New Paste Electrode Based on Copper and Gallium Mixed Metal Oxides-Decorated CNT for Highly Electrocatalyzed Hydrogen Evolution Reaction
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural, Surface, and Morphological Properties
2.2. Electrochemical Characterization
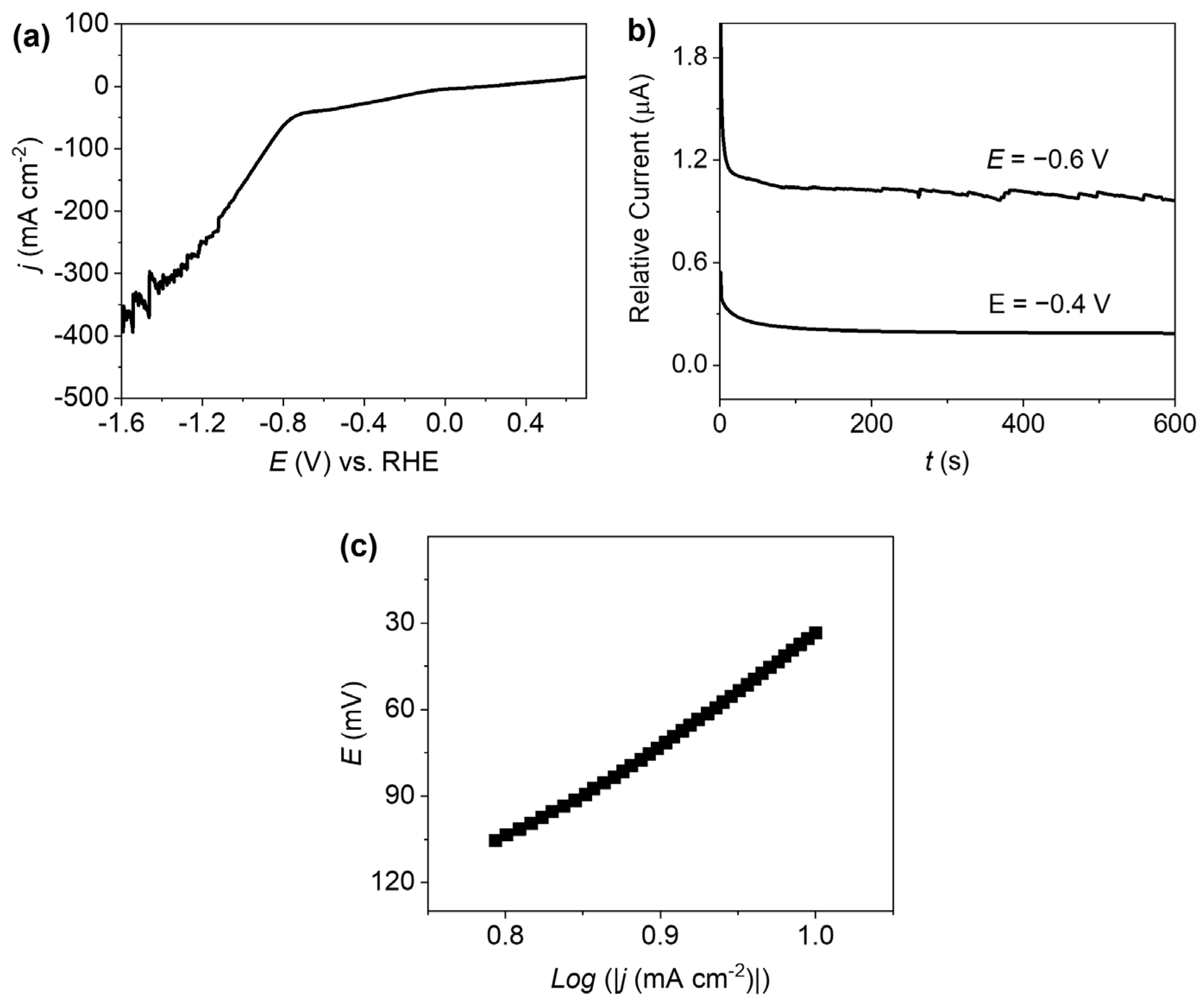
2.3. Electrocatalytic Study of the HER
2.4. Quantitative Study
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of Electrocatalysts
3.3. Paste Electrodes Preparation
3.4. Instrumentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Squadrito, G.; Maggio, G.; Nicita, A. The green hydrogen revolution. Renew. Energy 2023, 216, 119041. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A green hydrogen economy for a renewable energy society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Moses, J.K.; Tivfa, T.A.; Qasem, N.A.; Alquaity, A.B. Combustion characteristics of hydrogen, ammonia, and their blends: A review. Fuel 2025, 388, 134404. [Google Scholar] [CrossRef]
- Tran, D.T.; Tran, P.K.L.; Malhotra, D.; Nguyen, T.H.; Nguyen, T.T.A.; Duong, N.T.A.; Kim, N.H.; Lee, J.H. Current status of developed electrocatalysts for water splitting technologies: From experimental to industrial perspective. Nano Converg. 2025, 12, 9. [Google Scholar] [CrossRef]
- Greitzer, M. Iridium and Platinum Availability for Electrolyser Production up to 2030. In Powerfuels; Springer: Cham, Switzerland, 2025; pp. 257–279. [Google Scholar]
- Feidenhans’l, A.A.; Regmi, Y.N.; Wei, C.; Xia, D.; Kibsgaard, J.; King, L.A. Precious metal free hydrogen evolution catalyst design and application. Chem. Rev. 2024, 124, 5617–5667. [Google Scholar] [CrossRef] [PubMed]
- Al-Naggar, A.H.; Salah, A.; Al-Hejri, T.M.; Kamble, C.; Jadhav, V.V.; Shaikh, S.F.; Mane, R.S. Self-supported iron-doped nickel oxide multifunctional electrodes for highly efficient energy storage and overall water-splitting uses. J. Energy Storage 2024, 86, 111363. [Google Scholar] [CrossRef]
- Tu, J.; Zhang, M.; Li, M.; Li, J.; Zhi, L. Phosphorus-doped nickel cobalt oxide (NiCo2O4) wrapped in 3D hierarchical hollow N-doped carbon nanoflowers as highly efficient bifunctional electrocatalysts for overall water splitting. J. Colloid Interface Sci. 2024, 668, 243–251. [Google Scholar] [CrossRef]
- Sajid, M.; Qayyum, W.; Farhan, A.; Qamar, M.A.; Nawaz, H. Progress in the development of copper oxide-based materials for electrochemical water splitting. Int. J. Hydrogen Energy 2024, 62, 209–227. [Google Scholar] [CrossRef]
- Farooq, A.; Khalil, S.; Basha, B.; Habib, A.; Al-Buriahi, M.; Warsi, M.F.; Yousaf, S.; Shahid, M. Electrochemical investigation of C-doped CoFe2O4/Fe2O3 nanostructures for efficient electrochemical water splitting. Int. J. Hydrogen Energy 2024, 51, 1318–1332. [Google Scholar] [CrossRef]
- Gaur, A.; Sharma, J.; Lim, D.H.; Lee, H.I.; Han, H. Recent Advances in Electronic Structure Modifications of Layered Double Hydroxide (LDH) for the Water Splitting Application. ChemCatChem 2025, 17, e202401584. [Google Scholar] [CrossRef]
- Yang, C.; Lu, T.; Zhang, L. Probing the structural evolution of cobalt hydroxide in electrochemical water splitting. Chem. Commun. 2024, 60, 10326–10329. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, F.; Liu, Y.; Liu, W. Preparation of Cu (OH)2/Cu2S arrays for enhanced hydrogen evolution reaction. Battery Energy 2024, 3, 20230060. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, T.; Tang, T.; Li, J. Phytic acid treated nickel–iron hydroxide promotes efficient electrocatalytic overall water splitting. New J. Chem. 2024, 48, 10769–10775. [Google Scholar] [CrossRef]
- de Almeida, J.C.; Wang, Y.; Rodrigues, T.A.; Nunes, P.H.H.; de Mendonça, V.R.; Falsetti, P.H.E.; Savazi, L.V.; He, T.; Bardakova, A.V.; Rudakova, A.V.; et al. Copper-based Materials for Photo and Electrocatalytic Process: Advancing Renewable Energy and Environmental Applications. Adv. Funct. Mater. 2025, 2502901. [Google Scholar] [CrossRef]
- Jena, S.S.; Tripathy, S.K.; Mandre, N.R.; Venugopal, R.; Farrokhpay, S. Sustainable use of copper resources: Beneficiation of low-grade copper ores. Minerals 2022, 12, 545. [Google Scholar] [CrossRef]
- Kannimuthu, K.; Sangeetha, K.; Sankar, S.S.; Karmakar, A.; Madhu, R.; Kundu, S. Investigation on nanostructured Cu-based electrocatalysts for improvising water splitting: A review. Inorg. Chem. Front. 2021, 8, 234–272. [Google Scholar] [CrossRef]
- Awad, M.; Nawwar, M.; Zhitomirsky, I. Synergy of Charge Storage Properties of CuO and Polypyrrole in Composite CuO-Polypyrrole Electrodes for Asymmetric Supercapacitor Devices. ACS Appl. Energy Mater. 2024, 7, 5572–5581. [Google Scholar] [CrossRef]
- Lu, H.; Song, S.; Jia, Q.; Liu, G.; Jiang, L. Advances in Cu2O-based photocathodes for photoelectrochemical water splitting. Acta Phys.-Chim. Sin. 2024, 40, 2304035. [Google Scholar] [CrossRef]
- Seo, Y.J.; Arunachalam, M.; Ahn, K.-S.; Kang, S.H. Integrating heteromixtured Cu2O/CuO photocathode interface through a hydrogen treatment for photoelectrochemical hydrogen evolution reaction. Appl. Surf. Sci. 2021, 551, 149375. [Google Scholar]
- Son, H.; Lee, J.-H.; Uthirakumar, P.; Van Dao, D.; Soon, A.; Lee, I.-H. Facile synthesis of flexible and scalable Cu/Cu2O/CuO nanoleaves photoelectrodes with oxidation-induced self-initiated charge-transporting platform for photoelectrochemical water splitting enhancement. J. Alloys Compd. 2023, 942, 169094. [Google Scholar] [CrossRef]
- Mumtazah, N.; Chan, C.-H.; Catherine, S.; Pham, M.-T.H.; Choi, J.; Yoo, J.S.; Chiang, C.Y. Modulating surface chemistry of copper oxides through annealing environment for enhanced selective HMF oxidation: A DFT-electrochemical approach. J. Environ. Chem. Eng. 2024, 12, 113444. [Google Scholar] [CrossRef]
- Dong, N.; Wu, J.; Wu, D.; Jiang, G.; Shi, J.; Chang, S. Cu/Cu2O/CuO hierarchical nanosheets for electrocatalytic conversion of nitrate to ammonia. ACS Appl. Nano Mater. 2024, 7, 9269–9277. [Google Scholar] [CrossRef]
- Liu, C.; Ma, J.; Fu, Z.; Zhao, P.; Bai, M.; Gao, Y.; Zhao, M.; He, Y.; Xiao, H.; Jia, J. Gallium-based materials for electrocatalytic and photocatalytic hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 73, 490–509. [Google Scholar] [CrossRef]
- Kakoria, A.; Devi, B.; Anand, A.; Halder, A.; Koner, R.R.; Sinha-Ray, S. Gallium oxide nanofibers for hydrogen evolution and oxygen reduction. ACS Appl. Nano Mater. 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Hui, Z.X.; Zhou, H.Y.; Zai, S.F.; Wen, Z.; Li, J.C.; Yang, C.C.; Jiang, Q. Ga doping enables superior alkaline hydrogen evolution reaction performances of CoP. Chem. Eng. J. 2022, 429, 132012. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Fu, W.; Chen, J.; Wang, D.; Xiao, Y.; Xi, S.; Ji, Y.; Wang, L. Energy-efficient CO(2) conversion to multicarbon products at high rates on CuGa bimetallic catalyst. Nat. Commun. 2024, 15, 7053. [Google Scholar] [CrossRef]
- Saboor, F.H.; Ataei, A. Decoration of metal nanoparticles and metal oxide nanoparticles on carbon nanotubes. Adv. J. Chem. Sect. A 2024, 7, 122. [Google Scholar]
- Tafete, G.A.; Thothadri, G.; Abera, M.K. A review on carbon nanotube-based composites for electrocatalyst applications. Fuller. Nanotub. Carbon Nanostruct. 2022, 30, 1075–1083. [Google Scholar] [CrossRef]
- Noor, T.; Yaqoob, L.; Iqbal, N. Recent advances in electrocatalysis of oxygen evolution reaction using noble-metal, transition-metal, and carbon-based materials. ChemElectroChem 2021, 8, 447–483. [Google Scholar] [CrossRef]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Playford, H.Y.; Hannon, A.C.; Tucker, M.G.; Dawson, D.M.; Ashbrook, S.E.; Kastiban, R.J.; Sloan, J.; Walton, R.I. Characterization of structural disorder in γ-Ga2O3. J. Phys. Chem. C 2014, 118, 16188–16198. [Google Scholar] [CrossRef]
- Tunell, G.; Posnjak, Ε.; Ksanda, C. Geometrical and optical properties, and crystal structure of tenorite. Z. Für Krist.-Cryst. Mater. 1935, 90, 120–142. [Google Scholar] [CrossRef]
- Goh, K.; Jiang, W.; Karahan, H.E.; Zhai, S.; Wei, L.; Yu, D.; Fane, A.G.; Wang, R.; Chen, Y. All-carbon nanoarchitectures as high-performance separation membranes with superior stability. Adv. Funct. Mater. 2015, 25, 7348–7359. [Google Scholar] [CrossRef]
- Zhu, H.; Li, K.; Chen, M.; Cao, H.; Wang, F. A novel metal–organic framework route to embed Co nanoparticles into multi-walled carbon nanotubes for effective oxygen reduction in alkaline media. Catalysts 2017, 7, 364. [Google Scholar] [CrossRef]
- Abdullah, T.A.; Juzsakova, T.; Rasheed, R.T.; Salman, A.D.; Sebestyen, V.; Domokos, E.; Sluser, B.; Cretescu, I. Polystyrene-Fe3O4-MWCNTs nanocomposites for toluene removal from water. Materials 2021, 14, 5503. [Google Scholar] [CrossRef]
- Oke, J.A.; Idisi, D.O.; Sarma, S.; Moloi, S.J.; Ray, S.C.; Chen, K.H.; Ghosh, A.; Shelke, A.; Pong, W.F. Electronic, electrical, and magnetic behavioral change of SiO2-NP-decorated MWCNTs. ACS Omega 2019, 4, 14589–14598. [Google Scholar] [CrossRef]
- Domagała, K.; Borlaf, M.; Kata, D.; Graule, T. Synthesis of copper-based multi-walled carbon nanotube composites. Arch. Metall. Mater. 2020, 65, 157–162. [Google Scholar] [CrossRef]
- Lavalley, J.; Daturi, M.; Montouillout, V.; Clet, G.; Areán, C.O.; Delgado, M.R.; Sahibed-Dine, A. Unexpected similarities between the surface chemistry of cubic and hexagonal gallia polymorphs. Phys. Chem. Chem. Phys. 2003, 5, 1301–1305. [Google Scholar] [CrossRef]
- Khadivi, A.H.; Vahdati, K.J.; Haddad, S.M. Facile synthesis of copper oxide nanoparticles using copper hydroxide by mechanochemical process. J. Ultrafine Grained Nanostruct. Mater. 2015, 48, 37–44. [Google Scholar]
- Neuburger, M. Präzisionsmessung der Gitterkonstante von Cuprooxyd Cu2O. Z. Für Phys. 1931, 67, 845–850. [Google Scholar] [CrossRef]
- Gao, S.; Xing, H.; Li, Y.; Wang, H. Synthesis of Cu2O/multi-walled carbon nanotube hybrid material and its microwave absorption performance. Res. Chem. Intermed. 2018, 44, 3425–3435. [Google Scholar] [CrossRef]
- Gicha, B.B.; Tufa, L.T.; Choi, Y.; Lee, J. Amorphous Ni1–xFex Oxyhydroxide Nanosheets with Integrated Bulk and Surface Iron for a High and Stable Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2021, 4, 6833–6841. [Google Scholar] [CrossRef]
- Huseynzade, F.; Akbaba, Y.; Shawuti, S.; Xan, M.M. Investigating the photocatalytic efficacy of a porous cuprous gallate (CuGa2O4) thick film. Phys. Scr. 2024, 99, 125905. [Google Scholar] [CrossRef]
- Pecchi, G.; Campos, C.M.; Jiliberto, M.G.; Delgado, E.J.; Fierro, J.L.G. Effect of additive Ag on the physicochemical and catalytic properties of LaMn0.9Co0.1O3.5 perovskite. Appl. Catal. A Gen. 2009, 371, 78–84. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Ferdiana, N.A.; Bahti, H.H.; Kurnia, D.; Wyantuti, S. Synthesis, characterization, and electrochemical properties of rare earth element nanoparticles and its application in electrochemical nanosensor for the detection of various biomolecules and hazardous compounds: A review. Sens. Bio-Sens. Res. 2023, 41, 100573. [Google Scholar] [CrossRef]
- Wu, T.; Han, M.Y.; Xu, Z.J. Size effects of electrocatalysts: More than a variation of surface area. ACS Nano 2022, 16, 8531–8539. [Google Scholar] [CrossRef] [PubMed]
- Klingler, R.J.; Kochi, J.K. Electron-transfer kinetics from cyclic voltammetry. Quantitative description of electrochemical reversibility. J. Phys. Chem. 1981, 85, 1731–1741. [Google Scholar] [CrossRef]
- Martínez, R.; Ramírez, M.T.; González, I. Voltammetric characterization of carbon paste electrodes with a nonconducting binder. Part I: Evidence of the influence of electroactive species dissolution into the paste on the voltammetric response. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 1998, 10, 336–342. [Google Scholar] [CrossRef]
- Brett, C.M.; Brett, O. Principles, methods, and applications. Electrochemistry 1993, 67, 444. [Google Scholar]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Salinas-Torres, D.; Huerta, F.; Montilla, F.; Morallón, E. Study on electroactive and electrocatalytic surfaces of single walled carbon nanotube-modified electrodes. Electrochim. Acta 2011, 56, 2464–2470. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised Pourbaix diagrams for copper at 25 to 300 °C. J. Electrochem. Soc. 1997, 144, 3476. [Google Scholar] [CrossRef]
- Sakong, S.; Groß, A. Dissociative adsorption of hydrogen on strained Cu surfaces. Surf. Sci. 2003, 525, 107–118. [Google Scholar] [CrossRef]
- Zhong, G.; Cheng, T.; Shah, A.H.; Wan, C.; Huang, Z.; Wang, S.; Leng, T.; Huang, Y.; Goddard, W.A., III; Duan, X. Determining the hydronium pK α at platinum surfaces and the effect on pH-dependent hydrogen evolution reaction kinetics. Proc. Natl. Acad. Sci. USA 2022, 119, e2208187119. [Google Scholar] [CrossRef]
- Carneiro-Neto, E.B.; Lopes, M.C.; Pereira, E.C. Simulation of interfacial pH changes during hydrogen evolution reaction. J. Electroanal. Chem. 2016, 765, 92–99. [Google Scholar] [CrossRef]
- Munoz, A.I.; Espallargas, N.; Mischler, S. Tribocorrosion; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Shin, S.J.; Kim, J.Y.; An, S.; Chung, T.D. Recent advances in electroanalytical methods for electroorganic synthesis. Curr. Opin. Electrochem. 2022, 35, 101054. [Google Scholar] [CrossRef]
- Tang, C.; Sun, A.; Xu, Y.; Wu, Z.; Wang, D. High specific surface area Mo2C nanoparticles as an efficient electrocatalyst for hydrogen evolution. J. Power Sources 2015, 296, 18–22. [Google Scholar] [CrossRef]
- Conway, B.; Tilak, B. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. [Google Scholar] [CrossRef]
- Wan, C.; Ling, Y.; Wang, S.; Pu, H.; Huang, Y.; Duan, X. Unraveling and resolving the inconsistencies in tafel analysis for hydrogen evolution reactions. ACS Cent. Sci. 2024, 10, 658–665. [Google Scholar] [CrossRef]
- Hitz, C.; Lasia, A. Experimental study and modeling of impedance of the her on porous Ni electrodes. J. Electroanal. Chem. 2001, 500, 213–222. [Google Scholar] [CrossRef]
- Ahlberg, E.; Ásbjörnsson, J. Carbon paste electrodes in mineral processing: An electrochemical study of sphalerite. Hydrometallurgy 1994, 36, 19–37. [Google Scholar] [CrossRef]
- Austin, L. Tafel slopes for flooded diffusion electrodes. Trans. Faraday Soc. 1964, 60, 1319–1324. [Google Scholar] [CrossRef]
- Kemppainen, E.; Halme, J.; Hansen, O.; Seger, B.; Lund, P.D. Two-phase model of hydrogen transport to optimize nanoparticle catalyst loading for hydrogen evolution reaction. Int. J. Hydrogen Energy 2016, 41, 7568–7581. [Google Scholar] [CrossRef]
- Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Role of carbon nanotubes in electroanalytical chemistry: A review. Anal. Chim. Acta 2008, 622, 11–47. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Yan, Y.; Zhang, M.; Su, L.; Xiong, S.; Mao, L. Electrochemistry and electroanalytical applications of carbon nanotubes: A review. Anal. Sci. 2005, 21, 1383–1393. [Google Scholar] [CrossRef][Green Version]
- Gidi, L.; Arce, R.; Ibarra, J.; Isaacs, M.; Aguirre, M.; Ramírez, G. Hydrogen evolution reaction highly electrocatalyzed by MWCNT/N-octylpyridinum hexafluorophosphate metal-free system. Electrochim. Acta 2021, 372, 137859. [Google Scholar] [CrossRef]
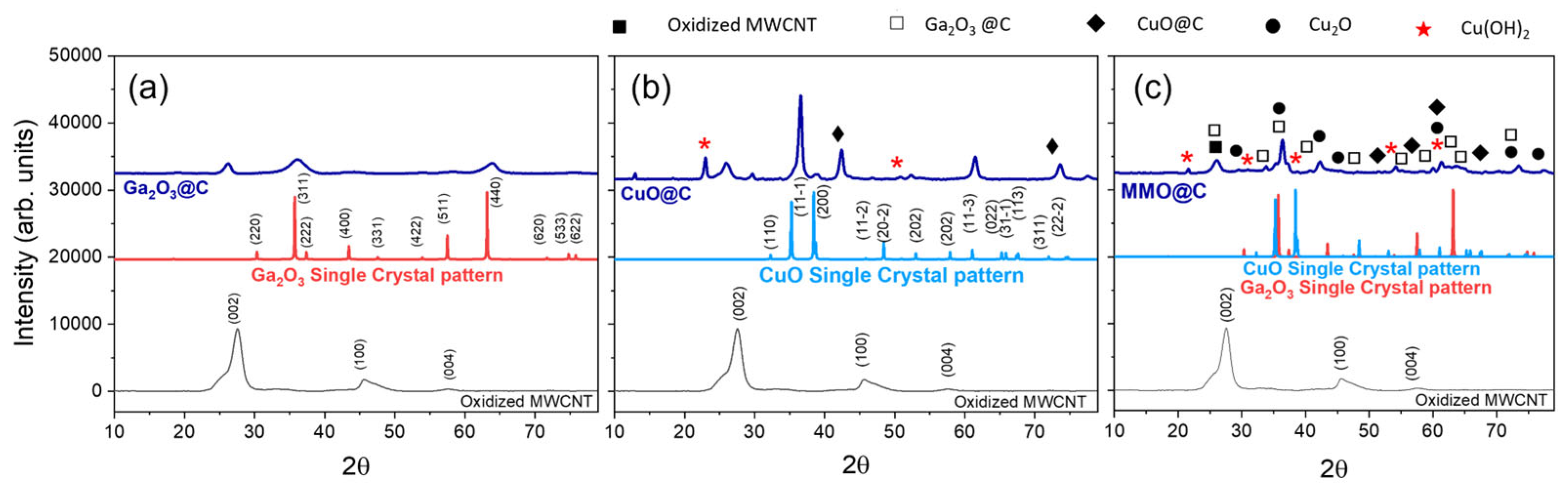
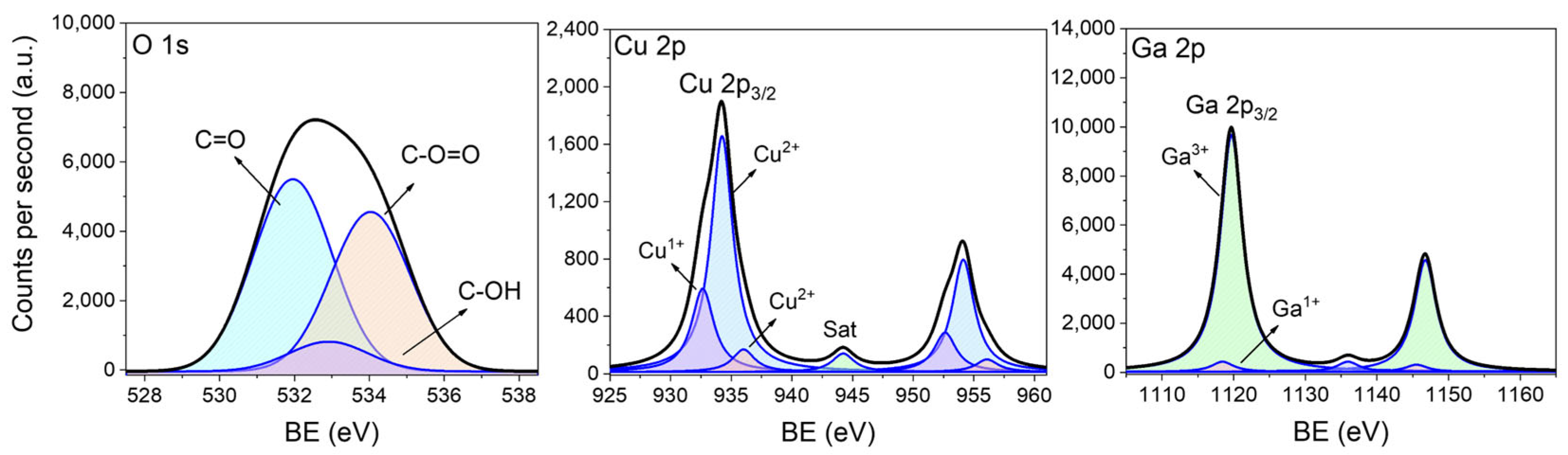

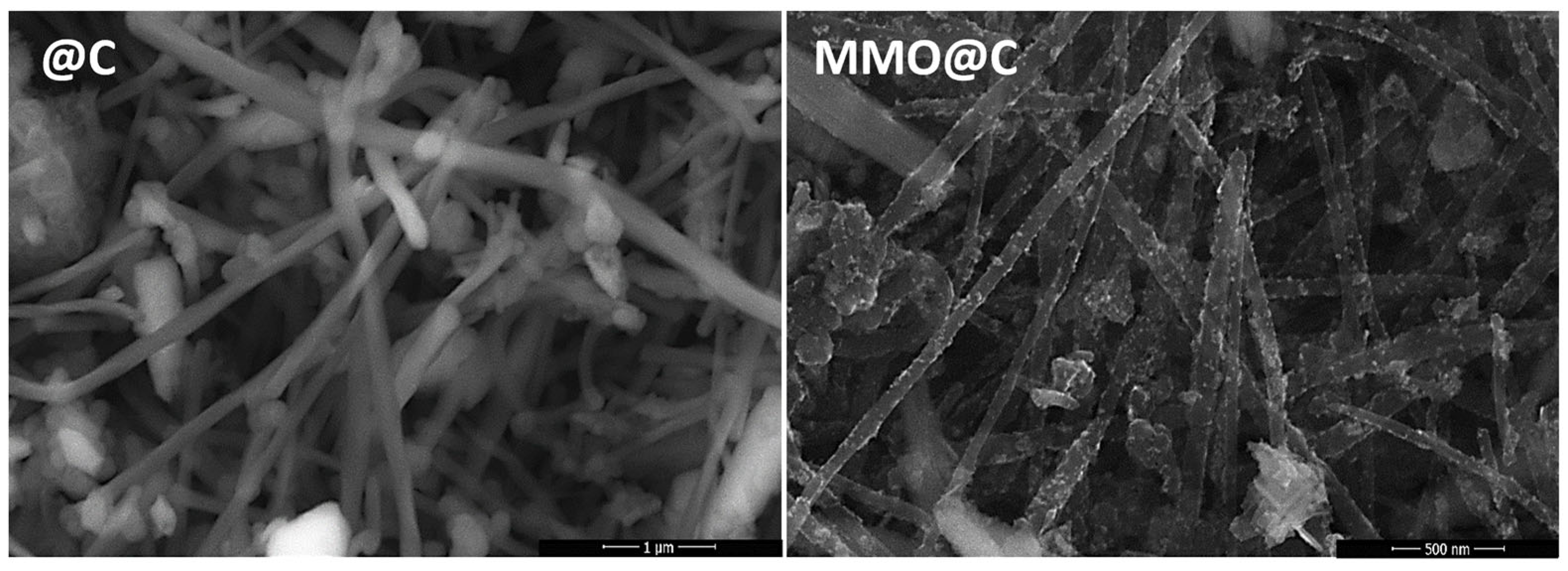
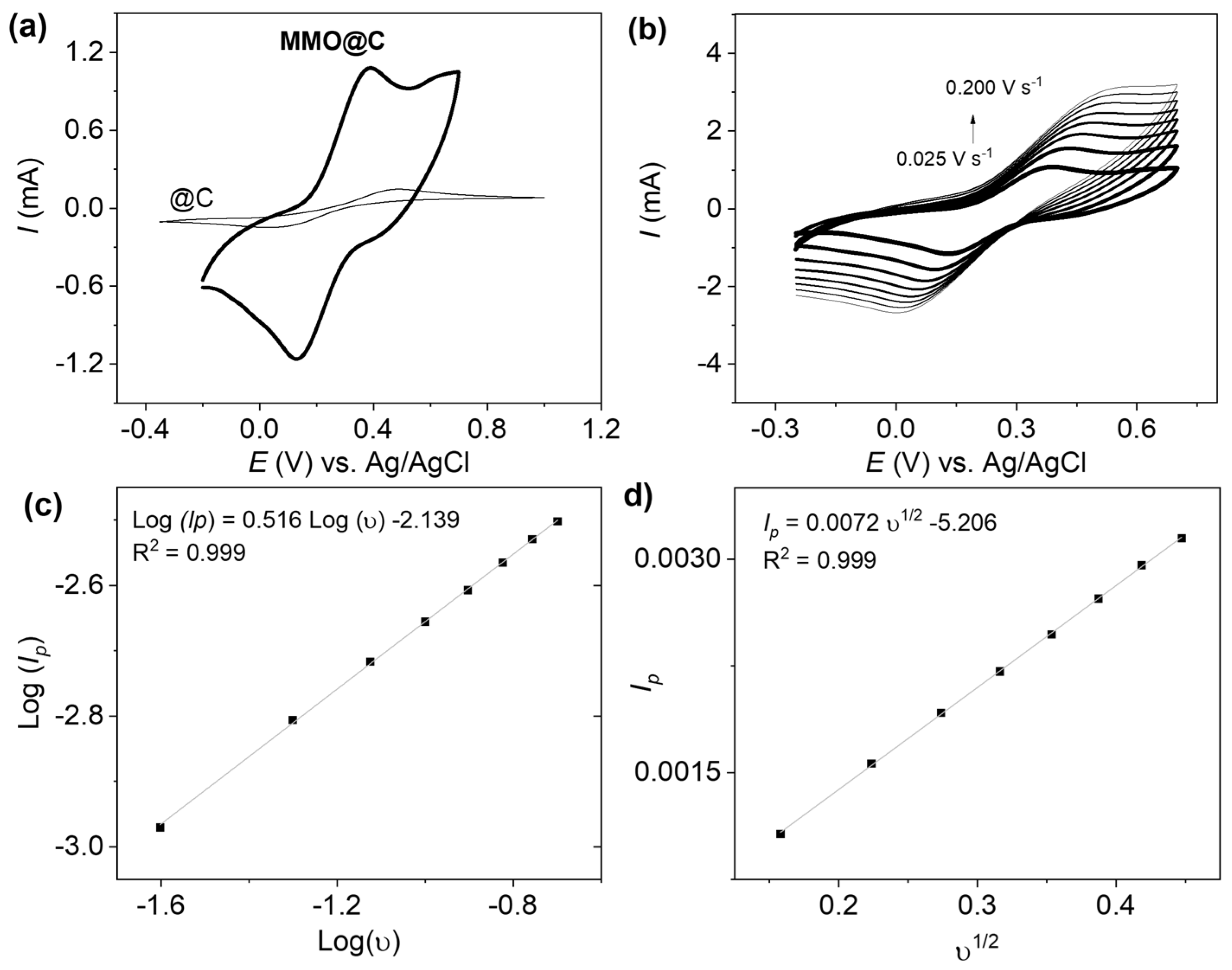

| Energy Level | Species | Binding Energy (eV) | Composition (%) |
|---|---|---|---|
| C 1s | C-C | 284.7 | 50 |
| C-O, C-OH | 286.2 | 28 | |
| C=O | 287.6 | 6 | |
| O-C=O | 288.9 | 16 | |
| O 1s | C=O | 532.0 | 57 |
| C-OH | 532.9 | 6 | |
| O-C=O | 534.0 | 37 | |
| Ga 2p | Ga1+ (Ga2O) | 1118.7 | 6 |
| Ga3+ (Ga2O3) | 1119.6 | 94 | |
| Cu 2p | Cu1+ (Cu2O) | 932.7 | 33 |
| Cu2+ (CuO) | 934.2 | 61 | |
| Cu2+ (Cu(OH)2) | 936.0 | 6 |
| C (%) | O (%) | Ga (%) | Cu (%) | Ga/Cu Atomic Ratio |
|---|---|---|---|---|
| 60 | 26 | 12 | 2 | 6 |
| Electrocatalyst | SBET (m2 g−1) | V Pore (cm3 g−1) | EO (V) vs. RHE, pH = 7.0 |
|---|---|---|---|
| @C | 13 | 0.017 | −0.90 |
| CuO@C | 22 | 0.047 | −0.38 |
| Ga2O3@C | 41 | 0.066 | −0.33 |
| MMO@C | 62 | 0.102 | +0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrientos, C.; Moris, S.; Arias, D.; Pecchi, G.; Ibarra, J.; Ramírez, G.; Gidi, L. New Paste Electrode Based on Copper and Gallium Mixed Metal Oxides-Decorated CNT for Highly Electrocatalyzed Hydrogen Evolution Reaction. Int. J. Mol. Sci. 2025, 26, 9057. https://doi.org/10.3390/ijms26189057
Barrientos C, Moris S, Arias D, Pecchi G, Ibarra J, Ramírez G, Gidi L. New Paste Electrode Based on Copper and Gallium Mixed Metal Oxides-Decorated CNT for Highly Electrocatalyzed Hydrogen Evolution Reaction. International Journal of Molecular Sciences. 2025; 26(18):9057. https://doi.org/10.3390/ijms26189057
Chicago/Turabian StyleBarrientos, Claudio, Silvana Moris, Dana Arias, Gina Pecchi, José Ibarra, Galo Ramírez, and Leyla Gidi. 2025. "New Paste Electrode Based on Copper and Gallium Mixed Metal Oxides-Decorated CNT for Highly Electrocatalyzed Hydrogen Evolution Reaction" International Journal of Molecular Sciences 26, no. 18: 9057. https://doi.org/10.3390/ijms26189057
APA StyleBarrientos, C., Moris, S., Arias, D., Pecchi, G., Ibarra, J., Ramírez, G., & Gidi, L. (2025). New Paste Electrode Based on Copper and Gallium Mixed Metal Oxides-Decorated CNT for Highly Electrocatalyzed Hydrogen Evolution Reaction. International Journal of Molecular Sciences, 26(18), 9057. https://doi.org/10.3390/ijms26189057







