Balancing Decidualization, Autophagy, and Cellular Senescence for Reproductive Success in Endometriosis Biology
Abstract
1. Introduction
2. Pathophysiology of Endometriosis
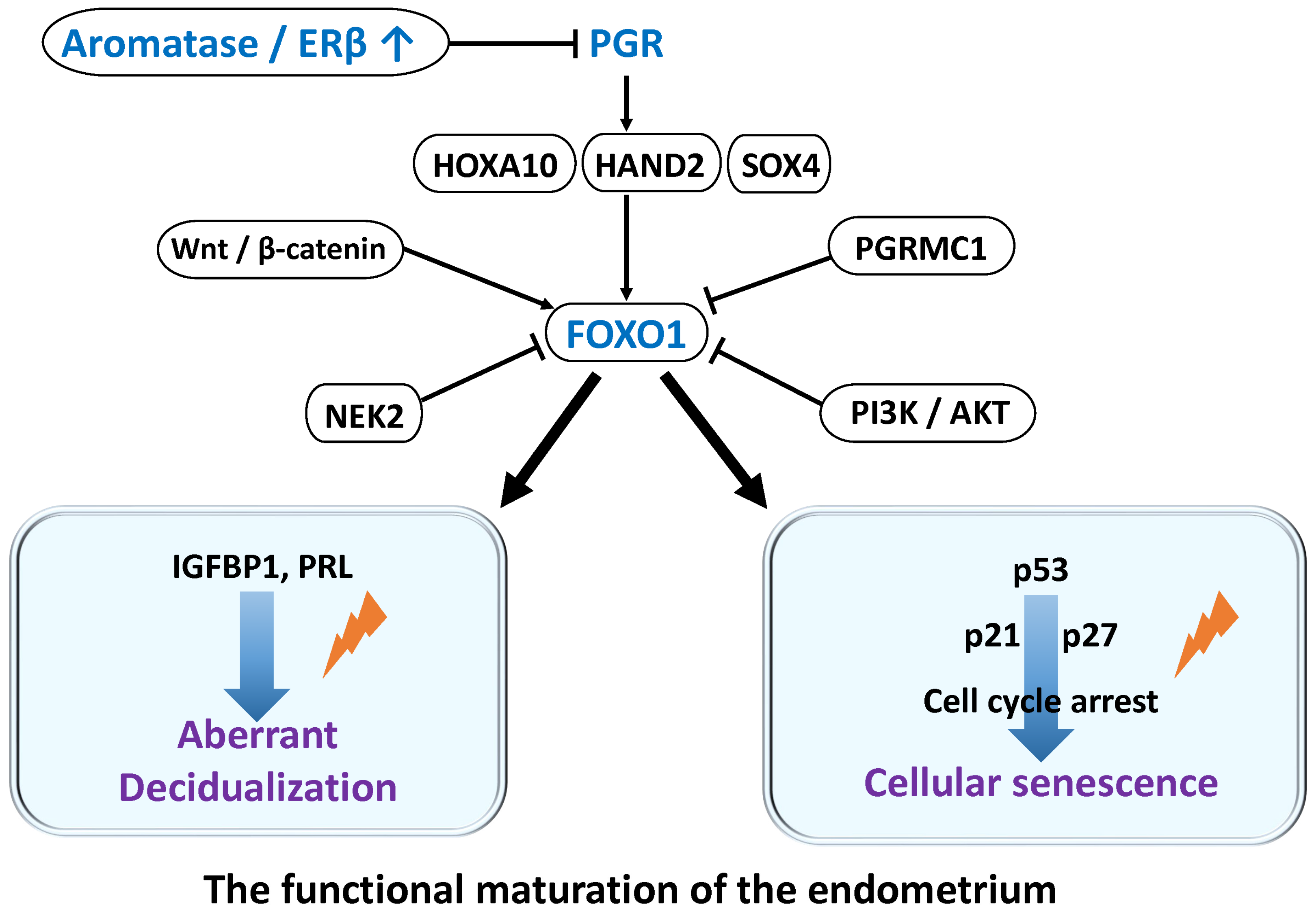
2.1. Hormonal Regulation of Decidualization
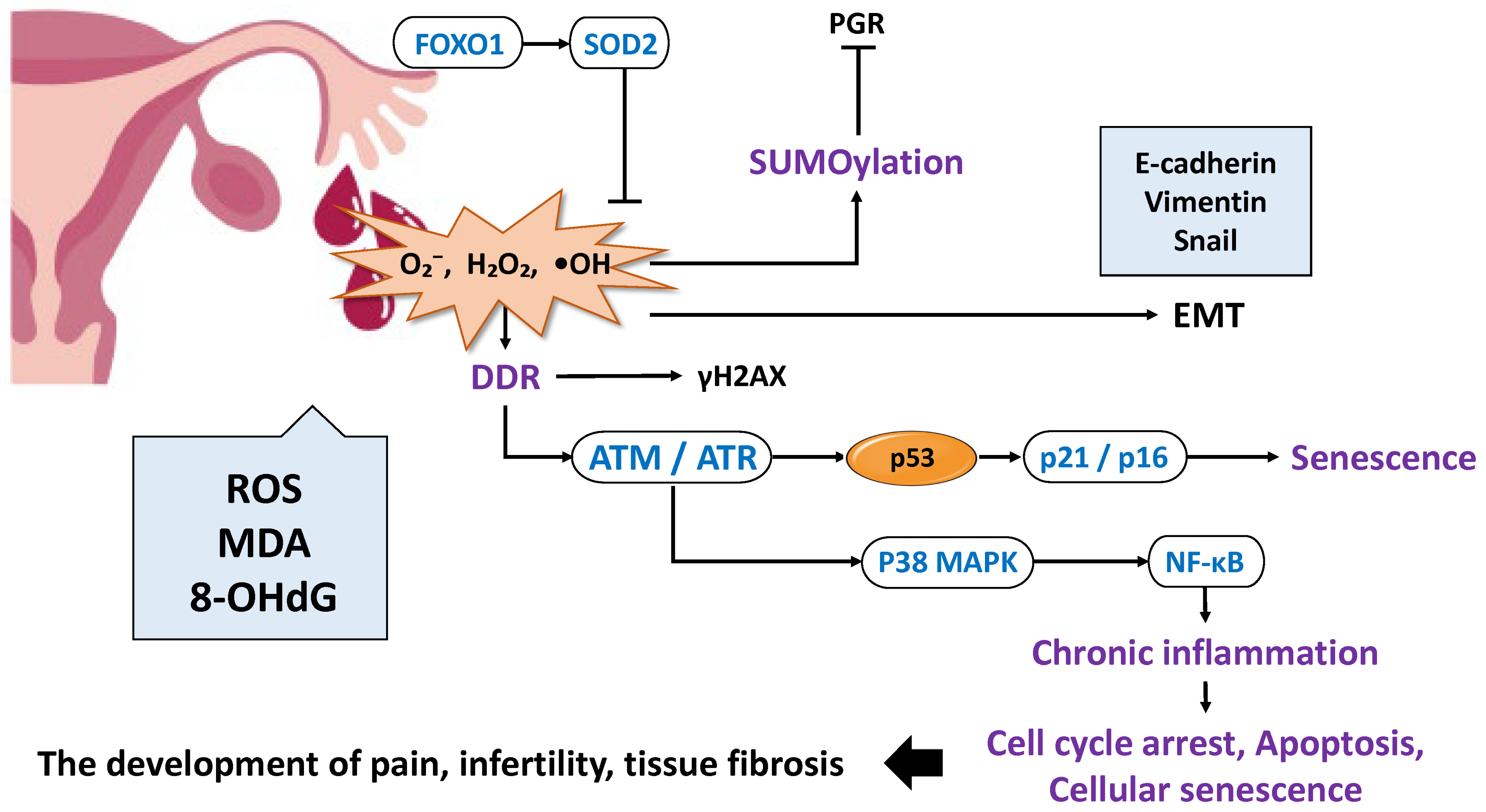
2.2. Oxidative Stress and Inflammation in Decidualization
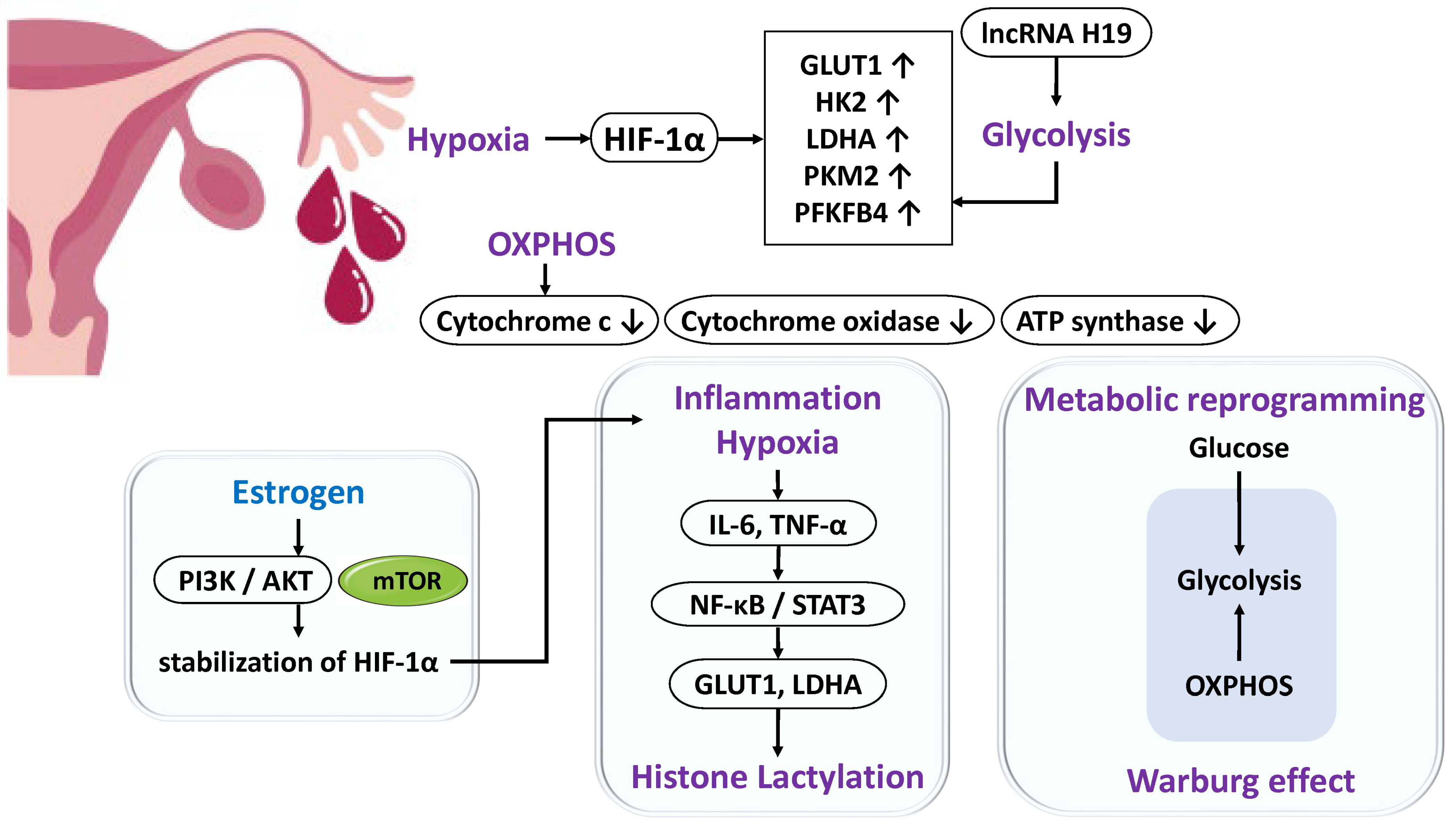
2.3. Metabolic Reprogramming
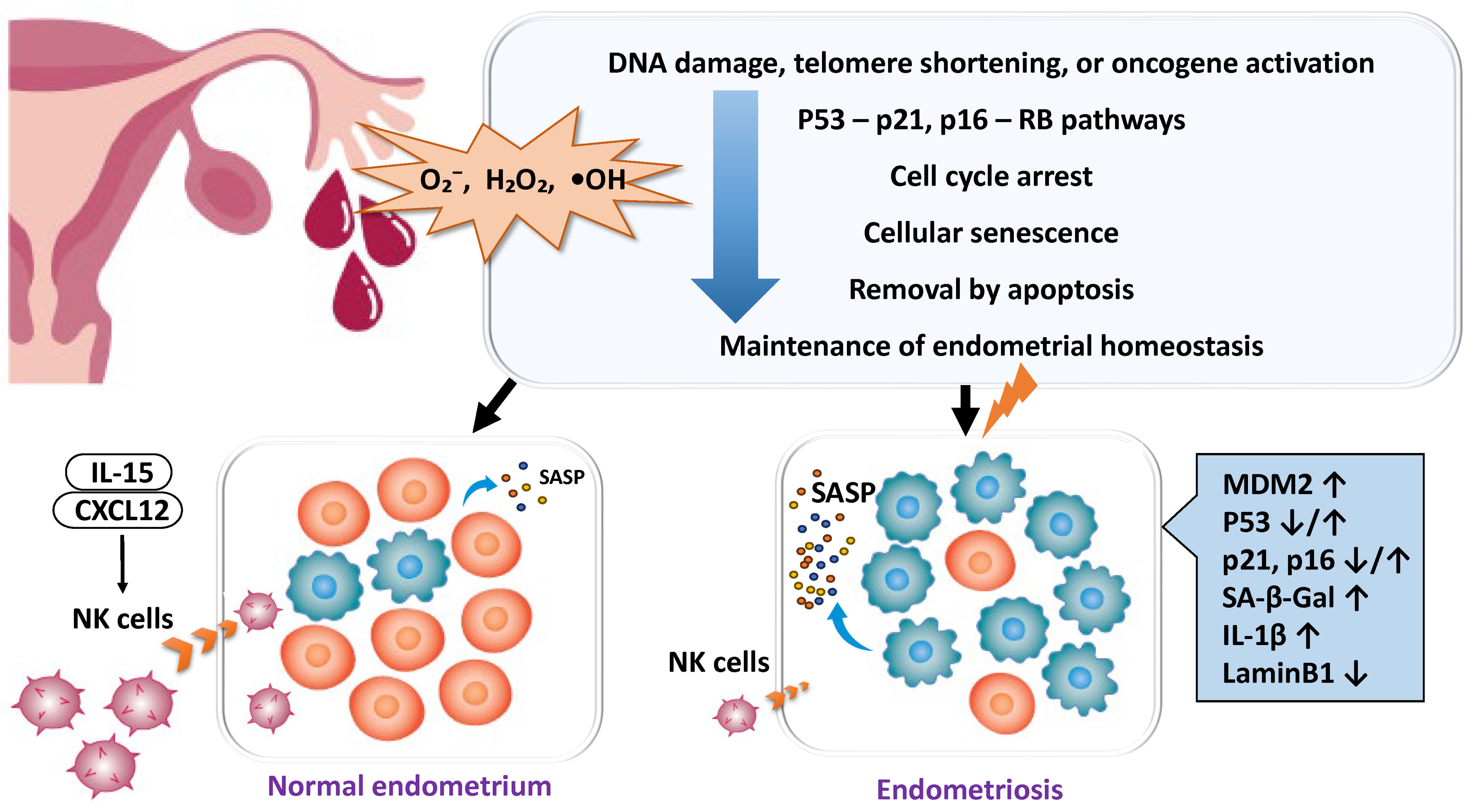
2.4. Disruption of Cellular Homeostasis in Endometriosis
2.4.1. Enhanced Cellular Senescence and Deficiency of Clearance Mechanisms
2.4.2. Evasion of Apoptosis and Enhancement of Survival Signals
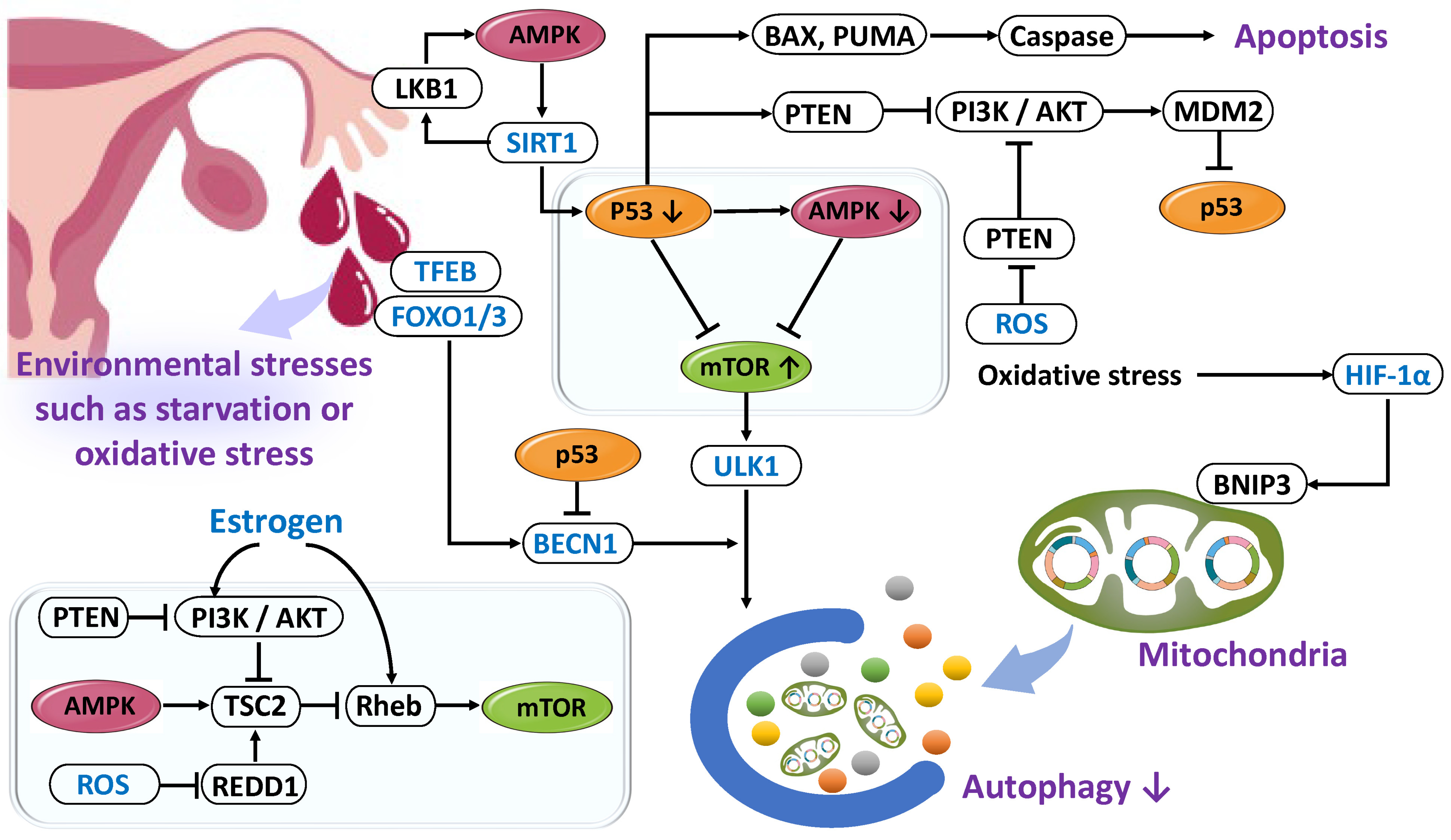
2.4.3. Dysfunction of Autophagy
Reduction in Autophagy
Enhancement of Autophagy
2.5. Genetic Regulation in Decidualization
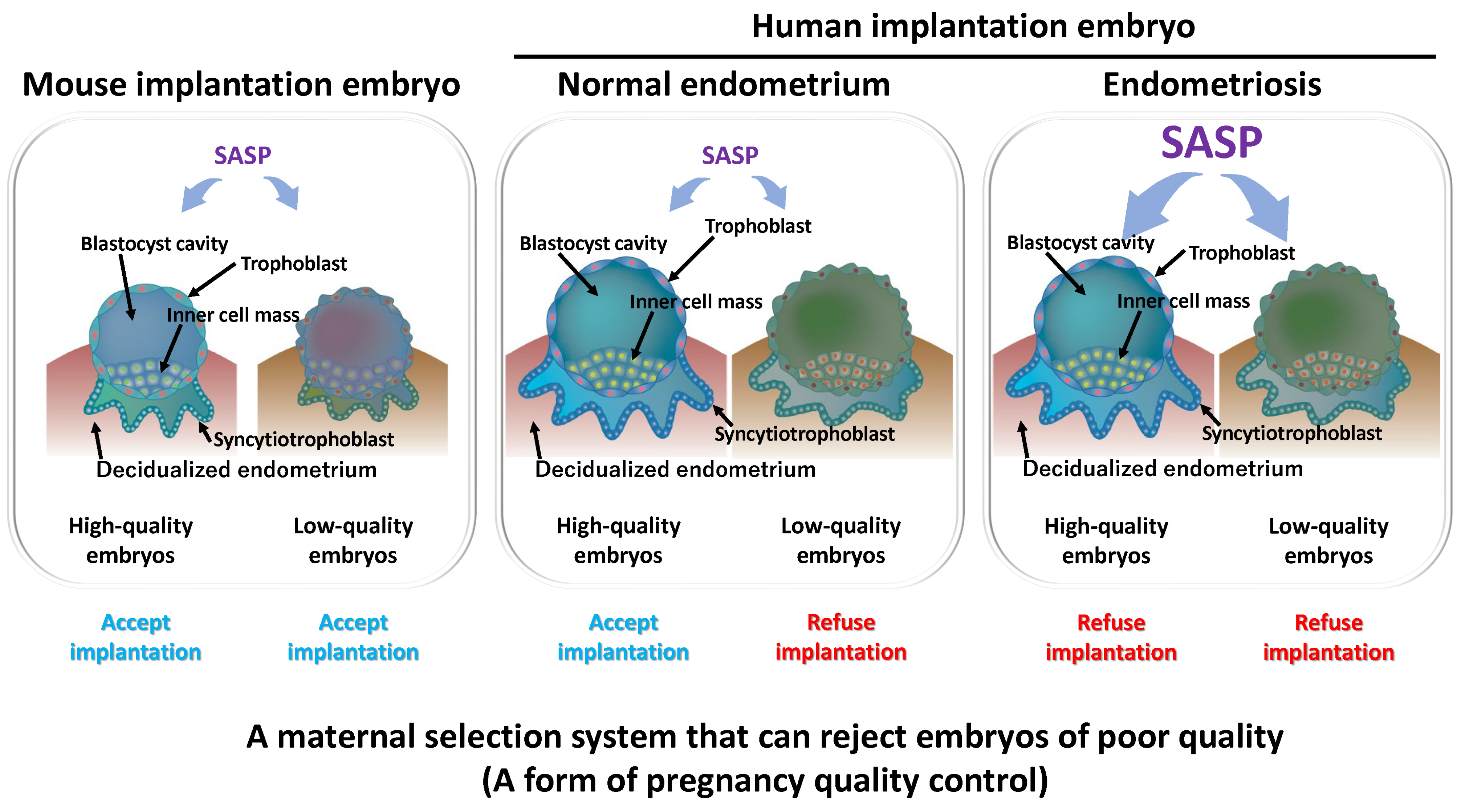
3. Decidualization as a Selection Barrier for Embryo Quality
4. Discussion and Future Perspectives
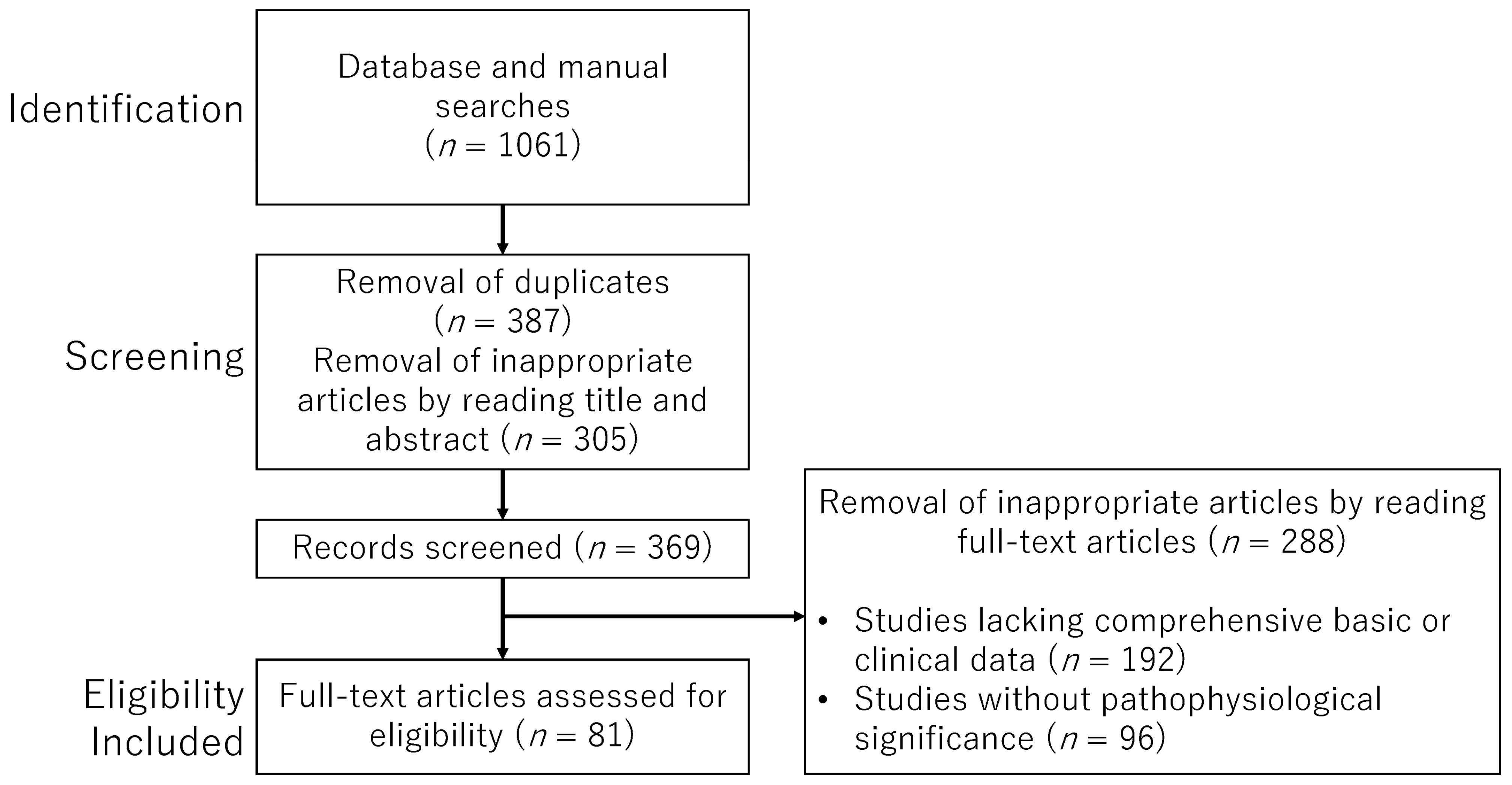
5. Methods
Search Strategy and Selection Criteria
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, T.; Tong, D.; Li, S.; Yu, X.; Liu, B.; Jiang, L.; Liu, K. Research advances in endometriosis-related signaling pathways: A review. Biomed. Pharmacother. 2023, 164, 114909. [Google Scholar] [CrossRef]
- Harada, T.; Kaponis, A.; Iwabe, T.; Taniguchi, F.; Makrydimas, G.; Sofikitis, N.; Paschopoulos, M.; Paraskevaidis, E.; Terakawa, N. Apoptosis in human endometrium and endometriosis. Hum. Reprod. Update 2004, 10, 29–38. [Google Scholar] [CrossRef]
- Allavena, G.; Carrarelli, P.; Del Bello, B.; Luisi, S.; Petraglia, F.; Maellaro, E. Autophagy is upregulated in ovarian endometriosis: A possible interplay with p53 and heme oxygenase-1. Fertil. Steril. 2015, 103, 1244–1251.e1. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Lu, S.; Wang, X.; Shi, X.; Mao, P. Autophagy-dependent ferroptosis is involved in the development of endometriosis. Gynecol. Endocrinol. 2023, 39, 2242962. [Google Scholar] [CrossRef]
- Kobayashi, H.; Umetani, M.; Nishio, M.; Shigetomi, H.; Imanaka, S.; Hashimoto, H. Molecular Mechanisms of Cellular Senescence in Age-Related Endometrial Dysfunction. Cells 2025, 14, 858. [Google Scholar] [CrossRef]
- Driva, T.S.; Schatz, C.; Sobočan, M.; Haybaeck, J. The Role of mTOR and eIF Signaling in Benign Endometrial Diseases. Int. J. Mol. Sci. 2022, 23, 3416. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Khanjani, S.; Saridogan, E. Endometriosis and In Vitro Fertilization. Medicina 2024, 60, 1358. [Google Scholar] [CrossRef]
- Shan, J.; Li, D.J.; Wang, X.Q. Towards a Better Understanding of Endometriosis-Related Infertility: A Review on How Endometriosis Affects Endometrial Receptivity. Biomolecules 2023, 13, 430. [Google Scholar] [CrossRef]
- Rawlings, T.M.; Makwana, K.; Taylor, D.M.; Molè, M.A.; Fishwick, K.J.; Tryfonos, M.; Odendaal, J.; Hawkes, A.; Zernicka-Goetz, M.; Hartshorne, G.M.; et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. eLife 2021, 10, e69603. [Google Scholar] [CrossRef] [PubMed]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 2017, 6, e31274. [Google Scholar] [CrossRef][Green Version]
- Deryabin, P.; Griukova, A.; Nikolsky, N.; Borodkina, A. The link between endometrial stromal cell senescence and decidualization in female fertility: The art of balance. Cell. Mol. Life Sci. 2020, 77, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Daniilidis, A.; Grigoriadis, G.; Kalaitzopoulos, D.R.; Angioni, S.; Kalkan, Ü.; Crestani, A.; Merlot, B.; Roman, H. Surgical Management of Ovarian Endometrioma: Impact on Ovarian Reserve Parameters and Reproductive Outcomes. J. Clin. Med. 2023, 12, 5324. [Google Scholar] [CrossRef]
- Agostinis, C.; Balduit, A.; Mangogna, A.; Zito, G.; Romano, F.; Ricci, G.; Kishore, U.; Bulla, R. Immunological Basis of the Endometriosis: The Complement System as a Potential Therapeutic Target. Front. Immunol. 2021, 11, 599117. [Google Scholar] [CrossRef]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.; Demir, R. Particular functions of estrogen and progesterone in establishment of uterine receptivity and embryo implantation. Histol. Histopathol. 2010, 25, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Szekeres-Barthó, J.; Kovács, G.L.; Sulyok, E.; Farkas, B.; Várnagy, Á.; Vértes, V.; Kovács, K.; Bódis, J. Key to Life: Physiological Role and Clinical Implications of Progesterone. Int. J. Mol. Sci. 2021, 22, 11039. [Google Scholar] [CrossRef]
- Chappell, P.E.; Lydon, J.P.; Conneely, O.M.; O’Malley, B.W.; Levine, J.E. Endocrine defects in mice carrying a null mutation for the progesterone receptor gene. Endocrinology 1997, 138, 4147–4152. [Google Scholar] [CrossRef][Green Version]
- Sang, Y.; Li, Y.; Xu, L.; Li, D.; Du, M. Regulatory Mechanisms of Endometrial Decidualization and Pregnancy-Related Diseases. Acta Biochim. Biophys. Sin. 2020, 52, 105–115. [Google Scholar] [CrossRef]
- Vázquez-Martínez, E.R.; Bello-Alvarez, C.; Hermenegildo-Molina, A.L.; Solís-Paredes, M.; Parra-Hernández, S.; Cruz-Orozco, O.; Silvestri-Tomassoni, J.R.; Escobar-Ponce, L.F.; Hernández-López, L.A.; Reyes-Mayoral, C.; et al. Expression of Membrane Progesterone Receptors in Eutopic and Ectopic Endometrium of Women with Endometriosis. BioMed Res. Int. 2020, 2020, 2196024. [Google Scholar] [CrossRef]
- Tamura, K.; Yoshie, M.; Kusama, K.; Tsuru, A. Mechanisms of Decidual Dysfunction and Infertility in Endometriosis: Roles of Prostaglandins and SASP. Reprod. Med. Biol. 2025, 24, e12663. [Google Scholar] [CrossRef]
- Jiang, L.; Fernando, S.R.; Kodithuwakku, S.P.; Cao, D.; Yeung, W.S.B.; Lee, K.F. Stromal cell decidualization and embryoimplantation: A vulnerable step leading tosuccessful pregnancy. Reprod. Dev. Med. 2024, 8, 101–110. [Google Scholar]
- Grinius, L.; Kessler, C.; Schroeder, J.; Handwerger, S. Forkhead transcription factor FOXO1A is critical for induction of human decidualization. J. Endocrinol. 2006, 189, 179–187. [Google Scholar] [CrossRef]
- Taylor, H.S.; Arici, A.; Olive, D.; Igarashi, P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J. Clin. Investig. 1998, 101, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kannan, A.; Das, A.; Demayo, F.J.; Hornsby, P.J.; Young, S.L.; Taylor, R.N.; Bagchi, M.K.; Bagchi, I.C. WNT4 acts downstream of BMP2 and functions via beta-catenin signaling pathway to regulate human endometrial stromal cell differentiation. Endocrinology 2013, 154, 446–457. [Google Scholar] [CrossRef]
- Essers, M.A.; de Vries-Smits, L.M.; Barker, N.; Polderman, P.E.; Burgering, B.M.; Korswagen, H.C. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 2005, 308, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Pazhohan, A.; Amidi, F.; Akbari-Asbagh, F.; Seyedrezazadeh, E.; Farzadi, L.; Khodarahmin, M.; Mehdinejadiani, S.; Sobhani, A. The Wnt/beta-catenin signaling in endometriosis, the expression of total and active forms of beta-catenin, total and inactive forms of glycogen synthase kinase-3beta, WNT7a and DICKKOPF-1. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 220, 1–5. [Google Scholar] [CrossRef]
- Tsuru, A.; Yoshie, M.; Kojima, J.; Yonekawa, R.; Azumi, M.; Kusama, K.; Nishi, H.; Tamura, K. PGRMC1 Regulates Cellular Senescence via Modulating FOXO1 Expression in Decidualizing Endometrial Stromal Cells. Biomolecules 2022, 12, 1046. [Google Scholar] [CrossRef]
- Mandal, R.; Kohoutova, K.; Petrvalska, O.; Horvath, M.; Srb, P.; Veverka, V.; Obsilova, V.; Obsil, T. FOXO4 interacts with p53 TAD and CRD and inhibits its binding to DNA. Protein Sci. 2022, 31, e4287. [Google Scholar] [CrossRef]
- Bourgeois, B.; Madl, T. Regulation of cellular senescence via the FOXO4-p53 axis. FEBS Lett. 2018, 592, 2083–2097. [Google Scholar] [CrossRef]
- Bunch, K.; Tinnemore, D.; Huff, S.; Hoffer, Z.S.; Burney, R.O.; Stallings, J.D. Expression patterns of progesterone receptor membrane components 1 and 2 in endometria from women with and without endometriosis. Reprod. Sci. 2014, 21, 190–197. [Google Scholar] [CrossRef]
- Retis-Resendiz, A.M.; Gómez-Suárez, S.K.; García-Gómez, E.; Vázquez-Martínez, E.R. Molecular Basis of Impaired Decidualization in the Eutopic Endometrium of Endometriosis Patients. Cells 2025, 14, 326. [Google Scholar] [CrossRef]
- Bulun, S.E.; Cheng, Y.H.; Pavone, M.E.; Xue, Q.; Attar, E.; Trukhacheva, E.; Tokunaga, H.; Utsunomiya, H.; Yin, P.; Luo, X.; et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin. Reprod. Med. 2010, 28, 36–43. [Google Scholar] [CrossRef]
- Inoue, T.; Kanzaki, H.; Iwai, M.; Imai, K.; Narukawa, S.; Higuchi, T.; Katsuragawa, H.; Mori, T. Tumour necrosis factor alpha inhibits in-vitro decidualization of human endometrial stromal cells. Hum. Reprod. 1994, 9, 2411–2417. [Google Scholar] [CrossRef]
- Wang, M.; Sun, F.; Zhang, S.; Zhang, X.; Sun, Y.; Yu, T.; Li, Y.; Jiang, A.; Qiao, P.; Ren, C.; et al. NEK2 promotes the development of ovarian endometriosis and impairs decidualization by phosphorylating FOXO1. Cell. Mol. Life Sci. 2024, 81, 237. [Google Scholar] [CrossRef]
- González-Ramos, R.; Van Langendonckt, A.; Defrère, S.; Lousse, J.C.; Colette, S.; Devoto, L.; Donnez, J. Involvement of the nuclear factor-kappaB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef]
- Cai, X.; Xu, M.; Zhang, H.; Zhang, M.; Wang, J.; Mei, J.; Zhang, Y.; Zhou, J.; Zhen, X.; Kang, N.; et al. Endometrial stromal PRMT5 plays a crucial role in decidualization by regulating NF-kappaB signaling in endometriosis. Cell Death Discov. 2022, 8, 408. [Google Scholar] [CrossRef]
- Madanes, D.; Bilotas, M.A.; Bastón, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I.; Ricci, A.G. PI3K/AKT pathway is altered in the endometriosis patient’s endometrium and presents differences according to severity stage. Gynecol. Endocrinol. 2020, 36, 436–440. [Google Scholar] [CrossRef]
- Yin, X.; Pavone, M.E.; Lu, Z.; Wei, J.; Kim, J.J. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J. Clin. Endocrinol. Metab. 2012, 97, E35–E43. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Shanoo, A.; Acharya, N. Endometriosis: A Comprehensive Exploration of Inflammatory Mechanisms and Fertility Implications. Cureus 2024, 16, e66128. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Endometriosis caused by retrograde menstruation: Now demonstrated by DNA evidence. Fertil. Steril. 2022, 118, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Wang, X.; Shi, Z.; Jiang, Y.; Zheng, B.; Xu, L.; Zhou, S. Understanding endometriosis from an immunomicroenvironmental perspective. Chin. Med. J. 2023, 136, 1897–1909. [Google Scholar] [CrossRef]
- Carli, C.; Metz, C.N.; Al-Abed, Y.; Naccache, P.H.; Akoum, A. Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: Involvement of novel kinase signaling pathways. Endocrinology 2009, 150, 3128–3137. [Google Scholar] [CrossRef]
- Yu, J.; Berga, S.L.; Zou, W.; Taylor, R.N. Interleukin-1β inhibits estrogen receptor-α, progesterone receptors A and B and biomarkers of human endometrial stromal cell differentiation: Implications for endometriosis. Mol. Hum. Reprod. 2019, 25, 625–637. [Google Scholar] [CrossRef]
- Lai, Z.Z.; Yang, H.L.; Ha, S.Y.; Chang, K.K.; Mei, J.; Zhou, W.J.; Qiu, X.M.; Wang, X.Q.; Zhu, R.; Li, D.J.; et al. Cyclooxygenase-2 in Endometriosis. Int. J. Biol. Sci. 2019, 15, 2783–2797. [Google Scholar] [CrossRef]
- Noble, L.S.; Takayama, K.; Zeitoun, K.M.; Putman, J.M.; Johns, D.A.; Hinshelwood, M.M.; Agarwal, V.R.; Zhao, Y.; Carr, B.R.; Bulun, S.E. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J. Clin. Endocrinol. Metab. 1997, 82, 600–606. [Google Scholar] [CrossRef]
- Laganà, A.S.; Salmeri, F.M.; Ban Frangež, H.; Ghezzi, F.; Vrtačnik-Bokal, E.; Granese, R. Evaluation of M1 and M2 macrophages in ovarian endometriomas from women affected by endometriosis at different stages of the disease. Gynecol. Endocrinol. 2020, 36, 441–444. [Google Scholar] [CrossRef]
- Lampiasi, N. Interactions between Macrophages and Mast Cells in the Female Reproductive System. Int. J. Mol. Sci. 2022, 23, 5414. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, X.; Guo, S.W. Platelets and Regulatory T Cells May Induce a Type 2 Immunity That Is Conducive to the Progression and Fibrogenesis of Endometriosis. Front. Immunol. 2020, 11, 610963. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Yamada, Y.; Kanayama, S.; Furukawa, N.; Noguchi, T.; Haruta, S.; Yoshida, S.; Sakata, M.; Sado, T.; Oi, H. The role of iron in the pathogenesis of endometriosis. Gynecol. Endocrinol. 2009, 25, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.R.; Park, J.E.; Juhn, K.M.; Ju, Y.J.; Jeong, J.; Kang, C.M.; Yun, H.J.; Yun, M.Y.; Shin, H.J.; Joo, H.Y.; et al. Cells with dysfunctional telomeres are susceptible to reactive oxygen species hydrogen peroxide via generation of multichromosomal fusions and chromosomal fragments bearing telomeres. Biochem. Biophys. Res. Commun. 2012, 417, 204–210. [Google Scholar] [CrossRef]
- Podhorecka, M.; Skladanowski, A.; Bozko, P. H2AX Phosphorylation: Its Role in DNA Damage Response and Cancer Therapy. J. Nucleic Acids. 2010, 2010, 920161. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Llopis, A.; Salvador, N.; Ercilla, A.; Guaita-Esteruelas, S.; Barrantes Idel, B.; Gupta, J.; Gaestel, M.; Davis, R.J.; Nebreda, A.R.; Agell, N. The stress-activated protein kinases p38alpha/beta and JNK1/2 cooperate with Chk1 to inhibit mitotic entry upon DNA replication arrest. Cell Cycle 2012, 11, 3627–3637. [Google Scholar] [CrossRef]
- Huang, C.; Ma, W.Y.; Maxiner, A.; Sun, Y.; Dong, Z. p38 kinase mediates UV-induced phosphorylation of p53 protein at serine 389. J. Biol. Chem. 1999, 274, 12229–12235. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Lv, L.; Liu, C.; Wang, L.; Sun, Y.N.; Zhao, Z.; Shi, B.; Li, Q.; Hao, G.M. Molecular regulation of DNA damage and repair in female infertility: A systematic review. Reprod. Biol. Endocrinol. 2024, 22, 103. [Google Scholar] [CrossRef]
- Li, Y.; Lv, X.; Jiang, M.; Jin, Z. Sitagliptin ameliorates hypoxia-induced damages in endometrial stromal cells: An implication in endometriosis. Bioengineered 2022, 13, 800–809. [Google Scholar] [CrossRef]
- Leitao, B.; Jones, M.C.; Fusi, L.; Higham, J.; Lee, Y.; Takano, M.; Goto, T.; Christian, M.; Lam, E.W.; Brosens, J.J. Silencing of the JNK pathway maintains progesterone receptor activity in decidualizing human endometrial stromal cells exposed to oxidative stress signals. FASEB J. 2010, 24, 1541–1551. [Google Scholar] [CrossRef]
- Kajihara, T.; Jones, M.; Fusi, L.; Takano, M.; Feroze-Zaidi, F.; Pirianov, G.; Mehmet, H.; Ishihara, O.; Higham, J.M.; Lam, E.W.; et al. Differential expression of FOXO1 and FOXO3a confers resistance to oxidative cell death upon endometrial decidualization. Mol. Endocrinol. 2006, 20, 2444–2455. [Google Scholar] [CrossRef]
- Ruan, J.; Tian, Q.; Li, S.; Zhou, X.; Sun, Q.; Wang, Y.; Xiao, Y.; Li, M.; Chang, K.; Yi, X. The IL-33-ST2 axis plays a vital role in endometriosis via promoting epithelial-mesenchymal transition by phosphorylating beta-catenin. Cell Commun. Signal. 2024, 22, 318. [Google Scholar] [CrossRef]
- Yang, Y.M.; Yang, W.X. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017, 8, 41679–41689. [Google Scholar] [CrossRef]
- Marquardt, R.M.; Tran, D.N.; Lessey, B.A.; Rahman, M.S.; Jeong, J.W. Epigenetic Dysregulation in Endometriosis: Implications for Pathophysiology and Therapeutics. Endocr. Rev. 2023, 44, 1074–1095. [Google Scholar] [CrossRef]
- Ito, F.; Yamada, Y.; Shigemitsu, A.; Akinishi, M.; Kaniwa, H.; Miyake, R.; Yamanaka, S.; Kobayashi, H. Role of Oxidative Stress in Epigenetic Modification in Endometriosis. Reprod. Sci. 2017, 24, 1493–1502. [Google Scholar] [CrossRef]
- Andrade, S.S.; Azevedo Ade, C.; Monasterio, I.C.; Paredes-Gamero, E.J.; Gonçalves, G.A.; Bonetti, T.C.; Albertoni, G.; Schor, E.; Barreto, J.A.; Luiza Oliva, M.; et al. 17beta-Estradiol and steady-state concentrations of H2O2: Antiapoptotic effect in endometrial cells from patients with endometriosis. Free Radic. Biol. Med. 2013, 60, 63–72. [Google Scholar] [CrossRef]
- Assaf, L.; Eid, A.A.; Nassif, J. Role of AMPK/mTOR, mitochondria, and ROS in the pathogenesis of endometriosis. Life Sci. 2022, 306, 120805. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, H.; Cestari, B.A.; Meola, J.; Podgaec, S. Higher Oxidative Stress in Endometriotic Lesions Upregulates Senescence-Associated p16ink4a and beta-Galactosidase in Stromal Cells. Int. J. Mol. Sci. 2023, 24, 914. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Imanaka, S. Recent progress in metabolomics for analyzing common infertility conditions that affect ovarian function. Reprod. Med. Biol. 2024, 23, e12609. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Matsubara, S.; Yoshimoto, C.; Shigetomi, H.; Imanaka, S. The role of mitochondrial dynamics in the pathophysiology of endometriosis. J. Obstet. Gynaecol. Res. 2023, 49, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Toniyan, K.A.; Malkov, A.A.; Biryukov, N.S.; Gorbacheva, E.Y.; Boyarintsev, V.V.; Ogneva, I.V. The Cellular Respiration of Endometrial Biopsies from Patients with Various Forms of Endometriosis. Int. J. Mol. Sci. 2024, 25, 3680. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Qiao, P.; Fu, R.; Wang, Y.; Lu, J.; Ling, X.; Liu, L.; Sun, Y.; Ren, C.; Yu, Z. Phosphorylation of PFKFB4 by PIM2 promotes anaerobic glycolysis and cell proliferation in endometriosis. Cell Death Dis. 2022, 13, 790, Correction in Cell Death Dis., 2024, 15, 469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiu, J.; Yang, T.; Ren, C.; Yu, Z. HSF1 promotes endometriosis development and glycolysis by up-regulating PFKFB3 expression. Reprod. Biol. Endocrinol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Atkins, H.M.; Bharadwaj, M.S.; O’Brien Cox, A.; Furdui, C.M.; Appt, S.E.; Caudell, D.L. Endometrium and endometriosis tissue mitochondrial energy metabolism in a nonhuman primate model. Reprod. Biol. Endocrinol. 2019, 17, 70. [Google Scholar] [CrossRef]
- Banerjee, S.; Xu, W.; Doctor, A.; Driss, A.; Nezhat, C.; Sidell, N.; Taylor, R.N.; Thompson, W.E.; Chowdhury, I. TNFalpha-Induced Altered miRNA Expression Links to NF-kappaB Signaling Pathway in Endometriosis. Inflammation 2023, 46, 2055–2070. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Pouly, J.L.; Canis, M. Persistent activation of signal transducer and activator of transcription 3 via interleukin-6 trans-signaling is involved in fibrosis of endometriosis. Hum. Reprod. 2022, 37, 1489–1504. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-kappaB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Mauro, C.; Leow, S.C.; Anso, E.; Rocha, S.; Thotakura, A.K.; Tornatore, L.; Moretti, M.; De Smaele, E.; Beg, A.A.; Tergaonkar, V.; et al. NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat. Cell Biol. 2011, 13, 1272–1279. [Google Scholar] [CrossRef]
- Londhe, P.; Yu, P.Y.; Ijiri, Y.; Ladner, K.J.; Fenger, J.M.; London, C.; Houghton, P.J.; Guttridge, D.C. Classical NF-kappaB Metabolically Reprograms Sarcoma Cells Through Regulation of Hexokinase 2. Front. Oncol. 2018, 8, 104. [Google Scholar] [CrossRef]
- Yang, J.G.; Wang, W.M.; Xia, H.F.; Yu, Z.L.; Li, H.M.; Ren, J.G.; Chen, G.; Wang, B.K.; Jia, J.; Zhang, W.; et al. Lymphotoxin-alpha promotes tumor angiogenesis in HNSCC by modulating glycolysis in a PFKFB3-dependent manner. Int. J. Cancer 2019, 145, 1358–1370. [Google Scholar] [CrossRef]
- Chen, J.; Qin, P.; Sun, Y.; Han, S. Histone lactylation promotes cell proliferation, migration and invasion through targeting HMGB1 in endometriosis. J. Biomed. Res. 2023, 37, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, J.; Xu, Z.; Li, M.; Dong, X.; Du, Y.; Xu, Z.; Yan, L. Highly expressed lncRNA H19 in endometriosis promotes aerobic glycolysis and histone lactylation. Reproduction 2024, 168, e240018. [Google Scholar] [CrossRef]
- Deryabin, P.I.; Borodkina, A.V. Stromal cell senescence contributes to impaired endometrial decidualization and defective interaction with trophoblast cells. Hum. Reprod. 2022, 37, 1505–1524. [Google Scholar] [CrossRef]
- Sang, L.; Fang, Q.J.; Zhao, X.B. A research on the protein expression of p53, p16, and MDM2 in endometriosis. Medicine 2019, 98, e14776. [Google Scholar] [CrossRef]
- Delenko, J.; Xue, X.; Chatterjee, P.K.; Hyman, N.; Shih, A.J.; Adelson, R.P.; Safaric Tepes, P.; Gregersen, P.K.; Metz, C.N. Quercetin enhances decidualization through AKT-ERK-p53 signaling and supports a role for senescence in endometriosis. Reprod. Biol. Endocrinol. 2024, 22, 100. [Google Scholar] [CrossRef]
- Malvezzi, H.; Dobo, C.; Filippi, R.Z.; Nascimento, H.M.D.; Sousa, L.P.d.S.e.; Meola, J.; Piccinato, C.A.; Podgaec, S. Altered p16(Ink4a), IL-1beta, and Lamin b1 Protein Expression Suggest Cellular Senescence in Deep Endometriotic Lesions. Int. J. Mol. Sci. 2022, 23, 2476. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Yoshimoto, C.; Shigetomi, H.; Kobayashi, H. Oxidative Stress and Antioxidant Defense in Endometriosis and Its Malignant Transformation. Oxidative Med. Cell. Longev. 2015, 2015, 848595. [Google Scholar] [CrossRef]
- Nousis, L.; Kanavaros, P.; Barbouti, A. Oxidative Stress-Induced Cellular Senescence: Is Labile Iron the Connecting Link? Antioxidants 2023, 12, 1250. [Google Scholar] [CrossRef]
- Taylor, R.N.; Berga, S.L.; Zou, E.; Washington, J.; Song, S.; Marzullo, B.J.; Bagchi, I.C.; Bagchi, M.K.; Yu, J. Interleukin-1beta induces and accelerates human endometrial stromal cell senescence and impairs decidualization via the c-Jun N-terminal kinase pathway. Cell Death Discov. 2024, 10, 288. [Google Scholar] [CrossRef]
- Kusama, K.; Yamauchi, N.; Yoshida, K.; Azumi, M.; Yoshie, M.; Tamura, K. Senolytic treatment modulates decidualization in human endometrial stromal cells. Biochem. Biophys. Res. Commun. 2021, 571, 174–180. [Google Scholar] [CrossRef]
- Harada, M. Cellular senescence in the pathogenesis of ovarian dysfunction. J. Obstet. Gynaecol. Res. 2024, 50, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.J.; Adelson, R.P.; Vashistha, H.; Khalili, H.; Nayyar, A.; Puran, R.; Herrera, R.; Chatterjee, P.K.; Lee, A.T.; Truskinovsky, A.M.; et al. Single-cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis. BMC Med. 2022, 20, 315. [Google Scholar] [CrossRef] [PubMed]
- Thiruchelvam, U.; Wingfield, M.; O’Farrelly, C. Natural Killer Cells: Key Players in Endometriosis. Am. J. Reprod. Immunol. 2015, 74, 291–301. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Moini, A.; Hosseini, R.; Fatehnejad, M.; Yekaninejad, M.S.; Javidan, M.; Changaei, M.; Feizisani, F.; Rajaei, S. A higher number of exhausted local PD1+, but not TIM3+, NK cells in advanced endometriosis. Heliyon 2023, 10, e23294. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Li, D.; Li, M. An Estrogen-NK Cells Regulatory Axis in Endometriosis, Related Infertility, and Miscarriage. Int. J. Mol. Sci. 2024, 25, 3362. [Google Scholar] [CrossRef]
- Lin, X.; Dai, Y.; Tong, X.; Xu, W.; Huang, Q.; Jin, X.; Li, C.; Zhou, F.; Zhou, H.; Lin, X.; et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox Biol. 2020, 30, 101431. [Google Scholar] [CrossRef]
- Palmieri, L.; Malvezzi, H.; Cestari, B.; Podgaec, S. Colocalization of senescent biomarkers in deep, superficial, and ovarian endometriotic lesions: A pilot study. Sci. Rep. 2022, 12, 17280. [Google Scholar] [CrossRef] [PubMed]
- Sonehara, R.; Nakamura, T.; Takeda, T.; Kaseki, S.; Seki, T.; Tanaka, H.; Yabuki, A.; Miyake, N.; Muraoka, A.; Osuka, S.; et al. A novel senotherapeutic strategy with azithromycin for preventing endometriosis progression. Reprod. Biol. Endocrinol. 2025, 23, 47. [Google Scholar] [CrossRef]
- Delbandi, A.A.; Mahmoudi, M.; Shervin, A.; Heidari, S.; Kolahdouz-Mohammadi, R.; Zarnani, A.H. Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls. BMC Women’s Health 2020, 20, 3. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, X. An Update on the Multifaceted Role of NF-kappaB in Endometriosis. Int. J. Biol. Sci. 2022, 18, 4400–4413. [Google Scholar] [CrossRef] [PubMed]
- Nasu, K.; Nishida, M.; Kawano, Y.; Tsuno, A.; Abe, W.; Yuge, A.; Takai, N.; Narahara, H. Aberrant expression of apoptosis-related molecules in endometriosis: A possible mechanism underlying the pathogenesis of endometriosis. Reprod. Sci. 2011, 18, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Imanaka, S.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H. Molecular mechanism of autophagy and apoptosis in endometriosis: Current understanding and future research directions. Reprod. Med. Biol. 2024, 23, e12577. [Google Scholar] [CrossRef]
- Harrath, A.H.; Rahman, M.A.; Bhajan, S.K.; Bishwas, A.K.; Rahman, M.D.H.; Alwasel, S.; Jalouli, M.; Kang, S.; Park, M.N.; Kim, B. Autophagy and Female Fertility: Mechanisms, Clinical Implications, and Emerging Therapies. Cells 2024, 13, 1354. [Google Scholar] [CrossRef]
- Pizzimenti, C.; Fiorentino, V.; Ruggeri, C.; Franchina, M.; Ercoli, A.; Tuccari, G.; Ieni, A. Autophagy Involvement in Non-Neoplastic and Neoplastic Endometrial Pathology: The State of the Art with a Focus on Carcinoma. Int. J. Mol. Sci. 2024, 25, 12118. [Google Scholar] [CrossRef]
- Adel, N.; Abdulghaffar, S.; Elmahdy, M.; Nabil, M.; Ghareeb, D.; Maghraby, H. Autophagy-related gene and protein expressions during blastocyst development. J. Assist. Reprod. Genet. 2023, 40, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef]
- Ling, N.X.Y.; Kaczmarek, A.; Hoque, A.; Davie, E.; Ngoei, K.R.W.; Morrison, K.R.; Smiles, W.J.; Forte, G.M.; Wang, T.; Lie, S.; et al. mTORC1 directly inhibits AMPK to promote cell proliferation under nutrient stress. Nat. Metab. 2020, 2, 41–49. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Russell, R.C.; Tian, Y.; Yuan, H.; Park, H.W.; Chang, Y.Y.; Kim, J.; Kim, H.; Neufeld, T.P.; Dillin, A.; Guan, K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013, 15, 741–750. [Google Scholar] [CrossRef]
- Jung, C.H.; Jun, C.B.; Ro, S.H.; Kim, Y.M.; Otto, N.M.; Cao, J.; Kundu, M.; Kim, D.H. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell 2009, 20, 1992–2003. [Google Scholar] [CrossRef]
- Stambolic, V.; MacPherson, D.; Sas, D.; Lin, Y.; Snow, B.; Jang, Y.; Benchimol, S.; Mak, T.W. Regulation of PTEN transcription by p53. Mol. Cell 2001, 8, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Chibaya, L.; Karim, B.; Zhang, H.; Jones, S.N. Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2003193118. [Google Scholar] [CrossRef]
- Tonnessen-Murray, C.A.; Lozano, G.; Jackson, J.G. The Regulation of Cellular Functions by the p53 Protein: Cellular Senescence. Cold Spring Harb. Perspect. Med. 2017, 7, a026112. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef]
- Guan, G.; Chen, Y.; Dong, Y. Unraveling the AMPK-SIRT1-FOXO Pathway: The In-Depth Analysis and Breakthrough Prospects of Oxidative Stress-Induced Diseases. Antioxidants 2025, 14, 70. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Shen, H.H.; Zhang, T.; Yang, H.L.; Lai, Z.Z.; Zhou, W.J.; Mei, J.; Shi, J.W.; Zhu, R.; Xu, F.Y.; Li, D.J.; et al. Ovarian hormones-autophagy-immunity axis in menstruation and endometriosis. Theranostics 2021, 11, 3512–3526. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, V.; Ciccarese, F.; Ciminale, V. Oncogenic pathways and the electron transport chain: A dangeROS liaison. Br. J. Cancer 2020, 122, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.X.; Wei, L.H.; Tu, Z.; Sun, P.M.; Wang, J.L.; Zhao, D.; Li, X.P.; Tang, J.M. 17 beta-estradiol activates PI3K/Akt signaling pathway by estrogen receptor (ER)-dependent and ER-independent mechanisms in endometrial cancer cells. J. Steroid Biochem. Mol. Biol. 2006, 99, 9–18. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. Kinases meet at TSC. Cell Res. 2007, 17, 971–973. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Luo, T.; Shi, H. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy. Mol. Biomed. 2022, 3, 47. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kwon, Y.G.; Kim, Y.M. The stress-responsive protein REDD1 and its pathophysiological functions. Exp. Mol. Med. 2023, 55, 1933–1944. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A. Molecular Interactions Between Reactive Oxygen Species and Autophagy in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3791. [Google Scholar] [CrossRef]
- Lin, Y.K.; Li, Y.Y.; Li, Y.; Li, D.J.; Wang, X.L.; Wang, L.; Yu, M.; Zhu, Y.Z.; Cheng, J.J.; Du, M.R. SCM-198 Prevents Endometriosis by Reversing Low Autophagy of Endometrial Stromal Cell via Balancing ERalpha and PR Signals. Front. Endocrinol. 2022, 13, 858176. [Google Scholar] [CrossRef]
- Yu, J.; Henske, E.P. Estrogen-induced activation of mammalian target of rapamycin is mediated via tuberin and the small GTPase Ras homologue enriched in brain. Cancer Res. 2006, 66, 9461–9466. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.C.; Rhodes, L.V.; Elliott, S.; Krebs, A.E.; Nephew, K.P.; Flemington, E.K.; Collins-Burow, B.M.; Burow, M.E. microRNA regulation of mammalian target of rapamycin expression and activity controls estrogen receptor function and RAD001 sensitivity. Mol. Cancer 2014, 13, 229. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. Estrogen biology: New insights into GPER function and clinical opportunities. Mol. Cell Endocrinol. 2014, 389, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Zdrojkowski, Ł.; Jasiński, T.; Ferreira-Dias, G.; Pawliński, B.; Domino, M. The Role of NF-kappaB in Endometrial Diseases in Humans and Animals: A Review. Int. J. Mol. Sci. 2023, 24, 2901. [Google Scholar] [CrossRef]
- Hung, S.W.; Zhang, R.; Tan, Z.; Chung, J.P.W.; Zhang, T.; Wang, C.C. Pharmaceuticals targeting signaling pathways of endometriosis as potential new medical treatment: A review. Med. Res. Rev. 2021, 41, 2489–2564. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shao, X. Nobiletin alleviates endometriosis via down-regulating NF-kappaB activity in endometriosis mouse model. Biosci. Rep. 2018, 38, BSR20180470. [Google Scholar] [CrossRef]
- Mei, J.; Zhu, X.Y.; Jin, L.P.; Duan, Z.L.; Li, D.J.; Li, M.Q. Estrogen promotes the survival of human secretory phase endometrial stromal cells via CXCL12/CXCR4 up-regulation-mediated autophagy inhibition. Hum. Reprod. 2015, 30, 1677–1689. [Google Scholar] [CrossRef]
- Kim, S.I.; Yeo, S.G.; Gen, Y.; Ju, H.R.; Kim, S.H.; Park, D.C. Differences in autophagy-associated mRNAs in peritoneal fluid of patients with endometriosis and gynecologic cancers. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 2, 100016. [Google Scholar] [CrossRef]
- Zhan, L.; Li, J.; Wei, B. Autophagy in endometriosis: Friend or foe? Biochem. Biophys. Res. Commun. 2018, 495, 60–63. [Google Scholar] [CrossRef]
- Kong, Z.; Yao, T. Role for autophagy-related markers Beclin-1 and LC3 in endometriosis. BMC Women’s Health 2022, 22, 264. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jo, M.; Lee, E.; Lee, D.Y.; Choi, D. Dienogest enhances autophagy induction in endometriotic cells by impairing activation of AKT, ERK1/2, and mTOR. Fertil. Steril. 2015, 104, 655–664.e1. [Google Scholar] [CrossRef]
- Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Salinaro, A.T.; Raffone, E.; Genovese, T.; et al. Autophagy and Mitophagy Promotion in a Rat Model of Endometriosis. Int. J. Mol. Sci. 2021, 22, 5074. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Xiong, W.; Zhang, L.; Xiong, Y.; Li, N.; He, H.; Du, Y.; Liu, Y. Hypoxia-inducible factor-1alpha promotes endometrial stromal cells migration and invasion by upregulating autophagy in endometriosis. Reproduction 2017, 153, 809–820. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Xiong, W.; He, H.; Fu, T.; Long, X.; Li, X.; Liang, J.; Ding, H.; Xu, Y.; et al. CircFOXO3 mediates hypoxia-induced autophagy of endometrial stromal cells in endometriosis. FASEB J. 2024, 38, e23515. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Interdonato, L.; Marino, Y.; Crupi, R.; Gugliandolo, E.; Macrì, F.; Di Paola, D.; et al. Complex Interplay between Autophagy and Oxidative Stress in the Development of Endometriosis. Antioxidants 2022, 11, 2484. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imanaka, S.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H. Role of autophagy and ferroptosis in the development of endometriotic cysts (Review). Int. J. Mol. Med. 2024, 54, 78. [Google Scholar] [CrossRef]
- Dong, X.; Xu, L.; Wang, S.; Jiao, X.; Yan, S.; Huang, Y.; Yuan, M.; Wang, G. Endometrial stromal cell autophagy-dependent ferroptosis caused by iron overload in ovarian endometriosis is inhibited by the ATF4-xCT pathway. Mol. Hum. Reprod. 2023, 30, gaad046. [Google Scholar] [CrossRef]
- Kobayashi, H.; Nishio, M.; Umetani, M.; Shigetomi, H.; Imanaka, S.; Hashimoto, H. Endometrial Aging and Reproductive Decline: The Central Role of Mitochondrial Dysfunction. Int. J. Mol. Sci. 2025, 26, 5060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xue, Z.; He, H.N.; Liu, X.; Yin, S.Y.; Wu, D.Y.; Zhang, X.; Schatten, H.; Miao, Y.L. Resveratrol delays postovulatory aging of mouse oocytes through activating mitophagy. Aging 2019, 11, 11504–11519. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.H.; Yu, H.Y.; Wang, P.; Mao, G.K.; Liu, W.X.; Li, M.N.; Wang, H.N.; Shang, Y.L.; Liu, C.; Xu, Z.L.; et al. Germ cell-specific Atg7 knockout results in primary ovarian insufficiency in female mice. Cell Death Dis. 2015, 6, e1589. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.Z.; Ouyang, Y.C.; Gao, S.C.; Gu, L.J.; Guo, J.N.; Sun, S.M.; Wang, Z.B.; Sun, Q.Y. Protein phosphatase 4 maintains the survival of primordial follicles by regulating autophagy in oocytes. Cell Death Dis. 2024, 15, 658. [Google Scholar] [CrossRef]
- Goh, H.H.; Ratnam, S.S. The LH surge in humans: Its mechanism and sex difference. Gynecol. Endocrinol. 1988, 2, 165–182. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, Q. Role of mTOR Signaling in Female Reproduction. Front. Endocrinol. 2019, 10, 692. [Google Scholar] [CrossRef]
- Wu, Y.; Strawn, E.; Basir, Z.; Halverson, G.; Guo, S.W. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 2006, 1, 106–111. [Google Scholar] [CrossRef]
- Attia, G.R.; Zeitoun, K.; Edwards, D.; Johns, A.; Carr, B.R.; Bulun, S.E. Progesterone receptor isoform A but not B is expressed in endometriosis. J. Clin. Endocrinol. Metab. 2000, 85, 2897–28902. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Christian, M.; Quenby, S.; Brosens, J.J. Expression of epigenetic effectors in decidualizing human endometrial stromal cells. Mol. Hum. Reprod. 2012, 18, 451–458. [Google Scholar] [CrossRef]
- Wu, Y.; Halverson, G.; Basir, Z.; Strawn, E.; Yan, P.; Guo, S.W. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am. J. Obstet. Gynecol. 2005, 193, 371–380. [Google Scholar] [CrossRef]
- Lin, X.; Dai, Y.; Gu, W.; Zhang, Y.; Zhuo, F.; Zhao, F.; Jin, X.; Li, C.; Huang, D.; Tong, X.; et al. The involvement of RNA N6-methyladenosine and histone methylation modification in decidualization and endometriosis-associated infertility. Clin. Transl. Med. 2024, 14, e1564. [Google Scholar] [CrossRef]
- Buzzio, O.L.; Lu, Z.; Miller, C.D.; Unterman, T.G.; Kim, J.J. FOXO1A differentially regulates genes of decidualization. Endocrinology 2006, 147, 3870–3876. [Google Scholar] [CrossRef]
- Rezk, N.A.; Lashin, M.B.; Sabbah, N.A. MiRNA 34-a regulate SIRT-1 and Foxo-1 expression in endometriosis. Non-Coding RNA Res. 2021, 6, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, J.; Long, X.; Weng, R.; Xiong, W.; Liang, J.; Liu, J.; Sun, J.; Cai, X.; Zhang, L.; et al. METTL3-regulated m6A modification impairs the decidualization of endometrial stromal cells by regulating YTHDF2-mediated degradation of FOXO1 mRNA in endometriosis-related infertility. Reprod. Biol. Endocrinol. 2023, 21, 99. [Google Scholar] [CrossRef]
- Su, R.W.; Strug, M.R.; Joshi, N.R.; Jeong, J.W.; Miele, L.; Lessey, B.A.; Young, S.L.; Fazleabas, A.T. Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J. Clin. Endocrinol. Metab. 2015, 100, E433–E442. [Google Scholar] [CrossRef]
- Yamagata, Y.; Asada, H.; Tamura, I.; Lee, L.; Maekawa, R.; Taniguchi, K.; Taketani, T.; Matsuoka, A.; Tamura, H.; Sugino, N. DNA methyltransferase expression in the human endometrium: Down-regulation by progesterone and estrogen. Hum. Reprod. 2009, 24, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- van Kaam, K.J.; Delvoux, B.; Romano, A.; D’Hooghe, T.; Dunselman, G.A.; Groothuis, P.G. Deoxyribonucleic acid methyltransferases and methyl-CpG-binding domain proteins in human endometrium and endometriosis. Fertil. Steril. 2011, 95, 1421–1427. [Google Scholar] [CrossRef]
- Wu, Y.; Strawn, E.; Basir, Z.; Halverson, G.; Guo, S.W. Aberrant expression of deoxyribonucleic acid methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertil. Steril. 2007, 87, 24–32. [Google Scholar] [CrossRef]
- Pei, T.; Liu, C.; Liu, T.; Xiao, L.; Luo, B.; Tan, J.; Li, X.; Zhou, G.; Duan, C.; Huang, W. miR-194-3p Represses the Progesterone Receptor and Decidualization in Eutopic Endometrium from Women With Endometriosis. Endocrinology 2018, 159, 2554–2562. [Google Scholar] [CrossRef]
- Yu, S.L.; Jeong, D.U.; Kang, Y.; Kim, T.H.; Lee, S.K.; Han, A.R.; Kang, J.; Park, S.R. Impairment of Decidualization of Endometrial Stromal Cells by hsa-miR-375 Through NOX4 Targeting. Reprod. Sci. 2022, 29, 3212–3221. [Google Scholar] [CrossRef]
- Joshi, N.R.; Miyadahira, E.H.; Afshar, Y.; Jeong, J.W.; Young, S.L.; Lessey, B.A.; Serafini, P.C.; Fazleabas, A.T. Progesterone Resistance in Endometriosis Is Modulated by the Altered Expression of MicroRNA-29c and FKBP4. J. Clin. Endocrinol. Metab. 2017, 102, 141–149. [Google Scholar] [CrossRef]
- Zhou, M.; Fu, J.; Xiao, L.; Yang, S.; Song, Y.; Zhang, X.; Feng, X.; Sun, H.; Xu, W.; Huang, W. miR-196a overexpression activates the MEK/ERK signal and represses the progesterone receptor and decidualization in eutopic endometrium from women with endometriosis. Hum. Reprod. 2016, 31, 2598–2608. [Google Scholar] [CrossRef]
- Szukiewicz, D.; Stangret, A.; Ruiz-Ruiz, C.; Olivares, E.G.; Soriţău, O.; Suşman, S.; Szewczyk, G. Estrogen- and Progesterone (P4)-Mediated Epigenetic Modifications of Endometrial Stromal Cells (EnSCs) and/or Mesenchymal Stem/Stromal Cells (MSCs) in the Etiopathogenesis of Endometriosis. Stem Cell Rev. Rep. 2021, 17, 1174–1193. [Google Scholar] [CrossRef]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.M.; Shi, J.W.; Wang, L.; Li, M.Q. NK cells: Shielding senescence homeostasis in the decidua during early pregnancy. Semin. Immunopathol. 2025, 47, 22. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kawahara, N.; Ogawa, K.; Yoshimoto, C. A Relationship Between Endometriosis and Obstetric Complications. Reprod. Sci. 2020, 27, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, G. Progesterone Resistance in Endometriosis: Current Evidence and Putative Mechanisms. Int. J. Mol. Sci. 2023, 24, 6992. [Google Scholar] [CrossRef]
- Poh, Q.H.; Rai, A.; Salamonsen, L.A.; Greening, D.W. Omics insights into extracellular vesicles in embryo implantation and their therapeutic utility. Proteomics 2023, 23, e2200107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lian, Y.; Jiang, J.; Wang, L.; Ren, L.; Li, Y.; Yan, X.; Chen, Q. Differential expression of microRNA in exosomes derived from endometrial stromal cells of women with endometriosis-associated infertility. Reprod. Biomed. Online 2020, 41, 170–181. [Google Scholar] [CrossRef]
- Fan, J.; Chen, C.; Zhong, Y. A cohort study on IVF outcomes in infertile endometriosis patients: The effects of rapamycin treatment. Reprod. Biomed. Online 2024, 48, 103319. [Google Scholar] [CrossRef]
- Arriola Apelo, S.I.; Lamming, D.W. Rapamycin: An InhibiTOR of Aging Emerges from the Soil of Easter Island. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 841–849. [Google Scholar] [CrossRef]
- Peters, A.E.; Mihalas, B.P.; Bromfield, E.G.; Roman, S.D.; Nixon, B.; Sutherland, J.M. Autophagy in female fertility: A role in oxidative stress and aging. Antioxid. Redox Signal. 2020, 32, 550–568. [Google Scholar] [CrossRef]
- Li, D.; You, Y.; Bi, F.F.; Zhang, T.N.; Jiao, J.; Wang, T.R.; Zhou, Y.M.; Shen, Z.Q.; Wang, X.X.; Yang, Q. Autophagy is activated in the ovarian tissue of polycystic ovary syndrome. Reproduction 2018, 155, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dou, Q.; Zhang, D.; Xiang, Y.; Tan, L. Melatonin regulates autophagy in granulosa cells from patients with premature ovarian insufficiency via activating Foxo3a. Aging 2024, 16, 844. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Aoki, A.; Kusabiraki, T.; Cheng, S.B.; Sharma, S.; Saito, S. Autophagy regulation in preeclampsia: Pros and cons. J. Reprod. Immunol. 2017, 123, 17–23. [Google Scholar] [CrossRef]
| Search Mode | The Keyword and Search Term Combinations |
|---|---|
| Search term 1 | p53 |
| Search term 2 | AMPK |
| Search term 3 | mTOR OR mTORC1 OR mTORC2 |
| Search term 4 | Endometriosis OR Ectopic endometrium |
| Search term 5 | Cellular senescence |
| Search term 6 | Decidualization |
| Search | (Search term 1 OR 2 OR 3) AND Search term 4 |
| (Search term 1 OR 2 OR 3) AND Search term 5 | |
| (Search term 1 OR 2 OR 3) AND Search term 6 | |
| (Search term 1 OR 2 OR 3) AND Search term 4 AND (Search term 5 OR Search term 6) | |
| Search term 4 AND Search term 5 | |
| Search term 4 AND Search term 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigetomi, H.; Nishio, M.; Umetani, M.; Imanaka, S.; Hashimoto, H.; Kobayashi, H. Balancing Decidualization, Autophagy, and Cellular Senescence for Reproductive Success in Endometriosis Biology. Int. J. Mol. Sci. 2025, 26, 9125. https://doi.org/10.3390/ijms26189125
Shigetomi H, Nishio M, Umetani M, Imanaka S, Hashimoto H, Kobayashi H. Balancing Decidualization, Autophagy, and Cellular Senescence for Reproductive Success in Endometriosis Biology. International Journal of Molecular Sciences. 2025; 26(18):9125. https://doi.org/10.3390/ijms26189125
Chicago/Turabian StyleShigetomi, Hiroshi, Miki Nishio, Mai Umetani, Shogo Imanaka, Hiratsugu Hashimoto, and Hiroshi Kobayashi. 2025. "Balancing Decidualization, Autophagy, and Cellular Senescence for Reproductive Success in Endometriosis Biology" International Journal of Molecular Sciences 26, no. 18: 9125. https://doi.org/10.3390/ijms26189125
APA StyleShigetomi, H., Nishio, M., Umetani, M., Imanaka, S., Hashimoto, H., & Kobayashi, H. (2025). Balancing Decidualization, Autophagy, and Cellular Senescence for Reproductive Success in Endometriosis Biology. International Journal of Molecular Sciences, 26(18), 9125. https://doi.org/10.3390/ijms26189125






