Apoptosis Inhibitor of Macrophage (AIM) Modulates Calcium Oxalate-Induced Ureteral Fibrosis in AIM-Felinized Mice
Abstract
1. Introduction
2. Results
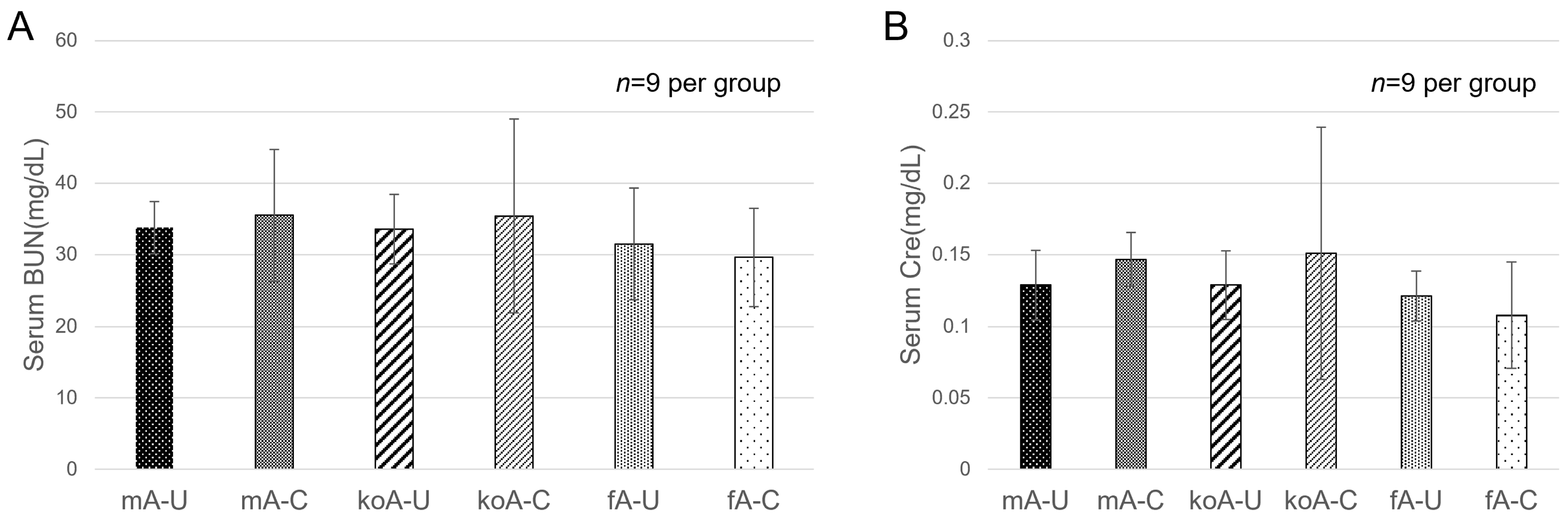
2.1. Blood Biochemistry Test
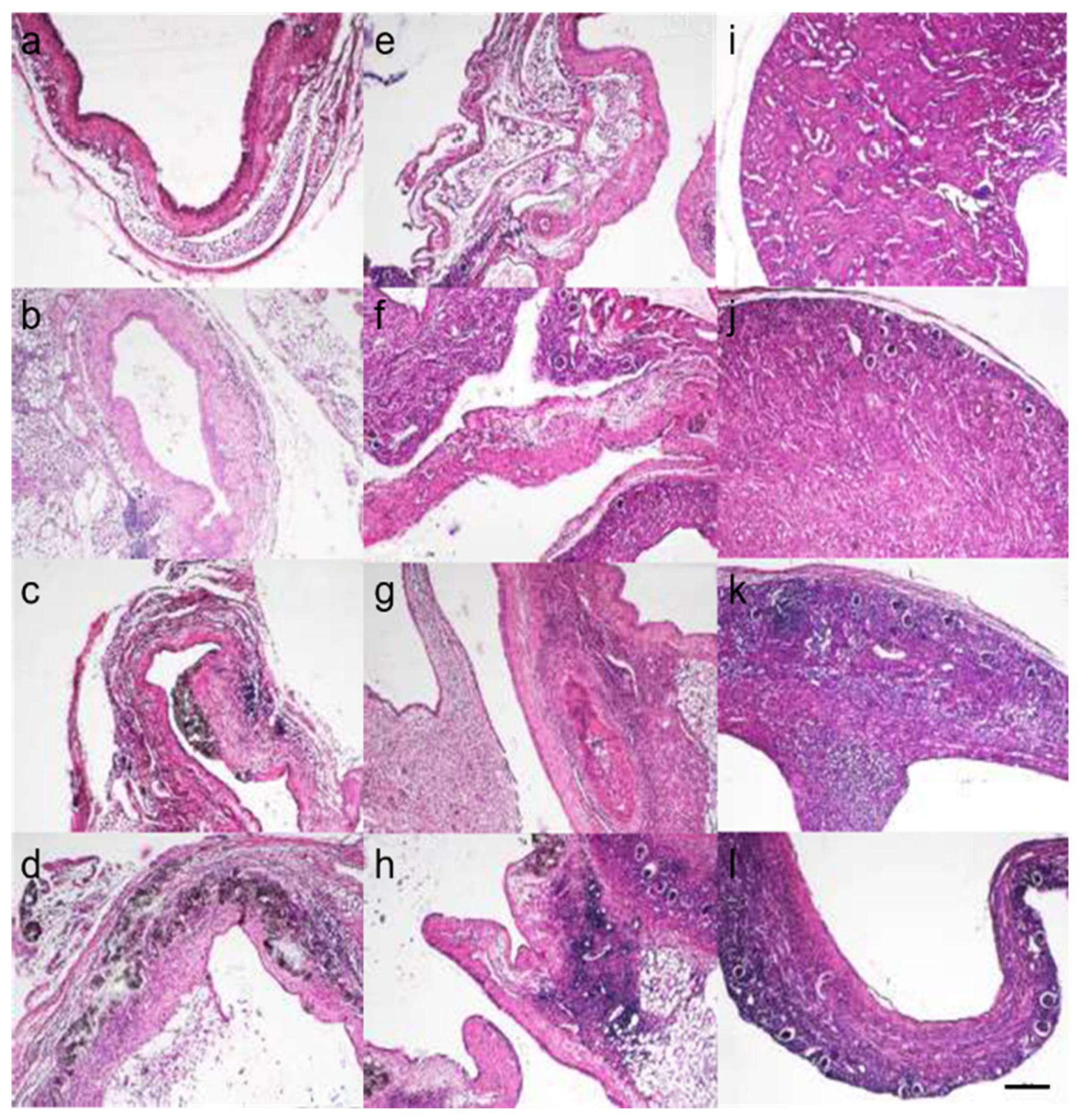
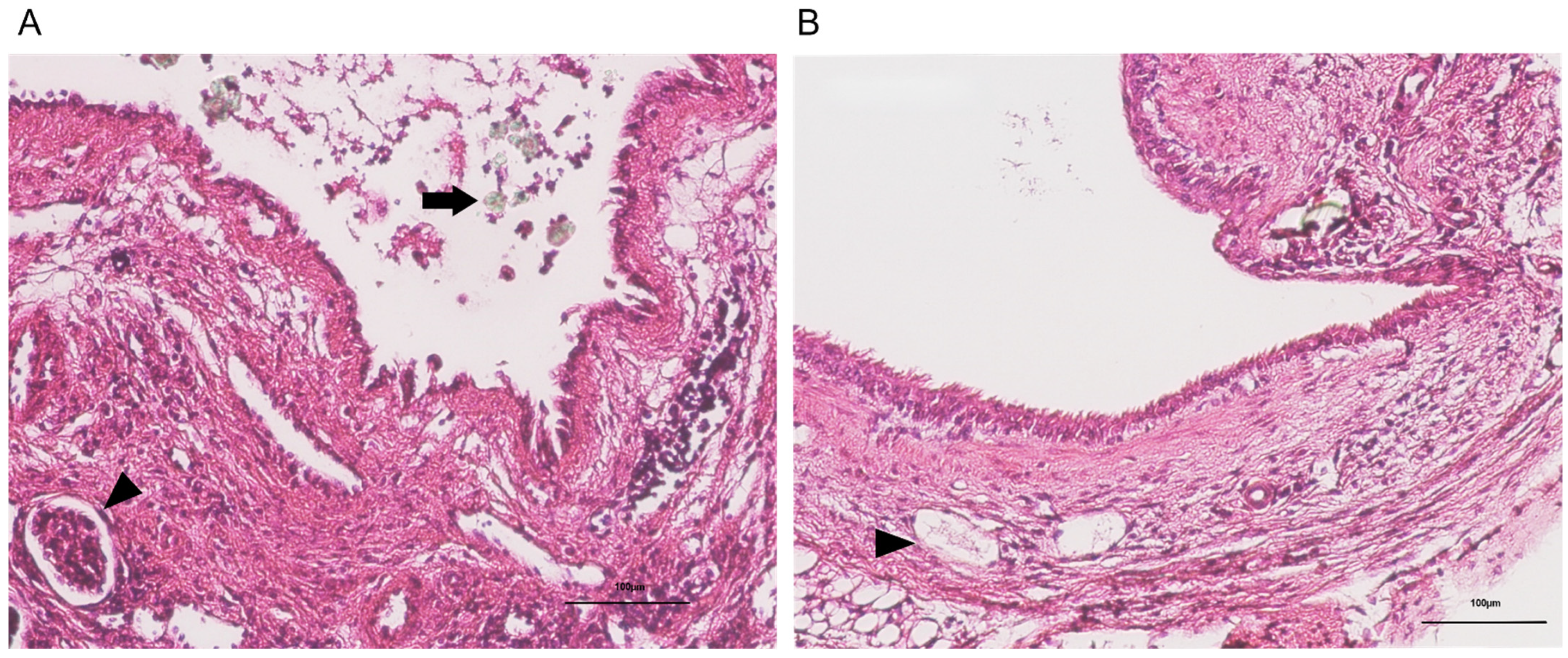
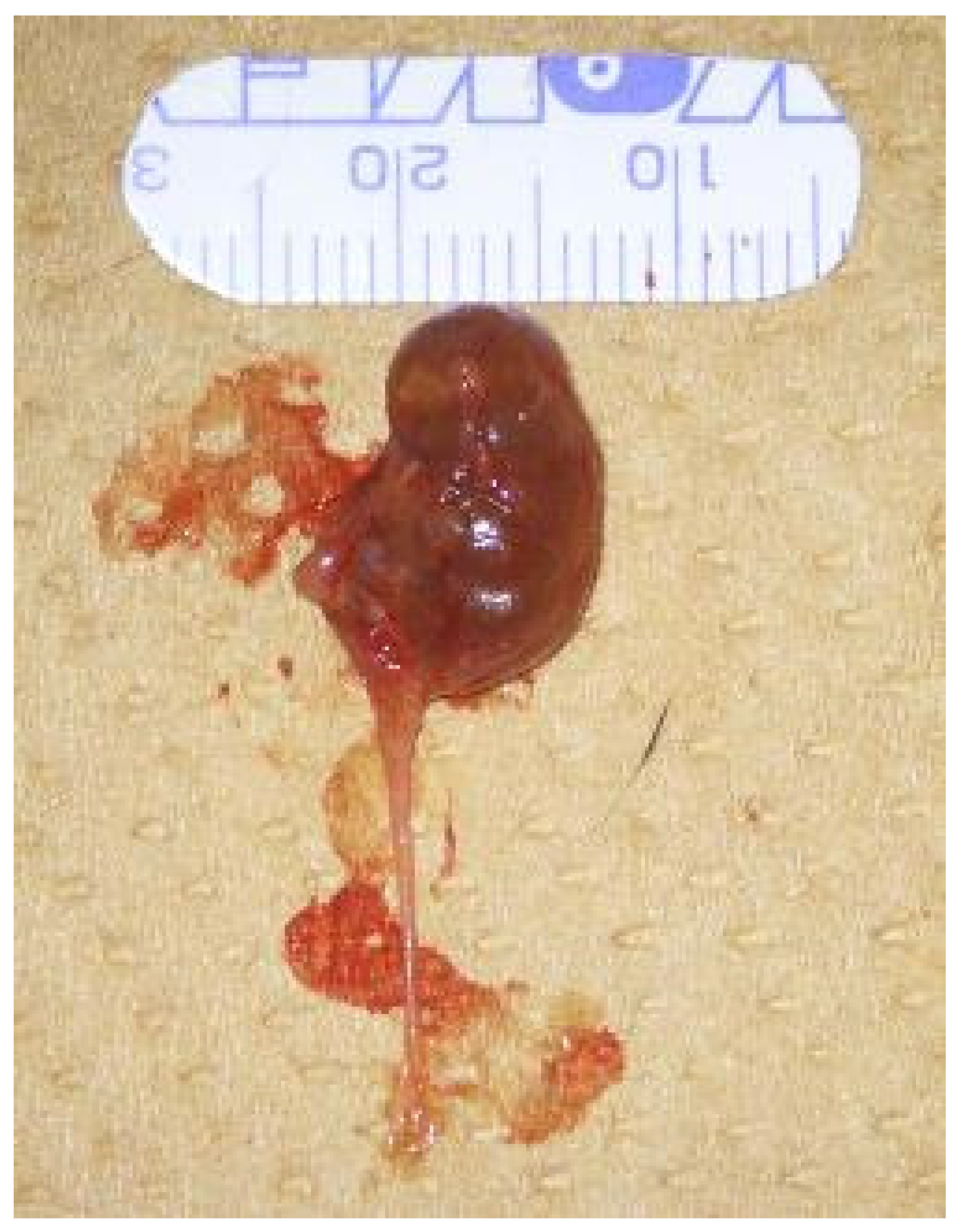
2.2. Histopathological Findings
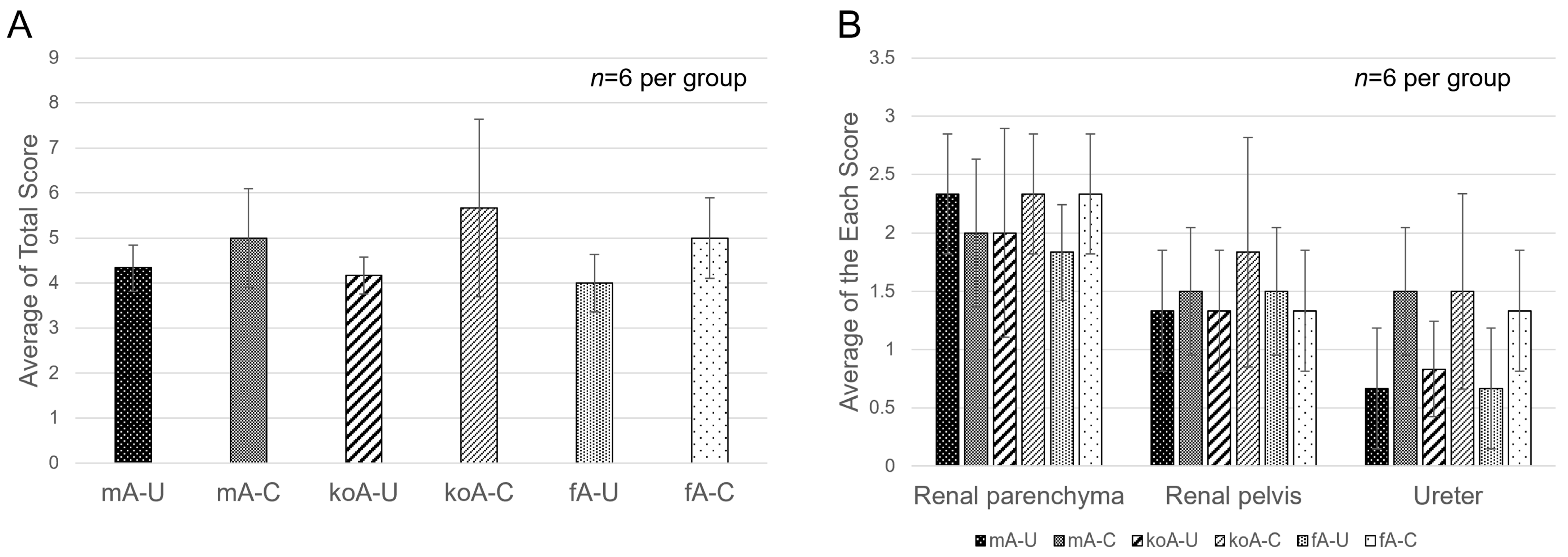
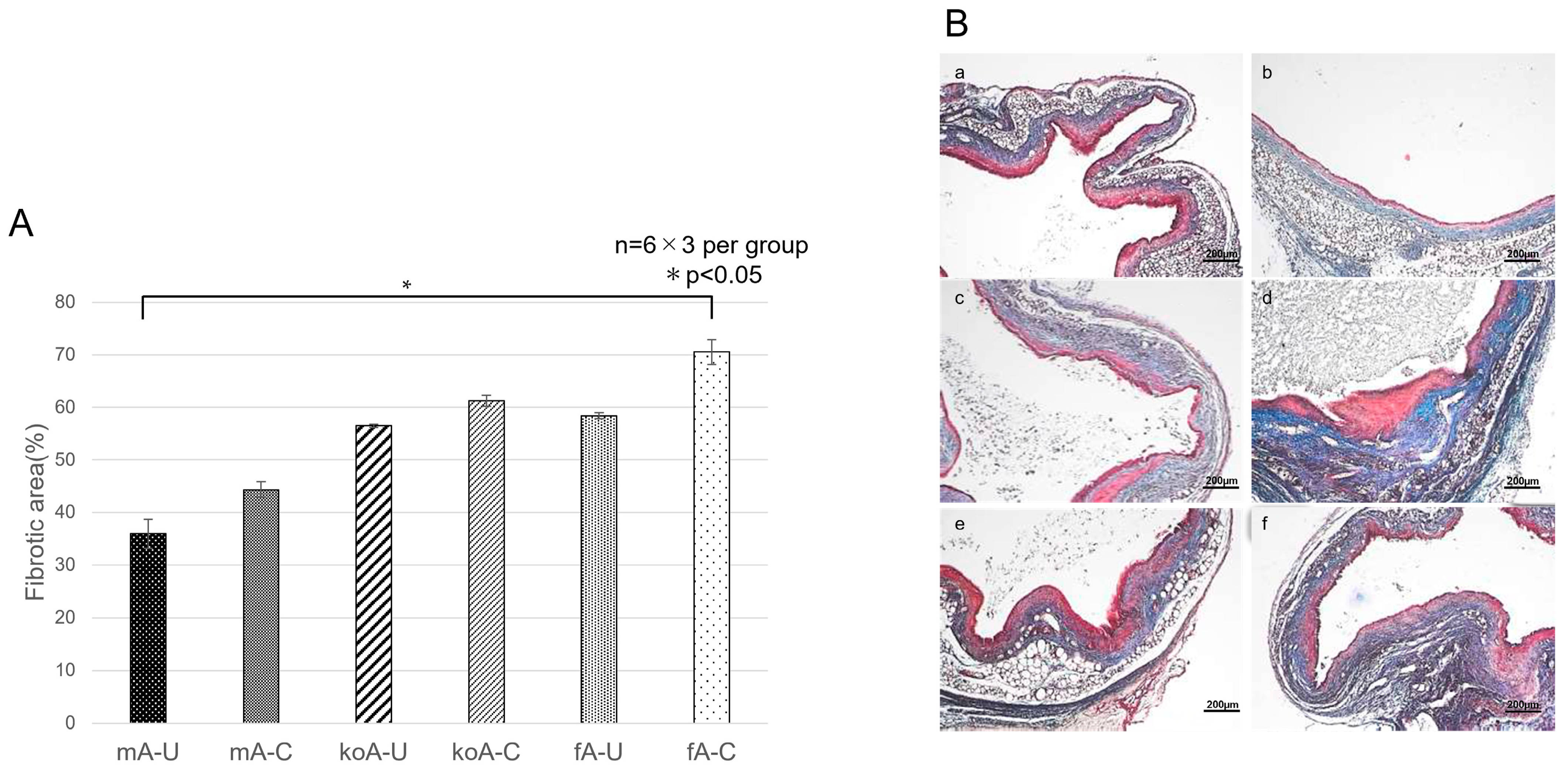
2.3. Quantification of Fibrosis
2.4. Immunohistochemistry (IHC) Staining
2.4.1. αSMA (Acta2)
2.4.2. AIM (CD5L)
3. Discussion
4. Materials and Methods
4.1. Test Animals
AIM-Knockout and AIM Felinized C57BL/6J Mice
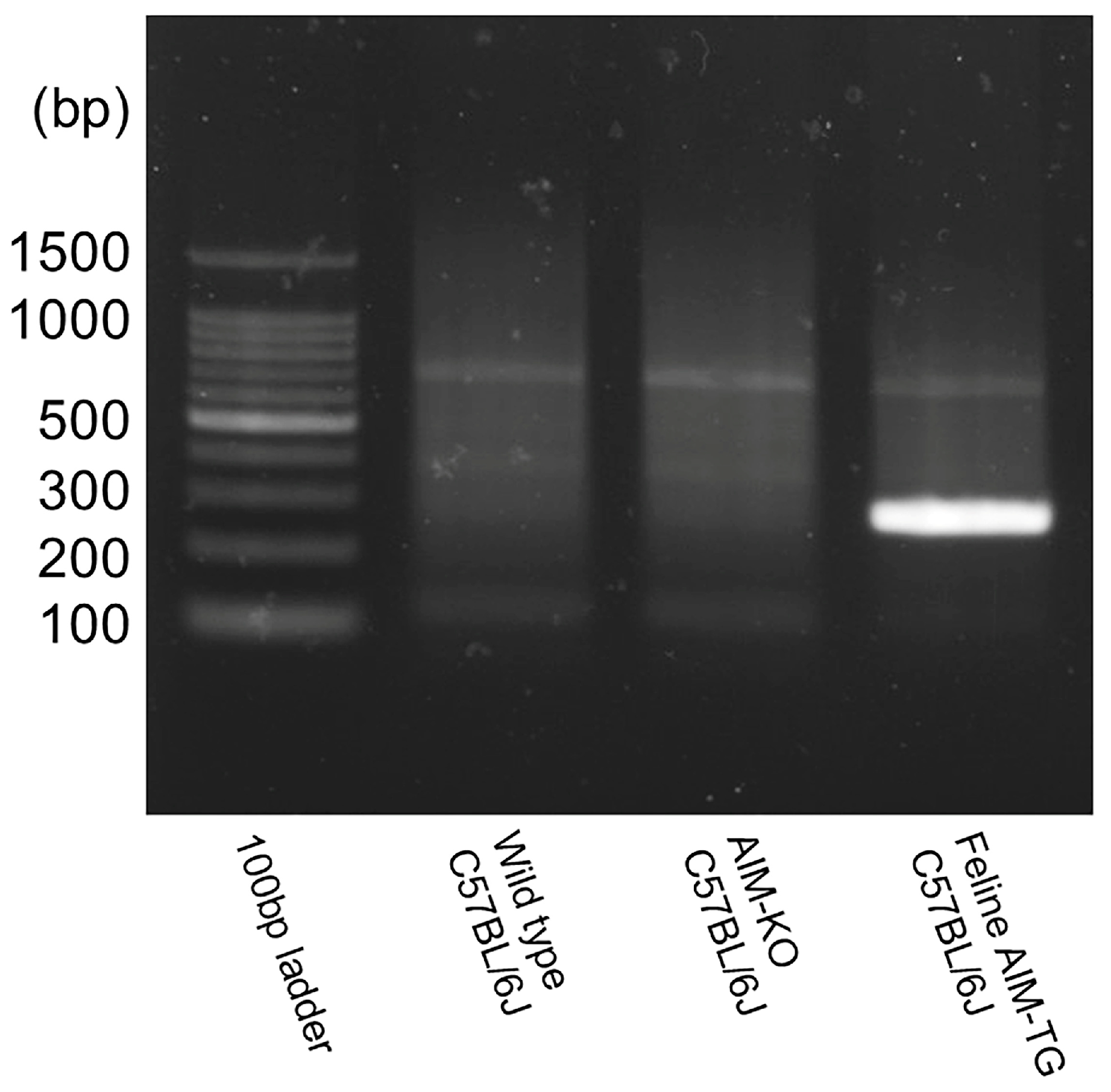
4.2. Experimental Groups
4.3. Ureteral Lumen Injury Model
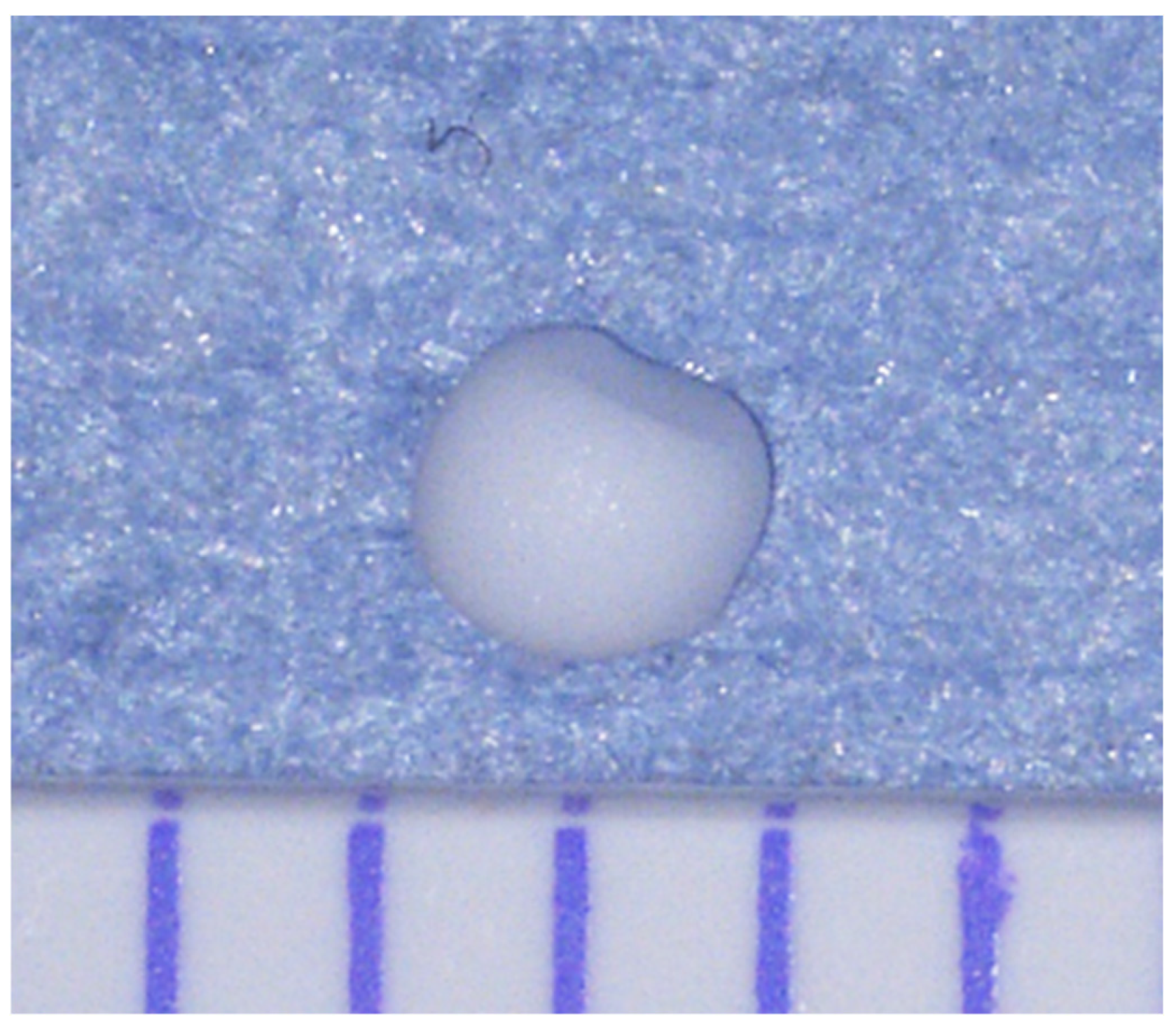
4.3.1. Preparation of a CaOx Beads
4.3.2. Anesthesia Protocol, Analgesic Management
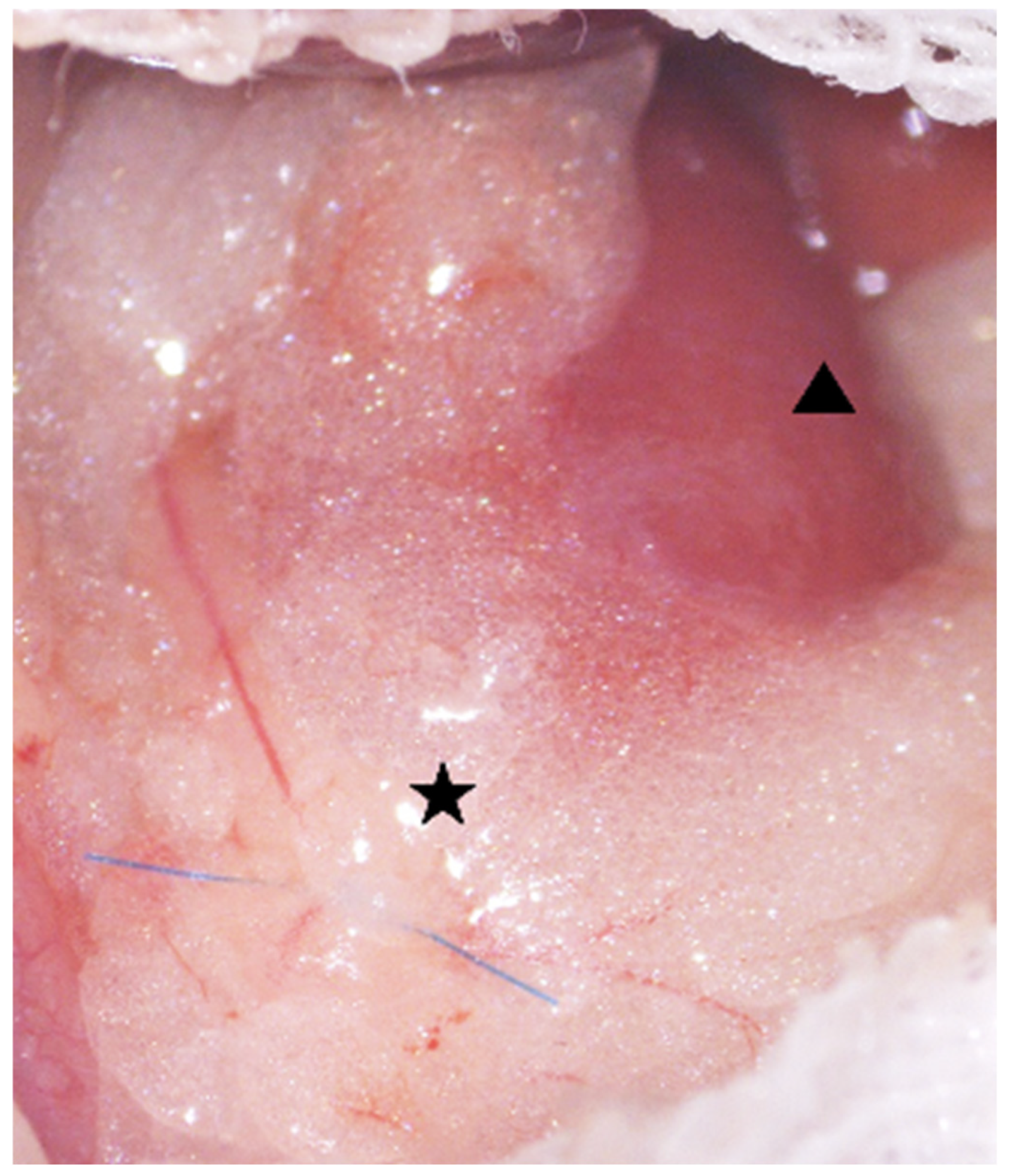
4.3.3. Ureteral Ligation and Insertion of CaOx Beads
4.4. Endpoint Criteria
4.5. Blood and Biochemical Tests
4.6. Histopathological Examination
4.6.1. Tissue Collection and Sectioning
4.6.2. Tissue Staining
4.6.3. Fibrosis Quantification
4.7. IHC Staining
4.7.1. αSMA (Acta2)
4.7.2. AIM (CD5L)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIM | Apoptosis inhibitor of macrophage |
| CKD | Chronic kidney disease |
| AKI | Acute kidney injury |
| CaOx | Calcium oxalate |
| UUO | Unilateral ureteral obstruction |
| HE | Hematoxylin and Eosin |
| MT | Masson’s Trichrome |
| αSMA | Alpha-smooth muscle actin |
| BUN | Blood urea nitrogen |
| CRE | Creatinine |
| TGF-β | Transforming growth factor-beta |
| ECM | Extracellular matrix |
| SRCR | Scavenger receptor cysteine-rich |
References
- Cannon, A.B.; Westropp, J.L.; Ruby, A.L.; Kass, P.H. Evaluation of trends in urolith composition in cats: 5230 cases (1985–2004). J. Am. Vet. Med. Assoc. 2007, 231, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Kyles, A.E.; Hardie, E.M.; Wooden, B.G.; Adin, C.A.; Stone, E.A.; Gregory, C.R.; Mathews, K.G.; Cowgill, L.D.; Vaden, S.; Nyland, T.G.; et al. Clinical, clinicopathologic, radiographic, and ultrasonographic abnormalities in cats with ureteral calculi: 163 cases (1984–2002). J. Am. Vet. Med. Assoc. 2005, 226, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Kyles, A.E.; Stone, E.A.; Gookin, J.; Spaulding, K.; Clary, E.M.; Wylie, K.; Spodnick, G. Diagnosis and surgical management of ob-structive ureteral calculi in cats: 11 cases (1993–1996). J. Am. Vet. Med. Assoc. 1998, 213, 1150–1556. [Google Scholar] [CrossRef]
- A Palm, C.; Westropp, J.L. Cats and Calcium Oxalate: Strategies for managing lower and upper tract stone disease. J. Feline Med. Surg. 2011, 13, 651–660. [Google Scholar] [CrossRef]
- Iwai, S. Ureterolithiasis in Cats. Jpn. J. Vet. Anesth. Surg. 2021, 52, 1–13. [Google Scholar] [CrossRef]
- Hardie, E.M.; E Kyles, A. Management of ureteral obstruction. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 989–1010. [Google Scholar] [CrossRef]
- Reicherz, A.; Eltit, F.; Scotland, K.; Almutairi, K.; Bell, R.; Mojtahedzadeh, B.; Cox, M.; Chew, B.; Lange, D. Indwelling stents cause severe inflammation and fibrosis of the ureter via urothelial–mesenchymal transition. Sci. Rep. 2023, 13, 5492. [Google Scholar] [CrossRef]
- Low, W.W.; Uhl, J.M.; Kass, P.H.; Ruby, A.L.; Westropp, J.L. Evaluation of trends in urolith composition and characteristics of dogs with urolithiasis: 25,499 cases (1985–2006). J. Am. Vet. Med. Assoc. 2010, 236, 193–200. [Google Scholar] [CrossRef]
- Wormser, C.; Clarke, D.L.; Aronson, L.R. Outcomes of ureteral surgery and ureteral stenting in cats: 117 cases (2006–2014). J. Am. Vet. Med. Assoc. 2016, 248, 518–525. [Google Scholar] [CrossRef]
- Culp, W.T.N.; Palm, C.A.; Hsueh, C.; Mayhew, P.D.; Hunt, G.B.; Johnson, E.G.; Drobatz, K.J. Outcome in cats with benign ureteral obstructions treated by means of ureteral stenting versus ureterotomy. J. Am. Vet. Med. Assoc. 2016, 249, 1292–1300. [Google Scholar] [CrossRef]
- Bartges, J.W.; Callens, A.J. Urolithiasis. Vet. Clin. N. Am. Small Anim. Pract. 2015, 45, 747–768. [Google Scholar] [CrossRef]
- Zaid, M.S.; Berent, A.C.; Weisse, C.; Caceres, A. Feline Ureteral Strictures: 10 Cases (2007–2009). J. Vet. Intern. Med. 2011, 25, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Berent, A.C.; Weisse, C.W.; Bagley, D.H.; Lamb, K. Use of a subcutaneous ureteral bypass device for treatment of benign ureteral obstruction in cats: 174 ureters in 134 cats (2009–2015). J. Am. Vet. Med. Assoc. 2018, 253, 1309–1327. [Google Scholar] [CrossRef] [PubMed]
- Kulendra, N.J.; Borgeat, K.; Syme, H.; Dirrig, H.; Halfacree, Z. Survival and complications in cats treated with subcutaneous ureteral bypass. J. Small Anim. Pract. 2020, 62, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Lulich, J.P.; Berent, A.C.; Adams, L.G.; Westropp, J.L.; Bartges, J.W.; Osborne, C.A. ACVIMSmall Animal Consensus Recommendations on the Treatment and Prevention of Uroliths in Dogs and Cats. J. Vet. Intern. Med. 2016, 30, 1564–1574. [Google Scholar] [CrossRef]
- Reicherz, A.; Eltit, F.; Almutairi, K.; Mojtahedzadeh, B.; Herout, R.; Chew, B.; Cox, M.; Lange, D. Ureteral Obstruction Promotes Ureteral Inflammation and Fibrosis. Eur. Urol. Focus 2023, 9, 371–380. [Google Scholar] [CrossRef]
- Kochin, E.J.; Gregory, C.R.; Wisner, E.; Cain, G.; Gourley, I.M. Evaluation of a method of ureteroneocystostomy in cats. J. Am. Vet. Med. Assoc. 1993, 202, 257–260. [Google Scholar] [CrossRef]
- Bohling, M.W. Wound Healing. Feline Soft Tissue and General Surgery; Langley-Hobbs, S.J., Demetrious, L., Ladlow, J.F., Eds.; Saunders Elsevier: New York, NY, USA, 2014; pp. 171–175. [Google Scholar]
- Chevalier, R.L.; Forbes, M.S.; Thornhill, B.A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009, 75, 1145–1152. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Chuang, W.L.; Huang, S.P.; Liu, K.M.; Huang, C.H. The temporal relationship between the severity of hydroureter and the dynamic changes of obstructed ureters in a rat model. Br. J. Urol. 1995, 76, 303–310. [Google Scholar] [CrossRef]
- Watanabe, M.; Ando, R.; Sugisawa, R.; Sasaki, N.; Iwai, S. A novel in vivo model of ureteral fibrosis induced by calcium oxalate beads in C57BL/6J mice. Urolithiasis 2023, 51, 119. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hirokami, Y.; Matsuhashi, N.; Takatsuka, H.; Naito, M. Increased Susceptibility of Thymocytes to Apoptosis in Mice Lacking AIM, a Novel Murine Macrophage-derived Soluble Factor Belonging to the Scavenger Receptor Cysteine-rich Domain Superfamily. J. Exp. Med. 1999, 189, 413–422. [Google Scholar] [CrossRef]
- Hiramoto, E.; Tsutsumi, A.; Suzuki, R.; Matsuoka, S.; Arai, S.; Kikkawa, M.; Miyazaki, T. The IgM pentamer is an asymmetric pentagon with an open groove that binds the AIM protein. Sci. Adv. 2018, 4, eaau1199. [Google Scholar] [CrossRef]
- Kai, T.; Yamazaki, T.; Arai, S.; Miyazaki, T.; Aguila, M.B. Stabilization and Augmentation of Circulating AIM in Mice by Synthesized IgM-Fc. PLoS ONE 2014, 9, e97037. [Google Scholar] [CrossRef]
- Chen, Q.; Ishii, K.; Mori, H.; Nishijima, A.; Arai, S.; Miyazaki, T.; Rosenthal, P.B. Cryo-EM reveals structural basis for human AIM/CD5L recognition of polymeric immunoglobulin M. Nat. Commun. 2024, 15, 9387. [Google Scholar] [CrossRef]
- Yasuda, K.; Nishijima, A.; Inoue, T.; Takagi, T.; Tanabe, K.; Minakuchi, J.; Arai, S.; Miyazaki, T. Release of Apoptosis Inhibitor of Macrophage (AIM) from Pentameric IgM in Serum Predicts Prognosis after Hemodialysis Initiation. Commun. Med. 2025, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Maehara, N.; Iwamura, Y.; Honda, S.-I.; Nakashima, K.; Kai, T.; Ogishi, M.; Morita, K.; Kurokawa, J.; Mori, M.; et al. Obesity-Associated Autoantibody Production Requires AIM to Retain the Immunoglobulin M Immune Complex on Follicular Dendritic Cells. Cell Rep. 2013, 3, 1187–1198. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Miyazaki, T. A scavenging system against internal pathogens promoted by the circulating protein apoptosis inhibitor of macrophage (AIM). Semin. Immunopathol. 2018, 40, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.G.; Escoda-Ferran, C.; Simões, I.T.; Arai, S.; Mascaró, M.O.; Carreras, E.; Martínez-Florensa, M.; Yelamos, J.; Miyazaki, T.; Lozano, F. The macrophage soluble receptor AIM/Api6/CD5L displays a broad pathogen recognition spectrum and is involved in early response to microbial aggression. Cell. Mol. Immunol. 2014, 11, 343–354. [Google Scholar] [CrossRef]
- Miyazaki, T.; Yamazaki, T.; Sugisawa, R.; Gershwin, M.E.; Arai, S. AIM associated with the IgM pentamer: Attackers on stand-by at aircraft carrier. Cell. Mol. Immunol. 2018, 15, 563–574. [Google Scholar] [CrossRef]
- Arai, S.; Miyazaki, T. Impacts of the apoptosis inhibitor of macrophage (AIM) on obesity-associated inflammatory diseases. Semin. Immunopathol. 2014, 36, 3–12. [Google Scholar] [CrossRef]
- Maehara, N.; Taniguchi, K.; Okuno, A.; Ando, H.; Hirota, A.; Li, Z.; Wang, C.-T.; Arai, S.; Miyazaki, T. AIM/CD5L attenuates DAMPs in the injured brain and thereby ameliorates ischemic stroke. Cell Rep. 2021, 36, 109693. [Google Scholar] [CrossRef]
- Arai, S.; Kitada, K.; Yamazaki, T.; Takai, R.; Zhang, X.; Tsugawa, Y.; Sugisawa, R.; Matsumoto, A.; Mori, M.; Yoshihara, Y.; et al. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat. Med. 2016, 22, 183–193. [Google Scholar] [CrossRef]
- Yamazaki, T.; Sugisawa, R.; Hiramoto, E.; Takai, R.; Matsumoto, A.; Senda, Y.; Nakashima, K.; Nelson, P.S.; Lucas, J.M.; Morgan, A.; et al. A proteolytic modification of AIM promotes its renal excretion. Sci. Rep. 2016, 6, 38762. [Google Scholar] [CrossRef]
- Komatsu, G.; Nonomura, T.; Sasaki, M.; Ishida, Y.; Arai, S.; Miyazaki, T. AIM-deficient mouse fed a high-trans fat, high-cholesterol diet: A new animal model for nonalcoholic fatty liver disease. Exp. Anim. 2019, 68, 147–158. [Google Scholar] [CrossRef]
- Kurokawa, J.; Arai, S.; Nakashima, K.; Nagano, H.; Nishijima, A.; Miyata, K.; Ose, R.; Mori, M.; Kubota, N.; Kadowaki, T.; et al. Macrophage-Derived AIM Is Endocytosed into Adipocytes and Decreases Lipid Droplets via Inhibition of Fatty Acid Synthase Activity. Cell Metab. 2010, 11, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, J.; Nagano, H.; Ohara, O.; Kubota, N.; Kadowaki, T.; Arai, S.; Miyazaki, T. Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 12072–12077. [Google Scholar] [CrossRef] [PubMed]
- Maehara, N.; Arai, S.; Mori, M.; Iwamura, Y.; Kurokawa, J.; Kai, T.; Kusunoki, S.; Taniguchi, K.; Ikeda, K.; Ohara, O.; et al. Circulating AIM Prevents Hepatocellular Carcinoma through Complement Activation. Cell Rep. 2014, 9, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Kurokawa, J.; Arai, S. AIMing at Metabolic Syndrome-Towards the Development of Novel Therapies for Metabolic Diseases via Apoptosis Inhibitor of Macrophage (AIM). Circ. J. 2011, 75, 2522–2531. [Google Scholar] [CrossRef]
- Okanoue, T.; Yamaguchi, K.; Shima, T.; Mitsumoto, Y.; Mizuno, M.; Katayama, T.; Seko, Y.; Moriguchi, M.; Umemura, A.; Itoh, Y.; et al. Serum levels of immunoglobulin M-free inhibitors of macrophage/CD5L as a predictive and early diagnostic marker for nonalcoholic steatohepatitis-associated hepatocellular carcinoma. Hepatol. Res. 2022, 52, 998–1008. [Google Scholar] [CrossRef]
- Sugisawa, R.; Komatsu, G.; Hiramoto, E.; Takeda, N.; Yamamura, K.-I.; Arai, S.; Miyazaki, T. Independent modes of disease repair by AIM protein distinguished in AIM-felinized mice. Sci. Rep. 2018, 8, 13157. [Google Scholar] [CrossRef]
- Matsuura, K.; Maehara, N.; Hirota, A.; Eguchi, A.; Yasuda, K.; Taniguchi, K.; Nishijima, A.; Matsuhashi, N.; Shiga, Y.; Ishii, R.; et al. Two independent modes of kidney stone suppression achieved by AIM/CD5L and KIM-1. Commun. Biol. 2022, 5, 783. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, H.; Honjo, M.; Arai, S.; Miyazaki, T.; Aihara, M. Apoptosis inhibitor of macrophages/CD5L enhances phagocytosis in the trabecular meshwork cells and regulates ocular hypertension. J. Cell. Physiol. 2023, 238, 2451–2467. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Maehara, N.; Kai, T.; Arai, S.; Miyazaki, T. Dietary fructose-induced hepatocellular carcinoma development manifested in mice lacking apoptosis inhibitor of macrophage (AIM). Genes Cells 2016, 21, 1320–1332. [Google Scholar] [CrossRef]
- Takahata, A.; Arai, S.; Hiramoto, E.; Kitada, K.; Kato, R.; Makita, Y.; Suzuki, H.; Nakata, J.; Araki, K.; Miyazaki, T.; et al. Crucial Role of AIM/CD5L in the Development of Glomerular Inflammation in IgA Nephropathy. J. Am. Soc. Nephrol. 2020, 31, 2013–2024. [Google Scholar] [CrossRef]
- Takimoto-Sato, M.; Suzuki, M.; Kimura, H.; Ge, H.; Matsumoto, M.; Makita, H.; Arai, S.; Miyazaki, T.; Nishimura, M.; Konno, S. Apoptosis inhibitor of macrophage (AIM)/CD5L is involved in the pathogenesis of COPD. Respir. Res. 2023, 24, 201. [Google Scholar] [CrossRef]
- Tomita, T.; Arai, S.; Kitada, K.; Mizuno, M.; Suzuki, Y.; Sakata, F.; Nakano, D.; Hiramoto, E.; Takei, Y.; Maruyama, S.; et al. Apoptosis inhibitor of macrophage ameliorates fungus-induced peritoneal injury model in mice. Sci. Rep. 2017, 7, 6450. [Google Scholar] [CrossRef]
- Wang, C.-T.; Tezuka, T.; Takeda, N.; Araki, K.; Arai, S.; Miyazaki, T.; Joles, J.A. High salt exacerbates acute kidney injury by disturbing the activation of CD5L/apoptosis inhibitor of macrophage (AIM) protein. PLoS ONE 2021, 16, e0260449. [Google Scholar] [CrossRef]
- Yamazaki, T.; Mori, M.; Arai, S.; Tateishi, R.; Abe, M.; Ban, M.; Nishijima, A.; Maeda, M.; Asano, T.; Kai, T.; et al. Circulating AIM as an Indicator of Liver Damage and Hepatocellular Carcinoma in Humans. PLoS ONE 2014, 9, e109123. [Google Scholar] [CrossRef]
- Yasuda, K.; Shimodan, S.; Maehara, N.; Hirota, A.; Iijima, R.; Nishijima, A.; Mori, H.; Toyama, R.; Ito, A.; Yoshikawa, Y.; et al. AIM/CD5L ameliorates autoimmune arthritis by promoting removal of inflammatory DAMPs at the lesions. J. Autoimmun. 2023, 142, 103149. [Google Scholar] [CrossRef]
- Sugisawa, R.; Hiramoto, E.; Matsuoka, S.; Iwai, S.; Takai, R.; Yamazaki, T.; Mori, N.; Okada, Y.; Takeda, N.; Yamamura, K.-I.; et al. Impact of feline AIM on the susceptibility of cats to renal disease. Sci. Rep. 2016, 6, 35251. [Google Scholar] [CrossRef]
- Le Clef, N.; Verhulst, A.; D’Haese, P.C.; A Vervaet, B. Unilateral Renal Ischemia-Reperfusion as a Robust Model for Acute to Chronic Kidney Injury in Mice. PLoS ONE 2016, 11, e0152153. [Google Scholar] [CrossRef]
- Capelouto, C.C.; Saltzman, B. The Pathophysiology of Ureteral Obstruction. J. Endourol. 1993, 7, 93–103. [Google Scholar] [CrossRef]
- Moe, O.W. Kidney stones: Pathophysiology and medical management. Lancet 2006, 367, 333–344. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor–β in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Mann, P.C.; Vahle, J.; Keenan, C.M.; Baker, J.F.; Bradley, A.E.; Goodman, D.G.; Harada, T.; Herbert, R.; Kaufmann, W.; Kellner, R.; et al. International Harmonization of Toxicologic Pathology Nomenclature: An overview and review of basic principles. Toxicol. Pathol. 2012, 40, 7S–13S. [Google Scholar] [CrossRef]


| Calcium Oxalate Group | Unilateral Ureteral Obstruction | ||
|---|---|---|---|
| C Group | U Group | ||
| C57BL/6 | mA | mA-C Group | mA-U Group |
| Mouse AIM-KO-C57BL/6 | koA | koA-C Group | koA-U Group |
| AIM Felinized C57BL/6 | fA | fA-C Group | fA-U Group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machida, Y.; Watanabe, M.; Suzuki, F.; Ando, R.; Watanabe, K.; Moriya, Y.; Maeda, K.; Okano, S.; Okamura, T.; Sugisawa, R.; et al. Apoptosis Inhibitor of Macrophage (AIM) Modulates Calcium Oxalate-Induced Ureteral Fibrosis in AIM-Felinized Mice. Int. J. Mol. Sci. 2025, 26, 9117. https://doi.org/10.3390/ijms26189117
Machida Y, Watanabe M, Suzuki F, Ando R, Watanabe K, Moriya Y, Maeda K, Okano S, Okamura T, Sugisawa R, et al. Apoptosis Inhibitor of Macrophage (AIM) Modulates Calcium Oxalate-Induced Ureteral Fibrosis in AIM-Felinized Mice. International Journal of Molecular Sciences. 2025; 26(18):9117. https://doi.org/10.3390/ijms26189117
Chicago/Turabian StyleMachida, Yuka, Masaki Watanabe, Fumi Suzuki, Ryo Ando, Koudai Watanabe, Yugo Moriya, Kenichi Maeda, Shozo Okano, Tadashi Okamura, Ryoichi Sugisawa, and et al. 2025. "Apoptosis Inhibitor of Macrophage (AIM) Modulates Calcium Oxalate-Induced Ureteral Fibrosis in AIM-Felinized Mice" International Journal of Molecular Sciences 26, no. 18: 9117. https://doi.org/10.3390/ijms26189117
APA StyleMachida, Y., Watanabe, M., Suzuki, F., Ando, R., Watanabe, K., Moriya, Y., Maeda, K., Okano, S., Okamura, T., Sugisawa, R., Sasaki, N., & Iwai, S. (2025). Apoptosis Inhibitor of Macrophage (AIM) Modulates Calcium Oxalate-Induced Ureteral Fibrosis in AIM-Felinized Mice. International Journal of Molecular Sciences, 26(18), 9117. https://doi.org/10.3390/ijms26189117







