Stress-Induced Membraneless Organelles in Neurons: Bridging Liquid–Liquid Phase Separation and Neurodevelopmental Dysfunction
Abstract
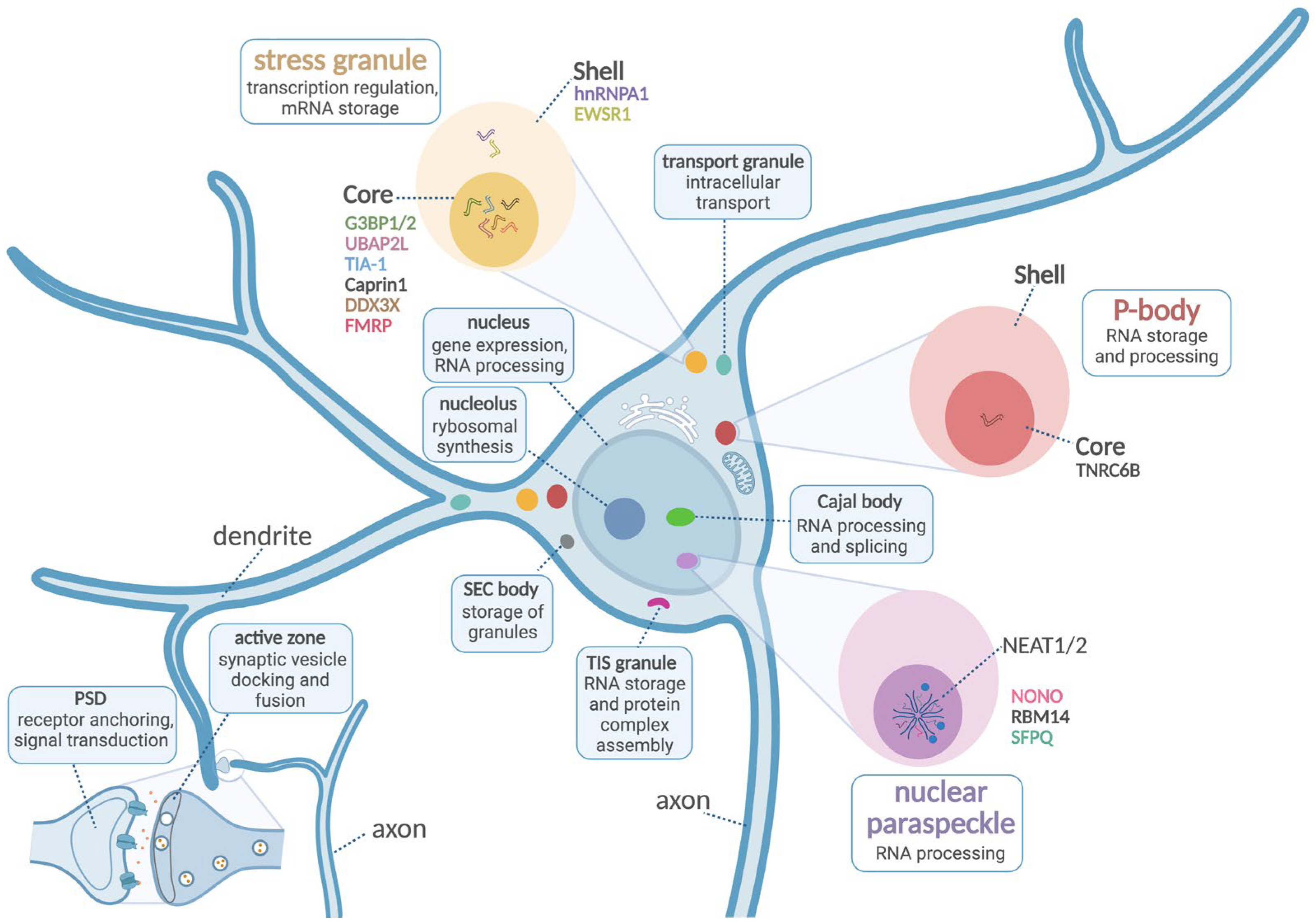
1. Introduction
2. Stress Granules
| Membraneless Organelle | UniProt Identifier | Protein Name | Protein Function | Type of NDD | Data Supporting NDD | Data Supporting LLPS | Protein Region(s) Mediating LLPS |
|---|---|---|---|---|---|---|---|
| stress granule | Q13283 | G3BP1 | DNA helicase | ataxia phenotype, ASD-like behavior | [52,53] | [48,49,50] | 1–142 NTFL2 domain; 143–226 IDR1; 410–466 IDR3 |
| stress granule | Q14157 | UBAP2L | ubiquitin-specific protease | speech–language problems, intellectual disability | [54] | [55,56] | disordered RGG/FG domains |
| stress granule | P31483 | TIA1 | RNA-binding protein | PTSD, anxiety disorder | [60,61] | [58,59] | 3 RRM domains and low-complexity regions |
| stress granule | Q14444 | CAPRIN1 | RNA-binding protein | fragile X-syndrome, autism spectrum disorder, ADHD, language delays | [65,66,67] | [62,64] | C-terminal low-complexity, disordered region of CAPRIN1 |
| stress granule | Q06787 | FMRP | RNA-binding protein | fragile X-syndrome, ASD | [68] | [69] | 445–632 C-terminal R/G-rich RGG motif-containing LC region |
| stress granule | O00571 | DDX3X | RNA helicase | intellectual disability, ASD-like phenotype; movement disorder | [70,71,72] | [34] | 1–168 N-terminal S/K-rich low-complexity region IDR containing RG motifs and the eIF4E-binding motif |
| stress granule | P09651 | hnRNPA1 | RNA-binding protein | ASID/autism spectrum—intellectual disability | [73] | [32,74,75] | 186–372 C-terminal G-rich prion-like low-complexity region |
| stress granule | Q01844 | EWSR1 | RNA-binding protein | ASD-like behavior | [76] | [77] | 1–285 N-terminal S/Y/Q/G-rich disordered domain; 286–360 disordered RGG repeats; 361–447 RNA binding region RRM |
| nuclear paraspeckle | Q15233 | NONO | RNA-binding protein | intellectual disability, global developmental delay | [78,79,80] | [81] | 218–272 NOPS domain for homodimerization or heterodimerization with SFPQ |
| nuclear paraspeckle | Q96PK6 | RBM14 | RNA-binding protein | autism spectrum disorder | [82] | [83] | 350–669 prion-like domain with 21 Y[G/N/A/S]AQ or [S/G]YG motifs |
| nuclear paraspeckle | P23246 | SFPQ | RNA-binding protein | autism spectrum disorder | [84] | [81,85] | GPM-rich disordered region |
| P-body | Q9UPQ9 | TNRC6B | RNA-binding protein | ASD, ADHD, developmental delay, intellectual disability | [86] | [87] | 437–1056 disordered GW-rich N-terminal Argonaute binding domain with tryptophan residues in motifs I and II |
3. Nuclear Paraspeckles
4. RNA-Based Processing Bodies (P-Bodies/GW-Bodies)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | Attention deficit hyperactivity disorder |
| ASD | Autistic spectrum disorders |
| ID | Intellectual disability |
| IDR | Intrinsically disordered region |
| LLPS | Liquid–liquid phase separation |
| MLO | Membraneless organelle |
| NDD | Neurodevelopmental disorder |
| PTSD | Post-traumatic stress disorder |
| SG | Stress granule |
References
- Morris-Rosendahl, D.J.; Crocq, M.A. Neurodevelopmental disorders-the history and future of a diagnostic concept. Dialogues Clin. Neurosci. 2020, 22, 65–72. [Google Scholar] [CrossRef]
- Cainelli, E.; Bisiacchi, P. Neurodevelopmental Disorders: Past, Present, and Future. Children 2022, 10, 31. [Google Scholar] [CrossRef]
- Tick, B.; Bolton, P.; Happe, F.; Rutter, M.; Rijsdijk, F. Heritability of autism spectrum disorders: A meta-analysis of twin studies. J. Child Psychol. Psychiatry 2016, 57, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Heyne, H.O.; Singh, T.; Stamberger, H.; Abou Jamra, R.; Caglayan, H.; Craiu, D.; De Jonghe, P.; Guerrini, R.; Helbig, K.L.; Koeleman, B.P.C.; et al. De novo variants in neurodevelopmental disorders with epilepsy. Nat. Genet. 2018, 50, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Lubrano, C.; Parisi, F.; Cetin, I. Impact of Maternal Environment and Inflammation on Fetal Neurodevelopment. Antioxidants 2024, 13, 453. [Google Scholar] [CrossRef]
- Fortin, O.; Mulkey, S.B. Neurodevelopmental outcomes in congenital and perinatal infections. Curr. Opin. Infect. Dis. 2023, 36, 405–413. [Google Scholar] [CrossRef]
- Nielsen-Saines, K.; Brasil, P.; Kerin, T.; Vasconcelos, Z.; Gabaglia, C.R.; Damasceno, L.; Pone, M.; Abreu de Carvalho, L.M.; Pone, S.M.; Zin, A.A.; et al. Delayed childhood neurodevelopment and neurosensory alterations in the second year of life in a prospective cohort of ZIKV-exposed children. Nat. Med. 2019, 25, 1213–1217. [Google Scholar] [CrossRef]
- Fitzgerald, E.; Boardman, J.P.; Drake, A.J. Preterm Birth and the Risk of Neurodevelopmental Disorders—Is There a Role for Epigenetic Dysregulation? Curr. Genom. 2018, 19, 507–521. [Google Scholar] [CrossRef]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- World Health Organization and United Nations Children’s Fund. Global Report on Children with Developmental Disabilities: From the Margins to the Mainstream; World Health Organization: Geneva, Switzerland, 2023; p. 116. [Google Scholar]
- Budimirovic, D.B.; Kaufmann, W.E. What can we learn about autism from studying fragile X syndrome? Dev. Neurosci. 2011, 33, 379–394. [Google Scholar] [CrossRef]
- Abbeduto, L.; McDuffie, A.; Thurman, A.J. The fragile X syndrome-autism comorbidity: What do we really know? Front. Genet. 2014, 5, 355. [Google Scholar] [CrossRef]
- El Khouri, E.; Ghoumid, J.; Haye, D.; Giuliano, F.; Drevillon, L.; Briand-Suleau, A.; De La Grange, P.; Nau, V.; Gaillon, T.; Bienvenu, T.; et al. Wnt/beta-catenin pathway and cell adhesion deregulation in CSDE1-related intellectual disability and autism spectrum disorders. Mol. Psychiatry 2021, 26, 3572–3585. [Google Scholar] [CrossRef]
- Ehret, F.; Moreno Traspas, R.; Neumuth, M.T.; Hamann, B.; Lasse, D.; Kempermann, G. Notch3-Dependent Effects on Adult Neurogenesis and Hippocampus-Dependent Learning in a Modified Transgenic Model of CADASIL. Front. Aging Neurosci. 2021, 13, 617733. [Google Scholar] [CrossRef] [PubMed]
- Memi, F.; Zecevic, N.; Radonjic, N. Multiple roles of Sonic Hedgehog in the developing human cortex are suggested by its widespread distribution. Brain Struct. Funct. 2018, 223, 2361–2375. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, J.; Zhang, H.; Khan, N.U.; Zhang, J.; Tang, X.; Cao, X.; Shen, L. Oxidative Stress in Autism Spectrum Disorder-Current Progress of Mechanisms and Biomarkers. Front. Psychiatry 2022, 13, 813304. [Google Scholar] [CrossRef] [PubMed]
- Tsang, B.; Pritisanac, I.; Scherer, S.W.; Moses, A.M.; Forman-Kay, J.D. Phase Separation as a Missing Mechanism for Interpretation of Disease Mutations. Cell 2020, 183, 1742–1756. [Google Scholar] [CrossRef]
- Wu, X.; Cai, Q.; Feng, Z.; Zhang, M. Liquid-Liquid Phase Separation in Neuronal Development and Synaptic Signaling. Dev. Cell 2020, 55, 18–29. [Google Scholar] [CrossRef]
- Brangwynne, C.P.; Eckmann, C.R.; Courson, D.S.; Rybarska, A.; Hoege, C.; Gharakhani, J.; Julicher, F.; Hyman, A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 2009, 324, 1729–1732. [Google Scholar] [CrossRef]
- Alberti, S.; Hyman, A.A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 196–213. [Google Scholar] [CrossRef]
- McDonald, N.A.; Fetter, R.D.; Shen, K. Assembly of synaptic active zones requires phase separation of scaffold molecules. Nature 2020, 588, 454–458. [Google Scholar] [CrossRef]
- Lautenschlager, J. Protein phase separation hotspots at the presynapse. Open Biol. 2022, 12, 210334. [Google Scholar] [CrossRef]
- Zeng, M.; Chen, X.; Guan, D.; Xu, J.; Wu, H.; Tong, P.; Zhang, M. Reconstituted Postsynaptic Density as a Molecular Platform for Understanding Synapse Formation and Plasticity. Cell 2018, 174, 1172–1187.E16. [Google Scholar] [CrossRef] [PubMed]
- Vistrup-Parry, M.; Chen, X.; Johansen, T.L.; Bach, S.; Buch-Larsen, S.C.; Bartling, C.R.O.; Ma, C.; Clemmensen, L.S.; Nielsen, M.L.; Zhang, M.; et al. Site-specific phosphorylation of PSD-95 dynamically regulates the postsynaptic density as observed by phase separation. iScience 2021, 24, 103268. [Google Scholar] [CrossRef] [PubMed]
- Ries, R.J.; Zaccara, S.; Klein, P.; Olarerin-George, A.; Namkoong, S.; Pickering, B.F.; Patil, D.P.; Kwak, H.; Lee, J.H.; Jaffrey, S.R. m(6)A enhances the phase separation potential of mRNA. Nature 2019, 571, 424–428. [Google Scholar] [CrossRef]
- Zbinden, A.; Perez-Berlanga, M.; De Rossi, P.; Polymenidou, M. Phase Separation and Neurodegenerative Diseases: A Disturbance in the Force. Dev. Cell 2020, 55, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Fagman, J.B.; Chen, C.; Alberti, S.; Liu, B. Protein phase separation and its role in tumorigenesis. eLife 2020, 9, e60264. [Google Scholar] [CrossRef]
- Darling, A.L.; Liu, Y.; Oldfield, C.J.; Uversky, V.N. Intrinsically Disordered Proteome of Human Membrane-Less Organelles. Proteomics 2018, 18, e1700193. [Google Scholar] [CrossRef]
- Uversky, V.N. Recent Developments in the Field of Intrinsically Disordered Proteins: Intrinsic Disorder-Based Emergence in Cellular Biology in Light of the Physiological and Pathological Liquid-Liquid Phase Transitions. Annu. Rev. Biophys. 2021, 50, 135–156. [Google Scholar] [CrossRef]
- Ghosh, A.; Mazarakos, K.; Zhou, H.X. Three archetypical classes of macromolecular regulators of protein liquid-liquid phase separation. Proc. Natl. Acad. Sci. USA 2019, 116, 19474–19483. [Google Scholar] [CrossRef]
- Nott, T.J.; Petsalaki, E.; Farber, P.; Jervis, D.; Fussner, E.; Plochowietz, A.; Craggs, T.D.; Bazett-Jones, D.P.; Pawson, T.; Forman-Kay, J.D.; et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 2015, 57, 936–947. [Google Scholar] [CrossRef]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P. Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef]
- Wheeler, J.R.; Matheny, T.; Jain, S.; Abrisch, R.; Parker, R. Distinct stages in stress granule assembly and disassembly. eLife 2016, 5, e18413. [Google Scholar] [CrossRef]
- Saito, M.; Hess, D.; Eglinger, J.; Fritsch, A.W.; Kreysing, M.; Weinert, B.T.; Choudhary, C.; Matthias, P. Acetylation of intrinsically disordered regions regulates phase separation. Nat. Chem. Biol. 2019, 15, 51–61. [Google Scholar] [CrossRef]
- Hosokawa, T.; Liu, P.W.; Cai, Q.; Ferreira, J.S.; Levet, F.; Butler, C.; Sibarita, J.B.; Choquet, D.; Groc, L.; Hosy, E.; et al. CaMKII activation persistently segregates postsynaptic proteins via liquid phase separation. Nat. Neurosci. 2021, 24, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Mateju, D.; Franzmann, T.M.; Patel, A.; Kopach, A.; Boczek, E.E.; Maharana, S.; Lee, H.O.; Carra, S.; Hyman, A.A.; Alberti, S. An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 2017, 36, 1669–1687. [Google Scholar] [CrossRef] [PubMed]
- Jeon, P.; Lee, J.A. Dr. Jekyll and Mr. Hyde? Physiology and Pathology of Neuronal Stress Granules. Front. Cell Dev. Biol. 2021, 9, 609698. [Google Scholar] [CrossRef] [PubMed]
- Riback, J.A.; Katanski, C.D.; Kear-Scott, J.L.; Pilipenko, E.V.; Rojek, A.E.; Sosnick, T.R.; Drummond, D.A. Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response. Cell 2017, 168, 1028–1040.E19. [Google Scholar] [CrossRef]
- Nakagawa, S.; Yamazaki, T.; Hirose, T. Molecular dissection of nuclear paraspeckles: Towards understanding the emerging world of the RNP milieu. Open Biol. 2018, 8, 180150. [Google Scholar] [CrossRef]
- Xing, W.; Muhlrad, D.; Parker, R.; Rosen, M.K. A quantitative inventory of yeast P body proteins reveals principles of composition and specificity. eLife 2020, 9, e56525. [Google Scholar] [CrossRef]
- van Leeuwen, W.; Rabouille, C. Cellular stress leads to the formation of membraneless stress assemblies in eukaryotic cells. Traffic 2019, 20, 623–638. [Google Scholar] [CrossRef]
- Wolozin, B.; Ivanov, P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 2019, 20, 649–666. [Google Scholar] [CrossRef]
- Marcelo, A.; Koppenol, R.; de Almeida, L.P.; Matos, C.A.; Nobrega, C. Stress granules, RNA-binding proteins and polyglutamine diseases: Too much aggregation? Cell Death Dis. 2021, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Wheeler, J.R.; Walters, R.W.; Agrawal, A.; Barsic, A.; Parker, R. ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure. Cell 2016, 164, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Kedersha, N.; Anderson, P.; Ivanov, P. Molecular mechanisms of stress granule assembly and disassembly. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118876. [Google Scholar] [CrossRef] [PubMed]
- Khong, A.; Matheny, T.; Jain, S.; Mitchell, S.F.; Wheeler, J.R.; Parker, R. The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules. Mol. Cell 2017, 68, 808–820.e5. [Google Scholar] [CrossRef]
- Niewidok, B.; Igaev, M.; Pereira da Graca, A.; Strassner, A.; Lenzen, C.; Richter, C.P.; Piehler, J.; Kurre, R.; Brandt, R. Single-molecule imaging reveals dynamic biphasic partition of RNA-binding proteins in stress granules. J. Cell Biol. 2018, 217, 1303–1318. [Google Scholar] [CrossRef]
- Yang, P.; Mathieu, C.; Kolaitis, R.M.; Zhang, P.; Messing, J.; Yurtsever, U.; Yang, Z.; Wu, J.; Li, Y.; Pan, Q.; et al. G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell 2020, 181, 325–345.e28. [Google Scholar] [CrossRef]
- Guillen-Boixet, J.; Kopach, A.; Holehouse, A.S.; Wittmann, S.; Jahnel, M.; Schlussler, R.; Kim, K.; Trussina, I.; Wang, J.; Mateju, D.; et al. RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell 2020, 181, 346–361.e17. [Google Scholar] [CrossRef]
- Sanders, D.W.; Kedersha, N.; Lee, D.S.W.; Strom, A.R.; Drake, V.; Riback, J.A.; Bracha, D.; Eeftens, J.M.; Iwanicki, A.; Wang, A.; et al. Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization. Cell 2020, 181, 306–324.e28. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Lee, S.J.; Jaiswal, P.B.; Alber, S.; Kar, A.N.; Miller-Randolph, S.; Taylor, E.E.; Smith, T.; Singh, B.; Ho, T.S.; et al. Axonal G3BP1 stress granule protein limits axonal mRNA translation and nerve regeneration. Nat. Commun. 2018, 9, 3358. [Google Scholar] [CrossRef]
- Zekri, L.; Chebli, K.; Tourriere, H.; Nielsen, F.C.; Hansen, T.V.; Rami, A.; Tazi, J. Control of fetal growth and neonatal survival by the RasGAP-associated endoribonuclease G3BP. Mol. Cell. Biol. 2005, 25, 8703–8716. [Google Scholar] [CrossRef]
- Martin, S.; Zekri, L.; Metz, A.; Maurice, T.; Chebli, K.; Vignes, M.; Tazi, J. Deficiency of G3BP1, the stress granules assembly factor, results in abnormal synaptic plasticity and calcium homeostasis in neurons. J. Neurochem. 2013, 125, 175–184. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, S.; Tan, S.; Du, B.; He, M.; Qin, H.; Chen, J.; Duan, X.; Luo, J.; Chen, F.; et al. De novo variants in genes regulating stress granule assembly associate with neurodevelopmental disorders. Sci. Adv. 2022, 8, eabo7112. [Google Scholar] [CrossRef]
- Guerber, L.; Pangou, E.; Sumara, I. Ubiquitin Binding Protein 2-Like (UBAP2L): Is it so NICE After All? Front. Cell Dev. Biol. 2022, 10, 931115. [Google Scholar] [CrossRef] [PubMed]
- Riggs, C.L.; Kedersha, N.; Amarsanaa, M.; Zubair, S.N.; Ivanov, P.; Anderson, P. UBAP2L contributes to formation of P-bodies and modulates their association with stress granules. J. Cell Biol. 2024, 223, e202307146. [Google Scholar] [CrossRef] [PubMed]
- Gilks, N.; Kedersha, N.; Ayodele, M.; Shen, L.; Stoecklin, G.; Dember, L.M.; Anderson, P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 2004, 15, 5383–5398. [Google Scholar] [CrossRef] [PubMed]
- Rayman, J.B.; Karl, K.A.; Kandel, E.R. TIA-1 Self-Multimerization, Phase Separation, and Recruitment into Stress Granules Are Dynamically Regulated by Zn(2). Cell Rep. 2018, 22, 59–71. [Google Scholar] [CrossRef]
- Ding, X.; Gu, S.; Xue, S.; Luo, S.Z. Disease-associated mutations affect TIA1 phase separation and aggregation in a proline-dependent manner. Brain Res. 2021, 1768, 147589. [Google Scholar] [CrossRef]
- Rayman, J.B.; Hijazi, J.; Li, X.; Kedersha, N.; Anderson, P.J.; Kandel, E.R. Genetic Perturbation of TIA1 Reveals a Physiological Role in Fear Memory. Cell Rep. 2019, 26, 2970–2983.e4. [Google Scholar] [CrossRef]
- Byres, L.P.; Mufteev, M.; Yuki, K.E.; Wei, W.; Piekna, A.; Wilson, M.D.; Rodrigues, D.C.; Ellis, J. Identification of TIA1 mRNA targets during human neuronal development. Mol. Biol. Rep. 2021, 48, 6349–6361. [Google Scholar] [CrossRef]
- Kim, T.H.; Payliss, B.J.; Nosella, M.L.; Lee, I.T.W.; Toyama, Y.; Forman-Kay, J.D.; Kay, L.E. Interaction hot spots for phase separation revealed by NMR studies of a CAPRIN1 condensed phase. Proc. Natl. Acad. Sci. USA 2021, 118, e2104897118. [Google Scholar] [CrossRef]
- Kim, T.H.; Tsang, B.; Vernon, R.M.; Sonenberg, N.; Kay, L.E.; Forman-Kay, J.D. Phospho-dependent phase separation of FMRP and CAPRIN1 recapitulates regulation of translation and deadenylation. Science 2019, 365, 825–829. [Google Scholar] [CrossRef]
- Chen, S.; Cao, X.; Zhang, J.; Wu, W.; Zhang, B.; Zhao, F. circVAMP3 Drives CAPRIN1 Phase Separation and Inhibits Hepatocellular Carcinoma by Suppressing c-Myc Translation. Adv. Sci. 2022, 9, e2103817. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, R.; Takao, K.; Miyakawa, T.; Shiina, N. Comprehensive behavioral analysis of RNG105 (Caprin1) heterozygous mice: Reduced social interaction and attenuated response to novelty. Sci. Rep. 2016, 6, 20775. [Google Scholar] [CrossRef] [PubMed]
- Pavinato, L.; Delle Vedove, A.; Carli, D.; Ferrero, M.; Carestiato, S.; Howe, J.L.; Agolini, E.; Coviello, D.A.; van de Laar, I.; Au, P.Y.B.; et al. CAPRIN1 haploinsufficiency causes a neurodevelopmental disorder with language impairment, ADHD and ASD. Brain 2023, 146, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, G.; Baldo, C.; Arado, A.; Martinheira da Silva, J.S.; Bocciardi, R.; Testa, B.; Baldassari, S.; Mancardi, M.M.; Zara, F.; Malacarne, M.; et al. Derivation of the IGGi006-A stem cell line from a patient with CAPRIN1 haploinsufficiency. Stem Cell Res. 2025, 85, 103696. [Google Scholar] [CrossRef]
- Richter, J.D.; Zhao, X. The molecular biology of FMRP: New insights into fragile X syndrome. Nat. Rev. Neurosci. 2021, 22, 209–222. [Google Scholar] [CrossRef]
- Tsang, B.; Arsenault, J.; Vernon, R.M.; Lin, H.; Sonenberg, N.; Wang, L.Y.; Bah, A.; Forman-Kay, J.D. Phosphoregulated FMRP phase separation models activity-dependent translation through bidirectional control of mRNA granule formation. Proc. Natl. Acad. Sci. USA 2019, 116, 4218–4227. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K.Y.; Yang, Z.L.; Li, X.H.; Xu, R.; Zhou, H. A de novo DDX3X Variant Is Associated With Syndromic Intellectual Disability: Case Report and Literature Review. Front. Pediatr. 2020, 8, 303. [Google Scholar] [CrossRef]
- Rosa, E.S.I.; Smetana, J.H.C.; de Oliveira, J.F. A comprehensive review on DDX3X liquid phase condensation in health and neurodevelopmental disorders. Int. J. Biol. Macromol. 2024, 259, 129330. [Google Scholar] [CrossRef]
- Kennis, M.G.P.; Rots, D.; Bouman, A.; Ockeloen, C.W.; Boelen, C.; Marcelis, C.L.M.; de Vries, B.B.A.; Elting, M.W.; Waisfisz, Q.; Suri, M.; et al. DDX3X-related neurodevelopmental disorder in males—Presenting a new cohort of 19 males and a literature review. Eur. J. Hum. Genet. 2025, 33, 980–988. [Google Scholar] [CrossRef]
- Gillentine, M.A.; Wang, T.; Hoekzema, K.; Rosenfeld, J.; Liu, P.; Guo, H.; Kim, C.N.; De Vries, B.B.A.; Vissers, L.; Nordenskjold, M.; et al. Rare deleterious mutations of HNRNP genes result in shared neurodevelopmental disorders. Genome Med. 2021, 13, 63. [Google Scholar] [CrossRef]
- Tsoi, P.S.; Quan, M.D.; Choi, K.J.; Dao, K.M.; Ferreon, J.C.; Ferreon, A.C.M. Electrostatic modulation of hnRNPA1 low-complexity domain liquid-liquid phase separation and aggregation. Protein Sci. 2021, 30, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Ritsch, I.; Lehmann, E.; Emmanouilidis, L.; Yulikov, M.; Allain, F.; Jeschke, G. Phase Separation of Heterogeneous Nuclear Ribonucleoprotein A1 upon Specific RNA-Binding Observed by Magnetic Resonance. Angew. Chem. Int. Ed. Engl. 2022, 61, e202204311. [Google Scholar] [CrossRef] [PubMed]
- Griesi-Oliveira, K.; Fogo, M.S.; Pinto, B.G.G.; Alves, A.Y.; Suzuki, A.M.; Morales, A.G.; Ezquina, S.; Sosa, O.J.; Sutton, G.J.; Sunaga-Franze, D.Y.; et al. Transcriptome of iPSC-derived neuronal cells reveals a module of co-expressed genes consistently associated with autism spectrum disorder. Mol. Psychiatry 2021, 26, 1589–1605. [Google Scholar] [CrossRef] [PubMed]
- Altmeyer, M.; Neelsen, K.J.; Teloni, F.; Pozdnyakova, I.; Pellegrino, S.; Grofte, M.; Rask, M.D.; Streicher, W.; Jungmichel, S.; Nielsen, M.L.; et al. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat. Commun. 2015, 6, 8088. [Google Scholar] [CrossRef]
- Mircsof, D.; Langouet, M.; Rio, M.; Moutton, S.; Siquier-Pernet, K.; Bole-Feysot, C.; Cagnard, N.; Nitschke, P.; Gaspar, L.; Znidaric, M.; et al. Mutations in NONO lead to syndromic intellectual disability and inhibitory synaptic defects. Nat. Neurosci. 2015, 18, 1731–1736. [Google Scholar] [CrossRef]
- Sewani, M.; Nugent, K.; Blackburn, P.R.; Tarnowski, J.M.; Hernandez-Garcia, A.; Amiel, J.; Whalen, S.; Keren, B.; Courtin, T.; Rosenfeld, J.A.; et al. Further delineation of the phenotypic spectrum associated with hemizygous loss-of-function variants in NONO. Am. J. Med. Genet. A 2020, 182, 652–658. [Google Scholar] [CrossRef]
- Carlston, C.M.; Bleyl, S.B.; Andrews, A.; Meyers, L.; Brown, S.; Bayrak-Toydemir, P.; Bale, J.F.; Botto, L.D. Expanding the genetic and clinical spectrum of the NONO-associated X-linked intellectual disability syndrome. Am. J. Med. Genet. A 2019, 179, 792–796. [Google Scholar] [CrossRef]
- Yamazaki, T.; Souquere, S.; Chujo, T.; Kobelke, S.; Chong, Y.S.; Fox, A.H.; Bond, C.S.; Nakagawa, S.; Pierron, G.; Hirose, T. Functional Domains of NEAT1 Architectural lncRNA Induce Paraspeckle Assembly through Phase Separation. Mol. Cell 2018, 70, 1038–1053.e7. [Google Scholar] [CrossRef]
- da Silva Montenegro, E.M.; Costa, C.S.; Campos, G.; Scliar, M.; de Almeida, T.F.; Zachi, E.C.; Silva, I.M.W.; Chan, A.J.S.; Zarrei, M.; Lourenco, N.C.V.; et al. Meta-Analyses Support Previous and Novel Autism Candidate Genes: Outcomes of an Unexplored Brazilian Cohort. Autism Res. 2020, 13, 199–206. [Google Scholar] [CrossRef]
- Hennig, S.; Kong, G.; Mannen, T.; Sadowska, A.; Kobelke, S.; Blythe, A.; Knott, G.J.; Iyer, K.S.; Ho, D.; Newcombe, E.A.; et al. Prion-like domains in RNA binding proteins are essential for building subnuclear paraspeckles. J. Cell Biol. 2015, 210, 529–539. [Google Scholar] [CrossRef]
- Takeuchi, A.; Iida, K.; Tsubota, T.; Hosokawa, M.; Denawa, M.; Brown, J.B.; Ninomiya, K.; Ito, M.; Kimura, H.; Abe, T.; et al. Loss of Sfpq Causes Long-Gene Transcriptopathy in the Brain. Cell Rep. 2018, 23, 1326–1341. [Google Scholar] [CrossRef]
- Marshall, A.C.; Cummins, J.; Kobelke, S.; Zhu, T.; Widagdo, J.; Anggono, V.; Hyman, A.; Fox, A.H.; Bond, C.S.; Lee, M. Different Low-complexity Regions of SFPQ Play Distinct Roles in the Formation of Biomolecular Condensates. J. Mol. Biol. 2023, 435, 168364. [Google Scholar] [CrossRef] [PubMed]
- Granadillo, J.L.; Stegmann, A.P.A.; Guo, H.; Xia, K.; Angle, B.; Bontempo, K.; Ranells, J.D.; Newkirk, P.; Costin, C.; Viront, J.; et al. Pathogenic variants in TNRC6B cause a genetic disorder characterised by developmental delay/intellectual disability and a spectrum of neurobehavioural phenotypes including autism and ADHD. J. Med. Genet. 2020, 57, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Sheu-Gruttadauria, J.; MacRae, I.J. Phase Transitions in the Assembly and Function of Human miRISC. Cell 2018, 173, 946–957.e16. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Ohashi, R.; Shinoda, Y.; Yamazaki, M.; Abe, M.; Fujikawa, A.; Shigenobu, S.; Futatsugi, A.; Noda, M.; Mikoshiba, K.; et al. RNG105/caprin1, an RNA granule protein for dendritic mRNA localization, is essential for long-term memory formation. eLife 2017, 6, e29677. [Google Scholar] [CrossRef]
- Korb, E.; Herre, M.; Zucker-Scharff, I.; Gresack, J.; Allis, C.D.; Darnell, R.B. Excess Translation of Epigenetic Regulators Contributes to Fragile X Syndrome and Is Alleviated by Brd4 Inhibition. Cell 2017, 170, 1209–1223.e20. [Google Scholar] [CrossRef]
- Goering, R.; Hudish, L.I.; Guzman, B.B.; Raj, N.; Bassell, G.J.; Russ, H.A.; Dominguez, D.; Taliaferro, J.M. FMRP promotes RNA localization to neuronal projections through interactions between its RGG domain and G-quadruplex RNA sequences. eLife 2020, 9, e52621. [Google Scholar] [CrossRef]
- Ferron, L.; Nieto-Rostro, M.; Cassidy, J.S.; Dolphin, A.C. Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nat. Commun. 2014, 5, 3628. [Google Scholar] [CrossRef]
- Kanai, Y.; Dohmae, N.; Hirokawa, N. Kinesin transports RNA: Isolation and characterization of an RNA-transporting granule. Neuron 2004, 43, 513–525. [Google Scholar] [CrossRef]
- Markmiller, S.; Soltanieh, S.; Server, K.L.; Mak, R.; Jin, W.; Fang, M.Y.; Luo, E.C.; Krach, F.; Yang, D.; Sen, A.; et al. Context-Dependent and Disease-Specific Diversity in Protein Interactions within Stress Granules. Cell 2018, 172, 590–604.e13. [Google Scholar] [CrossRef]
- Zhang, H.; Manan-Mejias, P.M.; Miles, H.N.; Putnam, A.A.; MacGillivray, L.R.; Ricke, W.A. DDX3X and Stress Granules: Emerging Players in Cancer and Drug Resistance. Cancers 2024, 16, 1131. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Chen, S.; Lu, W.; Luo, T.; Shi, R.; Li, J.; Shen, H. Liquid-liquid phase separation of DDX3X: Mechanisms, pathological implications, and therapeutic potential. Int. J. Biol. Macromol. 2025, 317, 144835. [Google Scholar] [CrossRef] [PubMed]
- Lennox, A.L.; Hoye, M.L.; Jiang, R.; Johnson-Kerner, B.L.; Suit, L.A.; Venkataramanan, S.; Sheehan, C.J.; Alsina, F.C.; Fregeau, B.; Aldinger, K.A.; et al. Pathogenic DDX3X Mutations Impair RNA Metabolism and Neurogenesis during Fetal Cortical Development. Neuron 2020, 106, 404–420.e8. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Kerner, B.; Snijders Blok, L.; Suit, L.; Thomas, J.; Kleefstra, T.; Sherr, E.H. DDX3X-Related Neurodevelopmental Disorder. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2020; pp. 1993–2021. [Google Scholar]
- Tang, L.; Levy, T.; Guillory, S.; Halpern, D.; Zweifach, J.; Giserman-Kiss, I.; Foss-Feig, J.H.; Frank, Y.; Lozano, R.; Belani, P.; et al. Prospective and detailed behavioral phenotyping in DDX3X syndrome. Mol. Autism 2021, 12, 36. [Google Scholar] [CrossRef]
- Tilliole, P.; Fix, S.; Godin, J.D. hnRNPs: Roles in neurodevelopment and implication for brain disorders. Front. Mol. Neurosci. 2024, 17, 1411639. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, N.C.; Wang, Y.D.; Scarborough, E.A.; Moore, J.; Diaz, Z.; MacLea, K.S.; Freibaum, B.; Li, S.; Molliex, A.; et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 2013, 495, 467–473. [Google Scholar] [CrossRef]
- Pinto, D.; Delaby, E.; Merico, D.; Barbosa, M.; Merikangas, A.; Klei, L.; Thiruvahindrapuram, B.; Xu, X.; Ziman, R.; Wang, Z.; et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am. J. Hum. Genet. 2014, 94, 677–694. [Google Scholar] [CrossRef]
- Reichert, S.C.; Li, R.; Turner, S.A.; van Jaarsveld, R.H.; Massink, M.P.G.; van den Boogaard, M.H.; Del Toro, M.; Rodriguez-Palmero, A.; Fourcade, S.; Schluter, A.; et al. HNRNPH1-related syndromic intellectual disability: Seven additional cases suggestive of a distinct syndromic neurodevelopmental syndrome. Clin. Genet. 2020, 98, 91–98. [Google Scholar] [CrossRef]
- Lee, J.; Nguyen, P.T.; Shim, H.S.; Hyeon, S.J.; Im, H.; Choi, M.H.; Chung, S.; Kowall, N.W.; Lee, S.B.; Ryu, H. EWSR1, a multifunctional protein, regulates cellular function and aging via genetic and epigenetic pathways. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1938–1945. [Google Scholar] [CrossRef]
- Yoon, Y.; Park, H.; Kim, S.; Nguyen, P.T.; Hyeon, S.J.; Chung, S.; Im, H.; Lee, J.; Lee, S.B.; Ryu, H. Genetic Ablation of EWS RNA Binding Protein 1 (EWSR1) Leads to Neuroanatomical Changes and Motor Dysfunction in Mice. Exp. Neurobiol. 2018, 27, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Sojitra, K.A.; Sohn, E.J.; Moreno-Romero, A.K.; Baudin, A.; Xu, X.; Mittal, J.; Libich, D.S. Insights into Molecular Diversity within the FUS/EWS/TAF15 Protein Family: Unraveling Phase Separation of the N-Terminal Low-Complexity Domain from RNA-Binding Protein EWS. J. Am. Chem. Soc. 2024, 146, 8071–8085. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Imamachi, N.; Akizuki, G.; Kumakura, M.; Kawaguchi, A.; Nagata, K.; Kato, A.; Kawaguchi, Y.; Sato, H.; Yoneda, M.; et al. Long Noncoding RNA NEAT1-Dependent SFPQ Relocation from Promoter Region to Paraspeckle Mediates IL8 Expression upon Immune Stimuli. Mol. Cell 2014, 54, 1055. [Google Scholar] [CrossRef]
- Fox, A.H.; Nakagawa, S.; Hirose, T.; Bond, C.S. Paraspeckles: Where Long Noncoding RNA Meets Phase Separation. Trends Biochem. Sci. 2018, 43, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Yamazaki, T.; Nakagawa, S. Molecular anatomy of the architectural NEAT1 noncoding RNA: The domains, interactors, and biogenesis pathway required to build phase-separated nuclear paraspeckles. Wiley Interdiscip. Rev. RNA 2019, 10, e1545. [Google Scholar] [CrossRef]
- Park, Y.; Lee, J.M.; Hwang, M.Y.; Son, G.H.; Geum, D. NonO binds to the CpG island of oct4 promoter and functions as a transcriptional activator of oct4 gene expression. Mol. Cells 2013, 35, 61–69. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, J.; Qi, S.; Shen, Q. NONO Regulates Cortical Neuronal Migration and Postnatal Neuronal Maturation. Neurosci. Bull. 2019, 35, 1097–1101. [Google Scholar] [CrossRef]
- Itai, T.; Sugie, A.; Nitta, Y.; Maki, R.; Suzuki, T.; Shinkai, Y.; Watanabe, Y.; Nakano, Y.; Ichikawa, K.; Okamoto, N.; et al. A novel NONO variant that causes developmental delay and cardiac phenotypes. Sci. Rep. 2023, 13, 975. [Google Scholar] [CrossRef]
- Thomas-Jinu, S.; Gordon, P.M.; Fielding, T.; Taylor, R.; Smith, B.N.; Snowden, V.; Blanc, E.; Vance, C.; Topp, S.; Wong, C.H.; et al. Non-nuclear Pool of Splicing Factor SFPQ Regulates Axonal Transcripts Required for Normal Motor Development. Neuron 2017, 94, 322–336.e5. [Google Scholar] [CrossRef]
- Chang, J.; Gilman, S.R.; Chiang, A.H.; Sanders, S.J.; Vitkup, D. Genotype to phenotype relationships in autism spectrum disorders. Nat. Neurosci. 2015, 18, 191–198. [Google Scholar] [CrossRef]
- Jakymiw, A.; Pauley, K.M.; Li, S.; Ikeda, K.; Lian, S.; Eystathioy, T.; Satoh, M.; Fritzler, M.J.; Chan, E.K. The role of GW/P-bodies in RNA processing and silencing. J. Cell Sci. 2007, 120, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Na, Z.; Slavoff, S.A. P-Bodies: Composition, Properties, and Functions. Biochemistry 2018, 57, 2424–2431. [Google Scholar] [CrossRef] [PubMed]
- Cassani, M.; Seydoux, G. Specialized germline P-bodies are required to specify germ cell fate in Caenorhabditis elegans embryos. Development 2022, 149, dev200920. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Schmich, F.; Srivatsa, S.; Weidner, J.; Beerenwinkel, N.; Spang, A. Context-dependent deposition and regulation of mRNAs in P-bodies. eLife 2018, 7, e29815, Correction in eLife 2018, 7, e41300. [Google Scholar] [CrossRef][Green Version]
- Harrison, A.F.; Shorter, J. RNA-binding proteins with prion-like domains in health and disease. Biochem. J. 2017, 474, 1417–1438. [Google Scholar] [CrossRef]
- Sprunger, M.L.; Jackrel, M.E. Prion-Like Proteins in Phase Separation and Their Link to Disease. Biomolecules 2021, 11, 1014. [Google Scholar] [CrossRef]
- Mensah, M.A.; Niskanen, H.; Magalhaes, A.P.; Basu, S.; Kircher, M.; Sczakiel, H.L.; Reiter, A.M.V.; Elsner, J.; Meinecke, P.; Biskup, S.; et al. Aberrant phase separation and nucleolar dysfunction in rare genetic diseases. Nature 2023, 614, 564–571. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bencsik, N.; Kimsanaliev, D.; Tárnok, K.; Schlett, K. Stress-Induced Membraneless Organelles in Neurons: Bridging Liquid–Liquid Phase Separation and Neurodevelopmental Dysfunction. Int. J. Mol. Sci. 2025, 26, 9068. https://doi.org/10.3390/ijms26189068
Bencsik N, Kimsanaliev D, Tárnok K, Schlett K. Stress-Induced Membraneless Organelles in Neurons: Bridging Liquid–Liquid Phase Separation and Neurodevelopmental Dysfunction. International Journal of Molecular Sciences. 2025; 26(18):9068. https://doi.org/10.3390/ijms26189068
Chicago/Turabian StyleBencsik, Norbert, Daniel Kimsanaliev, Krisztián Tárnok, and Katalin Schlett. 2025. "Stress-Induced Membraneless Organelles in Neurons: Bridging Liquid–Liquid Phase Separation and Neurodevelopmental Dysfunction" International Journal of Molecular Sciences 26, no. 18: 9068. https://doi.org/10.3390/ijms26189068
APA StyleBencsik, N., Kimsanaliev, D., Tárnok, K., & Schlett, K. (2025). Stress-Induced Membraneless Organelles in Neurons: Bridging Liquid–Liquid Phase Separation and Neurodevelopmental Dysfunction. International Journal of Molecular Sciences, 26(18), 9068. https://doi.org/10.3390/ijms26189068







