Targeting G6PD with Benzimidazole and Thiazole Derivatives Suppresses SIRT 2 and VEGF Expression and Induces Cytotoxicity in Glioma Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Candidate Compounds with Cytotoxic Activity in A172 and U87-MG Cell Lines
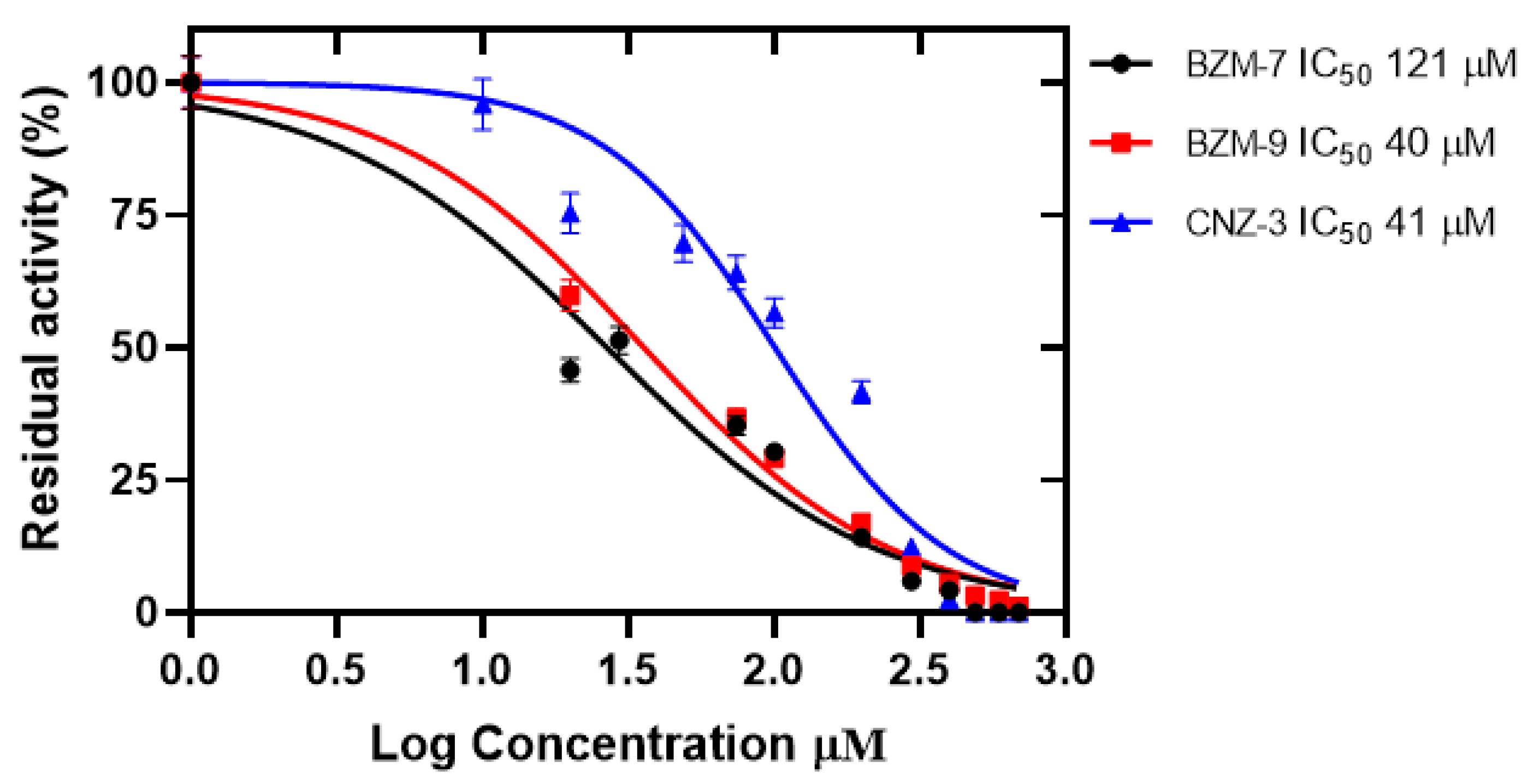
2.2. Determination of IC50 Values Under Normoxic and Hypoxic Conditions
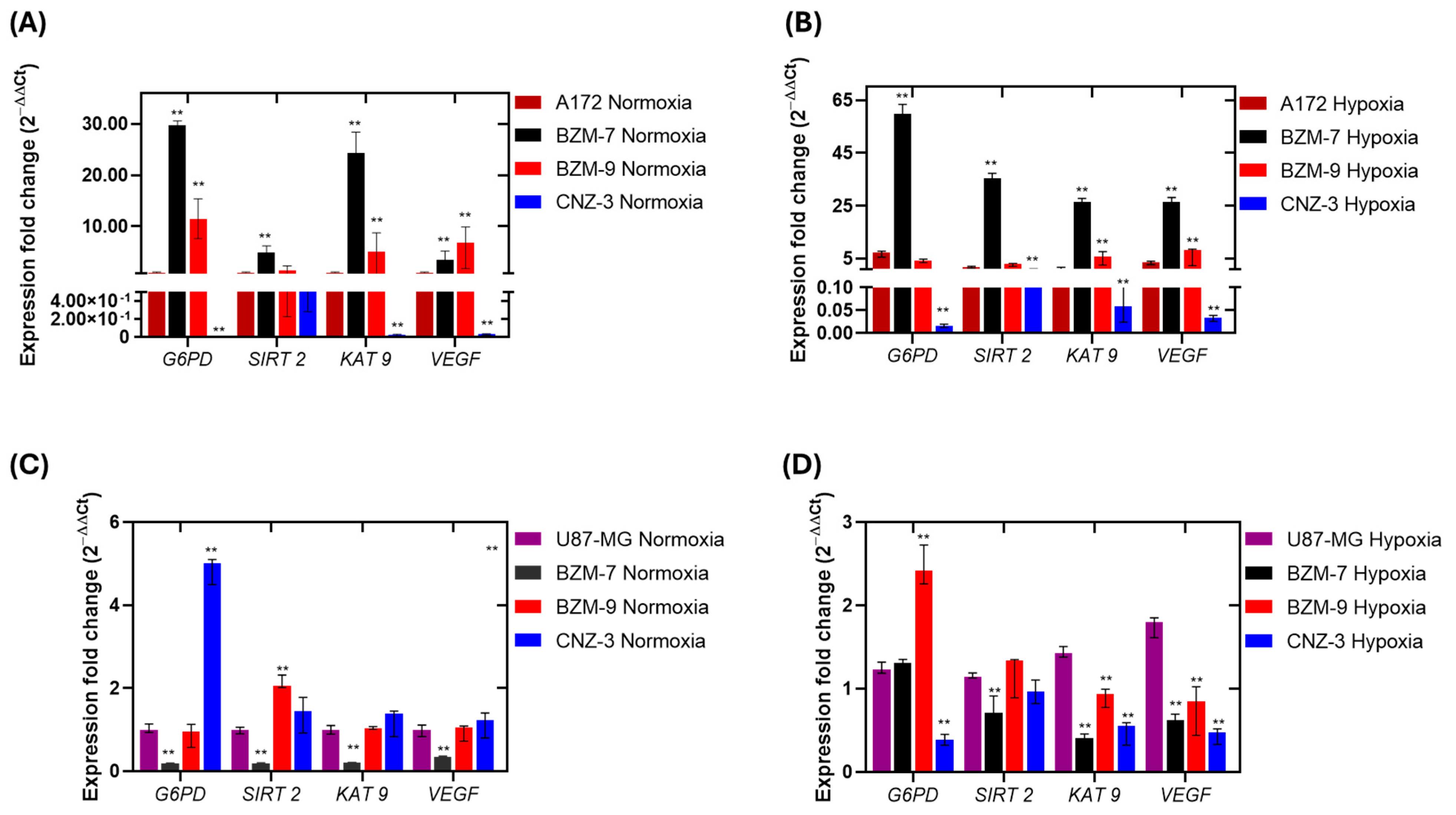
2.3. Gene Expression Analysis of SIRT2, KAT9, VEGF and G6PD in Glioblastoma Cells Under Normoxia and Hypoxia
2.4. Differential Regulation of G6PD, SIRT-2, KAT 9, and VEGF by Test Compounds Under Normoxic and Hypoxic Conditions
2.5. Effect of Compounds on the Recombinant Glucose-6-Phosphate Dehydrogenase (G6PD)
2.5.1. Determination of IC50 Values
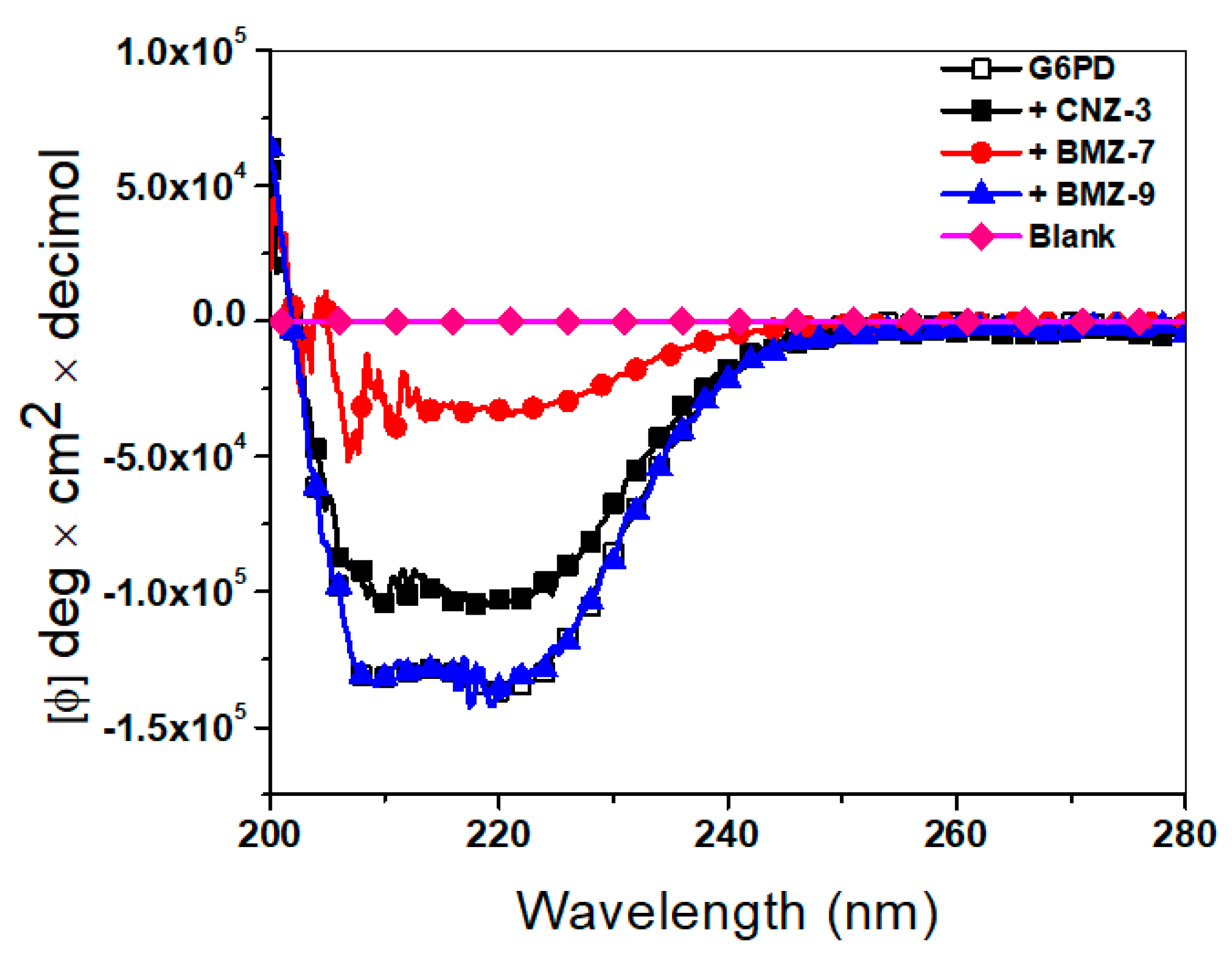
2.5.2. Structural Alterations by Circular Dichroism
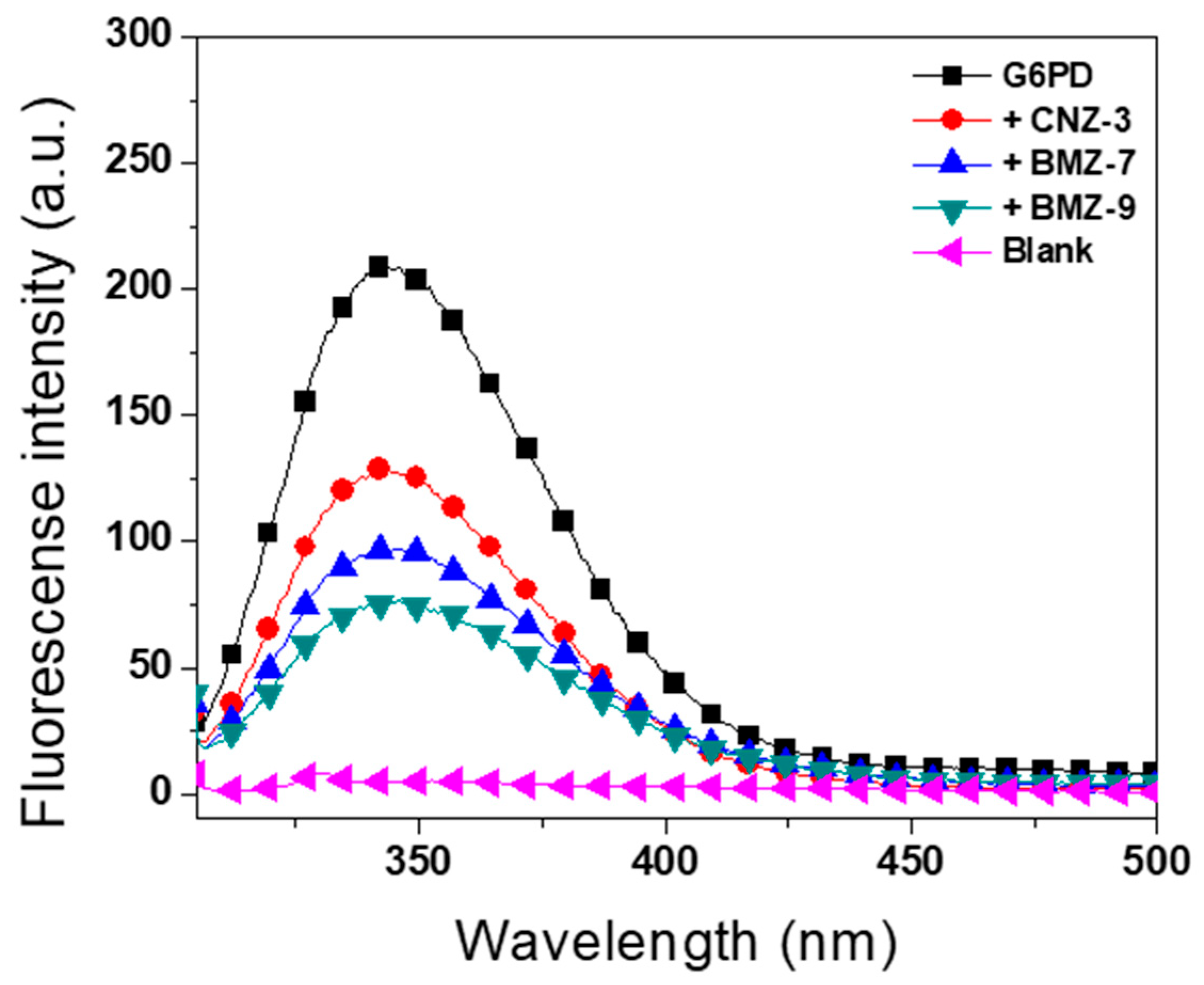
2.5.3. Intrinsic Fluorescence Assays
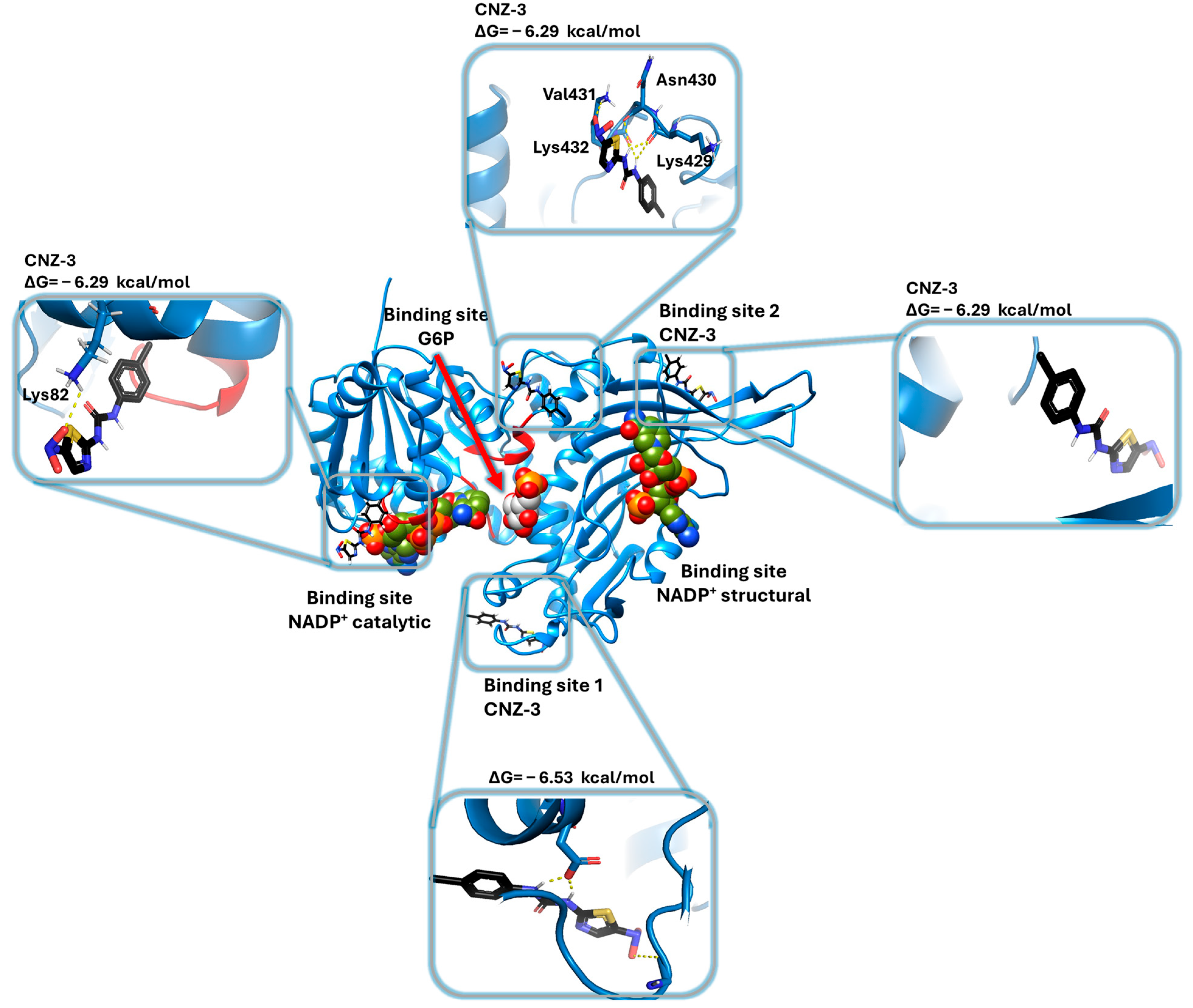
2.5.4. Molecular Docking of G6PD and Binding Site Prediction of BZM-7, BZM-9 and CNZ-3 Compounds on G6PD
3. Materials and Methods
3.1. Establishment of Monolayer Cell Cultures
3.2. Screening of Chemical Compounds (On Cell Viability)
3.3. Determination of IC50 of Selected Compounds
3.4. Evaluation of the Effect of Compounds on the Expression Levels of Glioblastoma Cell Lines Genes Using Quantitative RT-qPCR
3.5. Heterologous Expression of Human Glucose-6-Phosphate Dehydrogenase (G6PD)
3.6. Purification of Recombinant Human Glucose-6-Phosphate Dehydrogenase (G6PD)
3.7. Selection of Glucose-6-Phosphate Dehydrogenase (G6PD) Inhibitors
3.8. Concentration–Response Inactivation Assay and Determination of IC50 Values
3.9. Analysis of Secondary Structure by Circular Dichroism
3.10. Structural Analysis by Intrinsic Fluorescence
3.11. Molecular Docking Studies
3.11.1. Structure of the G6PD Protein and Ligands
3.11.2. Blind Molecular Docking Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPP | Pentose Phosphate Pathway |
| G6PD | Glucose-6-Phosphate Dehydrogenase |
| GBM | Glioblastoma |
| TMZ | Temozolomide |
| HIF-1α | Hypoxia-Inducible Factor 1-Alpha |
| VEGF | Vascular Endothelial Growth Factor |
| GSCs | Glioma Stem-Like Cells |
| PTMs | Post-Translational Modifications |
| KAT9 | Lysine Acetyltransferase |
| 6AN | 6-aminonicotinamide |
| ASA | Acetylsalicylic Acid |
| IC50 | Half-Maximal Inhibitory Concentration |
| CD | Circular Dichroism |
| G6P | Glucose-6-Phosphate |
| ATCC | American Type Culture Collection |
| EMEM | Eagle’s Minimum Essential Medium |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal Bovine Serum |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| cDNA | Complementary DNA |
| SIRT2 | Sirtuin 2 |
| SD | Standard Deviation |
| IPTG | isopropyl-β-D-1-thiogalactopyranoside |
References
- Abbruzzese, C.; Persico, M.; Matteoni, S.; Paggi, M.G. Molecular Biology in Glioblastoma Multiforme Treatment. Cells 2022, 11, 1850. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barnholtz-Sloan, J.S.; Ostrom, Q.T.; Cote, D. Epidemiology of Brain Tumors. Neurol. Clin. 2018, 36, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Eckenstein, M.; Thomas, A.A. Benign and malignant tumors of the central nervous system and pregnancy. Handb. Clin. Neurol. 2020, 172, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316, Erratum in: JAMA 2018, 319, 1824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Biserova, K.; Jakovlevs, A.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells 2021, 10, 621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barthel, L.; Hadamitzky, M.; Dammann, P.; Schedlowski, M.; Sure, U.; Thakur, B.K.; Hetze, S. Glioma: Molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 2022, 41, 53–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carrano, A.; Juarez, J.J.; Incontri, D.; Ibarra, A.; Cazares, H.G. Sex-Specific Differences in Glioblastoma. Cells 2021, 10, 1783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Lakomy, R.; Kazda, T.; Selingerova, I.; Poprach, A.; Pospisil, P.; Belanova, R.; Fadrus, P.; Vybihal, V.; Smrcka, M.; Jancalek, R.; et al. Real-World Evidence in Glioblastoma: Stupp’s Regimen After a Decade. Front. Oncol. 2020, 10, 840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 22, 351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaupel, P.; Thews, O.; Hoeckel, M. Treatment resistance of solid tumors: Role of hypoxia and anemia. Med. Oncol. 2001, 18, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Bratasz, A.; Kuppusamy, M.L.; Tazi, M.F.; Rivera, B.K.; Kuppusamy, P. Hypoxia induces chemoresistance in ovarian cancer cells by activation of signal transducer and activator of transcription 3. Int. J. Cancer 2009, 125, 2198–2204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelicano, H.; Martin, D.S.; Xu, R.H.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Pouysségur, J.; Dayan, F.; Mazure, N.M. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 2006, 441, 437–443. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of cancer cell metabolism by hypoxiainducible factor 1. Semin. Cancer Biol. 2009, 19, 12–16. [Google Scholar] [CrossRef]
- Acuña-Pilarte, K.; Koh, M.Y. The HIF axes in cancer: Angiogenesis, metabolism, and immune-modulation. Trends Biochem. Sci. 2025, 50, 677–694. [Google Scholar] [CrossRef]
- Kathagen, A.; Schulte, A.; Balcke, G.; Phillips, H.S.; Martens, T.; Matschke, J.; Günther, H.S.; Soriano, R.; Modrusan, Z.; Sandmann, T.; et al. Hypoxia and oxygenation induce a metabolic switch between pentose phosphate pathway and glycolysis in glioma stem-like cells. Acta Neuropathol. 2013, 126, 763–780. [Google Scholar] [CrossRef]
- Luzzatto, L.; Arese, P. Favism and Glucose-6-Phosphate Dehydrogenase Deficiency. N. Engl. J. Med. 2018, 378, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Suo, C.; Li, S.T.; Zhang, H.; Gao, P. Metabolic reprogramming for cancer cells and their microenvironment: Beyond the Warburg Effect. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, W.; Yang, Y.; Gu, C. Exploring the role of glucose 6 phosphate dehydrogenase in cancer (Review). Oncol. Rep. 2020, 44, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Y.; Wang, L.; Ji, S. ALKBH5 Promotes the Proliferation of Glioma Cells via Enhancing the mRNA Stability of G6PD. Neurochem. Res. 2021, 46, 3003–3011. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, M.; Pizzini, A.; Gatto, V.; Leonardi, L.; Gallo, M.; Brignardello, E.; Boccuzzi, G. Role of glucose-6-phosphate dehydrogenase inhibition in the antiproliferative effects of dehydroepiandrosterone on human breast cancer cells. Br. J. Cancer 1997, 75, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Zhang, Q.; Zhao, C.; Shi, L.; Wang, Y.; Wang, J.; Zhang, M. Overexpression of G6PD is associated with high risks of recurrent metastasis and poor progression-free survival in primary breast carcinoma. World J. Surg. Oncol. 2015, 13, 1–7. [Google Scholar] [CrossRef]
- Baba, M.; Yamamoto, R.; Iishi, H.; Tatsuta, M.; Wada, A. Role of glucose-6-phosphate dehydrogenase on enhanced proliferation of pre-neoplastic and neoplastic cells in rat liver induced by N-nitrosomorpholine. Int. J. Cancer 1989, 43, 892–895. [Google Scholar] [CrossRef]
- Ramão, A.; Gimenez, M.; Laure, H.J.; Izumi, C.; Vida, R.C.; Oba-Shinjo, S.; Marie, S.K.; Rosa, J.C. Changes in the expression of proteins associated with aerobic glycolysis and cell migration are involved in tumorigenic ability of two glioma cell lines. Proteome Sci. 2012, 10, 53. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, Y.; Hao, S.; Sun, H.; Liu, B.; Zhou, H.; Wang, Y.; Xu, Z.X. Recent findings in the regulation of G6PD and its role in diseases. Front. Pharmacol. 2022, 13, 932154. [Google Scholar] [CrossRef]
- Kim, S.C.; Sprung, R.; Chen, Y.; Xu, Y.; Ball, H.; Pei, J.; Cheng, T.; Kho, Y.; Xiao, H.; Xiao, L.; et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 2006, 23, 607–618. [Google Scholar] [CrossRef]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, W.; Jiang, W.; Yu, W.; Lin, Y.; Zhang, T.; Yao, J.; Zhou, L.; Zeng, Y.; Li, H.; et al. Regulation of cellular metabolism by protein lysine acetylation. Science 2010, 327, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat. Rev. Genet. 2009, 10, 32–42. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhou, L.S.; Zhao, Y.Z.; Wang, S.W.; Chen, L.L.; Liu, L.X.; Ling, Z.Q.; Hu, F.J.; Sun, Y.P.; Zhang, J.Y.; et al. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J. 2014, 33, 1304–1320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, S.N.; Wang, T.S.; Li, X.; Wang, Y.P. SIRT2 activates G6PD to enhance NADPH production and promote leukaemia cell proliferation. Sci. Rep. 2016, 6, 32734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arbe, M.F.; Agnetti, L.; Breininger, E.; Glikin, G.C.; Finocchiaro, L.M.E.; Villaverde, M.S. Glucose 6-phosphate dehydrogenase inhibition sensitizes melanoma cells to metformin treatment. Transl. Oncol. 2020, 13, 100842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varshney, R.; Dwarakanath, B.; Jain, V. Radiosensitization by 6-aminonicotinamide and 2-deoxy-D-glucose in human cancer cells. Int. J. Radiat. Biol. 2005, 81, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Shiff, S.J.; Koutsos, M.I.; Qiao, L.; Rigas, B. Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: Effects on cell cycle and apoptosis. Exp. Cell Res. 1996, 222, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; DuBois, R.N. Aspirin, NSAIDS, and colon cancer prevention: Mechanisms? Gastroenterology 1998, 114, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A. An introduction to aspirin, NSAids, and COX-2 inhibitors for the primary prevention of cardiovascular events and cancer and their potential preventive role in bladder carcinogenesis: Part II. Semin. Urol. Oncol. 2001, 19, 306–316. [Google Scholar] [PubMed]
- Rao, C.V.; Reddy, B.S. NSAIDs and chemoprevention. Curr. Cancer Drug Targets 2004, 4, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, S.; Zhong, X.; Huang, F.; Huang, J.; Xu, L. Oleanolic acid combined with aspirin plays antitumor roles in colorectal cancer via the Akt/NFκB/IκBα/COX2 pathway. Cell Death Discov. 2024, 10, 504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamoya, T.; Tomono, S.; Miyamoto, S.; Fujii, G.; Wakabayashi, K.; Mutoh, M. Theoretical basis validation and oxidative stress markers for cancer prevention clinical trials of aspirin. Sci. Rep. 2023, 13, 21883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gebauer, L.; Nist, A.; Mernberger, M.; Stiewe, T.; Moll, R.; Stabla, K.; Klinge, U.; Mack, E.; Brendel, C.; Neubauer, A. Superior Overall Survival in Patients with Colorectal Cancer, Regular Aspirin Use, and Combined Wild-Type PIK3CA and KRAS-Mutated Tumors. Cancers 2021, 13, 4959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sawaoka, H.; Tsuji, S.; Tsujii, M.; Gunawan, E.S.; Sasaki, Y.; Kawano, S.; Hori, M. Cyclooxygenase inhibitors suppress angiogenesis and reduce tumor growth in vivo. Lab. Investig. 1999, 79, 1469–1477. [Google Scholar]
- Abdelrahim, M.; Safe, S. Cyclooxygenase-2 inhibitors decrease vascular endothelial growth factor expression in colon cancer cells by enhanced degradation of Sp1 and Sp4 proteins. Mol. Pharmacol. 2005, 68, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Monga, J.; Ghosh, N.S.; Rani, I.; Singh, R.; Deswal, G.; Dhingra, A.K.; Grewal, A.S. Unlocking the Pharmacological Potential of Benzimidazole Derivatives: A Pathway to Drug Development. Curr. Top. Med. Chem. 2024, 24, 437–485. [Google Scholar] [CrossRef] [PubMed]
- Graff, B.T.; Palanivel, C.; Jenkins, C.B.; Baranowska-Kortylewicz, J.; Yan, Y. Benzimidazole carbamate induces cytotoxicity in breast cancer cells via two distinct cell death mechanisms. Cell Death Discov. 2023, 9, 162. [Google Scholar] [CrossRef]
- Musah-Eroje, A.; Watson, S. A novel 3D in vitro model of glioblastoma reveals resistance to temozolomide which was potentiated by hypoxia. J. Neurooncol. 2019, 142, 231–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro Oncol. 2023, 25 (Suppl. 2), iv1–iv99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, L.; Lin, C.; Wang, L.; Guo, H.; Wang, X. Hypoxia and hypoxia-inducible factors in glioblastoma multiforme progression and therapeutic implications. Exp. Cell Res. 2012, 318, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, P.; Wan, W.-W.; Xiong, S.-L.; Feng, H.; Wu, N. Cancer stem-like cells can be induced through dedifferentiation under hypoxic conditions in glioma, hepatoma and lung cancer. Cell Death Discov. 2017, 3, 16105. [Google Scholar] [CrossRef]
- Greenwald, A.C.; Darnell, N.G.; Hoefflin, R.; Simkin, D.; Mount, C.W.; Castro, L.N.G.; Harnik, Y.; Dumont, S.; Hirsch, D.; Nomura, M.; et al. Integrative spatial analysis reveals a multi-layered organization of glioblastoma. Cell 2024, 187, 2485–2501.e26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, A.; Crozet, M.D.; Rathelot, P.; Azas, N.; Vanelle, P. Synthesis and promising in vitro antiproliferative activity of sulfones of a 5-nitrothiazole series. Molecules 2012, 18, 97–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Altıntop, M.D.; Ciftci, H.I.; Radwan, M.O.; Sever, B.; Kaplancıklı, Z.A.; Ali, T.F.S.; Koga, R.; Fujita, M.; Otsuka, M.; Özdemir, A. Design, Synthesis, and Biological Evaluation of Novel 1,3,4-Thiadiazole Derivatives as Potential Antitumor Agents against Chronic Myelogenous Leukemia: Striking Effect of Nitrothiazole Moiety. Molecules 2017, 23, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wińska, P.; Wielechowska, M.; Milewski, Ł.; Siedlecki, P.; Łukowska-Chojnacka, E. Pro-Apoptotic Activity of 1-(4,5,6,7-Tetrabromo-1H-benzimidazol-1-yl)propan-2-one, an Intracellular Inhibitor of PIM-1 Kinase in Acute Lymphoblastic Leukemia and Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 5897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romero-Castro, A.; León-Rivera, I.; Avila-Rojas, L.C.; Navarrete-Vázquez, G.; Nieto-Rodríguez, A. Synthesis and preliminary evaluation of selected 2-aryl-5(6)-nitro-1H-benzimidazole derivatives as potential anticancer agents. Arch. Pharm. Res. 2011, 34, 181–189. [Google Scholar] [CrossRef]
- Lei, X.; Wang, Y.; Chen, Y.; Duan, J.; Gao, X.; Cong, Z. Fenbendazole Exhibits Antitumor Activity Against Cervical Cancer Through Dual Targeting of Cancer Cells and Cancer Stem Cells: Evidence from In Vitro and In Vivo Models. Molecules 2025, 30, 2377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernández-Ochoa, B.; Fernández-Rosario, F.; Castillo-Rodríguez, R.A.; Marhx-Bracho, A.; Cárdenas-Rodríguez, N.; Martínez-Rosas, V.; Morales-Luna, L.; González-Valdez, A.; Calderón-Jaimes, E.; de la Cruz, V.P.; et al. Validation and Selection of New Reference Genes for RT-qPCR Analysis in Pediatric Glioma of Different Grades. Genes 2021, 12, 1335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, H.C.; Wu, Y.H.; Yen, W.C.; Liu, H.Y.; Hwang, T.L.; Stern, A.; Chiu, D.T. The Redox Role of G6PD in Cell Growth, Cell Death, and Cancer. Cells 2019, 8, 1055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.H.; Lee, H.K. Current Understanding of Hypoxia in Glioblastoma Multiforme and Its Response to Immunotherapy. Cancers 2022, 14, 1176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, X.C.; Wang, J.; Shi, M.Y.; Liu, C.M.; Teng, Z.Q. Hypoxia-induced one-carbon metabolic reprogramming in glioma stem-like cells. Life Med. 2023, 2, lnad048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, A.; Yang, L.Y.; Liang, J.; Lu, D.; Zhang, J.L.; Cao, F.F.; Fu, J.Y.; Dai, W.J.; Zhang, J.F. SIRT2 modulates VEGFD-associated lymphangiogenesis by deacetylating EPAS1 in human head and neck cancer. Mol. Carcinog. 2020, 59, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Noch, E.; Bookland, M.; Khalili, K. Astrocyte-elevated gene-1 (AEG-1) induction by hypoxia and glucose deprivation in glioblastoma. Cancer Biol. Ther. 2011, 11, 32–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weathers, S.P.; de Groot, J. VEGF Manipulation in Glioblastoma. Oncology 2015, 29, 720–727. [Google Scholar] [PubMed]
- Vageli, D.P.; Doukas, P.G.; Goupou, K.; Benos, A.D.; Astara, K.; Zacharouli, K.; Sotiriou, S.; Ioannou, M. Hypoxia-inducible factor 1alpha and vascular endothelial growth factor in Glioblastoma Multiforme: A systematic review going beyond pathologic implications. Oncol. Res. 2024, 32, 1239–1256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rashed, F.B.; Diaz-Dussan, D.; Mashayekhi, F.; Macdonald, D.; Nation, P.N.; Yang, X.H.; Sokhi, S.; Stoica, A.C.; El-Saidi, H.; Ricardo, C.; et al. Cellular mechanism of action of 2-nitroimidazoles as hypoxia-selective therapeutic agents. Redox Biol. 2022, 52, 102300. [Google Scholar] [CrossRef]
- Funato, K.; Hayashi, T.; Echizen, K.; Negishi, L.; Shimizu, N.; Koyama-Nasu, R.; Nasu-Nishimura, Y.; Morishita, Y.; Tabar, V.; Todo, T.; et al. SIRT2-mediated inactivation of p73 is required for glioblastoma tumorigenicity. EMBO Rep. 2018, 19, e45587. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Outeiro, T.F.; Kontopoulos, E.; Altmann, S.M.; Kufareva, I.; Strathearn, K.E.; Amore, A.M.; Volk, C.B.; Maxwell, M.M.; Rochet, J.C.; McLean, P.J.; et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 2007, 317, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Roshdy, E.; Mustafa, M.; Shaltout, A.E.; Radwan, M.O.; Ibrahim, M.A.A.; Soliman, M.E.; Fujita, M.; Otsuka, M.; Ali, T.F.S. Selective SIRT2 inhibitors as promising anticancer therapeutics: An update from 2016 to 2020. Eur. J. Med. Chem. 2021, 224, 113709. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ye, H.; He, M.; Zhou, X.; Sun, N.; Guo, W.; Lin, X.; Huang, H.; Lin, Y.; Yao, R.; et al. LncRNA PDIA3P interacts with c-Myc to regulate cell proliferation via induction of pentose phosphate pathway in multiple myeloma. Biochem. Biophys. Res. Commun. 2018, 498, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huang, J.; Tian, Y.; Chen, Y.; Yang, Y.; Zhang, X.; Zhang, F.; Xue, L. Myc suppresses tumor invasion and cell migration by inhibiting JNK signaling. Oncogene 2017, 36, 3159–3167. [Google Scholar] [CrossRef] [PubMed]
- Farag, B.; Zaki, M.E.A.; Elsayed, D.A.; Gomha, S.M. Benzimidazole chemistry in oncology: Recent developments in synthesis, activity, and SAR analysis. RSC Adv. 2025, 15, 18593–18647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Othman, D.I.A.; Hamdi, A.; Tawfik, S.S.; Elgazar, A.A.; Mostafa, A.S. Identification of new benzimidazole-triazole hybrids as anticancer agents: Multi-target recognition, in vitro and in silico studies. J. Enzym. Inhib. Med. Chem. 2023, 38, 2166037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, W.; Zhang, W.; Peng, Y.; Jiang, Z.H.; Zhang, L.; Du, Z. Design, Synthesis and Anti-Tumor Activity of Novel Benzimidazole-Chalcone Hybrids as Non-Intercalative Topoisomerase II Catalytic Inhibitors. Molecules 2020, 25, 3180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Çevik, U.A.; Sağlık, B.N.; Osmaniye, D.; Levent, S.; Çavuşoğlu, B.K.; Karaduman, A.B.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis and docking study of benzimidazole-triazolothiadiazine hybrids as aromatase inhibitors. Arch. Pharm. 2020, 353, e2000008. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Nava, E.J.; Hernández-Ochoa, B.; Navarrete-Vázquez, G.; Arreguín-Espinosa, R.; Ortega-Cuellar, D.; González-Valdez, A.; Martínez-Rosas, V.; Morales-Luna, L.; Martínez-Miranda, J.; Sierra-Palacios, E.; et al. Novel inhibitors of human glucose-6-phosphate dehydrogenase (HsG6PD) affect the activity and stability of the protein. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129828. [Google Scholar] [CrossRef] [PubMed]
- Preuss, J.; Richardson, A.D.; Pinkerton, A.; Hedrick, M.; Sergienko, E.; Rahlfs, S.; Becker, K.; Bode, L. Identification and characterization of novel human glucose-6-phosphate dehydrogenase inhibitors. J. Biomol. Screen. 2013, 18, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Z.; Zhu, Y.; Qin, S. Glucose-6-phosphate dehydrogenase: A biomarker and potential therapeutic target for cancer. Anticancer Agents Med. Chem. 2014, 14, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.A.; Huang, H.Y.; Lin, C.L.; Chang, J.G. G6PD as a predictive marker for glioma risk, prognosis and chemosensitivity. J. Neurooncol. 2018, 139, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Q.; Lu, Y.X.; Wu, Q.N.; Liu, J.; Zeng, Z.L.; Mo, H.Y.; Chen, Y.; Tian, T.; Wang, Y.; Kang, T.B.; et al. Disrupting G6PD-mediated Redox homeostasis enhances chemosensitivity in colorectal cancer. Oncogene 2017, 36, 6282–6292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, T.; Li, B.; Shu, X.; Pang, J.; Wang, H.; Cai, X.; Liao, Y.; Xiao, X.; Chong, Y.; Gong, J.; et al. Pan-cancer analysis reveals that G6PD is a prognostic biomarker and therapeutic target for a variety of cancers. Front. Oncol. 2023, 13, 1183474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kotaka, M.; Gover, S.; Vandeputte-Rutten, L.; Au, S.W.; Lam, V.M.; Adams, M.J. Structural studies of glucose-6-phosphate and NADP+ binding to human glucose-6-phosphate dehydrogenase. Acta Crystallogr D Biol. Crystallogr. 2005, 61, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Vought, V.; Ciccone, T.; Davino, M.H.; Fairbairn, L.; Lin, Y.; Cosgrove, M.S.; Adams, M.J.; Levy, H.R. Delineation of the roles of amino acids involved in the catalytic functions of Leuconostoc mesenteroides glucose 6-phosphate dehydrogenase. Biochemistry 2000, 39, 15012–15021. [Google Scholar] [CrossRef]
- Gómez-Manzo, S.; Marcial-Quino, J.; Vanoye-Carlo, A.; Serrano-Posada, H.; González-Valdez, A.; Martínez-Rosas, V.; Hernández-Ochoa, B.; Sierra-Palacios, E.; Castillo-Rodríguez, R.A.; Cuevas-Cruz, M.; et al. Functional and Biochemical Characterization of Three Recombinant Human Glucose-6-Phosphate Dehydrogenase Mutants: Zacatecas, Vanua-Lava and Viangchan. Int. J. Mol. Sci. 2016, 17, 787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manco, L.; Gonçalves, P.; Macedo-Ribeiro, S.; Seabra, C.; Melo, P.; Ribeiro, M.L. Two new glucose-6-phosphate dehydrogenase mutations causing chronic hemolysis. Haematologica 2005, 90, 1135–1136. [Google Scholar] [PubMed]
- He, H.; Wang, X.; Shi, L.; Yin, W.; Yang, Z.; He, H.; Liang, Y. Synthesis, antitumor activity and mechanism of action of novel 1,3-thiazole derivatives containing hydrazide-hydrazone and carboxamide moiety. Bioorganic Med. Chem. Lett. 2016, 26, 3263–3270. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Jiang, H.; Meng, L.; Lang, B. Rosiglitazone induces apoptosis on human bladder cancer 5637 and T24 cell lines. Int. J. Clin. Exp. Pathol. 2017, 10, 10197–10204. [Google Scholar] [PubMed] [PubMed Central]
- Zhou, X.; Zou, L.; Chen, W.; Yang, T.; Luo, J.; Wu, K.; Shu, F.; Tan, X.; Yang, Y.; Cen, S.; et al. Flubendazole, FDA-approved anthelmintic, elicits valid antitumor effects by targeting P53 and promoting ferroptosis in castration-resistant prostate cancer. Pharmacol. Res. 2021, 164, 105305. [Google Scholar] [CrossRef] [PubMed]
- Koronkiewicz, M.; Kazimierczuk, Z.; Orzeszko, A. Antitumor activity of the protein kinase inhibitor 1-(β-D-2′-deoxyribofuranosyl)-4,5,6,7-tetrabromo-1H-benzimidazole in breast cancer cell lines. BMC Cancer. 2022, 22, 1069. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, S.; Wang, Y.; Yang, X.; Fang, H.; Hou, X. Discovery of a Novel Benzimidazole Derivative Targeting Histone Deacetylase to Induce Ferroptosis and Trigger Immunogenic Cell Death. J. Med. Chem. 2024, 67, 15098–15117. [Google Scholar] [CrossRef] [PubMed]
- Ai, G.; Dachineni, R.; Kumar, D.R.; Alfonso, L.F.; Marimuthu, S.; Bhat, G.J. Aspirin inhibits glucose-6-phosphate dehydrogenase activity in HCT 116 cells through acetylation: Identification of aspirin-acetylated sites. Mol. Med. Rep. 2016, 14, 1726–1732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nava-Zuazo, C.; Chávez-Silva, F.; Moo-Puc, R.; Chan-Bacab, M.J.; Ortega-Morales, B.O.; Moreno-Díaz, H.; Díaz-Coutiño, D.; Hernández-Núñez, E.; Navarrete-Vázquez, G. 2-acylamino-5-nitro-1,3-thiazoles: Preparation and in vitro bioevaluation against four neglected protozoan parasites. Bioorganic Med. Chem. 2014, 22, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Manzo, S.; Terrón-Hernández, J.; De la Mora-De la Mora, I.; González-Valdez, A.; Marcial-Quino, J.; García-Torres, I.; Vanoye-Carlo, A.; López-Velázquez, G.; Hernández-Alcántara, G.; Oria-Hernández, J.; et al. The stability of G6PD is affected by mutations with different clinical phenotypes. Int. J. Mol. Sci. 2014, 15, 21179–21201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-Rosas, V.; Hernández-Ochoa, B.; Navarrete-Vázquez, G.; Martínez-Conde, C.; Gómez-Chávez, F.; Morales-Luna, L.; González-Valdez, A.; Arreguin-Espinosa, R.; Enríquez-Flores, S.; de la Cruz, V.P.; et al. Kinetic and Molecular Docking Studies to Determine the Effect of Inhibitors on the Activity and Structure of Fused G6PD::6PGL Protein from Trichomonas vaginalis. Molecules 2022, 27, 1174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markwell, M.A.K.; Haas, S.M.; Tolbert, N.E.; Bieber, L.L. Protein Determination in Membrane and Lipoprotein Samples: Manual and Automated Procedures. Methods Enzymol. 1981, 72, 296–303. [Google Scholar]
- Hernández-Ochoa, B.; Navarrete-Vázquez, G.; Nava-Zuazo, C.; Castillo-Villanueva, A.; Méndez, S.T.; Torres-Arroyo, A.; Gómez-Manzo, S.; Marcial-Quino, J.; Ponce-Macotela, M.; Rufino-González, Y.; et al. Novel giardicidal compounds bearing proton pump inhibitor scaffold proceeding through triosephosphate isomerase inactivation. Sci. Rep. 2017, 7, 7810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Navarrete-Vázquez, G.; Chávez-Silva, F.; Colín-Lozano, B.; Estrada-Soto, S.; Hidalgo-Figueroa, S.; Guerrero-Álvarez, J.; Méndez, S.T.; Reyes-Vivas, H.; Oria-Hernández, J.; Canul-Canché, J.; et al. Synthesis of nitro(benzo)thiazole acetamides and in vitro antiprotozoal effect against amitochondriate parasites Giardia intestinalis and Trichomonas vaginalis. Bioorganic Med. Chem. 2015, 23, 2204–2210. [Google Scholar] [CrossRef]
- Medina-Franco, J.L.; Navarrete-Vázquez, G.; Méndez-Lucio, O. Activity and property landscape modeling is at the interface of chemoinformatics and medicinal chemistry. Future Med. Chem. 2015, 7, 1197–1211. [Google Scholar] [CrossRef]
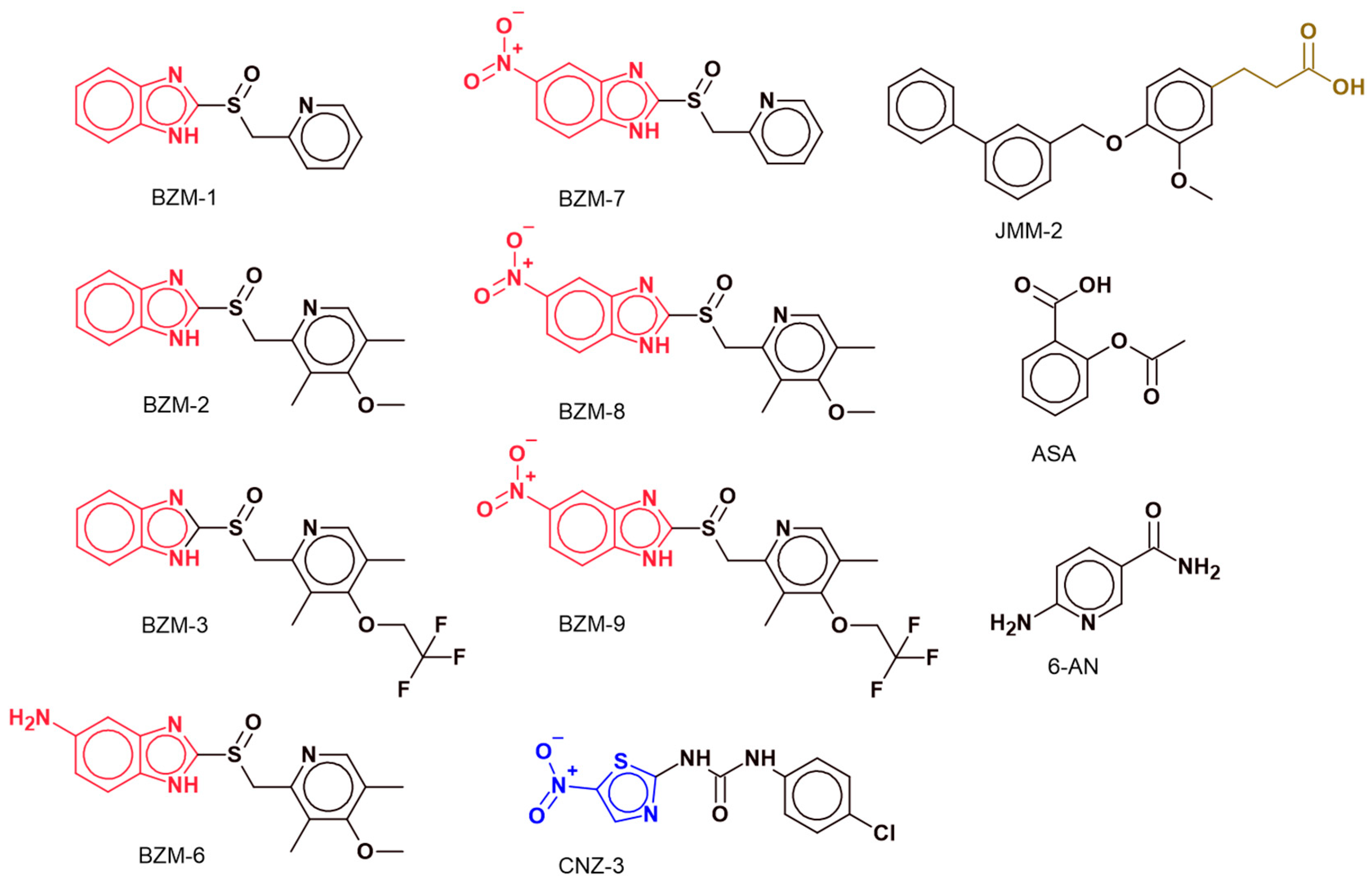
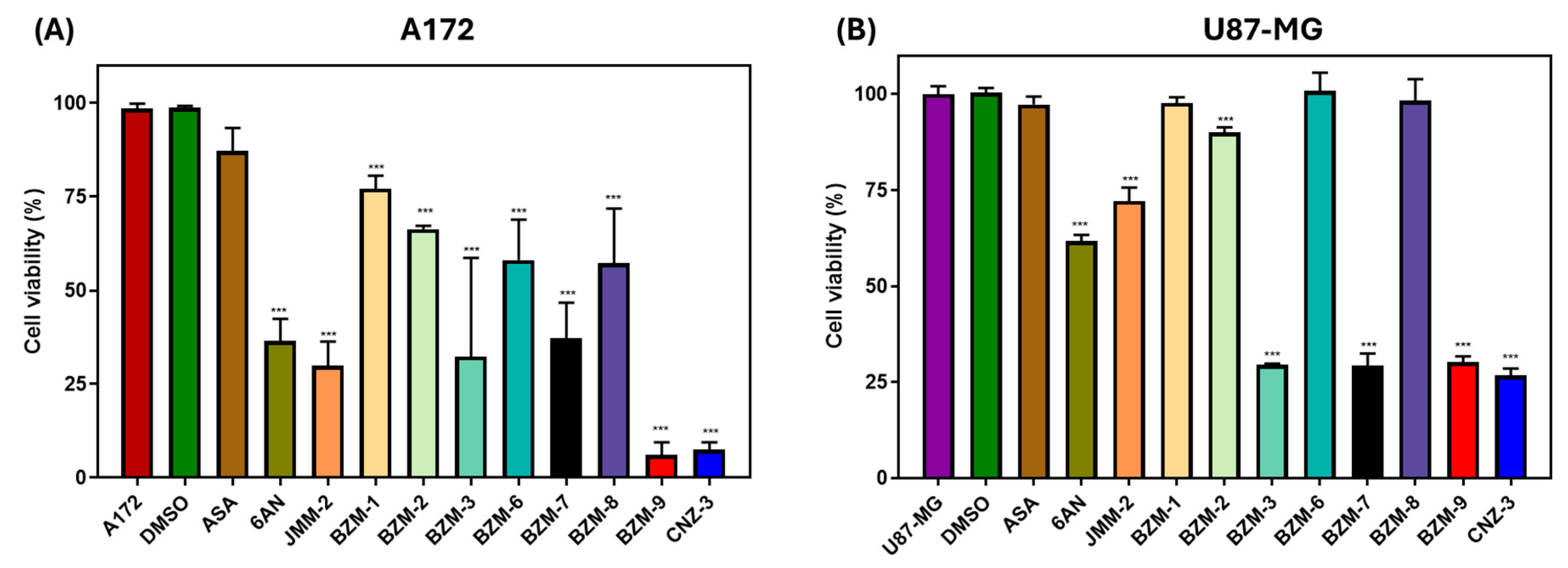
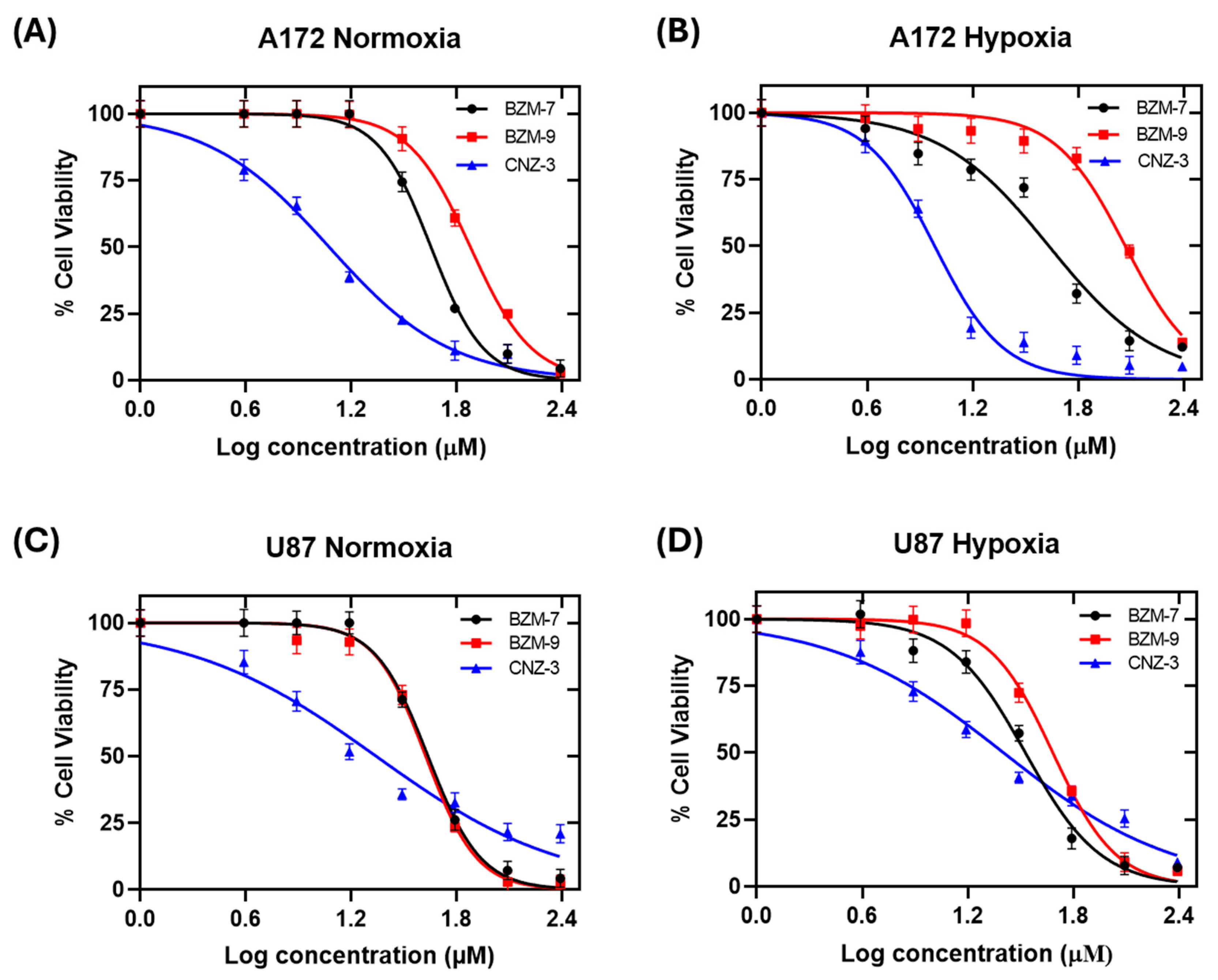
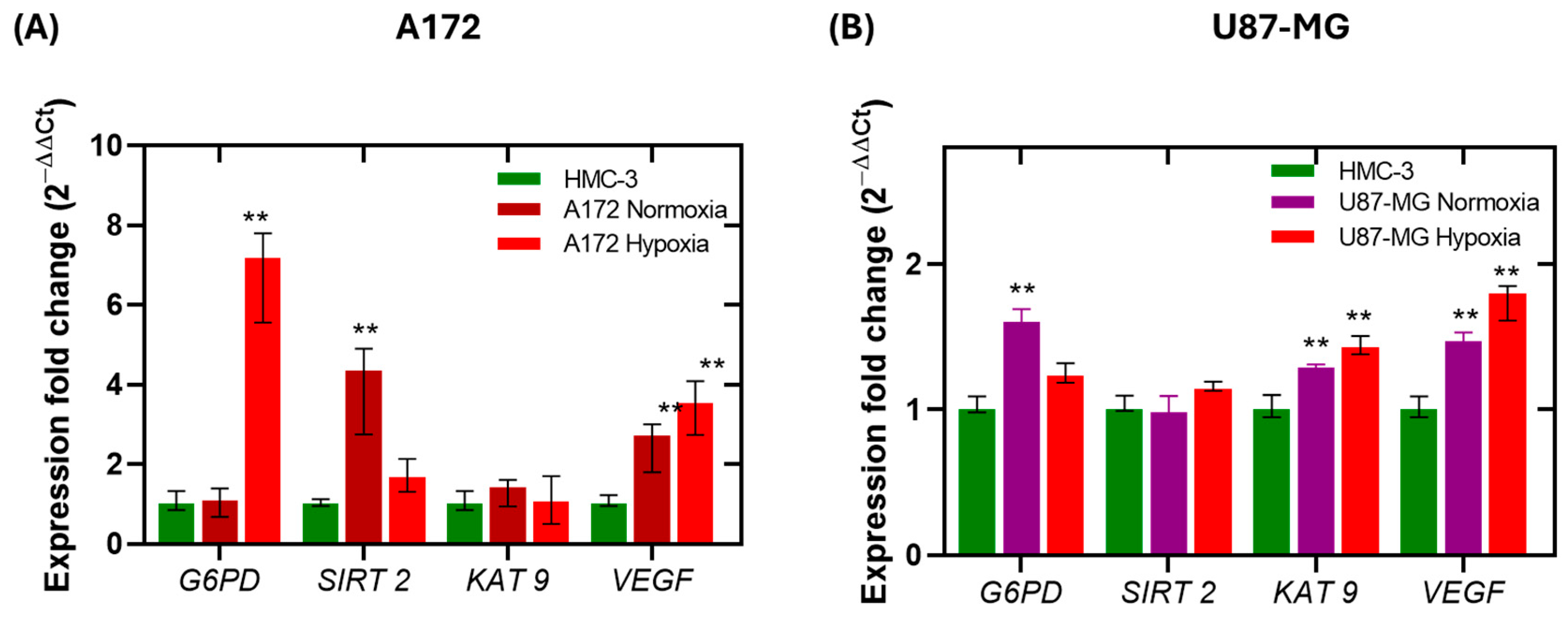

| A172 | U87-MG | |||||
|---|---|---|---|---|---|---|
| BZM-7 | BZM-9 | CNZ-3 | BZM-7 | BZM-9 | CNZ-3 | |
| Normoxia | 43 ± 1.6 µM | 35 ± 1.6 µM | 12 ± 1.0 µM | 38 ± 1.6 µM | 48 ± 1.6 µM | 12 ± 1.3 µM |
| Hypoxia | 43 ± 1.6 µM | 125 ± 2.6 µM | 9 ± 0.9 µM | 33 ± 1.5 µM | 44 ± 1.6 µM | 19 ± 1.4 µM |
| Gene | 5′-3′ Sequence | Length (bp) | Function |
|---|---|---|---|
| PKM, pyruvate kinase M1/2, variant 1 | Fw 5′-GGTTCGGAGGTTTGATGA-3′ Rv 5′-GGCTTCTTGATCATGCTCT-3′ | 186 | Glycolysis |
| G6PD, glucose-6-phosphate dehydrogenase variant 1 | Fw 5′-ATATTTATGGCAGCCGAGG-3′ Rv 5′-GTCAATGGTCCCGGTGT-3′ | 190 | Pentose phosphate pathway (PPP) |
| SIRT2, sirtuin 2 | Fw 5′-TTGGATGGAAGAAGGAGC-3′ Rv 5′-AGCTGTCACTGGGGTTTCT-3′ | 153 | Deacetylase involved in metabolism and stress response |
| ELP3, elongator acetyltransferase complex subunit 3 * | Fw 5′-TGCTAGTGGGATTGCTGT-3′ Rv 5′-TCAGAATCAGGTCCACCA-3′ | 90 | Lysine acetyltransferase, coactivator of transcription |
| VEGFA, vascular endothelial growth factor A | Fw 5′-TCTCTACCCCAGGTCAGACG-3′ Rv 5′-AGCAATGTCCTGAAGCTCCC-3′ | 98 | Angiogenesis and hypoxia response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Bautista, M.; Morales-Luna, L.; Pérez de la Cruz, V.; Castillo-Rodríguez, R.A.; Velázquez-Aragón, J.A.; Enríquez-Flores, S.; Flores-López, L.A.; Hernández-Urzúa, E.; Martínez-Rosas, V.; Wong-Baeza, C.; et al. Targeting G6PD with Benzimidazole and Thiazole Derivatives Suppresses SIRT 2 and VEGF Expression and Induces Cytotoxicity in Glioma Cells. Int. J. Mol. Sci. 2025, 26, 9092. https://doi.org/10.3390/ijms26189092
Vázquez-Bautista M, Morales-Luna L, Pérez de la Cruz V, Castillo-Rodríguez RA, Velázquez-Aragón JA, Enríquez-Flores S, Flores-López LA, Hernández-Urzúa E, Martínez-Rosas V, Wong-Baeza C, et al. Targeting G6PD with Benzimidazole and Thiazole Derivatives Suppresses SIRT 2 and VEGF Expression and Induces Cytotoxicity in Glioma Cells. International Journal of Molecular Sciences. 2025; 26(18):9092. https://doi.org/10.3390/ijms26189092
Chicago/Turabian StyleVázquez-Bautista, Montserrat, Laura Morales-Luna, Verónica Pérez de la Cruz, Rosa Angélica Castillo-Rodríguez, José Antonio Velázquez-Aragón, Sergio Enríquez-Flores, Luis Antonio Flores-López, Elizabeth Hernández-Urzúa, Víctor Martínez-Rosas, Carlos Wong-Baeza, and et al. 2025. "Targeting G6PD with Benzimidazole and Thiazole Derivatives Suppresses SIRT 2 and VEGF Expression and Induces Cytotoxicity in Glioma Cells" International Journal of Molecular Sciences 26, no. 18: 9092. https://doi.org/10.3390/ijms26189092
APA StyleVázquez-Bautista, M., Morales-Luna, L., Pérez de la Cruz, V., Castillo-Rodríguez, R. A., Velázquez-Aragón, J. A., Enríquez-Flores, S., Flores-López, L. A., Hernández-Urzúa, E., Martínez-Rosas, V., Wong-Baeza, C., Baeza-Ramírez, I., Navarrete-Vázquez, G., Pineda, B., Hernández-Ochoa, B., & Gómez-Manzo, S. (2025). Targeting G6PD with Benzimidazole and Thiazole Derivatives Suppresses SIRT 2 and VEGF Expression and Induces Cytotoxicity in Glioma Cells. International Journal of Molecular Sciences, 26(18), 9092. https://doi.org/10.3390/ijms26189092








