Therapeutic Targeting of Protein Lysine and Arginine Methyltransferases: Principles and Strategies for Inhibitor Design
Abstract
1. Introduction
1.1. Challenge to Treatment
1.2. Chemoresistance Mechanisms
2. Protein Methylation
2.1. Types and Function
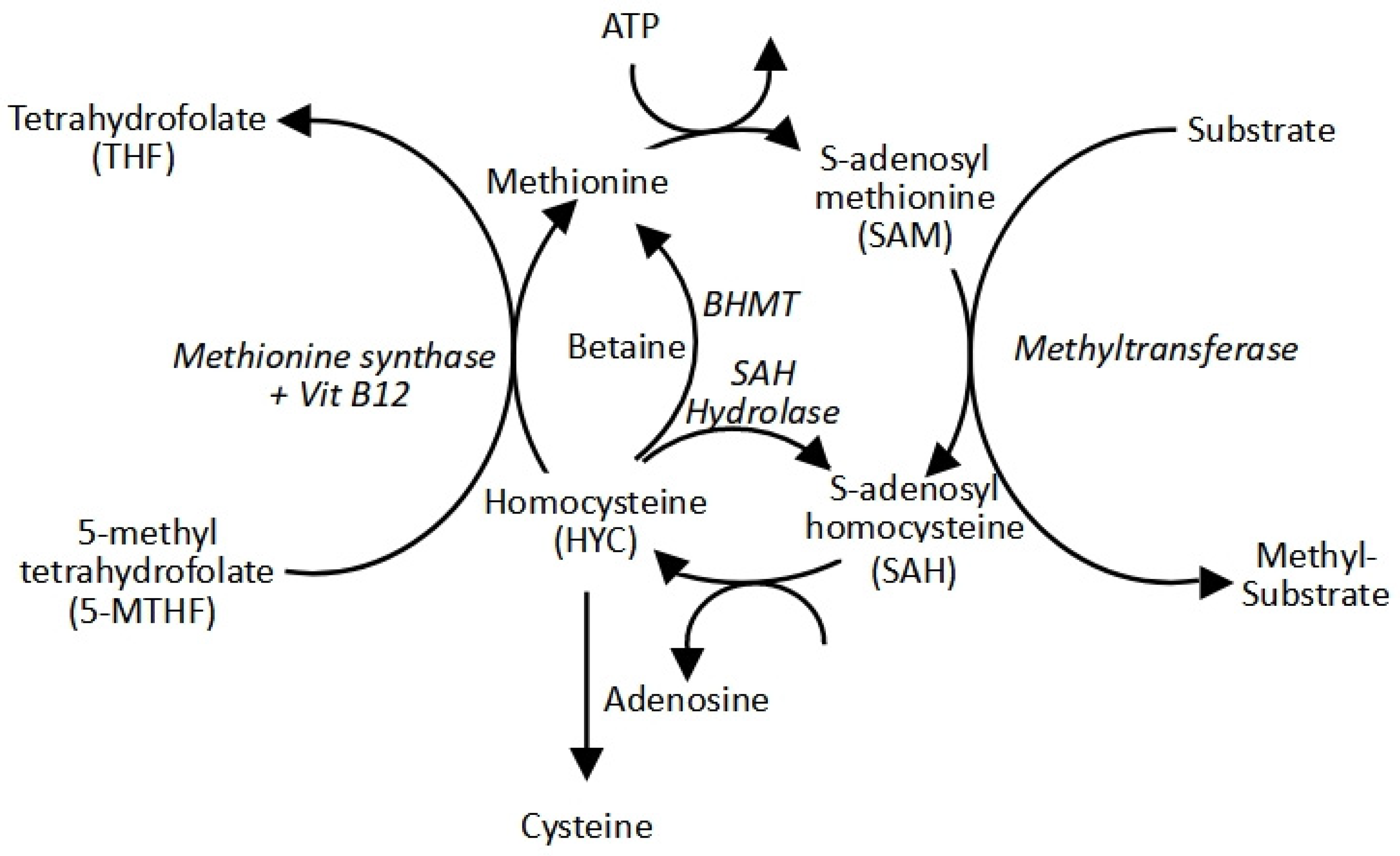
2.2. The Methyl Cycle: S-Adenosylmethionine Production
2.3. Protein Methyltransferases
2.3.1. Protein Lysine Methyltransferases (PKMTs)
2.3.2. Protein Arginine Methyltransferases (PRMTs)
2.3.3. Cancer Dysregulation
2.4. Classes of Methyltransferase Inhibitors
2.4.1. SAM Competitive Inhibitors
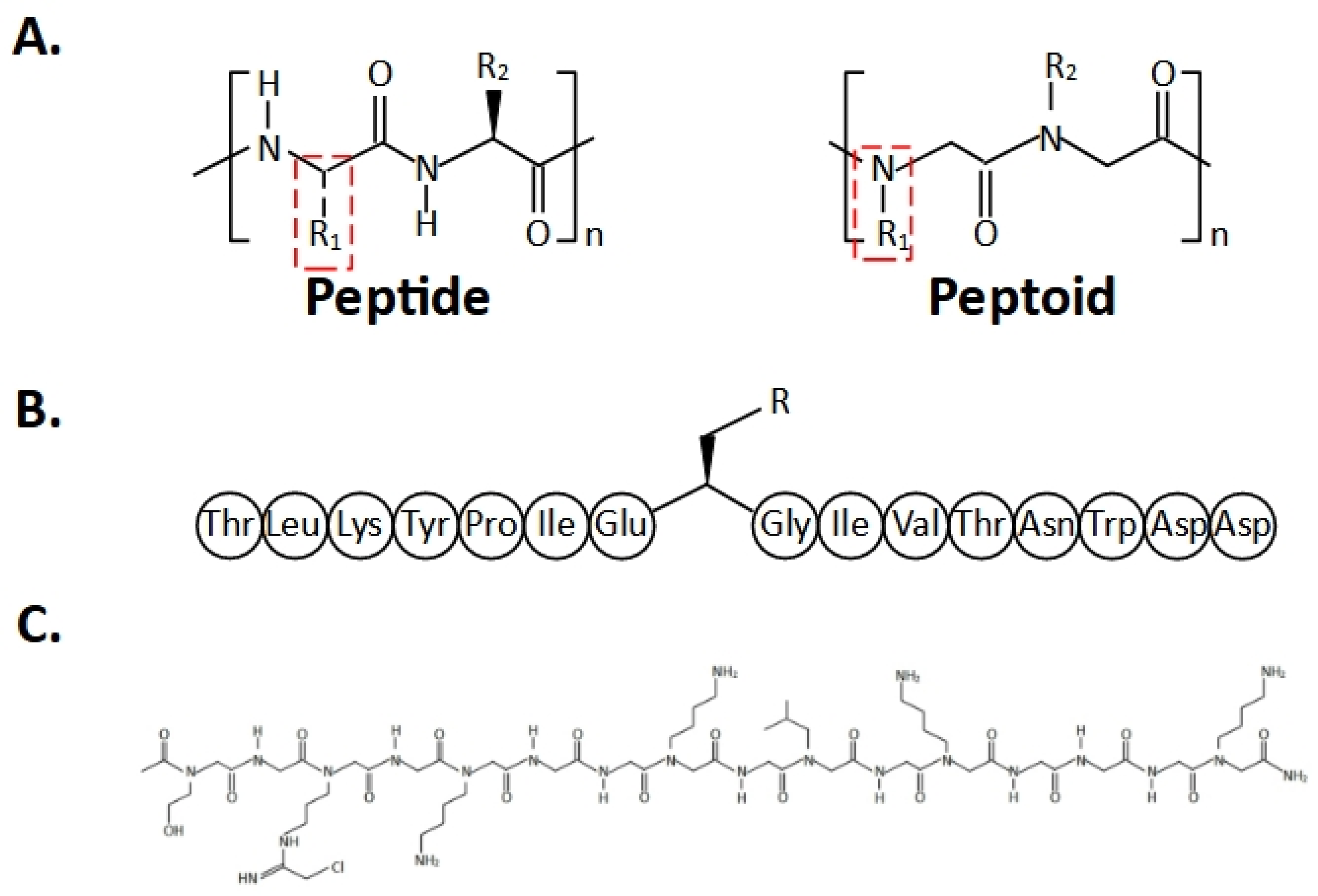
2.4.2. Substrate Competitive Inhibitors
2.4.3. Bisubstrate Inhibitors
2.4.4. Allosteric Inhibitors
2.4.5. Complex Disrupting Inhibitors
2.4.6. Covalent Inhibitors
2.4.7. PROTAC Inhibitors
2.4.8. Inhibitor Limitations
3. Failure to Clinic
4. Considerations for Designing Novel PMT Inhibitors
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-CdA | 2-chlorodeoxyadenosine |
| 5-FU | 5-fluorouracil |
| 5-MTHF | 5-methyltetrahydrafolate |
| 7βS | seven-β-strand |
| ABC | atp-binding cassette |
| ADMA/aRme2 | di-asymmetrical methyl arginine |
| ADME | absorption, distribution, metabolism, and excretion |
| AHcy | homocysteine |
| AHCY | adenosylhomocysteinase |
| AI | artificial intelligence |
| AKT | protein kinase B |
| Ala (A) | alanine |
| Ara-C | arabinoside (cytarabine) |
| ATP | adenosine triphosphate |
| AURKB | aurora kinase b |
| BHMT | betaine homocysteine methyltransferase |
| BRCA1 | breast cancer type 1 susceptibility protein |
| CDKN | cyclin-dependent kinase inhibitor |
| CRC | colorectal cancer |
| CRBN | cereblon |
| CSC | cancer stem cell |
| Cys (C) | cysteine |
| DELFIA | dissociation-enhanced lanthanide fluoroimmunoassays |
| DIA | data-independent acquisition |
| DOT1L | disruptor of telomeric silencing-1-like |
| EGFR | epidermal growth factor receptor |
| EHMT2 | euchromatic histone-lysine n-methyltransferase 2 |
| ELISA | enzyme-linked immunosorbent assays |
| ERK | extracellular signal-regulated kinase |
| EED | embryonic ectoderm development |
| EZH2 | enhancer of zeste 2 |
| GMS | 6′-methyleneamine sinefungin |
| GST | glutathione transferases |
| GSH | glutathione |
| HDAC2 | histone deacetylase 2 |
| His (H) | histidine |
| HSP70 or HSP90 | heat shock proteins 70 or 90 |
| K (Lys) | lysine |
| Kme1 | mono-methyl lysine |
| Kme2 | di-methyl lysine |
| Kme3 | tri-methyl lysine |
| MAPK | mitogen-activated protein kinase |
| MAT | methionine adenosyltransferase |
| M | methionine |
| MEP50 | methylosome protein 50 |
| METTL | methyltransferase-like |
| miRNAs | microRNAs |
| MMA/Rme1 | mono-methyl arginine |
| MLL1 | mixed-lineage leukaemia protein 1 |
| MRM | multiple reaction monitoring |
| MRP2 | multidrug resistance protein 2 |
| mTOR | mammalian target of rapamycin |
| MTR | methionine synthase |
| NF-κB | nuclear factor kappa B |
| N (Asn) | asparagine |
| P (Pro) | proline |
| p53 | tumour protein 53 |
| PARP | poly (ADP-ribose) polymerase |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed cell death ligand 1 |
| PGM | proline-glycine-methionine-rich |
| PI3K | phosphoinositide 3-kinases |
| pICln | chloride conductance regulatory protein |
| PMTs | protein methyltransferases |
| PKMTs | protein lysine methyltransferases |
| PRC2 | polycomb repressive complex 2 |
| PRMTs | protein arginine methyltransferases |
| PROTACs | proteolysis targeting chimeras |
| RB1 | Retinoblastoma 1 |
| RioK1 | RIO kinase 1 |
| R (Arg) | arginine |
| RSK4 | Ribosomal S6 kinase 4 |
| SAH/AdoHC | S-adenosylhomocysteine |
| SAM/AdoMet | S-adenosylmethionine |
| SDMA/sRme2 | di-symmetrical methyl arginine |
| SET | Su(var)3–9, enhancer of zeste (E(z)), and trithorax (trx) |
| SETD7 | SET domain containing 7 |
| SILAC | stable isotope labelling by amino acids in cell culture |
| SPOUT | SpoU-TrmD |
| SRM | selected reaction monitoring |
| STAT3 | signal transducer and activator of transcription 3 |
| SYMD2 | SET And MYND domain containing 2 |
| TKI | tyrosine kinase inhibitors |
| TMT | tandem mass tag |
| TR-FRET | time-resolved fluorescence resonance energy transfer |
| UPS | ubiquitin-proteasome system |
| VHL | Von Hippel−Lindau |
| WDR5 | WD repeat-containing protein 5 |
References
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Kannampuzha, S.; Gopalakrishnan, A.V. Cancer chemoresistance and its mechanisms: Associated molecular factors and its regulatory role. Med. Oncol. 2023, 40, 264. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef]
- Elton, T.S.; Ozer, H.G.; Yalowich, J.C. Effects of DNA topoisomerase IIα splice variants on acquired drug resistance. Cancer Drug Resist. 2020, 3, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Boggon, T.J.; Dayaram, T.; Jänne, P.A.; Kocher, O.; Meyerson, M.; Johnson, B.E.; Eck, M.J.; Tenen, D.G.; Halmos, B. EGFR mutation and resistance of non–small-cell lung cancer to gefitinib. N. Engl. J. Med. 2005, 352, 786–792. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Liu, Z.; Song, C. Overcoming the emerging drug resistance of smoothened: An overview of small-molecule SMO antagonists with antiresistance activity. Futur. Med. Chem. 2018, 10, 2855–2875. [Google Scholar] [CrossRef]
- Galmarini, C.M.; Mackey, J.R.; Dumontet, C. Nucleoside analogues: Mechanisms of drug resistance and reversal strategies. Leukemia 2001, 15, 875–890. [Google Scholar] [CrossRef]
- Damaraju, V.L.; Damaraju, S.; Young, J.D.; Baldwin, S.A.; Mackey, J.; Sawyer, M.B.; Cass, C.E. Nucleoside anticancer drugs: The role of nucleoside transporters in resistance to cancer chemotherapy. Oncogene 2003, 22, 7524–7536. [Google Scholar] [CrossRef]
- Gao, J.; Wang, C.; Wei, W. The effects of drug transporters on the efficacy of methotrexate in the treatment of rheumatoid arthritis. Life Sci. 2021, 268, 118907. [Google Scholar] [CrossRef]
- Vaidyanathan, A.; Sawers, L.; Gannon, A.L.; Chakravarty, P.; Scott, A.L.; Bray, S.E.; Ferguson, M.J.; Smith, G. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel-and olaparib-resistant ovarian cancer cells. Br. J. Cancer 2016, 115, 431–441. [Google Scholar] [CrossRef]
- Hui, R.C.-Y.; Francis, R.E.; Guest, S.K.; Costa, J.R.; Gomes, A.R.; Myatt, S.S.; Brosens, J.J.; Lam, E.W.-F. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol. Cancer Ther. 2008, 7, 670–678. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Farahani, M.V.; Hushmandi, K.; Zarrabi, A.; Goldman, A.; Ashrafizadeh, M.; Orive, G. Advances in understanding the role of P-gp in doxorubicin resistance: Molecular pathways, therapeutic strategies, and prospects. Drug Discov. Today 2022, 27, 436–455. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.G.; Mun, M.H.; Jeong, M.S.; Kim, W.T.; Lee, S.R.; Chung, J.W.; Kim, S.I.; Kim, T.N.; Nam, J.K.; Leem, S.H. Drug resistance of bladder cancer cells through activation of ABCG2 by FOXM1. BMB Rep. 2018, 51, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.H.; Chen, M.C.; Baskaran, R.; Lin, Y.M.; Day, C.H.; Lin, Y.J.; Tu, C.C.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J. Cell. Physiol. 2018, 233, 5458–5467. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.J.; Lee, H.I.; Jeong, S.H.; Nam, H.J.; Cho, J.H. NRF2 knockdown resensitizes 5-fluorouracil-resistant pancreatic cancer cells by suppressing HO-1 and ABCG2 expression. Int. J. Mol. Sci. 2020, 21, 4646. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, H.; Yang, S.; Su, D. Increased ABCC2 expression predicts cisplatin resistance in non-small cell lung cancer. Cell Biochem. Funct. 2021, 39, 277–286. [Google Scholar] [CrossRef]
- Biswas, R.; Bugde, P.; He, J.; Merien, F.; Lu, J.; Liu, D.X.; Myint, K.; Liu, J.; McKeage, M.; Li, Y. Transport-mediated oxaliplatin resistance associated with endogenous overexpression of MRP2 in Caco-2 and PANC-1 cells. Cancers 2019, 11, 1330. [Google Scholar] [CrossRef] [PubMed]
- Pljesa-Ercegovac, M.; Savic-Radojevic, A.; Matic, M.; Coric, V.; Djukic, T.; Radic, T.; Simic, T. Glutathione transferases: Potential targets to overcome chemoresistance in solid tumors. Int. J. Mol. Sci. 2018, 19, 3785. [Google Scholar] [CrossRef]
- Spitzwieser, M.; Pirker, C.; Koblmüller, B.; Pfeiler, G.; Hacker, S.; Berger, W.; Heffeter, P.; Cichna-Markl, M. Promoter methylation patterns of ABCB1, ABCC1 and ABCG2 in human cancer cell lines, multidrug-resistant cell models and tumor, tumor-adjacent and tumor-distant tissues from breast cancer patients. Oncotarget 2016, 7, 73347–73369. [Google Scholar] [CrossRef]
- Li, A.; Song, J.; Lai, Q.; Liu, B.; Wang, H.; Xu, Y.; Feng, X.; Sun, X.; Du, Z. Hypermethylation of ATP-binding cassette B1 (ABCB 1) multidrug resistance 1 (MDR 1) is associated with cisplatin resistance in the A549 lung adenocarcinoma cell line. Int. J. Exp. Pathol. 2016, 97, 412–421. [Google Scholar] [CrossRef]
- Sumarpo, A.; Ito, K.; Saiki, Y.; Ishizawa, K.; Wang, R.; Chen, N.; Sunamura, M.; Horii, A. Genetic and epigenetic aberrations of ABCB1 synergistically boost the acquisition of taxane resistance in esophageal squamous cancer cells. Biochem. Biophys. Res. Commun. 2020, 526, 586–591. [Google Scholar] [CrossRef]
- Mondal, P.; Meeran, S.M. microRNAs in cancer chemoresistance: The sword and the shield. Non-Coding RNA Res. 2021, 6, 200–210. [Google Scholar] [CrossRef]
- Arrigoni, E.; Galimberti, S.; Petrini, M.; Danesi, R.; Di Paolo, A. ATP-binding cassette transmembrane transporters and their epigenetic control in cancer: An overview. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Shui, X.; Tian, L.; Zhou, Y.; Zhao, B. Targeting ACSS2 activity suspends the formation of chemoresistance through suppressed histone H3 acetylation in human breast cancer. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Ye, P.; Xing, H.; Lou, F.; Wang, K.; Pan, Q.; Zhou, X.; Gong, L.; Li, D. Histone deacetylase 2 regulates doxorubicin (Dox) sensitivity of colorectal cancer cells by targeting ABCB1 transcription. Cancer Chemother. Pharmacol. 2016, 77, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, K.M.; Hariharan, S. Regulatory players of DNA damage repair mechanisms: Role in cancer chemoresistance. Biomed. Pharmacother. 2017, 93, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Abad, E.; Graifer, D.; Lyakhovich, A. DNA damage response and resistance of cancer stem cells. Cancer Lett. 2020, 474, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Guan, Y.D.; Chen, X.S.; Yang, J.M.; Cheng, Y. DNA repair pathways in cancer therapy and resistance. Front. Pharmacol. 2021, 11, 629266. [Google Scholar] [CrossRef]
- Pan, S.T.; Li, Z.L.; He, Z.X.; Qiu, J.X.; Zhou, S.F. Molecular mechanisms for tumour resistance to chemotherapy. Clin. Exp. Pharmacol. Physiol. 2016, 43, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Alimbetov, D.; Askarova, S.; Umbayev, B.; Davis, T.; Kipling, D. Pharmacological targeting of cell cycle, apoptotic and cell adhesion signaling pathways implicated in chemoresistance of cancer cells. Int. J. Mol. Sci. 2018, 19, 1690. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, Y.; Zhou, S.F. Role of apoptosis in cancer resistance to chemotherapy. In Current Understanding of Apoptosis—Programmed Cell Death; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Deng, W.; Wang, Y.; Ma, L.; Zhang, Y.; Ullah, S.; Xue, Y. Computational prediction of methylation types of covalently modified lysine and arginine residues in proteins. Brief Bioinform. 2017, 18, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, P.; Lavallée-Adam, M.; Faubert, D.; Blanchette, M.; Coulombe, B. A Newly Uncovered Group of Distantly Related Lysine Methyltransferases Preferentially Interact with Molecular Chaperones to Regulate Their Activity. PLoS Genet. 2013, 9, e1003210. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.G. Protein methylation at the surface and buried deep: Thinking outside the histone box. Trends Biochem. Sci. 2013, 38, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A. Protein methyltransferase inhibitors as precision cancer therapeutics: A decade of discovery. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170080. [Google Scholar] [CrossRef]
- Al-Hamashi, A.A.; Diaz, K.; Huang, R. Non-histone arginine methylation by protein arginine methyltransferases. Curr. Protein Pept. Sci. 2020, 21, 699–712. [Google Scholar] [CrossRef]
- Fulton, M.D.; Brown, T.; Zheng, Y.G. Mechanisms and inhibitors of histone arginine methylation. Chem. Rec. 2018, 18, 1792–1807. [Google Scholar] [CrossRef]
- Kaniskan, H.Ü.; Jin, J. Chemical probes of histone lysine methyltransferases. ACS Chem. Biol. 2015, 10, 40–50. [Google Scholar] [CrossRef]
- Kaniskan, H.U.; Konze, K.D.; Jin, J. Selective inhibitors of protein methyltransferases. J. Med. Chem. 2015, 58, 1596–1629. [Google Scholar] [CrossRef]
- Wei, H.; Mundade, R.; Lange, K.; Lu, T. Protein arginine methylation of non-histone proteins and its role in diseases. Cell Cycle 2014, 13, 32–41. [Google Scholar] [CrossRef]
- Wesche, J.; Kühn, S.; Kessler, B.M.; Salton, M.; Wolf, A. Protein arginine methylation: A prominent modification and its demethylation. Cell. Mol. Life Sci. 2017, 74, 3305–3315. [Google Scholar] [CrossRef]
- Lee, T.Y.; Chang, C.W.; Lu, C.T.; Cheng, T.H.; Chang, T.H. Identification and characterization of lysine-methylated sites on histones and non-histone proteins. Comput. Biol. Chem. 2014, 50, 11–18. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Shi, X. Emerging roles of lysine methylation on non-histone proteins. Cell. Mol. Life Sci. 2015, 72, 4257–4272. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, R.; Saloura, V.; Nakamura, Y. Critical roles of non-histone protein lysine methylation in human tumorigenesis. Nat. Rev. Cancer 2015, 15, 110–124. [Google Scholar] [CrossRef]
- Moore, K.E.; Gozani, O. An unexpected journey: Lysine methylation across the proteome. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2014, 1839, 1395–1403. [Google Scholar] [CrossRef]
- Biggar, K.K.; Li, S.S.C. Non-histone protein methylation as a regulator of cellular signalling and function. Nat. Rev. Mol. Cell Biol. 2015, 16, 5–17. [Google Scholar] [CrossRef]
- Mato, J.M.; Corrales, F.J.; Lu, S.C.; Avila, M.A. S-adenosylmethionine: A control switch that regulates liver function. FASEB J. 2002, 16, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Komoto, J.; Yamada, T.; Takata, Y.; Markham, G.D.; Takusagawa, F. Crystal structure of the S-adenosylmethionine synthetase ternary complex: A novel catalytic mechanism of S-adenosylmethionine synthesis from ATP and Met. Biochemistry 2004, 43, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Huang, Y.; Qiu, M.; Yin, C.; Ren, H.; Gan, H.; Li, H.; Zhou, Y.; Xia, J.; Li, W.; et al. Immunoassay of S-adenosylmethionine and S-adenosylhomocysteine: The methylation index as a biomarker for disease and health status. BMC Res. Notes 2016, 9, 498. [Google Scholar] [CrossRef]
- Pascale, R.M.; Simile, M.M.; Calvisi, D.F.; Feo, C.F.; Feo, F. S-adenosylmethionine: From the discovery of its inhibition of tumorigenesis to its use as a therapeutic agent. Cells 2022, 11, 409. [Google Scholar] [CrossRef]
- Nazki, F.H.; Sameer, A.S.; Ganaie, B.A. Folate: Metabolism, genes, polymorphisms and the associated diseases. Gene 2014, 533, 11–20. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Loenen, W.A. S-Adenosylmethionine: Jack of all trades and master of everything? Biochem. Soc. Trans. 2006, 34, 330–333. [Google Scholar] [CrossRef]
- Mosca, L.; Minopoli, M.; Pagano, M.; Vitiello, F.; Carriero, M.V.; Cacciapuoti, G.; Porcelli, M. Effects of S-adenosyl-L-methionine on the invasion and migration of head and neck squamous cancer cells and analysis of the underlying mechanisms. Int. J. Oncol. 2020, 56, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Z.; Szyf, M. S-adenosyl-methionine (SAM) alters the transcriptome and methylome and specifically blocks growth and invasiveness of liver cancer cells. Oncotarget 2017, 8, 111866–111881. [Google Scholar] [CrossRef]
- Mosca, L.; Pagano, M.; Borzacchiello, L.; Mele, L.; Russo, A.; Russo, G.; Cacciapuoti, G.; Porcelli, M. S-adenosylmethionine increases the sensitivity of human colorectal cancer cells to 5-fluorouracil by inhibiting P-glycoprotein expression and NF-κB activation. Int. J. Mol. Sci. 2021, 22, 9286. [Google Scholar] [CrossRef]
- Fernández-Ramos, D.; Lopitz-Otsoa, F.; Lu, S.C.; Mato, J.M. S-adenosylmethionine: A multifaceted regulator in cancer pathogenesis and therapy. Cancers 2025, 17, 535. [Google Scholar] [CrossRef]
- Richon, V.M.; Johnston, D.; Sneeringer, C.J.; Jin, L.; Majer, C.R.; Elliston, K.; Jerva, L.F.; Scott, M.P.; Copeland, R.A. Chemogenetic analysis of human protein methyltransferases. Chem. Biol. Drug Des. 2011, 78, 199–210. [Google Scholar] [CrossRef]
- Kaniskan, H.U.; Martini, M.L.; Jin, J. Inhibitors of protein methyltransferases and demethylases. Chem. Rev. 2018, 118, 989–1068. [Google Scholar] [CrossRef] [PubMed]
- Kozbial, P.Z.; Mushegian, A.R. Natural history of S-adenosylmethionine-binding proteins. BMC Struct. Biol. 2005, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, D.; Gozani, O. Histone lysine methyltransferases in biology and disease. Nat. Struct. Mol. Biol. 2019, 26, 880–889. [Google Scholar]
- Schapira, M.; de Freitas, R.F. Structural biology and chemistry of protein arginine methyltransferases. Medchemcomm 2014, 5, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Petrossian, T.C.; Clarke, S.G. Uncovering the human methyltransferasome. Mol. Cell Proteom. 2011, 10, M110.000976. [Google Scholar] [CrossRef]
- Tkaczuk, K.L.; Dunin-Horkawicz, S.; Purta, E.; Bujnicki, J.M. Structural and evolutionary bioinformatics of the SPOUT superfamily of methyltransferases. BMC Bioinform. 2007, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Young, B.D.; Weiss, D.I.; Zurita-Lopez, C.I.; Webb, K.J.; Clarke, S.G.; McBride, A.E. Identification of methylated proteins in the yeast small ribosomal subunit: A role for SPOUT methyltransferases in protein arginine methylation. Biochemistry 2012, 51, 5091–5104. [Google Scholar] [CrossRef]
- Carlson, S.M.; Gozani, O. Nonhistone lysine methylation in the regulation of cancer pathways. Cold Spring Harb. Perspect. Med. 2016, 6, a026435. [Google Scholar] [CrossRef]
- Yang, Y.; Bedford, M.T. Protein arginine methyltransferases and cancer. Nat. Rev. Cancer 2013, 13, 37–50. [Google Scholar] [CrossRef]
- Allis, C.D.; Berger, S.L.; Cote, J.; Dent, S.; Jenuwien, T.; Kouzarides, T.; Pillus, L.; Reinberg, D.; Shi, Y.; Shiekhattar, R.; et al. New Nomenclature for Chromatin-Modifying Enzymes. Cell 2007, 131, 633–636. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, H.; Shi, X. Lysine methylation: Beyond histones. Acta Biochim. Biophys. Sin. (Shanghai) 2012, 44, 14–27. [Google Scholar] [CrossRef]
- Cheng, X.; Collins, R.E.; Zhang, X. Structural and sequence motifs of protein (histone) methylation enzymes. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 267–294. [Google Scholar] [CrossRef]
- Falnes, P.; Jakobsson, M.E.; Davydova, E.; Ho, A.; Malecki, J. Protein lysine methylation by seven-β-strand methyltransferases. Biochem. J. 2016, 473, 1995–2009. [Google Scholar] [CrossRef]
- McGrath, J.; Trojer, P. Targeting histone lysine methylation in cancer. Pharmacol. Ther. 2015, 150, 1–22. [Google Scholar] [CrossRef]
- Wood, K.; Tellier, M.; Murphy, S. DOT1L and H3K79 methylation in transcription and genomic stability. Biomolecules 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Eirin-Lopez, J.M. Evolution of methyltransferase-like (METTL) proteins in metazoa: A complex gene family involved in epitranscriptomic regulation and other epigenetic processes. Mol. Biol. Evol. 2021, 38, 5309–5327. [Google Scholar] [CrossRef] [PubMed]
- Falnes, P.Ø.; Małecki, J.M.; Herrera, M.C.; Bengtsen, M.; Davydova, E. Human seven-β-strand (METTL) methyltransferases-conquering the universe of protein lysine methylation. J. Biol. Chem. 2023, 299, 104661. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Richard, S. Cellular pathways influenced by protein arginine methylation: Implications for cancer. Mol. Cell 2021, 81, 4357–4368. [Google Scholar] [CrossRef]
- Tewary, S.K.; Zheng, Y.G.; Ho, M.C. Protein arginine methyltransferases: Insights into the enzyme structure and mechanism at the atomic level. Cell Mol. Life Sci. 2019, 76, 2917–2932. [Google Scholar] [CrossRef]
- Yang, Y.; Hadjikyriacou, A.; Xia, Z.; Gayatri, S.; Kim, D.; Zurita-Lopez, C.; Kelly, R.; Guo, A.; Li, W.; Clarke, S.G.; et al. PRMT9 is a type II methyltransferase that methylates the splicing factor SAP145. Nat. Commun. 2015, 6, 6428. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Y.; Hu, X.; Zheng, Y.; Chen, X. Targeting PRMT5/Akt signalling axis prevents human lung cancer cell growth. J. Cell. Mol. Med. 2019, 23, 1333–1342. [Google Scholar] [CrossRef]
- Lin, H.; Wang, B.; Yu, J.; Wang, J.; Li, Q.; Cao, B. Protein arginine methyltransferase 8 gene enhances the colon cancer stem cell (CSC) function by upregulating the pluripotency transcription factor. J. Cancer 2018, 9, 1394–1402. [Google Scholar] [CrossRef]
- Shailesh, H.; Siveen, K.S.; Sif, S. Protein arginine methyltransferase 5 (PRMT5) activates WNT/β-catenin signalling in breast cancer cells via epigenetic silencing of DKK1 and DKK3. J. Cell. Mol. Med. 2021, 25, 1583–1600. [Google Scholar] [CrossRef]
- Chai, H.; Pan, C.; Zhang, M.; Huo, H.; Shan, H.; Wu, J. Histone methyltransferase SETD1A interacts with notch and promotes notch transactivation to augment ovarian cancer development. BMC Cancer 2023, 23, 96. [Google Scholar] [CrossRef]
- Hou, Z.; Sun, L.; Xu, F.; Hu, F.; Lan, J.; Song, D.; Feng, Y.; Wang, J.; Luo, X.; Hu, J.; et al. Blocking histone methyltransferase SETDB1 inhibits tumorigenesis and enhances cetuximab sensitivity in colorectal cancer. Cancer Lett. 2020, 487, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Vougiouklakis, T.; Bernard, B.J.; Nigam, N.; Burkitt, K.; Nakamura, Y.; Saloura, V. Clinicopathologic significance of protein lysine methyltransferases in cancer. Clin. Epigenet. 2020, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Chen, L.; Xiao, G.; Zeng, Y.; Shi, W.; Tanzhu, G.; Zhou, R. The protein arginine methyltransferase family (PRMTs) regulates metastases in various tumors: From experimental study to clinical application. Biomed. Pharmacother. 2023, 167, 115456. [Google Scholar] [CrossRef]
- Campagna-Slater, V.; Mok, M.W.; Nguyen, K.T.; Feher, M.; Najmanovich, R.; Schapira, M. Structural chemistry of the histone methyltransferases cofactor binding site. J. Chem. Inf. Model. 2011, 51, 612–623. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Q.; Paulk, J.; Kubicek, S.; Kemp, M.M.; Adams, D.J.; Shamji, A.F.; Wagner, B.K.; Schreiber, S.L. A small-molecule probe of the histone methyltransferase G9a induces cellular senescence in pancreatic adenocarcinoma. ACS Chem. Biol. 2012, 7, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.P.; Swewczyk, M.; Kennedy, S.; Trush, V.V.; Wu, H.; Zeng, H.; Dong, A.; de Freitas, R.F.; Tatlock, J.; Kumpf, R.A.; et al. Selective, small-molecule co-factor binding site inhibition of a Su (var) 3–9, enhancer of zeste, trithorax domain containing lysine methyltransferase. J. Med. Chem. 2019, 62, 7669–7683. [Google Scholar] [CrossRef]
- Wei, L.; Mei, D.; Hu, S.; Du, S. Dual-target EZH2 inhibitor: Latest advances in medicinal chemistry. Futur. Med. Chem. 2024, 16, 1561–1582. [Google Scholar] [CrossRef] [PubMed]
- Waters, N.J. Preclinical pharmacokinetics and pharmacodynamics of pinometostat (EPZ-5676), a first-in-class, small molecule S-adenosyl methionine competitive inhibitor of DOT1L. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 891–901. [Google Scholar] [CrossRef]
- Lin, H.; Luengo, J.I. Nucleoside protein arginine methyltransferase 5 (PRMT5) inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 1264–1269. [Google Scholar] [CrossRef]
- Ferreira de Freitas, R.; Ivanochko, D.; Schapira, M. Methyltransferase inhibitors: Competing with, or exploiting the bound cofactor. Molecules 2019, 24, 4492. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Barsyte-Lovejoy, D.; Li, F.; Xiong, Y.; Korboukh, V.; Huang, X.P.; Allali-Hassani, A.; Janzen, W.P.; Roth, B.L.; Frye, S.V.; et al. Discovery of an in vivo chemical probe of the lysine methyltransferases G9a and GLP. J. Med. Chem. 2013, 56, 8931–8942. [Google Scholar] [CrossRef] [PubMed]
- Konze, K.D.; Pattenden, S.G.; Liu, F.; Barsyte-Lovejoy, D.; Li, F.; Simon, J.M.; Davis, I.J.; Vedadi, M.; Jin, J. A chemical tool for in vitro and in vivo precipitation of lysine methyltransferase G9a. ChemMedChem 2014, 9, 549–553. [Google Scholar] [CrossRef]
- Sweis, R.F.; Pliushchev, M.; Brown, P.J.; Guo, J.; Li, F.; Maag, D.; Petros, A.M.; Soni, N.B.; Tse, C.; Vedadi, M.; et al. Discovery and development of potent and selective inhibitors of histone methyltransferase g9a. ACS Med. Chem. Lett. 2014, 5, 205–209. [Google Scholar] [CrossRef]
- Wang, S.E.; Xiong, Y.; Jang, M.A.; Park, K.S.; Donahue, M.; Velez, J.; Jin, J.; Jiang, Y.H. Newly developed oral bioavailable EHMT2 inhibitor as a potential epigenetic therapy for Prader-Willi syndrome. Mol. Ther. 2024, 32, 2662–2675. [Google Scholar] [CrossRef]
- Konze, K.D.; Ma, A.; Li, F.; Barsyte-Lovejoy, D.; Parton, T.; MacNevin, C.J.; Liu, F.; Gao, C.; Huang, X.P.; Kuznetsova, E.; et al. An orally bioavailable chemical probe of the lysine methyltransferases EZH2 and EZH1. ACS Chem. Biol. 2013, 8, 1324–1334. [Google Scholar] [CrossRef]
- Vaswani, R.G.; Gehling, V.S.; Dakin, L.A.; Cook, A.S.; Nasveschuk, C.G.; Duplessis, M.; Iyer, P.; Balasubramanian, S.; Zhao, F.; Good, A.C.; et al. Identification of (R)-N-((4-Methoxy-6-methyl-2-oxo-1, 2-dihydropyridin-3-yl) methyl)-2-methyl-1-(1-(1-(2, 2, 2-trifluoroethyl) piperidin-4-yl) ethyl)-1 H-indole-3-carboxamide (CPI-1205), a Potent and Selective Inhibitor of Histone Methyltransferase EZH2, Suitable for Phase I Clinical Trials for B-Cell Lymphomas. J. Med. Chem. 2016, 59, 9928–9941. [Google Scholar]
- Kung, P.P.; Bingham, P.; Brooun, A.; Collins, M.; Deng, Y.L.; Dinh, D.; Fan, C.; Gajiwala, K.S.; Grantner, R.; Gukasyan, H.J.; et al. Optimization of orally bioavailable enhancer of zeste homolog 2 (EZH2) inhibitors using ligand and property-based design strategies: Identification of development candidate (R)-5, 8-Dichloro-7-(methoxy (oxetan-3-yl) methyl)-2-((4-methoxy-6-methyl-2-oxo-1, 2-dihydropyridin-3-yl) methyl)-3, 4-dihydroisoquinolin-1 (2 H)-one (PF-06821497). J. Med. Chem. 2018, 61, 650–665. [Google Scholar]
- Lu, B.; Shen, X.; Zhang, L.; Liu, D.; Zhang, C.; Cao, J.; Shen, R.; Zhang, J.; Wang, D.; Wan, H.; et al. Discovery of EBI-2511: A highly potent and orally active EZH2 inhibitor for the treatment of non-hodgkin’s lymphoma. ACS Med. Chem. Lett. 2018, 9, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Barsyte-Lovejoy, D.; Li, F.; Oudhoff, M.J.; Tatlock, J.H.; Dong, A.; Zeng, H.; Wu, H.; Freeman, S.A.; Schapira, M.; Senisterra, G.A.; et al. (R)-PFI-2 is a potent and selective inhibitor of SETD7 methyltransferase activity in cells. Proc. Natl. Acad. Sci. USA 2014, 111, 12853–12858. [Google Scholar] [CrossRef]
- Takemoto, Y.; Ito, A.; Niwa, H.; Okamura, M.; Fujiwara, T.; Hirano, T.; Handa, N.; Umehara, T.; Sonoda, T.; Ogawa, K.; et al. Identification of cyproheptadine as an inhibitor of SET domain containing lysine methyltransferase 7/9 (Set7/9) that regulates estrogen-dependent transcription. J. Med. Chem. 2016, 59, 3650–3660. [Google Scholar] [CrossRef]
- Eggert, E.; Hillig, R.C.; Koehr, S.; Stöckigt, D.; Weiske, J.; Barak, N.; Mowat, J.; Brumby, T.; Christ, C.D.; Ter Laak, A.; et al. Discovery and characterization of a highly potent and selective aminopyrazoline-based in vivo probe (BAY-598) for the protein lysine methyltransferase SMYD2. J. Med. Chem. 2016, 59, 4578–4600. [Google Scholar] [CrossRef]
- Mitchell, L.H.; Boriack-Sjodin, P.A.; Smith, S.; Thomenius, M.; Rioux, N.; Munchhof, M.; Mills, J.E.; Klaus, C.; Totman, J.; Riera, T.V.; et al. Novel oxindole sulfonamides and sulfamides: EPZ031686, the first orally bioavailable small molecule SMYD3 inhibitor. ACS Med. Chem. Lett. 2016, 7, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Sweis, R.F.; Wang, Z.; Algire, M.; Arrowsmith, C.H.; Brown, P.J.; Chiang, G.G.; Guo, J.; Jakob, C.G.; Kennedy, S.; Li, F.; et al. Discovery of A-893, a new cell-active benzoxazinone inhibitor of lysine methyltransferase SMYD2. ACS Med. Chem. Lett. 2015, 6, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Thomenius, M.J.; Totman, J.; Harvey, D.; Mitchell, L.H.; Riera, T.V.; Cosmopoulos, K.; Grassian, A.R.; Klaus, C.; Foley, M.; Admirand, E.A.; et al. Small molecule inhibitors and CRISPR/Cas9 mutagenesis demonstrate that SMYD2 and SMYD3 activity are dispensable for autonomous cancer cell proliferation. PLoS ONE 2018, 13, e0197372. [Google Scholar] [CrossRef] [PubMed]
- Peserico, A.; Germani, A.; Sanese, P.; Barbosa, A.J.; Di Virgilio, V.; Fittipaldi, R.; Fabini, E.; Bertucci, C.; Varchi, G.; Moyer, M.P.; et al. A SMYD3 small-molecule inhibitor impairing cancer cell growth. J. Cell. Physiol. 2015, 230, 2447–2460. [Google Scholar] [CrossRef]
- Gradl, S.; Steuber, H.; Weiske, J.; Schmees, N.; Siegel, S.; Stoeckigt, D.; Christ, C.D.; Li, F.; Organ, S.; Barsyte-Lovejoy, D.; et al. Abstract 1646: Discovery and characterization of BAY-6035, a novel benzodiazepine-based SMYD3 inhibitor. Cancer Res. 2018, 78 (Suppl. S13), 1646. [Google Scholar] [CrossRef]
- Ma, A.; Yu, W.; Li, F.; Bleich, R.M.; Herold, J.M.; Butler, K.V.; Norris, J.L.; Korboukh, V.; Tripathy, A.; Janzen, W.P.; et al. Discovery of a selective, substrate-competitive inhibitor of the lysine methyltransferase SETD8. J. Med. Chem. 2014, 57, 6822–6833. [Google Scholar] [CrossRef]
- Blum, G.; Ibanez, G.; Rao, X.; Shum, D.; Radu, C.; Djaballah, H.; Rice, J.C.; Luo, M. Small-Molecule Inhibitors of SETD8 with Cellular Activity. ACS Chem. Biol. 2014, 9, 2471–2478. [Google Scholar] [CrossRef]
- Butler, K.V.; Ma, A.; Yu, W.; Li, F.; Tempel, W.; Babault, N.; Pittella-Silva, F.; Shao, J.; Wang, J.; Luo, M.; et al. Structure-based design of a covalent inhibitor of the SET domain-containing protein 8 (SETD8) lysine methyltransferase. J. Med. Chem. 2016, 59, 9881–9889. [Google Scholar] [CrossRef]
- Gerhart, S.V.; Kellner, W.A.; Thompson, C.; Pappalardi, M.B.; Zhang, X.P.; de Oca, R.M.; Penebre, E.; Duncan, K.; Boriack-Sjodin, A.; Le, B.; et al. Activation of the p53-MDM4 regulatory axis defines the anti-tumour response to PRMT5 inhibition through its role in regulating cellular splicing. Sci. Rep. 2018, 8, 9711. [Google Scholar] [CrossRef]
- Chan-Penebre, E.; Kuplast, K.G.; Majer, C.R.; Boriack-Sjodin, P.A.; Wigle, T.J.; Johnston, L.D.; Rioux, N.; Munchhof, M.J.; Jin, L.; Jacques, S.L.; et al. A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat. Chem. Biol. 2015, 11, 432–437. [Google Scholar] [CrossRef]
- Duncan, K.W.; Rioux, N.; Boriack-Sjodin, P.A.; Munchhof, M.J.; Reiter, L.A.; Majer, C.R.; Jin, L.; Johnston, L.D.; Chan-Penebre, E.; Kuplast, K.G.; et al. Structure and property guided design in the identification of PRMT5 tool compound EPZ015666. ACS Med. Chem. Lett. 2016, 7, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Iwase, K.; Iwanami, N.; Tanaka, Y.; Kagechika, H.; Hirano, T. Development of novel bisubstrate-type inhibitors of histone methyltransferase SET7/9. Bioorg. Med. Chem. 2010, 18, 8158–8166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qian, K.; Yan, C.; He, M.; Jassim, B.A.; Ivanov, I.; Zheng, Y.G. Discovery of decamidine as a new and potent PRMT1 inhibitor. MedChemComm 2017, 8, 440–444. [Google Scholar] [CrossRef]
- Alinari, L.; Mahasenan, K.V.; Yan, F.; Karkhanis, V.; Chung, J.H.; Smith, E.M.; Quinion, C.; Smith, P.L.; Kim, L.; Patton, J.T.; et al. Selective inhibition of protein arginine methyltransferase 5 blocks initiation and maintenance of B-cell transformation. Blood 2015, 125, 2530–2543. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zheng, W.; Eram, M.S.; Vhuiyan, M.; Dong, A.; Zeng, H.; He, H.; Brown, P.; Frankel, A.; Vedadi, M.; et al. Structural basis of arginine asymmetrical dimethylation by PRMT6. Biochem. J. 2016, 473, 3049–3063. [Google Scholar] [CrossRef]
- Kaniskan, H.Ü.; Szewczyk, M.M.; Yu, Z.; Eram, M.S.; Yang, X.; Schmidt, K.; Luo, X.; Dai, M.; He, F.; Zang, I.; et al. A potent, selective and cell-active allosteric inhibitor of protein arginine methyltransferase 3 (PRMT3). Angew. Chem. Int. Ed. 2015, 54, 5166–5170. [Google Scholar] [CrossRef]
- Talibov, V.O.; Fabini, E.; FitzGerald, E.A.; Tedesco, D.; Cederfelt, D.; Talu, M.J.; Rachman, M.M.; Mihalic, F.; Manoni, E.; Naldi, M.; et al. Discovery of an allosteric ligand binding site in SMYD3 lysine methyltransferase. ChemBioChem 2021, 22, 1597–1608. [Google Scholar] [CrossRef]
- Liu, K.L.; Zhu, K.; Zhang, H. An overview of the development of EED inhibitors to disable the PRC2 function. RSC Med. Chem. 2022, 13, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Bird, G.H.; Neff, T.; Guo, G.; Kerenyi, M.A.; Walensky, L.D.; Orkin, S.H. Targeted disruption of the EZH2-EED complex inhibits EZH2-dependent cancer. Nat. Chem. Biol. 2013, 9, 643–650. [Google Scholar] [CrossRef]
- Qi, W.; Zhao, K.; Gu, J.; Huang, Y.; Wang, Y.; Zhang, H.; Zhang, M.; Zhang, J.; Yu, Z.; Li, L.; et al. An allosteric PRC2 inhibitor targeting the H3K27me3 binding pocket of EED. Nat. Chem. Biol. 2017, 13, 381–388. [Google Scholar] [CrossRef]
- Crunkhorn, S. Allosteric histone methyltransferase modulators block tumour growth. Nat. Rev. Drug Discov. 2017, 16, 165. [Google Scholar] [CrossRef]
- Dong, H.; Liu, S.; Zhang, X.; Chen, S.; Kang, L.; Chen, Y.; Ma, S.; Fu, X.; Liu, Y.; Zhang, H.; et al. An allosteric PRC2 inhibitor targeting EED suppresses tumor progression by modulating the immune response. Cancer Res. 2019, 79, 5587–5596. [Google Scholar] [CrossRef]
- Rej, R.K.; Wang, C.; Lu, J.; Wang, M.; Petrunak, E.; Zawacki, K.P.; McEachern, D.; Fernandez-Salas, E.; Yang, C.Y.; Wang, L.; et al. EEDi-5285: An exceptionally potent, efficacious, and orally active small-molecule inhibitor of embryonic ectoderm development. J. Med. Chem. 2020, 63, 7252–7267. [Google Scholar] [CrossRef]
- Yokoyama, A.; Somervaille, T.C.; Smith, K.S.; Rozenblatt-Rosen, O.; Meyerson, M.; Cleary, M.L. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell 2005, 123, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Alicea-Velázquez, N.L.; Shinsky, S.A.; Loh, D.M.; Lee, J.H.; Skalnik, D.G.; Cosgrove, M.S. Targeted disruption of the interaction between WD-40 repeat protein 5 (WDR5) and mixed lineage leukemia (MLL)/SET1 family proteins specifically inhibits MLL1 and SETd1A methyltransferase complexes. J. Biol. Chem. 2016, 291, 22357–22372. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Aguilar, A.; Huang, L.; Xu, T.; Zheng, K.; McEachern, D.; Przybranowski, S.; Foster, C.; Zawacki, K.; Liu, Z.; et al. Discovery of M-808 as a highly potent, covalent, small-molecule inhibitor of the Menin–MLL interaction with strong in vivo antitumor activity. J. Med. Chem. 2020, 63, 4997–5010. [Google Scholar] [CrossRef]
- Trojer, P. Disrupting histone lysine methylation. Nat. Chem. Biol. 2015, 11, 552–554. [Google Scholar] [CrossRef]
- Chen, W.L.; Chen, X.; Li, D.D.; Wang, X.H.; Long, G.L.; Jiang, Z.Y.; You, Q.D.; Guo, X.K. Discovery of a potent MLL1 and WDR5 protein-protein interaction inhibitor with in vivo antitumor activity. Eur. J. Med. Chem. 2021, 223, 113677. [Google Scholar] [CrossRef] [PubMed]
- Stopa, N.; Krebs, J.E.; Shechter, D. The PRMT5 arginine methyltransferase: Many roles in development, cancer and beyond. Cell. Mol. Life Sci. 2015, 72, 2041–2059. [Google Scholar] [CrossRef]
- Antonysamy, S. The structure and function of the PRMT5:MEP50 complex. In Macromolecular Protein Complexes; Springer: Berlin/Heidelberg, Germany, 2017; pp. 185–194. [Google Scholar]
- Mulvaney, K.M.; Blomquist, C.; Acharya, N.; Li, R.; Ranaghan, M.J.; O’Keefe, M.; Rodriguez, D.J.; Young, M.J.; Kesar, D.; Pal, D.; et al. Molecular basis for substrate recruitment to the PRMT5 methylosome. Mol. Cell 2021, 81, 3481–3495. [Google Scholar] [CrossRef] [PubMed]
- Asberry, A.M.; Cai, X.; Deng, X.; Santiago, U.; Liu, S.; Sims, H.S.; Liang, W.; Xu, X.; Wan, J.; Jiang, W.; et al. Discovery and biological characterization of PRMT5:MEP50 protein–protein interaction inhibitors. J. Med. Chem. 2022, 65, 13793–13812. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowski, A.; Esser, L.M.; Willaume, A.; Prudent, R.; Peter, C.; ‘t Hart, P.; Waldmann, H. Development of macrocyclic PRMT5–adaptor protein interaction inhibitors. J. Med. Chem. 2022, 65, 15300–15311. [Google Scholar] [CrossRef]
- Powers, J.C.; Asgian, J.L.; Ekici, Ö.D.; James, K.E. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem. Rev. 2002, 102, 4639–4750. [Google Scholar] [CrossRef]
- Li, B.; Rong, D.; Wang, Y. Targeting protein-protein interaction with covalent small-molecule inhibitors. Curr. Top. Med. Chem. 2019, 19, 1872–1876. [Google Scholar] [CrossRef]
- Gehringer, M.; Laufer, S.A. Emerging and re-emerging warheads for targeted covalent inhibitors: Applications in medicinal chemistry and chemical biology. J. Med. Chem. 2018, 62, 5673–5724. [Google Scholar] [CrossRef]
- Chan, A.M.; Goodis, C.C.; Pommier, E.G.; Fletcher, S. Recent applications of covalent chemistries in protein–protein interaction inhibitors. RSC Med. Chem. 2022, 13, 921–928. [Google Scholar] [CrossRef]
- Ábrányi-Balogh, P.; Petri, L.; Imre, T.; Szijj, P.; Scarpino, A.; Hrast, M.; Mitrović, A.; Fonovič, U.P.; Németh, K.; Barreteau, H.; et al. A road map for prioritizing warheads for cysteine targeting covalent inhibitors. Eur. J. Med. Chem. 2018, 160, 94–107. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Hu, X.; Duan, X.; Wan, G.; Li, L.; Feng, Q.; Zhang, Y.; Wang, N.; Yu, L. Covalent inhibitors of EZH2: Design, synthesis and evaluation. Biomed. Pharmacother. 2022, 147, 112617. [Google Scholar] [CrossRef]
- Park, K.S.; Xiong, Y.; Yim, H.; Velez, J.; Babault, N.; Kumar, P.; Liu, J.; Jin, J. Discovery of the first-in-class G9a/GLP covalent inhibitors. J. Med. Chem. 2022, 65, 10506–10522. [Google Scholar] [CrossRef]
- Lin, H.; Wang, M.; Zhang, Y.W.; Tong, S.; Leal, R.A.; Shetty, R.; Vaddi, K.; Luengo, J.I. Discovery of potent and selective covalent protein arginine methyltransferase 5 (PRMT5) inhibitors. ACS Med. Chem. Lett. 2019, 10, 1033–1038. [Google Scholar] [CrossRef]
- Shen, Y.; Li, F.; Szewczyk, M.M.; Halabelian, L.; Park, K.S.; Chau, I.; Dong, A.; Zeng, H.; Chen, H.; Meng, F.; et al. Discovery of a first-in-class protein arginine methyltransferase 6 (PRMT6) covalent inhibitor. J. Med. Chem. 2020, 63, 5477–5487. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef]
- Diehl, C.J.; Ciulli, A. Discovery of small molecule ligands for the von Hippel-Lindau (VHL) E3 ligase and their use as inhibitors and PROTAC degraders. Chem. Soc. Rev. 2022, 51, 8216–8257. [Google Scholar] [CrossRef]
- Velez, J.; Han, Y.; Yim, H.; Yang, P.; Deng, Z.; Park, K.S.; Kabir, M.; Kaniskan, H.Ü.; Xiong, Y.; Jin, J. Discovery of the first-in-class G9a/GLP PROTAC degrader. J. Med. Chem. 2024, 67, 6397–6409. [Google Scholar] [CrossRef]
- Mukherjee, A.; Yamashita, Y.; Maeda, R.; Akiyama, T.; Endo, K.; Takada, Y.; Tsumoto, H.; Moriyama, Y.; Ito, A.; Shirai, F.; et al. A Novel PROTAC G9a/GLP Degrader that Inhibits, Similar to G9a siRNA, the Migration of MCF-7 Breast-Cancer Cells without Affecting Proliferation. J. Med. Chem. 2025, 68, 18258–18271. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Shen, Q.; Long, R.; Mao, Y.; Tong, S.; Yang, Y.; Gao, J.; Zhou, H.; Chen, Y.; Zhou, B. Discovery of potent and selective G9a degraders for the treatment of pancreatic cancer. J. Med. Chem. 2024, 67, 13271–13285. [Google Scholar] [CrossRef] [PubMed]
- Velez, J.; Dale, B.; Park, K.S.; Kaniskan, H.Ü.; Yu, X.; Jin, J. Discovery of a novel, highly potent EZH2 PROTAC degrader for targeting non-canonical oncogenic functions of EZH2. Eur. J. Med. Chem. 2024, 267, 116154. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.; Anderson, C.; Park, K.S.; Kaniskan, H.U.; Ma, A.; Shen, Y.; Zhang, C.; Xie, L.; Chen, X.; Yu, X.; et al. Targeting triple-negative breast cancer by a novel proteolysis targeting chimera degrader of enhancer of zeste homolog 2. ACS Pharmacol. Transl. Sci. 2022, 5, 491–507. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.; Gong, W.; Liu, X.; Park, K.S.; Ma, A.; Tsai, Y.H.; Shen, Y.; Onikubo, T.; Pi, W.C.; et al. EZH2 noncanonically binds cMyc and p300 through a cryptic transactivation domain to mediate gene activation and promote oncogenesis. Nat. Cell Biol. 2022, 24, 384–399. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Liu, X.; Lu, D.; Li, S.; Qu, L.; Yin, F.; Luo, H.; Zhang, Y.; Luo, Z.; et al. Discovery of precision targeting EZH2 degraders for triple-negative breast cancer. Eur. J. Med. Chem. 2022, 238, 114462. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Sun, Y.; Qiao, S.; Luo, Y.; Liu, P.; Jiang, Z.X.; Hu, Y.; Wang, Z.; Huang, P.; Wen, S. Design, synthesis, and evaluation of VHL-based EZH2 degraders to enhance therapeutic activity against lymphoma. J. Med. Chem. 2021, 64, 10167–10184. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Stratikopoulos, E.; Park, K.S.; Wei, J.; Martin, T.C.; Yang, X.; Schwarz, M.; Leshchenko, V.; Rialdi, A.; Dale, B.; et al. Discovery of a first-in-class EZH2 selective degrader. Nat. Chem. Biol. 2020, 16, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, X.; Wang, Q.; Wu, X.; Zhang, Q.; Wei, W.; Su, X.; He, H.; Zhou, S.; Hu, R.; et al. Design and synthesis of EZH2-based PROTACs to degrade the PRC2 complex for targeting the noncatalytic activity of EZH2. J. Med. Chem. 2021, 64, 2829–2848. [Google Scholar] [CrossRef]
- Xu, C.; Meng, F.; Park, K.S.; Storey, A.J.; Gong, W.; Tsai, Y.H.; Gibson, E.; Byrum, S.D.; Li, D.; Edmondson, R.D.; et al. A NSD3-targeted PROTAC suppresses NSD3 and cMyc oncogenic nodes in cancer cells. Cell Chem. Biol. 2022, 29, 386–397. [Google Scholar] [CrossRef]
- Meng, F.; Xu, C.; Park, K.S.; Kaniskan, H.U.; Wang, G.G.; Jin, J. Discovery of a first-in-class degrader for nuclear receptor binding SET domain protein 2 (NSD2) and Ikaros/Aiolos. J. Med. Chem. 2022, 65, 10611–10625. [Google Scholar] [CrossRef]
- Liu, L.; Parolia, A.; Liu, Y.; Hou, C.; He, T.; Qiao, Y.; Eyunni, S.; Luo, J.; Li, C.; Wang, Y.; et al. Discovery of LLC0424 as a Potent and Selective in Vivo NSD2 PROTAC Degrader. J. Med. Chem. 2024, 67, 6938–6951. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Chen, X.; Yu, A.; Du, W.; Huang, Y.; Wu, F.; Yu, L.; Li, J.; Wen, C.; et al. Discovery of a potent and selective proteolysis targeting chimera (PROTAC) degrader of NSD3 histone methyltransferase. Eur. J. Med. Chem. 2022, 239, 114528. [Google Scholar] [CrossRef]
- Legaard Andersson, J.; Christensen, J.; Kleine-Kohlbrecher, D.; Comet, I.V.; Støier, J.F.; Antoku, Y.; Poljak, V.; Moretti, L.; Dolberg, J.; Jacso, T.; et al. Discovery of NSD2-degraders from novel and selective DEL hits. Chembiochem 2023, 24, e202300515. [Google Scholar]
- Zou, W.; Li, M.; Wan, S.; Ma, J.; Lian, L.; Luo, G.; Zhou, Y.; Li, J.; Zhou, B. Discovery of PRMT3 degrader for the treatment of acute leukemia. Adv. Sci. 2024, 11, e2405963. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Song, H.; He, Y.; Lo, Y.; Fan, Z.; Lu, J. Development of a Selective and Potent PRMT4 PROTAC Degrader with Efficacy against Multiple Myeloma in Vitro and in Vivo. J. Med. Chem. 2025, 68, 13973–13989. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gao, G.; Yu, X.; Kim, H.; Wang, L.; Xie, L.; Schwarz, M.; Chen, X.; Guccione, E.; Liu, J.; et al. Discovery of first-in-class protein arginine methyltransferase 5 (PRMT5) degraders. J. Med. Chem. 2020, 63, 9977–9989. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Kim, H.; Qian, C.; Xie, L.; Chen, X.; Xiong, Y.; Hu, J.; Chen, M.; Guccione, E.; Shen, Y.; et al. Discovery of a Potent and Selective Protein Arginine Methyltransferase 5 (PRMT5) PROTAC Degrader. J. Med. Chem. 2025, 68, 8543–8563. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Zhou, Z.; Hou, L.; Liu, W.; Ren, W.; Mi, D.; Sun, J.; Dai, X.; Wu, Y.; et al. Targeting PRMT5 through PROTAC for the treatment of triple-negative breast cancer. J. Exp. Clin. Cancer Res. 2024, 43, 314. [Google Scholar] [CrossRef]
- Potjewyd, F.; Turner, A.M.W.; Beri, J.; Rectenwald, J.M.; Norris-Drouin, J.L.; Cholensky, S.H.; Margolis, D.M.; Pearce, K.H.; Herring, L.E.; James, L.I. Degradation of polycomb repressive complex 2 with an EED-targeted bivalent chemical degrader. Cell Chem. Biol. 2020, 27, 47–56. [Google Scholar] [CrossRef]
- Hsu, J.H.R.; Rasmusson, T.; Robinson, J.; Pachl, F.; Read, J.; Kawatkar, S.; O’Donovan, D.H.; Bagal, S.; Code, E.; Rawlins, P.; et al. EED-targeted PROTACs degrade EED, EZH2, and SUZ12 in the PRC2 complex. Cell Chem. Biol. 2020, 27, 41–46. [Google Scholar] [CrossRef]
- Bashore, F.M.; Foley, C.A.; Ong, H.W.; Rectenwald, J.M.; Hanley, R.P.; Norris-Drouin, J.L.; Cholensky, S.H.; Mills, C.A.; Pearce, K.H.; Herring, L.E.; et al. PROTAC linkerology leads to an optimized bivalent chemical degrader of polycomb repressive complex 2 (PRC2) components. ACS Chem. Biol. 2023, 18, 494–507. [Google Scholar] [CrossRef]
- Dölle, A.; Adhikari, B.; Krämer, A.; Weckesser, J.; Berner, N.; Berger, L.M.; Diebold, M.; Szewczyk, M.M.; Barsyte-Lovejoy, D.; Arrowsmith, C.H.; et al. Design, synthesis, and evaluation of WD-repeat-containing protein 5 (WDR5) degraders. J. Med. Chem. 2021, 64, 10682–10710. [Google Scholar] [CrossRef]
- Yu, X.; Li, D.; Kottur, J.; Shen, Y.; Kim, H.S.; Park, K.S.; Tsai, Y.H.; Gong, W.; Wang, J.; Suzuki, K.; et al. A selective WDR5 degrader inhibits acute myeloid leukemia in patient-derived mouse models. Sci. Transl. Med. 2021, 13, eabj1578. [Google Scholar] [CrossRef]
- Li, D.; Yu, X.; Kottur, J.; Gong, W.; Zhang, Z.; Storey, A.J.; Tsai, Y.H.; Uryu, H.; Shen, Y.; Byrum, S.D.; et al. Discovery of a dual WDR5 and Ikaros PROTAC degrader as an anti-cancer therapeutic. Oncogene 2022, 41, 3328–3340. [Google Scholar] [CrossRef]
- Yu, X.; Li, D.; Kottur, J.; Kim, H.S.; Herring, L.E.; Yu, Y.; Xie, L.; Hu, X.; Chen, X.; Cai, L.; et al. Discovery of potent and selective WDR5 proteolysis targeting chimeras as potential therapeutics for pancreatic cancer. J. Med. Chem. 2023, 66, 16168–16186. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.L.; Pérez-Areales, F.J.; Rao, S.V.; Walsh, S.J.; Carroll, J.S.; Spring, D.R. Towards the targeted protein degradation of PRMT1. ChemMedChem 2024, 19, e202400269. [Google Scholar] [CrossRef]
- Wang, M.Y.; Liow, P.; Guzman, M.I.T.; Qi, J. Exploring methods of targeting histone methyltransferases and their applications in cancer therapeutics. ACS Chem. Biol. 2022, 17, 744–755. [Google Scholar] [CrossRef]
- He, H.; Li, X.; Su, F.; Jin, H.; Zhang, J.; Wang, Y. Current and Emerging Approaches Targeting G9a for the Treatment of Various Diseases. J. Med. Chem. 2024, 68, 1068–1089. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, A.; Mukherjee, A.; Bhattacharya, D. Fascinating transformation of SAM-competitive protein methyltransferase inhibitors from nucleoside analogues to non-nucleoside analogues. J. Med. Chem. 2022, 65, 1662–1684. [Google Scholar] [CrossRef]
- Dempke, W.C.; Desole, M.; Chiusolo, P.; Sica, S.; Schmidt-Hieber, M. Targeting the undruggable: Menin inhibitors ante portas. J. Cancer Res. Clin. Oncol. 2023, 149, 9451–9459. [Google Scholar] [CrossRef]
- Rossi, A.; Zacchi, F.; Reni, A.; Rota, M.; Palmerio, S.; Menis, J.; Zivi, A.; Milleri, S.; Milella, M. Progresses and Pitfalls of Epigenetics in Solid Tumors Clinical Trials. Int. J. Mol. Sci. 2024, 25, 11740. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Chang, X.; Qu, F.; Bian, J.; Wang, J.; Li, Z.; Xu, X. Overview of the development of protein arginine methyltransferase modulators: Achievements and future directions. Eur. J. Med. Chem. 2024, 267, 116212. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, T.; Chen, D.Q.; Xiong, X.; Shi, L.; Zuo, Y.; Xiao, H.; Liu, L. Promising role of protein arginine methyltransferases in overcoming anti-cancer drug resistance. Drug Resist. Updat. 2024, 72, 101016. [Google Scholar] [CrossRef]
- Micallef, I.; Fenech, K.; Baron, B. Therapeutic targeting potential of the protein lysine and arginine methyltransferases to reverse cancer chemoresistance. Front. Mol. Biosci. 2024, 11, 1455415. [Google Scholar] [CrossRef]
- Brekker, M.A.; Sartawi, T.; Sawatzky, T.M.; Causey, C.P.; Rehman, F.K.; Knuckley, B. A peptoid-based inhibitor of protein arginine methyltransferase 1 (PRMT1) induces apoptosis and autophagy in cancer cells. J. Biol. Chem. 2022, 298, 102205. [Google Scholar] [CrossRef]
- Hintzen, J.C.J.; Moesgaard, L.; Kwiatkowski, S.; Drozak, J.; Kongsted, J.; Mecinović, J. β-Actin Peptide-Based Inhibitors of Histidine Methyltransferase SETD3. Chem. Med. Chem. 2021, 16, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Luo, M. Current chemical biology approaches to interrogate protein methyltransferases. ACS Chem. Biol. 2012, 7, 443–463. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, W. Enzymatic assays of histone methyltransferase enzymes. In Epigenetic Technological Applications; Academic Press: New York, NY, USA, 2015; pp. 333–361. [Google Scholar]
- Aebersold, R.; Agar, J.N.; Amster, I.J.; Baker, M.S.; Bertozzi, C.R.; Boja, E.S.; Costello, C.E.; Cravatt, B.F.; Fenselau, C.; Garcia, B.A.; et al. How many human proteoforms are there? Nat. Chem. Biol. 2018, 14, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Kelleher, N.L. Proteoforms as the next proteomics currency. Science 2018, 359, 1106–1107. [Google Scholar] [CrossRef]
- Marx, V. Inside the chase after those elusive proteoforms. Nat. Methods 2024, 21, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Naryzhny, S. Puzzle of proteoform variety—Where is a key? Proteomes 2024, 12, 15. [Google Scholar] [CrossRef]
- Baker, T.; Nerle, S.; Pritchard, J.; Zhao, B.; Rivera, V.M.; Garner, A.; Gonzalvez, F. Acquisition of a single EZH2 D1 domain mutation confers acquired resistance to EZH2-targeted inhibitors. Oncotarget 2015, 6, 32646–32655. [Google Scholar] [CrossRef] [PubMed]
- Gibaja, V.; Shen, F.; Harari, J.; Korn, J.; Ruddy, D.; Saenz-Vash, V.; Zhai, H.; Rejtar, T.; Paris, C.G.; Yu, Z.; et al. Development of secondary mutations in wild-type and mutant EZH2 alleles cooperates to confer resistance to EZH2 inhibitors. Oncogene 2016, 35, 558–566. [Google Scholar] [CrossRef]
- Bisserier, M.; Wajapeyee, N. Mechanisms of resistance to EZH2 inhibitors in diffuse large B-cell lymphomas. Blood 2018, 131, 2125–2137. [Google Scholar] [CrossRef]
- Drosos, Y.; Myers, J.A.; Xu, B.; Mathias, K.M.; Beane, E.C.; Radko-Juettner, S.; Mobley, R.J.; Larsen, M.E.; Piccioni, F.; Ma, X.; et al. NSD1 mediates antagonism between SWI/SNF and polycomb complexes and is required for transcriptional activation upon EZH2 inhibition. Mol. Cell 2022, 82, 2472–2489. [Google Scholar] [CrossRef] [PubMed]
- Pang, F.; Zhang, L.; Li, M.; Yi, X.; Wang, Y.; Yang, P.; Wen, B.; Jiang, J.; Teng, Y.; Yang, X.; et al. Ribosomal S6 protein kinase 4 promotes resistance to EZH2 inhibitors in glioblastoma. Cancer Gene Ther. 2023, 30, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Kazansky, Y.; Cameron, D.; Mueller, H.S.; Demarest, P.; Zaffaroni, N.; Arrighetti, N.; Zuco, V.; Kuwahara, Y.; Somwar, R.; Ladanyi, M.; et al. Overcoming clinical resistance to EZH2 inhibition using rational epigenetic combination therapy. Cancer Discov. 2024, 14, 965–981. [Google Scholar] [CrossRef]
- Huang, X.; Yan, J.; Zhang, M.; Wang, Y.; Chen, Y.; Fu, X.; Wei, R.; Zheng, X.L.; Liu, Z.; Zhang, X.; et al. Targeting epigenetic crosstalk as a therapeutic strategy for EZH2-aberrant solid tumors. Cell 2018, 175, 186–199. [Google Scholar] [CrossRef]
- Baron, B. Comprehensive mass spectrometric investigation strategies of the human methylproteome. J. Proteome Data Methods 2021, 3, 1. [Google Scholar]
- Micallef, I.; Baron, B. Proteomic strategies for methylation analysis in colorectal cancer chemoresistance. J. Proteome Data Methods 2023, 5, 16. [Google Scholar]
- Levy, D. Lysine methylation signaling of non-histone proteins in the nucleus. Cell. Mol. Life Sci. 2019, 76, 2873–2883. [Google Scholar] [CrossRef]
- Wang, D.; Quiros, J.; Mahuron, K.; Pai, C.C.; Ranzani, V.; Young, A.; Silveria, S.; Harwin, T.; Abnousian, A.; Pagani, M.; et al. Targeting EZH2 reprograms intratumoral regulatory T cells to enhance cancer immunity. Cell Rep. 2018, 23, 3262–3274. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ren, S.; Chen, Y.; Liu, A.; Wu, Q.; Jiang, T.; Lv, P.; Song, D.; Hu, F.; Lan, J.; et al. PD-L1 methylation restricts PD-L1/PD-1 interactions to control cancer immune surveillance. Sci. Adv. 2023, 9, eade4186. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.L.; Lin, H.; Li, Y.H.; Yang, T.Y.; Chen, M.C. RAGE potentiates EGFR signaling and interferes with the anticancer effect of gefitinib on NSCLC cells. Am. J. Physiol.-Cell Physiol. 2023, 325, C1313–C1325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huo, X.; Guo, H.; Xue, L. Combined inhibition of PARP and EZH2 for cancer treatment: Current status, opportunities, and challenges. Front. Pharmacol. 2022, 13, 965244. [Google Scholar] [CrossRef]
- O’Brien, S.; Butticello, M.; Thompson, C.; Wilson, B.; Wyce, A.; Mahajan, V.; Kruger, R.; Mohammad, H.; Fedoriw, A. Inhibiting PRMT5 induces DNA damage and increases anti-proliferative activity of Niraparib, a PARP inhibitor, in models of breast and ovarian cancer. BMC Cancer 2023, 23, 775. [Google Scholar] [CrossRef]
| Substrate | Inhibitor | Reference |
|---|---|---|
| EHMT2 | UNC0642 | [94] |
| UNC0925 | [95] | |
| A-366 | [96] | |
| MS152 | [97] | |
| EZH2 | UNC1999 | [98] |
| CPI-1205 | [99] | |
| PF-06821497 | [100] | |
| EBI-2511 | [101] | |
| SETD7 | (R)-PFI-2 | [102] |
| Cyproheptadine | [103] | |
| SMYD2 | BAY-598 | [104] |
| EPZ030456 | [105] | |
| A-893 | [106] | |
| EPZ033294 | [107] | |
| SMYD3 | BCI-121 | [108] |
| EPZ028862 | [107] | |
| BAY-6035 | [109] | |
| SETD8 | UNC0379 | [110] |
| SPS8I1 | [111] | |
| MS2177 | [112] | |
| PRMT5 | GSK3326595/EPZ015938 (Pemrametostat) | [113] |
| GSK3235025/EPZ015666 | [114] | |
| GSK3203591/EPZ015866 | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micallef, I.; Baron, B. Therapeutic Targeting of Protein Lysine and Arginine Methyltransferases: Principles and Strategies for Inhibitor Design. Int. J. Mol. Sci. 2025, 26, 9038. https://doi.org/10.3390/ijms26189038
Micallef I, Baron B. Therapeutic Targeting of Protein Lysine and Arginine Methyltransferases: Principles and Strategies for Inhibitor Design. International Journal of Molecular Sciences. 2025; 26(18):9038. https://doi.org/10.3390/ijms26189038
Chicago/Turabian StyleMicallef, Isaac, and Byron Baron. 2025. "Therapeutic Targeting of Protein Lysine and Arginine Methyltransferases: Principles and Strategies for Inhibitor Design" International Journal of Molecular Sciences 26, no. 18: 9038. https://doi.org/10.3390/ijms26189038
APA StyleMicallef, I., & Baron, B. (2025). Therapeutic Targeting of Protein Lysine and Arginine Methyltransferases: Principles and Strategies for Inhibitor Design. International Journal of Molecular Sciences, 26(18), 9038. https://doi.org/10.3390/ijms26189038







