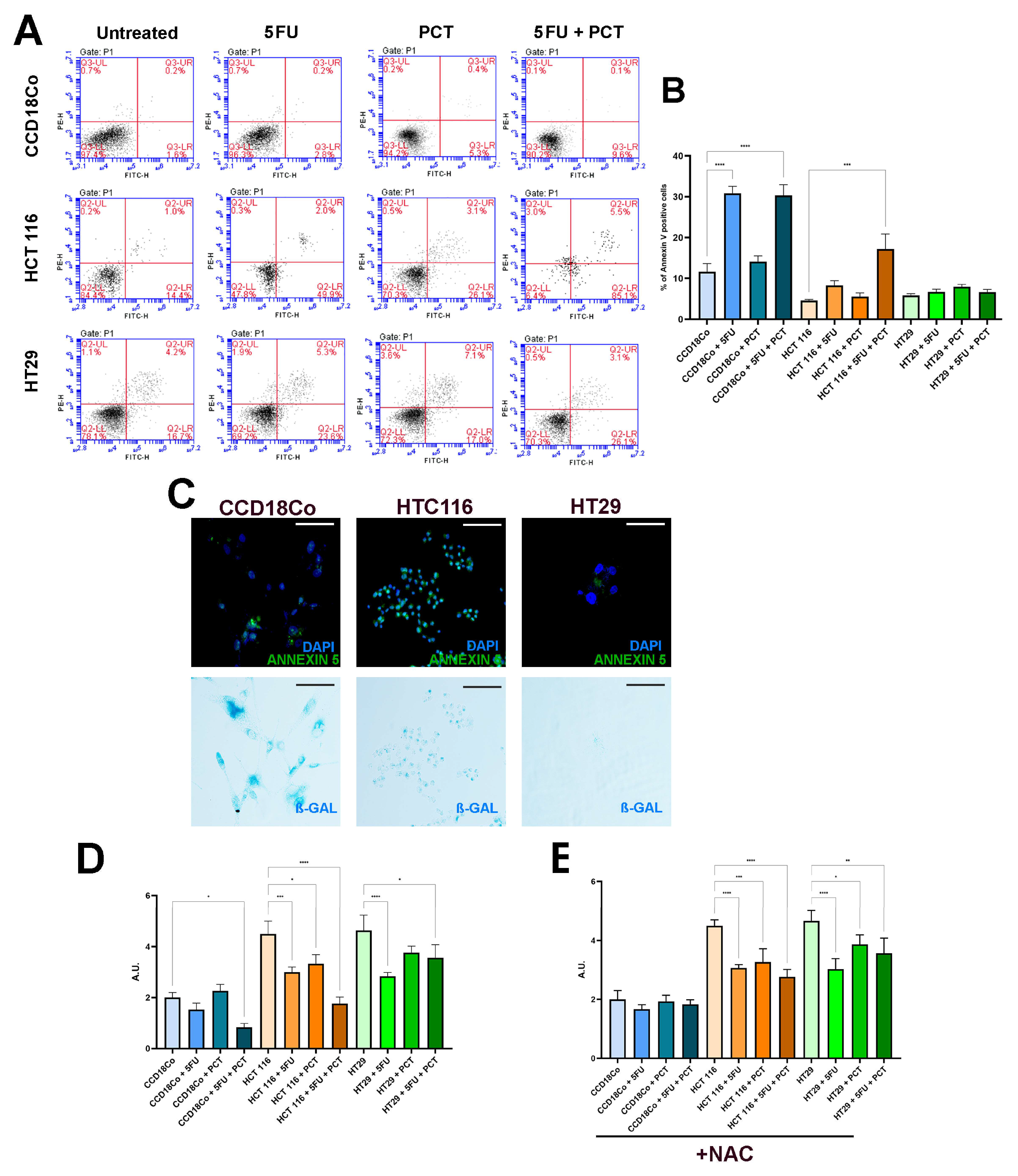
2.2. Piceatannol Selectively Eliminates 5FU-Induced Senescent Cells
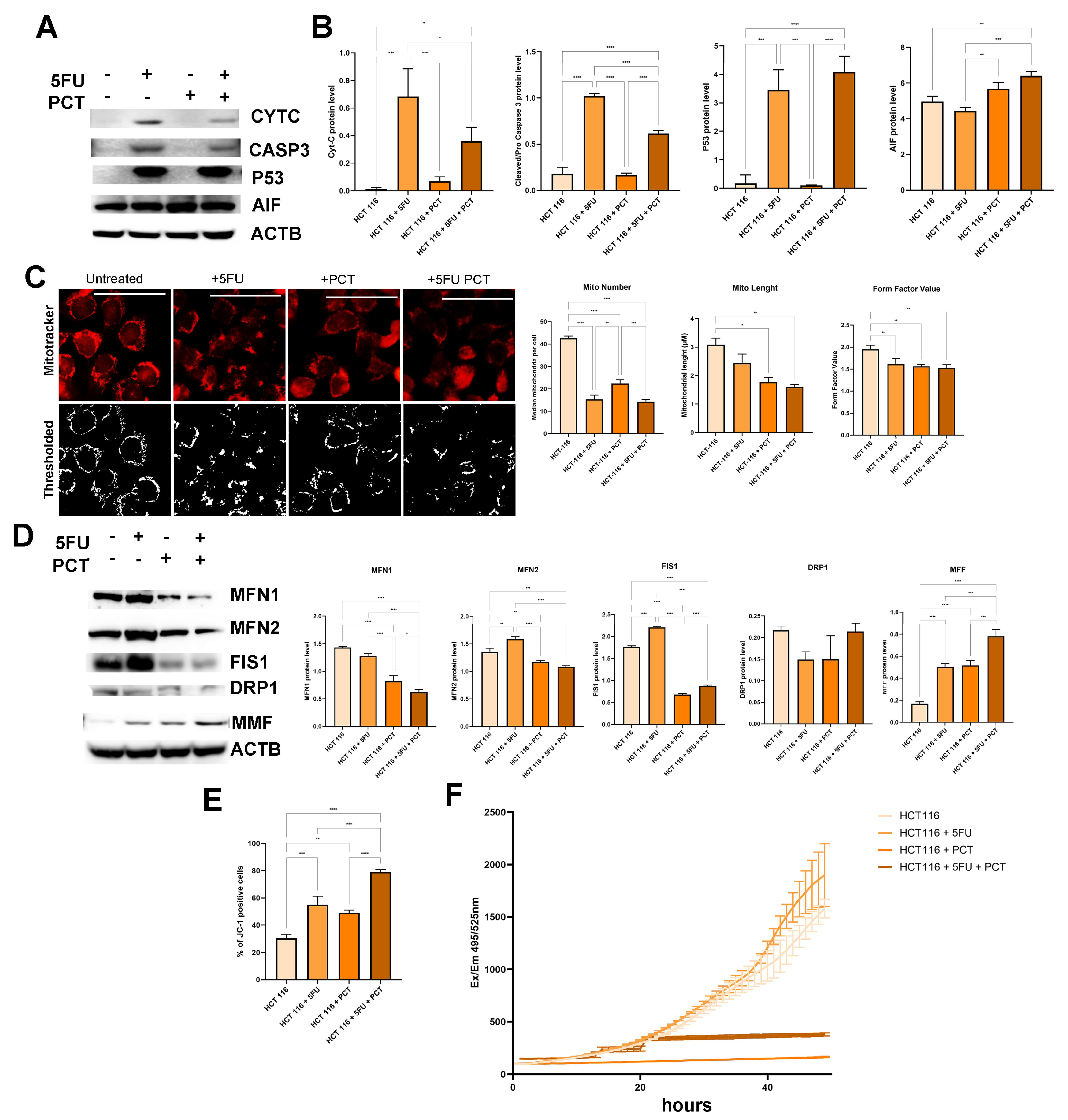
2.3. PCT Induces AIF-Dependent Apoptosis Without Increasing ROS
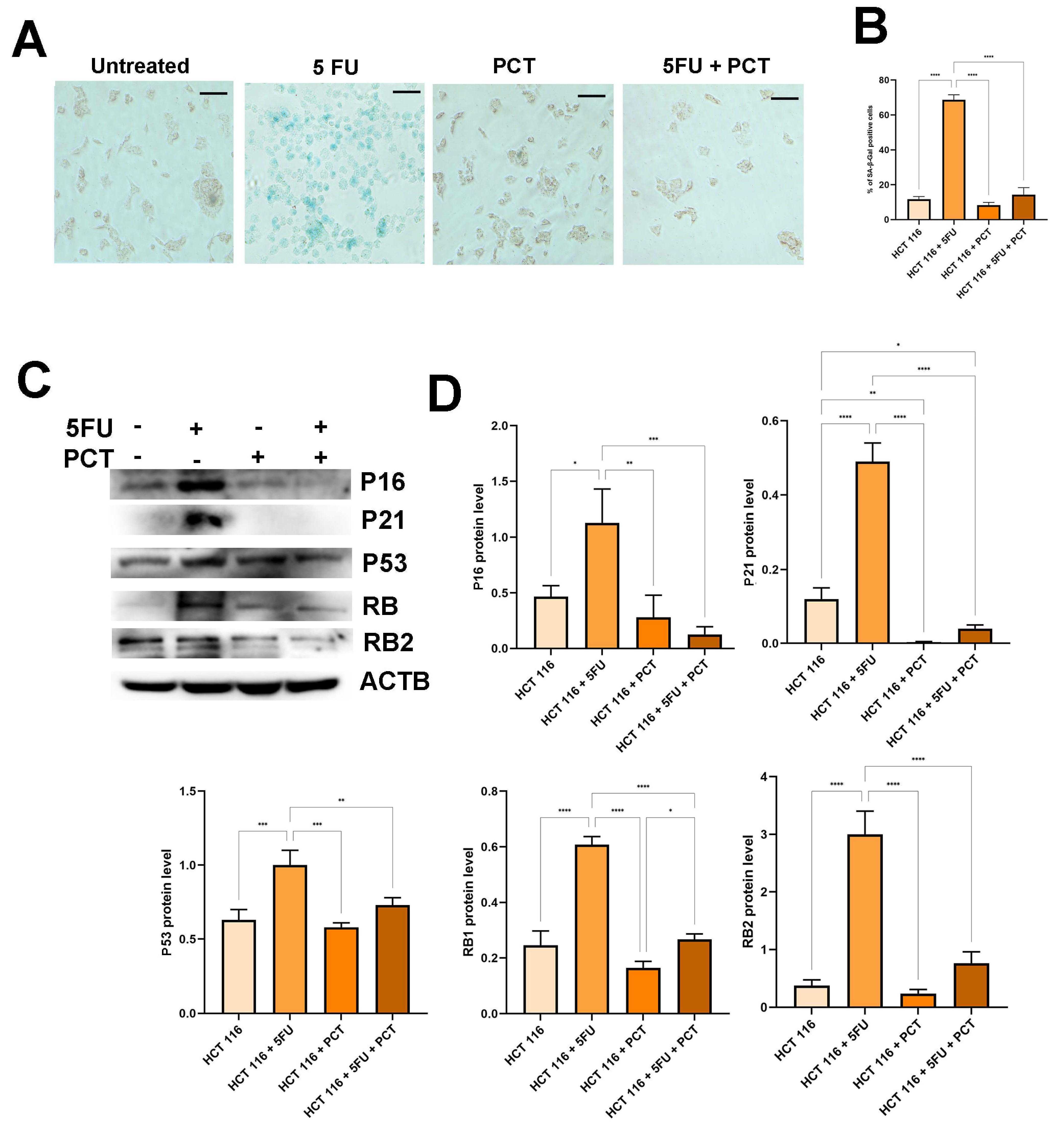
2.4. PCT Promotes Long-Term Resolution of the Senescent Phenotype
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Senescence Induction and Treatment Schedule
4.3. SA-β-galactosidase Staining
4.4. Immunocytochemistry
4.5. Apoptosis Assay (Annexin V/PI)
4.6. Mitochondrial Membrane Potential (JC-1)
4.7. Reactive Oxygen Species Measurement (DCF-DA)
4.8. Cell Viability (CCK-8 Assay)
4.9. Mitochondrial Morphology Analysis
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolpin, B.M.; Mayer, R.J. Systemic Treatment of Colorectal Cancer. Gastroenterology 2008, 134, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Azwar, S.; Seow, H.F.; Abdullah, M.; Jabar, M.F.; Mohtarrudin, N. Recent Updates on Mechanisms of Resistance to 5-Fluorouracil and Reversal Strategies in Colon Cancer Treatment. Biology 2021, 10, 854. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef]
- Herranz, N.; Gil, J. Mechanisms and Functions of Cellular Senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. eBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lelarge, V.; Capelle, R.; Oger, F.; Mathieu, T.; Le Calvé, B. Senolytics: From Pharmacological Inhibitors to Immunotherapies, a Promising Future for Patients’ Treatment. npj Aging 2024, 10, 12. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B.; et al. Identification of a Novel Senolytic Agent, Navitoclax, Targeting the Bcl-2 Family of Anti-Apoptotic Factors. Aging Cell 2016, 15, 428–435. [Google Scholar] [CrossRef]
- Al Mamun, A.; Sufian, M.A.; Uddin, M.S.; Sumsuzzman, D.M.; Jeandet, P.; Islam, M.S.; Zhang, H.J.; Kong, A.N.; Sarwar, M.S. Exploring the Role of Senescence Inducers and Senotherapeutics as Targets for Anticancer Natural Products. Eur. J. Pharmacol. 2022, 928, 174991. [Google Scholar] [CrossRef]
- Alessio, N.; Squillaro, T.; Lettiero, I.; Galano, G.; De Rosa, R.; Peluso, G.; Galderisi, U.; Di Bernardo, G. Biomolecular Evaluation of Piceatannol’s Effects in Counteracting the Senescence of Mesenchymal Stromal Cells: A New Candidate for Senotherapeutics? Int. J. Mol. Sci. 2021, 22, 11619. [Google Scholar] [CrossRef]
- Maruki-Uchida, H.; Kurita, I.; Sugiyama, K.; Sai, M.; Maeda, K.; Ito, T. The Protective Effects of Piceatannol from Passion Fruit (Passiflora edulis) Seeds in UVB-Irradiated Keratinocytes. Biol. Pharm. Bull. 2013, 36, 845–849. [Google Scholar] [CrossRef]
- Kershaw, J.; Kim, K.H. The Therapeutic Potential of Piceatannol, a Natural Stilbene, in Metabolic Diseases: A Review. J. Med. Food. 2017, 20, 427–438. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, E.J.; Park, H.; Park, S.Y.; Jun, J.G.; Park, J.H.Y. The Grape Component Piceatannol Induces Apoptosis in Du145 Human Prostate Cancer Cells via the Activation of Extrinsic and Intrinsic Pathways. J. Med. Food 2009, 12, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Çınar Ayan, İ.; Güçlü, E.; Vural, H.; Dursun, H.G. Piceatannol Induces Apoptotic Cell Death through Activation of Caspase-Dependent Pathway and Upregulation of ROS-Mediated Mitochondrial Dysfunction in Pancreatic Cancer Cells. Mol. Biol. Rep. 2022, 49, 11947–11957. [Google Scholar] [CrossRef] [PubMed]
- Bunz, F.; Dutriaux, A.; Lengauer, C.; Waldman, T.; Zhou, S.; Brown, J.P.; Sedivy, J.M.; Kinzler, K.W.; Vogelstein, B. Requirement for P53 and P21 to Sustain G2 Arrest after DNA Damage. Science 1998, 282, 1497–1501. [Google Scholar] [CrossRef]
- Alessio, N.; Acar, M.B.; Squillaro, T.; Aprile, D.; Ayaz-Güner, Ş.; Di Bernardo, G.; Peluso, G.; Özcan, S.; Galderisi, U. Progression of Irradiated Mesenchymal Stromal Cells from Early to Late Senescence: Changes in SASP Composition and Anti-Tumour Properties. Cell Prolif. 2023, 56, e13401. [Google Scholar] [CrossRef]
- Song, K.X.; Wang, J.X.; Huang, D. Therapy-Induced Senescent Tumor Cells in Cancer Relapse. J. Natl. Cancer Cent. 2023, 3, 273–278. [Google Scholar] [CrossRef]
- Fitsiou, E.; Soto-Gamez, A.; Demaria, M. Biological Functions of Therapy-Induced Senescence in Cancer. Semin. Cancer Biol. 2022, 81, 5–13. [Google Scholar] [CrossRef]
- te Poele, R.H.; Okorokov, A.L.; Jardine, L.; Cummings, J.; Joel, S.P. DNA Damage Is Able to Induce Senescence in Tumor Cells in Vitro and in vivo. Cancer Res. 2002, 62, 1876–1883. [Google Scholar]
- Schmitt, C.A. Senescence, Apoptosis and Therapy—Cutting the Lifelines of Cancer. Nat. Rev. Cancer 2003, 3, 286–295. [Google Scholar]
- Boccellino, M.; Donniacuo, M.; Bruno, F.; Rinaldi, B.; Quagliuolo, L.; Ambruosi, M.; Pace, S.; De Rosa, M.; Olgaç, A.; Banoglu, E.; et al. Protective Effect of Piceatannol and Bioactive Stilbene Derivatives against Hypoxia-Induced Toxicity in H9c2 Cardiomyocytes and Structural Elucidation as 5-LOX Inhibitors. Eur. J. Med. Chem. 2019, 180, 637–647. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting Cellular Senescence with Senotherapeutics: Senolytics and Senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.A. Apoptosis-Inducing Factor (AIF) at the Crossroad of Cell Survival and Cell Death: Implications for Cancer and Mitochondrial Diseases. Cell Commun. Sing. 2025, 23, 264. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, V.; Alessio, N.; Aprile, D.; Galano, G.; De Rosa, R.; Schiraldi, C.; Di Bernardo, G.; Galderisi, U. Terpenes: Natural compounds found in plants as potential senotherapeutics targeting senescent mesenchymal stromal cells and promoting apoptosis. Stem Cell Res. Ther. 2025, 16, 231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seo, J.H.; Agarwal, E.; Chae, Y.C.; Lee, Y.G.; Garlick, D.S.; Storaci, A.M.; Ferrero, S.; Gaudioso, G.; Gianelli, U.; Vaira, V.; et al. Mitochondrial fission factor is a novel Myc-dependent regulator of mitochondrial permeability in cancer. eBioMedicine 2019, 48, 353–363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Liu, K.; Wang, N.; Zhang, H. N-acetylcysteine induces apoptosis via the mitochondria-dependent pathway but not via endoplasmic reticulum stress in H9c2 cells. Mol. Med. Rep. 2017, 16, 6626–6633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mlejnek, P. Direct Interaction between N-Acetylcysteine and Cytotoxic Electrophile-An Overlooked In Vitro Mechanism of Protection. Antioxidants 2022, 11, 1485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally Occurring P16 Ink4a-Positive Cells Shorten Healthy Lifespan. Nature 2016, 530, 184–189. [Google Scholar] [CrossRef]
- Jin, C.Y.; Molagoda, I.M.N.; Park, C.; Kwon, T.K.; Yun, S.J.; Kim, W.J.; Kim, G.Y.; Choi, Y.H. Piceatannol-Induced Apoptosis Is Reversed by N-Acetyl-L-cysteine through Restoration of XIAP Expression. Biol. Pharm. Bull. 2018, 41, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosino, A.; Patrone, D.; Moriello, C.; Al-Sammarraie, S.H.A.; Lettiero, I.; Finicelli, M.; Siniscalco, D.; Alessio, N. Selective Senolysis of 5FU-Induced CRC Senescent Cells by Piceatannol Through Mitochondrial Depolarization and AIF-Dependent Apoptosis. Int. J. Mol. Sci. 2025, 26, 9134. https://doi.org/10.3390/ijms26189134
Ambrosino A, Patrone D, Moriello C, Al-Sammarraie SHA, Lettiero I, Finicelli M, Siniscalco D, Alessio N. Selective Senolysis of 5FU-Induced CRC Senescent Cells by Piceatannol Through Mitochondrial Depolarization and AIF-Dependent Apoptosis. International Journal of Molecular Sciences. 2025; 26(18):9134. https://doi.org/10.3390/ijms26189134
Chicago/Turabian StyleAmbrosino, Alessia, Deanira Patrone, Claudia Moriello, Sura Hilal Ahmed Al-Sammarraie, Ida Lettiero, Mauro Finicelli, Dario Siniscalco, and Nicola Alessio. 2025. "Selective Senolysis of 5FU-Induced CRC Senescent Cells by Piceatannol Through Mitochondrial Depolarization and AIF-Dependent Apoptosis" International Journal of Molecular Sciences 26, no. 18: 9134. https://doi.org/10.3390/ijms26189134
APA StyleAmbrosino, A., Patrone, D., Moriello, C., Al-Sammarraie, S. H. A., Lettiero, I., Finicelli, M., Siniscalco, D., & Alessio, N. (2025). Selective Senolysis of 5FU-Induced CRC Senescent Cells by Piceatannol Through Mitochondrial Depolarization and AIF-Dependent Apoptosis. International Journal of Molecular Sciences, 26(18), 9134. https://doi.org/10.3390/ijms26189134







