Extended PRF: Impact of Heat on Gene Expression in Gingival Fibroblasts
Abstract
1. Introduction
2. Results
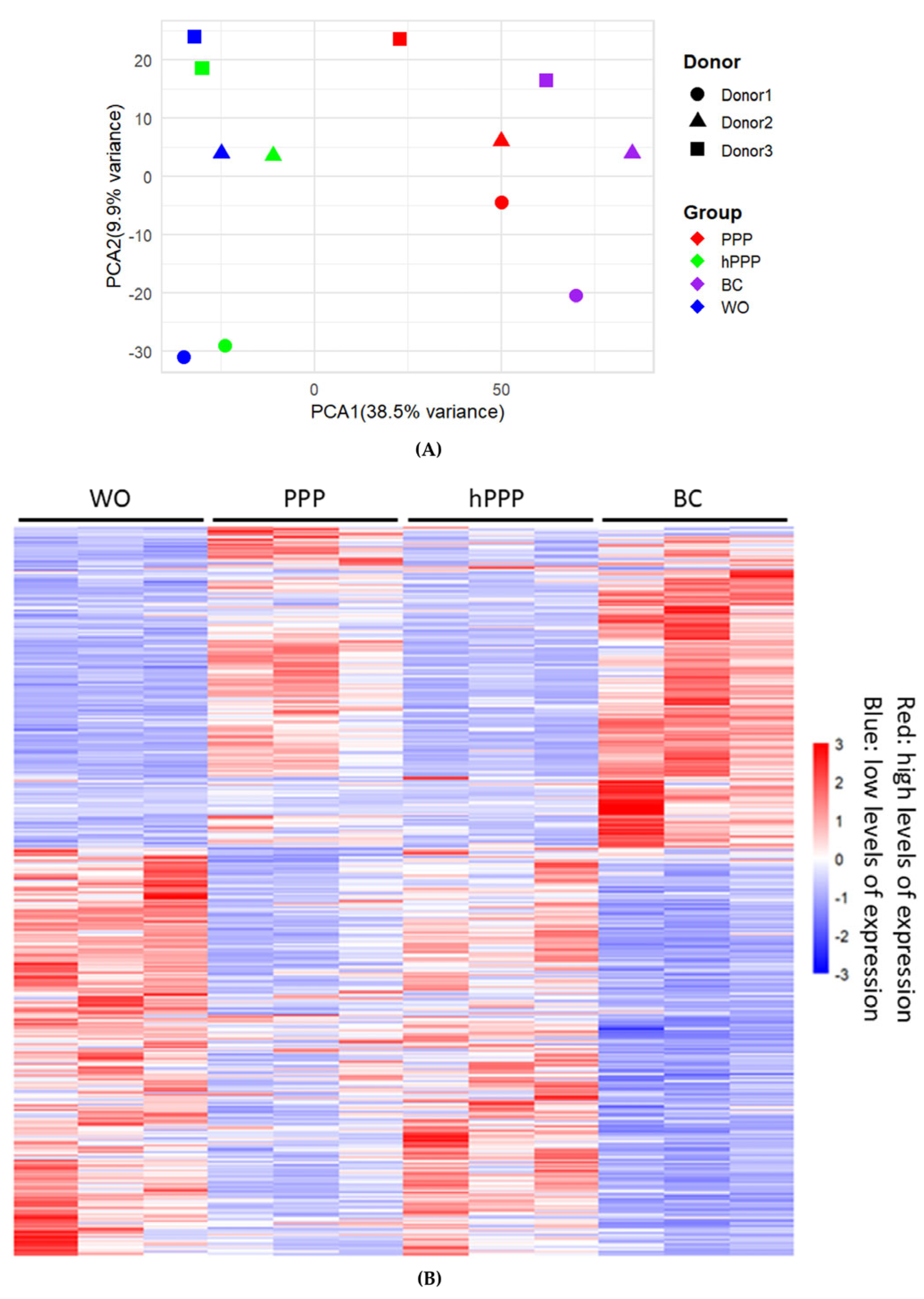
2.1. Principal Component Analysis and Heat Map of Gene Expression by PPP, hPPP, and BC
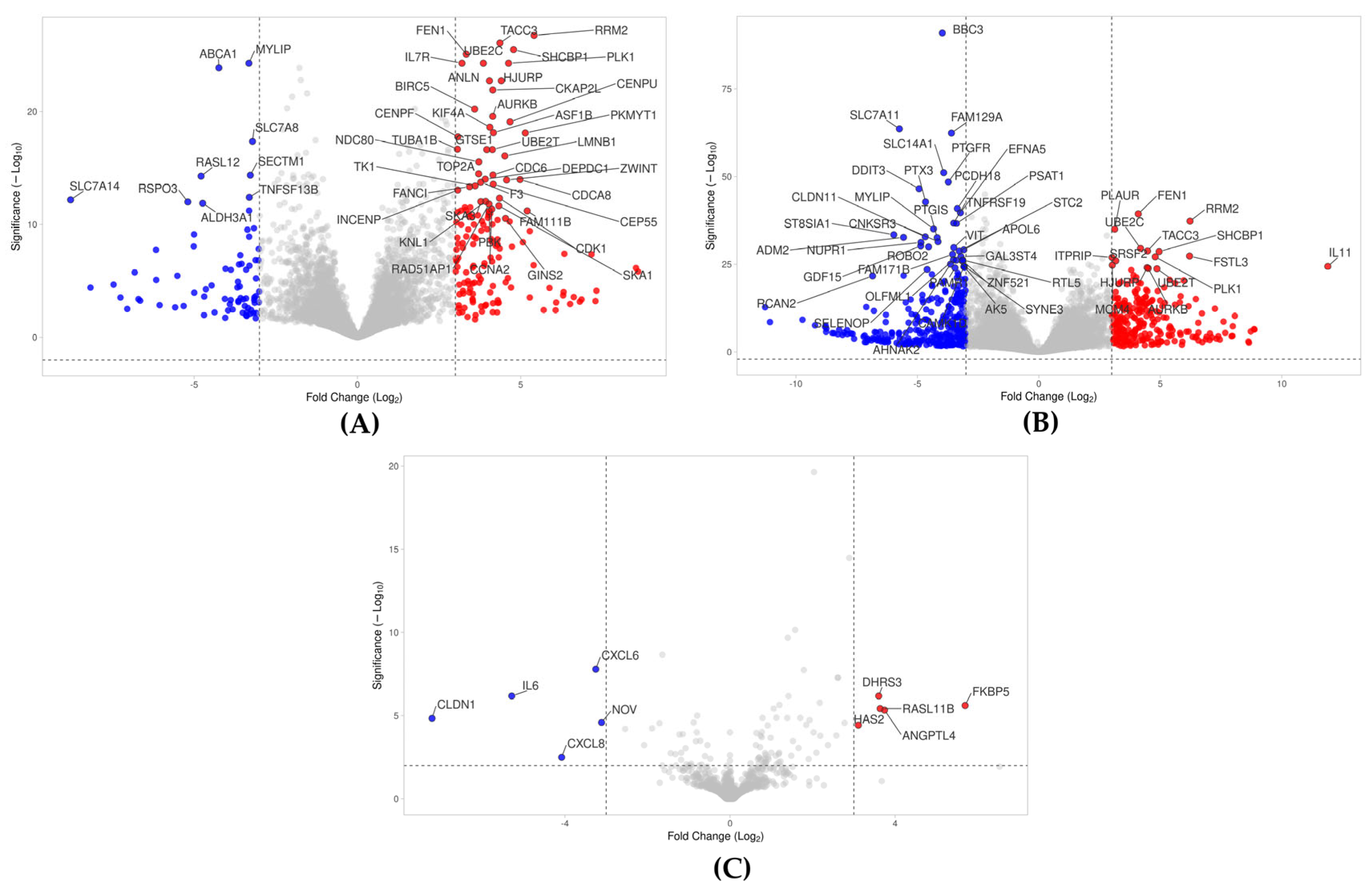
2.2. Volcano Analysis of Gene Expression Changes by PPP, hPPP, and BC
2.3. Venn and UpSet Analyses of Genes Regulated by PPP, hPPP, and BC
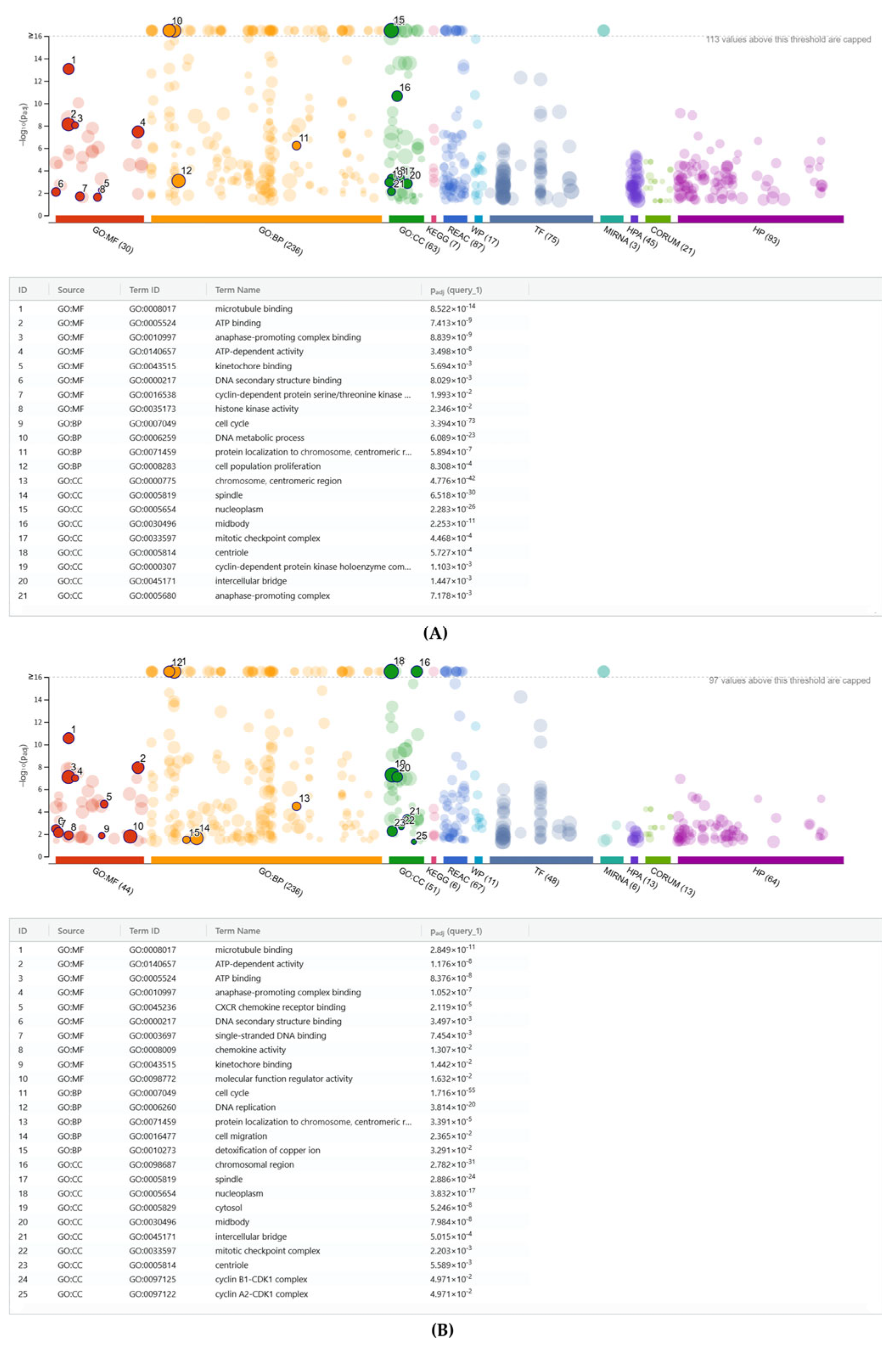
2.4. G:Profiler Analysis of Gene Expression Changes by PPP, hPP, P and BC
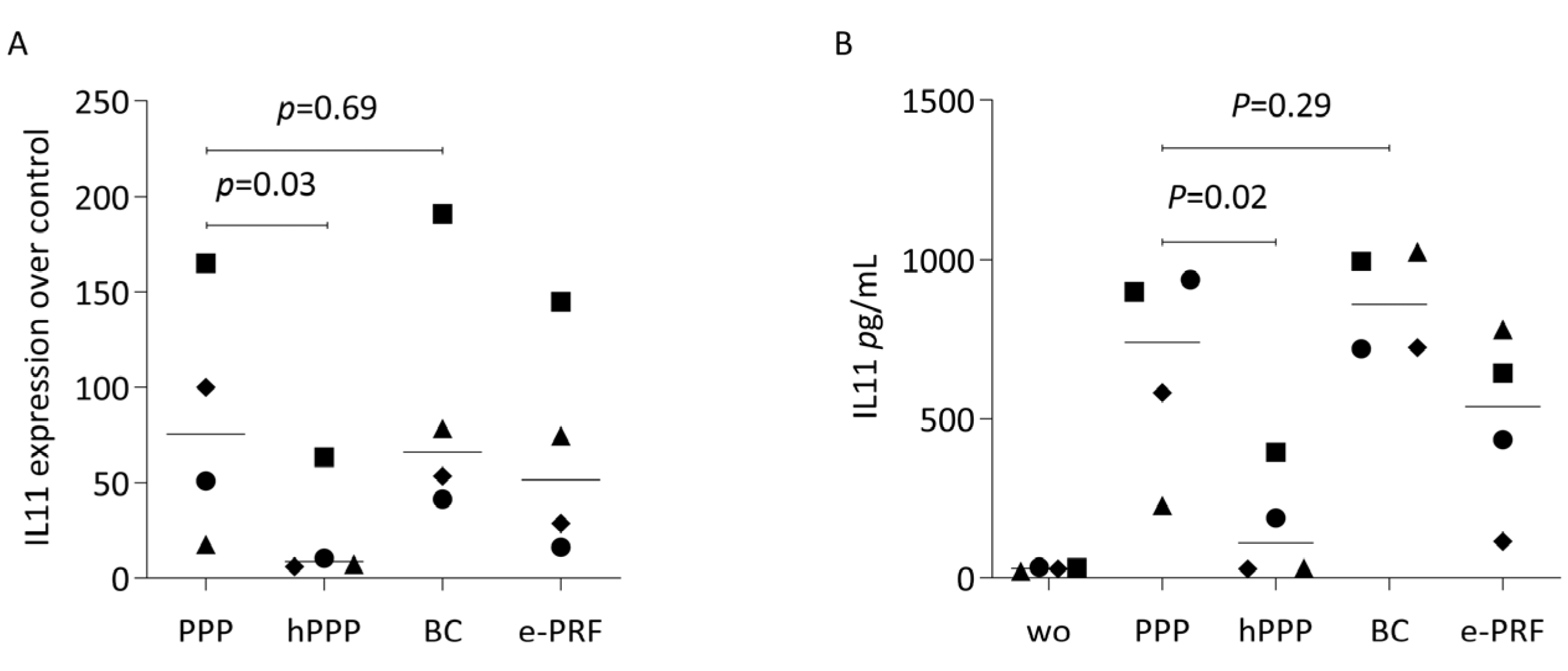
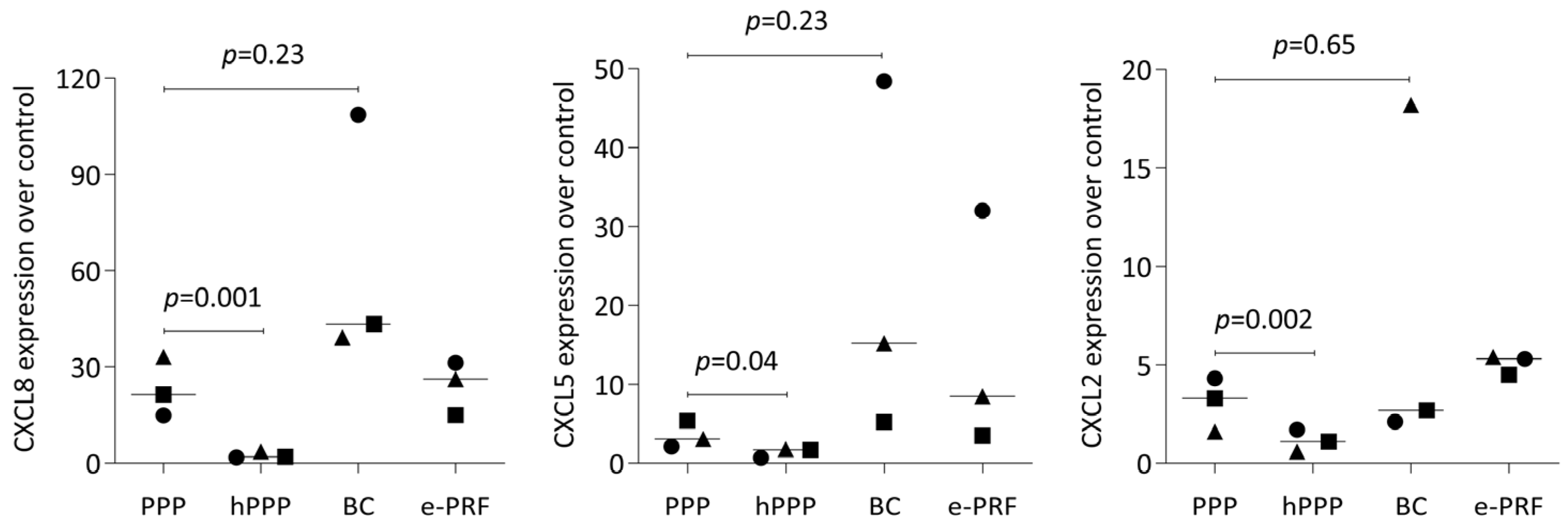
2.5. PPP, Buffy Coat, and E-PRF Lysates, but Not Heated PPP, Enhance Gene Expression
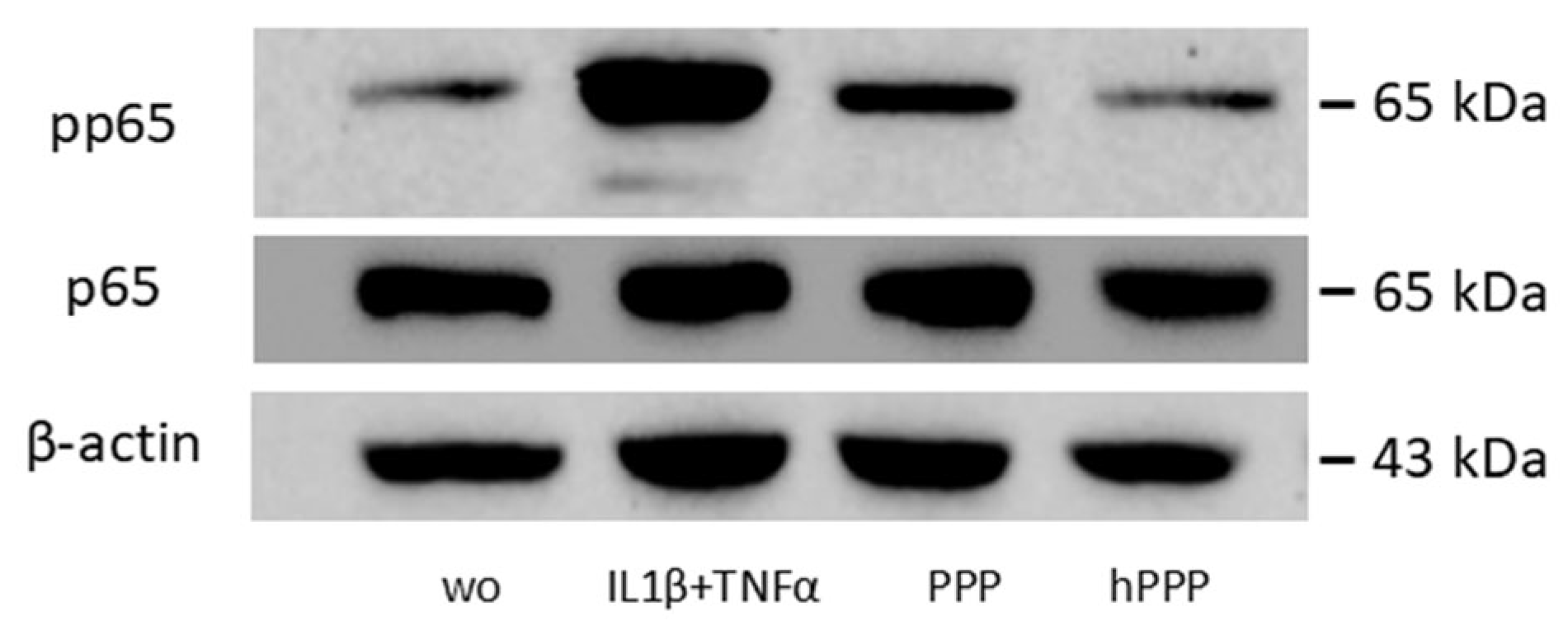
2.6. Lysates of PPP, but Not Heated PPP, Induced the Phosphorylation of p65
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preparation of PPP, Heated PPP, Buffy Coat, and E-PRF Lysate
4.3. RNA Sequencing
4.4. Heat Map of Gene Expression Changes, Volcano Plot, Venn Diagram, and Gene Set Enrichment Analysis
4.5. Reverse-Transcription Quantitative Real-Time PCR (RT-qPCR) and Immunoassay
4.6. Western Blot
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Li, Y.; Ling, J.; Jiang, Q. Inflammasomes in alveolar bone loss. Front. Immunol. 2021, 12, 691013. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, L.; Li, X.; Liu, S.; Du, J.; Xu, J.; Hu, J.; Liu, Y. Challenges and tissue engineering strategies of periodontal-guided tissue regeneration. Tissue Eng. Part C Methods 2022, 28, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Robin, M.; Mouloungui, E.; Castillo Dali, G.; Wang, Y.; Saffar, J.L.; Pavon-Djavid, G.; Divoux, T.; Manneville, S.; Behr, L.; Cardi, D.; et al. Mineralized collagen plywood contributes to bone autograft performance. Nature 2024, 636, 100–107. [Google Scholar] [CrossRef]
- Shanbhag, S.; Kampleitner, C.; Al-Sharabi, N.; Mohamed-Ahmed, S.; Apaza Alccayhuaman, K.A.; Heimel, P.; Tangl, S.; Beinlich, A.; Rana, N.; Sanz, M.; et al. Functionalizing Collagen Membranes with MSC-Conditioned Media Promotes Guided Bone Regeneration in Rat Calvarial Defects. Cells 2023, 12, 767. [Google Scholar] [CrossRef]
- Strauss, F.J.; Schneider, D.; Jung, R.E.; Kraus, R.; Thoma, D.S.; Naenni, N. Hard and soft tissue contour changes following simultaneous guided bone regeneration at single peri-implant dehiscence defects using either resorbable or non-resorbable membranes: A 6-month secondary analysis of a randomized controlled trial. Clin. Oral. Investig. 2025, 29, 231. [Google Scholar] [CrossRef]
- Chhaya, D.; Vaidya, N.; Patel, V.; Chudasama, K.; Doshi, S.H.; Kumar, P. Evaluation and comparison of mechanical properties of platelet-rich fibrin membrane, fish collagen membrane, bovine collagen membrane and chorionic membrane—An SEM study. Indian J. Dent. Res. 2022, 33, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef]
- Goswami, P.; Chaudhary, V.; Arya, A.; Verma, R.; Vijayakumar, G.; Bhavani, M. Platelet-Rich Fibrin (PRF) and its application in dentistry: A literature review. J. Pharm. Bioallied Sci. 2024, 16, S5–S7. [Google Scholar] [CrossRef]
- Karkle, A.; Neimane, L.; Zolovs, M.; Dambergs, M.; Meistere, D.; Vaskevica, A.; Slaidina, A. Impact of Advanced Platelet-Rich Fibrin on Early Bone Healing After Endodontic Microsurgery: A Randomized Controlled Trial. Diagnostics 2025, 15, 516. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; He, D.; Ren, S.; Fan, L.; Sun, J. Platelet-rich fibrin in dentistry. J. Appl. Biomater. Funct. Mater. 2024, 22, 22808000241299588. [Google Scholar] [CrossRef]
- Acerra, A.; Caggiano, M.; Chiacchio, A.; Scognamiglio, B.; D‘Ambrosio, F. PRF and PRP in Dentistry: An Umbrella Review. J. Clin. Med. 2025, 14, 3224. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; de Ruvo, E.; Campanelli, M.; Palermo, A.; Fabbro, M.D.; Blasio, M.D.; Inchingolo, A.D.; Dipalma, G. Guided Bone Regeneration: CGF and PRF Combined With Various Types of Scaffolds-A Systematic Review. Int. J. Dent. 2024, 2024, 4990295. [Google Scholar] [CrossRef]
- Quirynen, M.; Siawasch, S.A.M.; Yu, J.; Miron, R.J. Essential principles for blood centrifugation. Periodontol. 2000 2025, 97, 43–51. [Google Scholar] [CrossRef]
- Soderstrom, A.C.; Nybo, M.; Nielsen, C.; Vinholt, P.J. The effect of centrifugation speed and time on pre-analytical platelet activation. Clin. Chem. Lab. Med. 2016, 54, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Hantgan, R.R.; Taylor, R.G.; Lewis, J.C. Platelets interact with fibrin only after activation. Blood 1985, 65, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Bannish, B.E.; Paynter, B.; Risman, R.A.; Shroff, M.; Tutwiler, V. The effect of plasmin-mediated degradation on fibrinolysis and tissue plasminogen activator diffusion. Biophys. J. 2024, 123, 610–621. [Google Scholar] [CrossRef]
- Castellino, F.J.; Ploplis, V.A.; Powell, J.R.; Strickland, D.K. The existence of independent domain structures in human Lys77-plasminogen. J. Biol. Chem. 1981, 256, 4778–4782. [Google Scholar] [CrossRef]
- Risman, R.A.; Kirby, N.C.; Bannish, B.E.; Hudson, N.E.; Tutwiler, V. Fibrinolysis: An illustrated review. Res. Pract. Thromb. Haemost. 2023, 7, 100081. [Google Scholar] [CrossRef]
- Miron, R.J.; Pikos, M.A.; Estrin, N.E.; Kobayashi-Fujioka, M.; Espinoza, A.R.; Basma, H.; Zhang, Y. Extended platelet-rich fibrin. Periodontol. 2000 2024, 94, 114–130. [Google Scholar] [CrossRef]
- Zeng, L.; Zeng, J.; He, J.; Zhou, Y.; Li, Y.; Li, C.; Lin, Z.; Chen, G.; Wu, H.; Zhou, L. ALB-PRF facilitates chondrogenesis by promoting chondrocytes migration, proliferation and differentiation. Platelets 2024, 35, 2414792. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Duan, Q.; Bao, H.; Li, A.; Li, W.; Chen, J.; He, Y. A comparative study of the effects of platelet-rich fibrin, concentrated growth factor and platelet-poor plasma on the healing of tooth extraction sockets in rabbits. BMC Oral Health 2022, 22, 87. [Google Scholar] [CrossRef]
- Mehdipour Chari, K.; Enderami, S.E.; Mansour, R.N.; Hasanzadeh, E.; Amini Mahabadi, J.; Abazari, M.; Asadi, P.; Hojjat, A. Applications of blood plasma derivatives for cutaneous wound healing: A mini-review of clinical studies. Regen. Ther. 2024, 27, 251–258. [Google Scholar] [CrossRef]
- Coucke, B.; Dilissen, E.; Cremer, J.; Schrijvers, R.; Theys, T.; Van Gerven, L. Leukocyte-and Platelet-Rich Fibrin for enhanced tissue repair: An in vitro study characterizing cellular composition, growth factor kinetics and transcriptomic insights. Mol. Biol. Rep. 2024, 51, 954. [Google Scholar] [CrossRef] [PubMed]
- Iao, S.; Ouyang, X.; Qiao, J.; Liu, J.; Liu, W.; Zhong, J. Growth factors release in concentrated growth factor and advanced platelet-rich fibrin sticky bone. Oral Dis. 2025, 31, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Zwittnig, K.; Kirnbauer, B.; Jakse, N.; Schlenke, P.; Mischak, I.; Ghanaati, S.; Al-Maawi, S.; Vegh, D.; Payer, M.; Zrnc, T.A. Growth factor release within liquid and solid prf. J. Clin. Med. 2022, 11, 5070. [Google Scholar] [CrossRef]
- Kargarpour, Z.; Nasirzade, J.; Panahipour, L.; Miron, R.J.; Gruber, R. Liquid platelet-rich fibrin and heat-coagulated albumin gel: Bioassays for TGF-beta activity. Materials 2020, 13, 3466. [Google Scholar] [CrossRef]
- Estrin, N.E.; Basma, H.; Espinoza, A.R.; Pinto, M.A.C.; Pikos, M.A.; Miron, R.J. Extended Platelet-Rich Fibrin as a Membrane for Lateral Window Sinus Lifts: A Case Series. Clin. Implant. Dent. Relat. Res. 2025, 27, e13427. [Google Scholar] [CrossRef]
- Imani, A.; Panahipour, L.; Kuhtreiber, H.; Mildner, M.; Gruber, R. RNAseq of Gingival Fibroblasts Exposed to PRF Membrane Lysates and PRF Serum. Cells 2024, 13, 1308. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Schaller, B.; Mourao, C.; Zhang, Y.; Sculean, A.; Miron, R.J. Biological characterization of an injectable platelet-rich fibrin mixture consisting of autologous albumin gel and liquid platelet-rich fibrin (Alb-PRF). Platelets 2021, 32, 74–81. [Google Scholar] [CrossRef]
- Kargarpour, Z.; Nasirzade, J.; Panahipour, L.; Miron, R.J.; Gruber, R. Liquid PRF Reduces the Inflammatory Response and Osteoclastogenesis in Murine Macrophages. Front. Immunol. 2021, 12, 636427. [Google Scholar] [CrossRef]
- Kargarpour, Z.; Nasirzade, J.; Di Summa, F.; Panahipour, L.; Miron, R.J.; Gruber, R. Platelet-Rich Fibrin Can Neutralize Hydrogen Peroxide-Induced Cell Death in Gingival Fibroblasts. Antioxidants 2020, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Varga, F.; Fischer, M.B.; Watzek, G. Platelets stimulate proliferation of bone cells: Involvement of platelet-derived growth factor, microparticles and membranes. Clin. Oral. Implants. Res. 2002, 13, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; Duchez, A.C.; Audoux, E.; Ebermeyer, T.; Arthaud, C.A.; Prier, A.; Eyraud, M.A.; Mismetti, P.; Garraud, O.; Bertoletti, L.; et al. Platelets as key factors in inflammation: Focus on CD40L/CD40. Front. Immunol. 2022, 13, 825892. [Google Scholar] [CrossRef]
- Sempowski, G.D.; Chess, P.R.; Moretti, A.J.; Padilla, J.; Phipps, R.P.; Blieden, T.M. CD40 mediated activation of gingival and periodontal ligament fibroblasts. J. Periodontol. 1997, 68, 284–292. [Google Scholar] [CrossRef]
- Altenburg, A.; Baldus, S.E.; Smola, H.; Pfister, H.; Hess, S. CD40 ligand-CD40 interaction induces chemokines in cervical carcinoma cells in synergism with IFN-gamma. J. Immunol. 1999, 162, 4140–4147. [Google Scholar] [CrossRef]
- Yang, R.; Roshani, D.; Gao, B.; Li, P.; Shang, N. Metallothionein: A Comprehensive Review of Its Classification, Structure, Biological Functions, and Applications. Antioxidants 2024, 13, 825. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, M.; Wang, H.; Wu, S.; Yao, J.; Ge, Y.; Lu, Y.; Hu, Q. Identification of Potential Biomarkers From Hepatocellular Carcinoma With MT1 Deletion. Pathol. Oncol. Res. 2021, 27, 597527. [Google Scholar] [CrossRef] [PubMed]
- Dean, K.M.; Qin, Y.; Palmer, A.E. Visualizing metal ions in cells: An overview of analytical techniques, approaches, and probes. Biochim. Biophys. Acta 2012, 1823, 1406–1415. [Google Scholar] [CrossRef]
- Rodriguez, R.; Muller, S.; Colombeau, L.; Solier, S.; Sindikubwabo, F.; Caneque, T. Metal ion signaling in biomedicine. Chem. Rev. 2025, 125, 660–744. [Google Scholar] [CrossRef] [PubMed]
- Panahipour, L.; Micucci, C.; Gelmetti, B.; Gruber, R. In Vitro Bioassay for Damage-Associated Molecular Patterns Arising from Injured Oral Cells. Bioengineering 2024, 11, 687. [Google Scholar] [CrossRef]
- Gheno, E.; Mourao, C.; Mello-Machado, R.C.; Stellet Lourenco, E.; Miron, R.J.; Catarino, K.F.F.; Alves, A.T.; Alves, G.G.; Calasans-Maia, M.D. In vivo evaluation of the biocompatibility and biodegradation of a new denatured plasma membrane combined with liquid PRF (Alb-PRF). Platelets 2021, 32, 542–554. [Google Scholar] [CrossRef]
- Cortellini, S.; Castro, A.B.; Temmerman, A.; Van Dessel, J.; Pinto, N.; Jacobs, R.; Quirynen, M. Leucocyte- and platelet-rich fibrin block for bone augmentation procedure: A proof-of-concept study. J. Clin. Periodontol. 2018, 45, 624–634. [Google Scholar] [CrossRef]
- Panahipour, L.; Kargarpour, Z.; Mildner, M.; Kuhtreiber, H.; Gruber, R. RNAseq of peripheral blood mononucleated cells exposed to platelet-rich fibrin and enamel matrix derivatives. Sci. Rep. 2025, 15, 3661. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.; Yu, J.; Koirala, S.; Mourao, C.F.; Andrade, C.; Rescigno, E.; Zamora, Y.; Pinto, D.; Quirynen, M. L-PRF in extra-oral wound care. Periodontol. 2000 2025, 97, 342–362. [Google Scholar] [CrossRef]
- Ogéus, T. Platelet-Rich Fibrin in Combination with Heat Coagulated Albumin Gel as Treatment for Lateral Epicondylitis, A Retrospective 1-Year Follow-Up Study. J. Orthop. Sports Med. 2024, 6, 219–226. [Google Scholar] [CrossRef]
- Ogéus, T. Treatment of Osgood-Schlatter disease in an adolescent athlete with liquid platelet-rich fibrin and heat-coagulated albumin gel: A case report. J. Orthop. Sports Med. 2024, 8, 53–56. [Google Scholar] [CrossRef]
- Ogéus, T. Stromal vascular fraction with platelet-rich fibrin for osteoarthritis management in knee and hip osteoarthritis: A retrospective 2-year follow-up study. J. Orthop. Sports Med. 2024, 6, 135–143. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Ibrahim, J.G.; Love, M.I. Heavy-tailed prior distributions for sequence count data: Removing the noise and preserving large differences. Bioinformatics 2019, 35, 2084–2092. [Google Scholar] [CrossRef]
- Shouib, R.; Eitzen, G.; Steenbergen, R. A Guide to Basic RNA Sequencing Data Processing and Transcriptomic Analysis. Bio-Protocol 2025, 15, e5295. [Google Scholar] [CrossRef]
- Gu, Z. Complex heatmap visualization. Imeta 2022, 1, e43. [Google Scholar] [CrossRef]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Lex, A.; Gehlenborg, N.; Strobelt, H.; Vuillemot, R.; Pfister, H. UpSet: Visualization of Intersecting Sets. IEEE Trans. Vis. Comput. Graph. 2014, 20, 1983–1992. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. g:Profiler-interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef]

| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| CXCL2 | CCCATGGTTAAGAAAATCATCG | CTTCAGGAACAGCCACCAAT |
| CXCL5 | AGCTGCGTTGCGTTTGTTTAC | TGGCGAACACTTGCAGATTAC |
| CXCL8 | AACTTCTCCACAACCCTCTG | TTGGCAGCCTTCCTGATTTC |
| IL11 | AAATAAGGCACAGATGCC | CCTTCCAAAGCCAGATC |
| GAPDH | AGCCACATCGCTCAGACAC | GCCCAATACGACCAAATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Panahipour, L.; Rassi Faghihi, D.; Miron, R.J.; Gruber, R. Extended PRF: Impact of Heat on Gene Expression in Gingival Fibroblasts. Int. J. Mol. Sci. 2025, 26, 9120. https://doi.org/10.3390/ijms26189120
Huang X, Panahipour L, Rassi Faghihi D, Miron RJ, Gruber R. Extended PRF: Impact of Heat on Gene Expression in Gingival Fibroblasts. International Journal of Molecular Sciences. 2025; 26(18):9120. https://doi.org/10.3390/ijms26189120
Chicago/Turabian StyleHuang, Xiaoyu, Layla Panahipour, Dorna Rassi Faghihi, Richard J. Miron, and Reinhard Gruber. 2025. "Extended PRF: Impact of Heat on Gene Expression in Gingival Fibroblasts" International Journal of Molecular Sciences 26, no. 18: 9120. https://doi.org/10.3390/ijms26189120
APA StyleHuang, X., Panahipour, L., Rassi Faghihi, D., Miron, R. J., & Gruber, R. (2025). Extended PRF: Impact of Heat on Gene Expression in Gingival Fibroblasts. International Journal of Molecular Sciences, 26(18), 9120. https://doi.org/10.3390/ijms26189120







