Optogenetic and Endogenous Modulation of Ca2+ Signaling in Schwann Cells: Implications for Autocrine and Paracrine Neurotrophic Regulation
Abstract
1. Introduction
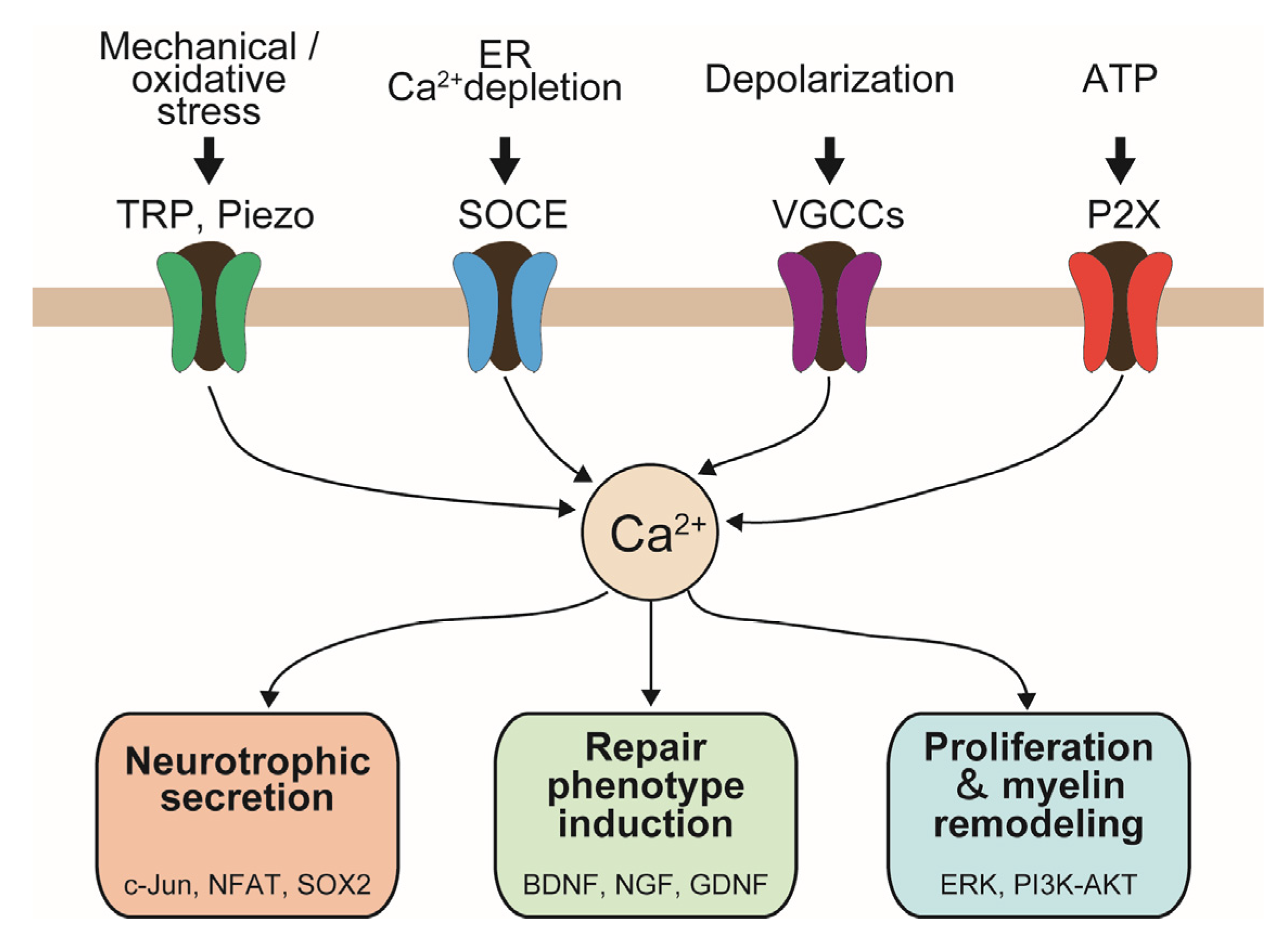
2. Endogenous Ca2+-Permeable Channels in SCs
2.1. Voltage-Gated Ca2+ Channels in SCs
2.2. TRP Channels
2.3. Piezo Channels
2.4. P2X Channels
3. Functional Implications of Ca2+ Signaling in Regenerating SCs
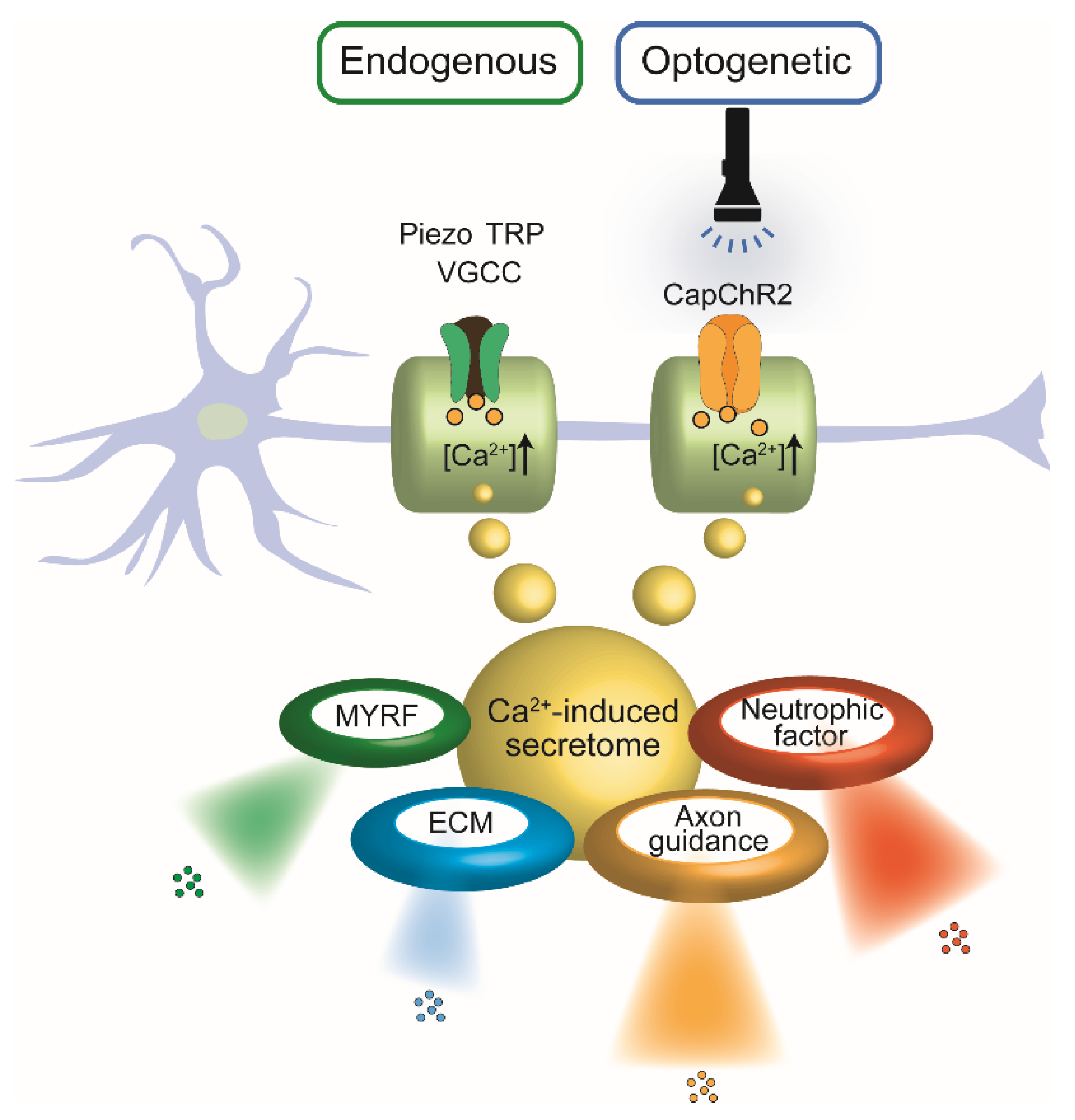
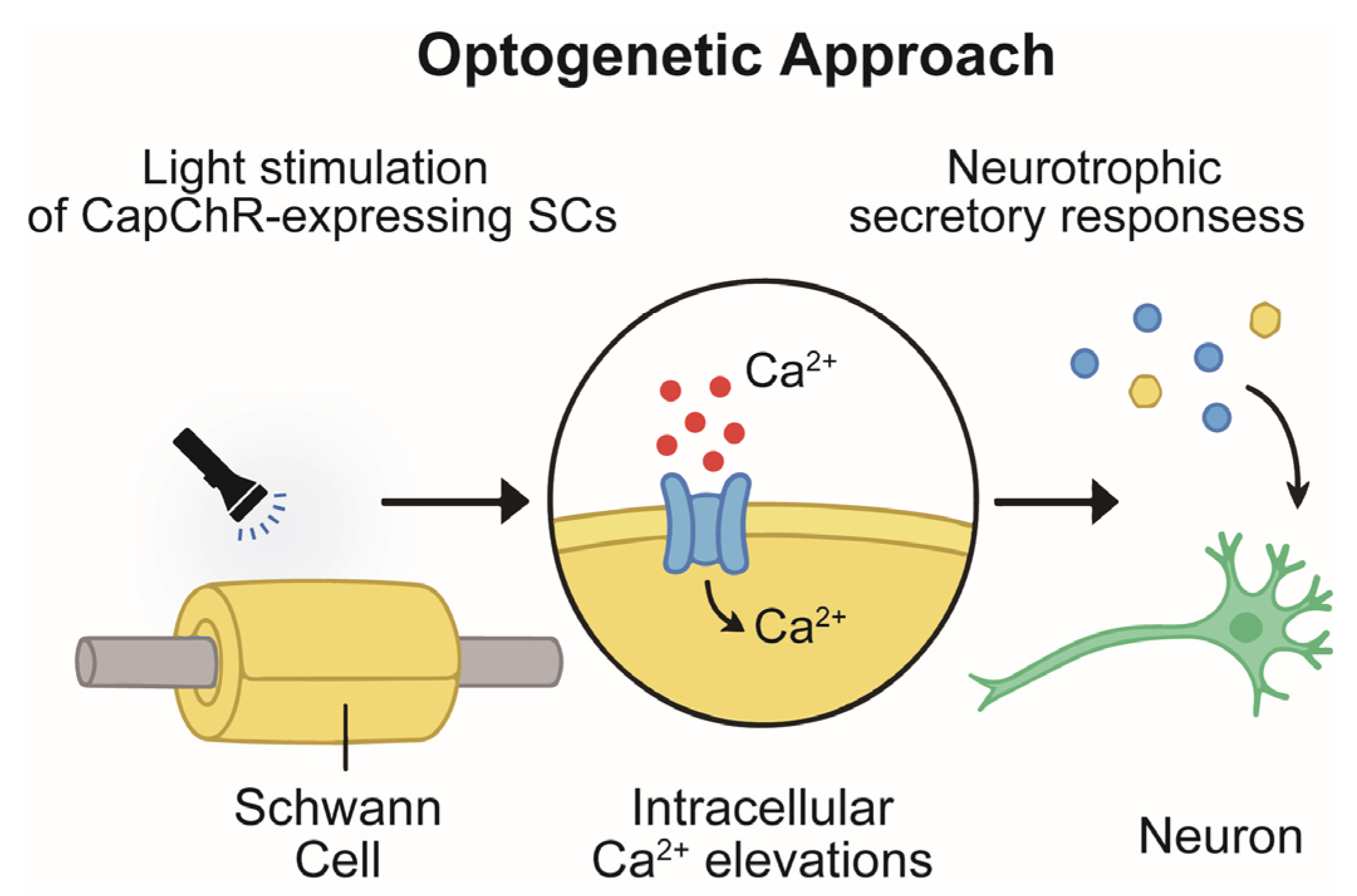
4. Optogenetic Control of Ca2+ Signaling in SCs and Neurite Outgrowth
5. Calcium-Triggered Secretory Programs in Schwann Cells: Implications for Nerve Regeneration
5.1. Axon Guidance
5.2. Neurotrophic Factors
5.3. Extracellular Matrix (ECM) Molecules
5.4. SC-Autonomous Regulation via MYRF and Associated Intracellular Signaling Cascades
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| AGRIN | Agrin |
| BDNF | Brain-derived neurotrophic factor |
| Ca2+ | Calcium ion |
| CapChR2 | Calcium-permeable Channelrhodopsin-2 |
| COL9A1 | Collagen type IX alpha 1 chain |
| CXCL12 (SDF-1) | Stromal cell-derived factor-1 |
| DVL1 | Disheveled segment polarity protein 1 |
| ECM | Extracellular matrix |
| EPHA2 | Eph receptor A2 |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| GDF7 | Growth differentiation factor 7 |
| GSK3β | Glycogen synthase kinase 3 beta |
| LIF | Leukemia inhibitory factor |
| MAPK | Mitogen-activated protein kinase |
| MACF1 | Microtubule-actin crosslinking factor 1 |
| MMP9 | Matrix metalloproteinase 9 |
| MYRF | Myelin regulatory factor |
| NFAT | Nuclear factor of activated T cells |
| NMJ | Neuromuscular junction |
| NRP2 | Neuropilin-2 |
| NTN1 | Netrin-1 |
| OPN | Osteopontin |
| PI3K | Phosphoinositide 3-kinase |
| PLXNA4 | Plexin-A4 |
| PNN | Perineuronal net |
| PTEN | Phosphatase and tensin homolog |
| ROBO1 | Roundabout guidance receptor 1 |
| SCs | Schwann cells |
| SEMA | Semaphorin |
| SOCE | Store-operated calcium entry |
| TGF-β1 | Transforming growth factor beta 1 |
| TRP | Transient receptor potential |
| VGCC | Voltage-gated calcium channel |
| VEGF-A | Vascular endothelial growth factor A |
| WNT5A | Wnt family member 5A |
References
- Zhang, S.; Huang, M.; Zhi, J.; Wu, S.; Wang, Y.; Pei, F. Research Hotspots and Trends of Peripheral Nerve Injuries Based on Web of Science From 2017 to 2021: A Bibliometric Analysis. Front. Neurol. 2022, 13, 872261. [Google Scholar] [CrossRef]
- Magnéli, M.; Axenhus, M. Epidemiology and regional variance of traumatic peripheral nerve injuries in Sweden: A 15-year observational study. PLoS ONE 2024, 19, e0310988. [Google Scholar] [CrossRef]
- Bergmeister, K.D.; Große-Hartlage, L.; Daeschler, S.C.; Rhodius, P.; Böcker, A.; Beyersdorff, M.; Kern, A.O.; Kneser, U.; Harhaus, L. Acute and long-term costs of 268 peripheral nerve injuries in the upper extremity. PLoS ONE 2020, 15, e0229530. [Google Scholar] [CrossRef]
- Gomez-Sanchez, J.A.; Pilch, K.S.; van der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R.; Lloyd, A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015, 7, a020487. [Google Scholar] [CrossRef] [PubMed]
- Fex Svennigsen, A.; Dahlin, L.B. Repair of the Peripheral Nerve-Remyelination that Works. Brain Sci. 2013, 3, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Radtke, C.; Vogt, P.M. Peripheral nerve regeneration: A current perspective. Eplasty 2009, 9, e47. [Google Scholar] [PubMed]
- Ino, D.; Sagara, H.; Suzuki, J.; Kanemaru, K.; Okubo, Y.; Iino, M. Neuronal Regulation of Schwann Cell Mitochondrial Ca2+ Signaling during Myelination. Cell Rep. 2015, 12, 1951–1959. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Role of c-Jun and Autocrine Signaling Loops in the Control of Repair Schwann Cells and Regeneration. Front. Cell. Neurosci. 2021, 15, 820216. [Google Scholar] [CrossRef]
- Heredia, D.J.; Feng, C.Y.; Hennig, G.W.; Renden, R.B.; Gould, T.W. Activity-induced Ca(2+) signaling in perisynaptic Schwann cells of the early postnatal mouse is mediated by P2Y(1) receptors and regulates muscle fatigue. eLife 2018, 7, e30839. [Google Scholar] [CrossRef]
- De Logu, F.; Nassini, R.; Materazzi, S.; Carvalho Gonçalves, M.; Nosi, D.; Rossi Degl’Innocenti, D.; Marone, I.M.; Ferreira, J.; Li Puma, S.; Benemei, S.; et al. Schwann cell TRPA1 mediates neuroinflammation that sustains macrophage-dependent neuropathic pain in mice. Nat. Commun. 2017, 8, 1887. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Takayama, Y.; Ohno, N.; Kanda, H.; Dai, Y.; Sokabe, T.; Tominaga, M. Increased TRPV4 expression in non-myelinating Schwann cells is associated with demyelination after sciatic nerve injury. Commun. Biol. 2020, 3, 716. [Google Scholar] [CrossRef] [PubMed]
- Numata, T.; Shimizu, T.; Okada, Y. TRPM7 is a stretch- and swelling-activated cation channel involved in volume regulation in human epithelial cells. Am. J. Physiol. Cell Physiol. 2007, 292, C460–C467. [Google Scholar] [CrossRef]
- Numata, T.; Shimizu, T.; Okada, Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell. Physiol. Biochem. 2007, 19, 1–8. [Google Scholar] [CrossRef]
- Fila, M.; Przyslo, L.; Derwich, M.; Sobczuk, P.; Pawlowska, E.; Blasiak, J. The TRPA1 Ion Channel Mediates Oxidative Stress-Related Migraine Pathogenesis. Molecules 2024, 29, 3385. [Google Scholar] [CrossRef] [PubMed]
- Darby, W.G.; Grace, M.S.; Baratchi, S.; McIntyre, P. Modulation of TRPV4 by diverse mechanisms. Int. J. Biochem. Cell Biol. 2016, 78, 217–228. [Google Scholar] [CrossRef]
- Suttinont, C.; Maeno, K.; Yano, M.; Sato-Numata, K.; Numata, T.; Tsutsumi, M. Role of Piezo2 in Schwann Cell Volume Regulation and Its Impact on Neurotrophic Release Regulation. Cell. Physiol. Biochem. 2024, 58, 292–310. [Google Scholar] [CrossRef]
- Acheta, J.; Bhatia, U.; Haley, J.; Hong, J.; Rich, K.; Close, R.; Bechler, M.E.; Belin, S.; Poitelon, Y. Piezo channels contribute to the regulation of myelination in Schwann cells. Glia 2022, 70, 2276–2289. [Google Scholar] [CrossRef]
- Vanoye, C.G.; Sakakura, M.; Follis, R.M.; Trevisan, A.J.; Narayan, M.; Li, J.; Sanders, C.R.; Carter, B.D. Peripheral myelin protein 22 modulates store-operated calcium channel activity, providing insights into Charcot-Marie-Tooth disease etiology. J. Biol. Chem. 2019, 294, 12054–12065. [Google Scholar] [CrossRef]
- Amédée, T.; Ellie, E.; Dupouy, B.; Vincent, J.D. Voltage-dependent calcium and potassium channels in Schwann cells cultured from dorsal root ganglia of the mouse. J. Physiol. 1991, 441, 35–56. [Google Scholar] [CrossRef]
- Kramer, M.M.; Lataster, L.; Weber, W.; Radziwill, G. Optogenetic Approaches for the Spatiotemporal Control of Signal Transduction Pathways. Int. J. Mol. Sci. 2021, 22, 5300. [Google Scholar] [CrossRef]
- Kwon, E.; Heo, W.D. Optogenetic tools for dissecting complex intracellular signaling pathways. Biochem. Biophys. Res. Commun. 2020, 527, 331–336. [Google Scholar] [CrossRef]
- Zhang, K.; Cui, B. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. 2015, 33, 92–100. [Google Scholar] [CrossRef]
- Heck, J.; Palmeira Do Amaral, A.C.; Weißbach, S.; El Khallouqi, A.; Bikbaev, A.; Heine, M. More than a pore: How voltage-gated calcium channels act on different levels of neuronal communication regulation. Channels 2021, 15, 322–338. [Google Scholar] [CrossRef]
- Campiglio, M.; Flucher, B.E. The role of auxiliary subunits for the functional diversity of voltage-gated calcium channels. J. Cell. Physiol. 2015, 230, 2019–2031. [Google Scholar] [CrossRef]
- Beaudu-Lange, C.; Despeyroux, S.; Marcaggi, P.; Coles, J.A.; Amédée, T. Functional Ca2+ and Na+ channels on mouse Schwann cells cultured in serum-free medium: Regulation by a diffusible factor from neurons and by cAMP. Eur. J. Neurosci. 1998, 10, 1796–1809. [Google Scholar] [CrossRef]
- Huang, J.; Ye, Z.; Hu, X.; Lu, L.; Luo, Z. Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells. Glia 2010, 58, 622–631. [Google Scholar] [CrossRef]
- Inoue, I.; Tsutsui, I.; Abbott, N.J.; Brown, E.R. Ionic currents in isolated and in situ squid Schwann cells. J. Physiol. 2002, 541 Pt 3, 769–778. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef]
- Numata, T.; Kiyonaka, S.; Kato, K.; Takahashi, N.; Mori, Y. Activation of TRP Channels in Mammalian Systems. In TRP Channels; Zhu, M.X., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Okada, Y.; Numata, T.; Sabirov, R.Z.; Kashio, M.; Merzlyak, P.G.; Sato-Numata, K. Cell death induction and protection by activation of ubiquitously expressed anion/cation channels. Part 3: The roles and properties of TRPM2 and TRPM7. Front. Cell Dev. Biol. 2023, 11, 1246955. [Google Scholar] [CrossRef]
- Chun, Y.L.; Kim, M.; Kim, Y.H.; Kim, N.; Yang, H.; Park, C.; Huh, Y.; Jung, J. Carvacrol effectively protects demyelination by suppressing transient receptor potential melastatin 7 (TRPM7) in Schwann cells. Anat. Sci. Int. 2020, 95, 230–239. [Google Scholar] [CrossRef]
- Potier, M.; Trebak, M. New developments in the signaling mechanisms of the store-operated calcium entry pathway. Pflügers Arch. 2008, 457, 405–415. [Google Scholar] [CrossRef]
- Landouré, G.; Zdebik, A.A.; Martinez, T.L.; Burnett, B.G.; Stanescu, H.C.; Inada, H.; Shi, Y.; Taye, A.A.; Kong, L.; Munns, C.H.; et al. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat. Genet. 2010, 42, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Martinac, B.; Poole, K. Mechanically activated ion channels. Int. J. Biochem. Cell Biol. 2018, 97, 104–107. [Google Scholar] [CrossRef]
- Fang, X.Z.; Zhou, T.; Xu, J.Q.; Wang, Y.X.; Sun, M.M.; He, Y.J.; Pan, S.W.; Xiong, W.; Peng, Z.K.; Gao, X.H.; et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci. 2021, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Shan, Y.; Guo, X.; Zhang, M.; Chen, M.; Li, Y.; Zhang, M.; Pei, D. Structure of human PIEZO1 and its slow inactivating channelopathy mutants. eLife 2025, 13, RP101923. [Google Scholar] [CrossRef]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef]
- Ranade, S.S.; Qiu, Z.; Woo, S.H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B.; et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10347–10352. [Google Scholar] [CrossRef]
- Pardo-Pastor, C.; Rubio-Moscardo, F.; Vogel-González, M.; Serra, S.A.; Afthinos, A.; Mrkonjic, S.; Destaing, O.; Abenza, J.F.; Fernández-Fernández, J.M.; Trepat, X.; et al. Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc. Natl. Acad. Sci. USA 2018, 115, 1925–1930. [Google Scholar] [CrossRef]
- Katsuta, E.; Takabe, K.; Vujcic, M.; Gottlieb, P.A.; Dai, T.; Mercado-Perez, A.; Beyder, A.; Wang, Q.; Opyrchal, M. Mechano-Sensing Channel PIEZO2 Enhances Invasive Phenotype in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2022, 23, 9909. [Google Scholar] [CrossRef]
- Numata, T.; Sato-Numata, K.; Hermosura, M.C.; Mori, Y.; Okada, Y. TRPM7 is an essential regulator for volume-sensitive outwardly rectifying anion channel. Commun. Biol. 2021, 4, 599. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Schmid, R.; Evans, R.J. ATP-Gated P2X Receptor Channels: Molecular Insights into Functional Roles. Annu. Rev. Physiol. 2019, 81, 43–62. [Google Scholar] [CrossRef]
- Hattori, M.; Gouaux, E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 2012, 485, 207–212. [Google Scholar] [CrossRef]
- Faroni, A.; Smith, R.J.P.; Procacci, P.; Castelnovo, L.F.; Puccianti, E.; Reid, A.J.; Magnaghi, V.; Verkhratsky, A. Purinergic signaling mediated by P2X7 receptors controls myelination in sciatic nerves. J. Neurosci. Res. 2014, 92, 1259–1269. [Google Scholar] [CrossRef]
- Song, X.M.; Xu, X.H.; Zhu, J.; Guo, Z.; Li, J.; He, C.; Burnstock, G.; Yuan, H.; Xiang, Z. Up-regulation of P2X7 receptors mediating proliferation of Schwann cells after sciatic nerve injury. Purinergic Signal. 2015, 11, 203–213. [Google Scholar] [CrossRef]
- Su, W.; He, X.; Lin, Z.; Xu, J.; Shangguan, J.; Wei, Z.; Zhao, Y.; Xing, L.; Gu, Y.; Chen, G. Activation of P2X7R Inhibits Proliferation and Promotes the Migration and Differentiation of Schwann Cells. Mol. Neurobiol. 2025, 62, 3067–3081. [Google Scholar] [CrossRef]
- Su, W.F.; Wu, F.; Jin, Z.H.; Gu, Y.; Chen, Y.T.; Fei, Y.; Chen, H.; Wang, Y.X.; Xing, L.Y.; Zhao, Y.Y.; et al. Overexpression of P2X4 receptor in Schwann cells promotes motor and sensory functional recovery and remyelination via BDNF secretion after nerve injury. Glia 2019, 67, 78–90. [Google Scholar] [CrossRef]
- Sohn, E.J.; Nam, Y.K.; Park, H.T. Involvement of the miR-363-5p/P2RX4 Axis in Regulating Schwann Cell Phenotype after Nerve Injury. Int. J. Mol. Sci. 2021, 22, 11601. [Google Scholar] [CrossRef]
- Min, Q.; Parkinson, D.B.; Dun, X.P. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 2021, 69, 235–254. [Google Scholar] [CrossRef]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Napoli, I.; Noon, L.A.; Ribeiro, S.; Kerai, A.P.; Parrinello, S.; Rosenberg, L.H.; Collins, M.J.; Harrisingh, M.C.; White, I.J.; Woodhoo, A.; et al. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron 2012, 73, 729–742. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Y.; Wang, Y. Sam68 promotes Schwann cell proliferation by enhancing the PI3K/Akt pathway and acts on regeneration after sciatic nerve crush. Biochem. Biophys. Res. Commun. 2016, 473, 1045–1051. [Google Scholar] [CrossRef]
- Balice-Gordon, R.J.; Bone, L.J.; Scherer, S.S. Functional gap junctions in the schwann cell myelin sheath. J. Cell Biol. 1998, 142, 1095–1104. [Google Scholar] [CrossRef]
- Wagstaff, L.J.; Gomez-Sanchez, J.A.; Fazal, S.V.; Otto, G.W.; Kilpatrick, A.M.; Michael, K.; Wong, L.Y.N.; Ma, K.H.; Turmaine, M.; Svaren, J.; et al. Failures of nerve regeneration caused by aging or chronic denervation are rescued by restoring Schwann cell c-Jun. eLife 2021, 10, e62232. [Google Scholar] [CrossRef]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell. Mol. Life Sci. 2020, 77, 3977–3989. [Google Scholar] [CrossRef]
- McMorrow, L.A.; Kosalko, A.; Robinson, D.; Saiani, A.; Reid, A.J. Advancing Our Understanding of the Chronically Denervated Schwann Cell: A Potential Therapeutic Target? Biomolecules 2022, 12, 1128. [Google Scholar] [CrossRef]
- Janke, E.K.; Chalmers, S.B.; Roberts-Thomson, S.J.; Monteith, G.R. Intersection between calcium signalling and epithelial-mesenchymal plasticity in the context of cancer. Cell Calcium 2023, 112, 102741. [Google Scholar] [CrossRef]
- Norgard, R.J.; Pitarresi, J.R.; Maddipati, R.; Aiello-Couzo, N.M.; Balli, D.; Li, J.; Yamazoe, T.; Wengyn, M.D.; Millstein, I.D.; Folkert, I.W.; et al. Calcium signaling induces a partial EMT. EMBO Rep. 2021, 22, e51872. [Google Scholar] [CrossRef]
- Dong, Q.; Guo, Y.; Lv, C.; Ren, L.; Chen, B.; Wang, Y.; Liu, Y.; Liu, M.; Liu, K.; Zhang, N.; et al. Unveiling a novel cancer hallmark by evaluation of neural infiltration in cancer. Brief. Bioinform. 2025, 26, bbaf082. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, G.; Li, S.; Li, J.; Wang, W.; Xue, J.; Wang, Y.; Fang, M.; Zhou, N. Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J. Nanobiotechnol. 2023, 21, 10, Erratum in J. Nanobiotechnol. 2024, 22, 81. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Tan, S.; Li, Z.; Sun, J.; Li, Y.; Xie, Z.; Li, Z.; Han, F.; Liu, Y. Design Strategies of PEDOT:PSS-Based Conductive Hydrogels and Their Applications in Health Monitoring. Polymers 2025, 17, 1192. [Google Scholar] [CrossRef]
- Lopez-Buenafe, G.d.R.; Alonso-Cabrera, J.A.; Marcuello, C.; Ortiz-Perez, M.; Benito-Lopez, F.; Colom, A.; Basabe-Desmonts, L.; Saez, J. Fabrication and Characterization of PEDOT:PSS-Based Microstructured Electrodes for In Vitro Cell Culture. Adv. Mater. Interfaces 2025, n/a, 2500097. [Google Scholar] [CrossRef]
- Kleinlogel, S.; Feldbauer, K.; Dempski, R.E.; Fotis, H.; Wood, P.G.; Bamann, C.; Bamberg, E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat. Neurosci. 2011, 14, 513–518. [Google Scholar] [CrossRef]
- Berndt, A.; Schoenenberger, P.; Mattis, J.; Tye, K.M.; Deisseroth, K.; Hegemann, P.; Oertner, T.G. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc. Natl. Acad. Sci. USA 2011, 108, 7595–7600. [Google Scholar] [CrossRef]
- Liang, R.; Yu, J.K.; Meisner, J.; Liu, F.; Martinez, T.J. Electrostatic Control of Photoisomerization in Channelrhodopsin 2. J. Am. Chem. Soc. 2021, 143, 5425–5437. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Sato-Numata, K.; Suttinont, C.; Numata, T. Optogenetic Stimulation of Ca2+ Influx via Channelrhodopsin CapChR2 in Schwann Cells Promotes Neurite Outgrowth in Co-cultured PC12 Cells: A Neuronal Model. Cureus 2025, 17, e90023. [Google Scholar] [CrossRef]
- Volkov, O.; Kovalev, K.; Polovinkin, V.; Borshchevskiy, V.; Bamann, C.; Astashkin, R.; Marin, E.; Popov, A.; Balandin, T.; Willbold, D.; et al. Structural insights into ion conduction by channelrhodopsin 2. Science 2017, 358, eaan8862. [Google Scholar] [CrossRef]
- Fernandez Lahore, R.G.; Pampaloni, N.P.; Schiewer, E.; Heim, M.M.; Tillert, L.; Vierock, J.; Oppermann, J.; Walther, J.; Schmitz, D.; Owald, D.; et al. Calcium-permeable channelrhodopsins for the photocontrol of calcium signalling. Nat. Commun. 2022, 13, 7844, Erratum in Nat. Commun. 2024, 15, 3958. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, W.; Yuan, S.; Dou, Y. Mechanism of Calcium Ion-Selective Channel Opening in the ChR2_L132C Mutant: A Molecular Dynamics Simulation. Processes 2024, 12, 494. [Google Scholar] [CrossRef]
- Jung, K.; Park, J.H.; Kim, S.-Y.; Jeon, N.L.; Cho, S.-R.; Hyung, S. Optogenetic stimulation promotes Schwann cell proliferation, differentiation, and myelination in vitro. Sci. Rep. 2019, 9, 3487. [Google Scholar] [CrossRef]
- Kennedy, T.E.; Serafini, T.; de la Torre, J.R.; Tessier-Lavigne, M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 1994, 78, 425–435. [Google Scholar] [CrossRef]
- Liu, G.; Beggs, H.; Jürgensen, C.; Park, H.-T.; Tang, H.; Gorski, J.; Jones, K.R.; Reichardt, L.F.; Wu, J.; Rao, Y. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat. Neurosci. 2004, 7, 1222–1232. [Google Scholar] [CrossRef]
- Yamagishi, S.; Bando, Y.; Sato, K. Involvement of Netrins and Their Receptors in Neuronal Migration in the Cerebral Cortex. Front. Cell. Dev. Biol. 2020, 8, 590009. [Google Scholar] [CrossRef]
- Bielle, F.; Marcos-Mondéjar, P.; Leyva-Díaz, E.; Lokmane, L.; Mire, E.; Mailhes, C.; Keita, M.; García, N.; Tessier-Lavigne, M.; Garel, S.; et al. Emergent growth cone responses to combinations of Slit1 and Netrin 1 in thalamocortical axon topography. Curr. Biol. 2011, 21, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Dupin, I.; Lokmane, L.; Dahan, M.; Garel, S.; Studer, V. Subrepellent doses of Slit1 promote Netrin-1 chemotactic responses in subsets of axons. Neural Dev. 2015, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Brose, K.; Bland, K.S.; Wang, K.H.; Arnott, D.; Henzel, W.; Goodman, C.S.; Tessier-Lavigne, M.; Kidd, T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 1999, 96, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, R.J. Getting neural circuits into shape with semaphorins. Nat. Rev. Neurosci. 2012, 13, 605–618. [Google Scholar] [CrossRef]
- Winberg, M.L.; Noordermeer, J.N.; Tamagnone, L.; Comoglio, P.M.; Spriggs, M.K.; Tessier-Lavigne, M.; Goodman, C.S. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell 1998, 95, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Koropouli, E.; Kolodkin, A.L. Semaphorins and the dynamic regulation of synapse assembly, refinement, and function. Curr. Opin. Neurobiol. 2014, 27, 1–7. [Google Scholar] [CrossRef]
- Harreguy, M.B.; Tanvir, Z.; Shah, E.; Simprevil, B.; Tran, T.S.; Haspel, G. Semaphorin signaling restricts neuronal regeneration in C. elegans. Front. Cell. Dev. Biol. 2022, 10, 814160. [Google Scholar] [CrossRef] [PubMed]
- Majumder, T.; Khot, B.; Suriyaarachchi, H.; Nathan, A.; Liu, G. MYC regulation of the miR-92-Robo1 axis in Slit-mediated commissural axon guidance. Mol. Biol. Cell 2025, 36, ar50. [Google Scholar] [CrossRef]
- Yang, T.; Huang, H.; Shao, Q.; Yee, S.; Majumder, T.; Liu, G. miR-92 Suppresses Robo1 Translation to Modulate Slit Sensitivity in Commissural Axon Guidance. Cell Rep. 2018, 24, 2694–2708.e6. [Google Scholar] [CrossRef]
- Wong, K.; Park, H.T.; Wu, J.Y.; Rao, Y. Slit proteins: Molecular guidance cues for cells ranging from neurons to leukocytes. Curr. Opin. Genet. Dev. 2002, 12, 583–591. [Google Scholar] [CrossRef]
- Suto, F.; Ito, K.; Uemura, M.; Shimizu, M.; Shinkawa, Y.; Sanbo, M.; Shinoda, T.; Tsuboi, M.; Takashima, S.; Yagi, T.; et al. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J. Neurosci. 2005, 25, 3628–3637. [Google Scholar] [CrossRef]
- Leighton, P.A.; Mitchell, K.J.; Goodrich, L.V.; Lu, X.; Pinson, K.; Scherz, P.; Skarnes, W.C.; Tessier-Lavigne, M. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature 2001, 410, 174–179. [Google Scholar] [CrossRef]
- Huot, J. Ephrin signaling in axon guidance. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Klein, R. Bidirectional Eph–ephrin signaling during axon guidance. Trends Cell Biol. 2007, 17, 230–238. [Google Scholar] [CrossRef]
- Weth, F.; Kania, A. Chapter 6—Ephrin/Eph signaling in axon guidance. In Cellular Migration and Formation of Axons and Dendrites, 2nd ed.; Rubenstein, J., Rakic, P., Chen, B., Kwan, K.Y., Kolodkin, A., Anton, E., Eds.; Academic Press: Amsterdam, The Netherlands, 2020; pp. 123–146. [Google Scholar]
- Giger, R.J.; Cloutier, J.-F.; Sahay, A.; Prinjha, R.K.; Levengood, D.V.; Moore, S.E.; Pickering, S.; Simmons, D.; Rastan, S.; Walsh, F.S.; et al. Neuropilin-2 Is Required In Vivo for Selective Axon Guidance Responses to Secreted Semaphorins. Neuron 2000, 25, 29–41. [Google Scholar] [CrossRef]
- Chen, H.; Bagri, A.; Zupicich, J.A.; Zou, Y.; Stoeckli, E.; Pleasure, S.J.; Lowenstein, D.H.; Skarnes, W.C.; Chédotal, A.; Tessier-Lavigne, M. Neuropilin-2 Regulates the Development of Select Cranial and Sensory Nerves and Hippocampal Mossy Fiber Projections. Neuron 2000, 25, 43–56. [Google Scholar] [CrossRef]
- Jin, K.; Zhu, Y.; Sun, Y.; Mao, X.O.; Xie, L.; Greenberg, D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11946–11950. [Google Scholar] [CrossRef]
- Rosenstein, J.M.; Krum, J.M.; Ruhrberg, C. VEGF in the nervous system. Organogenesis 2010, 6, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.M.; Obuchowicz, E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: A new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides 2012, 46, 1–10. [Google Scholar] [CrossRef]
- Mackenzie, F.; Ruhrberg, C. Diverse roles for VEGF-A in the nervous system. Development 2012, 139, 1371–1380. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Henderson, C.E. Role of neurotrophic factors in neuronal development. Curr. Opin. Neurobiol. 1996, 6, 64–70. [Google Scholar] [CrossRef]
- Lipsky, R.H.; Marini, A.M. Brain-Derived Neurotrophic Factor in Neuronal Survival and Behavior-Related Plasticity. Ann. N. Y. Acad. Sci. 2007, 1122, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Moya-Alvarado, G.; Gonzalez-Billaut, C.; Bronfman, F.C. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton 2016, 73, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.K.; Hoffer, B.J.; Wang, Y. Stroke and TGF-β proteins: Glial cell line-derived neurotrophic factor and bone morphogenetic protein. Pharmacol. Ther. 2005, 105, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Mendelsohn, M.; Jessell, T.M. Neuronal patterning by BMPs: A requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998, 12, 3394–3407. [Google Scholar] [CrossRef]
- Ardelt, A.A.; Bhattacharyya, B.J.; Belmadani, A.; Ren, D.; Miller, R.J. Stromal derived growth factor-1 (CXCL12) modulates synaptic transmission to immature neurons during post-ischemic cerebral repair. Exp. Neurol. 2013, 248, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Heskamp, A.; Leibinger, M.; Andreadaki, A.; Gobrecht, P.; Diekmann, H.; Fischer, D. CXCL12/SDF-1 facilitates optic nerve regeneration. Neurobiol. Dis. 2013, 55, 76–86, Erratum in Neurobiol. Dis. 2013, 58, 1–2. [Google Scholar] [CrossRef]
- Li, M.; Ransohoff, R.M. Multiple roles of chemokine CXCL12 in the central nervous system: A migration from immunology to neurobiology. Prog. Neurobiol. 2008, 84, 116–131. [Google Scholar] [CrossRef]
- Oentaryo, M.J.; Tse, A.C.; Lee, C.W. Neuronal MT1-MMP mediates ECM clearance and Lrp4 cleavage for agrin deposition and signaling in presynaptic development. J. Cell. Sci. 2020, 133, jcs246710. [Google Scholar] [CrossRef]
- Hilgenberg, L.G.W.; Ho, K.D.; Lee, D.; O’Dowd, D.K.; Smith, M.A. Agrin Regulates Neuronal Responses to Excitatory Neurotransmitters in Vitro and in Vivo. Mol. Cell. Neurosci. 2002, 19, 97–110. [Google Scholar] [CrossRef]
- Bose, C.M.; Qiu, D.; Bergamaschi, A.; Gravante, B.; Bossi, M.; Villa, A.; Rupp, F.; Malgaroli, A. Agrin controls synaptic differentiation in hippocampal neurons. J. Neurosci. 2000, 20, 9086–9095. [Google Scholar] [CrossRef]
- Sann, S.B.; Xu, L.; Nishimune, H.; Sanes, J.R.; Spitzer, N.C. Neurite outgrowth and in vivo sensory innervation mediated by a Ca(V)2.2-laminin beta 2 stop signal. J. Neurosci. 2008, 28, 2366–2374. [Google Scholar] [CrossRef]
- Hunter, D.D.; Brunken, W.J. β2 Laminins Modulate Neuronal Phenotype in the Rat Retina. Mol. Cell. Neurosci. 1997, 10, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, Y.; Niikura, M.; Watanabe, M.; Onishi, K.; Tanabe, S.; Moriwaki, Y.; Okuda, T.; Ohara, S.; Murayama, S.; Takao, M.; et al. Selective Expression of Osteopontin in ALS-resistant Motor Neurons is a Critical Determinant of Late Phase Neurodegeneration Mediated by Matrix Metalloproteinase-9. Sci. Rep. 2016, 6, 27354. [Google Scholar] [CrossRef]
- Al-Dalahmah, O.; Lam, M.; McInvale, J.J.; Qu, W.; Nguyen, T.; Mun, J.-Y.; Kwon, S.; Ifediora, N.; Mahajan, A.; Humala, N.; et al. Osteopontin drives neuroinflammation and cell loss in MAPT-N279K frontotemporal dementia patient neurons. Cell Stem Cell 2024, 31, 676–693.e10. [Google Scholar] [CrossRef]
- Reinhard, S.M.; Razak, K.; Ethell, I.M. A delicate balance: Role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front. Cell. Neurosci. 2015, 9, 280. [Google Scholar] [CrossRef] [PubMed]
- Seitz-Holland, J.; Seethaler, M.; Makris, N.; Rushmore, J.; Cho, K.-I.K.; Rizzoni, E.; Vangel, M.; Sahin, O.S.; Heller, C.; Pasternak, O.; et al. The association of matrix metalloproteinase 9 (MMP9) with hippocampal volume in schizophrenia: A preliminary MRI study. Neuropsychopharmacology 2022, 47, 524–530. [Google Scholar] [CrossRef]
- Murase, S.; McKay, R.D. Matrix Metalloproteinase-9 Regulates Survival of Neurons in Newborn Hippocampus*. J. Biol. Chem. 2012, 287, 12184–12194. [Google Scholar] [CrossRef]
- Su, J.; Gorse, K.; Ramirez, F.; Fox, M.A. Collagen XIX is expressed by interneurons and contributes to the formation of hippocampal synapses. J. Comp. Neurol. 2010, 518, 229–253. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Cole, J.; Fox, M.A. Loss of Interneuron-Derived Collagen XIX Leads to a Reduction in Perineuronal Nets in the Mammalian Telencephalon. ASN Neuro 2017, 9, 1759091416689020. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ho, M. Role of glypican-1 in regulating multiple cellular signaling pathways. Am. J. Physiol. Cell Physiol. 2021, 321, C846–C858. [Google Scholar] [CrossRef] [PubMed]
- Malavé, C.; Villegas, G.M.; Hernández, M.; Martínez, J.C.; Castillo, C.; Suárez de Mata, Z.; Villegas, R. Role of glypican-1 in the trophic activity on PC12 cells induced by cultured sciatic nerve conditioned medium: Identification of a glypican-1–neuregulin complex. Brain Res. 2003, 983, 74–83. [Google Scholar] [CrossRef]
- Chernousov, M.A.; Rothblum, K.; Stahl, R.C.; Evans, A.; Prentiss, L.; Carey, D.J. Glypican-1 and alpha4(V) collagen are required for Schwann cell myelination. J. Neurosci. 2006, 26, 508–517. [Google Scholar] [CrossRef]
- Moffat, J.J.; Ka, M.; Jung, E.M.; Smith, A.L.; Kim, W.Y. The role of MACF1 in nervous system development and maintenance. Semin. Cell Dev. Biol. 2017, 69, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ka, M.; Jung, E.M.; Mueller, U.; Kim, W.Y. MACF1 regulates the migration of pyramidal neurons via microtubule dynamics and GSK-3 signaling. Dev. Biol. 2014, 395, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Weng, J.; Yuan, Y.-S.; Kou, Y.-H.; Han, N.; Jiang, B.-G.; Zhang, P.-X.; Shi, Q. Wnt5a Affects Schwann Cell Proliferation and Regeneration via Wnt/c-Jun and PTEN Signaling Pathway. Chin. Med. J. 2018, 131, 2623–2625. [Google Scholar] [CrossRef]
- van Vliet, A.C.; Lee, J.; van der Poel, M.; Mason, M.R.J.; Noordermeer, J.N.; Fradkin, L.G.; Tannemaat, M.R.; Malessy, M.J.A.; Verhaagen, J.; De Winter, F. Coordinated changes in the expression of Wnt pathway genes following human and rat peripheral nerve injury. PLoS ONE 2021, 16, e0249748. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Vetrugno, C.; Cossa, L.G.; Marsigliante, S. TGF-β1 activates RSC96 Schwann cells migration and invasion through MMP-2 and MMP-9 activities. J. Neurochem. 2020, 153, 525–538. [Google Scholar] [CrossRef]
- Matsuoka, I.; Nakane, A.; Kurihara, K. Induction of LIF-mRNA by TGF-β1 in Schwann cells. Brain Res. 1997, 776, 170–180. [Google Scholar] [CrossRef]
- Awatramani, R.; Shumas, S.; Kamholz, J.; Scherer, S.S. TGFbeta1 modulates the phenotype of Schwann cells at the transcriptional level. Mol. Cell. Neurosci. 2002, 19, 307–319. [Google Scholar] [CrossRef]
- Parkinson, D.B.; Dong, Z.; Bunting, H.; Whitfield, J.; Meier, C.; Marie, H.; Mirsky, R.; Jessen, K.R. Transforming growth factor beta (TGFbeta) mediates Schwann cell death in vitro and in vivo: Examination of c-Jun activation, interactions with survival signals, and the relationship of TGFbeta-mediated death to Schwann cell differentiation. J. Neurosci. 2001, 21, 8572–8585. [Google Scholar] [CrossRef]
- Einheber, S.; Hannocks, M.J.; Metz, C.N.; Rifkin, D.B.; Salzer, J.L. Transforming growth factor-beta 1 regulates axon/Schwann cell interactions. J. Cell Biol. 1995, 129, 443–458. [Google Scholar] [CrossRef]
- Tao, H.-Y.; He, B.; Liu, S.-Q.; Wei, A.-L.; Tao, F.-H.; Tao, H.-L.; Deng, W.-X.; Li, H.-H.; Chen, Q. Effect of carboxymethylated chitosan on the biosynthesis of NGF and activation of the Wnt/β-catenin signaling pathway in the proliferation of Schwann cells. Eur. J. Pharmacol. 2013, 702, 85–92. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Torii, T.; Takada, S.; Ohno, N.; Saitoh, Y.; Nakamura, K.; Ito, A.; Ogata, T.; Terada, N.; Tanoue, A.; et al. Involvement of the Tyro3 receptor and its intracellular partner Fyn signaling in Schwann cell myelination. Mol. Biol. Cell 2015, 26, 3489–3503. [Google Scholar] [CrossRef]
- Jesuraj, N.J.; Marquardt, L.M.; Kwasa, J.A.; Sakiyama-Elbert, S.E. Glial cell line-derived neurotrophic factor promotes increased phenotypic marker expression in femoral sensory and motor-derived Schwann cell cultures. Exp. Neurol. 2014, 257, 10–18. [Google Scholar] [CrossRef]
- Saijilafu; Hur, E.-M.; Liu, C.-M.; Jiao, Z.; Xu, W.-L.; Zhou, F.-Q. PI3K–GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat. Commun. 2013, 4, 2690. [Google Scholar] [CrossRef]
- Ren, C.; Chen, X.; Du, N.; Geng, S.; Hu, Y.; Liu, X.; Wu, X.; Lin, Y.; Bai, X.; Yin, W.; et al. Low-intensity pulsed ultrasound promotes Schwann cell viability and proliferation via the GSK-3β/β-catenin signaling pathway. Int. J. Biol. Sci. 2018, 14, 497–507. [Google Scholar] [CrossRef]
- Keng, V.W.; Rahrmann, E.P.; Watson, A.L.; Tschida, B.R.; Moertel, C.L.; Jessen, W.J.; Rizvi, T.A.; Collins, M.H.; Ratner, N.; Largaespada, D.A. PTEN and NF1 inactivation in Schwann cells produces a severe phenotype in the peripheral nervous system that promotes the development and malignant progression of peripheral nerve sheath tumors. Cancer Res. 2012, 72, 3405–3413. [Google Scholar] [CrossRef]
- Zhang, S.J.; Li, X.X.; Yu, Y.; Chiu, A.P.; Lo, L.H.; To, J.C.; Rowlands, D.K.; Keng, V.W. Schwann cell-specific PTEN and EGFR dysfunctions affect neuromuscular junction development by impairing Agrin signaling and autophagy. Biochem. Biophys. Res. Commun. 2019, 515, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Koppes, A.N.; Nordberg, A.L.; Paolillo, G.M.; Goodsell, N.M.; Darwish, H.A.; Zhang, L.; Thompson, D.M. Electrical stimulation of schwann cells promotes sustained increases in neurite outgrowth. Tissue Eng. Part A 2014, 20, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Azees, A.A.; Thompson, A.C.; Thomas, R.; Zhou, J.; Ruther, P.; Wise, A.K.; Ajay, E.A.; Garrett, D.J.; Quigley, A.; Fallon, J.B.; et al. Spread of activation and interaction between channels with multi-channel optogenetic stimulation in the mouse cochlea. Hear. Res. 2023, 440, 108911. [Google Scholar] [CrossRef]
- Thompson, A.C.; Wise, A.K.; Hart, W.L.; Needham, K.; Fallon, J.B.; Gunewardene, N.; Stoddart, P.R.; Richardson, R.T. Hybrid optogenetic and electrical stimulation for greater spatial resolution and temporal fidelity of cochlear activation. J. Neural. Eng. 2020, 17, 056046. [Google Scholar] [CrossRef] [PubMed]
- Hajimirzaei, P.; Tabatabaei, F.S.A.; Nasibi-Sis, H.; Razavian, R.S.; Nasirinezhad, F. Schwann cell transplantation for remyelination, regeneration, tissue sparing, and functional recovery in spinal cord injury: A systematic review and meta-analysis of animal studies. Exp. Neurol. 2025, 384, 115062. [Google Scholar] [CrossRef]
- Yu, Z.; Men, Y.; Dong, P. Schwann cells promote the capability of neural stem cells to differentiate into neurons and secret neurotrophic factors. Exp. Ther. Med. 2017, 13, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, J.; Hain, S.; Progatzky, F. Glial-immune interactions in barrier organs. Mucosal Immunol. 2025, 18, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Queralt, M.; Fledrich, R.; Stassart, R.M. Schwann cell functions in peripheral nerve development and repair. Neurobiol. Dis. 2023, 176, 105952. [Google Scholar] [CrossRef]
- Bray, E.R.; Chéret, J.; Yosipovitch, G.; Paus, R. Schwann cells as underestimated, major players in human skin physiology and pathology. Exp. Dermatol. 2020, 29, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tzekova, N.; Heinen, A.; Küry, P. Molecules involved in the crosstalk between immune- and peripheral nerve Schwann cells. J. Clin. Immunol. 2014, 34 (Suppl. 1), S86–S104. [Google Scholar] [CrossRef]
- Haykal, D.; Berardesca, E.; Kabashima, K.; Dréno, B. Beyond beauty: Neurocosmetics, the skin-brain axis, and the future of emotionally intelligent skincare. Clin. Dermatol. 2025, 43, 523–527. [Google Scholar] [CrossRef]
- Chen, Z.L.; Strickland, S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J. Cell Biol. 2003, 163, 889–899. [Google Scholar] [CrossRef]
- Patton, B.L.; Miner, J.H.; Chiu, A.Y.; Sanes, J.R. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J. Cell Biol. 1997, 139, 1507–1521. [Google Scholar] [CrossRef]
- Chernousov, M.A.; Carey, D.J. Schwann cell extracellular matrix molecules and their receptors. Histol. Histopathol. 2000, 15, 593–601. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, G.; Hou, B.; Song, E.; Wen, J.; Ba, Y.; Zhu, D.; Wang, G.; Qin, F. Effects of ECM proteins (laminin, fibronectin, and type IV collagen) on the biological behavior of Schwann cells and their roles in the process of remyelination after peripheral nerve injury. Front. Bioeng. Biotechnol. 2023, 11, 1133718. [Google Scholar] [CrossRef]
- McMahan, U.J. The agrin hypothesis. Cold Spring Harb. Symp. Quant. Biol. 1990, 55, 407–418. [Google Scholar] [CrossRef]
- Chabas, D.; Baranzini, S.E.; Mitchell, D.; Bernard, C.C.; Rittling, S.R.; Denhardt, D.T.; Sobel, R.A.; Lock, C.; Karpuj, M.; Pedotti, R.; et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 2001, 294, 1731–1735. [Google Scholar] [CrossRef]
- Peng, K.; Sant, D.; Andersen, N.; Silvera, R.; Camarena, V.; Piñero, G.; Graham, R.; Khan, A.; Xu, X.M.; Wang, G.; et al. Magnetic separation of peripheral nerve-resident cells underscores key molecular features of human Schwann cells and fibroblasts: An immunochemical and transcriptomics approach. Sci. Rep. 2020, 10, 18433. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Lin, Z.; Song, P.; Quan, D.; Bai, Y. Biomaterial-Based Schwann Cell Transplantation and Schwann Cell-Derived Biomaterials for Nerve Regeneration. Front. Cell. Neurosci. 2022, 16, 926222. [Google Scholar] [CrossRef]
- Patel, N.P.; Lyon, K.A.; Huang, J.H. An update-tissue engineered nerve grafts for the repair of peripheral nerve injuries. Neural Regen. Res. 2018, 13, 764–774. [Google Scholar] [CrossRef]
- Yi, S.; Xu, L.; Gu, X. Scaffolds for peripheral nerve repair and reconstruction. Exp. Neurol. 2019, 319, 112761. [Google Scholar] [CrossRef]
- Balkowiec, A.; Katz, D.M. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J. Neurosci. 2002, 22, 10399–10407. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, A.; Staiger, V. Neurotrophin secretion from hippocampal neurons evoked by long-term-potentiation-inducing electrical stimulation patterns. Proc. Natl. Acad. Sci. USA 2002, 99, 6386–6391. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Huang, J.; Lu, L.; Hu, X.; Luo, Z.; Li, M. Electrically induced brain-derived neurotrophic factor release from Schwann cells. J. Neurosci. Res. 2014, 92, 893–903. [Google Scholar] [CrossRef]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Xiao, B. Levering Mechanically Activated Piezo Channels for Potential Pharmacological Intervention. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Iqbal, J. Purinergic receptors modulators: An emerging pharmacological tool for disease management. Med. Res. Rev. 2022, 42, 1661–1703. [Google Scholar] [CrossRef]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Shin, S.; Baek, M.C.; Yea, K. Modification of immune cell-derived exosomes for enhanced cancer immunotherapy: Current advances and therapeutic applications. Exp. Mol. Med. 2024, 56, 19–31. [Google Scholar] [CrossRef] [PubMed]

| Protein Name | Gene Symbol | Function Category | References |
|---|---|---|---|
| Netrin-1 | NTN1 | Axon Guidance | [75,76,77] |
| Slit1 | SLIT1 | Axon Guidance | [78,79,80] |
| Semaphorin | SEMA | Axon Guidance | [81,82,83,84] |
| ROBO1 | ROBO1 | Axon Guidance | [85,86,87] |
| Plexin-A4 | PLXNA4 | Axon Guidance | [88,89] |
| EphA2 | EPHA2 | Axon Guidance | [90,91,92] |
| Neuropilin-2 | NRP2 | Axon Guidance | [93,94] |
| VEGF-A | VEGFA | Neurotrophic Factor | [95,96,97,98] |
| BDNF | BDNF | Neurotrophic Factor | [99,100,101,102] |
| GDF7 | GDF7 | Neurotrophic Factor | [103,104] |
| CXCL12 | CXCL12 | Neurotrophic Factor | [105,106,107] |
| Agrin | AGRN | ECM | [108,109,110] |
| Laminin β2 | LAMB2 | ECM | [111,112] |
| Osteopontin | OPN | ECM | [113,114] |
| MMP9 | MMP9 | ECM | [115,116,117] |
| COL9A1 | COL9A1 | ECM | [118,119] |
| Glypican-1 | GPC1 | ECM | [120,121,122] |
| MACF1 | MACF1 | ECM | [123,124] |
| Wnt5a | WNT5A | SC Regulation | [125,126] |
| TGFβ1 | TGFB1 | SC Regulation | [127,128,129,130,131] |
| DVL1 | DVL1 | SC Regulation | [126,132] |
| Fyn | FYN | SC Regulation | [133,134] |
| GSK3B | GSK3B | SC Regulation | [135,136] |
| PTEN | PTEN | SC Regulation | [125,137,138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Numata, T.; Tsutsumi, M.; Sato-Numata, K. Optogenetic and Endogenous Modulation of Ca2+ Signaling in Schwann Cells: Implications for Autocrine and Paracrine Neurotrophic Regulation. Int. J. Mol. Sci. 2025, 26, 9082. https://doi.org/10.3390/ijms26189082
Numata T, Tsutsumi M, Sato-Numata K. Optogenetic and Endogenous Modulation of Ca2+ Signaling in Schwann Cells: Implications for Autocrine and Paracrine Neurotrophic Regulation. International Journal of Molecular Sciences. 2025; 26(18):9082. https://doi.org/10.3390/ijms26189082
Chicago/Turabian StyleNumata, Tomohiro, Moe Tsutsumi, and Kaori Sato-Numata. 2025. "Optogenetic and Endogenous Modulation of Ca2+ Signaling in Schwann Cells: Implications for Autocrine and Paracrine Neurotrophic Regulation" International Journal of Molecular Sciences 26, no. 18: 9082. https://doi.org/10.3390/ijms26189082
APA StyleNumata, T., Tsutsumi, M., & Sato-Numata, K. (2025). Optogenetic and Endogenous Modulation of Ca2+ Signaling in Schwann Cells: Implications for Autocrine and Paracrine Neurotrophic Regulation. International Journal of Molecular Sciences, 26(18), 9082. https://doi.org/10.3390/ijms26189082







