Hybrid Electrospun Conductive Nanofibers for Emerging Organic Contaminants’ Degradation in Visible Light Photocatalysis: A Review
Abstract
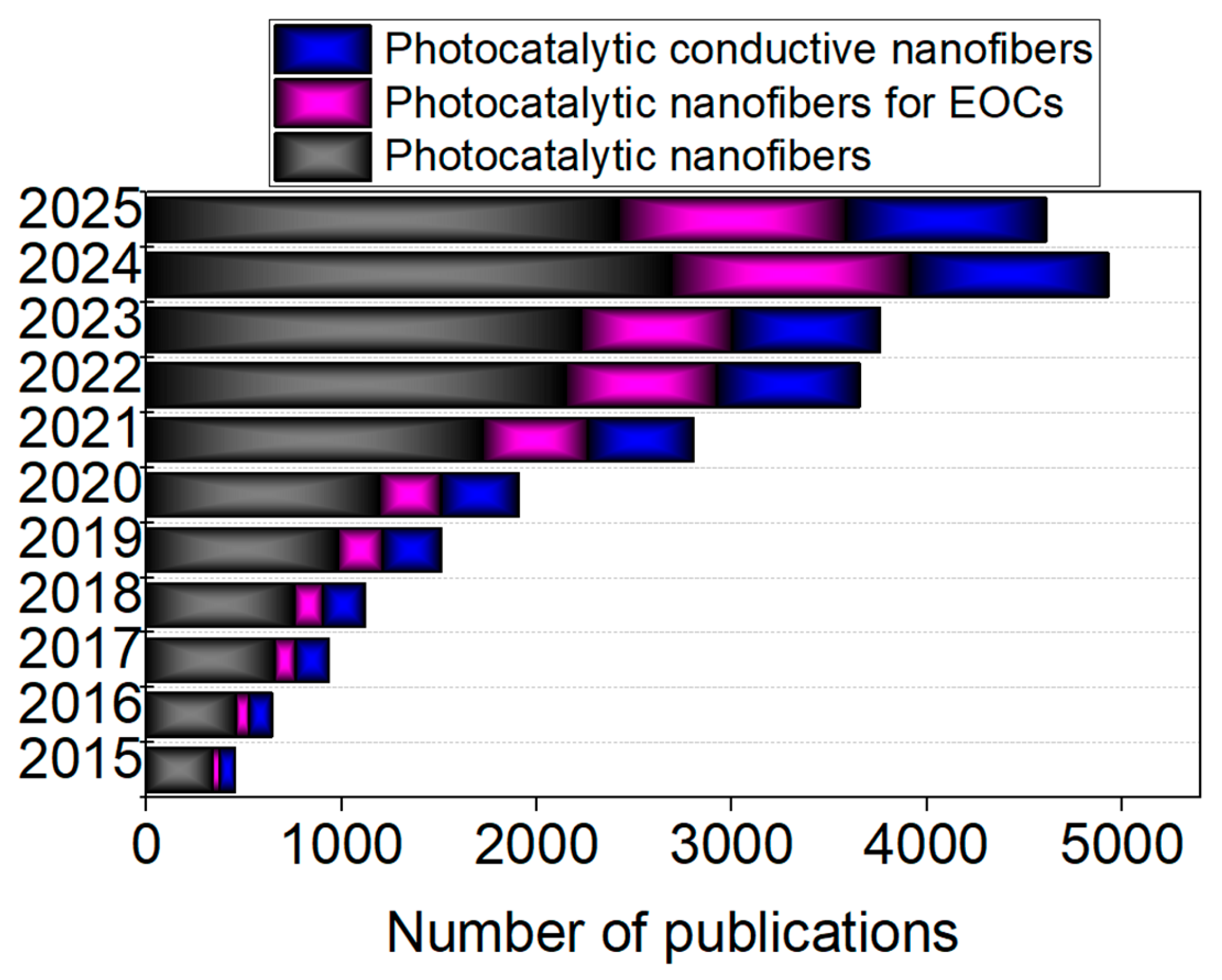
1. Introduction
2. Hybrid Electrospun Conductive Nanofibers for Photocatalysis Under VIS Light
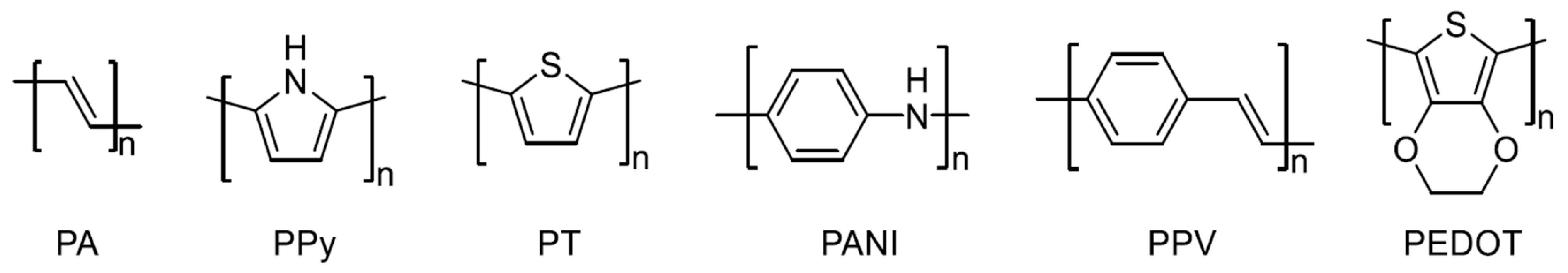
2.1. Conductive Polymer Semiconductors
2.2. Conductive-Polymer-Integrated Photocatalysts
2.3. Fabrication of Hybrid Electrospun Conductive Nanofibers
2.3.1. Electrospinning Process
2.3.2. Post-Electrospinning Surface Modification
2.3.3. Vacuum Self-Assembly
3. Photocatalytic Degradation Mechanism
3.1. Type-II Heterojunction Structure
3.2. Z-Scheme Heterojunction Structure
3.3. Schottky Junction
4. Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinç, B.; Çelebi, A.; Avaz, G.; Canl, O.; Güzel, B.; Eren, B.; Yetis, U. Spatial distribution and source identification of persistent organic pollutants in the sediments of the Yesilirmak River and coastal area in the Black Sea. Mar. Pollut. Bull. 2021, 172, 112884. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Duttagupta, S.; Mukherjee, A. Emerging organic contaminants in global community drinking water sources and supply: A review of occurrence, processes and remediation. J. Environ. Chem. Eng. 2022, 10, 107560. [Google Scholar] [CrossRef]
- Sudarsan, J.; Dogra, K.; Kumar, R.; Raval, N.; Leifels, M.; Mukherjee, S.; Trivedi, M.; Jain, M.; Zang, J.; Barceló, D.; et al. Tricks and tracks of prevalence, occurrences, treatment technologies, and challenges of mixtures of emerging contaminants in the environment: With special emphasis on microplastic. J. Contam. Hydrol. 2024, 265, 104389. [Google Scholar] [CrossRef]
- Kumar, M.; Bolan, N.; Hoang, S.; Sawarkar, A.; Jasemizad, T.; Gao, B.; Keerthanan, S.; Padhye, L.; Singh, L.; Kumar, S.; et al. Remediation of soils and sediments polluted with polycyclic aromatic hydrocarbons: To immobilize, mobilize, or degrade? J. Hazard. Mater. 2021, 420, 126534. [Google Scholar] [CrossRef]
- Balint, A.; Matei, E.; Rapa, M.; Saulean, A.; Mates, I. Human Exposure Estimation of Polycyclic Aromatic Hydrocarbons (PAHs) Resulting from Bucharest Landfill Leakages. Sustainability 2025, 17, 1356. [Google Scholar] [CrossRef]
- Rapa, M.; Darie-Nita, R.N.; Matei, E.; Predescu, A.M.; Berbecaru, A.C.; Predescu, C. Insights into Anthropogenic Micro- and Nanoplastic Accumulation in Drinking Water Sources and Their Potential Effects on Human Health. Polymers 2023, 15, 2425. [Google Scholar] [CrossRef] [PubMed]
- Ziyaei, K.; Mokhtari, M.; Hashemi, M.; Rezaei, K.; Abdi, F. Association between exposure to water sources contaminated with polycyclic aromatic hydrocarbons and cancer risk: A systematic review. Sci. Total Environ. 2024, 924, 2425. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Lorestani, B.; Ardakani, S.; Cheraghi, M.; Sadr, M. Source identification and health risk assessment of PAHs in surface soils from the vicinity of Arad-Kouh processing and disposal complex, Tehran, Iran. Int. J. Environ. Anal. Chem. 2023, 103, 9647–9660. [Google Scholar] [CrossRef]
- Yasir, M.; Ngwabebhoh, F.; Sopik, T.; Ali, H.; Sedlarik, V. Electrospun polyurethane nanofibers coated with polyaniline/polyvinyl alcohol as ultrafiltration membranes for the removal of ethinylestradiol hormone micropollutant from aqueous phase. J. Environ. Chem. Eng. 2022, 10, 107811. [Google Scholar] [CrossRef]
- Yu, X.; Tian, Y.; Wei, Y.; Wang, K.; Liu, Z.; Yang, F.; Chen, L.; Zhang, J. Constructing p-n heterojunctions of Cl-Cu2O and PANI on three-dimensional nanofiber networks for enhanced photocatalytic degradation of oxytetracycline. J. Alloys Compd. 2024, 1005, 175998. [Google Scholar] [CrossRef]
- Kumar, A.; Mittal, H.; Nagar, R.; Khanuja, M. The synergistic effect of acid-etched g-C3N4 nanosheets and polyaniline nanofibers for the adsorption and photocatalytic degradation of textile dyes: A study of charge transfer mechanism and intermediate products. Mater. Adv. 2022, 3, 5325–5336. [Google Scholar] [CrossRef]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of textile dyes in aquatic environment: Adverse impacts on aquatic ecosystem and human health, and its management using bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Duan, D.; Zhang, Z.; Liu, C.; Cai, W.; Zhao, Z. Environmental Impacts and Biological Technologies Toward Sustainable Treatment of Textile Dyeing Wastewater: A Review. Sustainability 2024, 16, 10867. [Google Scholar] [CrossRef]
- Kayani, K.; Mohammed, S.; Mustafa, M.; Aziz, S. Dyes and their toxicity: Removal from wastewater using carbon dots/metal oxides as hybrid materials: A review. Mater. Adv. 2025, 6, 5391–5409. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stud. Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Anirudhan, T.; Shainy, F.; Mohan, A. Fabrication of zinc oxide nanorod incorporated carboxylic graphene/polyaniline composite and its photocatalytic activity for the effective degradation of diuron from aqueous solutions. Sol. Energy 2018, 171, 534–546. [Google Scholar] [CrossRef]
- Pathak, V.; Verma, V.; Rawat, B.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.; Seibert, D.; Quesada, H.; Bassetti, F.; Fagundes-Klen, M.; Bergamasco, R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Singer, A.; Shaw, H.; Rhodes, V.; Hart, A. Review of Antimicrobial Resistance in the Environment and Its Relevance to Environmental Regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef]
- Horvat, O.; Kovacevic, Z. Human and Veterinary Medicine Collaboration: Synergistic Approach to Address Antimicrobial Resistance Through the Lens of Planetary Health. Antibiotics 2025, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Mogashane, T.; Maree, J.; Mokoena, L. Adsorption of Polycyclic Aromatic Hydrocarbons from Wastewater Using Iron Oxide Nanomaterials Recovered from Acid Mine Water: A Review. Minerals 2024, 14, 826. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, L.; Moghaddam, T.; Chen, M.; Wu, S.; Yuan, X. Adsorption mechanism of polycyclic aromatic hydrocarbons using wood waste-derived biochar. J. Hazard. Mater. 2022, 425, 128003. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizade-shirvan, A.; Mohammadi, M.; Mazaheri, Y.; Fallahizadeh, S.; Ghorbani, H.; Shokri, S.; Shariatifar, N.; Darroudi, M.; Shamloo, E. Employing a magnetic chitosan/molybdenum disulfide nanocomposite for efficiently removing polycyclic aromatic hydrocarbons from milk samples. Sci. Rep. 2024, 14, 15054. [Google Scholar] [CrossRef]
- Chen, W.; Jia, Y.; Liu, A.; Zhou, Q.; Song, L. Simultaneous elimination of cyanotoxins and PCBs via mechanical collection of cyanobacterial blooms: An application of “green-bioadsorption concept”. J. Environ. Sci. 2017, 57, 118–126. [Google Scholar] [CrossRef]
- Badea, S.; Niculescu, V. Recent Progress in the Removal of Legacy and Emerging Organic Contaminants from Wastewater Using Metal-Organic Frameworks: An Overview on Adsorption and Catalysis Processes. Materials 2022, 15, 3850. [Google Scholar] [CrossRef]
- Kathi, S.; Mahmoud, A. Trends in effective removal of emerging contaminants from wastewater: A comprehensive review. Desalination Water Treat. 2024, 317, 100258. [Google Scholar] [CrossRef]
- Matei, E.; Covaliu-Mierla, C.I.; Turcanu, A.A.; Rapa, M.; Predescu, A.M.; Predescu, C. Multifunctional Membranes-A Versatile Approach for Emerging Pollutants Removal. Membranes 2022, 12, 67. [Google Scholar] [CrossRef]
- Bobirica, C.; Bobirica, L.; Rapa, M.; Matei, E.; Predescu, A.M.; Orbeci, C. Photocatalytic Degradation of Ampicillin Using PLA/TiO2 Hybrid Nanofibers Coated on Different Types of Fiberglass. Water 2020, 12, 176. [Google Scholar] [CrossRef]
- Magro, C.; Mateus, E.; Paz-Garcia, J.; Ribeiro, A. Emerging organic contaminants in wastewater: Understanding electrochemical reactors for triclosan and its by-products degradation. Chemosphere 2020, 247, 125758. [Google Scholar] [CrossRef]
- Rayaroth, M.; Marchel, M.; Boczkaj, G. Advanced oxidation processes for the removal of mono and polycyclic aromatic hydrocarbons—A review. Sci. Total Environ. 2023, 857, 159043. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, Y.; Yao, J.; Kong, D.; Chu, M.; Zhang, G.; Wang, Y. Enhancing dechlorination and inhibiting H2 evolution: Surface N-hydroxymethylation of Pd-loaded carbon nitride for photocatalytic elimination of polychlorinated aromatics. J. Catal. 2024, 438, 115732. [Google Scholar] [CrossRef]
- Yan, Z.; Örmeci, B.; Han, Y.; Zhang, J. Supercritical water oxidation for treatment of wastewater sludge and recalcitrant organic contaminants. Environ. Technol. Innov. 2020, 18, 100728. [Google Scholar] [CrossRef]
- Jing, R.; Fusi, S.; Kjellerup, B. Remediation of Polychlorinated Biphenyls (PCBs) in Contaminated Soils and Sediment: State of Knowledge and Perspectives. Front. Environ. Sci. 2018, 6, 189–202. [Google Scholar] [CrossRef]
- Divine, C.; March, L.; Kalra, S.; Hurst, J. Sonolysis and Super Critical Water Oxidation (SCWO): Development Maturity and Potential for Destroying PFAS. Ground Water Monit. Remediat. 2023, 43, 18–33. [Google Scholar] [CrossRef]
- Tufail, A.; Price, W.; Mohseni, M.; Pramanik, B.; Hai, F. A critical review of advanced oxidation processes for emerging trace organic contaminant degradation: Mechanisms, factors, degradation products, and effluent toxicity. J. Water Process Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Olasupo, A.; Corbin, D.; Shiflett, M. Trends in low temperature and non-thermal technologies for the degradation of persistent organic pollutants. J. Hazard. Mater. 2024, 468, 133830. [Google Scholar] [CrossRef]
- Bensadi, L.; Azzoug, M.; Benlaribi, R. Occurrence, sources and risk assessment of 12 dioxin-like polychlorinated biphenyls (DL-PCBs) in sediments from the Soummam River protected area, Algeria: A potential threat to the Mediterranean Sea. Mar. Pollut. Bull. 2024, 208, 117003. [Google Scholar] [CrossRef]
- Qu, X.; Niu, Q.; Sheng, C.; Xia, M.; Zhang, C.; Qu, X.; Yang, C. Co-toxicity and co-contamination remediation of polycyclic aromatic hydrocarbons and heavy metals: Research progress and future perspectives. Environ. Res. 2024, 263, 120211. [Google Scholar] [CrossRef]
- Rodríguez, C.; Amaya-Chávez, A.; Roa-Morales, G.; Barrera-Díaz, C.; Ureña-Núñez, F. An Integrated Electrocoagulation-Phytoremediation Process for the Treatment of Mixed Industrial Wastewater. Int. J. Phytoremediation 2010, 12, 772–784. [Google Scholar] [CrossRef]
- Peterson, E.; Summers, R. Removal of effluent organic matter with biofiltration for potable reuse: A review and meta-analysis. Water Res. 2021, 199, 117180. [Google Scholar] [CrossRef]
- Guo, W.; Ren, H.; Jin, Y.; Chai, Z.; Liu, B. The bioremediation of the typical persistent organic pollutants (POPs) by microalgae-bacteria consortia: A systematic review. Chemosphere 2024, 355, 141852. [Google Scholar] [CrossRef] [PubMed]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Ramísio, P.; Puga, H. A Comprehensive Review on Various Phases of Wastewater Technologies: Trends and Future Perspectives. Eng 2024, 5, 2633–2661. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Chen, C.; Zhou, X.; Liu, Y.; Yang, J.; Geng, Q.; Chen, G.; Ding, Y.; Yang, F. Current Progress in Natural Degradation and Enhanced Removal Techniques of Antibiotics in the Environment: A Review. Int. J. Environ. Res. Public health 2022, 19, 10919. [Google Scholar] [CrossRef]
- Huang, S.; Yu, J.; Li, C.; Zhu, Q.; Zhang, Y.; Lichtfouse, E.; Marmier, N. The Effect Review of Various Biological, Physical and Chemical Methods on the Removal of Antibiotics. Water 2022, 14, 3138. [Google Scholar] [CrossRef]
- Chakraborty, U.; Kaur, G.; Rubahn, H.; Kaushik, A.; Chaudhary, G.; Mishra, Y. Advanced metal oxides nanostructures to recognize and eradicate water pollutants. Prog. Mater. Sci. 2023, 139, 101169. [Google Scholar] [CrossRef]
- Moradeeya, P.G.; Kumar, M.A.; Sharma, A.; Basha, S. Conductive polymer layered semiconductor for degradation of triclopyr acid and 2,4-dichlorophenoxyacetic acid from aqueous stream using coalesce adsorption-photocatalysis technique. Chemosphere 2022, 298, 134360. [Google Scholar] [CrossRef]
- Wang, W.; Yang, R.; Li, T.; Komarneni, S.; Liu, B. Advances in recyclable and superior photocatalytic fibers: Material, construction, application and future perspective. Compos. Part B-Eng. 2021, 205, 108512. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, F.; Shen, Y.; Duan, X.; Zhao, S.; Chen, X.; Liang, J. Removal of Emerging Organic Pollutants by Zeolite Mineral (Clinoptilolite) Composite Photocatalysts in Drinking Water and Watershed Water. Catalysts 2024, 14, 216, Correction in Catalysts 2024, 24, 310. [Google Scholar] [CrossRef]
- Feng, H.; Liang, L.; Wu, W.; Huang, Z.; Liu, Y. Architecting epitaxial-lattice-mismatch-free (LMF) zinc oxide/bismuth oxyiodide nano-heterostructures for efficient photocatalysis. J. Mater. Chem. C 2020, 8, 11263–11273. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Conlan, X.; Laleh, M.; Kong, L.; O’Dell, L.; Dumee, L.; Merenda, A. Atomically-thin Schottky-like photo-electrocatalytic cross-flow membrane reactors for ultrafast remediation of persistent organic pollutants. Water Res. 2022, 218, 118519. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Li, Y.; Li, D.; Qiu, J.; Xu, X.; Yang, H. A review of metal-organic framework (MOF) materials as an effective photocatalyst for degradation of organic pollutants. Nanoscale Adv. 2023, 5, 6318–6348. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Lu, X.; Deka, B.; Shang, J.; Wu, H.; Sun, J.; Yi, C.; Farid, M.; An, A.; Guo, J. Research progress in the preparation of electrospinning MOF nanofiber membranes and applications in the field of photocatalysis. Sep. Purif. Technol. 2025, 356, 129948. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, C.; Li, X.; Yang, Q.; Wang, D.; Duan, A.; Ding, J.; Rong, S.; Chen, Z.; Luo, J.; et al. Enhanced photodegradation of tetracycline hydrochloride by hexameric AgBr/Zn-Al MMO S-scheme heterojunction photocatalysts: Low metal leaching, degradation mechanism and intermediates. Chem. Eng. J. 2022, 446, 137371. [Google Scholar] [CrossRef]
- Wang, X.; Yu, G.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Acosta, M.; Santiago, M.; Irvin, J. Electrospun Conducting Polymers: Approaches and Applications. Materials 2022, 15, 8820. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Sundriyal, S.; Maheshwari, P. Sustainable Nanoporous Metal-Organic Framework/Conducting Polymer Composites for Supercapacitor Applications. ACS Appl. Nano Mater. 2024, 7, 18554–18565. [Google Scholar] [CrossRef]
- Folorunso, O.; Olukanmi, P.; Thokozani, S. Conductive polymers’ electronic structure modification for multifunctional applications. Mater. Today Commun. 2023, 35, 106308. [Google Scholar] [CrossRef]
- Massaglia, G.; Chiodoni, A.; Marasso, S.; Pirri, C.; Quaglio, M. Electrical Conductivity Modulation of Crosslinked Composite Nanofibers Based on PEO and PEDOT:PSS. J. Nanomater. 2018, 2018, 3286901. [Google Scholar] [CrossRef]
- Dulgerbaki, C.; Maslakci, N.; Komur, A.; Oksuz, A. Electrochromic strategy for tungsten oxide/polypyrrole hybrid nanofiber materials. Eur. Polym. J. 2018, 107, 173–180. [Google Scholar] [CrossRef]
- Chen, C.; Huang, S.; Wan, H.; Chen, Y.; Yu, S.; Wu, H.; Yang, T. Electrospun Hydrophobic Polyaniline/Silk Fibroin Electrochromic Nanofibers with Low Electrical Resistance. Polymers 2020, 12, 2102. [Google Scholar] [CrossRef]
- Capilli, G.; Sartori, D.; Gonzalez, M.; Laurenti, E.; Minero, C.; Calza, P. Non-purified commercial multiwalled carbon nanotubes supported on electrospun polyacrylonitrile@polypyrrole nanofibers as photocatalysts for water decontamination. RSC Adv. 2021, 11, 9911–9920. [Google Scholar] [CrossRef]
- Capilli, G.; Calza, P.; Minero, C.; Cerruti, M. Electrospun core-sheath PAN@PPY nanofibers decorated with ZnO: Photo-induced water decontamination enhanced by a semiconducting support. J. Mater. Chem. A 2019, 7, 26429–26441. [Google Scholar] [CrossRef]
- Armakovic, S.; Armakovic, S.; Savanovic, M. Photocatalytic Application of Polymers in Removing Pharmaceuticals from Water: A Comprehensive Review. Catalysts 2024, 14, 447. [Google Scholar] [CrossRef]
- Wang, A.; Wang, R.; Pan, Y.; Ni, J.; Liang, X.; Du, M.; Zhang, J.; Liu, D.; Ma, J.; Wang, J.; et al. High conductive polymer PANI link Bi2MoO6 and PBA to establish “tandem hybrid catalysis system” by coupling photocatalysis and PMS activation technology. Appl. Catal. B-Environ. Energy 2024, 344, 123621. [Google Scholar] [CrossRef]
- Ajmal, Z.; Naciri, Y.; Ahmad, M.; Hsini, A.; Bouziani, A.; Laabd, M.; Raza, W.; Murtaza, A.; Kumar, A.; Ullah, S.; et al. Use of conductive polymer-supported oxide-based photocatalysts for efficient VOCs & SVOCs removal in gas/liquid phase. J. Environ. Chem. Eng. 2023, 11, 108935. [Google Scholar] [CrossRef]
- Ali, F.; Dawood, A.; Hussain, A.; Koka, N.; Khan, M.; Khan, M.; Asim, M.; Janjua, N.; Nasir, M.; Jabeen, Z.; et al. PANI-based nanocomposites synthetic methods, properties, and catalytic applications. Inorg. Chem. Commun. 2024, 161, 112077. [Google Scholar] [CrossRef]
- Hajjaoui, H.; Soufi, A.; Boumya, W.; Abdennouri, M.; Barka, N. Polyaniline/Nanomaterial Composites for the Removal of Heavy Metals by Adsorption: A Review. J. Compos. Sci. 2021, 5, 233. [Google Scholar] [CrossRef]
- Cui, Z.; Yuan, R.; Chen, H.; Zhou, B.; Zhu, B.; Zhang, C. Application of polyaniline-based photocatalyst in photocatalytic degradation of micropollutants in water: A review. J. Water Process Eng. 2024, 59, 104900. [Google Scholar] [CrossRef]
- Liu, Z.; Miao, Y.; Liu, M.; Ding, Q.; Tjiu, W.; Cui, X.; Liu, T. Flexible polyaniline-coated TiO2/SiO2 nanofiber membranes with enhanced visible-light photocatalytic degradation performance. J. Colloid Interface Sci. 2014, 424, 49–55. [Google Scholar] [CrossRef]
- Liu, J.; McCarthy, D.; Tong, L.; Cowan, M.; Kinsley, J.; Sonnenberg, L.; Skorenko, K.; Boyer, S.; DeCoste, J.; Bernier, W.; et al. Poly(3,4-ethylenedioxythiophene) (PEDOT) infused TiO2 nanofibers: The role of hole transport layer in photocatalytic degradation of phenazopyridine as a pharmaceutical contaminant. RSC Adv. 2016, 6, 113884–113892. [Google Scholar] [CrossRef]
- Visan, A.; Covaliu, L.; Covaliu-Mierla, C. Removal from Wastewater of Benzethonium Chloride (Bzt) by Photocatalysis. Univ. Politeh. Buchar. Sci. Bull. Ser. B-Chem. Mater. Sci. 2024, 86, 139–146. [Google Scholar]
- Coman, V.; Deak, G.; Matei, E.; Predescu, A.M.; Râpă, M.; Berbecaru, A.; Predescu, C. A way to reduce the emerging pollutants impact on environment using nano-tio2 as photocatalyst. UPB Sci. Bull. Ser. B: Chem. Mater. Sci. 2021, 83, 245–252. [Google Scholar]
- Irodia, R. Enhanced Photocatalytic Degradation of Tetracycline by TiO2 Nanotubes Coated with TiO2 Nanofibers. Univ. Politeh. Buchar. Sci. Bull. Ser. B-Chem. Mater. Sci. 2023, 85, 63–76. [Google Scholar]
- Yang, Y.; Wen, J.; Wei, J.; Xiong, R.; Shi, J.; Pan, C. Polypyrrole-Decorated Ag-TiO2 Nanofibers Exhibiting Enhanced Photocatalytic Activity under Visible-Light Illumination. ACS Appl. Mater. Interfaces 2013, 5, 6201–6207. [Google Scholar] [CrossRef]
- Liu, S.; Wu, D.; Hu, J.; Zhao, L.; Zhao, L.; Yang, M.; Feng, Q. Electrospun flexible core-sheath PAN/PU/β-CD@Ag nanofiber membrane decorated with ZnO: Enhance the practical ability of semiconductor photocatalyst. Environ. Sci. Pollut. Res. 2022, 29, 39638–39648. [Google Scholar] [CrossRef]
- Kalikeri, S.; Kamath, N.; Gadgil, D.; Kodialbail, V. Visible light-induced photocatalytic degradation of Reactive Blue-19 over highly efficient polyaniline-TiO2 nanocomposite: A comparative study with solar and UV photocatalysis. Environ. Sci. Pollut. Res. 2018, 25, 3731–3744. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Y.; Wang, J.; Zhang, M.; Luo, W.; Zhu, Y. Separation-Free Polyaniline/TiO2 3D Hydrogel with High Photocatalytic Activity. Adv. Mater. Interfaces 2016, 3, 1500502. [Google Scholar] [CrossRef]
- Wang, P.; Shan, Q.; Liu, L.; Zhao, C.; Chen, L. Preparation, characterization and photocatalytic performance of polyoxometalate/polyaniline/titania ternary composite. J. Coord. Chem. 2018, 71, 457–467. [Google Scholar] [CrossRef]
- Cui, W.; He, J.; Wang, H.; Hu, J.; Liu, L.; Liang, Y. Polyaniline hybridization promotes photo-electro-catalytic removal of organic contaminants over 3D network structure of rGH-PANI/TiO2 hydrogel. Appl. Catal. B-Environ. 2018, 232, 232–245. [Google Scholar] [CrossRef]
- Alenizi, M.; Kumar, R.; Aslam, M.; Alseroury, F.; Barakat, M. Construction of a ternary g-C3N4/TiO2@polyaniline nanocomposite for the enhanced photocatalytic activity under solar light. Sci. Rep. 2019, 9, 12091. [Google Scholar] [CrossRef]
- Faisal, M.; Rashed, M.; Ahmed, J.; Alsaiari, M.; Alkorbi, A.; Jalalah, M.; Alsareii, S.; Harraz, F. Rapid photodegradation of linezolid antibiotic and methylene blue dye over Pt nanoparticles/polypyrrole-carbon black/ZnO novel visible light photocatalyst. J. Environ. Chem. Eng. 2021, 9, 106773. [Google Scholar] [CrossRef]
- Zhou, T.; Zhao, L.; Wu, D.; Feng, Q.; Zhao, B. Uniformly assembled polypyrrole-covered bacterial cellulose/g-C3N4 flexible nanofiber membrane for catalytic degradation of tetracycline hydrochloride. J. Water Process Eng. 2022, 47, 102775. [Google Scholar] [CrossRef]
- Asadpoor, R.; Habibi, D.; Aghababaei, N. Integrated dual Z-scheme n-n-p/Schottky junctions upon Ni-ZnO/Bi2WO6/PANI for increased photocatalytic ciprofloxacin degradation under visible light. Sep. Purif. Technol. 2025, 363, 132060. [Google Scholar] [CrossRef]
- Ghosh, S.; Mallik, A.; Basu, R. Enhanced photocatalytic activity and photoresponse of poly(3,4-ethylenedioxythiophene) nanofibers decorated with gold nanoparticle under visible light. Sol. Energy 2018, 159, 548–560. [Google Scholar] [CrossRef]
- Liu, F.; Nguyen, T.; Wang, Q.; Massuyeau, F.; Dan, Y.; Jiang, L. Construction of Z-scheme g-C3N4/Ag/P3HT heterojunction for enhanced visible-light photocatalytic degradation of tetracycline (TC) and methyl orange (MO). Appl. Surf. Sci. 2019, 496, 143653. [Google Scholar] [CrossRef]
- Lin, L.; He, Q.; Chen, Y.; Wang, B.; Zhang, L.; Dai, X.; Jiang, Y.; Chen, H.; Liao, J.; Mao, Y.; et al. MoS2/polyaniline (PANI)/polyacrylonitrile (PAN)@BiFeO3 bilayer hollow nanofiber membrane: Photocatalytic filtration and piezoelectric effect enhancing degradation and disinfection. J. Colloid Interface Sci. 2023, 644, 29–41. [Google Scholar] [CrossRef]
- Mao, Y.; Lin, L.; Chen, Y.; Yang, M.; Zhang, L.; Dai, X.; He, Q.; Jiang, Y.; Chen, H.; Liao, J.; et al. Preparation of site-specific Z-scheme g-C3N4/PAN/PANI@LaFeO3 cable nanofiber membranes by coaxial electrospinning: Enhancing filtration and photocatalysis performance. Chemosphere 2023, 328, 138553. [Google Scholar] [CrossRef]
- Li, L.; Wang, P.; Wang, M.; Yan, H.; Liu, X.; Tong, L.; Li, Y.; Sun, Y.; Li, K.; Yang, X.; et al. Facile dual-functionalization of PEN nanofibrous membranes with solar evaporation and photocatalysis for efficient dye wastewater purification. J. Environ. Chem. Eng. 2024, 12, 113585. [Google Scholar] [CrossRef]
- Asgari, S.; Ziarani, G.; Badiei, A.; Vasseghian, Y. A ternary composite nanofibrous photocatalyst: FcLR-gC3N4/polyisothianaphthene/polyacrylonitrile for degradation of organic dyes. J. Taiwan Inst. Chem. Eng. 2024, 163, 105672. [Google Scholar] [CrossRef]
- Sun, F.; Xie, Y.; Xu, D.; Liu, F.; Qi, H.; Ma, Q.; Yang, Y.; Yu, H.; Yu, W.; Dong, X. Electrospun self-supporting double Z-scheme tricolor-typed microfiber oriented-heterostructure photocatalyst with highly effective hydrogen evolution and organic pollutants degradation. J. Environ. Chem. Eng. 2023, 11, 109169. [Google Scholar] [CrossRef]
- Zia, J.; Riaz, U. Photocatalytic degradation of water pollutants using conducting polymer-based nanohybrids: A review on recent trends and future prospects. J. Mol. Liq. 2021, 340, 117162. [Google Scholar] [CrossRef]
- Omer, S.; Forgách, L.; Zelkó, R.; Sebe, I. Scale-up of Electrospinning: Market Overview of Products and Devices for Pharmaceutical and Biomedical Purposes. Pharmaceutics 2021, 13, 286. [Google Scholar] [CrossRef]
- Forgie, J.; Leclinche, F.; Dréan, E.; Dolez, P. Electrospinning of High-Performance Nanofibres: State of the Art and Insights into the Path Forward. Appl. Sci. 2023, 13, 12476. [Google Scholar] [CrossRef]
- Badmus, M.; Liu, J.; Wang, N.; Radacsi, N.; Zhao, Y. Hierarchically electrospun nanofibers and their applications: A review. Nano Mater. Sci. 2021, 3, 213–232. [Google Scholar] [CrossRef]
- Cordoba, A.; Saldias, C.; Urzúa, M.; Montalti, M.; Guernelli, M.; Focarete, M.; Leiva, A. On the Versatile Role of Electrospun Polymer Nanofibers as Photocatalytic Hybrid Materials Applied to Contaminated Water Remediation: A Brief Review. Nanomaterials 2022, 12, 756. [Google Scholar] [CrossRef]
- Arshad, Z.; Ali, M.; Lee, E.; Alshareef, M.; Alsowayigh, M.; Shahid, K.; Shahid, R.; Lee, K. Comparison of Electrospun Titania and Zinc Oxide Nanofibers for Perovskite Solar Cells and Photocatalytic Degradation of Methyl Orange Dye. Catalysts 2023, 13, 1062. [Google Scholar] [CrossRef]
- Yar, A.; Haspulat, B.; Üstün, T.; Eskizeybek, V.; Avci, A.; Kamis, H.; Achour, S. Electrospun TiO2/ZnO/PAN hybrid nanofiber membranes with efficient photocatalytic activity. RSC Adv. 2017, 7, 29806–29814. [Google Scholar] [CrossRef]
- Samadi, M.; Moshfegh, A. Recent Developments of Electrospinning-Based Photocatalysts in Degradation of Organic Pollutants: Principles and Strategies. ACS Omega 2022, 7, 45867–45881. [Google Scholar] [CrossRef] [PubMed]
- Mapukata, S.; Shingange, K.; Mokhena, T. Review of the recent advances on the fabrication, modification and application of electrospun TiO2 and ZnO nanofibers for the treatment of organic pollutants in wastewater. Front. Chem. Eng. 2023, 5, 1304128. [Google Scholar] [CrossRef]
- Regmi, D.; Choi, J.; Xu, J. Electrospinning of Heterogeneous Nanofibers: A Review. ECS Adv. 2024, 3, 041001. [Google Scholar] [CrossRef]
- Nayl, A.; Abd-Elhamid, A.; Awwad, N.; Abdelgawad, M.; Wu, J.; Mo, X.; Gomha, S.; Aly, A.; Bräse, S. Review of the Recent Advances in Electrospun Nanofibers Applications in Water Purification. Polymers 2022, 14, 1594. [Google Scholar] [CrossRef]
- Wei, X.; Guo, Q.; Li, Y.; Zheng, L.; Li, Z.; Zhang, K.; Yuan, C. Reusable electrospun carbon nanofiber composite for selective removal of inorganic arsenic species in water. Environ. Pollut. Bioavailab. 2021, 33, 184–193. [Google Scholar] [CrossRef]
- Fahanwi, A.; Yasir, M.; Nguyen, H.; Saha, N.; Saha, T.; Sedlarik, V.; Saha, P. In situ polyaniline polymerization on electrospun cellulose acetate nanofibers derived from recycled waste filter butts of cigarettes for the enhanced removal of methyl orange and rhodamine. Chem. Eng. Res. Des. 2024, 201, 18–30. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Conductive Electrospun Nanofiber Mats. Materials 2020, 13, 152. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, Q.; Yang, Y.; Wu, T.; Qiang, M.; Que, W. Elastic and Conductive Photocatalytic Membrane. Adv. Sustain. Syst. 2025, 9, 2401024. [Google Scholar] [CrossRef]
- Shakiba, M.; Nabavi, S.; Emadi, H.; Faraji, M. Development of a superhydrophilic nanofiber membrane for oil/water emulsion separation via modification of polyacrylonitrile/polyaniline composite. Polym. Adv. Technol. 2021, 32, 1301–1316. [Google Scholar] [CrossRef]
- Bhadra, J.; Parangusan, H.; Popelka, A.; Lehocky, M.; Humpolicek, P.; Al-Thani, N. Electrospun Polystyrene/PANI-Ag fibers for organic dye removal and antibacterial application. J. Environ. Chem. Eng. 2020, 8, 103746. [Google Scholar] [CrossRef]
- Jiang, M.; Li, M.; Zhang, X.; Zhu, W.; Liang, X. Microenvironment regulation of electropolymerized Thiophene-Based conductive polymers for enhanced electrocatalyzed hydrogen evolutions and oxygen reductions. J. Electroanal. Chem. 2024, 965, 118344. [Google Scholar] [CrossRef]
- Badsha, I.; Rasal, R.; Gangasalam, A.; Thiyagarajan, D. Modification of electrospun polyacrylonitrile nanofiber membranes with curcumin quantum dots for enhanced self-cleaning, antifouling and photocatalytic performance for water treatment. J. Water Process Eng. 2024, 60, 105251. [Google Scholar] [CrossRef]
- Jiang, B.R.; Kauffman, A.E.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; Scott, G.I.; Xiao, S. Health impacts of environmental contamination of micro- and nanoplastics: A review. Environ. Health Prev. Med. 2020, 25, 29. [Google Scholar] [CrossRef]
- Râpa, M.; Cârstea, E.M.; Saulean, A.A.; Popa, C.L.; Matei, E.; Predescu, A.M.; Predescu, C.; Dontu, S.I.; Dinca, A.G. An Overview of the Current Trends in Marine Plastic Litter Management for a Sustainable Development. Recycling 2024, 9, 30. [Google Scholar] [CrossRef]
- Stancu, A.; Râpa, M.; Popa, C.; Dontu, S.; Matei, E.; Covaliu-Mirela, C. Degradation of Emerging Plastic Pollutants from Aquatic Environments Using TiO2 and Their Composites in Visible Light Photocatalysis. Molecules 2025, 30, 3186. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C-Photochem. Rev. 2017, 31, 1–35. [Google Scholar] [CrossRef]


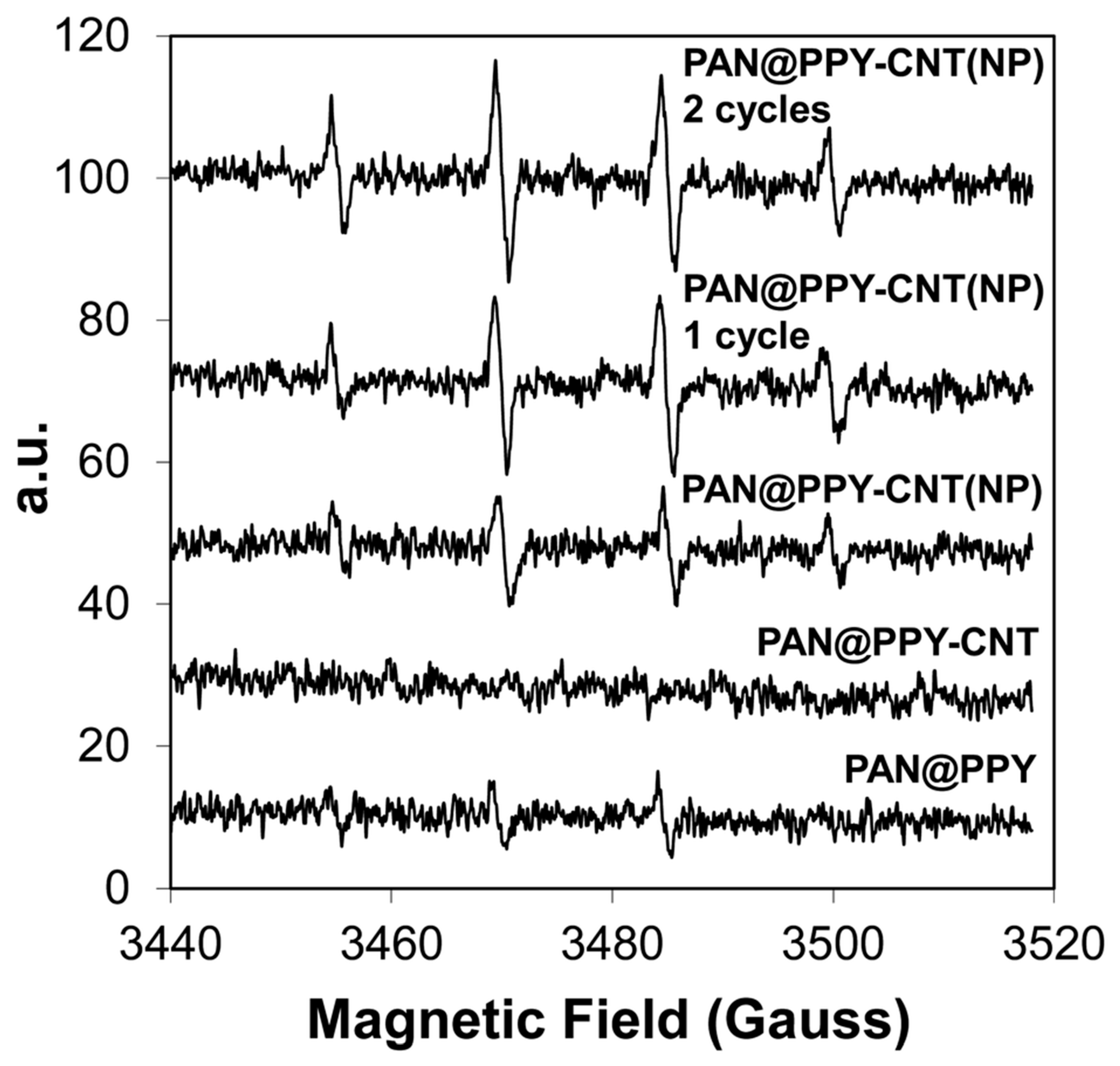


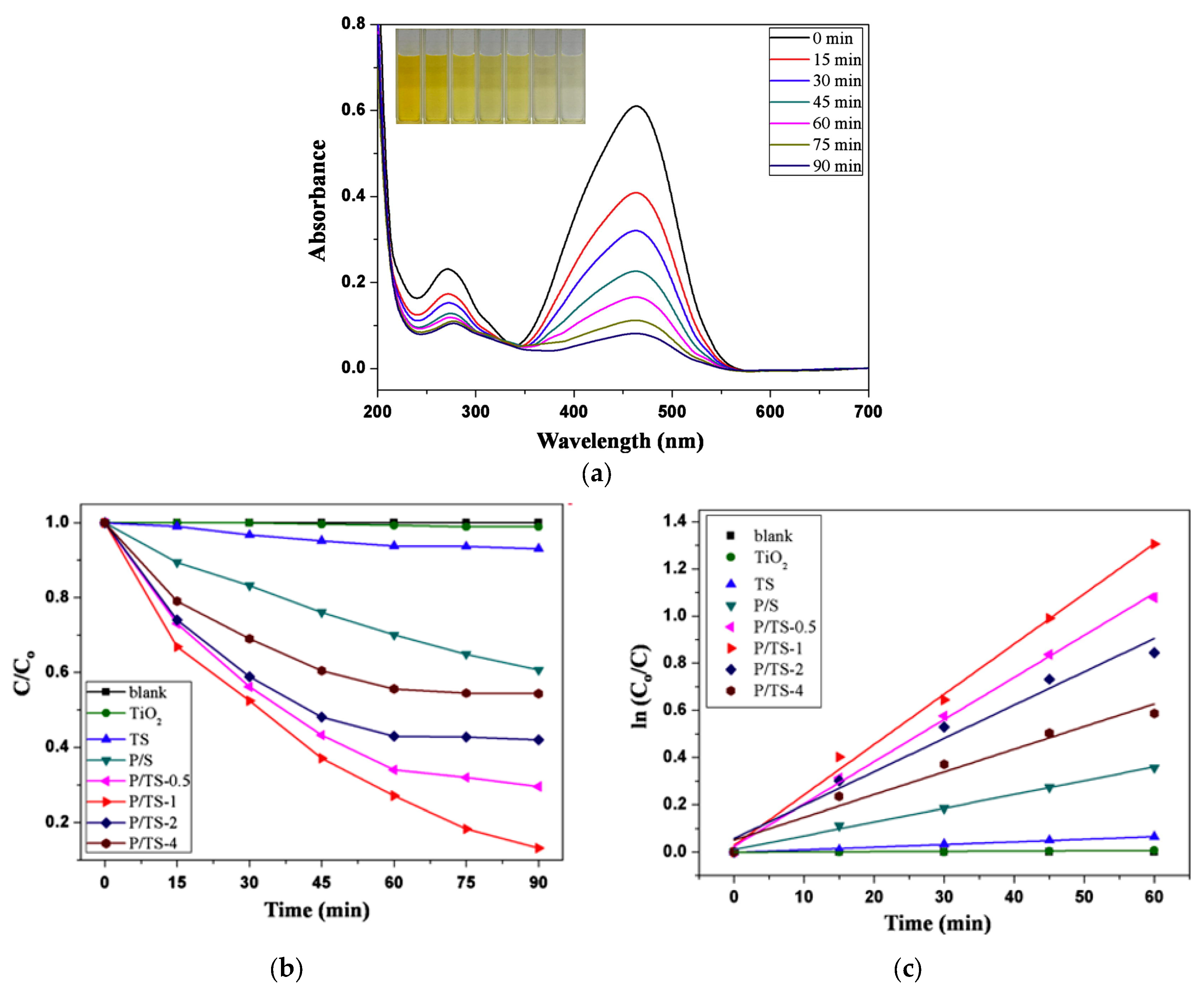
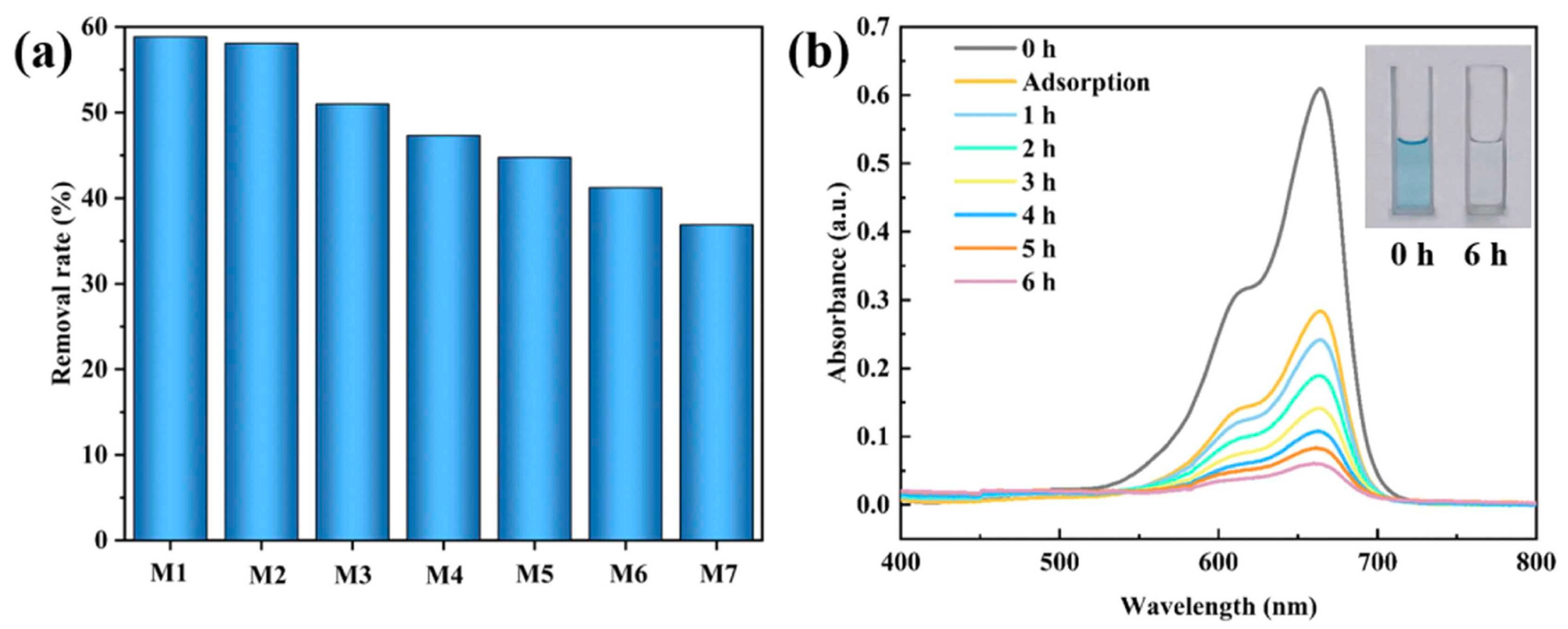
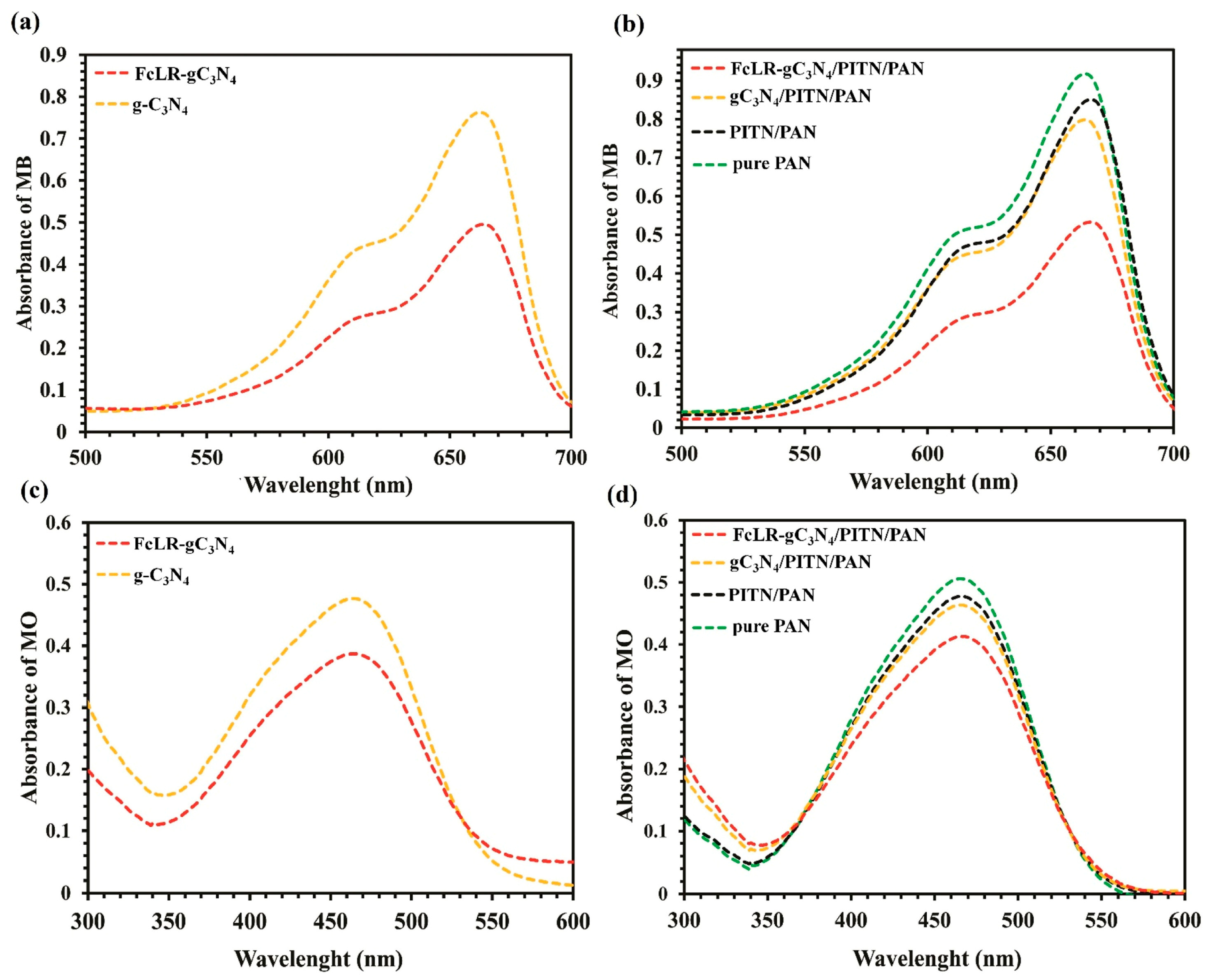





| Conductive Composite | Electrospinning Conditions | EOC Type | Optimized Adsorption/Photocatalysis Conditions | Efficacity | Ref. | |
|---|---|---|---|---|---|---|
| Processing Conditions | Solution | |||||
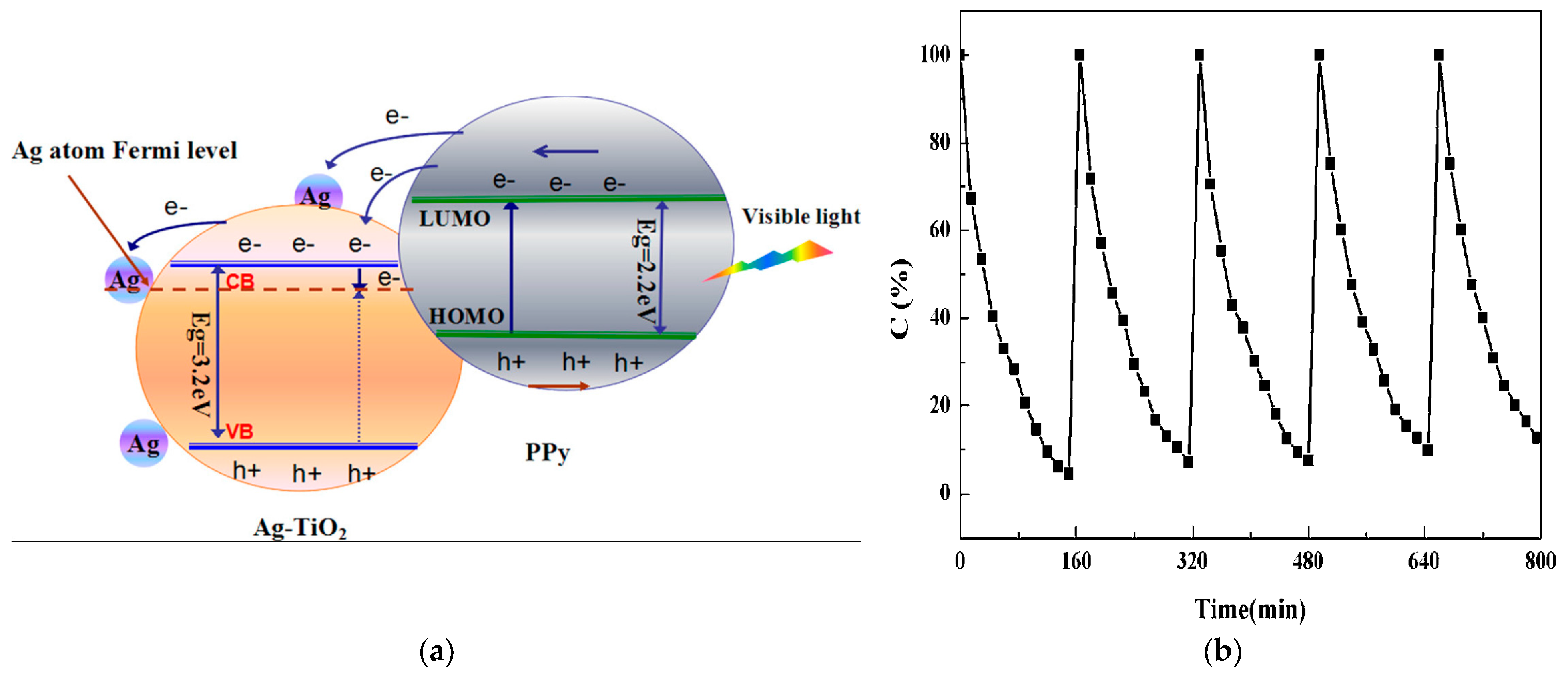
| PPy-Ag-TiO2 nanofibers | Voltage, flow rate, and distance from tip of needle: 10.0 kV–12.0 kV, 1 mL h−1, and 10.0 cm, respectively | Ag-TiO2 solution: 1 g of tetrabutyl titanate (TBT); 4 g of PVP, 0.1 M AgNO3, and 0.1 M sodium bis (2-ethylhexyl); 5 mM PPy in surfactant sodium dodecyl sulfonate (SDS) | Gaseous acetone | 125 W high-pressure Hg lamp with cut-off 400 nm filter; photocatalyst powder 0.5 g; adsorption–desorption equilibrium: at 2 h in dark | Complete degradation; k = 0.087 min−1 | [78] |
| PAN@PPy–CNT(NP) mats | For PAN mats: Voltage 16 kV; Distance from needle-collector: 20 cm; flow rate: 0.8–0.65 mL h−1; relative humidity: 40–48%; temperature: 22–25 °C; solution of PPY-impregnated PAN mats at 0.12 mL cm−2 | Core solution: 20% w/w solution of PAN in DMF; shell solution: 50 mM PPY solution dissolved in 4-dodecylbenzene sulfonic acid (DBSA) in presence of 100 mM, ammonium peroxydisulfate (APS) | Model contaminants: MO (10−5 M), RhB (10−5 M), and naphthalene (5 ppm) | Sunlight simulator device with 550 W xenon lamp; irradiation time: 120 min; static conditions; adsorption–desorption equilibrium: at 2 h in dark | Adsorption efficiency of RB and naphthalene: 40 ± 10% and 35 ± 10%, respectively (2 removal cycles); TOC: 0.16 mg cm−2 for 60 min irradiation time | [65] |
| PANI-coated TiO2/SiO2 nanofiber membranes | Voltage, flow rate, and distance from tip of needle were: 15 kV, 2.0 mL h−1, and 20 cm, respectively | TiO2/SiO2 solution: 0.002 mol of TEOS and 0.01 mol of Ti(OBu)4; 4 wt% (PVP) in ethanol solution | MO solution (1.5 mg L−1) | 500 W xenon lamp with cut-off 420 nm glass filter; photocatalyst membrane dimensions 1.5 cm × 0.8 cm | Degradation rate: 87% during 90 min; | [73] |
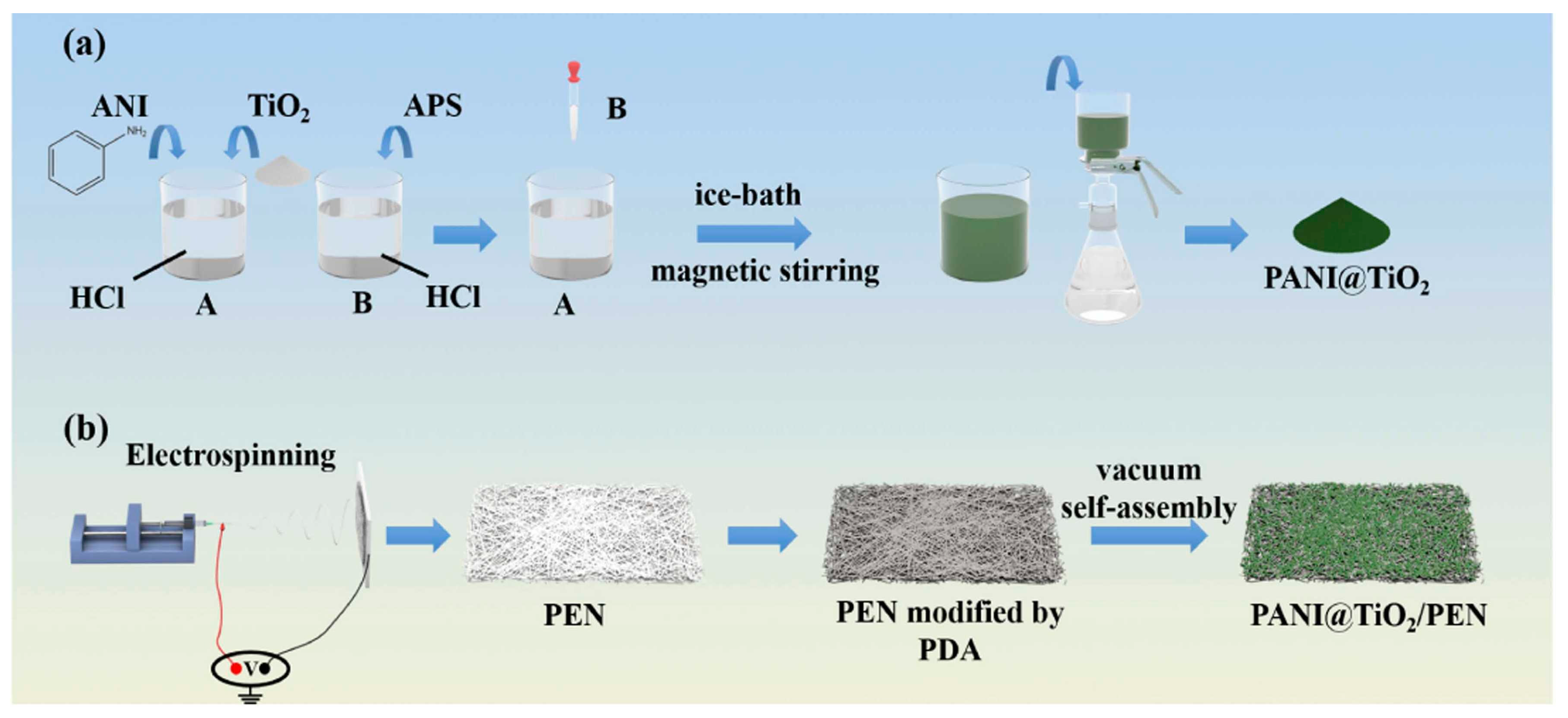
| PANI@TiO2/PEN composite nanofibrous superhydrophilic membrane | Voltage, flow rate, and rotational speed: 18 kV, 0.0009 mm s−1, and 300 rpm, respectively | 2 g of PEN powder was dissolved in 5 mL of DMF solvent | MB (3 mg L−1) | Xenon lamp for 1 h and 1 kW m− 2 sunlight intensity | Degradation rate: 92.18% during 6 h | [92] |
| Ferrocenyl dithiophosphonic acid (FcLR)-gC3N4/polyisothianaphthene (PITN)/PAN ternary composite nanofiber | Voltage and flow rate: 16.5 kV and 0.5 mL h−1; needle diameter of 21-gauge; collector rotation speed of 100 rpm | 0.3 wt% FcLR-gC3N4, 1.2 wt% PITN, and 12.0 wt% PAN were dissolved in DMF | MB (cationic dye) and MO (anionic dye), 10 mL, 5 mg L−1 | 55 W xenon lamp source, for 2 h, at room temperature and slowly stirring; photocatalytic nanofiber: 0.5 g | Degradation rates: 92% for MB and 29% for MO; MB mineralization: 65% by TOC, 60% by COD, and 55% by BOD tests after 2 h and 30 min | [93] |
| MoS2/PANI/PAN@BiFeO3 bilayer hollow nanofiber membrane (PPBM-H) | Coaxial electrospinning technique; voltage, aluminum foil collector distance, and flow were 17 kV, 15 cm, and 0.5 mL h−1, respectively | DMF as solvent | TCH (25 mg⋅L−1) and MO (15 mg⋅L−1) from wastewater (50 mL) | 50 mg of photocatalyst was immersed in hand-made membrane filtration device containing water pump for filtering wastewater | Degradation rates: 99.5% for TCH and 99.9% for MO | [90] |
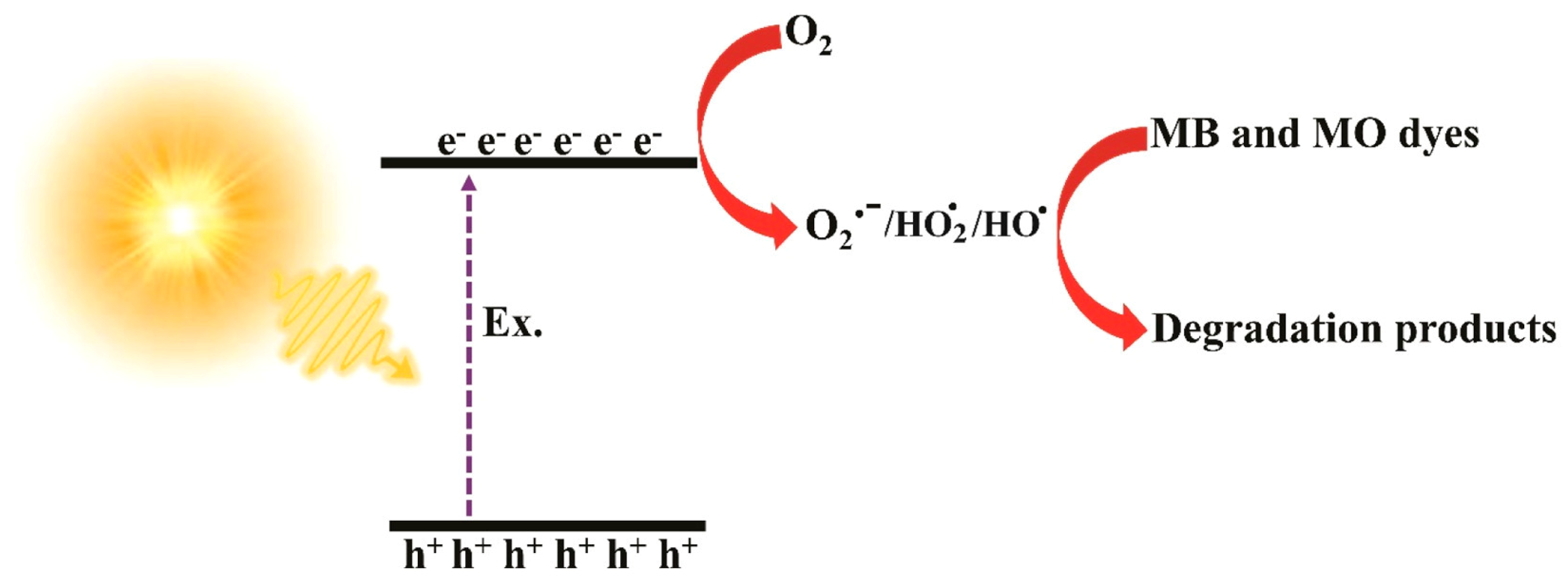
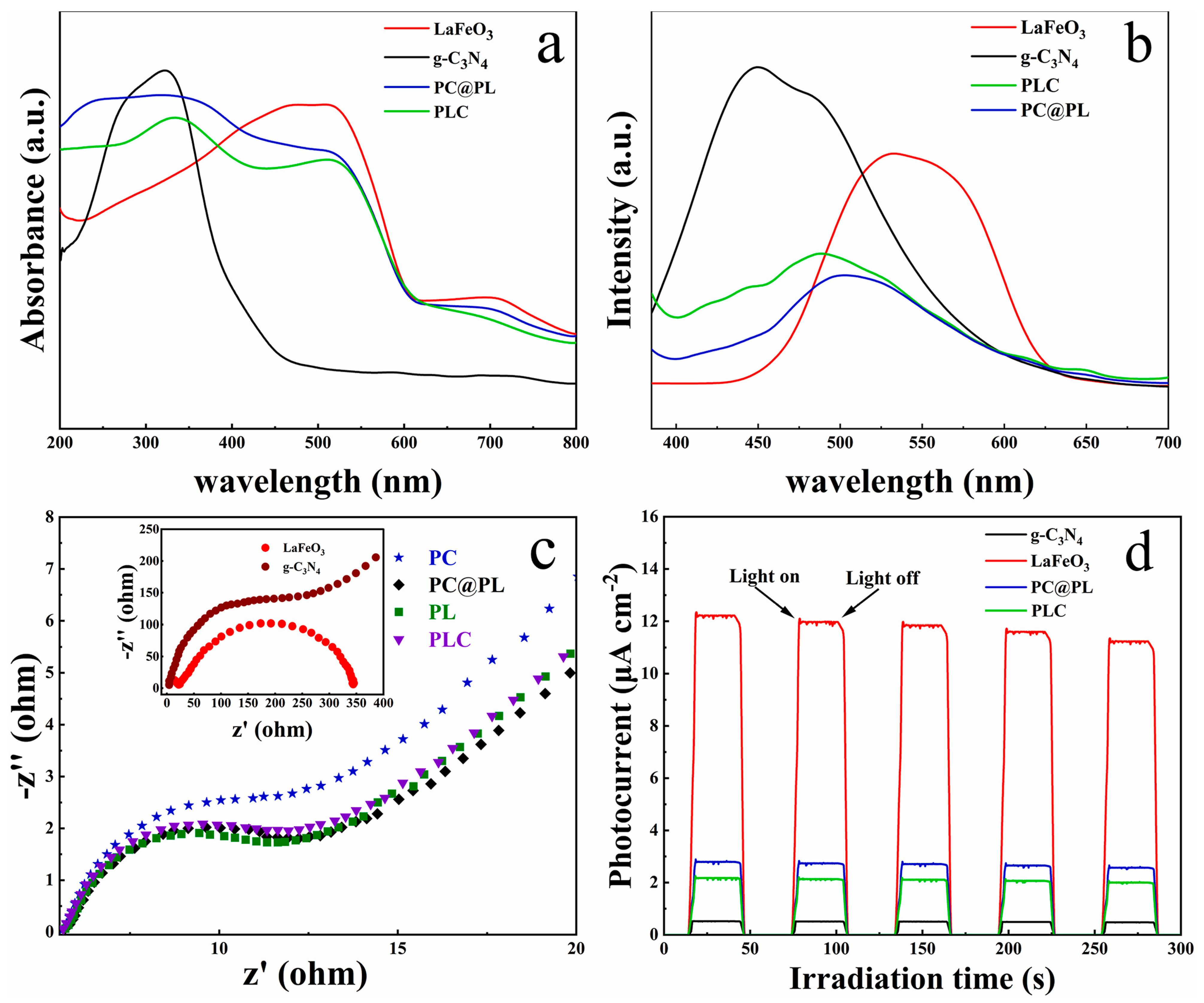
| g-C3N4/PAN/PANI@LaFeO3 cable nanofiber membranes (PC@PL) | Coaxial electrospinning technique; voltage, flow rate, and distance from tip of needle: 18 kV, 1.5 mL h−1, and 15 cm, respectively | Volume ratio of 5.068:1 between mixed solution for shell sheath and core sheath; DMF as solvent | Methyl violet (MV), CIP, and acetamiprid (AP) (all 20 mg L−1) | 500 W spherical xenon lamp, wavelength of 300–800 nm, 30 cm distance from solution, and 37,600–39,600 Lux sunlight intensity | Degradation rates: 97.0% for MB, 94.3% for MV, 87.6% for CIP, and 88.9% for AP, within 75 min | [91] |
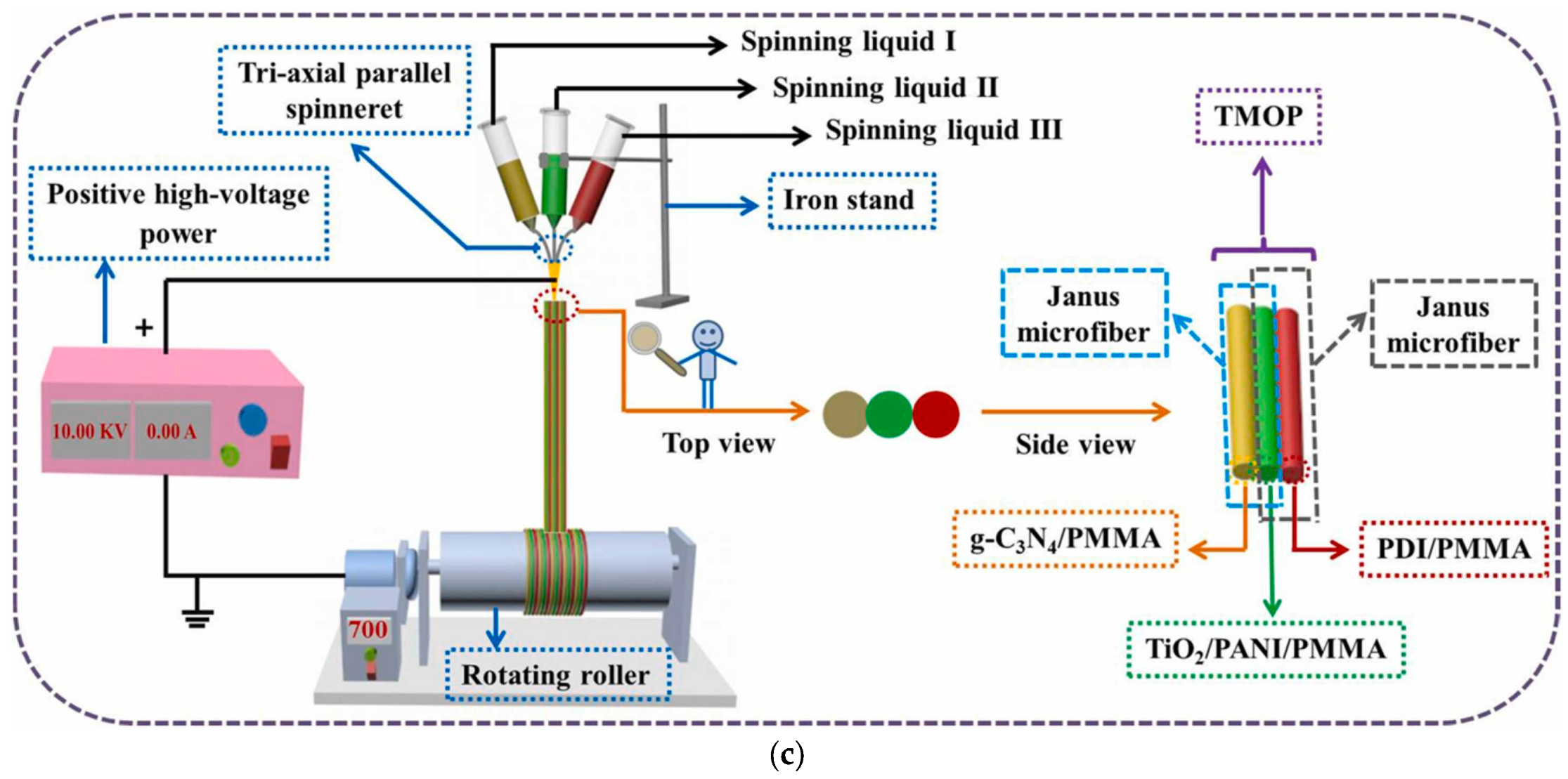
| g-C3N4/PMMA/TiO2/PANI/PMMA/self-assembled 3,4,9,10-PDI/PMMA tricolor-typed microfiber oriented-heterostructure photocatalyst (TMOP) | Voltage, flow rate, and the distance from the tip of the needle were: 10 kV, 0.8 mL h−1, and 15 cm; Rotation rate: 700 rpm | g-C3N4/PMMA (MP1); TiO2/PANI/PMMA (MP2); PDI/PMMA (MP3) (triaxial parallel electrospinning); TiO2 amount varied: 0.320 g, 0.480 g, and 0.640 g | CIP, TCH, chlortetracycline hydrochloride (CTC), levofloxacin (LEV), and MB | Simulated sunlight | Degradation rates: 88.99% for CIP within 90 min, 91.15% for TCH within 80 min, 77.55% for CTC within 150 min, 69.51% for LEV within 150 min, and 92.50% for MB within 50 min | [94] |
| Conductive Composite | Fiber’s Characteristics | Mechanical Robustness | Recycling Cycles | Perspectives for Development | Ref. |
|---|---|---|---|---|---|
| PPy-Ag-TiO2 nanofibers | Diameter size of TiO2 nanofibers: ~50−200 nm; Average size of Ag NPs: 18.02 nm; Length of the fibers: a few millimeters; thickness of PPy: ~10 nm; appearance of PPy-Ag-TiO2 nanofibers: rather uneven | - | Photocatalytic activity decreased by ~10% after five successive reaction cycles | Promising pathway for engineering composite photocatalysts for uses beyond traditional photocatalysis | [78] |
| PAN@PPy–CNT(NP) mats | Thickness: 30 ± 10 μm; CNT (NT) detached from fibers soon after PAN@PPY–CNT(NP) mats were immersed in water | Ultimate tensile strength 10 ± 3 MPa; Elongation at break 50 ± 15%; Young’s modulus 200 ± 70 MPa (at a tensile rate of 0.01 mm s −1) | Two cycles; rinsed with abundant water before subsequent cycle | Potential candidate for future large-scale photocatalytic applications | [65] |
| PANI-coated TiO2/SiO2 nanofiber membranes | Self-standing, flexible, and porous membranes (90% porosity) | - | Photocatalytic activity decreased by ~20% after five successive reaction cycles | Prospective uses in photocatalysis and aquatic pollution remediation | [73] |
| PANI@TiO2/PEN composite nanofibrous superhydrophilic membrane | Average diameter of bare PEN fibers: roughly 170 nm; agglomeration of PANI@TiO2 on PEN fibers; uniform polymerization of PANI on surface of TiO2 NPs; higher hydrophilicity | - | Water evaporation rates up to 3.23 kg m−2 h−1 (stability up to eight cycles); thermal and corrosion resistance | Simple and green approach to water purification | [92] |
| FcLR-gC3N4/PITN/PAN ternary composite nanofiber | Initial decomposition during temperature ranging from 295 °C to 350 °C (by TGA); higher hydrophobicity | Tensile strength: 2.17 MPa (pure PAN: 0.97 MPa); Elongation at break: 51.25% (pure PAN: 33.22%) | Good photocatalytic activity during three recycling cycles: in the case of MB, it decreased from 92% to 88% | Advanced candidate for sustainable water and wastewater treatment | [93] |
| PPBM-H bilayer hollow nanofiber membrane | Double-layer hollow structure; nanofibers are uniform without agglomeration, with rough surface; good hydrophilicity (low contact angle of 11.6°) | Young’s modulus: 81.13 MPa; Tensile strength: 2.34 MPa | After ten cycles, degradation efficiency decreased to 85.5% | Advanced self-cleaning multifunctional membranes exhibited outstanding performance, including water flux of 1248 L·m−2·h−1, BSA rejection of 98.8%, piezo-photocatalytic degradation of TCH (99.2% within 2 h), and complete disinfection of E. coli within 60 min | [90] |
| PC@PL cable nanofiber membranes | Average pore size of 0.92 μm; thickness of ~283 μm | Tensile strength: 2.7 MPa (meeting the requirements for filtration membranes) | After five reuse cycles, degradation efficiency was sustained at 94% | Exceptional adsorption and filtration capacity, offering practical solution for handling wastewater effluents containing wide range of contaminants such as dyes, pesticides, antibiotics, and bacteria | [91] |
| TMOP tricolor-typed microfiber oriented-heterostructure photocatalyst | Diameter sizes of photocatalyst containing different amounts of TiO2: 1.214 ± 0.031 µm, 1.077 ± 0.029 µm, and 1.096 ± 0.032 µm; specific surface area of 31.907 m2 g−1 for TMOP-2 | - | After three consecutive cycles, photodegradation efficiency of TMOP-2 for CIP slightly decreased; stability of TMOP-2 for four reusability cycles in the case of photocatalytic hydrogen evolution | High-performance photocatalyst for concurrent removal of organic contaminants and photocatalytic hydrogen evolution; hydrogen generation efficiency: 536.7 μmol h−1g−1 | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Râpă, M.; Alhalaili, B.; Dincă, F.A.; Predescu, A.M.; Matei, E.; Vidu, R. Hybrid Electrospun Conductive Nanofibers for Emerging Organic Contaminants’ Degradation in Visible Light Photocatalysis: A Review. Int. J. Mol. Sci. 2025, 26, 9055. https://doi.org/10.3390/ijms26189055
Râpă M, Alhalaili B, Dincă FA, Predescu AM, Matei E, Vidu R. Hybrid Electrospun Conductive Nanofibers for Emerging Organic Contaminants’ Degradation in Visible Light Photocatalysis: A Review. International Journal of Molecular Sciences. 2025; 26(18):9055. https://doi.org/10.3390/ijms26189055
Chicago/Turabian StyleRâpă, Maria, Badriyah Alhalaili, Florin Aurel Dincă, Andra Mihaela Predescu, Ecaterina Matei, and Ruxandra Vidu. 2025. "Hybrid Electrospun Conductive Nanofibers for Emerging Organic Contaminants’ Degradation in Visible Light Photocatalysis: A Review" International Journal of Molecular Sciences 26, no. 18: 9055. https://doi.org/10.3390/ijms26189055
APA StyleRâpă, M., Alhalaili, B., Dincă, F. A., Predescu, A. M., Matei, E., & Vidu, R. (2025). Hybrid Electrospun Conductive Nanofibers for Emerging Organic Contaminants’ Degradation in Visible Light Photocatalysis: A Review. International Journal of Molecular Sciences, 26(18), 9055. https://doi.org/10.3390/ijms26189055










