Efficacy of 2,4-Dinitrobenzenesulfonic Acid (DNBS) in the Maintenance of a Model of Inflammatory Bowel Disease in Pigs (Sus scrofa domestica)
Abstract
1. Introduction
2. Results
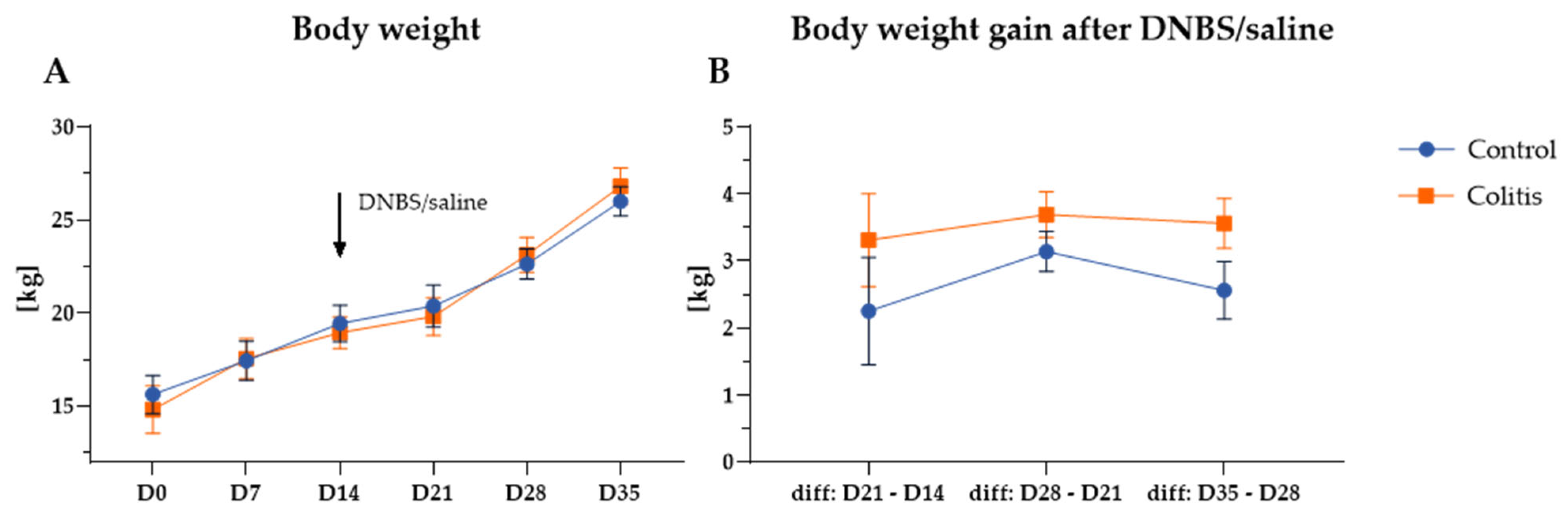
2.1. Body Weight and Body Weight Gain
2.2. Clinical Condition of Animals in the Experimental Period
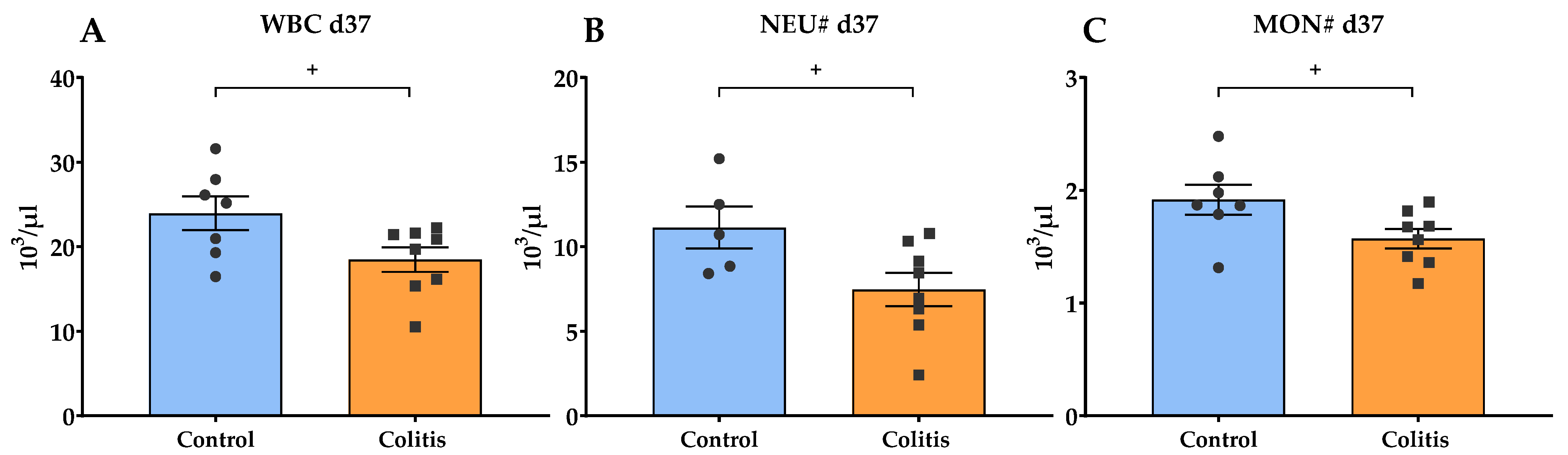
2.3. Hematological and Biochemical Examination of Blood
2.4. Plasma Cytokines Concentrations
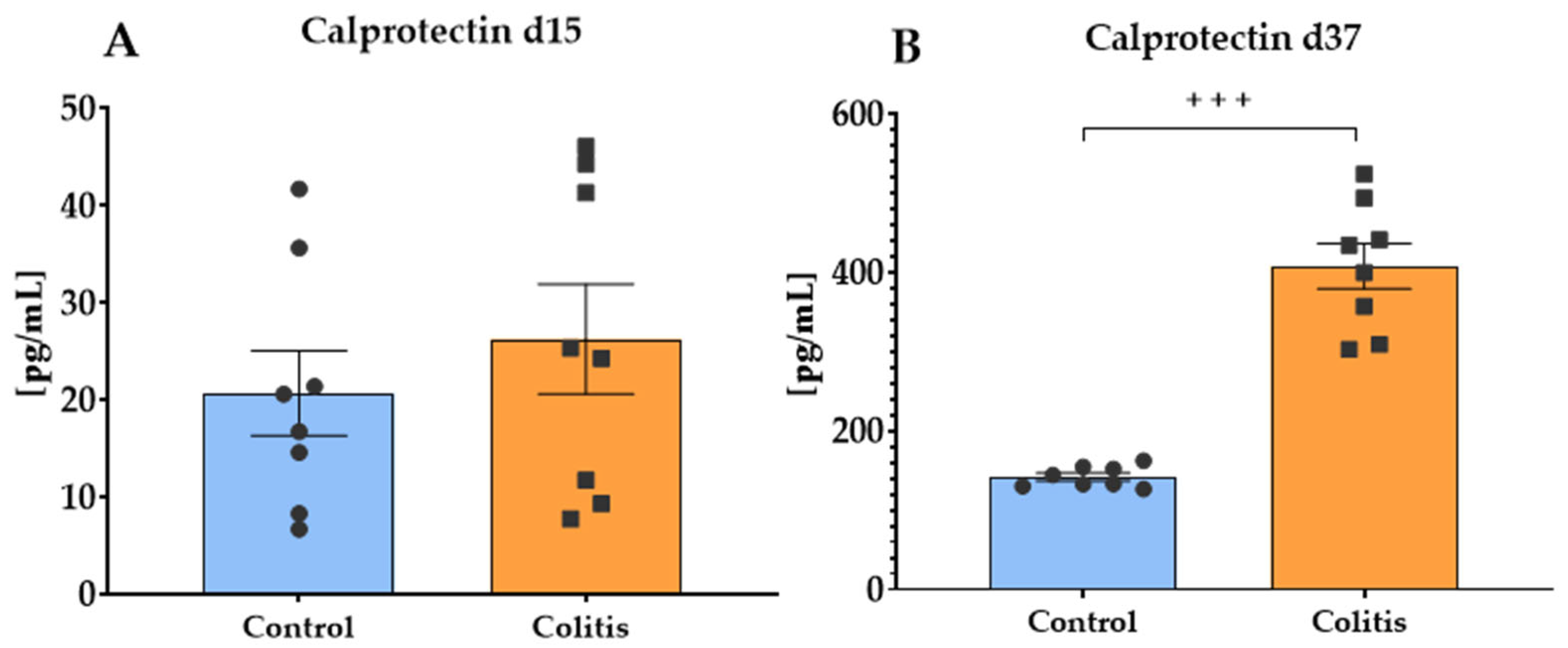
2.5. Fecal Calprotectin Concentration
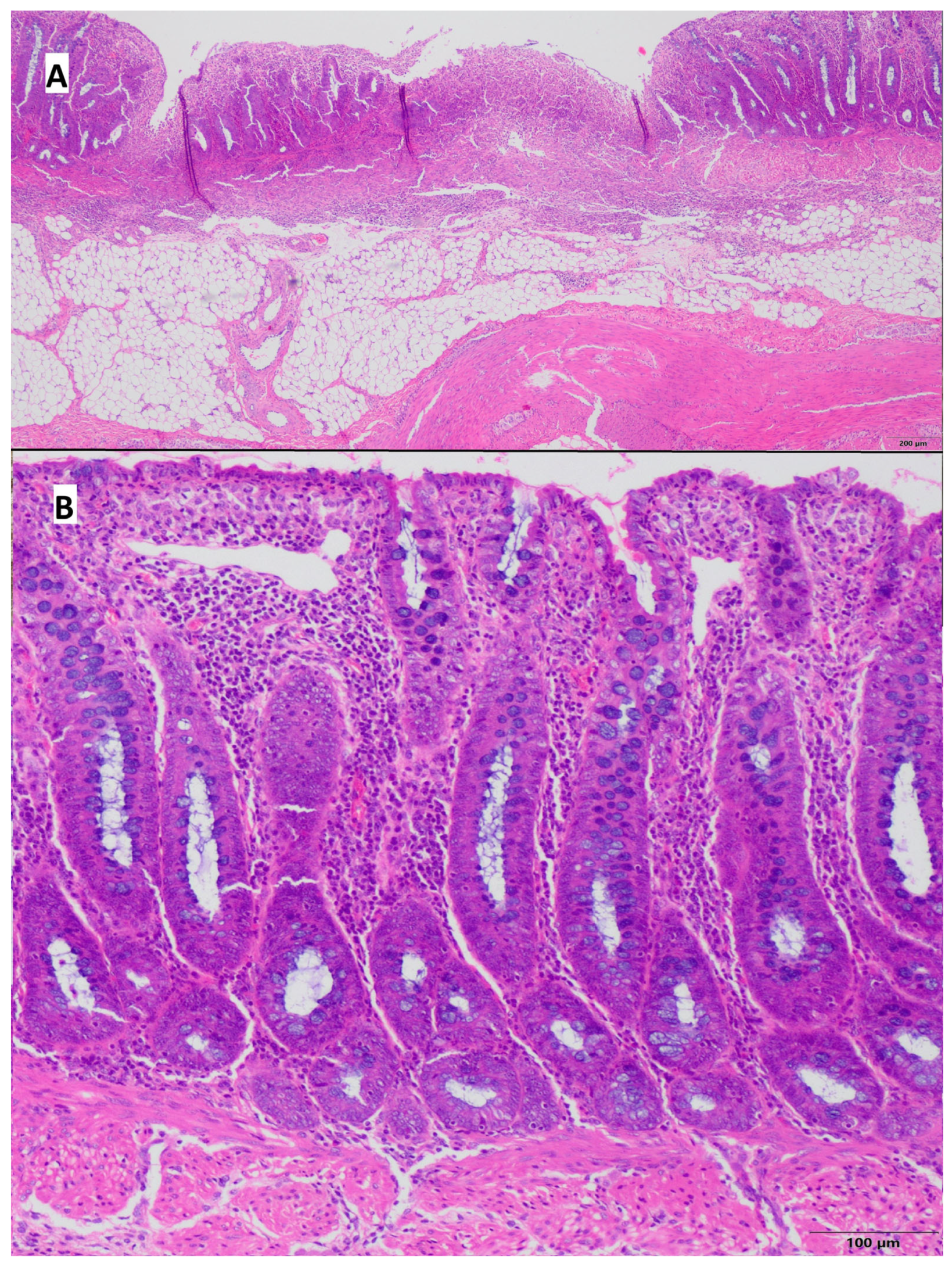
2.6. Histopathological Evaluation of Porcine Colon
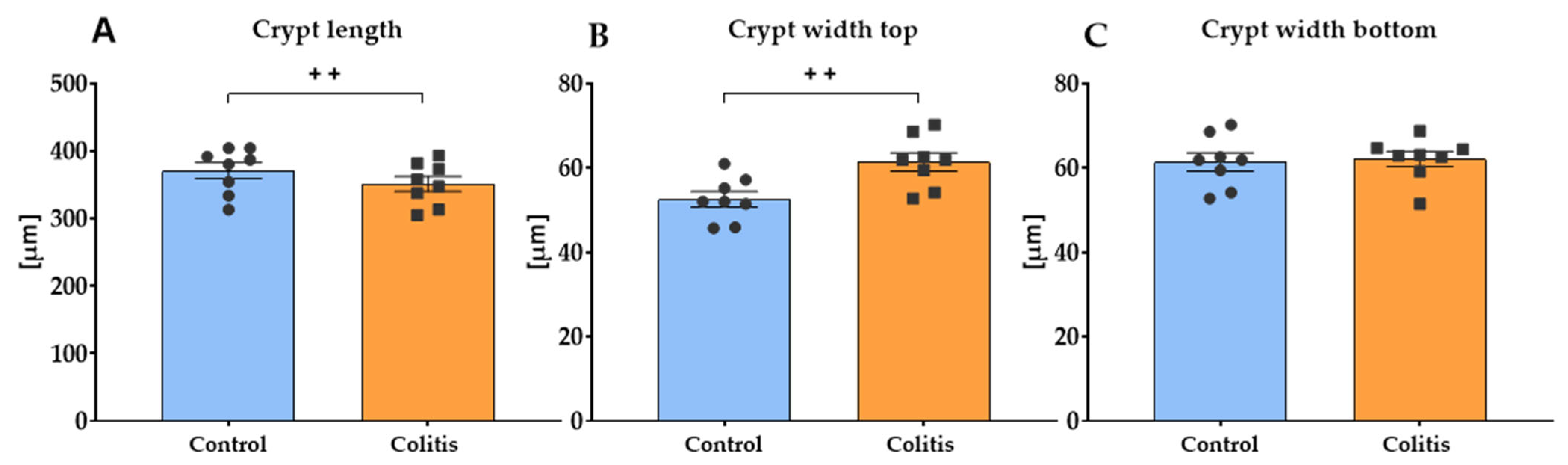
2.7. Histomorphometric Analysis of the Small Intestine
3. Discussion
4. Materials and Methods
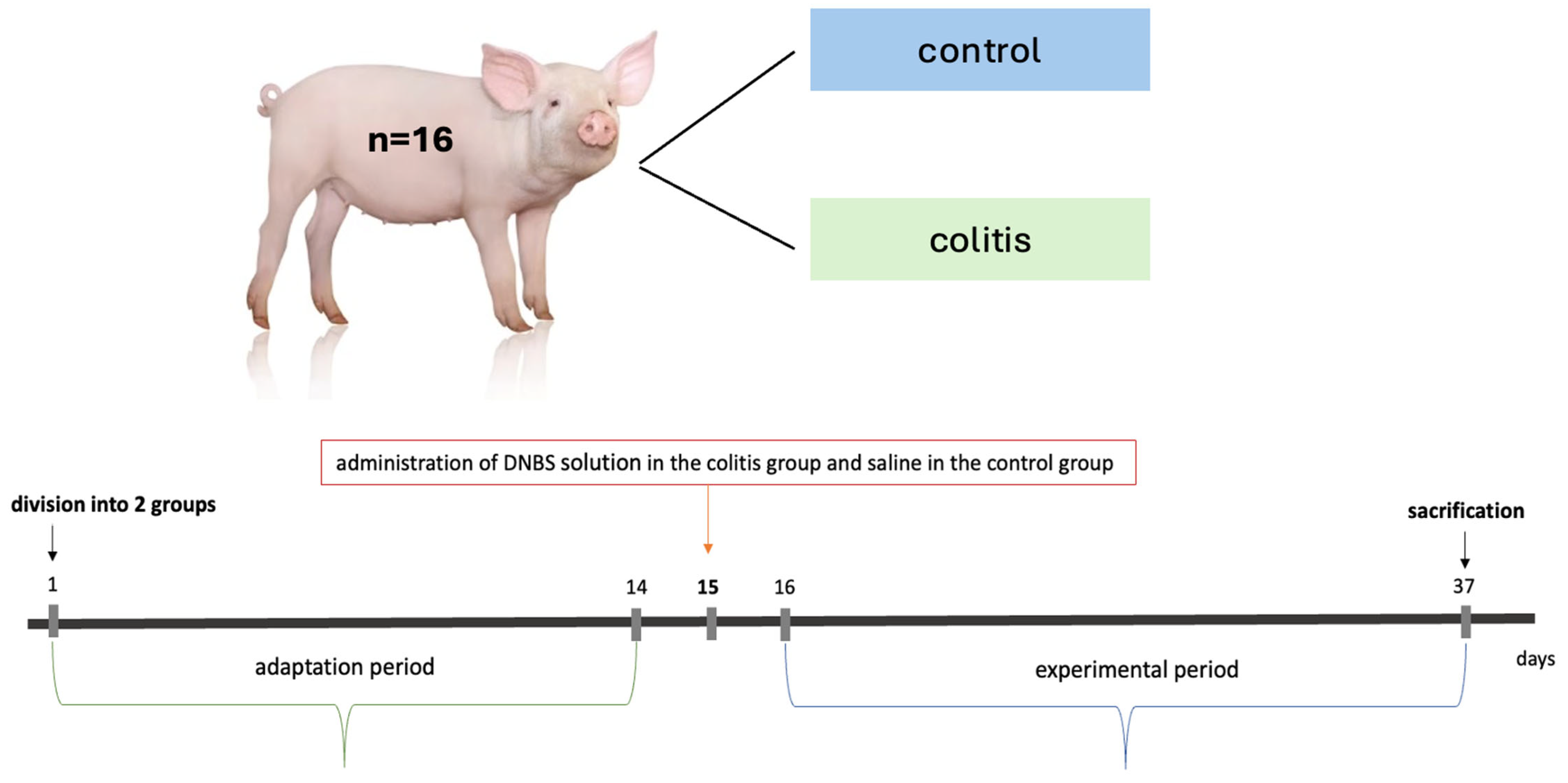
4.1. Animal Experiment
4.1.1. Evaluation of the Clinical Condition of Animals
4.1.2. Sampling Procedure and Autopsy
4.2. Hematological and Biochemical Examination of the Blood
4.3. Assessment of Plasma Cytokine Levels
4.4. Examine Fecal Calprotectin Levels
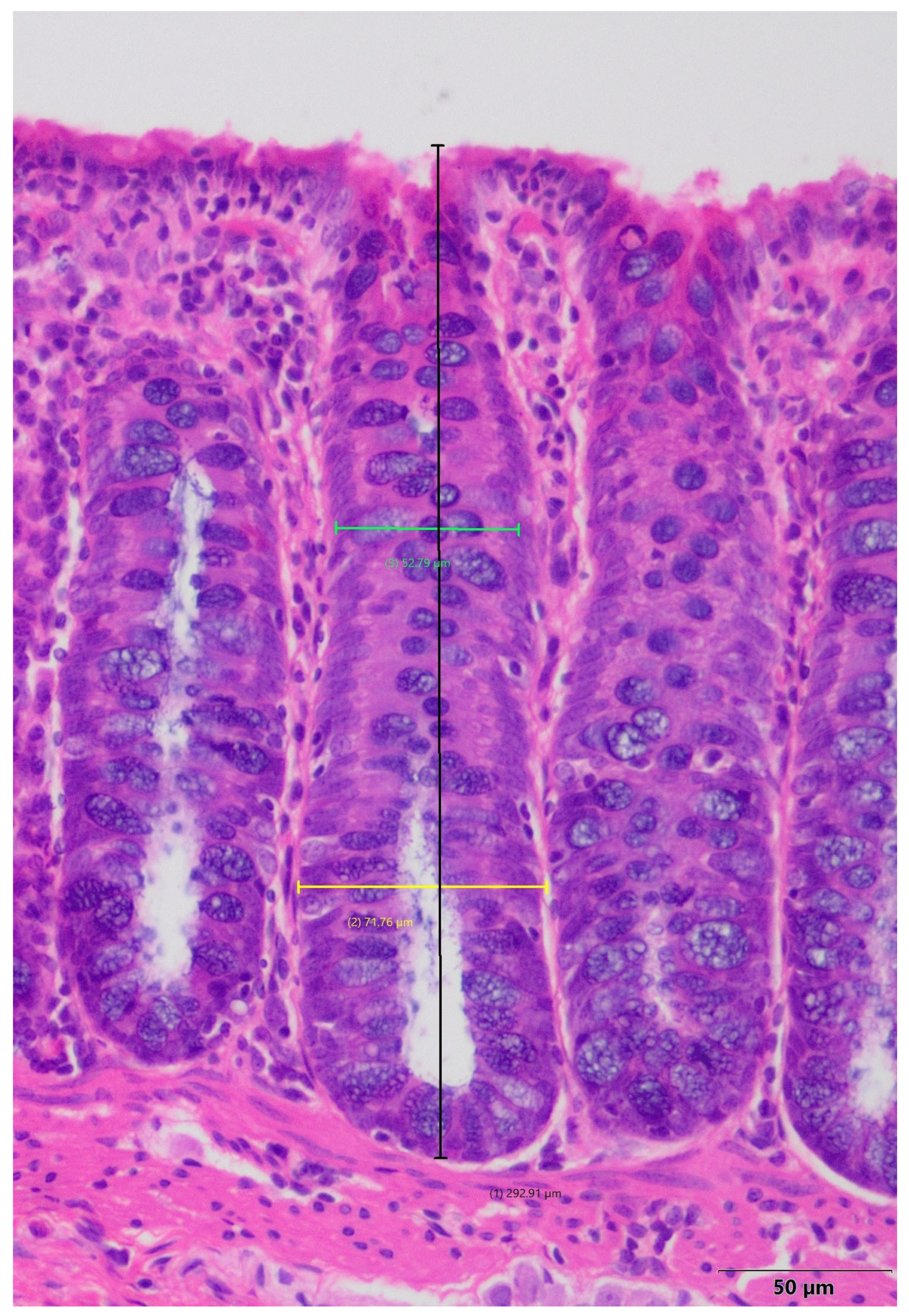
4.5. Histopathological Evaluation of Porcine Colon and Small Intestine
4.6. Statistical Analysis
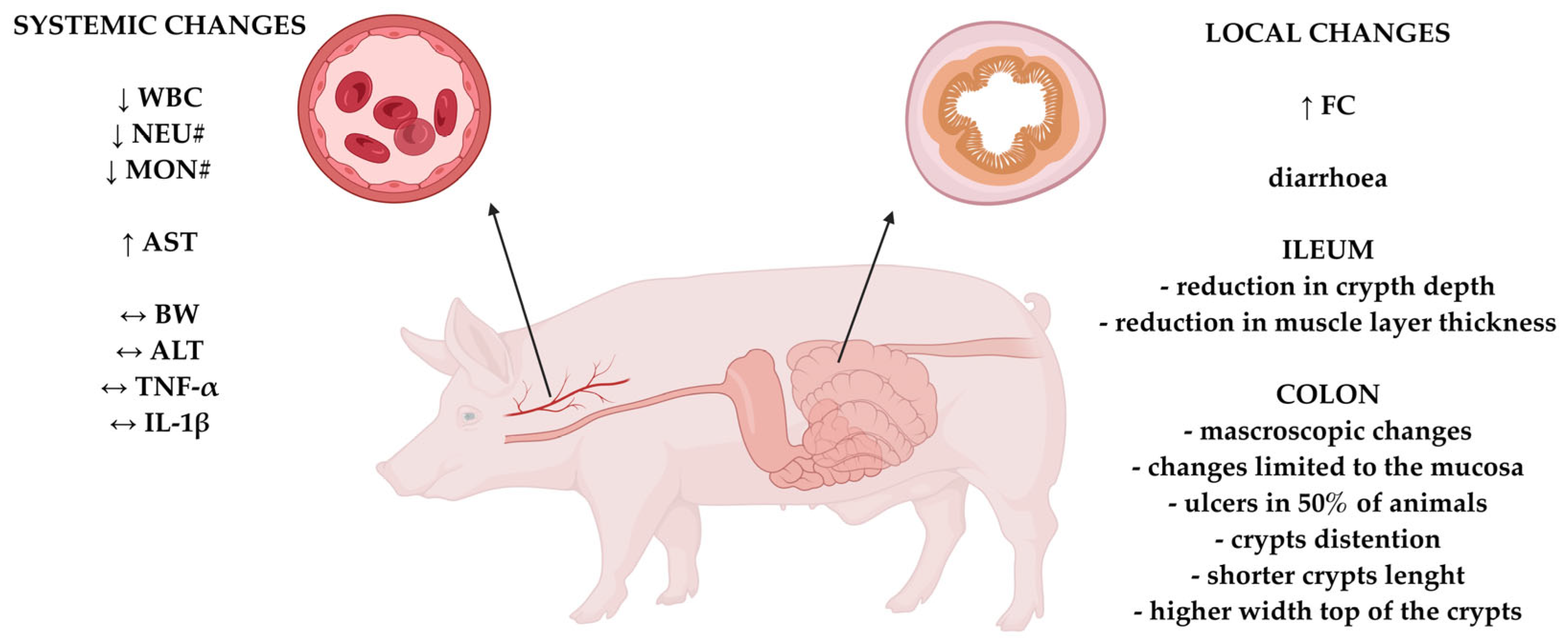
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | Chronic inflammatory bowel disease |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| GIT | Gastrointestinal tract |
| DNBS | 2,4-dinitrobenzenesulfonic acid |
| TNBS | 2,4,6-trinitrobenzene sulfonic acid |
| DSS | Dextran sulfate sodium |
| BW | Body weight |
| BWG | Body weight gain |
| AP | Abdominal pain |
| FQF | Fecal quality and frequency |
| WBC | White blood cells |
| NEU# | Absolute value of neutrophils |
| MON# | Absolute value of monocytes |
| IETs | Intraepithelial layer |
| FC | Fecal calprotectin |
| TNF-α | Tumor necrosis factor-alpha |
| IL-1β | Interleukin-1 beta |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, diagnosis, and management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Sanat, Z.M.; Vahedi, H.; Malekzadeh, R.; Fanni, Z. Epidemiologic profile of inflammatory bowel disease in Eastern Mediterranean Region (EMRO) countries: A systematic review and meta-analysis. BMC Public Health 2024, 24, 1395. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C. Changing Global Epidemiology of Inflammatory Bowel Diseases: Sustaining Health Care Delivery Into the 21st Century. Clin. Gastroenterol. Hepatol. 2020, 18, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Windsor, J.W.; Kalpan, G.G. Evolving epidemiology of IBD. Curr. Gastroenterol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Dollinger, M.; Torres, J.; Vermeire, S. Crohn’s disease. Lancet 2024, 403, 1177–1191. [Google Scholar] [CrossRef]
- Plevris, N.; Lees, C.W. Disease monitoring in inflammatory bowel disease: Evolving principles and possibilities. Gastroenterology 2022, 162, 1456–1475. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Disease-a-Month 2018, 64, 20–57. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Disease-a-Month 2019, 65, 100851. [Google Scholar] [CrossRef]
- Silva, I.; Pinto, R.; Mateus, V. Preclinical study in vivo for new pharmacological approaches in inflammatory bowel disease: A systematic review of chronic model of TNBS-induced colitis. J. Clin. Med. 2019, 8, 1574. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Yadav, D.; Katiyar, S.; Jain, S.; Yadav, H. Postbiotics as Mitochondrial Modulators in Inflammatory Bowel Disease: Mechanistic Insights and Therapeutic Potential. Biomolecules 2025, 15, 954. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Roy, S. Prospective therapeutic targets and recent advancements in the treatment of inflammatory bowel disease. Immunopharmacol. Immunotoxicol. 2024, 46, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO guidelines on therapeutics in Crohn’s disease: Medical treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Anka, D.; Senhaji, N.; Aouiss, A.; Khalki, L.; Tijani, Y.; Zaid, N.; Marhoume, F.Z.; Naya, A.; Oudghiri, M.; Kabine, M.; et al. IL-1 and CD40/CD40L platelet complex: Elements of induction of Crohn’s disease and new therapeutic targets. Arch. Pharmacal Res. 2021, 44, 117–132. [Google Scholar] [CrossRef]
- Baydi, Z.; Limami, Y.; Khalki, L.; Zaid, N.; Naya, A.; Mtairag, E.M.; Oudghri, M.; Zaid, Y. An update of research animal models of inflammatory bowel disease. Sci. World J. 2021, 2021, 7479540. [Google Scholar] [CrossRef]
- Ma, S.; Yeom, J.; Lim, Y.H. Dairy Propionibacterium freudenreichii ameliorates acute colitis by stimulating MUC2 expression in intestinal goblet cell in a DSS-induced colitis rat model. Sci. Rep. 2020, 10, 5523. [Google Scholar] [CrossRef]
- Morampudi, V.; Bhinder, G.; Wu, X.; Dai, C.; Sham, H.P.; Vallance, B.A.; Jacobson, K. DNBS/TNBS colitis models: Providing insights into inflammatory bowel disease and effects of dietary fat. J. Vis. Exp. 2014, 84, 51297. [Google Scholar] [CrossRef]
- Patterson, J.K.; Lei, X.G.; Miller, D.D. The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Exp. Biol. Med. 2008, 233, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Heinritz, S.N.; Mosenthin, R.; Weiss, E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr. Res. Rev. 2013, 26, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Palenca, I.; Seguella, L.; Del Re, A.; Franzin, S.B.; Corpetti, C.; Pesce, M.; Rurgo, S.; Steardo, L.; Sarnelli, G.; Esposito, G. N-Palmitoyl-D-Glucosamine Inhibits TLR-4/NLRP3 and Improves DNBS-Induced Colon Inflammation through a PPAR-α-Dependent Mechanism. Biomolecules 2022, 12, 1163. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.S.; Fredborg, M.; Theil, P.K.; Yue, Y.; Bruhn, L.V.; Andersen, V.; Purup, S. Dietary red meat adversely affects disease severity in a pig model of DSS-induced colitis despite reduction in colonic pro-inflammatory gene expression. Nutrients 2020, 12, 1728. [Google Scholar] [CrossRef]
- Wen, C.; Chen, D.; Zhong, R.; Peng, X. Animal models of inflammatory bowel disease: Category and evaluation indexes. Gastroenterol. Rep. 2024, 12, goae021. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Geboes, K.; Riddell, R.; Öst, A.; Jensfelt, B.; Persson, T.; Löfberg, R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef]
- Wang, M.; Donovan, S.M. Human microbiota-associated swine: Current progress and future opportunities. ILAR J. 2015, 56, 63–73. [Google Scholar] [CrossRef]
- Yin, L.; Yang, H.; Li, J.; Li, Y.; Ding, X.; Wu, G.; Yin, Y. Pig models on intestinal development and therapeutics. Amino Acids 2017, 49, 2099–2106. [Google Scholar] [CrossRef]
- Szkopek, D.; Wychowański, P.; Zaworski, K.; Seklecka, B.; Starzyński, R.; Lipiński, P.; Pierzynowska, K.; Pierzynowski, S.G.; Donaldson, J.; Paczewski, Ł.; et al. Investigating the Influence of a Tooth Absence on Facial Bone Growth Using a Porcine Model. Int. J. Mol. Sci. 2024, 25, 12509. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Manser, C.; Pittet, V.; Vavricka, S.R.; Biedermann, L. Gender differences in inflammatory bowel disease. Digestion 2020, 101, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Zagórowicz, E.; Walkiewicz, D.; Kucha, P.; Perwieniec, J.; Maluchnik, M.; Wieszczy, P.; Reguła, J. Nationwide data on epidemiology of inflammatory bowel disease in Polan between 2009 and 2020. Pol. Arch. Intern. Med. 2022, 132, 16194. [Google Scholar] [CrossRef] [PubMed]
- Breugelmans, T.; Van Spaendonk, H.; De Man, J.G.; De Schepper, H.U.; Jauregui-Amezaga, A.; Macken, E.; Linden, S.K.; Pintelon, I.; Timmermans, J.; De Winter, B.Y.; et al. In-depth study of transmembrane mucins in association with intestinal barrier dysfunction during the course of T cell transfer and DSS-induces colitis. J. Crohn’s Colitis 2020, 14, 974–994. [Google Scholar] [CrossRef]
- Xiao, H.; Li, H.; Wen, Y.; Jiang, D.; Zhu, S.; He, X.; Xiong, Q.; Gao, J.; Hou, S.; Huang, S.; et al. Tremella fuciformis polysaccharides ameliorated ulcerative colitis via inhibiting inflammation and enhancing intestinal epithelial barrier function. Int. J. Biol. Macromol. 2021, 180, 633–642. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di Carlo, M.; Serio, R. Preventive effects of guanosine on intestinal inflammation in 2, 4-dinitrobenzene sulfonic acid (DNBS)-induced colitis in rats. Inflammopharmacology 2019, 27, 349–359. [Google Scholar] [CrossRef]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Valatas, V.; Bamias, G.; Kolios, G. Experimental colitis models: Insights into the pathogenesis of inlammatory bowel disease and translational issues. Eur. J. Pharmacol. 2015, 759, 253–264. [Google Scholar] [CrossRef]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran-sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Gancarcikova, S.; Lauko, S.; Hrckova, G.; Andrejcakova, Z.; Hajduckova, V.; Madar, M.; Fecskeova, L.K.; Mudronova, D.; Mravcova, K.; Strkolcova, G.; et al. Innovative Animal Model of DSS-Induced Ulcerative Colitis in Pseudo Germ-Free Mice. Cells 2020, 9, 2571. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Adedara, I.A.; Awoyem, O.V.; Njoku, C.R.; Micah, G.O.; Esogwa, C.U.; Owumi, S.E.; Olopade, J.O. Dietary protocatechuic acid ameliorates dextran sulphate sodium-induced ulcerative colitis and hepatotoxicity in rats. Food Funct. 2016, 7, 913–921. [Google Scholar] [CrossRef]
- Żyła, E.; Dziendzikowska, K.; Gajewska, M.; Wilczak, J.; Harasym, J.; Gromadzka-Ostrowska, J. Beneficial effects of oat beta-glucan dietary supplementation in colitis depend on its molecular weight. Molecules 2019, 24, 3591. [Google Scholar] [CrossRef]
- Sadik, C.D.; Kim, N.D.; Luster, A.D. Neutrophils cascading their way to inflammation. Trends Immunol. 2011, 32, 452–460. [Google Scholar] [CrossRef]
- Kopiasz, Ł.; Dziendzikowska, K.; Gajewska, M.; Wilczak, J.; Harasym, J.; Żyła, E.; Kamola, D.; Oczkowski, M.; Królikowski, T.; Gromadzka-Ostrowska, J. Time-Dependent Indirect Antioxidative Effects of Oat Beta-Glucans on Peripheral Blood Parameters in the Animal Model of Colon Inflammation. Antioxidants 2020, 9, 375. [Google Scholar] [CrossRef]
- Nathan, C. Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 2006, 6, 173–182. [Google Scholar] [CrossRef]
- Fournier, B.M.; Parkos, C.A. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012, 5, 354–366. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar] [CrossRef]
- Grijó, N.N.; Borra, R.C.; Sdepanian, V.L. Proinflammatory and Anti-inflammatory Cytokines Present in the Acute Phase of Experimental Colitis Treated with Saccharomyces boulardii. Dig. Dis. Sci. 2010, 55, 2498–2504. [Google Scholar] [CrossRef]
- Neilly, P.J.D.; Gardiner, K.R.; Kirk, S.J.; Jennings, G.; Anderson, N.H.; Elia, M.; Rowlands, B.J. Endotoxaemia and cytokine production in experimental colitis. Br. J. Surg. 1995, 82, 1479–1482. [Google Scholar] [CrossRef]
- Elissa, N.; Kermarrec, L.; Hussein, H.; Bernstein, C.N.; Ghia, J. Appropriateness of reference genes for normalizing messenger RNA in mouse 2,4-dinitrobenzene sulfonic acid (DNBS)-induced colitis using quantitative real time PCR. Sci. Rep. 2017, 7, 42427. [Google Scholar] [CrossRef] [PubMed]
- Siggins, R.W.; Melvan, J.N.; Welsh, D.A.; Bagby, G.J.; Nelson, S.; Zhang, P. Alcohol suppresses the granulopoietic response to pulmonary Streptococcus pneumoniae infection with enhancement of STAT3 signaling. J. Immunol. 2012, 186, 4306–4313. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lin, Y.; Gao, B.; Zhang, P. Impairment of hematopoietic precursor cell activation during the granulopoietic response to bacteriemia in mice with chronić-plus-binge alcohol administration. Infect. Immun. 2017, 85, e00369-17. [Google Scholar] [CrossRef] [PubMed]
- Akbar, H.; Khan, M.A.; Khan, M.A.; Khan, M.S.; Aslam, S.; Nasir, A.; Anjum, A.A. Effects of different doses of medetomidine on clinical and hematological parameters in dogs. J. Anim. Plant Sci. 2014, 24, 730–737. [Google Scholar]
- Samimi, A.S.; Sakhaee, E.; Iranmanesh, F. Evaluation of sedative, analgesic, physiological, and laboratory effects of two doses off medetomidine and xylazine in dromedary calves. J. Vet. Pharmacol. Ther. 2019, 42, 411–419. [Google Scholar] [CrossRef]
- Atalan, G. Effect of detomidine-butorphanol-ketamine and atipamezole on clinical, cardiorespiratory, haematological parameters in sheep. Erciyes Üniv. Vet. Fak. Derg. 2019, 16, 1–7. [Google Scholar] [CrossRef]
- Sato, S.; Yamano, Y.; Kanno, C.; Maeda, Y.; Takahashi, F. Cardiopulmonary function, anesthetic effects, quality of arousal, hematology, and blood biochemistry during continuous intravenous infusion of a combination solution of xylazine, butorphanol, and propofol in calves. Vet. Res. Commun. 2024, 48, 2295–2308. [Google Scholar] [CrossRef]
- Kuhl, A.A.; Kakirman, H.; Janotta, M.; Dreher, S.; Cremer, P.; Pawlowski, N.N.; Loddenkemper, C.; Heimesaat, M.M.; Grollich, K.; Zeitz, M.; et al. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology 2007, 133, 1882–1892. [Google Scholar] [CrossRef]
- Zhang, R.; Ito, S.; Nishio, N.; Cheng, Z.; Suzuki, H.; Isobe, K.-I. Up-regulation of Gr1+CD11b+ population in spleen of dextran sulfate sodium administered mice works to repair colitis. Inflamm. Allergy-Drug Targets 2011, 10, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Natsui, M.; Kawasaki, K.; Takizawa, H.; Hayashi, S.; Matsuda, Y.; Sugimura, K.; Seki, K.; Narisawa, R.; Sendo, F.; Asakura, H. Selective depletion of neutrophils by a monoclonal antibody, RP-3, suppresses dextran sulphate sodium-induced colitis in rats. J. Gastroenterol. Hepatol. 2008, 12, 801–808. [Google Scholar] [CrossRef]
- Buell, M.G.; Berin, M.C. Neutrophil-independence of the initiation of colonic injury: Comparison of results from three models of experimental colitis in the rat. Dig. Dis. Sci. 1994, 39, 2575–2588. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.A.; Rodrigues, L.A.; Columbus, D.A.; Aguirre, J.C.P.; Harding, J.C.S.; Cantarelli, V.S.; Costa, M.d.O. Experimental infectious challenge in pigs leads to elevated fecal calprotectin levels following colitis, but not enteritis. Porc. Health Manag. 2021, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Lallès, J.-P.; Fagerhol, M.K. Faecal calprotectin: A non invasive marker of inflammation in pigs? ISAH 2005, 1, 405–408. [Google Scholar]
- Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S.; Elbeltagi, R. Fecal calprotectin in pediatric gastrointestinal diseases: Pros and cons. World J. Clin. Pediatr. 2024, 13, 93341. [Google Scholar] [CrossRef]
- Bjarnason, I. The use of fecal calprotectin in inflammatory bowel disease. Gastroenterol. Hepatol. 2017, 13, 53–56. [Google Scholar] [PubMed] [PubMed Central]
- de Magalhães Costa, M.H.; Sassaki, L.Y.; Chebli, J.M.F. Fecal calprotectin and endoscopic scores: The cornerstones in clinical practice for evaluating mucosal healing in inflammatory bowel diasease. World J. Gastroenterol. 2024, 30, 3022–3035. [Google Scholar] [CrossRef]
- Kalla, R.; Kennedy, N.A.; Ventham, N.T.; Boyapati, R.K.; Adams, A.T.; Nimmo, E.R.; Visconti, M.R.; Drummond, H.; Ho, G.; Pattenden, R.; et al. Serum calprotectin: A novel diagnostic and prognostic marker in inflammatory bowel diseases. Am. J. Gastroenterol. 2016, 111, 1796–1805. [Google Scholar] [CrossRef]
- Riccutio, A.; Griffiths, A.M. Clinical value of fecal calprotectin. Crit. Rev. Clin. Lab. Sci. 2019, 56, 307–320. [Google Scholar] [CrossRef]
- Amara, J.; Saliba, Y.; Hajal, J.; Smayra, V.; Bakhos, J.; Sayegh, R.; Fares, N. Cicardian rhythm disruption aggravates DSS-induced colitis in mice with fecal calprotectin as a marker of colitis severity. Dig. Dis. Sci. 2019, 64, 3122–3133. [Google Scholar] [CrossRef]
- Wang, W.; Cao, W.; Zhang, S.; Chen, D.; Liu, L. The role of calprotectin in the diagnosis and treatment of inflammatory bowel disease. Int. J. Mol. Sci. 2025, 26, 1996. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Jeong, J.; Kim, J.; Han, M.J.; Kim, D. Simultaneous amelioratation of colitis and liver injury in mice by Bifidobacterium longum LC67 and Lactobacillus plantarum LC27. Sci. Rep. 2018, 8, 7500. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.; Harris, D.C.; Whitington, P.F. Relative elevations of serum alanine and aspartate aminotransferase in muscular dystrophy. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zong, X.; Xiao, X.; Cheng, Y.; Fu, J.; Lu, Z.; Jin, M.; Wang, F.; Wang, Y. Multi-omics analysis of the gut-liver axis reveals the mechanism of liver injury in colitis mice. Front. Immunol. 2022, 12, 773070. [Google Scholar] [CrossRef]
- Koller, T.; Galambosova, M.; Filakovska, S.; Kubincova, M.; Hlavaty, T.; Toth, J.; Krajcovicova, A.; Payer, J. Drug-induced liver injury in inflammatory bowel disease: 1-year prospective observational study. World J. Gastroenterol. 2017, 23, 4102–4111. [Google Scholar] [CrossRef]
- Scott, J.A.; Mysko, C.; Purssell, H.; Athwal, V.S. Investigation of abnormal liver blood tests in patients with inflammatory bowel disease. Frontline Gastroenterol. 2024, 15, 443–444. [Google Scholar] [CrossRef]
- Pai, R.K.; Jairath, V. What is the role of histopathology in the evaluation of disease activity in Crohn’s disease? Best Pract. Res. Clin. Gastroenterol. 2019, 38, 101601. [Google Scholar] [CrossRef]
- Neri, B.; Mossa, M.; Scucchi, L.; Sena, G.; Palmieri, G.; Biancone, L. Histological scores in inflammatory bowel disease. J. Dig. Dis. 2020, 22, 9–22. [Google Scholar] [CrossRef]
- Erben, U.; Loddenkemper, C.; Spieckermann, S.; Heimesaat, M.M.; Siegmund, B.; Kuhl, A.A. Histomorphology of intestinal inflammation in inflammatory bowel diseases (IBD) mouse models and its relevance for IBD in men. Int. J. Clin. Exp. Pathol. 2016, 9, 408–442. [Google Scholar]
- Strobel, D.; Goertz, R.S.; Bernatik, T. Diagnostics in inflammatory bowel disease: Ultrasound. World J. Gastroenterol. 2011, 17, 3192–3197. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–179. [Google Scholar] [CrossRef]
- Clevers, H. The intestinal crypt, a prototype stem cell compartment. Cell 2013, 154, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Szkopek, D.; Mendel, M.; Kinsner, M.; Ognik, K.; Szyryńska, N.; Lewczuk, B.; Kozłowski, K.; Kos, I.; Konieczka, P. Cannabidiol and nano-selenium mediate intestinal barrier function by affecting mucosal microstructures, and gut-associated immunological and oxidative stress response in the gut of chickens infected with C. perfringens. Front. Immunol. 2025, 16, 1529449. [Google Scholar] [CrossRef] [PubMed]
- Zaworski, K.; Wychowański, P.; Szkopek, D.; Woliński, J.; Donaldson, J.; Pierzynowski, S.; Pierzynowska, K. The Regulatory Role of Pancreatic Enzymes in the Maintenance of Small Intestinal Structure and Enterocyte Turnover with Special Reference to Alpha Amylase. Int. J. Mol. Sci. 2025, 26, 249. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLOS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Feldman, A.T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. In Histopathology; Methods in Molecular Biology; Day, C., Ed.; Humana Press: New York, NY, USA, 2014; Volume 1180, pp. 31–43. [Google Scholar]

| Control Group | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pig Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||
| Days After Administration of NaCl | AP | FQF | AP | FQF | AP | FQF | AP | FQF | AP | FQF | AP | FQF | AP | FQF | AP | FQF |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 2 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 |
| 5 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Colitis Group | ||||||||||||||||
| Pig Number | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||||||
| Days After Administration of DNBS | AP | FQF | AP | FQF | AP | FQF | AP | FQF | AP | FQF | AP | FQF | AP | FQF | AP | FQF |
| 1 | 0 | 2 | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 3 | 0 | 2 | 1 | 0 |
| 2 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 2 |
| 3 | 0 | 2 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 |
| 4 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 |
| 5 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 2 |
| 6 | 0 | 3 | 0 | 2 | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 2 |
| 7 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 |
| 8 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 |
| 9 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 |
| 11 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 |
| 12 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 0 |
| 13 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 0 |
| 14 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 0 |
| 15 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 |
| 16 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 |
| 17 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 0 |
| 18 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 1 |
| 19 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 |
| 20 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 |
| 21 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Feature | Experimental Group | Statistical Significance | |||
|---|---|---|---|---|---|
| Control 15 d | Colitis 15 d | Control 37 d | Colitis 37 d | ||
| AST [mU/mL] | 35.98 ± 4.54 | 37.19 ± 4.42 ## | 38.32 ± 3.56 | 54.75 ± 4.79 ** | ** p = 0.0153 |
| ALT [mU/mL] | 35.55 ± 3.80 | 32.92 ± 3.40 | 56.11 ± 10.28 | 65.44 ± 10.33 | NS |
| Feature | Experimental Group | Statistical Significance | |||
|---|---|---|---|---|---|
| Control 15 d | Colitis 15 d | Control 37 d | Colitis 37 d | ||
| TNF-α [pg/mL] | 29.93 ± 9.44 | 25.49 ± 7.50 | 23.75 ± 5.15 | 20.65 ± 4.40 | NS |
| IL-1β [pg/mL] | 85.54 ± 23.67 | 81.08 ± 25.17 | 68.35 ± 16.50 | 54.25 ± 10.11 | NS |
| Pig Number | General Architecture | Chronic Inflammatory Infiltrate | Lamina Propria Neutrophils | Lamina Propria Eosinophils | Neutrophils in Epithelium | Crypt Destruction | Erosion and Ulceration |
|---|---|---|---|---|---|---|---|
| Control Group | |||||||
| 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| 2 | 0 | 1 | 0 | 2 | 1 | 0 | 0 |
| 3 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| 4 | 1 crypt distension | 1 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| 6 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| 7 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Colitis Group | |||||||
| 9 | 1 crypt distension | 1 | 0 | 2 | 1 | 0 | 0 |
| 10 | 1 crypt distension | 2 | 0 | 2 | 1 | 0 | 1 |
| 11 | 1 crypt distension | 2 | 1 | 1 | 1 | 0 | 3 |
| 12 | 1 crypt distension | 2 | 1 | 1 | 1 | 0 | 3 |
| 13 | 1 crypt distension | 2 | 1 | 1 | 1 | 0 | 3 |
| 14 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| 15 | 0 | 2 | 0 | 1 | 0 | 0 | 0 |
| 16 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Parameter/Group | Control Group | Colitis Group |
|---|---|---|
| Duodenum | ||
| Villus length [μm] | 1128.11 ± 10.92 | 1104.64 ± 12.60 |
| Mucosa thickness [μm] | 935.19 ± 4.52 | 910.66 ± 14.24 |
| Crypt depth [μm] | 320.26 ± 3.46 | 311.73 ± 4.00 |
| Muscularis thickness [μm] | 653.15 ± 3.71 | 642.64 ± 8.72 |
| Proximal Jejunum | ||
| Villus length [μm] | 984.01 ± 6.32 | 993.55 ± 15.92 |
| Mucosa thickness [μm] | 914.42 ± 5.70 | 903.10 ± 10.75 |
| Crypt depth [μm] | 254.78 ± 2.48 | 252.70 ± 2.83 |
| Muscularis thickness [μm] | 594.75 ± 7.55 | 577.00 ± 12.66 |
| Middle Jejunum | ||
| Villus length [μm] | 949.42 ± 6.59 | 941.34 ± 16.94 |
| Mucosa thickness [μm] | 842.68 ± 9.78 | 831.30 ± 7.53 |
| Crypt depth [μm] | 250.91 ± 1.69 | 250.15 ± 3.53 |
| Muscularis thickness [μm] | 568.00 ± 5.48 a | 559.31 ± 9.70 b |
| Distal Jejunum | ||
| Villus length [μm] | 916.30 ± 7.19 | 907.15 ± 9.37 |
| Mucosa thickness [μm] | 812.72 ± 6.88 | 805.45 ± 12.62 |
| Crypt depth [μm] | 249.25 ± 2.29 | 246.12 ± 3.24 |
| Muscularis thickness [μm] | 534.41 ± 3.89 | 530.36 ± 5.19 |
| Ileum | ||
| Villus length [μm] | 864.32 ± 7.64 | 851.66 ± 12.93 |
| Mucosa thickness [μm] | 760.08 ± 6.56 | 738.85 ± 10.23 |
| Crypt depth [μm] | 236.38 ± 2.14 a | 229.16 ± 2.30 b |
| Muscularis thickness [μm] | 521.55 ± 3.68 a | 503.71 ± 6.29 b |
| Abdominal Pain | |
|---|---|
| Points | Description |
| 0 | No abdominal pain |
| 1 | Moderate abdominal pain |
| 2 | Severe abdominal pain |
| Fecal quality and frequency | |
| 0 | Feces properly formed, without pathological additions, frequency normal |
| 1 | Feces properly formed, but too soft, or feces partially abnormally formed, without pathological additions, frequency normal |
| 2 | Diarrhea, without pathological additions, normal or increased frequency |
| 3 | Diarrhea with blood or with a lot of mucus, increased frequency |
| General Architecture | |
|---|---|
| Points | Description |
| 0 | No abnormality |
| 1 | Mild abnormality |
| 2 | Mild or moderate diffuse or multifocal abnormalities |
| 3 | Severe diffuse or multifocal abnormalities |
| Chronic inflammatory infiltrate | |
| 0 | No increase |
| 1 | Mild but unequivocal increase |
| 2 | Moderate increase |
| 3 | Marked increase |
| Lamina propria neutrophils | |
| 0 | None |
| 1 | Mild but unequivocal increase |
| 2 | Moderate increase |
| 3 | Marked increase |
| Lamina propria eosinophils | |
| 0 | None |
| 1 | Mild but unequivocal increase |
| 2 | Moderate increase |
| 3 | Marked increase |
| Epithelium neutrophils | |
| 0 | None |
| 1 | <5% crypts involved |
| 2 | <50% crypts involved |
| 3 | >50% crypts involved |
| Crypt destruction | |
| 0 | None |
| 1 | Probable—local excess of neutrophils in part of crypt |
| 2 | Probable—marked attenuation |
| 3 | Unequivocal crypt destruction |
| Erosion or ulceration | |
| 0 | No erosion, ulceration, or granulation tissue |
| 1 | Recovering epithelium + adjacent inflammation |
| 2 | Probable erosion—focally stripped |
| 3 | Unequivocal erosion |
| 4 | Ulcer or granulation tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkopek, D.; Woliński, J.; Kopiasz, Ł.; Dziendzikowska, K.; Zaworski, K.; Sapierzyński, R.; Gromadzka-Ostrowska, J. Efficacy of 2,4-Dinitrobenzenesulfonic Acid (DNBS) in the Maintenance of a Model of Inflammatory Bowel Disease in Pigs (Sus scrofa domestica). Int. J. Mol. Sci. 2025, 26, 9115. https://doi.org/10.3390/ijms26189115
Szkopek D, Woliński J, Kopiasz Ł, Dziendzikowska K, Zaworski K, Sapierzyński R, Gromadzka-Ostrowska J. Efficacy of 2,4-Dinitrobenzenesulfonic Acid (DNBS) in the Maintenance of a Model of Inflammatory Bowel Disease in Pigs (Sus scrofa domestica). International Journal of Molecular Sciences. 2025; 26(18):9115. https://doi.org/10.3390/ijms26189115
Chicago/Turabian StyleSzkopek, Dominika, Jarosław Woliński, Łukasz Kopiasz, Katarzyna Dziendzikowska, Kamil Zaworski, Rafał Sapierzyński, and Joanna Gromadzka-Ostrowska. 2025. "Efficacy of 2,4-Dinitrobenzenesulfonic Acid (DNBS) in the Maintenance of a Model of Inflammatory Bowel Disease in Pigs (Sus scrofa domestica)" International Journal of Molecular Sciences 26, no. 18: 9115. https://doi.org/10.3390/ijms26189115
APA StyleSzkopek, D., Woliński, J., Kopiasz, Ł., Dziendzikowska, K., Zaworski, K., Sapierzyński, R., & Gromadzka-Ostrowska, J. (2025). Efficacy of 2,4-Dinitrobenzenesulfonic Acid (DNBS) in the Maintenance of a Model of Inflammatory Bowel Disease in Pigs (Sus scrofa domestica). International Journal of Molecular Sciences, 26(18), 9115. https://doi.org/10.3390/ijms26189115







