Vaccination Against Extracellular Vimentin Plus Doxorubicin for Canine Hemangiosarcoma
Abstract
1. Introduction
2. Results
2.1. Study Population
2.2. Immunohistochemical Analysis
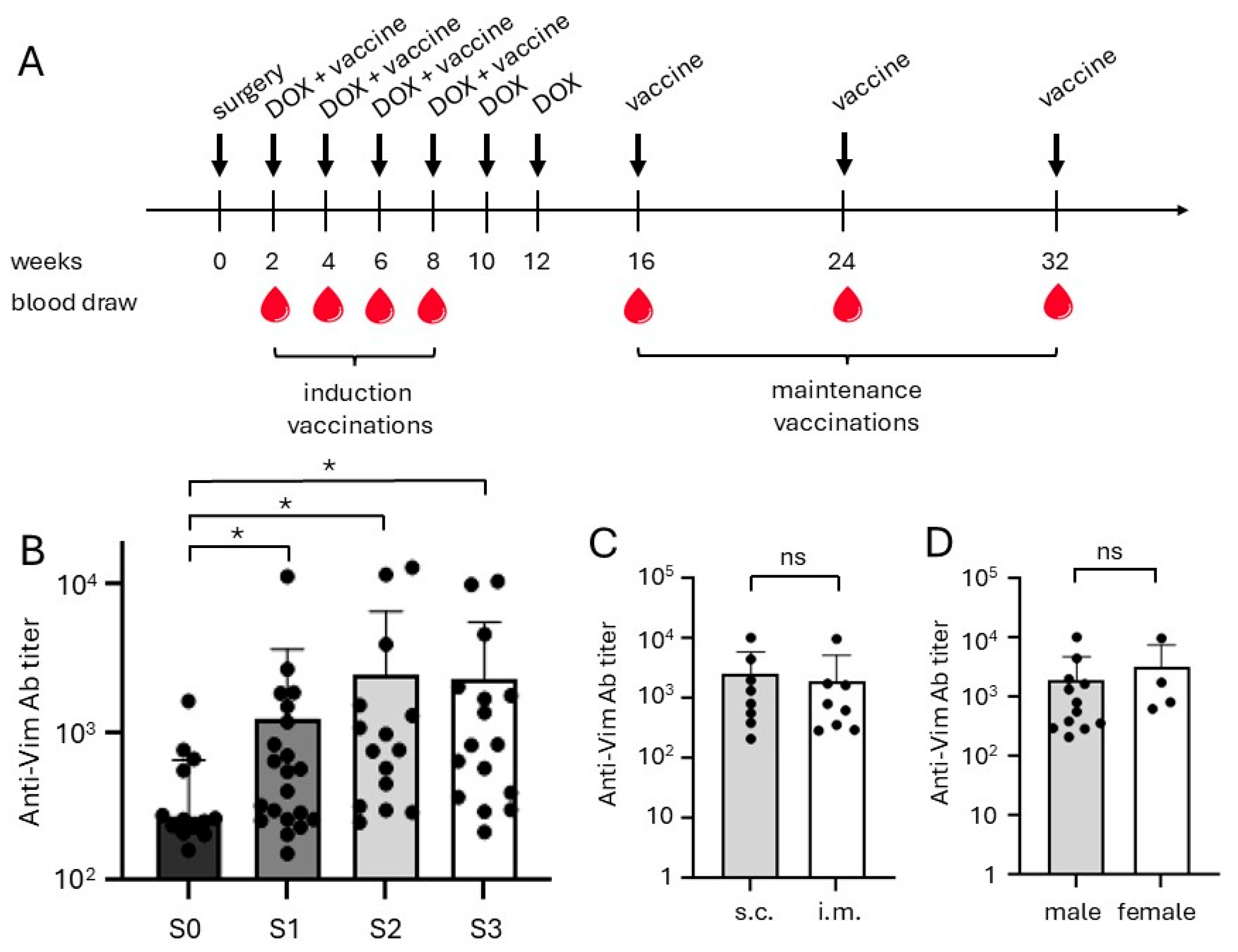
2.3. Vaccination and Antibody Responses
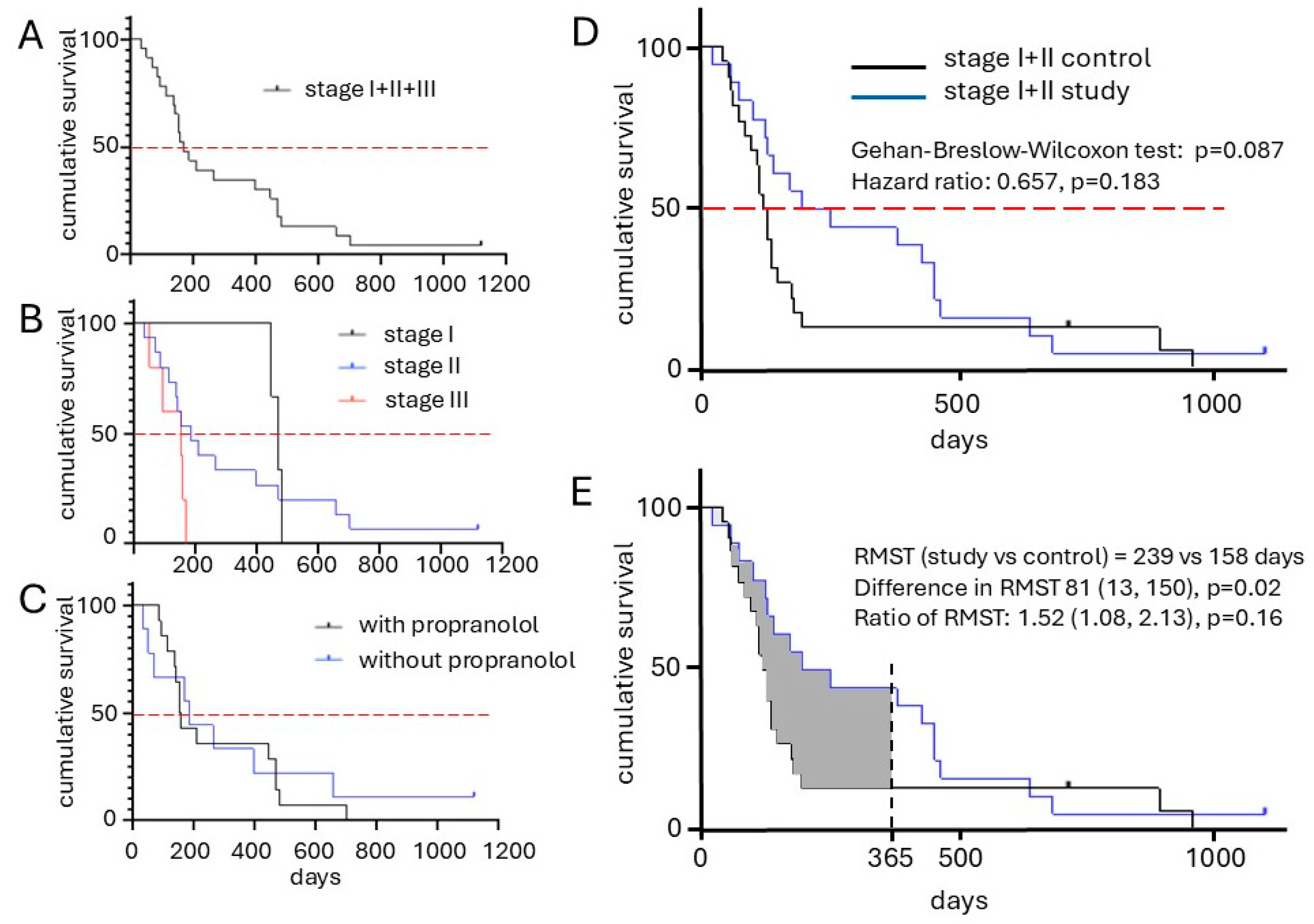
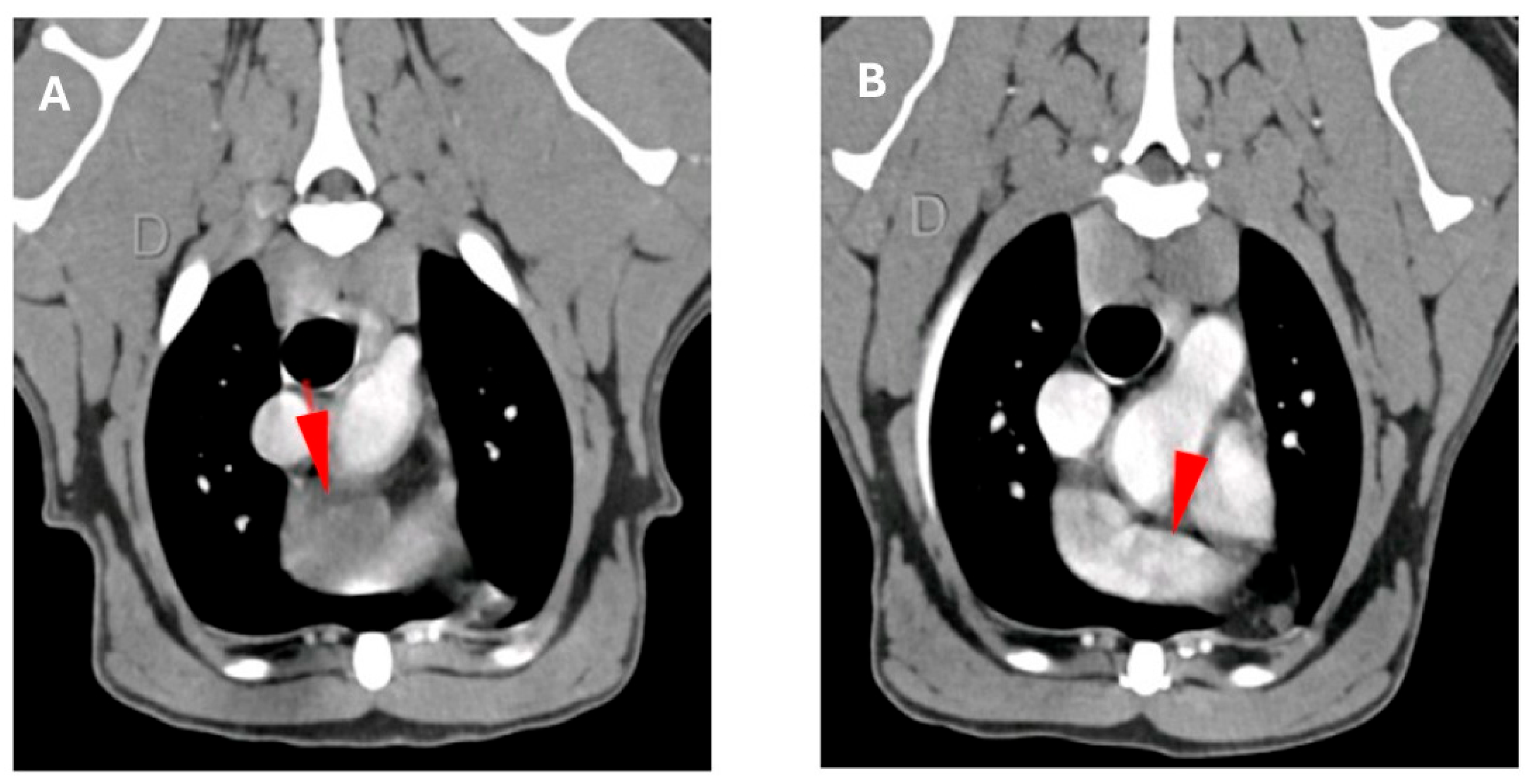
2.4. Clinical Outcome
2.5. Safety and Tolerability
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Immune Response Measurement
4.3. Anti-eVim Antibody ELISA
4.4. Patient Monitoring and Evaluation
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- WHO. Soft Tissue and Bone Tumours WHO Classification of Tumours, 5th ed.; IARC Press: Lyon, France, 2020. [Google Scholar]
- Young, R.J.; Brown, N.J.; Reed, M.W.; Hughes, D.; Woll, P.J. Angiosarcoma. Lancet Oncol. 2010, 11, 983–991. [Google Scholar] [CrossRef]
- Sturm, E.C.; Marasco, I.S.; Katz, S.C. Multidisciplinary Management of Angiosarcoma—A Review. J. Surg. Res. 2021, 257, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Megquier, K.; Turner-Maier, J.; Swofford, R.; Kim, J.-H.; Sarver, A.L.; Wang, C.; Sakthikumar, S.; Johnson, J.; Koltookian, M.; Lewellen, M.; et al. Comparative genomics reveals shared mutational landscape in canine hemangiosarcoma and human angiosarcoma. Mol. Cancer Res. 2019, 17, 2410–2421. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wu, M.; Durham, A.C.; Radaelli, E.; Mason, N.J.; Xu, X.; Roth, D.B.; Birkeland, A. Molecular subtypes in canine hemangiosarcoma reveal similarities with human angiosarcoma. PLoS ONE 2020, 15, e0229728. [Google Scholar] [CrossRef] [PubMed]
- Käcker, C.; Marx, A.; Mössinger, K.; Svehla, F.; Schneider, U.; Hogendoorn, P.C.W.; Nielsen, O.S.; Küffer, S.; Sauer, C.; Fisher, C.; et al. High frequency of MYC gene amplification is a common feature of radiation-induced sarcomas. Further results from EORTC STBSG TL 01/01. Genes Chromosomes Cancer 2013, 52, 93–98. [Google Scholar] [CrossRef]
- Schlemmer, M.; Reichardt, P.; Verweij, J.; Hartmann, J.; Judson, I.; Thyss, A.; Hogendoorn, P.; Marreaud, S.; Van Glabbeke, M.; Blay, J. Paclitaxel in patients with advanced angiosarcomas of soft tissue: A retrospective study of the EORTC soft tissue and bone sarcoma group. Eur. J. Cancer 2008, 44, 2433–2436. [Google Scholar] [CrossRef]
- Estabrooks, T.; Gurinovich, A.; Pietruska, J.; Lewis, B.; Harvey, G.; Post, G.; Lambert, L.; Miller, A.; Rodrigues, L.; White, M.E.; et al. Identification of genomic alterations with clinical impact in canine splenic hemangiosarcoma. Vet. Comp. Oncol. 2023, 21, 623–633. [Google Scholar] [CrossRef]
- Mullin, C.; Clifford, C.A. Histiocytic Sarcoma and Hemangiosarcoma Update. Vet. Clin. N. Am. Small Anim. Pr. 2019, 49, 855–879. [Google Scholar] [CrossRef]
- De Nardi, A.B.; Gomes, C.d.O.M.S.; Fonseca-Alves, C.E.; de Paiva, F.N.; Linhares, L.C.M.; Carra, G.J.U.; Horta, R.d.S.; Sueiro, F.A.R.; Jark, P.C.; Nishiya, A.T.; et al. Diagnosis, Prognosis, and Treatment of Canine Hemangiosarcoma: A Review Based on a Consensus Organized by the Brazilian Association of Veterinary Oncology, ABROVET. Cancers 2023, 15, 2025. [Google Scholar] [CrossRef]
- Wendelburg, K.M.; Price, L.L.; Burgess, K.E.; Lyons, J.A.; Lew, F.H.; Berg, J. Survival time of dogs with splenic hemangiosarcoma treated by splenectomy with or without adjuvant chemotherapy: 208 cases (2001–2012). J. Am. Vet. Med. Assoc. 2015, 247, 393–403. [Google Scholar] [CrossRef]
- Batschinski, K.; Nobre, A.; Vargas-Mendez, E.; Tedardi, M.; Cirillo, J.; Cestari, G.; Ubukata, R.; Dagli, M.L.Z. Canine visceral hemangiosarcoma treated with surgery alone or surgery and doxorubicin: 37 cases (2005–2014). Can. Vet. J. 2018, 59, 967–972. [Google Scholar]
- Kim, S.E.; Liptak, J.M.; Gall, T.T.; Monteith, G.J.; Woods, J.P. Epirubicin in the adjuvant treatment of splenic hemangiosarcoma in dogs: 59 cases (1997–2004). J. Am. Vet. Med. Assoc. 2007, 231, 1550–1557. [Google Scholar] [CrossRef]
- Faulhaber, E.A.; Janik, E.; Thamm, D.H. Adjuvant carboplatin for treatment of splenic hemangiosarcoma in dogs: Retrospective evaluation of 18 cases (2011–2016) and comparison with doxorubicin-based chemotherapy. J. Vet. Intern. Med. 2021, 35, 1929–1934. [Google Scholar] [CrossRef]
- Pritchard, C.; Al-Nadaf, S.; Rebhun, R.B.; Willcox, J.L.; Skorupski, K.A.; Lejeune, A. Efficacy and toxicity of carboplatin in the treatment of macroscopic mesenchymal neoplasia in dogs. Vet. Comp. Oncol. 2023, 21, 717–725. [Google Scholar] [CrossRef]
- Vail, D.M.; MacEwen, E.G.; Kurzman, I.D.; Dubielzig, R.R.; Helfand, S.C.; Kisseberth, W.C.; London, C.A.; Obradovich, J.E.; Madewell, B.R.; Rodriguez, C.O., Jr. Liposome-encapsulated muramyl tripeptide phosphatidylethanolamine adjuvant immunotherapy for splenic hemangiosarcma in the dog: A randomized mul-ti-institutional clinical trial. Clin. Cancer Res. 1995, 1, 1165–1170. [Google Scholar]
- Léauté-Labrèze, C.; de la Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef]
- Wagner, M.J.; Cranmer, L.D.; Loggers, E.T.; Pollack, S.M. Propranolol for the treatment of vascular sarcomas. J. Exp. Pharmacol. 2018, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Saha, J.; Kim, J.H.; Amaya, C.N.; Witcher, C.; Khammanivong, A.; Korpela, D.M.; Brown, D.R.; Taylor, J.; Bryan, B.A.; Dickerson, E.B. Propranolol Sensitizes Vascular Sarcoma Cells to Doxorubicin by Altering Lysosomal Drug Sequestration and Drug Efflux. Front. Oncol. 2021, 10, 614288. [Google Scholar] [CrossRef]
- Terauchi, M.; Fujii, Y.; Goto, S.; Iwasaki, R.; Yoshikawa, R.; Mori, T. Efficacy and adverse events of anthracycline and propranolol combination in five dogs with stage 3 hemangiosarcoma. Open Vet. J. 2023, 13, 801–806. [Google Scholar] [CrossRef]
- Konduri, V.; Halpert, M.M.; Baig, Y.C.; Coronado, R.; Rodgers, J.R.; Levitt, J.M.; Cerroni, B.; Piscoya, S.; Wilson, N.; DiBernardi, L.; et al. Dendritic cell vaccination plus low-dose doxorubicin for the treatment of spontaneous canine hemangiosarcoma. Cancer Gene Ther. 2019, 26, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Musser, M.L.; Coto, G.M.; Lingnan, Y.; Mochel, J.P.; Johannes, C.M.; Sabattini, S. Pilot safety evaluation of doxorubicin chemotherapy combined with non-specific immunotherapy (Immunocidin®) for canine splenic hemangiosarcoma. PLoS ONE 2022, 17, e0279594. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Tiraboschi, L.; Aralla, M.; Sabattini, S.; Melacarne, A.; Agnoli, C.; Balboni, A.; Salvi, M.; Foglia, A.; Punzi, S.; et al. A Phase 2, Single-Arm, Open-Label Clinical Trial on Adjuvant Peptide-Based Vaccination in Dogs with Aggressive Hemangiosarcoma Undergoing Surgery and Chemotherapy. Cancers 2023, 15, 4209. [Google Scholar] [CrossRef] [PubMed]
- Borgatti, A.; Dickerson, E.B.; Lawrence, J. Emerging therapeutic approaches for canine sarcomas: Pushing the boundaries beyond the conventional. Vet. Comp. Oncol. 2020, 18, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Kapturska, K.M.; Pawlak, A. New molecular targets in canine hemangiosarcoma—Comparative review and future of the precision medicine. Vet. Comp. Oncol. 2023, 21, 357–377. [Google Scholar] [CrossRef]
- Moretti, G.; Bufalari, A. Editorial: A review of canine soft tissue sarcomas: New insights in diagnostic and treatment measures. Front. Vet. Sci. 2024, 11, 1454513. [Google Scholar] [CrossRef]
- Bryan, J.N.; Maitz, C.A. Translational History and Hope of Immunotherapy of Canine Tumors. Clin. Cancer Res. 2024, 30, 4272–4285. [Google Scholar] [CrossRef]
- Huinen, Z.R.; Huijbers, E.J.M.; van Beijnum, J.R.; Nowak-Sliwinska, P.; Griffioen, A.W. Anti-angiogenic agents—Overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. 2021, 18, 527–540. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; van Beijnum, J.R.; Griffioen, C.J.; Huinen, Z.R.; Sopesens, N.G.; Schulz, R.; Jenkins, S.V.; Dings, R.P.; Groenendijk, F.H.; Huijbers, E.J.; et al. Proinflammatory activity of VEGF-targeted treatment through reversal of tumor endothelial cell anergy. Angiogenesis 2023, 26, 279–293. [Google Scholar] [CrossRef]
- van Beijnum, J.R.; Huijbers, E.J.; van Loon, K.; Blanas, A.; Akbari, P.; Roos, A.; Wong, T.J.; Denisov, S.S.; Hackeng, T.M.; Jimenez, C.R.; et al. Extracellular vimentin mimics VEGF and is a target for anti-angiogenic immunotherapy. Nat. Commun. 2022, 13, 2842. [Google Scholar] [CrossRef]
- Huijbers, E.J.; van Beijnum, J.R.; Lê, C.T.; Langman, S.; Nowak-Sliwinska, P.; Mayo, K.H.; Griffioen, A.W. An improved conjugate vaccine technology; induction of antibody responses to the tumor vasculature. Vaccine 2018, 36, 3054–3060, Erratum in Vaccine 2019, 37, 4231–4232. [Google Scholar] [CrossRef]
- van Loon, K.; Huijbers, E.J.M.; de Haan, J.D.; Griffioen, A.W. Cancer Vaccination against Extracellular Vimentin Efficiently Adju-vanted with Montanide ISA 720/CpG. Cancers 2022, 14, 2593. [Google Scholar] [CrossRef]
- Engbersen, D.J.M.; van Beijnum, J.R.; Roos, A.; van Beelen, M.; de Haan, J.D.; Grinwis, G.C.M.; Schalken, J.A.; Witjes, J.A.; Griffioen, A.W.; Huijbers, E.J.M. Vaccination against Extracellular Vimentin for Treatment of Urothelial Cancer of the Bladder in Client-Owned Dogs. Cancers 2023, 15, 3958. [Google Scholar] [CrossRef]
- Frenz, M.; Kaup, F.-J.; Neumann, S. Serum vascular endothelial growth factor in dogs with haemangiosarcoma and haematoma. Res. Vet. Sci. 2014, 97, 257–262. [Google Scholar] [CrossRef]
- Ravi, V.; Subramaniam, A.; Zheng, J.; Amini, B.; Trinh, V.A.; Joseph, J.; Mennel, R.G.; Bishop, A.J.; Sturgis, E.M.; Goepfert, R.P.; et al. Clinical activity of checkpoint inhibitors in angiosarcoma: A retrospective cohort study. Cancer 2022, 128, 3383–3391. [Google Scholar] [CrossRef]
- Subramaniam, A.; Giani, C.; Napolitano, A.; Ravi, V.; Frezza, A.M.; Jones, R.L. Management of Vascular Sarcoma. Surg. Oncol. Clin. N. Am. 2022, 31, 485–510. [Google Scholar] [CrossRef]
- Fiste, O.; Dimos, A.; Kardara, V.E.; Ballasis, K.; Karampeazis, A. Propranolol and Weekly Paclitaxel in the Treatment of Metastatic Heart Angiosarcoma. Cureus 2020, 12, e12262. [Google Scholar] [CrossRef]
- Luczynska, E.; Rudnicki, W.; Kargol, J.; Szpor, J.; Hodorowicz-Zaniewska, D.; Wysocki, P.J.; Gorski, M.; Popiela, T.J. Primary bilateral angiosarcoma of the breast treated with neoadjuvant chemotherapy combined with propranolol. Breast J. 2021, 27, 781–786. [Google Scholar] [CrossRef]
- Goerdt, L.V.; Schneider, S.W.; Booken, N. Cutaneous Angiosarcomas: Molecular Pathogenesis Guides Novel Therapeutic Approaches. JDDG J. Dtsch. Dermatol. Ges. 2022, 20, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Embaby, A.; van Merendonk, L.; Steeghs, N.; Beijnen, J.; Huitema, A. Beta-adrenergic receptor blockade in angiosarcoma: Which beta-blocker to choose? Front. Oncol. 2022, 12, 940582. [Google Scholar] [CrossRef] [PubMed]
- Nyers, E. Oral Propranolol Used as Adjunct Therapy in Cutaneous Angiosarcoma. Cutis 2022, 110, E33–E36. [Google Scholar] [CrossRef] [PubMed]
- Embaby, A.; Heinhuis, K.M.; Ijzerman, N.S.; Koenen, A.M.; van der Kleij, S.; Hofland, I.; van Boven, H.; Sanders, J.; van der Graaf, W.T.; Haas, R.L.; et al. Propranolol monotherapy in angiosarcoma—A window-of-opportunity study (PropAngio). Eur. J. Cancer 2024, 202, 113974. [Google Scholar] [CrossRef] [PubMed]
- Massalee, R.; Cao, X. Repurposing beta-blockers for combinatory cancer treatment: Effects on conventional and immune therapies. Front. Pharmacol. 2024, 14, 1325050. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; Varela-Ramirez, A.; Dickerson, E.; Pasquier, E.; Torabi, A.; Aguilera, R.; Nahleh, Z.; Bryan, B. The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed. J. 2019, 42, 155–165. [Google Scholar] [CrossRef]
- O’lOgbon, J.; Tarantola, L.; Williams, N.R.; Mehta, S.; Ahmed, A.; Davies, E.A. Does propranolol have a role in cancer treatment? A systematic review of the epidemiological and clinical trial literature on beta-blockers. J. Cancer Res. Clin. Oncol. 2025, 151, 212. [Google Scholar] [CrossRef]
- Budde, J.A.; McCluskey, D.M. Plumb’s Veterinary Drug Handbook, 10th ed.; Wiley Blackwell: Hoboken, NJ, USA, 2023. [Google Scholar]
- Huijbers, E.J.; van Beijnum, J.R.; van Loon, K.; Griffioen, C.J.; Volckmann, R.; Bassez, A.; Lambrechts, D.; Nunes Monteiro, M.; Jimenez, C.R.; Hogendoorn, P.C.; et al. Embryonic reprogramming of the tumor vasculature reveals targets for cancer therapy. Proc. Natl. Acad. Sci. USA 2025, 122, e2424730122. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumours in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group—Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef]

| Dogs (n) | Gender (n) | Breed * (n) | Weight (kg) | Age (yr) | Clinical Stage (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Single | Mixed | Median | Mean | I | II | II | ||||
| Intact | Spayed | Intact | Castrated | |||||||||
| % | % | % | % | Range | Range | % | % | % | ||||
| Study | 23 | 0 | 7 | 9 | 7 | 15 | 8 | 28.9 | 9.2 | 3 | 15 | 5 |
| 0% | 30% | 39% | 30% | 65% | 35% | 5.2–64.0 | 1.9–13.6 | 13% | 65% | 22% | ||
| Control | 22 | 0 | 11 | 2 | 9 | 15 | 7 | 25 | N.R. | 7 | 15 | 0 |
| 0% | 50% | 9% | 41% | 68% | 32% | 8.4–42.0 | N.R. | 32% | 68% | 0% | ||
| Overall Survival Time (d) | Study | Control | Significance *** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Range | 1-yr OS * | n | Median | Range | 1-yr OS | p-Value ** | Y/N | |
| Stage I | 3 | 469 | 446–348 | 100% | 7 | 139 | 70–911 | 14% | 0.0333 | Y |
| Stage II | 15 | 186 | 34–235 | 33% | 15 | 128 | 54–975 | 13% | 0.1949 | N |
| Stage I + II | 18 | 235 | 34–1119 | 44% | 22 | 136 | 54–975 | 14% | 0.0344 | Y |
| Stage III | 5 | 153 | 34–168 | 0% | ||||||
| Stage I–III | 23 | 168 | 24–1119 | 35% | ||||||
| Treatment Related Adverse Events | Events/Dogs (n = 23) | |||||||
|---|---|---|---|---|---|---|---|---|
| (n) | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||||
| s.c. * (n = 12) | i.m. ** (n = 11) | s.c. (n = 12) | i.m. (n = 11) | s.c. (n = 12) | i.m. (n = 11) | s.c. (n = 12) | i.m. (n = 11) | |
| Injection side adverse events | ||||||||
| -Injection site reactions | 8/4 | 0/0 | 3/2 | 1/1 | 0/0 | 0/0 | 0/0 | 0/0 |
| -Lameness local extremity | 0/0 | 4/2 | 2/2 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Systemic adverse events | ||||||||
| -Diarrhoea | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 | 0/0 | 0/0 |
| -Lethargy/fatigue/performance | 1/1 | 0/0 | 0/1 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| -Neutropenia | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 |
| -Vomiting | 0/0 | 1/1 | 0/0 | 1/1 | 0/0 | 0/0 | 0/0 | 0/0 |
| Total adverse events | 9/6 | 5/3 | 6/5 | 2/2 | 0/0 | 1/1 | 0/0 | 1/1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engbersen, D.J.M.; van Bergen, L.; Bos, E.N.; Hansen, Q.; Roos, A.; Huijbers, E.J.M.; Faulhaber, E.A.; Hogendoorn, P.C.W.; Thamm, D.H.; Griffioen, A.W. Vaccination Against Extracellular Vimentin Plus Doxorubicin for Canine Hemangiosarcoma. Int. J. Mol. Sci. 2025, 26, 9096. https://doi.org/10.3390/ijms26189096
Engbersen DJM, van Bergen L, Bos EN, Hansen Q, Roos A, Huijbers EJM, Faulhaber EA, Hogendoorn PCW, Thamm DH, Griffioen AW. Vaccination Against Extracellular Vimentin Plus Doxorubicin for Canine Hemangiosarcoma. International Journal of Molecular Sciences. 2025; 26(18):9096. https://doi.org/10.3390/ijms26189096
Chicago/Turabian StyleEngbersen, Diederik J. M., Lobke van Bergen, Emma N. Bos, Quinty Hansen, Arno Roos, Elisabeth J. M. Huijbers, Erica A. Faulhaber, Pancras C. W. Hogendoorn, Douglas H. Thamm, and Arjan W. Griffioen. 2025. "Vaccination Against Extracellular Vimentin Plus Doxorubicin for Canine Hemangiosarcoma" International Journal of Molecular Sciences 26, no. 18: 9096. https://doi.org/10.3390/ijms26189096
APA StyleEngbersen, D. J. M., van Bergen, L., Bos, E. N., Hansen, Q., Roos, A., Huijbers, E. J. M., Faulhaber, E. A., Hogendoorn, P. C. W., Thamm, D. H., & Griffioen, A. W. (2025). Vaccination Against Extracellular Vimentin Plus Doxorubicin for Canine Hemangiosarcoma. International Journal of Molecular Sciences, 26(18), 9096. https://doi.org/10.3390/ijms26189096







