The Mechanisms of Resistance to JAK Inhibitors in Lymphoid Leukemias: A Scoping Review of Evidence from Preclinical Models and Case Reports
Abstract
1. Introduction
2. Rationale for the Scoping Review and Main Objective
3. Materials and Methods
3.1. Information Sources and Search Strategy
3.2. Eligibility Criteria and Study Selection
3.3. Definitions
3.4. Data Collection, Charting, and Synthesis of Results
3.5. Critical Appraisal
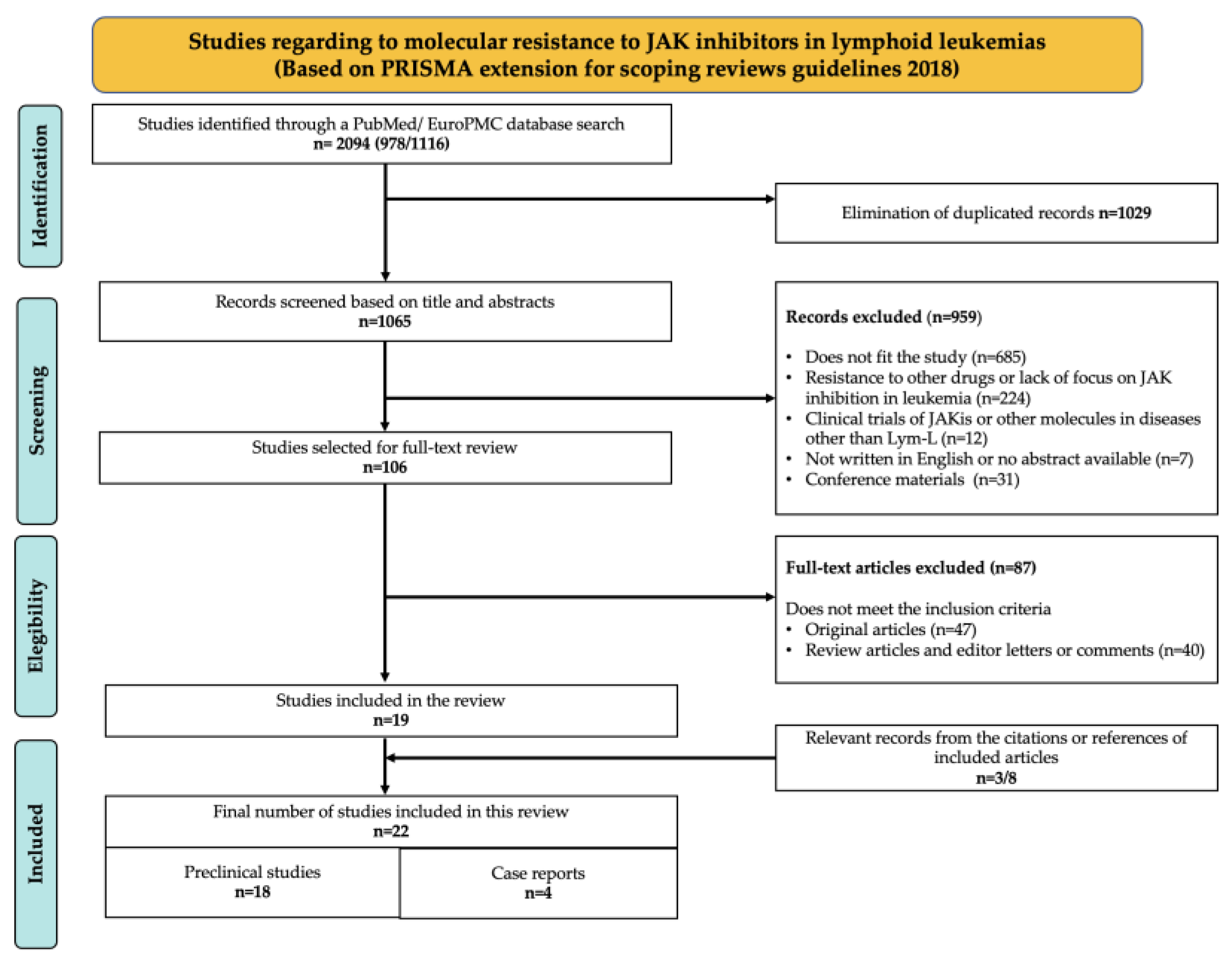
4. Results
4.1. Selection of Sources of Evidence
4.2. Sources of Evidence
- (1)
- Preclinical studies with JAKi-resistant models generated in vitro or in vivo, which recapitulate Lym-L features to simulate potential clinical scenarios. This category included a total of 18 articles (see Table S1).
- (2)
- Case reports, which described the outcomes of JAK-mutated Lym-L patients who experienced chemotherapy failure, consented to be treated with a JAKi therapeutic regimen, and developed JAKi clinical resistance. This category included a total of 4 articles (see Table S2).
4.3. Critical Appraisal of Sources of Evidence
4.4. Synthesis of the Results
5. Discussion
5.1. JAK Normal Protein Structure, Regulation, and Role in Lymphoid Development
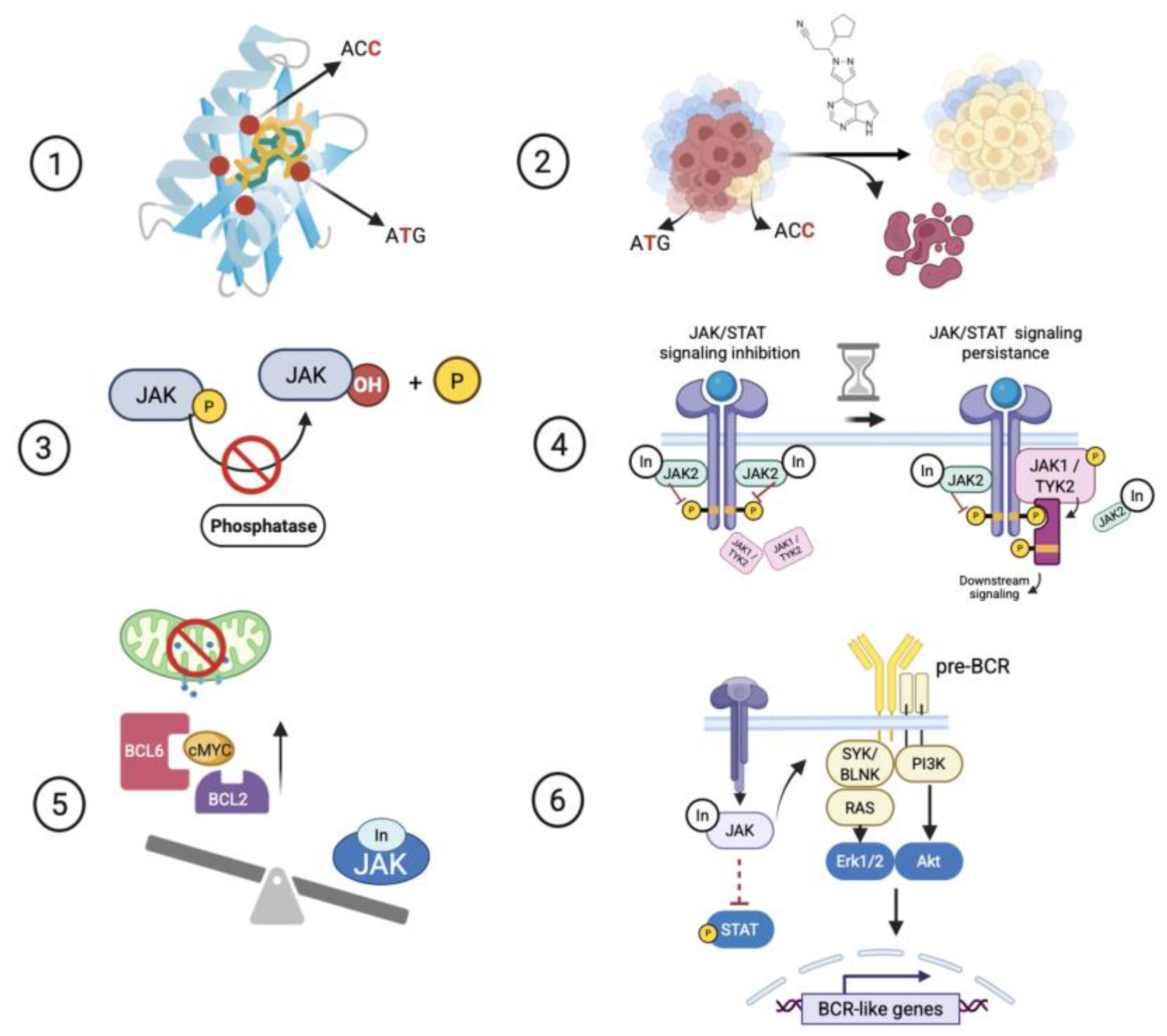
5.2. Point Mutations Around the ATP-Binding Site of the Kinase Domain Confer Genetic Resistance to JAKis
5.2.1. Some Preclinically Characterized JAKi-Resistant Mutations Have Been Identified as Somatic Variants in Patients with Lym-L
5.2.2. The Clinical Relevance of JAKi-Resistance Mutations in Patients with Lym-L
5.3. The Cooperation Between Double JAK Mutants Confers Genetic Resistance to JAKi
5.4. The Inactivation of JAK-Negative Regulator Phosphatases Confers Functional Resistance to JAKi
5.5. The Persistence of JAK/STAT Signaling Confers Functional Resistance to JAKi
5.6. Upregulation of Prosurvival Proteins Confers Functional Resistance to JAKi
5.7. The Adaptation Through a Shift Towards Pre-BCR Cellular Identity Confers Functional Resistance to JAKi
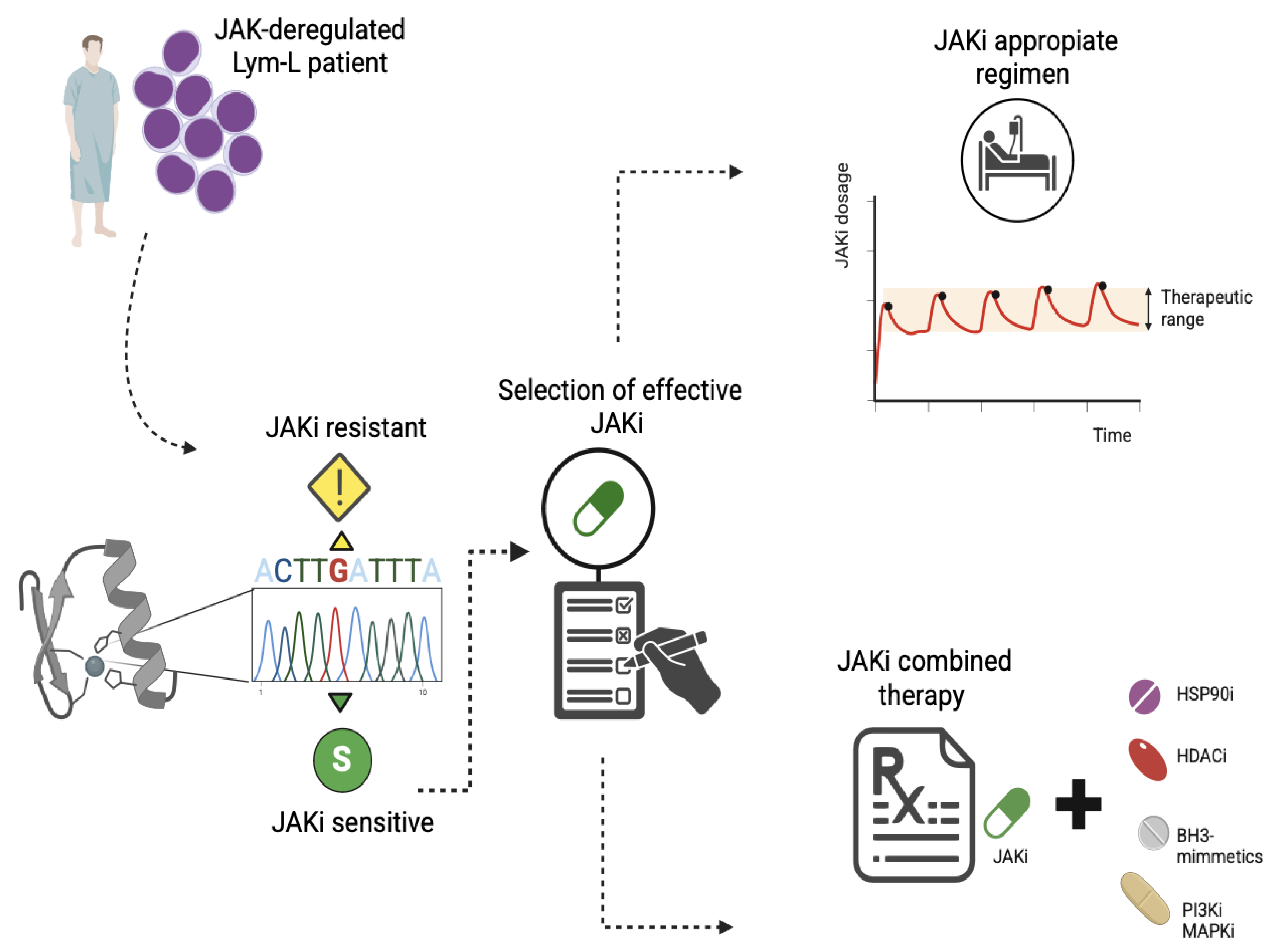
5.8. Strategies to Overcome the JAKi-Resistance in Patients with Lym-L
- HSP90 inhibition: The HSP90 ATPase is a molecular chaperone necessary for the conformational maturation of normal and mutated forms of JAK proteins. Thus, HSP90 is a promising therapeutic target against JAK-deregulated cancers. There are clinical trials with promising results in patients with advanced hematological malignancies [65]. In this context, Weigert O et al. (2012) demonstrated that the HSP90 inhibitor NVP_AUY922 promotes the degradation of both wild-type and mutant JAK2, improves survival in murine models of human and mice CRLF2-rearranged B-ALL, and reduces the proliferation of BaF3 CRLF2-positive cells harboring kinase domain point mutations. These observations identify the HSP90 inhibitors as promising therapeutic agents for overcoming resistance to JAKi [34].
- HDAC inhibitors: Histone deacetylases (HDACs) play an essential role in regulating HSP90 activity and the transcriptional activity of STATs. Thus, HDAC inhibitors are an attractive target for treating deregulated JAK in Lym-L, overcoming JAKi resistance. Consistent with this, Tavakoli Shirazi P et al. (2021) demonstrated that HDAC inhibitors, such as vorinostat or panobinostat, were effective against TYK2-rearranged B-ALL cells that had acquired cerdulatinib (a TYK2 inhibitor) resistance, demonstrating their potential therapeutic use [8].
- Inhibition of antiapoptotic proteins: Based on the evidence provided from preclinical models of B or T cell precursor ALL and two patients with refractory T-PLL (Table S1), JAKi enhances the dependency on antiapoptotic proteins in JAK-deregulated Lym-L cells (Figure 2). Consequently, combined therapy with JAKi and BH3-mimetics, such as FX1, ABT-737, or venetoclax, can overcome resistance to JAK inhibition [21].
- Inhibition of crosstalk signaling pathways: Studies by Hurtz C. et al. (2020) and Sasaki K et al. (2022) highlight the inefficacy of JAKi as a monotherapy. These investigations emphasize the concept of combined TKI therapy against multiple crosstalk kinases, including PI3K, BLNK, and RAS-MAPK, to overcome the JAKi resistance in CRLF2-rearranged B-ALL [27,38].
6. Strengths and Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABT-737 | A BCL-2/BCL-xL inhibitor |
| ALL | Acute Lymphoblastic Leukemia |
| AMP/ASCO/CAP | Association for Molecular Pathology/American Society of Clinical Oncology/College of American Pathologists |
| ATP | Adenosine Triphosphate |
| AUY922 | An HSP90 inhibitor |
| B-ALL | B-cell Acute Lymphoblastic Leukemia |
| BCL2 | B-cell Lymphoma 2 (anti-apoptotic protein) |
| BH3 | Bcl-2 Homology Domain 3 |
| BSA | Bovine Serum Albumin |
| BCR | B-cell Receptor |
| CD45 | Cluster of Differentiation 45 |
| CHZ868 | A type-II JAK inhibitor |
| CMP6 | A type-I JAK inhibitor |
| CRLF2 | Cytokine Receptor-Like Factor 2 |
| c-Myc | Cellular Myelocytomatosis oncogene |
| CyTOF | Cytometry by Time of Flight |
| DFG | Asp-Phe-Gly motif |
| DMSO | Dimethyl Sulfoxide |
| FDR | False Discovery Rate |
| FDA | Food and Drug Administration U.S. |
| FERM | Four-point-one, Ezrin, Radixin, Moesin domain |
| FX1 | A BCL6 inhibitor |
| HSP90 | HSP90Heat Shock Protein 90 |
| HDAC | Histone Deacetylase |
| HSCT | Hematopoietic Stem Cell Transplant |
| JAK | Janus Kinase |
| JAKi | Janus Kinase Inhibitor |
| KD | Kinase Domain |
| Lym-L | Lymphoid Leukemias |
| MeSH | Medical Subject Headings |
| MPN | Myeloproliferative Neoplasms |
| NES | Normalized Enrichment Score |
| PDB | Protein Data Bank |
| PI3K | Phosphoinositide 3-Kinase |
| PKD | Pseudokinase Domain |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PTPN2 | Protein Tyrosine Phosphatase, Non-Receptor Type 2 |
| PTPRC | Protein Tyrosine Phosphatase, Receptor Type C |
| RAS-MAPK | Rat Sarcoma–Mitogen-Activated Protein Kinase Pathway |
| SHP1/2 | SHP1/2Src Homology 2 Domain-Containing Phosphatase ½ |
| SH2 | Src Homology 2 Domain |
| SOCS | Suppressor of Cytokine Signaling |
| STAT | Signal Transducer and Activator of Transcription |
| T-ALL | T-cell Acute Lymphoblastic Leukemia |
| T-PLL | T-cell Prolymphocytic Leukemia |
| TKI | Tyrosine Kinase Inhibitor |
| TP53 | Tumor Protein p53 |
| TYK2 | Tyrosine Kinase 2 |
| VAF | Variant Allele Fraction |
References
- Shawky, A.M.; Almalki, F.A.; Abdalla, A.N.; Abdelazeem, A.H.; Gouda, A.M. A Comprehensive Overview of Globally Approved JAK Inhibitors. Pharmaceutics 2022, 14, 1001. [Google Scholar] [CrossRef]
- Alicea-Velázquez, N.L.; Boggon, T.J. The use of structural biology in Janus kinase targeted drug discovery. Curr. Drug Targets 2011, 12, 546–555. [Google Scholar] [CrossRef]
- Springuel, L.; Renauld, J.-C.; Knoops, L. JAK kinase targeting in hematologic malignancies: A sinuous pathway from identification of genetic alterations towards clinical indications. Haematologica 2015, 100, 1240–1253. [Google Scholar] [CrossRef]
- Bellanger, D.; Jacquemin, V.; Chopin, M.; Pierron, G.; Bernard, O.A.; Ghysdael, J.; Stern, M.-H. Recurrent JAK1 and JAK3 somatic mutations in T-cell prolymphocytic leukemia. Leukemia 2014, 28, 417–419. [Google Scholar] [CrossRef]
- Gutierrez, M.; Bladek, P.; Goksu, B.; Murga-Zamalloa, C.; Bixby, D.; Wilcox, R. T-Cell Prolymphocytic Leukemia: Diagnosis, Pathogenesis, and Treatment. Int. J. Mol. Sci. 2023, 24, 12106. [Google Scholar] [CrossRef]
- Schwab, C.; Harrison, C.J. Advances in B-cell Precursor Acute Lymphoblastic Leukemia Genomics. HemaSphere 2018, 2, e53. [Google Scholar] [CrossRef]
- Kiel, M.J.; Velusamy, T.; Rolland, D.; Sahasrabuddhe, A.A.; Chung, F.; Bailey, N.G.; Schrader, A.; Li, B.; Li, J.Z.; Ozel, A.B.; et al. Integrated genomic sequencing reveals mutational landscape of T-cell prolymphocytic leukemia. Blood 2014, 124, 1460–1472. [Google Scholar] [CrossRef]
- Tavakoli Shirazi, P.; Eadie, L.N.; Page, E.C.; Heatley, S.L.; Bruning, J.B.; White, D.L. Constitutive JAK/STAT signaling is the primary mechanism of resistance to JAKi in TYK2-rearranged acute lymphoblastic leukemia. Cancer Lett. 2021, 512, 28–37. [Google Scholar] [CrossRef]
- Kwon, S. Molecular dissection of Janus kinases as drug targets for inflammatory diseases. Front. Immunol. 2022, 13, 1075192. [Google Scholar] [CrossRef]
- Alghandour, R.; Sakr, D.H.; Shaaban, Y. Philadelphia-like acute lymphoblastic leukemia: The journey from molecular background to the role of bone marrow transplant-review article. Ann. Hematol. 2023, 102, 1287–1300. [Google Scholar] [CrossRef]
- Meyer, S.C. Mechanisms of Resistance to JAK2 Inhibitors in Myeloproliferative Neoplasms. Hematol. Oncol. Clin. N. Am. 2017, 31, 627–642. [Google Scholar] [CrossRef]
- Fowlkes, S.; Murray, C.; Fulford, A.; De Gelder, T.; Siddiq, N. Myeloproliferative neoplasms (MPNs)—Part 1: An overview of the diagnosis and treatment of the “classical” MPNs. Can. Oncol. Nurs. J. Rev. Can. Nurs. Oncol. 2018, 28, 262–268. [Google Scholar] [CrossRef]
- Leroy, E.; Constantinescu, S.N. Rethinking JAK2 inhibition: Towards novel strategies of more specific and versatile Janus kinase inhibition. Leukemia 2017, 31, 1023–1038. [Google Scholar] [CrossRef]
- Kong, X.; Sun, H.; Pan, P.; Li, D.; Zhu, F.; Chang, S.; Xu, L.; Li, Y.; Hou, T. How Does the L884P Mutation Confer Resistance to Type-II Inhibitors of JAK2 Kinase: A Comprehensive Molecular Modeling Study. Sci. Rep. 2017, 7, 9088. [Google Scholar] [CrossRef]
- Downes, C.E.J.; McClure, B.J.; Bruning, J.B.; Page, E.; Breen, J.; Rehn, J.; Yeung, D.T.; White, D.L. Acquired JAK2 mutations confer resistance to JAK inhibitors in cell models of acute lymphoblastic leukemia. NPJ Precis. Oncol. 2021, 5, 75. [Google Scholar] [CrossRef]
- Maude, S.L.; Tasian, S.K.; Vincent, T.; Hall, J.W.; Sheen, C.; Roberts, K.G.; Seif, A.E.; Barrett, D.M.; Chen, I.-M.; Collins, J.R.; et al. Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood 2012, 120, 3510–3518. [Google Scholar] [CrossRef]
- Herbaux, C.; Poulain, S.; Roos-Weil, D.; Bay, J.-O.; Guillermin, Y.; Lemonnier, F.; Laribi, K.; Visanica, S.; Moreaux, J.; Gonzalez, H.; et al. Preliminary Study of Ruxolitinib and Venetoclax for Treatment of Patients with T-Cell Prolymphocytic Leukemia Refractory to, or Ineligible for Alemtuzumab. Blood 2021, 138, 1201. [Google Scholar] [CrossRef]
- Appelmann, I.; Rillahan, C.D.; de Stanchina, E.; Carbonetti, G.; Chen, C.; Lowe, S.W.; Sherr, C.J. Janus kinase inhibition by ruxolitinib extends dasatinib- and dexamethasone-induced remissions in a mouse model of Ph+ ALL. Blood 2015, 125, 1444–1451. [Google Scholar] [CrossRef]
- Mayfield, J.R.; Czuchlewski, D.R.; Gale, J.M.; Matlawska-Wasowska, K.; Vasef, M.A.; Nickl, C.; Pickett, G.; Ness, S.A.; Winter, S.S. Integration of ruxolitinib into dose-intensified therapy targeted against a novel JAK2 F694L mutation in B-precursor acute lymphoblastic leukemia. Pediatr. Blood Cancer 2017, 64, e26328. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Stern, J.W.; Jubelirer, T.F.; Wertheim, G.B.; Lin, F.; Chang, F.; Gu, Z.; Mullighan, C.G.; Li, Y.; Harvey, R.C.; et al. Clinical efficacy of ruxolitinib and chemotherapy in a child with Philadelphia chromosome-like acute lymphoblastic leukemia with GOLGA5-JAK2 fusion and induction failure. Haematologica 2018, 103, e427–e431. [Google Scholar] [CrossRef]
- Herbaux, C.; Kornauth, C.; Poulain, S.; Chong, S.J.F.; Collins, M.C.; Valentin, R.; Hackett, L.; Tournilhac, O.; Lemonnier, F.; Dupuis, J.; et al. BH3 profiling identifies ruxolitinib as a promising partner for venetoclax to treat T-cell prolymphocytic leukemia. Blood 2021, 137, 3495–3506. [Google Scholar] [CrossRef]
- Kesarwani, M.; Huber, E.; Kincaid, Z.; Evelyn, C.R.; Biesiada, J.; Rance, M.; Thapa, M.B.; Shah, N.P.; Meller, J.; Zheng, Y.; et al. Targeting substrate-site in Jak2 kinase prevents emergence of genetic resistance. Sci. Rep. 2015, 5, 14538. [Google Scholar] [CrossRef]
- Losdyck, E.; Hornakova, T.; Springuel, L.; Degryse, S.; Gielen, O.; Cools, J.; Constantinescu, S.N.; Flex, E.; Tartaglia, M.; Renauld, J.-C.; et al. Distinct Acute Lymphoblastic Leukemia (ALL)-associated Janus Kinase 3 (JAK3) Mutants Exhibit Different Cytokine-Receptor Requirements and JAK Inhibitor Specificities. J. Biol. Chem. 2015, 290, 29022–29034. [Google Scholar] [CrossRef]
- Wu, S.-C.; Li, L.S.; Kopp, N.; Montero, J.; Chapuy, B.; Yoda, A.; Christie, A.L.; Liu, H.; Christodoulou, A.; van Bodegom, D.; et al. Activity of the Type II JAK2 Inhibitor CHZ868 in B Cell Acute Lymphoblastic Leukemia. Cancer Cell 2015, 28, 29–41. [Google Scholar] [CrossRef]
- Moskowitz, A.J.; Ghione, P.; Jacobsen, E.; Ruan, J.; Schatz, J.H.; Noor, S.; Myskowski, P.; Vardhana, S.; Ganesan, N.; Hancock, H.; et al. A phase 2 biomarker-driven study of ruxolitinib demonstrates effectiveness of JAK/STAT targeting in T-cell lymphomas. Blood 2021, 138, 2828–2837. [Google Scholar] [CrossRef]
- Tasian, S.K.; Doral, M.Y.; Borowitz, M.J.; Wood, B.L.; Chen, I.-M.; Harvey, R.C.; Gastier-Foster, J.M.; Willman, C.L.; Hunger, S.P.; Mullighan, C.G.; et al. Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia. Blood 2012, 120, 833–842. [Google Scholar] [CrossRef]
- Hurtz, C.; Wertheim, G.B.; Loftus, J.P.; Blumenthal, D.; Lehman, A.; Li, Y.; Bagashev, A.; Manning, B.; Cummins, K.D.; Burkhardt, J.K.; et al. Oncogene-independent BCR-like signaling adaptation confers drug resistance in Ph-like ALL. J. Clin. Investig. 2020, 130, 3637–3653. [Google Scholar] [CrossRef]
- Hornakova, T.; Springuel, L.; Devreux, J.; Dusa, A.; Constantinescu, S.N.; Knoops, L.; Renauld, J.-C. Oncogenic JAK1 and JAK2-activating mutations resistant to ATP-competitive inhibitors. Haematologica 2011, 96, 845–853. [Google Scholar] [CrossRef][Green Version]
- Lahera, A.; Vela-Martín, L.; Fernández-Navarro, P.; Llamas, P.; López-Lorenzo, J.L.; Cornago, J.; Santos, J.; Fernández-Piqueras, J.; Villa-Morales, M. The JAK3Q988P mutation reveals oncogenic potential and resistance to ruxolitinib. Mol. Carcinog. 2024, 63, 5–10. [Google Scholar] [CrossRef]
- Arwood, M.L.; Liu, Y.; Harkins, S.K.; Weinstock, D.M.; Yang, L.; Stevenson, K.E.; Plana, O.D.; Dong, J.; Cirka, H.; Jones, K.L.; et al. New scaffolds for type II JAK2 inhibitors overcome the acquired G993A resistance mutation. Cell Chem. Biol. 2023, 30, 618–631.e12. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Yasuda, T.; Goto, H.; Maeda, N.; Akahane, K.; Inukai, T.; Yamamoto, H.; Karnan, S.; Ota, A.; Hyodo, T.; et al. BCL6 inhibition ameliorates resistance to ruxolitinib in CRLF2-rearranged acute lymphoblastic leukemia. Haematologica 2023, 108, 394–408. [Google Scholar] [CrossRef]
- Waibel, M.; Solomon, V.S.; Knight, D.A.; Ralli, R.A.; Kim, S.-K.; Banks, K.-M.; Vidacs, E.; Virely, C.; Sia, K.C.S.; Bracken, L.S.; et al. Combined targeting of JAK2 and Bcl-2/Bcl-xL to cure mutant JAK2-driven malignancies and overcome acquired resistance to JAK2 inhibitors. Cell Rep. 2013, 5, 1047–1059. [Google Scholar] [CrossRef]
- Weigert, O.; Lane, A.A.; Bird, L.; Kopp, N.; Chapuy, B.; van Bodegom, D.; Toms, A.V.; Marubayashi, S.; Christie, A.L.; McKeown, M.; et al. Genetic resistance to JAK2 enzymatic inhibitors is overcome by HSP90 inhibition. J. Exp. Med. 2012, 209, 259–273. [Google Scholar] [CrossRef]
- Sadras, T.; Heatley, S.L.; Kok, C.H.; McClure, B.J.; Yeung, D.; Hughes, T.P.; Sutton, R.; Ziegler, D.S.; White, D.L. A novel somatic JAK2 kinase-domain mutation in pediatric acute lymphoblastic leukemia with rapid on-treatment development of LOH. Cancer Genet. 2017, 216–217, 86–90. [Google Scholar] [CrossRef]
- Marit, M.R.; Chohan, M.; Matthew, N.; Huang, K.; Kuntz, D.A.; Rose, D.R.; Barber, D.L. Random mutagenesis reveals residues of JAK2 critical in evading inhibition by a tyrosine kinase inhibitor. PLoS ONE 2012, 7, e43437. [Google Scholar] [CrossRef][Green Version]
- Springuel, L.; Hornakova, T.; Losdyck, E.; Lambert, F.; Leroy, E.; Constantinescu, S.N.; Flex, E.; Tartaglia, M.; Knoops, L.; Renauld, J.-C. Cooperating JAK1 and JAK3 mutants increase resistance to JAK inhibitors. Blood 2014, 124, 3924–3931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sasaki, K.; Yamauchi, T.; Semba, Y.; Nogami, J.; Imanaga, H.; Terasaki, T.; Nakao, F.; Akahane, K.; Inukai, T.; Verhoeyen, E.; et al. Genome-wide CRISPR-Cas9 screen identifies rationally designed combination therapies for CRLF2-rearranged Ph-like ALL. Blood 2022, 139, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.N.; Schaap, N.; Mesa, R.A. Management of myelofibrosis after ruxolitinib failure. Ann. Hematol. 2020, 99, 1177–1191. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. JMD 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Li, Q.; Ren, Z.; Cao, K.; Li, M.M.; Wang, K.; Zhou, Y. CancerVar: An artificial intelligence-empowered platform for clinical interpretation of somatic mutations in cancer. Sci. Adv. 2022, 8, eabj1624. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arteaga, A.; Margolskee, E.; Wei, M.T.; van Besien, K.; Inghirami, G.; Horwitz, S. Combined use of tofacitinib (pan-JAK inhibitor) and ruxolitinib (a JAK1/2 inhibitor) for refractory T-cell prolymphocytic leukemia (T-PLL) with a JAK3 mutation. Leuk. Lymphoma 2019, 60, 1626–1631. [Google Scholar] [CrossRef]
- Kim, S.-K.; Knight, D.A.; Jones, L.R.; Vervoort, S.; Ng, A.P.; Seymour, J.F.; Bradner, J.E.; Waibel, M.; Kats, L.; Johnstone, R.W. JAK2 is dispensable for maintenance of JAK2 mutant B-cell acute lymphoblastic leukemias. Genes Dev. 2018, 32, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Kleppe, M.; Soulier, J.; Asnafi, V.; Mentens, N.; Hornakova, T.; Knoops, L.; Constantinescu, S.; Sigaux, F.; Meijerink, J.P.; Vandenberghe, P.; et al. PTPN2 negatively regulates oncogenic JAK1 in T-cell acute lymphoblastic leukemia. Blood 2011, 117, 7090–7098. [Google Scholar] [CrossRef]
- Raivola, J.; Haikarainen, T.; Abraham, B.G.; Silvennoinen, O. Janus Kinases in Leukemia. Cancers 2021, 13, 800. [Google Scholar] [CrossRef]
- Zhong, J.; Sharma, J.; Raju, R.; Palapetta, S.M.; Prasad, T.S.K.; Huang, T.-C.; Yoda, A.; Tyner, J.W.; van Bodegom, D.; Weinstock, D.M.; et al. TSLP signaling pathway map: A platform for analysis of TSLP-mediated signaling. Database J. Biol. Databases Curation 2014, 2014, bau007. [Google Scholar] [CrossRef]
- Persky, N.S.; Hernandez, D.; Do Carmo, M.; Brenan, L.; Cohen, O.; Kitajima, S.; Nayar, U.; Walker, A.; Pantel, S.; Lee, Y.; et al. Defining the landscape of ATP-competitive inhibitor resistance residues in protein kinases. Nat. Struct. Mol. Biol. 2020, 27, 92–104. [Google Scholar] [CrossRef]
- Miller, G.D.; Bruno, B.J.; Lim, C.S. Resistant mutations in CML and Ph(+)ALL—Role of ponatinib. Biol Targets Ther. 2014, 8, 243–254. [Google Scholar] [CrossRef]
- Lucet, I.S.; Fantino, E.; Styles, M.; Bamert, R.; Patel, O.; Broughton, S.E.; Walter, M.; Burns, C.J.; Treutlein, H.; Wilks, A.F.; et al. The structural basis of Janus kinase 2 inhibition by a potent and specific pan-Janus kinase inhibitor. Blood 2006, 107, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Zhang, J.; Harvey, R.C.; Collins-Underwood, J.R.; Schulman, B.A.; Phillips, L.A.; Tasian, S.K.; Loh, M.L.; Su, X.; Liu, W.; et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 2009, 106, 9414–9418. [Google Scholar] [CrossRef]
- Veevers, R.; Cawley, G.; Hayward, S. Investigation of sequence features of hinge-bending regions in proteins with domain movements using kernel logistic regression. BMC Bioinform. 2020, 21, 137. [Google Scholar] [CrossRef]
- Hoemberger, M.; Pitsawong, W.; Kern, D. Cumulative mechanism of several major imatinib-resistant mutations in Abl kinase. Proc. Natl. Acad. Sci. USA 2020, 117, 19221–19227. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Zhai, Y.; Wang, J. Non-small cell lung cancer harboring a rare EGFR L747P mutation showing intrinsic resistance to both gefitinib and osimertinib (AZD9291): A case report. Thorac. Cancer 2018, 9, 745–749. [Google Scholar] [CrossRef]
- Pagliarini, R.; Shao, W.; Sellers, W.R. Oncogene addiction: Pathways of therapeutic response, resistance, and road maps toward a cure. EMBO Rep. 2015, 16, 280–296. [Google Scholar] [CrossRef]
- Gaikwad, A.; Rye, C.L.; Devidas, M.; Heerema, N.A.; Carroll, A.J.; Izraeli, S.; Plon, S.E.; Basso, G.; Pession, A.; Rabin, K.R. Prevalence and clinical correlates of JAK2 mutations in Down syndrome acute lymphoblastic leukaemia. Br. J. Haematol. 2009, 144, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Downes, C.E.; McClure, B.J.; McDougal, D.P.; Heatley, S.L.; Bruning, J.B.; Thomas, D.; Yeung, D.T.; White, D.L. JAK2 Alterations in Acute Lymphoblastic Leukemia: Molecular Insights for Superior Precision Medicine Strategies. Front. Cell Dev. Biol. 2022, 10, 942053. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Wall, M.; Corboy, G.P.; Taubenheim, N.; Gregory, G.P.; Opat, S.; Shortt, J. Failure of tofacitinib to achieve an objective response in a DDX3X-MLLT10 T-lymphoblastic leukemia with activating JAK3 mutations. Cold Spring Harb. Mol. Case Stud. 2020, 6, a004994. [Google Scholar] [CrossRef]
- Greenplate, A.; Wang, K.; Tripathi, R.M.; Palma, N.; Ali, S.M.; Stephens, P.J.; Miller, V.A.; Shyr, Y.; Guo, Y.; Reddy, N.M.; et al. Genomic Profiling of T-Cell Neoplasms Reveals Frequent JAK1 and JAK3 Mutations with Clonal Evasion from Targeted Therapies. JCO Precis. Oncol. 2018, 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Porcu, M.; Kleppe, M.; Gianfelici, V.; Geerdens, E.; De Keersmaecker, K.; Tartaglia, M.; Foà, R.; Soulier, J.; Cauwelier, B.; Uyttebroeck, A.; et al. Mutation of the receptor tyrosine phosphatase PTPRC (CD45) in T-cell acute lymphoblastic leukemia. Blood 2012, 119, 4476–4479. [Google Scholar] [CrossRef]
- Koppikar, P.; Bhagwat, N.; Kilpivaara, O.; Manshouri, T.; Adli, M.; Hricik, T.; Liu, F.; Saunders, L.M.; Mullally, A.; Abdel-Wahab, O.; et al. Heterodimeric JAK-STAT activation as a mechanism of persistence to JAK2 inhibitor therapy. Nature 2012, 489, 155–159. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lovén, J.; Rahl, P.B.; Paranal, R.M.; Burge, C.B.; Bradner, J.E.; Lee, T.I.; Young, R.A. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 2012, 151, 56–67. [Google Scholar] [CrossRef]
- Chen, J.A.; Hou, Y.; Roskin, K.M.; Arber, D.A.; Bangs, C.D.; Baughn, L.B.; Cherry, A.M.; Ewalt, M.D.; Fire, A.Z.; Fresard, L.; et al. Lymphoid blast transformation in an MPN with BCR-JAK2 treated with ruxolitinib: Putative mechanisms of resistance. Blood Adv. 2021, 5, 3492–3496. [Google Scholar] [CrossRef]
- Gisslinger, H.; Schalling, M.; Gisslinger, B.; Skrabs, C.; Müllauer, L.; Kralovics, R. Restoration of response to ruxolitinib upon brief withdrawal in two patients with myelofibrosis. Am. J. Hematol. 2014, 89, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Shank, K.; Dunbar, A.; Koppikar, P.; Kleppe, M.; Teruya-Feldstein, J.; Csete, I.; Bhagwat, N.; Keller, M.; Kilpivaara, O.; Michor, F.; et al. Mathematical modeling reveals alternative JAK inhibitor treatment in myeloproliferative neoplasms. Haematologica 2020, 105, e91–e94. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Joshi, A.; Sato, N.; Lee, S.; Lee, M.-J.; Trepel, J.B.; Neckers, L. An update on the status of HSP90 inhibitors in cancer clinical trials. Cell Stress Chaperones 2024, 29, 519–539. [Google Scholar] [CrossRef] [PubMed]

| Ref. | JAK/STAT Initial Activating Lesion | Resistance Mutation | Tested JAKi | IC50 Control (µM) | IC50 JAK Mutated (µM) | Molecular Mechanism of Resistance |
|---|---|---|---|---|---|---|
| N lobe and P-loop regions | ||||||
| [36] | TEL::JAK2 | JAK2 p.G831R JAK2 p.E864K JAK2 p.V881A | JAK inhibitor 1 | 0.25 <0.01 <0.01 | 10 R >0.1 R >0.1 R | Non reported. |
| [34] | JAK2 p.R683G | JAK2 p.E864K | BVB808 BSK805 JAK inhibitor 1 Ruxolitinib C Tofacitinib C TG101348 | 0.09 3.1 0.19 0.08 0.48 0.60 | 0.42 R 8.70 R 4.67 R 0.08 S 0.50 S 0.81 S | The mutation produces the occlusion of the ATP/JAKi pocket by destabilizing of the P-loop due to a steric clash induced by the charge of the new amino acid side chain. |
| Allosteric site | ||||||
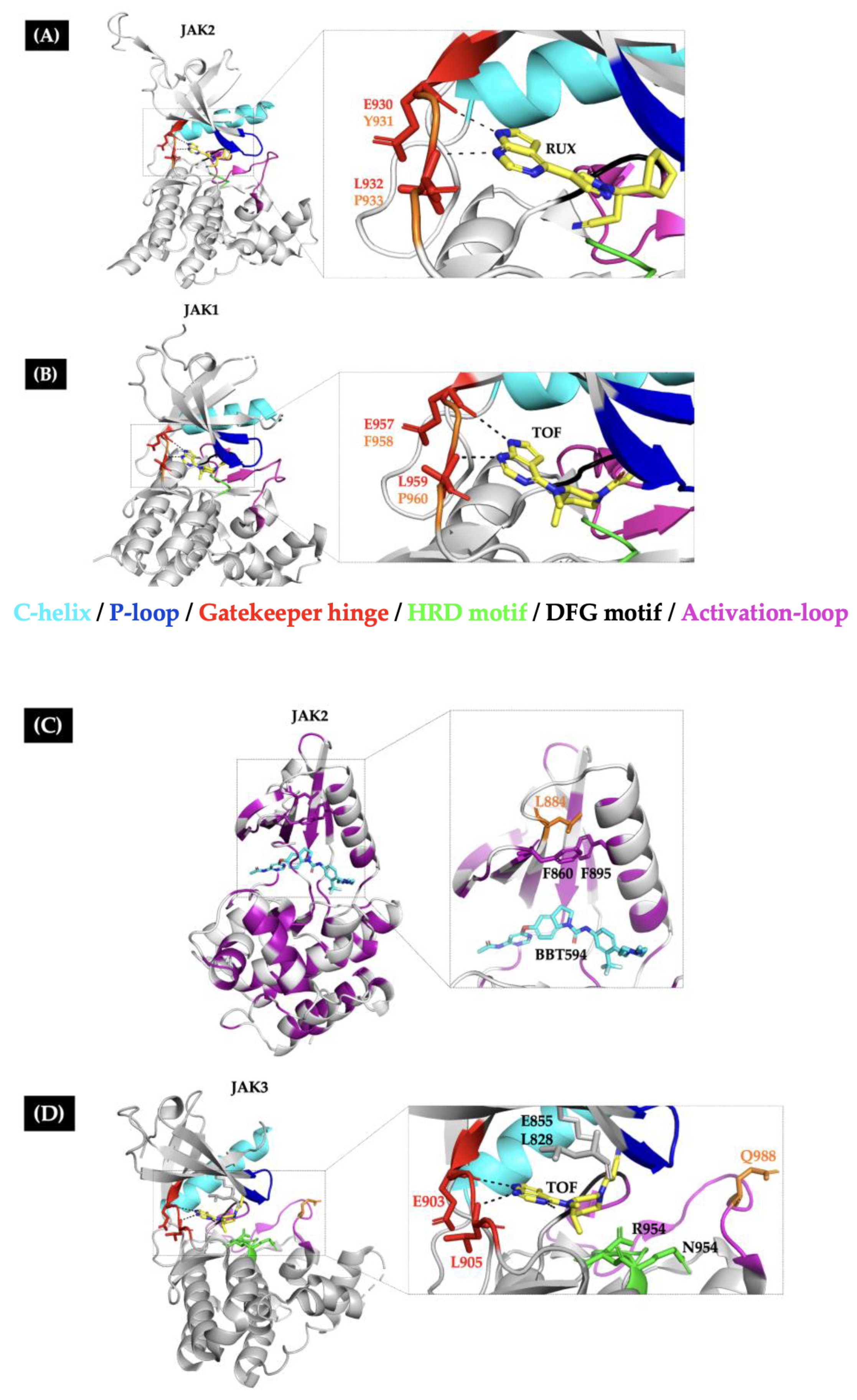
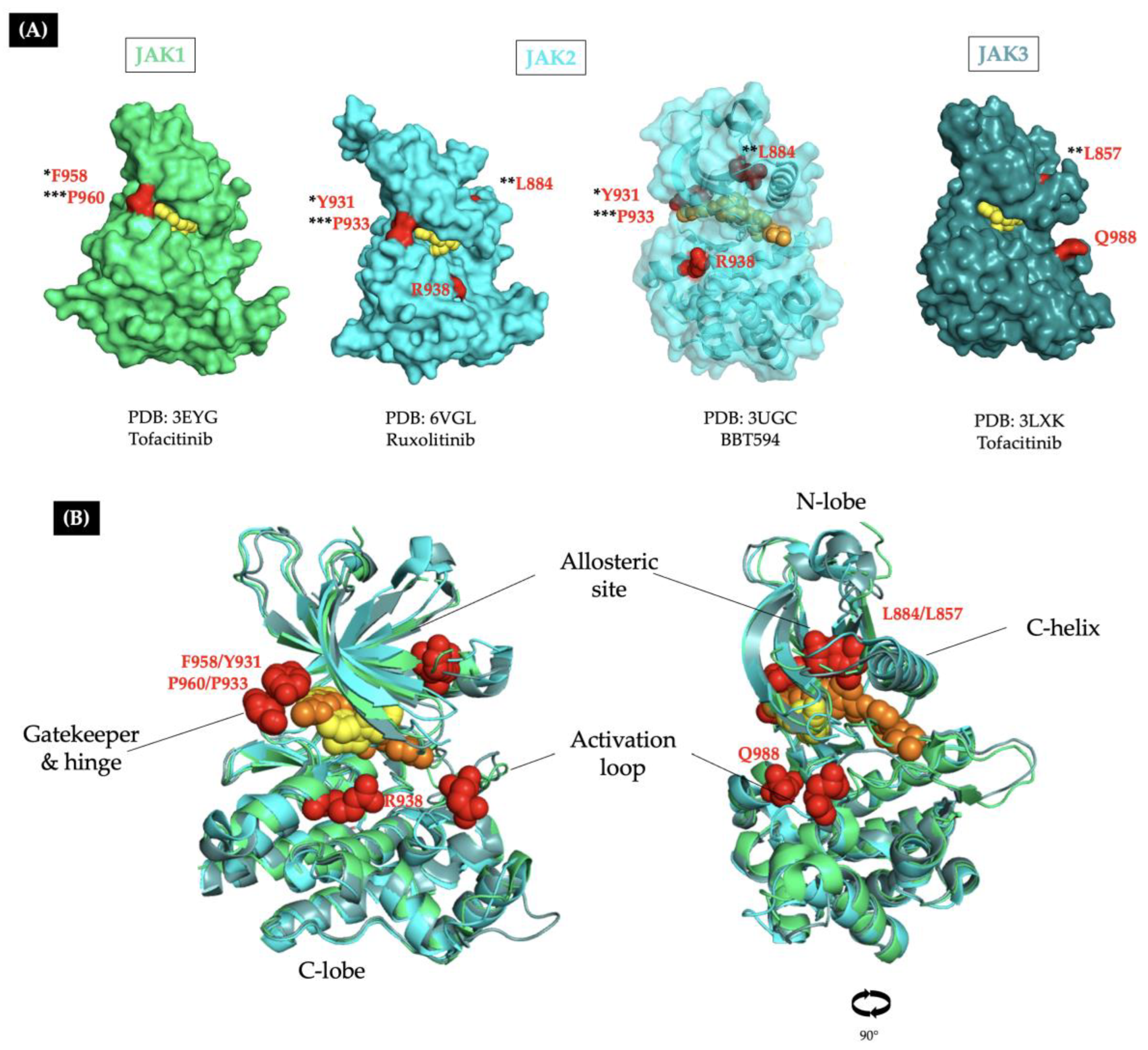
| [24] | JAK2 p.R683G | JAK2 p.L884P | BBT594 II and CHZ-868 II Ruxolitinib C Fedratinib C | <0.5 <0.5 <1.0 | > 0.5 R <0.5 S <1.0 S | This mutation disrupts the interaction between the Phe895 and Phe860, leading to the destabilization of the BBT594 binding pocket. |
| [23] | JAK3 p.Y100A | JAK3 p.L857P | Ruxolitinib Tofacitinib NIBR3049 | 1.26 0.17 3.42 | 2.33 R 0.32 S 1.15 S | The mutated residue is homologous to JAK2 L884P and mediates the active/inactive conformational change in the KD. |
| Gatekeeper, hinge and activation loop regions | ||||||
| [36] | TEL::JAK2 | JAK2 p.N909K | JAK inhibitor 1 | 0.25 | >10 | The mutation produces a steric clash that may push the neighboring V911 residue into the inhibitor binding pocket. |
| JAK2 p.Y918H | JAK inhibitor 1 | 0.25 | >10 | Not reported | ||
| JAK2 p.M929I | JAK inhibitor 1 | 0.25 | >10 | The side chain of M929 may facilitate the proper positioning of JAKi. The M929I mutation is homologous to the ABL1p.T315I resistance mutation. | ||
| [15,34] | JAK2 p.R683G ATF7IP::JAK2 | JAK2 p.Y931C JAK2 p.Y931C | BVB808 BSK805 JAK inhibitor 1 Ruxolitinib C Tofacitinib C TG101348 Ruxolitinib C BMS-911543 AZD-1480 Fedratinib C | 0.09 3.1 0.19 0.08 0.48 0.59 0.43 0.66 0.51 0.83 | 0.42 R 8.70 R 4.67 R 0.38 R 5.24 R 1.1 R 1.98 R >5.0 R 3.45 R 1.77 R | This mutation causes the occlusion of the ATP/JAKi pocket by destabilizing the P-loop due to a steric clash induced by the charge of the new aminoacidic side chain. |
| [43] | JAK2 p.R683G | JAK2 p.P933R | Ruxolitinib C | IC50 non reported | IC50 non reported (pJAK2 levels higher than control) | The P933 residue is adjacent to the ATP-binding site and is thought to impart the rigidity necessary for the inhibitor to anchor to the hinge region. |
| [34,36] | TEL::JAK2 JAK2 p.R683G | JAK2 p.G935R JAK2 p.G935R | JAK inhibitor 1 JAK inhibitor 1 BVB808 BSK805 TG101348 Ruxolitinib C Tofacitinib C | 0.25 0.19 0.09 0.31 0.60 0.08 0.48 | >10 R 0.51 R 0.46 R 8.72 R 1.29 R 0.29 R 0.44 S | The mutation introduces a large, positively charged side chain that may hinder drug binding due to steric hindrance. |
| [35] | CRLF2::P2RY8 | JAK2 p.R938Q | Ruxolitinib C | IC50 non reported | IC50 non reported (pJAK2 levels higher than control) | This mutation may result in allosteric changes within the ATP-binding pocket. |
| [28] | None | JAK1 p.F958V/C/S/L JAK1 p.P960S/T | CMP6 Ruxolitinib C | 0.1 <0.1 | >1 R ≥0.1 R | The mutations abolish the molecular interactions between the hinge region and the JAKi through changes in aminoacidic side chain orientation. |
| [36] | TEL::JAK2 | JAK2 p.R975G | JAK inhibitor 1 | 0.25 | >10 R | Replacing Arg 975 with Gly increases the flexibility of the main chain, which may promote opening of the JAKi pocket. |
| [15] | ATF7IP::JAK2 | JAK2 p.R983F | Ruxolitinib C BMS-911543 AZD-1480 Fedratinib C | 0.43 0.66 0.51 0.83 | >2.0 R >5.0 R 3.86 R 0.71 S | The mutation abolishes the ruxolitinib binding, as the bulkier Phe residue would sterically hinder interaction. |
| [29] | None | JAK3 p.Q988P | Ruxolitinib C Tofacitinib C | <1.0 <1.0 | >1.0 R <1.0 | Not reported |
| [15] | ATF7IP::JAK2 | JAK2 p.G993A | Ruxolitinib C BMS-911543 AZD-1480 Fedratinib C CHZ-868 | 0.43 0.66 0.51 0.83 <2.0 | >2.0 R >5.0 R 2.83 R 1.71 R >5.0 R | A steric clash would occur between the methyl group of Ala933 and the nitrile group of ruxolitinib, which would destabilize the binding of the benzimidazole ring of CHZ-868. |
| [30] | JAK2 p.R683G | JAK2 p.G993A | Ruxolitinib C Fedratinib C CHZ-868 II BBT-594 II MFH-6-7-1 II YLIU-5-162 II | 0.37 0.60 0.21 0.17 0.24 0.26 | 2.30 R 1.19 R 2.30 R 0.27 R 0.22 S 0.24 S | |
| [36] | TEL::JAK2 | JAK2 p.P1057S JAK2 p.R1127K | JAK inhibitor 1 | 0.25 | >10 R | Not reported |
| Reference | JAKi Resistance Mutation | CancerVar Classification * | Patients Annotated in Genomic Databases | Sample ID and Features | Mutation Features |
|---|---|---|---|---|---|
| Allosteric site | |||||
| [24] | JAK2 p.L884P | Tier 3 Uncertain clinical significance. | A case of CRLF2 rearranged B-ALL | SJBALL021004_D2 Non specified | Somatic VAF 0.26 |
| [23] | JAK3 p.L857P | Tier 2 Potential clinical significance. | A child diagnosed with T-ALL | COSS2770751 Tumor sample obtained after chemotherapy | Somatic, heterozygous |
| An adult with T-ALL | COSS2321345 Non specified | Non specified | |||
| A pediatric patient with T-ALL | COSS2770784 Sample collected at diagnosis | Non specified | |||
| A pediatric patient with T-ALL | COSS2770828 Non specified | Non specified | |||
| A child with T-ALL | COSS2770874 Tumor sample collected at diagnosis | Non specified | |||
| A child with T-ALL | COSS2770886 Tumor sample collected at diagnosis | Non specified | |||
| A 9-years old white male with T-ALL | COSS2730627 Tumor primary sample | Somatic | |||
| A 12-years old white male with T-ALL | COSS2730644 Tumor primary sample | Somatic | |||
| An 8.53-years old female with T-ALL | COSS2940234 Tumor primary sample | Somatic | |||
| A patient with T-ALL | SJTALL079673_D1 Tumor sample collected at diagnosis | Somatic VAF 50% | |||
| A patient with T-ALL | SJTALL015689_D1 Tumor sample collected at diagnosis | Somatic VAF 49% | |||
| A patient with T-ALL | SJALL016445_D1 Tumor sample collected at diagnosis. | Somatic VAF 32% | |||
| A patient with T-ALL | SJTALL07967_D1 Tumor sample collected at diagnosis. | Somatic VAF 50% | |||
| Gatekeeper, hinge, activation loop and DGF regions | |||||
| [28] | JAK1 p.F958C | Tier 2 Potential clinical significance | An adult with T-cell Lymphoma/Leukemia | COSS2488735 Non specified | Somatic |
| JAK1 p.P960S | Tier 3 Uncertain clinical significance | An 8.6 years-female patient with B-ALL iAMP21 positive | SJBALL030072_D1 Primary tumor sample analyzed before treatment | Somatic VAF 0.35 | |
| A 42-year-old male with T-ALL | COSS2629905 Tumor sample | Somatic heterozygous | |||
| [15,34] | JAK2 p.Y931C | Tier 2 Potential clinical significance | A 14-year-old female patient with B-ALL | COSS2843904 Primary tumor sample | Somatic |
| [43] | JAK2 p.P933R | Tier 3 Uncertain clinical significance | An 8.7-years old child with CRLF2::IGH positive B-ALL | COSS1715748 Primary tumor sample | Somatic |
| An 8.7-years old female patient with B-ALL | COSS1231802 Non specified | Heterozygous somatic mutation | |||
| A case of pediatric B-ALL | SJBALL021439_D2 Diagnosis sample | Somatic mutation VAF 0.25 | |||
| [35] | JAK2 p.R938Q | Tier 3 Uncertain clinical significance | A female child diagnosed with B-ALL | COSS2873651 Relapse tumor sample | Somatic |
| [29] | JAK3 p.Q988P | Tier 3 Uncertain clinical significance | A 40-years old male with T-ALL | COSS2513936 Tumor sample collected before chemotherapy | Non specified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez Anaya, D.; Coyotecatl, M.V.; Navarrete Meneses, M.d.P.; Enríquez Flores, S.; Pérez-Vera, P. The Mechanisms of Resistance to JAK Inhibitors in Lymphoid Leukemias: A Scoping Review of Evidence from Preclinical Models and Case Reports. Int. J. Mol. Sci. 2025, 26, 9111. https://doi.org/10.3390/ijms26189111
Martínez Anaya D, Coyotecatl MV, Navarrete Meneses MdP, Enríquez Flores S, Pérez-Vera P. The Mechanisms of Resistance to JAK Inhibitors in Lymphoid Leukemias: A Scoping Review of Evidence from Preclinical Models and Case Reports. International Journal of Molecular Sciences. 2025; 26(18):9111. https://doi.org/10.3390/ijms26189111
Chicago/Turabian StyleMartínez Anaya, Daniel, Marian Valladares Coyotecatl, Maria del Pilar Navarrete Meneses, Sergio Enríquez Flores, and Patricia Pérez-Vera. 2025. "The Mechanisms of Resistance to JAK Inhibitors in Lymphoid Leukemias: A Scoping Review of Evidence from Preclinical Models and Case Reports" International Journal of Molecular Sciences 26, no. 18: 9111. https://doi.org/10.3390/ijms26189111
APA StyleMartínez Anaya, D., Coyotecatl, M. V., Navarrete Meneses, M. d. P., Enríquez Flores, S., & Pérez-Vera, P. (2025). The Mechanisms of Resistance to JAK Inhibitors in Lymphoid Leukemias: A Scoping Review of Evidence from Preclinical Models and Case Reports. International Journal of Molecular Sciences, 26(18), 9111. https://doi.org/10.3390/ijms26189111






