Shared Genetic Architecture Between Atopic Dermatitis and Autoimmune Diseases
Abstract
1. Introduction
2. Results
2.1. Genetic Correlations Between Atopic Dermatitis and Autoimmune Disorders
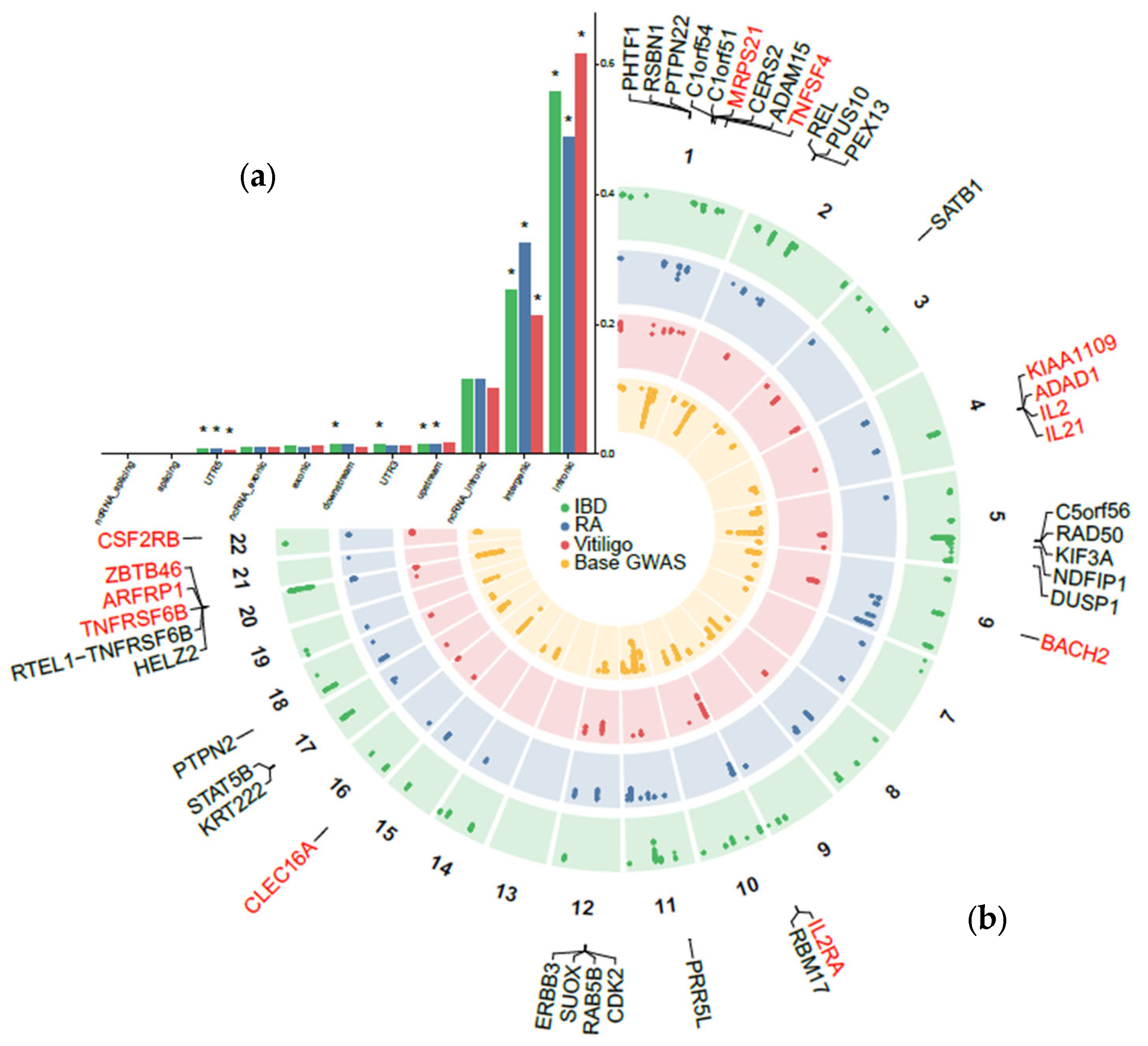
2.2. Pleiotropic Loci Between Atopic Dermatitis and Autoimmune Disorders
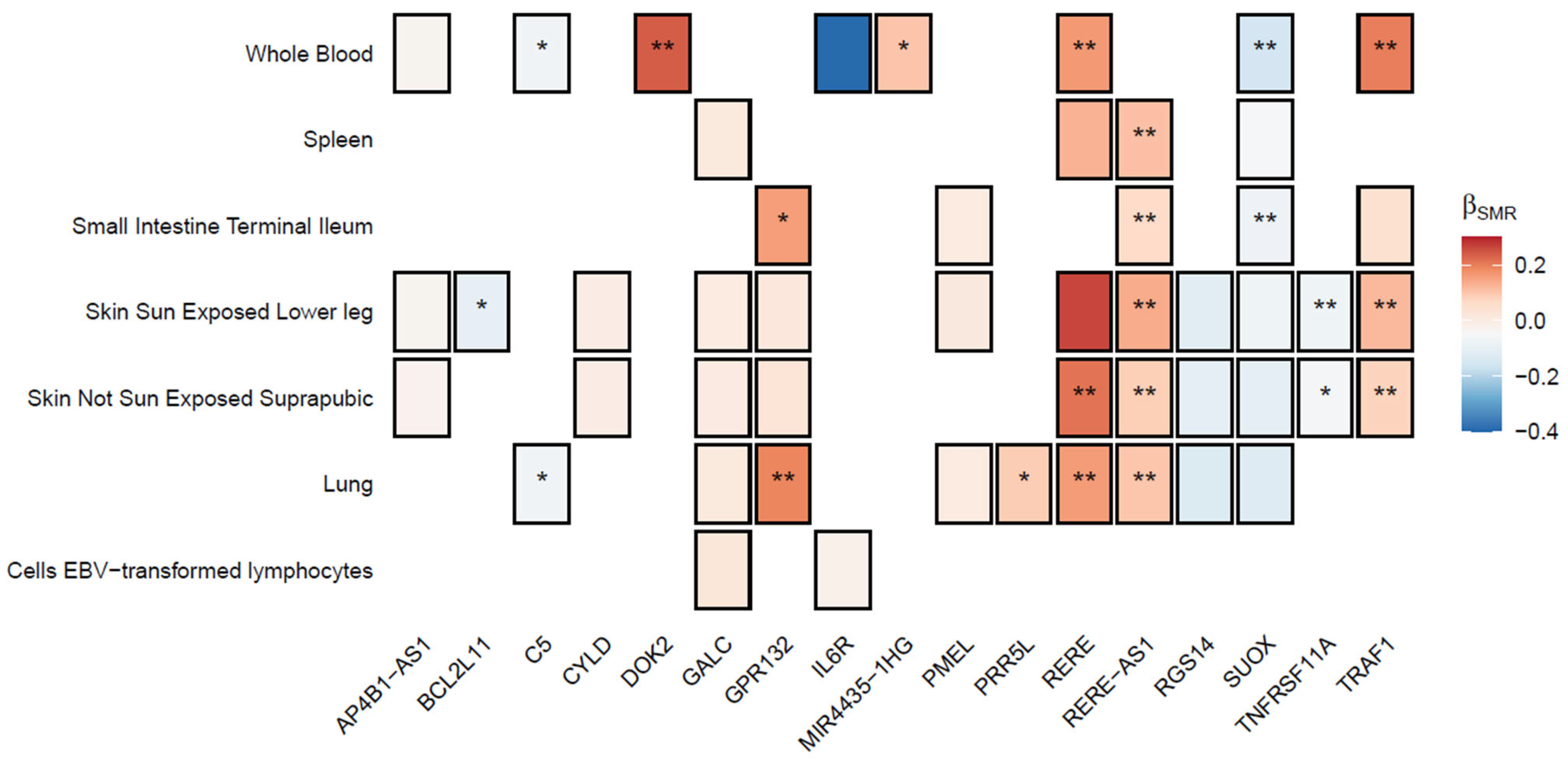
2.3. Prioritization of Candidate Pleiotropic Genes and Pathways with Tissue Specificity
2.4. Multi-Trait Colocalization Analysis
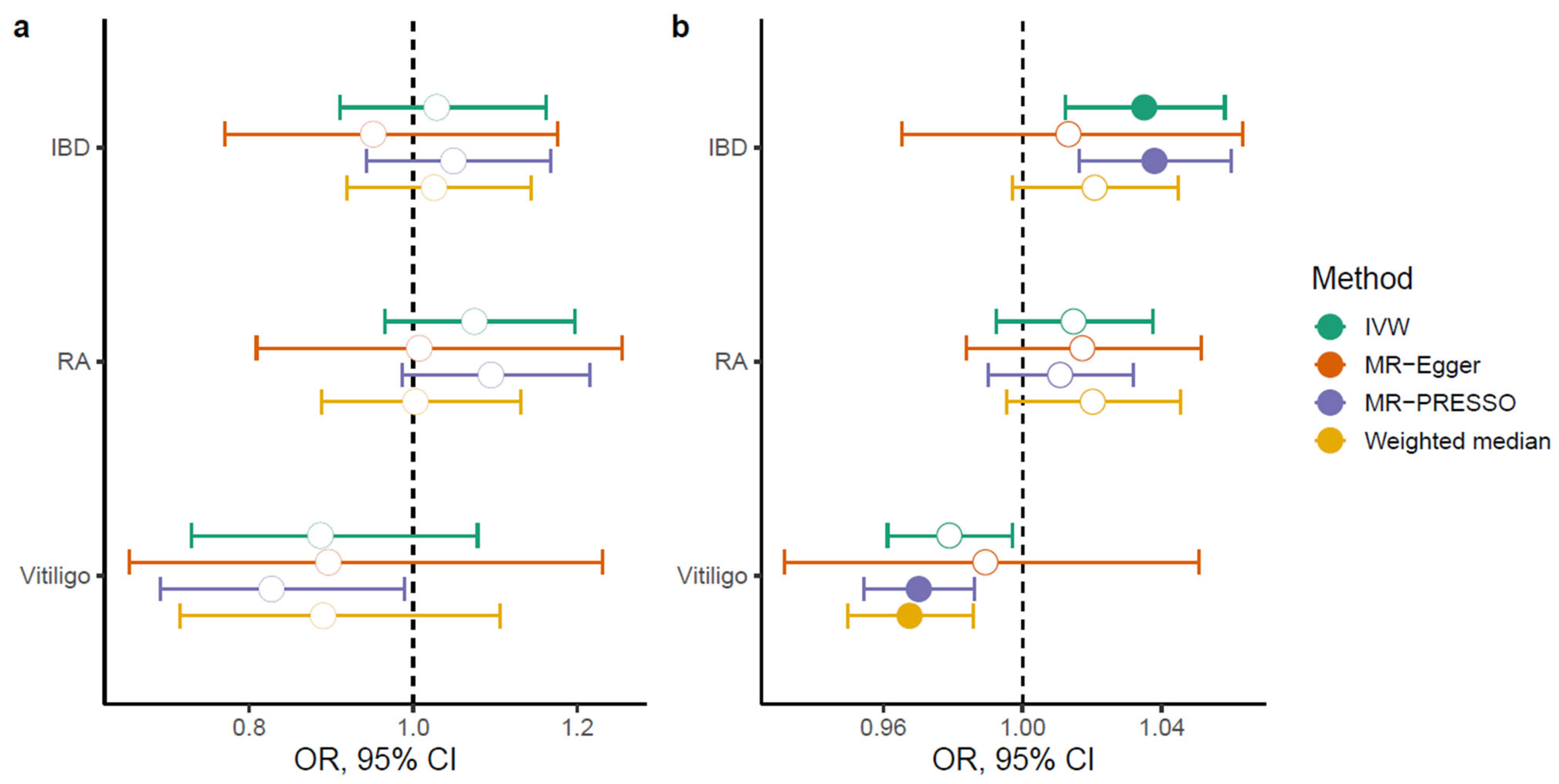
2.5. Bidirectional Causal Relationships
3. Discussion
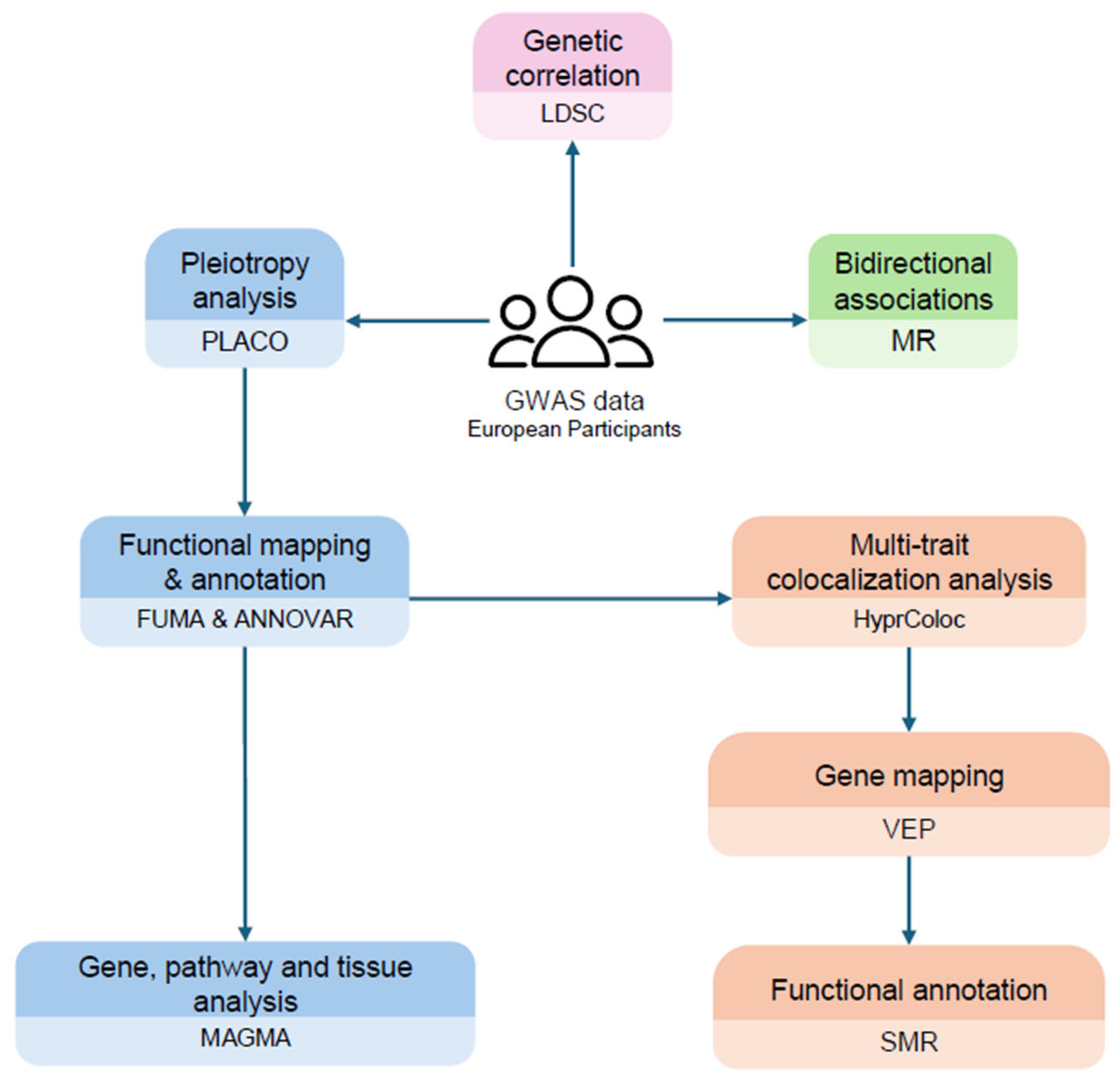
4. Methods
4.1. Data Sources
4.2. Genetic Correlation
4.3. Pleiotropy Analysis
4.4. Functional Annotation
4.5. Multi-Trait Colocalization Analysis
4.6. Bidirectional Causal Relationships
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Tian, J.; Zhang, D.; Yang, Y.; Huang, Y.; Wang, L.; Yao, X.; Lu, Q. Global Epidemiology of Atopic Dermatitis: A Comprehensive Systematic Analysis and Modelling Study. Br. J. Dermatol. 2023, 190, 55–61. [Google Scholar] [CrossRef]
- Antonatos, C.; Pontikas, A.; Akritidis, A.; Georgiou, S.; Stratigos, A.J.; Kleidona, I.A.; Gregoriou, S.; Grafanaki, K.; Vasilopoulos, Y. Neuroticism and Inflammatory Skin Diseases: A Bidirectional Mendelian Randomization Study. Arch. Dermatol. Res. 2024, 316, 213. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Løset, M.; Brown, S.J.; Saunes, M.; Hveem, K. Genetics of Atopic Dermatitis: From DNA Sequence to Clinical Relevance. Dermatology 2019, 235, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Pontikas, A.; Antonatos, C.; Evangelou, E.; Vasilopoulos, Y. Candidate Gene Association Studies in Atopic Dermatitis in Participants of European and Asian Ancestry: A Systematic Review and Meta-Analysis. Genes 2023, 14, 1456. [Google Scholar] [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic Dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef]
- Budu-Aggrey, A.; Kilanowski, A.; Sobczyk, M.K.; 23andMe Research Team; Shringarpure, S.S.; Mitchell, R.; Reis, K.; Reigo, A.; Estonian Biobank Research Team; Mägi, R.; et al. European and Multi-Ancestry Genome-Wide Association Meta-Analysis of Atopic Dermatitis Highlights Importance of Systemic Immune Regulation. Nat. Commun. 2023, 14, 6172. [Google Scholar] [CrossRef] [PubMed]
- Schuler, C.F.; Tsoi, L.C.; Billi, A.C.; Harms, P.W.; Weidinger, S.; Gudjonsson, J.E. Genetic and Immunological Pathogenesis of Atopic Dermatitis. J. Investig. Dermatol. 2024, 144, 954–968. [Google Scholar] [CrossRef]
- Ferreira, M.A.; Vonk, J.M.; Baurecht, H.; Marenholz, I.; Tian, C.; Hoffman, J.D.; Helmer, Q.; Tillander, A.; Ullemar, V.; van Dongen, J.; et al. Shared Genetic Origin of Asthma, Hay Fever and Eczema Elucidates Allergic Disease Biology. Nat. Genet. 2017, 49, 1752–1757. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Halling, A.-S.; Schmid-Grendelmeier, P.; Guttman-Yassky, E.; Silverberg, J.I. Comorbidities of Atopic Dermatitis—What Does the Evidence Say? J. Allergy Clin. Immunol. 2023, 151, 1155–1162. [Google Scholar] [CrossRef]
- Antonatos, C.; Pontikas, A.; Akritidis, A.; Mitsoudi, D.; Georgiou, S.; Stratigos, A.J.; Zacharopoulou, A.; Gregoriou, S.; Grafanaki, K.; Vasilopoulos, Y. A Genome-Wide Pleiotropy Study between Atopic Dermatitis and Neuropsychiatric Disorders. Hum. Genom. 2025, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Li, A.; Su, F.; Wang, Y.; Zhou, L.; Tang, C.; Feng, R.; Mao, R.; Chen, M.; Chen, L.; et al. An Atlas of the Shared Genetic Architecture between Atopic and Gastrointestinal Diseases. Commun. Biol. 2024, 7, 1696. [Google Scholar] [CrossRef] [PubMed]
- Elhage, K.G.; Kranyak, A.; Jin, J.Q.; Haran, K.; Spencer, R.K.; Smith, P.L.; Davis, M.S.; Hakimi, M.; Bhutani, T.; Liao, W. Mendelian Randomization Studies in Atopic Dermatitis: A Systematic Review. J. Investig. Dermatol. 2024, 144, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Ivert, L.U.; Wahlgren, C.-F.; Lindelöf, B.; Dal, H.; Bradley, M.; Johansson, E.K. Association between Atopic Dermatitis and Autoimmune Diseases: A Population-Based Case–Control Study. Br. J. Dermatol. 2021, 185, 335–342. [Google Scholar] [CrossRef]
- Schmitt, J.; Schwarz, K.; Baurecht, H.; Hotze, M.; Fölster-Holst, R.; Rodríguez, E.; Lee, Y.A.E.; Franke, A.; Degenhardt, F.; Lieb, W.; et al. Atopic Dermatitis Is Associated with an Increased Risk for Rheumatoid Arthritis and Inflammatory Bowel Disease, and a Decreased Risk for Type 1 Diabetes. J. Allergy Clin. Immunol. 2016, 137, 130–136. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, T. Beyond Skin White Spots: Vitiligo and Associated Comorbidities. Front. Med. 2023, 10, 1072837. [Google Scholar] [CrossRef]
- Shirai, Y.; Nakanishi, Y.; Suzuki, A.; Konaka, H.; Nishikawa, R.; Sonehara, K.; Namba, S.; Tanaka, H.; Masuda, T.; Yaga, M.; et al. Multi-Trait and Cross-Population Genome-Wide Association Studies across Autoimmune and Allergic Diseases Identify Shared and Distinct Genetic Component. Ann. Rheum. Dis. 2022, 81, 1301–1312. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Gooderham, M.J.; Paller, A.S.; Deleuran, M.; Bunick, C.G.; Gold, L.F.S.; Hijnen, D.; Calimlim, B.M.; Lee, W.-J.; Teixeira, H.D.; et al. Early and Sustained Improvements in Symptoms and Quality of Life with Upadacitinib in Adults and Adolescents with Moderate-to-Severe Atopic Dermatitis: 52-Week Results from Two Phase III Randomized Clinical Trials (Measure Up 1 and Measure Up 2). Am. J. Clin. Dermatol. 2024, 25, 485–496. [Google Scholar] [CrossRef]
- Dhar, S.; De, A.; Sarda, A.; Godse, K.; Lahiri, K. Real-World Efficacy and Safety of Oral Tofacitinib in Patients with Refractory Moderate to Severe Atopic Dermatitis: A Multicenter Retrospective Study. Indian J. Dermatol. 2024, 69, 292–295. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwon, J.E.; Cho, M.-L. Immunological Pathogenesis of Inflammatory Bowel Disease. Intest. Res. 2018, 16, 26–42. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J.; Takemura, S.; Kurtin, P.J. Cell-Cell Interactions in Synovitis: Interactions between T Cells and B Cells in Rheumatoid Arthritis. Arthritis Res. Ther. 2000, 2, 457. [Google Scholar] [CrossRef] [PubMed]
- Marchioro, H.Z.; Silva de Castro, C.C.; Fava, V.M.; Sakiyama, P.H.; Dellatorre, G.; Miot, H.A. Update on the Pathogenesis of Vitiligo. An. Bras. Dermatol. 2022, 97, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, M.; Sollis, E.; Ji, Y.; Lewis, E.; Abid, A.; Bircan, K.O.; Hall, P.; Hayhurst, J.; John, S.; Mosaku, A.; et al. The NHGRI-EBI GWAS Catalog: Standards for Reusability, Sustainability and Diversity. Nucleic Acids Res. 2025, 53, D998–D1005. [Google Scholar] [CrossRef] [PubMed]
- de Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.-G.; et al. Genome-Wide Association Study Implicates Immune Activation of Multiple Integrin Genes in Inflammatory Bowel Disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef]
- Ishigaki, K.; Sakaue, S.; Terao, C.; Luo, Y.; Sonehara, K.; Yamaguchi, K.; Amariuta, T.; Too, C.L.; Laufer, V.A.; Scott, I.C.; et al. Multi-Ancestry Genome-Wide Association Analyses Identify Novel Genetic Mechanisms in Rheumatoid Arthritis. Nat. Genet. 2022, 54, 1640–1651. [Google Scholar] [CrossRef]
- Jin, Y.; Andersen, G.; Yorgov, D.; Ferrara, T.M.; Ben, S.; Brownson, K.M.; Holland, P.J.; Birlea, S.A.; Siebert, J.; Hartmann, A.; et al. Genome-Wide Association Studies of Autoimmune Vitiligo Identify 23 New Risk Loci and Highlight Key Pathways and Regulatory Variants. Nat. Genet. 2016, 48, 1418–1424. [Google Scholar] [CrossRef]
- Antonatos, C.; Grafanaki, K.; Georgiou, S.; Evangelou, E.; Vasilopoulos, Y. Disentangling the Complexity of Psoriasis in the Post-Genome-Wide Association Era. Genes Immun. 2023, 24, 236–247. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Moss, A.C.; Farraye, F.A. Ulcerative Colitis. Mayo Clin. Proc. 2019, 94, 1357–1373. [Google Scholar] [CrossRef]
- Xin, Y.; Yuan, T.; Wang, J. Circulating Immune Cells and Vitiligo: A Bidirectional Two-Sample Mendelian Randomization Study. Front. Immunol. 2024, 15, 1391186. [Google Scholar] [CrossRef]
- Antonatos, C.; Budu-Aggrey, A.; Pontikas, A.; Akritidis, A.; Pasmatzi, E.; Tsiogka, A.; Gregoriou, S.; Grafanaki, K.; Paternoster, L.; Vasilopoulos, Y. Polygenic Transcriptome Risk Scores Enhance Predictive Accuracy in Atopic Dermatitis. J. Transl. Med. 2025, 23, 575. [Google Scholar] [CrossRef]
- Wu, L.-C.; Hwang, C.-Y.; Chung, P.-I.; Hua, T.-C.; Chen, Y.-D.; Chu, S.-Y.; Lee, D.-D.; Chang, Y.-T.; Wang, W.-J.; Liu, H.-N.; et al. Autoimmune Disease Comorbidities in Patients with Atopic Dermatitis: A Nationwide Case–Control Study in Taiwan. Pediatr. Allergy Immunol. 2014, 25, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Narla, S.; Silverberg, J.I. Association between Atopic Dermatitis and Autoimmune Disorders in US Adults and Children: A Cross-Sectional Study. J. Am. Acad. Dermatol. 2019, 80, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Freuer, D. Causal Association Between Atopic Dermatitis and Inflammatory Bowel Disease: A 2-Sample Bidirectional Mendelian Randomization Study. Inflamm. Bowel Dis. 2022, 28, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Diluvio, L.; Cannizzaro, M.V.; Grelli, S.; Galluzzo, M.; Talamonti, M.; Annicchiarico-Petruzzelli, M.; Mancini, M.; Melino, G.; et al. Skin Immunity and Its Dysregulation in Atopic Dermatitis, Hidradenitis Suppurativa and Vitiligo. Cell Cycle 2020, 19, 257–267. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Croft, M.; Geng, B.; Rynkiewicz, N.; Lucchesi, D.; Peakman, M.; van Krinks, C.; Valdecantos, W.; Xing, H.; Weidinger, S. The Role of OX40 Ligand/OX40 Axis Signalling in Atopic Dermatitis. Br. J. Dermatol. 2024, 191, 488–496. [Google Scholar] [CrossRef]
- Hsu, T.-L.; Chang, Y.-C.; Chen, S.-J.; Liu, Y.-J.; Chiu, A.W.; Chio, C.-C.; Chen, L.; Hsieh, S.-L. Modulation of Dendritic Cell Differentiation and Maturation by Decoy Receptor 3. J. Immunol. 2002, 168, 4846–4853. [Google Scholar] [CrossRef]
- Ellinghaus, D.; Baurecht, H.; Esparza-Gordillo, J.; Rodríguez, E.; Matanovic, A.; Marenholz, I.; Hübner, N.; Schaarschmidt, H.; Novak, N.; Michel, S.; et al. High-Density Genotyping Study Identifies Four New Susceptibility Loci for Atopic Dermatitis. Nat. Genet. 2013, 45, 808–812. [Google Scholar] [CrossRef]
- Albers, H.M.; Kurreeman, F.A.S.; Stoeken-Rijsbergen, G.; Brinkman, D.M.C.; Kamphuis, S.S.M.; van Rossum, M.A.J.; Girschick, H.J.; Wouters, C.; Saurenmann, R.K.; Hoppenreijs, E.; et al. Association of the Autoimmunity Locus 4q27 with Juvenile Idiopathic Arthritis. Arthritis Rheum. 2009, 60, 901–904. [Google Scholar] [CrossRef]
- Wang, J.; Wicker, L.S.; Santamaria, P. IL-2 and Its High-Affinity Receptor: Genetic Control of Immunoregulation and Autoimmunity. Semin. Immunol. 2009, 21, 363–371. [Google Scholar] [CrossRef]
- Kumar, V.; Gutierrez-Achury, J.; Kanduri, K.; Almeida, R.; Hrdlickova, B.; Zhernakova, D.V.; Westra, H.-J.; Karjalainen, J.; Ricaño-Ponce, I.; Li, Y.; et al. Systematic Annotation of Celiac Disease Loci Refines Pathological Pathways and Suggests a Genetic Explanation for Increased Interferon-Gamma Levels. Hum. Mol. Genet. 2015, 24, 397–409. [Google Scholar] [CrossRef]
- Antonatos, C.; Mitsoudi, D.; Pontikas, A.; Akritidis, A.; Xiropotamos, P.; Georgakilas, G.K.; Georgiou, S.; Tsiogka, A.; Gregoriou, S.; Grafanaki, K.; et al. Transcriptome-Wide Analyses Delineate the Genetic Architecture of Expression Variation in Atopic Dermatitis. Hum. Genet. Genom. Adv. 2025, 6, 100422. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Mukherjee, A.; Varghese, A.P.; Yang, X.-L.; Chen, S.; Zhang, H. Roles of G Protein-Coupled Receptors in Inflammatory Bowel Disease. World J. Gastroenterol. 2020, 26, 1242–1261. [Google Scholar] [CrossRef] [PubMed]
- Demela, P.; Pirastu, N.; Soskic, B. Cross-Disorder Genetic Analysis of Immune Diseases Reveals Distinct Gene Associations That Converge on Common Pathways. Nat. Commun. 2023, 14, 2743. [Google Scholar] [CrossRef] [PubMed]
- Mucha, S.; Baurecht, H.; Novak, N.; Rodríguez, E.; Bej, S.; Mayr, G.; Emmert, H.; Stölzl, D.; Gerdes, S.; Jung, E.S.; et al. Protein-Coding Variants Contribute to the Risk of Atopic Dermatitis and Skin-Specific Gene Expression. J. Allergy Clin. Immunol. 2020, 145, 1208–1218. [Google Scholar] [CrossRef]
- Watanabe, K.; Stringer, S.; Frei, O.; Umićević Mirkov, M.; de Leeuw, C.; Polderman, T.J.C.; van der Sluis, S.; Andreassen, O.A.; Neale, B.M.; Posthuma, D. A Global Overview of Pleiotropy and Genetic Architecture in Complex Traits. Nat. Genet. 2019, 51, 1339–1348. [Google Scholar] [CrossRef]
- The GTEx Consortium. The GTEx Consortium Atlas of Genetic Regulatory Effects across Human Tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Bulik-Sullivan, B.; Finucane, H.K.; Anttila, V.; Gusev, A.; Day, F.R.; Loh, P.-R.; ReproGen Consortium; Psychiatric Genomics Consortium; Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control Consortium 3; Duncan, L.; et al. An Atlas of Genetic Correlations across Human Diseases and Traits. Nat. Genet. 2015, 47, 1236–1241. [Google Scholar] [CrossRef]
- Ray, D.; Chatterjee, N. A Powerful Method for Pleiotropic Analysis under Composite Null Hypothesis Identifies Novel Shared Loci between Type 2 Diabetes and Prostate Cancer. PLoS Genet. 2020, 16, e1009218. [Google Scholar] [CrossRef]
- Watanabe, K.; Taskesen, E.; van Bochoven, A.; Posthuma, D. Functional Mapping and Annotation of Genetic Associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional Annotation of Genetic Variants from High-Throughput Sequencing Data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- de Leeuw, C.A.; Mooij, J.M.; Heskes, T.; Posthuma, D. MAGMA: Generalized Gene-Set Analysis of GWAS Data. PLoS Comput. Biol. 2015, 11, e1004219. [Google Scholar] [CrossRef]
- Foley, C.N.; Staley, J.R.; Breen, P.G.; Sun, B.B.; Kirk, P.D.W.; Burgess, S.; Howson, J.M.M. A Fast and Efficient Colocalization Algorithm for Identifying Shared Genetic Risk Factors across Multiple Traits. Nat. Commun. 2021, 12, 764. [Google Scholar] [CrossRef]
- Hunt, S.E.; Moore, B.; Amode, R.M.; Armean, I.M.; Lemos, D.; Mushtaq, A.; Parton, A.; Schuilenburg, H.; Szpak, M.; Thormann, A.; et al. Annotating and Prioritizing Genomic Variants Using the Ensembl Variant Effect Predictor—A Tutorial. Hum. Mutat. 2022, 43, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, F.; Hu, H.; Bakshi, A.; Robinson, M.R.; Powell, J.E.; Montgomery, G.W.; Goddard, M.E.; Wray, N.R.; Visscher, P.M.; et al. Integration of Summary Data from GWAS and eQTL Studies Predicts Complex Trait Gene Targets. Nat. Genet. 2016, 48, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Thompson, S.G.; CRP CHD Genetics Collaboration. Avoiding Bias from Weak Instruments in Mendelian Randomization Studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian Randomization Analysis with Multiple Genetic Variants Using Summarized Data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting Findings from Mendelian Randomization Using the MR-Egger Method. Eur. J. Epidemiol. 2017, 32, 377–389, Erratum in Eur. J. Epidemiol. 2017, 32, 391–392. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred from Mendelian Randomization between Complex Traits and Diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
| Disease | Sample Size | Cases | Controls | Ancestry | Authors |
|---|---|---|---|---|---|
| AD [7] | 864,982 | 60,653 | 804,329 | European | Budu Aggrey et al., 2023 |
| IBD [24] | 59,957 | 25,042 | 34,915 | European | de Lange et al., 2017 |
| RA [25] | 97,173 | 22,350 | 74,823 | European | Ishigaki et al., 2022 |
| Vitiligo [26] | 44,266 | 4680 | 39,586 | European | Jin et al., 2016 |
| Genomic Coordinates | Colocalized Traits | PP | SNP | SNP.PP | Mapped Genes | AD GWAS p-Value |
|---|---|---|---|---|---|---|
| chr1:7900638-8739302 | RA, vitiligo, AD | 0.7127 | rs301799 | 0.3816 | RERE, RERE-AS1 | 1.43 × 10−7 |
| chr1:114053808-114573318 | IBD, RA, vitiligo, AD | 0.8452 | rs6679677 | 0.7535 | PTPN22, AP4B1-AS1, RSBN1, PHTF1 | 7.18 × 10−6 |
| chr1:154170087-154675456 | RA, AD | 0.8875 | rs12133641 | 0.5913 | IL6R | 1.72 × 10−21 |
| chr2:111658567-112183001 | IBD, RA, vitiligo, AD | 0.9422 | rs72837826 | 0.9039 | BCL2L11, MIR4435-2HG, ENSG00000295185 | 2.82 × 10−6 |
| chr5:176544191-177044191 | IBD, AD | 0.7366 | rs12654812 | 0.6639 | RGS14 | 7.66 × 10−8 |
| chr6:90680513-91226768 | IBD, vitiligo, AD | 0.7597 | rs17513531 | 0.1207 | BACH2 | 3.85 × 10−10 |
| chr6:106417535-106917535 | RA, AD | 0.8917 | rs9372120 | 0.34 | ATG5, U4, ENSG00000303838, RN7SL47P | 1.08 × 10−7 |
| chr6:159222295-159739791 | RA, AD | 0.8782 | rs212389 | 0.8069 | ENSG00000285492, TAGAP-AS1, TAGAP | 1.82 × 10−11 |
| chr8:21519432-22019432 | IBD, AD | 0.9431 | rs56094005 | 0.5426 | DOK2 | 9.35 × 10−9 |
| chr8:126341392-126858642 | RA, AD | 0.7486 | rs28550378 | 0.3259 | ENSG00000302425 | 3.91 × 10−15 |
| chr8:129302491-129802540 | IBD, RA, vitiligo, AD | 0.7118 | rs938650 | 0.5337 | LINC00824, ENSG00000298619, ENSG00000298640 | 3.75 × 10−5 |
| chr9:123441237-123941237 | RA, AD | 0.6172 | rs1930785 | 0.097 | TRAF1, C5, PHF19, ENSG00000306516 | 3.41 × 10−5 |
| chr11:36187868-36735919 | IBD, AD | 0.6701 | rs28520436 | 0.316 | PRR5L | 1.22 × 10−24 |
| chr11:118492800-118992800 | RA, AD | 0.9434 | rs74541740 | 0.1599 | ENSG00000306274, ENSG00000306318 | 2.19 × 10−10 |
| chr12:56134804-56694632 | RA, AD | 0.984 | rs705699 | 0.6027 | CDK2, RAB5B, PMEL, SUOX | 3.31 × 10−9 |
| chr12:111634608-112257756 | IBD, RA, vitiligo, AD | 0.9443 | rs3184504 | 0.7639 | SH2B3, ATXN2 | 1.56 × 10−8 |
| chr14:75731856-76231856 | RA, AD | 0.9868 | rs175714 | 0.8197 | ENSG00000297883 | 1.44 × 10−7 |
| chr14:88176297-88676297 | IBD, AD | 0.9252 | rs4462528 | 0.4761 | GALC, SHLD2P2 | 5.99 × 10−6 |
| chr14:105273663-105773663 | IBD, AD | 0.7636 | rs7147439 | 0.5047 | LINC02298, GPR132, ENSG00000307140 | 4.71 × 10−8 |
| chr16:50763434-51263434 | IBD, AD | 0.6399 | rs12324931 | 1 | CYLD | 0.000261 |
| chr17:38507789-39007789 | IBD, AD | 0.8969 | rs1358175 | 0.6417 | ENSG00000279775 | 1.99 × 10−11 |
| chr18:59759814-60272811 | RA, AD | 0.9242 | rs4574025 | 0.5308 | TNFRSF11A | 1.48 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazanas, P.; Antonatos, C.; Tsoumani, K.T.; Sgourou, A.; Vasilopoulos, Y. Shared Genetic Architecture Between Atopic Dermatitis and Autoimmune Diseases. Int. J. Mol. Sci. 2025, 26, 9124. https://doi.org/10.3390/ijms26189124
Lazanas P, Antonatos C, Tsoumani KT, Sgourou A, Vasilopoulos Y. Shared Genetic Architecture Between Atopic Dermatitis and Autoimmune Diseases. International Journal of Molecular Sciences. 2025; 26(18):9124. https://doi.org/10.3390/ijms26189124
Chicago/Turabian StyleLazanas, Panagiotis, Charalabos Antonatos, Konstantina T. Tsoumani, Argyro Sgourou, and Yiannis Vasilopoulos. 2025. "Shared Genetic Architecture Between Atopic Dermatitis and Autoimmune Diseases" International Journal of Molecular Sciences 26, no. 18: 9124. https://doi.org/10.3390/ijms26189124
APA StyleLazanas, P., Antonatos, C., Tsoumani, K. T., Sgourou, A., & Vasilopoulos, Y. (2025). Shared Genetic Architecture Between Atopic Dermatitis and Autoimmune Diseases. International Journal of Molecular Sciences, 26(18), 9124. https://doi.org/10.3390/ijms26189124







