Possible Roles for Purinergic Receptor P2RX4 in Breast and Prostate Cancers
Abstract
1. Introduction
2. Purinergic Receptors
3. P2X Receptors and Their Roles in Disease and Cancer
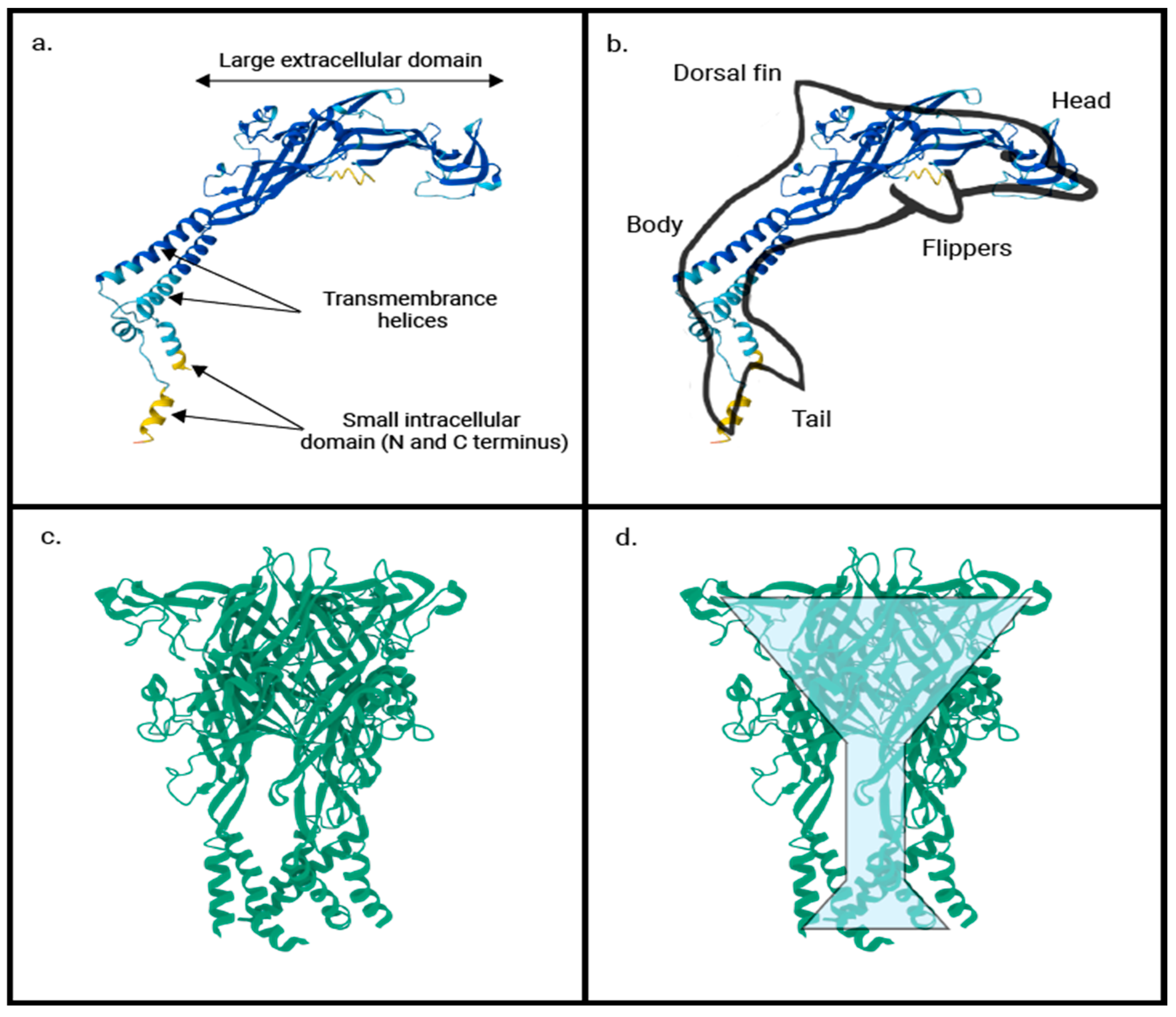
4. P2RX4: A Dual-Function Purinergic Receptor
5. P2RX4 in Breast Cancer Aggressiveness, Epithelial–Mesenchymal Transition & Autophagy
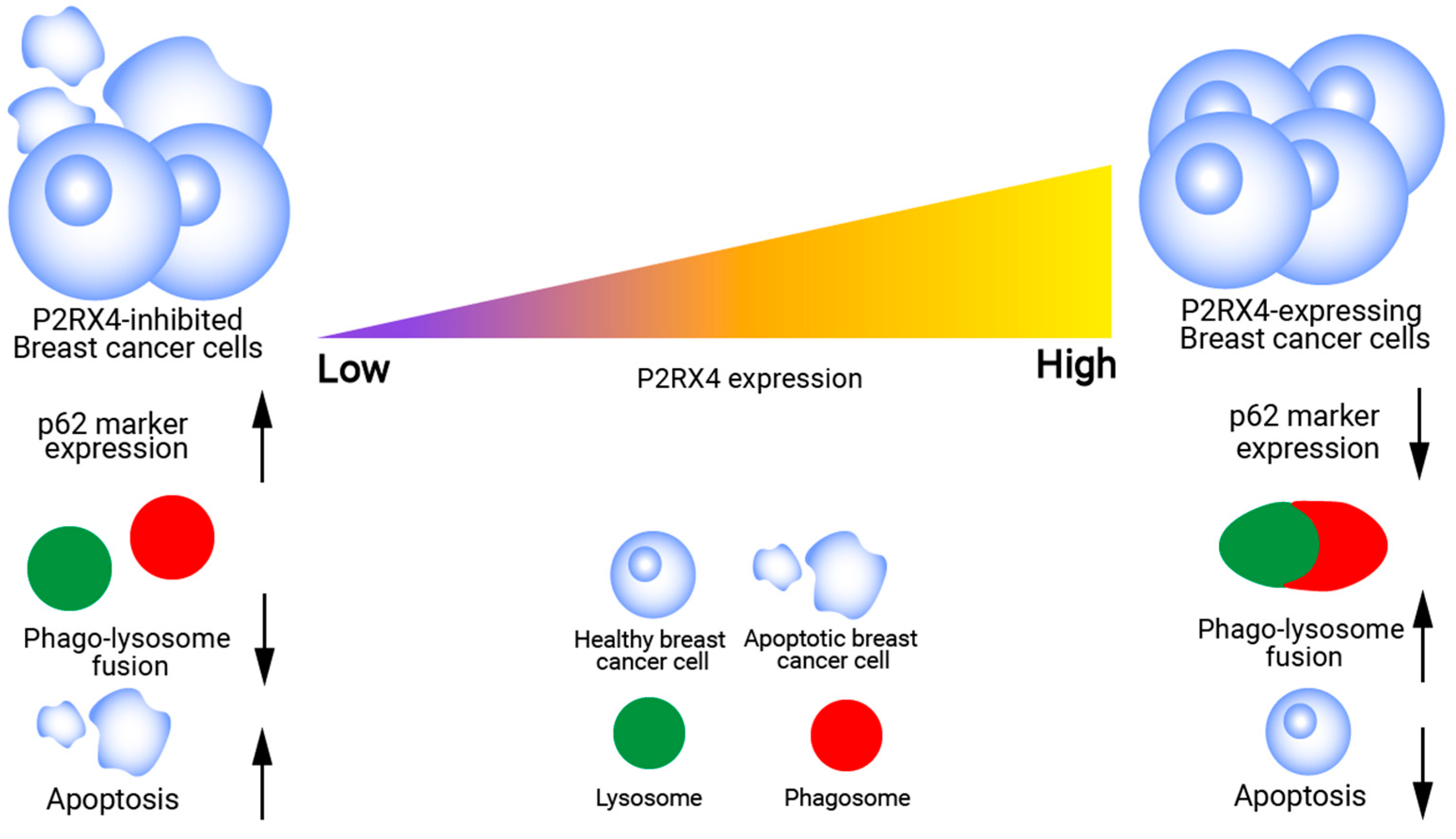
6. P2RX4-Mediated Lysosomal Exocytosis in Breast Cancer
7. P2RX4 and Prostate Cancer Aggressiveness and Tumor Growth
8. P2RX4 Modulates Oncogenes and Tumor Suppressor Genes in Prostate Cancer
9. P2RX4 Variants and Risk of Breast and Prostate Cancer
10. P2X-Related Cancer Therapeutics
11. Concluding Thoughts
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lee, E.Y.; Muller, W.J. Oncogenes and Tumor Suppressor Genes. Cold Spring Harb. Perspect. Biol. 2010, 2, a003236. [Google Scholar] [CrossRef] [PubMed]
- Holstege, H.; Joosse, S.A.; van Oostrom, C.T.M.; Nederlof, P.M.; de Vries, A.; Jonkers, J. High Incidence of Protein-Truncating TP53 Mutations in BRCA1-Related Breast Cancer. Cancer Res. 2009, 69, 3625–3633. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signalling: From discovery to current developments. Exp. Physiol. 2014, 99, 16–34. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Vultaggio-Poma, V.; Sarti, A.C. P2X receptors in cancer growth and progression. Biochem. Pharmacol. 2021, 187, 114350. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Di Virgilio, F. Purinergic signalling and cancer. Purinergic Signal. 2013, 9, 491–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, S.; Tan, S.; Zeng, Y.; Zeng, H. The P2 purinoceptors in prostate cancer. Purinergic Signal. 2023, 19, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ren, S.; Yiu, M.K.; Fai, N.C.; Cheng, W.S.; Ian, L.H.; Naito, S.; Matsuda, T.; Kehinde, E.; Kural, A.; et al. Prostate cancer in Asia: A collaborative report. Asian J. Urol. 2014, 1, 15–29. [Google Scholar] [CrossRef]
- Lim, Y.X.; Lim, Z.L.; Ho, P.J.; Li, J. Breast Cancer in Asia: Incidence, Mortality, Early Detection, Mammography Programs, and Risk-Based Screening Initiatives. Cancers 2022, 14, 4218. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Burnstock, G. Biology of purinergic signalling: Its ancient evolutionary roots, its omnipresence and its multiple functional significance. BioEssays 2014, 36, 697–705. [Google Scholar] [CrossRef]
- North, R.A. Molecular Physiology of P2X Receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E. Cellular Distribution and Functions of P2 Receptor Subtypes in Different Systems. In International Review of Cytology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 240, pp. 31–304. [Google Scholar]
- Kawate, T.; Michel, J.C.; Birdsong, W.T.; Gouaux, E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature 2009, 460, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.; Kadima, S.; Kyle, B.; Hollywood, M.A.; Thornbury, K.D.; McHale, N.G.; Sergeant, G.P. P2X Receptor Currents in Smooth Muscle Cells Contribute to Nerve Mediated Contractions of Rabbit Urethral Smooth Muscle. J. Urol. 2011, 186, 745–752. [Google Scholar] [CrossRef]
- Khakh, B.S.; North, R.A. Neuromodulation by Extracellular ATP and P2X Receptors in the CNS. Neuron 2012, 76, 51–69. [Google Scholar] [CrossRef]
- Hattori, M.; Gouaux, E. Crystal Structure of the ATP-Gated P2X4 Ion Channel in the Closed, Apo State at 2.9 Ang-Stroms: 4dw0. 2012. Available online: https://www.wwpdb.org/pdb?id=pdb_00004dw0 (accessed on 16 June 2025).
- Burnstock, G. Physiology and Pathophysiology of Purinergic Neurotransmission. Physiol. Rev. 2007, 87, 659–797. [Google Scholar] [CrossRef]
- Surprenant, A.; North, R.A. Signaling at Purinergic P2X Receptors. Annu. Rev. Physiol. 2009, 71, 333–359. [Google Scholar] [CrossRef]
- Afework, M.; Burnstock, G. Distribution of P2X receptors in the rat adrenal gland. Cell Tissue Res. 1999, 298, 449–456. [Google Scholar] [CrossRef]
- Kaczmarek-Hájek, K.; Lörinczi, É.; Hausmann, R.; Nicke, A. Molecular and functional properties of P2X receptors—Recent progress and persisting challenges. Purinergic Signal. 2012, 8, 375–417. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.L.; Troncoso, M.F.; Espelt, M.V. Extracellular ATP and adenosine in tumor microenvironment: Roles in epithelial–mesenchymal transition, cell migration, and invasion. J. Cell. Physiol. 2022, 237, 389–400. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Adinolfi, E. Extracellular purines, purinergic receptors and tumor growth. Oncogene 2017, 36, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, A. Extracellular ATP in the Immune System: More Than Just a “Danger Signal”. Sci. Signal. 2009, 2, pe6. [Google Scholar] [CrossRef]
- Burnstock, G. P2X ion channel receptors and inflammation. Purinergic Signal. 2016, 12, 59–67. [Google Scholar] [CrossRef]
- Myrtek, D.; Idzko, M. Chemotactic activity of extracellular nucleotideson human immune cells. Purinergic Signal. 2007, 3, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Mulryan, K.; Gitterman, D.P.; Lewis, C.J.; Vial, C.; Leckie, B.J.; Cobb, A.L.; Brown, J.E.; Conley, E.C.; Buell, G.; Pritchard, C.A.; et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature 2000, 403, 86–89. [Google Scholar] [CrossRef]
- Guan, Z.; Osmond, D.A.; Inscho, E.W. P2X receptors as regulators of the renal microvasculature. Trends Pharmacol. Sci. 2007, 28, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Inscho, E.W.; Cook, A.K.; Imig, J.D.; Vial, C.; Evans, R.J. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J. Clin. Investig. 2003, 112, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Cockayne, D.A.; Dunn, P.M.; Zhong, Y.; Rong, W.; Hamilton, S.G.; Knight, G.E.; Ruan, H.; Ma, B.; Yip, P.; Nunn, P.; et al. P2X2 knockout mice and P2X2 /P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J. Physiol. 2005, 567, 621–639. [Google Scholar] [CrossRef]
- Finger, T.E.; Danilova, V.; Barrows, J.; Bartel, D.L.; Vigers, A.J.; Stone, L.; Hellekant, G.; Kinnamon, S.C. ATP Signaling Is Crucial for Communication from Taste Buds to Gustatory Nerves. Science 2005, 310, 1495–1499. [Google Scholar] [CrossRef]
- Eddy, M.C.; Eschle, B.K.; Barrows, J.; Hallock, R.M.; Finger, T.E.; Delay, E.R. Double P2X2/P2X3 Purinergic Receptor Knockout Mice Do Not Taste NaCl or the Artificial Sweetener SC45647. Chem. Senses 2009, 34, 789–797. [Google Scholar] [CrossRef]
- Cockayne, D.A.; Hamilton, S.G.; Zhu, Q.-M.; Dunn, P.M.; Zhong, Y.; Novakovic, S.; Malmberg, A.B.; Cain, G.; Berson, A.; Kassotakis, L.; et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 2000, 407, 1011–1015. [Google Scholar] [CrossRef]
- Souslova, V.; Cesare, P.; Ding, Y.; Akopian, A.N.; Stanfa, L.; Suzuki, R.; Carpenter, K.; Dickenson, A.; Boyce, S.; Hill, R.; et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 2000, 407, 1015–1017, Correction in Nature 2001, 409, 743. [Google Scholar] [CrossRef]
- Bian, X.; Ren, J.; De Vries, M.; Schnegelsberg, B.; Cockayne, D.A.; Ford, A.P.; Galligan, J.J. Peristalsis is impaired in the small intestine of mice lacking the P2X3 subunit. J. Physiol. 2003, 551, 309–322. [Google Scholar] [CrossRef]
- Ulmann, L.; Hatcher, J.P.; Hughes, J.P.; Chaumont, S.; Green, P.J.; Conquet, F.; Buell, G.N.; Reeve, A.J.; Chessell, I.P.; Rassendren, F. Up-Regulation of P2X4 Receptors in Spinal Microglia after Peripheral Nerve Injury Mediates BDNF Release and Neuropathic Pain. J. Neurosci. 2008, 28, 11263–11268. [Google Scholar] [CrossRef]
- Tsuda, M.; Kuboyama, K.; Inoue, T.; Nagata, K.; Tozaki-Saitoh, H.; Inoue, K. Behavioral Phenotypes of Mice Lacking Purinergic P2X4 Receptors in Acute and Chronic Pain Assays. Mol. Pain 2009, 5, 28. [Google Scholar] [CrossRef]
- Ulmann, L.; Hirbec, H.; Rassendren, F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010, 29, 2290–2300. [Google Scholar] [CrossRef]
- Overes, I.M.; De Rijke, B.; Van Horssen-Zoetbrood, A.; Fredrix, H.; De Graaf, A.O.; Jansen, J.H.; Van Krieken, J.H.J.M.; Raymakers, R.A.P.; Van Der Voort, R.; De Witte, T.M.; et al. Expression of P2X5 in lymphoid malignancies results in LRH-1-specific cytotoxic T-cell-mediated lysis. Br. J. Haematol. 2008, 141, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Walsh, M.C.; Takegahara, N.; Middleton, S.A.; Shin, H.-I.; Kim, J.; Choi, Y. The purinergic receptor P2X5 regulates inflammasome activity and hyper-multinucleation of murine osteoclasts. Sci. Rep. 2017, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Banfi, C.; Ferrario, S.; De Vincenti, O.; Ceruti, S.; Fumagalli, M.; Mazzola, A.; D’AMbrosi, N.; Volontè, C.; Fratto, P.; Vitali, E.; et al. P2 receptors in human heart: Upregulation of P2X6 in patients undergoing heart transplantation, interaction with TNFα and potential role in myocardial cell death. J. Mol. Cell. Cardiol. 2005, 39, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Labasi, J.M.; Petrushova, N.; Donovan, C.; McCurdy, S.; Lira, P.; Payette, M.M.; Brissette, W.; Wicks, J.R.; Audoly, L.; Gabel, C.A. Absence of the P2X7 Receptor Alters Leukocyte Function and Attenuates an Inflammatory Response. J. Immunol. 2002, 168, 6436–6445. [Google Scholar] [CrossRef]
- Solle, M.; Labasi, J.; Perregaux, D.G.; Stam, E.; Petrushova, N.; Koller, B.H.; Griffiths, R.J.; Gabel, C.A. Altered Cytokine Production in Mice Lacking P2X7 Receptors. J. Biol. Chem. 2001, 276, 125–132. [Google Scholar] [CrossRef]
- Takenouchi, T.; Sekiyama, K.; Sekigawa, A.; Fujita, M.; Waragai, M.; Sugama, S.; Iwamaru, Y.; Kitani, H.; Hashimoto, M. P2X7 Receptor Signaling Pathway as a Therapeutic Target for Neurodegenerative Diseases. Arch. Immunol. Ther. Exp. 2010, 58, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.R.; Perez-Neut, M.; Kaja, S.; Gentile, S. Voltage-Gated Ion Channels in Cancer Cell Proliferation. Cancers 2015, 7, 849–875. [Google Scholar] [CrossRef]
- Asif, A.; Khalid, M.; Manzoor, S.; Ahmad, H.; Rehman, A.U. Role of purinergic receptors in hepatobiliary carcinoma in Pakistani population: An approach towards proinflammatory role of P2X4 and P2X7 receptors. Purinergic Signal. 2019, 15, 367–374. [Google Scholar] [CrossRef]
- Zhang, W.-J. Effect of P2X purinergic receptors in tumor progression and as a potential target for anti-tumor therapy. Purinergic Signal. 2021, 17, 151–162. [Google Scholar] [CrossRef]
- Adinolfi, E.; Capece, M.; Amoroso, F.; Marchi, E.; Franceschini, A. Emerging Roles of P2X Receptors in Cancer. Curr. Med. Chem. 2015, 22, 878–890. [Google Scholar] [CrossRef]
- North, R.A.; Jarvis, M.F. P2X Receptors as Drug Targets. Mol. Pharmacol. 2013, 83, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Bidula, S.; Dhuna, K.; Helliwell, R.; Stokes, L. Positive allosteric modulation of P2X7 promotes apoptotic cell death over lytic cell death responses in macrophages. Cell Death Dis. 2019, 10, 882. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, P.; Wang, B.; Kovacevic, K.; Jalilian, I.; Bosman, G.J.; Wiley, J.S.; Sluyter, R. P2X7 receptor activation induces cell death and microparticle release in murine erythroleukemia cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 1797–1804. [Google Scholar] [CrossRef]
- Mehta, N.; Kaur, M.; Singh, M.; Chand, S.; Vyas, B.; Silakari, P.; Bahia, M.S.; Silakari, O. Purinergic receptor P2X7: A novel target for anti-inflammatory therapy. Bioorg. Med. Chem. 2014, 22, 54–88. [Google Scholar] [CrossRef]
- Keystone, E.C.; Wang, M.M.; Layton, M.; Hollis, S.; McInnes, I.B. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann. Rheum. Dis. 2012, 71, 1630–1635. [Google Scholar] [CrossRef]
- Gilbert, S.M.; Gidley Baird, A.; Glazer, S.; Barden, J.A.; Glazer, A.; Teh, L.C.; King, J. A phase I clinical trial demonstrates that nfP2X7-targeted antibodies provide a novel, safe and tolerable topical therapy for basal cell carcinoma. Br. J. Dermatol. 2017, 177, 117–124. [Google Scholar] [CrossRef]
- Chong, J.-H.; Zheng, G.-G.; Zhu, X.-F.; Guo, Y.; Wang, L.; Ma, C.-H.; Liu, S.-Y.; Xu, L.-L.; Lin, Y.-M.; Wu, K.-F. Abnormal expression of P2X family receptors in Chinese pediatric acute leukemias. Biochem. Biophys. Res. Commun. 2010, 391, 498–504. [Google Scholar] [CrossRef]
- Chadet, S.; Allard, J.; Brisson, L.; Lopez-Charcas, O.; Lemoine, R.; Heraud, A.; Lerondel, S.; Guibon, R.; Fromont, G.; Le Pape, A.; et al. P2x4 receptor promotes mammary cancer progression by sustaining autophagy and associated mesenchymal transition. Oncogene 2022, 41, 2920–2931. [Google Scholar] [CrossRef]
- Maynard, J.P.; Lu, J.; Vidal, I.; Hicks, J.; Mummert, L.; Ali, T.; Kempski, R.; Carter, A.M.; Sosa, R.Y.; Peiffer, L.B.; et al. P2X4 purinergic receptors offer a therapeutic target for aggressive prostate cancer. J. Pathol. 2022, 256, 149–163. [Google Scholar] [CrossRef]
- Suurväli, J.; Boudinot, P.; Kanellopoulos, J.; Rüütel Boudinot, S. P2X4: A fast and sensitive purinergic receptor. Biomed. J. 2017, 40, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Kennedy, C. P2X Receptors in Health and Disease. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 61, pp. 333–372. [Google Scholar]
- Qureshi, O.S.; Paramasivam, A.; Yu, J.C.H.; Murrell-Lagnado, R.D. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 2007, 120, 3838–3849. [Google Scholar] [CrossRef]
- Murrell-Lagnado, R.D.; Frick, M. P2X4 and lysosome fusion. Curr. Opin. Pharmacol. 2019, 47, 126–132. [Google Scholar] [CrossRef]
- Kanellopoulos, J.M.; Almeida-Da-Silva, C.L.C.; Boudinot, S.R.; Ojcius, D.M. Structural and Functional Features of the P2X4 Receptor: An Immunological Perspective. Front. Immunol. 2021, 12, 645834. [Google Scholar] [CrossRef] [PubMed]
- Fois, G.; Föhr, K.J.; Kling, C.; Fauler, M.; Wittekindt, O.H.; Dietl, P.; Frick, M. P2X4 receptor re-sensitization depends on a protonation/deprotonation cycle mediated by receptor internalization and recycling. J. Physiol. 2018, 596, 4893–4907. [Google Scholar] [CrossRef] [PubMed]
- Sophocleous, R.A.; Ooi, L.; Sluyter, R. The P2X4 Receptor: Cellular and Molecular Characteristics of a Promising Neuroin-flammatory Target. Int. J. Mol. Sci. 2022, 23, 5739. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhu, M.X. Regulation of lysosomal ion homeostasis by channels and transporters. Sci. China Life Sci. 2016, 59, 777–791. [Google Scholar] [CrossRef]
- Miklavc, P.; Frick, M. Vesicular calcium channels as regulators of the exocytotic post-fusion phase. Commun. Integr. Biol. 2011, 4, 796–798. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hattori, M.; Gouaux, E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 2012, 485, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef]
- Szczurek, E.; Krüger, T.; Klink, B.; Beerenwinkel, N.; Traulsen, A. A mathematical model of the metastatic bottleneck predicts patient outcome and response to cancer treatment. PLoS Comput. Biol. 2020, 16, e1008056. [Google Scholar] [CrossRef]
- Primeaux, M.; Gowrikumar, S.; Dhawan, P. Role of CD44 isoforms in epithelial-mesenchymal plasticity and metastasis. Clin. Exp. Metastasis 2022, 39, 391–406. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef]
- Rakesh, R.; PriyaDharshini, L.C.; Sakthivel, K.M.; Rasmi, R.R. Role and regulation of autophagy in cancer. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166400. [Google Scholar] [CrossRef]
- Hashemi, M.; Paskeh, M.D.A.; Orouei, S.; Abbasi, P.; Khorrami, R.; Dehghanpour, A.; Esmaeili, N.; Ghahremanzade, A.; Zandieh, M.A.; Peymani, M.; et al. Towards dual function of autophagy in breast cancer: A potent regulator of tumor progression and therapy response. Biomed. Pharmacother. 2023, 161, 114546. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Pankiv, S.; Øvervatn, A.; Brech, A.; Johansen, T. Chapter 12 Monitoring Autophagic Degradation of p62/SQSTM1. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 452, pp. 181–197. [Google Scholar]
- Liu, W.J.; Ye, L.; Huang, W.F.; Guo, L.J.; Xu, Z.G.; Wu, H.L.; Yang, C.; Liu, H.F. p62 links the autophagy pathway and the ubiqutin–proteasome system upon ubiquitinated protein degradation. Cell. Mol. Biol. Lett. 2016, 21, 29. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Song, D.; Zen, R.; Zhang, L.; Wang, Y.; Yang, H.; Zhang, D.; Jia, J.; Zhang, J.; et al. Lysosomal dysfunction and autophagy blockade contribute to autophagy-related cancer suppressing peptide-induced cytotoxic death of cervical cancer cells through the AMPK/mTOR pathway. J. Exp. Clin. Cancer Res. 2020, 39, 197. [Google Scholar]
- Patra, S.; Praharaj, P.P.; Klionsky, D.J.; Bhutia, S.K. Vorinostat in autophagic cell death: A critical insight into autophagy-mediated, -associated and -dependent cell death for cancer prevention. Drug Discov. Today 2022, 27, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef]
- Tancini, B.; Buratta, S.; Delo, F.; Sagini, K.; Chiaradia, E.; Pellegrino, R.M.; Emiliani, C.; Urbanelli, L. Lysosomal Exocytosis: The Extracellular Role of an Intracellular Organelle. Membranes 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Machado, E.; White-Gilbertson, S.; van de Vlekkert, D.; Janke, L.; Moshiach, S.; Campos, Y.; Finkelstein, D.; Gomero, E.; Mosca, R.; Qiu, X.; et al. Regulated lysosomal exocytosis mediates cancer progression. Sci. Adv. 2015, 1, e1500603. [Google Scholar] [CrossRef]
- Geisslinger, F.; Müller, M.; Vollmar, A.M.; Bartel, K. Targeting Lysosomes in Cancer as Promising Strategy to Overcome Chemoresistance—A Mini Review. Front. Oncol. 2020, 10, 1156. [Google Scholar] [CrossRef]
- Vasiljeva, O.; Hostetter, D.R.; Moore, S.J.; Winter, M.B. The multifaceted roles of tumor-associated proteases and harnessing their activity for prodrug activation. Biol. Chem. 2019, 400, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yao, J.; Jin, Q.; Wang, X.; Zhu, H.; Huang, F.; Wang, W.; Qiang, J.; Ni, Q. LAMP1 expression is associated with poor prognosis in breast cancer. Oncol. Lett. 2017, 14, 4729–4735. [Google Scholar] [CrossRef]
- Machado, E.R.; Annunziata, I.; van de Vlekkert, D.; Grosveld, G.C.; D’aZzo, A. Lysosomes and Cancer Progression: A Malignant Liaison. Front. Cell Dev. Biol. 2021, 9, 642494. [Google Scholar] [CrossRef]
- Kanada, M.; Bachmann, M.H.; Contag, C.H. Signaling by Extracellular Vesicles Advances Cancer Hallmarks. Trends Cancer 2016, 2, 84–94. [Google Scholar] [CrossRef]
- Sung, B.H.; Weaver, A.M. Exosome secretion promotes chemotaxis of cancer cells. Cell Adhes. Migr. 2017, 11, 187–195. [Google Scholar] [CrossRef]
- Zhong, X.Z.; Cao, Q.; Sun, X.; Dong, X. Activation of lysosomal P2X4 by ATP transported into lysosomes via VNUT/SLC17A9 using V-ATPase generated voltage gradient as the driving force. J. Physiol. 2016, 594, 4253–4266. [Google Scholar] [CrossRef]
- Miklavc, P.; Mair, N.; Wittekindt, O.H.; Haller, T.; Dietl, P.; Felder, E.; Timmler, M.; Frick, M. Fusion-activated Ca2+ entry via vesicular P2X4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 14503–14508. [Google Scholar] [CrossRef]
- Huang, P.; Zou, Y.; Zhong, X.Z.; Cao, Q.; Zhao, K.; Zhu, M.X.; Murrell-Lagnado, R.; Dong, X.-P. P2X4 Forms Functional ATP-activated Cation Channels on Lysosomal Membranes Regulated by Luminal pH. J. Biol. Chem. 2014, 289, 17658–17667. [Google Scholar] [CrossRef]
- Tidd-Johnson, A.; Sebastian, S.A.; Co, E.L.; Afaq, M.; Kochhar, H.; Sheikh, M.; Mago, A.; Poudel, S.; Fernandez, J.A.; Rodriguez, I.D.; et al. Prostate cancer screening: Continued controversies and novel biomarker advancements. Curr. Urol. 2022, 16, 197–206. [Google Scholar] [CrossRef]
- Kimura, T. East meets West: Ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin. J. Cancer 2012, 31, 421–429. [Google Scholar] [CrossRef]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018, 25, 524–531. [Google Scholar] [CrossRef]
- He, J.; Zhou, Y.; Carrera, H.M.A.; Sprules, A.; Neagu, R.; Zarkesh, S.A.; Eaton, C.; Luo, J.; Gartland, A.; Wang, N. Inhibiting the P2X4 Receptor Suppresses Prostate Cancer Growth In Vitro and In Vivo, Suggesting a Potential Clinical Target. Cells 2020, 9, 2511. [Google Scholar] [CrossRef]
- Ghalali, A.; Ye, Z.-W.; Högberg, J.; Stenius, U. PTEN and PHLPP crosstalk in cancer cells and in TGFβ-activated stem cells. Biomed. Pharmacother. 2020, 127, 110112. [Google Scholar] [CrossRef]
- Ahearn, T.U.; Pettersson, A.; Ebot, E.M.; Gerke, T.; Graff, R.E.; Morais, C.L.; Hicks, J.L.; Wilson, K.M.; Rider, J.R.; Sesso, H.D.; et al. A Prospective Investigation of PTEN Loss and ERG Expression in Lethal Prostate Cancer. J. Natl. Cancer Inst. 2015, 108, djv346. [Google Scholar] [CrossRef]
- Adamo, P.; Ladomery, M.R. The oncogene ERG: A key factor in prostate cancer. Oncogene 2016, 35, 403–414. [Google Scholar] [CrossRef]
- Chen, Q.; Gu, M.; Cai, Z.-K.; Zhao, H.; Sun, S.-C.; Liu, C.; Zhan, M.; Chen, Y.-B.; Wang, Z. TGF-β1 promotes epithelial-to-mesenchymal transition and stemness of prostate cancer cells by inducing PCBP1 degradation and alternative splicing of CD44. Cell. Mol. Life Sci. 2021, 78, 949–962. [Google Scholar] [CrossRef]
- Hashemi, M.; Taheriazam, A.; Daneii, P.; Hassanpour, A.; Kakavand, A.; Rezaei, S.; Hejazi, E.S.; Aboutalebi, M.; Gholamrezaie, H.; Saebfar, H.; et al. Targeting PI3K/Akt signaling in prostate cancer therapy. J. Cell Commun. Signal. 2023, 17, 423–443. [Google Scholar] [CrossRef]
- Alharbi, A.F.; Parrington, J. The role of genetic polymorphisms in endolysosomal ion channels TPC2 and P2RX4 in cancer pathogenesis, prognosis, and diagnosis: A genetic association in the UK Biobank. NPJ Genom. Med. 2021, 6, 58. [Google Scholar] [CrossRef]
- Stokes, L.; Scurrah, K.; Ellis, J.A.; Cromer, B.A.; Skarratt, K.K.; Gu, B.J.; Harrap, S.B.; Wiley, J.S. A Loss-of-Function Polymorphism in the Human P2X4 Receptor Is Associated with Increased Pulse Pressure. Hypertension 2011, 58, 1086–1092. [Google Scholar] [CrossRef]
- Sluyter, R.; McEwan, T.B.-D.; Sophocleous, R.A.; Stokes, L. Methods for studying P2X4 receptor ion channels in immune cells. J. Immunol. Methods 2024, 526, 113626. [Google Scholar] [CrossRef]
- Williams, W.A.; Linley, J.E.; Jones, C.A.; Shibata, Y.; Snijder, A.; Button, J.; Hatcher, J.P.; Huang, L.; Taddese, B.; Thornton, P.; et al. Antibodies binding the head domain of P2X4 inhibit channel function and reverse neuropathic pain. Pain 2019, 160, 1989–2003. [Google Scholar] [CrossRef]
- Ascione, L.; Guidi, L.; Prakash, A.; Trapani, D.; LoRusso, P.; Lou, E.; Curigliano, G. Unlocking the Potential: Biomarkers of Response to Antibody-Drug Conjugates. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e431766. [Google Scholar] [CrossRef]
- Adair, J.R.; Howard, P.W.; Hartley, J.A.; Williams, D.G.; Chester, K.A. Antibody–drug conjugates—A perfect synergy. Expert Opin. Biol. Ther. 2012, 12, 1191–1206. [Google Scholar] [CrossRef]
- Sievers, E.L.; Senter, P.D. Antibody-Drug Conjugates in Cancer Therapy. Annu. Rev. Med. 2013, 64, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Brinkmann, U.; Reichert, J.M.; Kontermann, R.E. The present and future of bispecific antibodies for cancer therapy. Nat. Rev. Drug Discov. 2024, 23, 301–319. [Google Scholar] [CrossRef]
- Sun, D.; Shi, X.; Li, S.; Wang, X.; Yang, X.; Wan, M. CAR-T cell therapy: A breakthrough in traditional cancer treatment strategies (Review). Mol. Med. Rep. 2024, 29, 47. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- De Marco, R.C.; Monzo, H.J.; Ojala, P.M. CAR T Cell Therapy: A Versatile Living Drug. Int. J. Mol. Sci. 2023, 24, 6300. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Hasanali, Z.S.; Zhao, Y.; Das, A.; Lavaert, M.; Roman, C.J.; Londregan, J.; Allman, D.; Bhandoola, A. Bone marrow plasma cells require P2RX4 to sense extracellular ATP. Nature 2024, 626, 1102–1107. [Google Scholar] [CrossRef]


| P2X Receptor | Tissue Distribution | References |
|---|---|---|
| P2RX1 | Smooth muscle, platelets, cerebellum, dorsal horn spinal neurons | [11,12,14,17,18] |
| P2RX2 | Smooth muscle, CNS, retina, chromaffin cells, autonomic and sensory ganglia | [11,12,15,17,18,19,20] |
| P2RX3 | Sensory neurons | [11,12,15,17,18,19,20] |
| P2RX4 | Central nervous system, epithelial cells, endothelial cells, cardiac myocytes, immune cells | [11,12,15,17,18,20] |
| P2RX5 | Skeletal muscle cells, bone neurons and epithelial cells | [11,12,17] |
| P2RX6 | Central nervous system | [11,12,17,19] |
| P2RX7 | Immune cells, central nervous system | [11,12,15,17,18,20] |
| P2X Receptor | Disease Implication | References |
|---|---|---|
| P2RX1 | Impaired kidney function, male infertility | [26,27,28] |
| P2RX2 | Bladder hyporeflexia, impaired taste sensing | [29,30,31] |
| P2RX3 | Reduce pain sensing, impaired gut peristalsis | [32,33,34] |
| P2RX4 | Inflammatory diseases, neuropathic pain | [35,36,37] |
| P2RX5 | Lymphoid malignancies, bone loss | [38,39] |
| P2RX6 | Chronic heart diseases | [40] |
| P2RX7 | Inflammatory diseases, neurodegenerative diseases | [41,42,43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, W.H.J.; Lam, K.-P. Possible Roles for Purinergic Receptor P2RX4 in Breast and Prostate Cancers. Int. J. Mol. Sci. 2025, 26, 9043. https://doi.org/10.3390/ijms26189043
Ho WHJ, Lam K-P. Possible Roles for Purinergic Receptor P2RX4 in Breast and Prostate Cancers. International Journal of Molecular Sciences. 2025; 26(18):9043. https://doi.org/10.3390/ijms26189043
Chicago/Turabian StyleHo, Walfred H. J., and Kong-Peng Lam. 2025. "Possible Roles for Purinergic Receptor P2RX4 in Breast and Prostate Cancers" International Journal of Molecular Sciences 26, no. 18: 9043. https://doi.org/10.3390/ijms26189043
APA StyleHo, W. H. J., & Lam, K.-P. (2025). Possible Roles for Purinergic Receptor P2RX4 in Breast and Prostate Cancers. International Journal of Molecular Sciences, 26(18), 9043. https://doi.org/10.3390/ijms26189043




