Dietary Mannan Oligosaccharides Enhance Lactational Performance, Nutrient Metabolism, Plasma Metabolomics, and Gut Microbiota in Dezhou Donkeys
Abstract
1. Introduction
2. Results
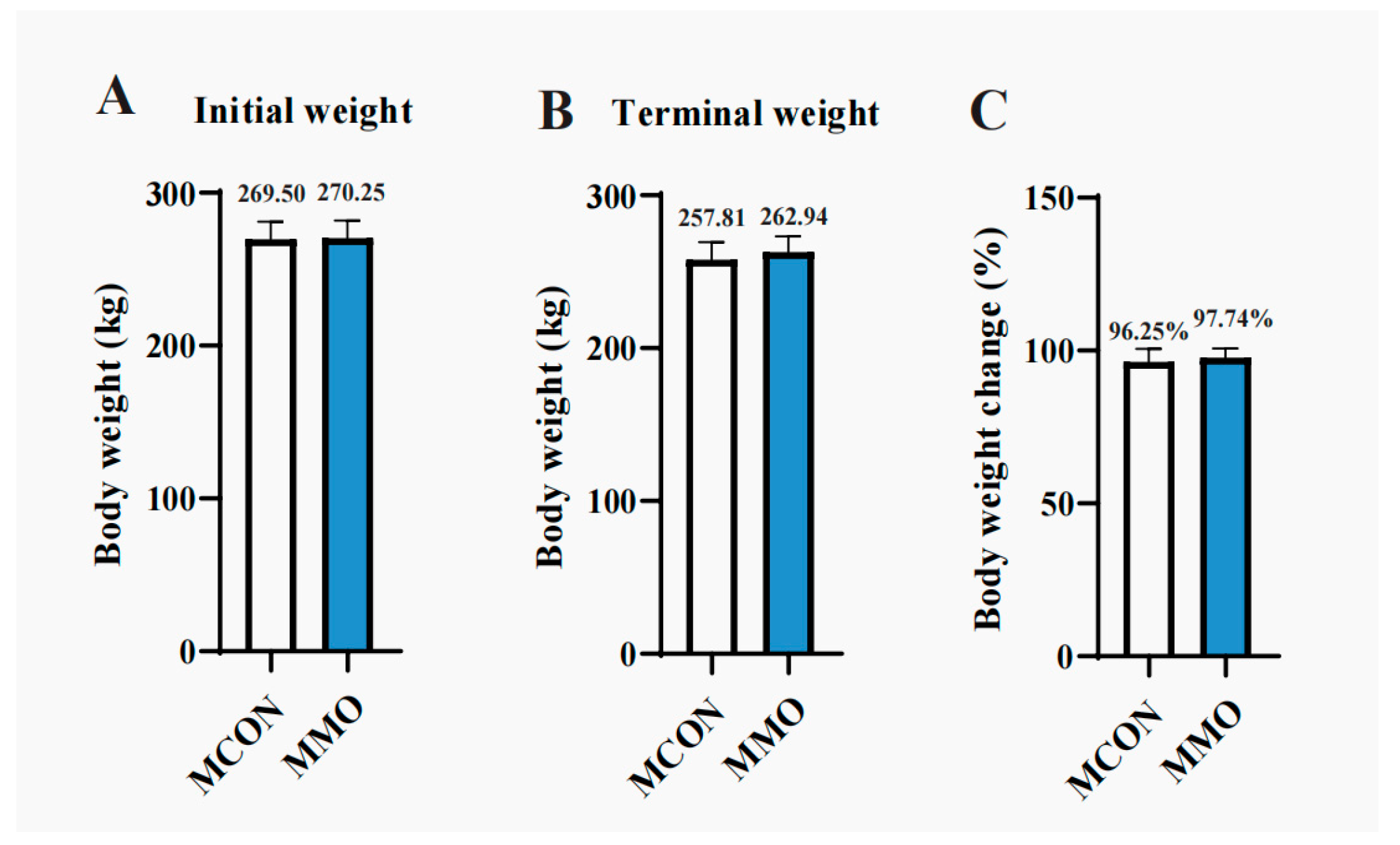
2.1. Effects of Mannan Oligosaccharides on Growth Performance in Dezhou Donkeys
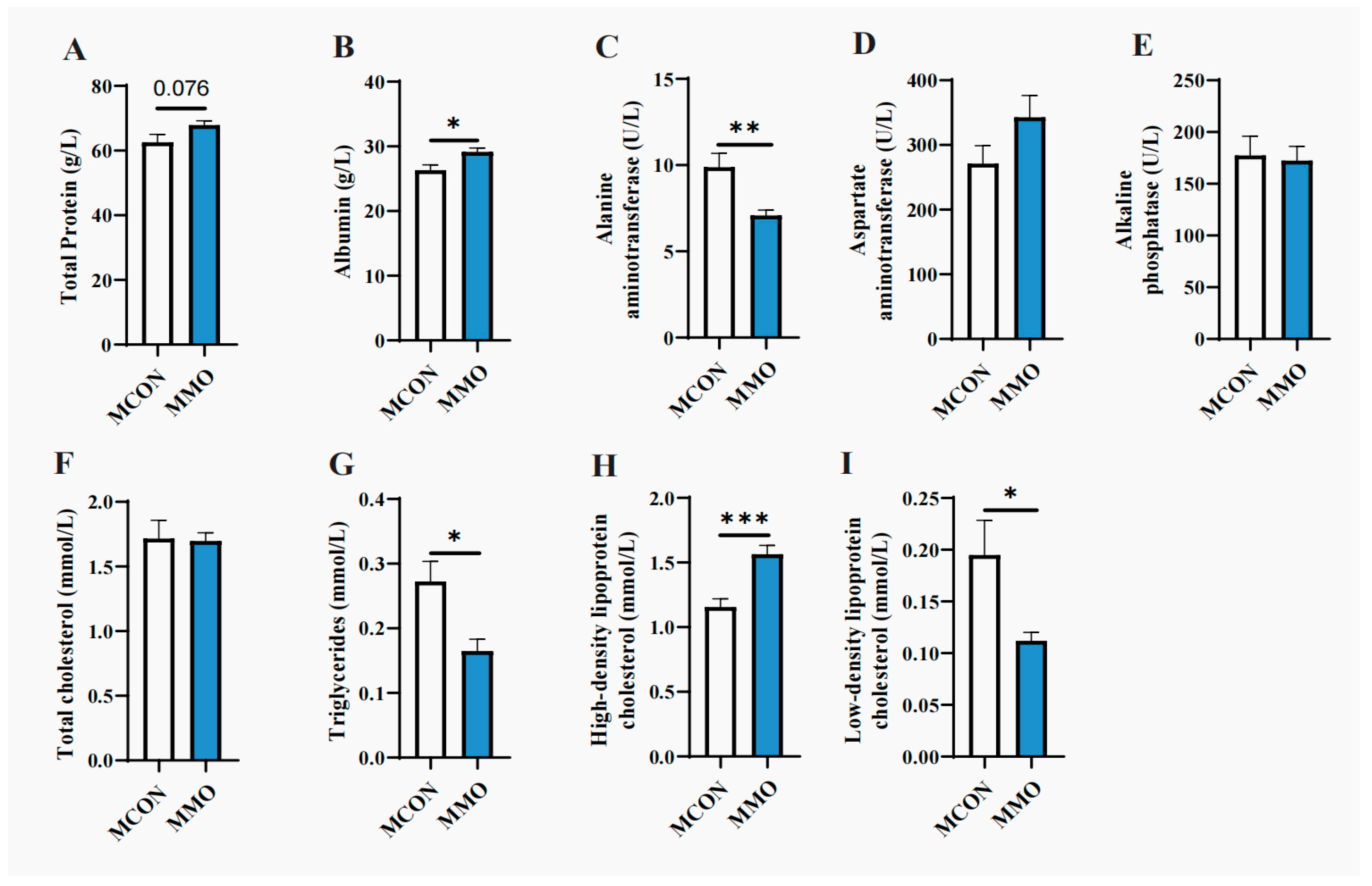
2.2. Effects of Mannan Oligosaccharides on Blood Biochemical Parameters in Dezhou Donkeys
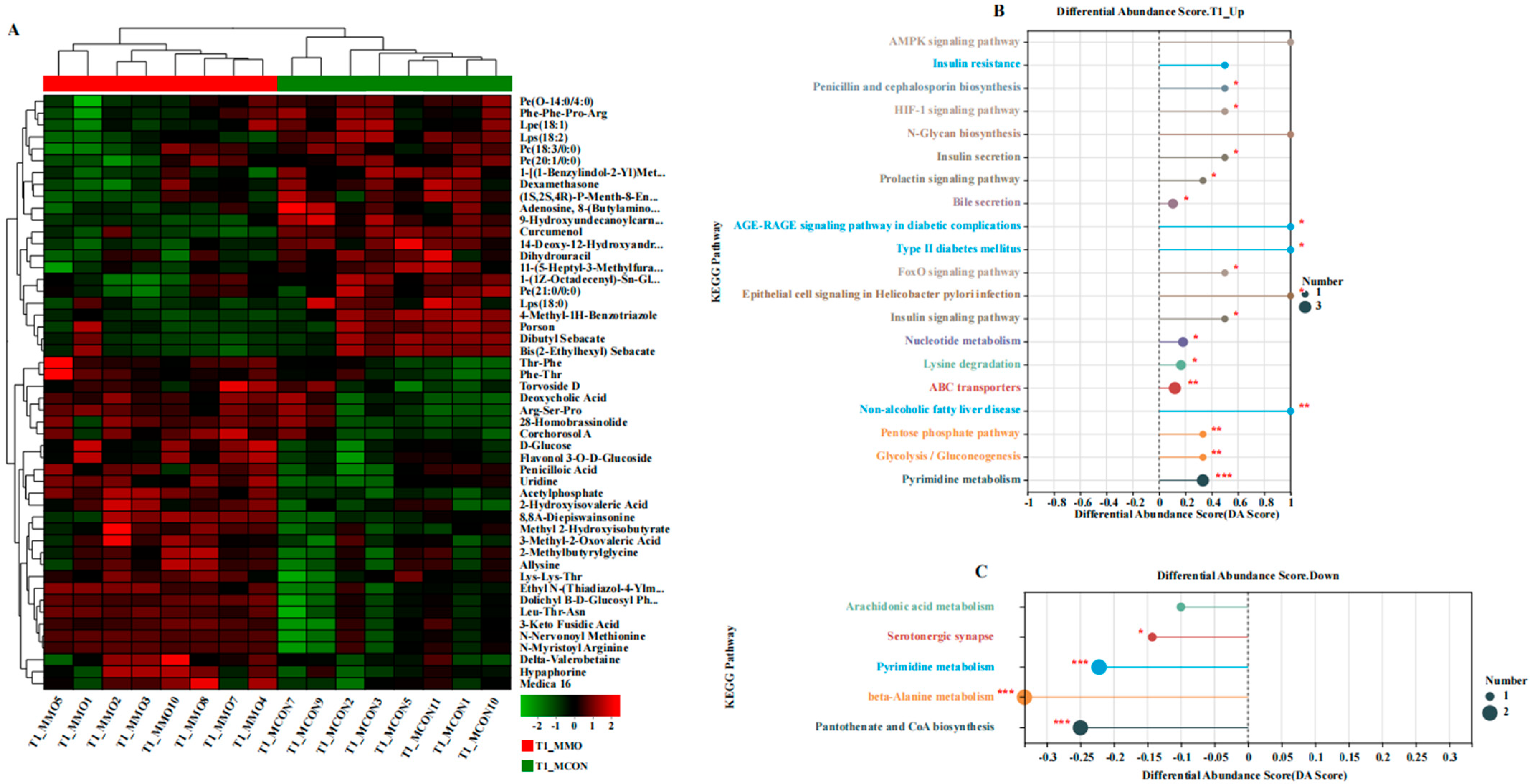
2.3. Effects of Mannan Oligosaccharides on Serum Metabolome in Dezhou Donkeys
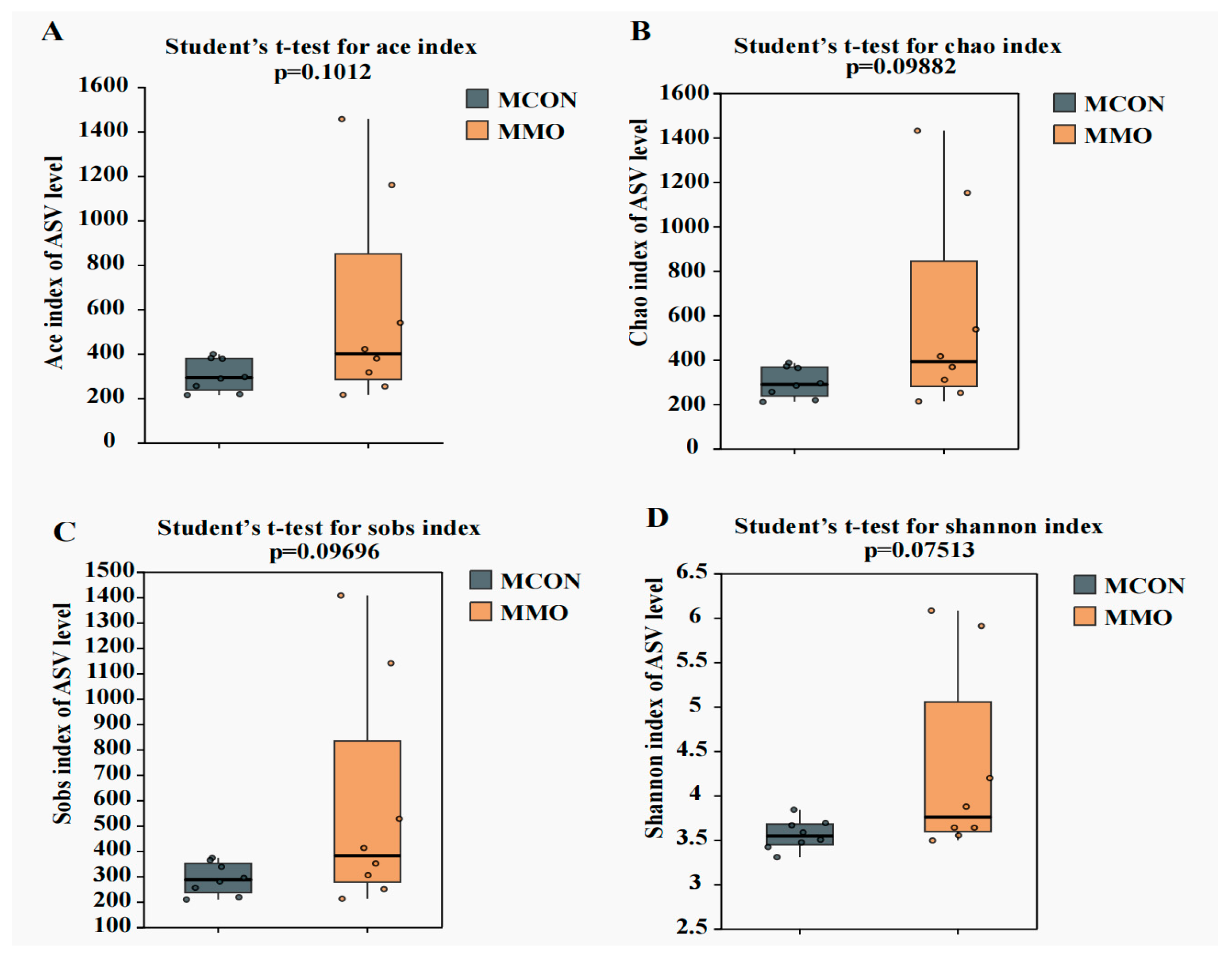
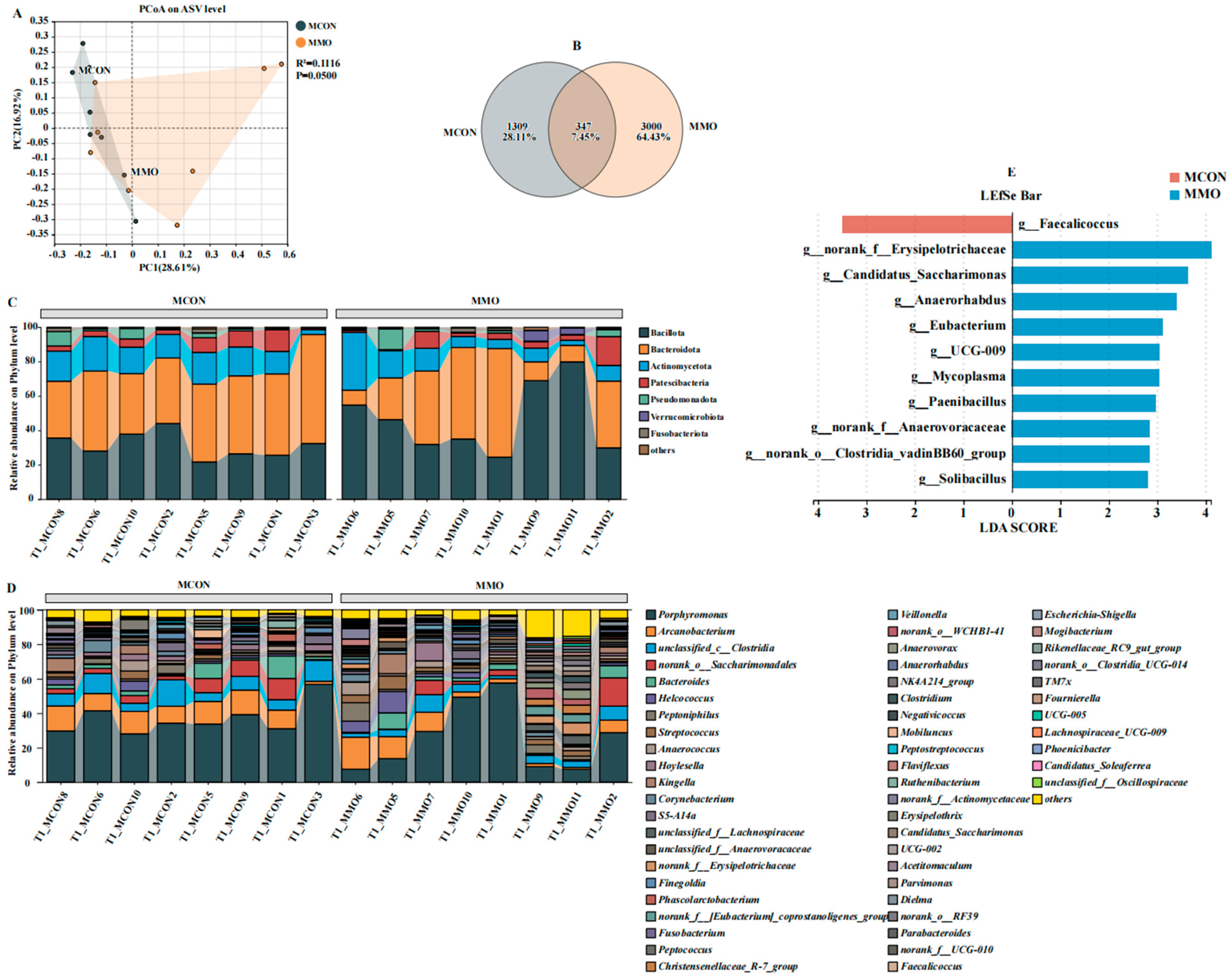
2.4. Effects of Mannan Oligosaccharide Supplementation on the Fecal Microbial Community in Dezhou Donkeys
2.5. Effects of Mannan Oligosaccharide Supplementation on the Differential Fecal Microbial Community in Dezhou Donkeys
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Sample Collection
4.3. Serum Biochemical Indices
4.4. Plasma Metabolomics Analysis
4.5. Microbiota Sequencing and Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Alpha diversity indices |
| AhR | aryl hydrocarbon receptor |
| ABCA1 | ATP-binding cassette sub-family A member 1 |
| ABCG1 | ATP-binding cassette sub-family G member 1 |
| ASV | Amplicon Sequence Variant |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ALP | alkaline phosphatase |
| ALB | albumin |
| ADG | average daily gain |
| BI | Class B Type I |
| CD4+ | Cluster of Differentiation 4-positive T lymphocyte |
| CD8+ | Cluster of Differentiation 8-positive T lymphocyte |
| CA | cholic acid |
| DA | the Differential Abundance |
| FCR | feed conversion ratio |
| FXR | Farnesoid X Receptor |
| GSH | glutathione |
| Gly-Trp | Glycyl-Tryptophan |
| HDL | High-Density Lipoprotein |
| HMDB | The Human Metabolome Database |
| LDL | Low-Density Lipoprotein |
| IDO | Indoleamine 2,3-Dioxygenase |
| I3P | indole-3-pyruvate |
| IPA | indolepropionic acid |
| IL-1β | Interleukin-1 beta |
| IL-8 | Interleukin-8 |
| IL-6 | Interleukin-6 |
| IBD | inflammatory bowel disease |
| IgY | immunoglobulin Y |
| IgM | immunoglobulin M |
| LPS | lipopolysaccharide |
| LDA | Linear Discriminant Analysis |
| MMO | the mannan oligosaccharide-treated group |
| MUC2 | Mucin 2 |
| MCON | the not MOS-supplemented group |
| MOS | Mannan oligosaccharides |
| NADH | Nicotinamide Adenine Dinucleotide |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| OPLS-DA | orthogonal partial least squares discriminant analysis |
| PCA | principal component analysis |
| PCoA | principal coordinate analysis |
| PC1 | Principal Component 1 |
| PLA2 | pancreatic lipase |
| pTreg | peripheral T regulatory |
| QC | quality control |
| RCT | reverse cholesterol transport |
| SOD | Superoxide Dismutase |
| SD | standard deviation |
| TNF-α | Tumor Necrosis Factor-alpha |
| TC | total cholesterol |
| TG | total triglyceride |
| TPH | tryptophan hydroxylase |
| TP | total protein |
| Thr | threonine |
| TCA | tricarboxylic acid |
| VFAs | volatile fatty acids |
| VIP | Variable Importance in Projection |
References
- Jana, U.K.; Suryawanshi, R.K.; Prajapati, B.P.; Kango, N. Prebiotic mannooligosaccharides: Synthesis, characterization and bioactive properties. Food Chem. 2021, 342, 128328. [Google Scholar] [CrossRef] [PubMed]
- Faustino, M.; Durão, J.; Pereira, C.F.; Pintado, M.E.; Carvalho, A.P. Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae—A sustainable source of functional ingredients. Carbohydr. Polym. 2021, 272, 118467. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Sun, F.; Lin, G.; Guo, Y.; Zhao, J. Molecular actions of different functional oligosaccharides on intestinal integrity, immune function and microbial community in weanling pigs. Food Funct. 2022, 13, 12303–12315. [Google Scholar] [CrossRef]
- Zhang, N.; Jin, M.; Wang, K.; Zhang, Z.; Shah, N.P.; Wei, H. Functional oligosaccharide fermentation in the gut: Improving intestinal health and its determinant factors—A review. Carbohydr. Polym. 2022, 284, 119043. [Google Scholar] [CrossRef]
- Wang, T.; Wu, H.X.; Li, W.J.; Xu, R.; Qiao, F.; Du, Z.Y.; Zhang, M.L. Effects of dietary mannan oligosaccharides (MOS) supplementation on metabolism, inflammatory response and gut microbiota of juvenile Nile tilapia (Oreochromis niloticus) fed with high carbohydrate diet. Fish Shellfish. Immunol. 2022, 130, 550–559. [Google Scholar] [CrossRef]
- Tang, J.; Liu, J.; Yan, Q.; Gu, Z.; August, A.; Huang, W.; Jiang, Z. Konjac Glucomannan Oligosaccharides Prevent Intestinal Inflammation Through SIGNR1-Mediated Regulation of Alternatively Activated Macrophages. Mol. Nutr. Food Res. 2021, 65, e2001010. [Google Scholar] [CrossRef]
- Ravichandran, T.; Perumal, R.K.; Vijayalakshmy, K.; Raw, Z.; Cooke, F.; Baltenweck, I.; Rahman, H. Means of Livelihood, Clean Environment to Women Empowerment: The Multi-Faceted Role of Donkeys. Animals 2023, 13, 1927. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Shi, X.; Zhang, Z.; Li, Y.; Huang, B.; Ren, W.; Wang, X.; Wang, C.; Chai, W. An investigation of genetic diversity in three Dezhou donkey original breeding farms. Sci. Rep. 2023, 13, 11203. [Google Scholar] [CrossRef]
- Bertino, E.; Agosti, M.; Peila, C.; Corridori, M.; Pintus, R.; Fanos, V. The Donkey Milk in Infant Nutrition. Nutrients 2022, 14, 403. [Google Scholar] [CrossRef]
- Thiemann, A.K.; Sullivan, R.J.E. Gastrointestinal Disorders of Donkeys and Mules. Vet. Clin. N. Am. Equine Pract. 2019, 35, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Hsu, H.S.; Chiang, M.T. Influence of Varied Dietary Cholesterol Levels on Lipid Metabolism in Hamsters. Nutrients 2024, 16, 2472. [Google Scholar] [CrossRef]
- Shahid, M.; Gao, J.; Zhou, Y.; Liu, G.; Ali, T.; Deng, Y.; Sabir, N.; Su, J.; Han, B. Prototheca zopfii isolated from bovine mastitis induced oxidative stress and apoptosis in bovine mammary epithelial cells. Oncotarget 2017, 8, 31938–31947. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aicha, S.; Badimon, L.; Vilahur, G. Advances in HDL: Much More than Lipid Transporters. Int. J. Mol. Sci. 2020, 21, 732. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Rilwan, H.B.; Adebisi, S.S.; Timbuak, J.A.; Oladele, S.B.; Muhammad, A.; Makena, W.; Sadeeq, A.A. Attenuation of streptozotocin induced high fat diet exacerbated dyslipidemia mediated hepatic and aortic injuries in male pigs by camel milk. Egypt. J. Basic Appl. Sci. 2023, 10, 107–122. [Google Scholar] [CrossRef]
- Gallagher, K.; Bernstein, I.; Collings, C.; Main, D.; Ahmad, G.; Naughton, S.; Daddam, J.; Mavangira, V.; Vandehaar, M.; Zhou, Z. Abomasal infusion of branched-chain amino acids or branched-chain keto-acids alter lactation performance and liver triglycerides in fresh cows. J. Anim. Sci. Biotechnol. 2024, 15, 13. [Google Scholar] [CrossRef]
- Xiao, F.; Shao, S.; Zhang, H.; Li, G.; Piao, S.; Zhao, D.; Li, G.; Yan, M. Neuroprotective effect of Ziziphi Spinosae Semen on rats with p-chlorophenylalanine-induced insomnia via activation of GABA(A) receptor. Front. Pharmacol. 2022, 13, 965308. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Qin, Y.J.; Xu, C.; Xia, T.; Du, Z.W.; Zheng, L.P.; Li, A.A.; Meng, F.; Zhang, Y.; Zhang, J.; et al. Design of fast-onset antidepressant by dissociating SERT from nNOS in the DRN. Science 2022, 378, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.C.; Ardura-Fabregat, A.; de Lima, K.A.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O.; et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef]
- Xu, Y.; Hong, Y.; Yan, T.; Sun, Q.; Yuan, F.; Liang, S.; Ye, L.; Geng, R.; Qi, Y.; Ye, Q.; et al. Tryptophan Metabolic Enzyme IL4I1 Inhibits Ferroptosis by Decreasing Ubiquitination of Nrf2 via I3P in Glioblastoma. Cell Prolif. 2025, 58, e13816. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef] [PubMed]
- Keskey, R.C.; Xiao, J.; Hyoju, S.; Lam, A.; Kim, D.; Sidebottom, A.M.; Zaborin, A.; Dijkstra, A.; Meltzer, R.; Thakur, A.; et al. Enterobactin inhibits microbiota-dependent activation of AhR to promote bacterial sepsis in mice. Nat. Microbiol. 2025, 10, 388–404. [Google Scholar] [CrossRef]
- Hu, D.; Hou, M.; Song, P.; Chen, Q.; Feng, Y.; Wu, X.; Ni, Y. Dietary bile acids supplementation improves the growth performance and alleviates fatty liver in broilers fed a high-fat diet via improving the gut microbiota. Poult. Sci. 2024, 103, 103270. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, X.; Zhao, Y.; Guo, Y.; Shi, B.; Zhou, Y.; Zhang, Y.; Yan, S. Cecal Microbial Diversity and Metabolome Reveal a Reduction in Growth Due to Oxidative Stress Caused by a Low-Energy Diet in Donkeys. Antioxidants 2024, 13, 1377. [Google Scholar] [CrossRef]
- Lee, M.H.; Nuccio, S.P.; Mohanty, I.; Hagey, L.R.; Dorrestein, P.C.; Chu, H.; Raffatellu, M. How bile acids and the microbiota interact to shape host immunity. Nat. Rev. Immunol. 2024, 24, 798–809. [Google Scholar] [CrossRef]
- Chen, L.Z.; Sun, W.W.; Bo, L.; Wang, J.Q.; Xiu, C.; Tang, W.J.; Shi, J.B.; Zhou, H.P.; Liu, X.H. New arylpyrazoline-coumarins: Synthesis and anti-inflammatory activity. Eur. J. Med. Chem. 2017, 138, 170–181. [Google Scholar] [CrossRef]
- Alexander, C.; Swanson, K.S.; Fahey, G.C.; Garleb, K.A. Perspective: Physiologic Importance of Short-Chain Fatty Acids from Nondigestible Carbohydrate Fermentation. Adv. Nutr. 2019, 10, 576–589. [Google Scholar] [CrossRef]
- Behr, C.; Slopianka, M.; Haake, V.; Strauss, V.; Sperber, S.; Kamp, H.; Walk, T.; Beekmann, K.; Rietjens, I.; van Ravenzwaay, B. Analysis of metabolome changes in the bile acid pool in feces and plasma of antibiotic-treated rats. Toxicol. Appl. Pharmacol. 2019, 363, 79–87. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Chi, Z.; Zhang, M.; Fu, B.; Wang, X.; Yang, H.; Fang, X.; Li, Z.; Teng, T.; Shi, B. Branched Short-Chain Fatty Acid-Rich Fermented Protein Food Improves the Growth and Intestinal Health by Regulating Gut Microbiota and Metabolites in Young Pigs. J. Agric. Food Chem. 2024, 72, 21594–21609. [Google Scholar] [CrossRef]
- Wu, J.; Wang, J.; Lin, Z.; Liu, C.; Zhang, Y.; Zhang, S.; Zhou, M.; Zhao, J.; Liu, H.; Ma, X. Clostridium butyricum alleviates weaned stress of piglets by improving intestinal immune function and gut microbiota. Food Chem. 2023, 405, 135014. [Google Scholar] [CrossRef]
- Liao, X.D.; Ma, G.; Cai, J.; Fu, Y.; Yan, X.Y.; Wei, X.B.; Zhang, R.J. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult. Sci. 2015, 94, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, Z.; Gao, Z.; Li, Q.; Qiu, X.; Wu, F.; Guan, T.; Cao, B.; Su, H. Effects of compound probiotics on growth performance, rumen fermentation, blood parameters, and health status of neonatal Holstein calves. J. Dairy. Sci. 2022, 105, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Roushdy, E.M.; Zaglool, A.W.; El-Tarabany, M.S. Effects of chronic thermal stress on growth performance, carcass traits, antioxidant indices and the expression of HSP70, growth hormone and superoxide dismutase genes in two broiler strains. J. Therm. Biol. 2018, 74, 337–343. [Google Scholar] [CrossRef]
- Lee, D.; Jo, H.; Choi, J.I. Molecular Hydrogen Modulates T Cell Differentiation and Enhances Neuro-Regeneration in a Vascular Dementia Mouse Model. Antioxidants 2025, 14, 111. [Google Scholar] [CrossRef]
- Endo, H.; Niioka, M.; Kobayashi, N.; Tanaka, M.; Watanabe, T. Butyrate-producing probiotics reduce nonalcoholic fatty liver disease progression in rats: New insight into the probiotics for the gut-liver axis. PLoS ONE 2013, 8, e63388. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235. [Google Scholar] [CrossRef]
- Duan, W.; Zheng, B.; Li, T.; Liu, R.H. Effects of Polyphenols from Oat and Oat Bran on Anti-Inflammatory Activity and Intestinal Barrier Function in Raw264.7 and Caco-2 Models. Nutrients 2025, 17, 1962. [Google Scholar] [CrossRef]

| Ingredients | Content (%) | Nutrients | Nutrient Level (%) |
|---|---|---|---|
| Corn | 31.75 | Dry matter | 88.53 |
| Middlings bran | 12.00 | Crude protein | 17.18 |
| Wheat flour middling | 21.00 | Ash | 8.37 |
| DDGS | 5.00 | Crude fiber | 4.27 |
| Wheat bran | 18.00 | Ether extract | 4.59 |
| Soybean meal | 6.65 | Calcium | 1.27 |
| NaCl | 0.63 | Phosphorus | 0.52 |
| CaCO3 | 3.72 | Lysine | 0.75 |
| CaHPO4 | 0.25 | ||
| Premix1 | 1.00 | ||
| Total | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Wang, Y.; Li, P.; Liu, J.; Mao, X.; Li, Z.; Wen, Z.; Yin, Y.; Li, Y.; Lin, G.; et al. Dietary Mannan Oligosaccharides Enhance Lactational Performance, Nutrient Metabolism, Plasma Metabolomics, and Gut Microbiota in Dezhou Donkeys. Int. J. Mol. Sci. 2025, 26, 9105. https://doi.org/10.3390/ijms26189105
Wang T, Wang Y, Li P, Liu J, Mao X, Li Z, Wen Z, Yin Y, Li Y, Lin G, et al. Dietary Mannan Oligosaccharides Enhance Lactational Performance, Nutrient Metabolism, Plasma Metabolomics, and Gut Microbiota in Dezhou Donkeys. International Journal of Molecular Sciences. 2025; 26(18):9105. https://doi.org/10.3390/ijms26189105
Chicago/Turabian StyleWang, Tianzheng, Yaru Wang, Pengshuai Li, Jiaxin Liu, Xinyi Mao, Zuowei Li, Zhangxinhan Wen, Yuhan Yin, Yan Li, Gang Lin, and et al. 2025. "Dietary Mannan Oligosaccharides Enhance Lactational Performance, Nutrient Metabolism, Plasma Metabolomics, and Gut Microbiota in Dezhou Donkeys" International Journal of Molecular Sciences 26, no. 18: 9105. https://doi.org/10.3390/ijms26189105
APA StyleWang, T., Wang, Y., Li, P., Liu, J., Mao, X., Li, Z., Wen, Z., Yin, Y., Li, Y., Lin, G., Zhang, H., Qu, H., Ma, Q., & Huang, S. (2025). Dietary Mannan Oligosaccharides Enhance Lactational Performance, Nutrient Metabolism, Plasma Metabolomics, and Gut Microbiota in Dezhou Donkeys. International Journal of Molecular Sciences, 26(18), 9105. https://doi.org/10.3390/ijms26189105







