Transcriptional Consequences of MeCP2 Knockdown and Overexpression in Mouse Primary Cortical Neurons
Abstract
1. Introduction
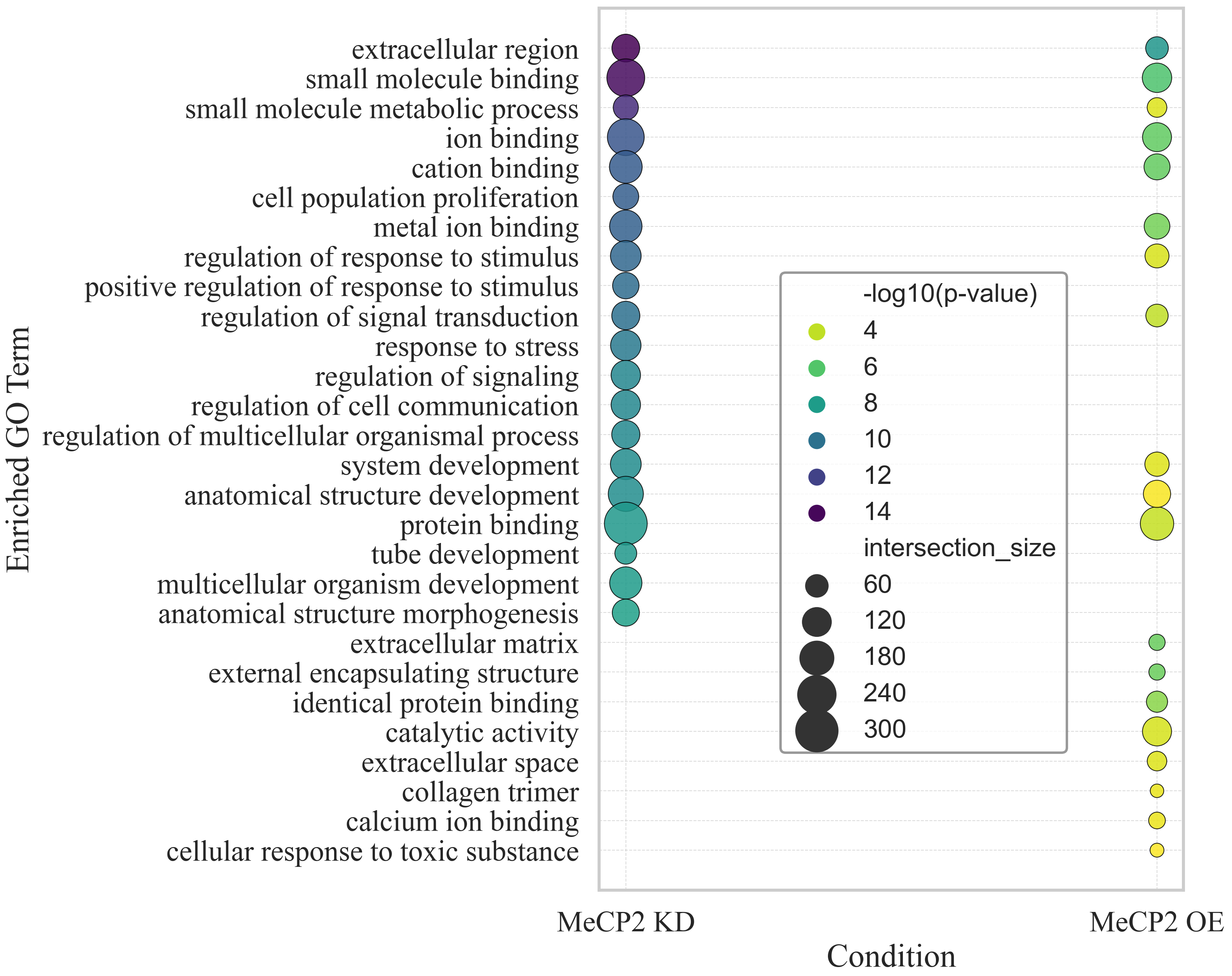
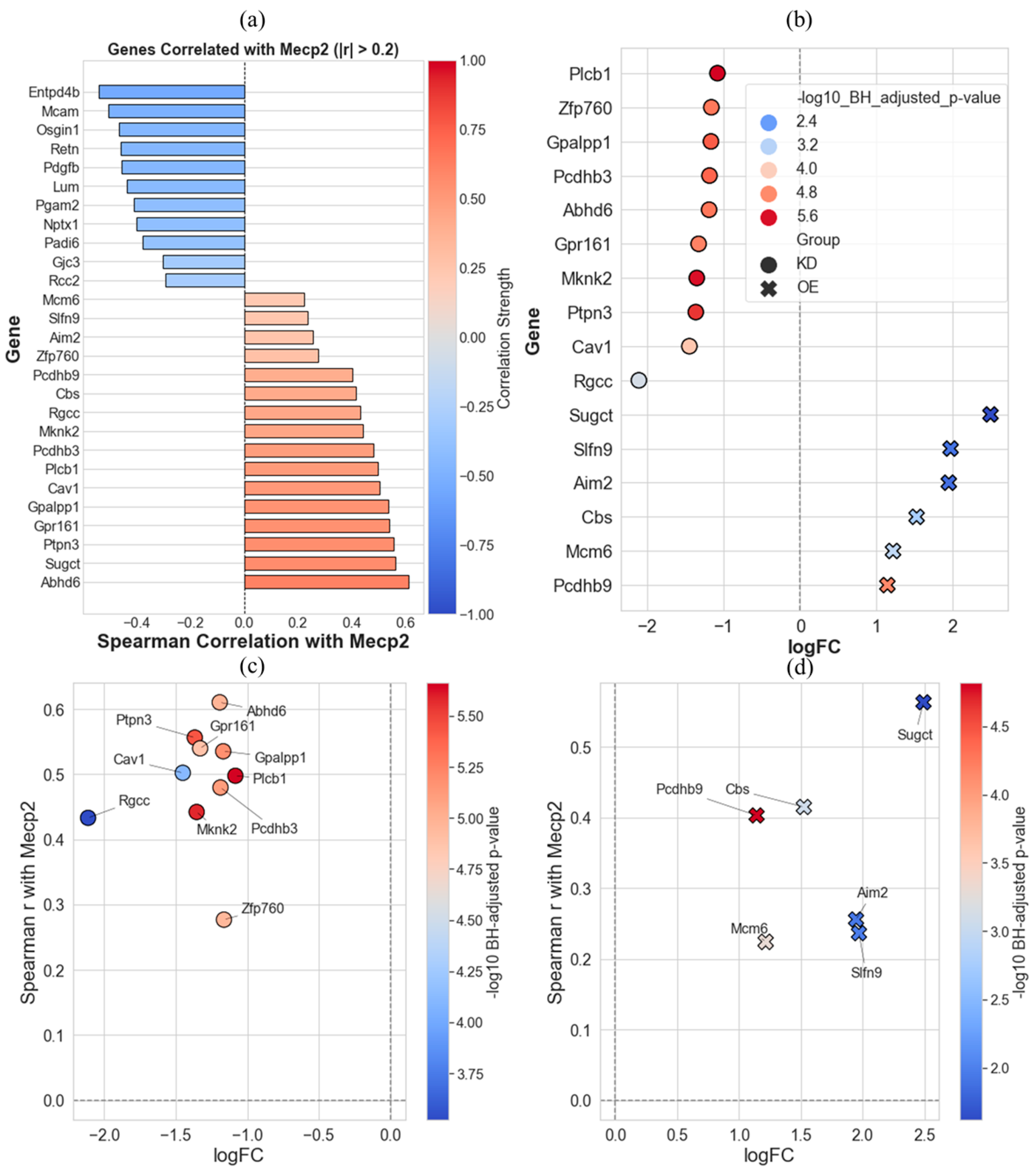
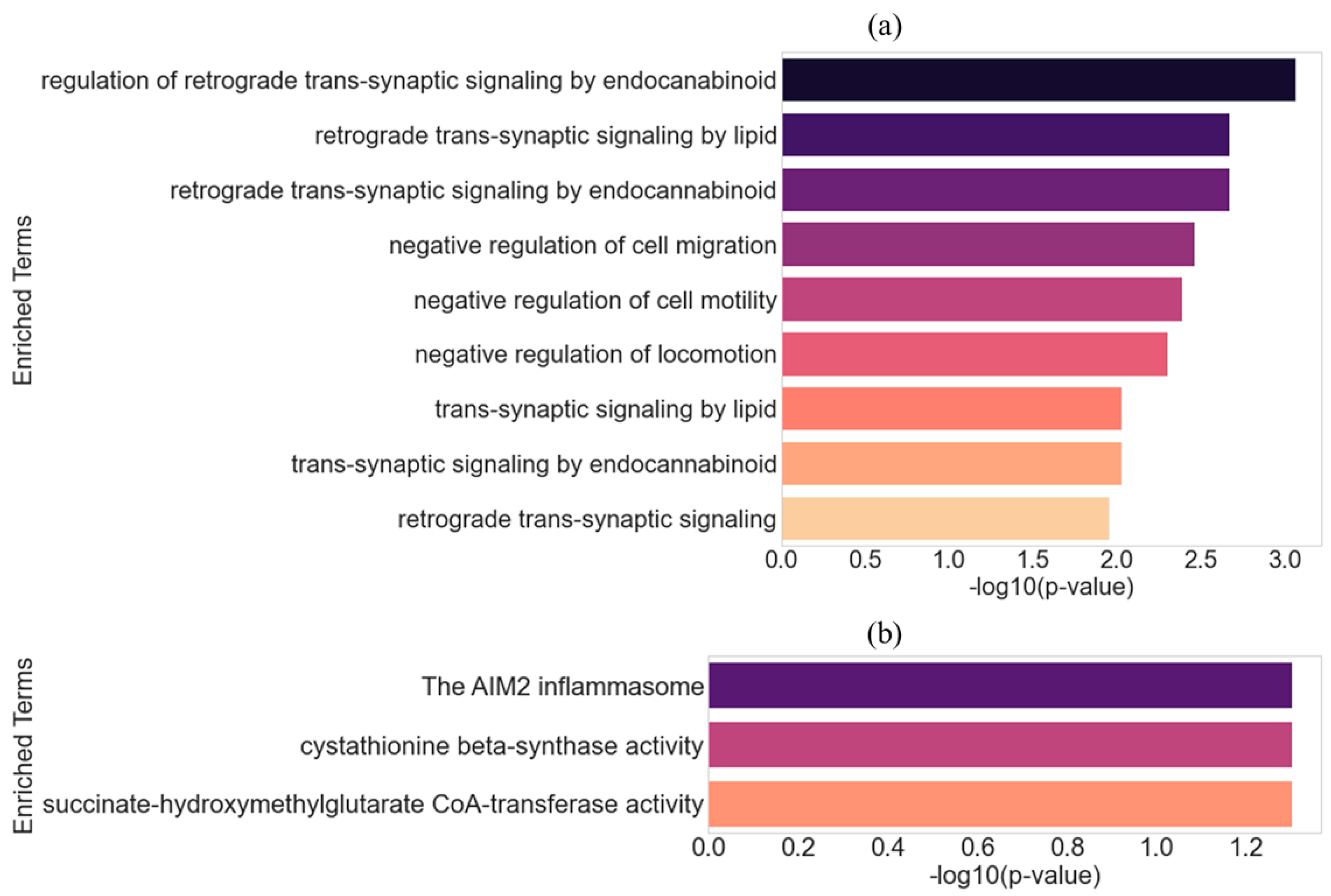
2. Results
3. Discussion
4. Materials and Methods
4.1. Dataset Description
4.2. Statistical Methods
4.3. Gene Ontology (GO) Analysis
5. Conclusions
Limitations of Study and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yasui, D.H.; Xu, H.; Dunaway, K.W.; LaSalle, J.M.; Jin, L.-W.; Maezawa, I. MeCP2 modulates gene expression pathways in astrocytes. Mol. Autism 2013, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.M.; Baker, S.A.; Zoghbi, H.Y. MECP2 disorders: From the clinic to mice and back. J. Clin. Investig. 2015, 125, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Kinde, B.; Wu, D.Y.; Greenberg, M.E.; Gabel, H.W. DNA methylation in the gene body influences MeCP2-mediated gene repression. Proc. Natl. Acad. Sci. USA 2016, 113, 15114–15119. [Google Scholar] [CrossRef] [PubMed]
- Nettles, S.A.; Ikeuchi, Y.; Lefton, K.B.; Abbasi, L.; Erickson, A.; Agwu, C.; Papouin, T.; Bonni, A.; Gabel, H.W. MeCP2 represses the activity of topoisomerase IIβ in long neuronal genes. Cell Rep. 2023, 42, 113538. [Google Scholar] [CrossRef]
- Papapietro, O.; Nejentsev, S. Topoisomerase 2β and DNA topology during B cell development. Front. Immunol. 2022, 13, 982870. [Google Scholar] [CrossRef]
- Nur-E-Kamal, A.; Meiners, S.; Ahmed, I.; Azarova, A.; Lin, C.-P.; Lyu, Y.L.; Liu, L.F. Role of DNA topoisomerase IIβ in neurite outgrowth. Brain Res. 2007, 1154, 50–60. [Google Scholar] [CrossRef]
- Khazeem, M.M.; Casement, J.W.; Schlossmacher, G.; Kenneth, N.S.; Sumbung, N.K.; Chan, J.Y.T.; McGow, J.F.; Cowell, I.G.; Austin, C.A. TOP2B Is Required to Maintain the Adrenergic Neural Phenotype and for ATRA-Induced Differentiation of SH-SY5Y Neuroblastoma Cells. Mol. Neurobiol. 2022, 59, 5987–6008. [Google Scholar] [CrossRef]
- Horvath, P.M.; Monteggia, L.M. MeCP2 as an Activator of Gene Expression. Trends Neurosci. 2018, 41, 72–74. [Google Scholar] [CrossRef]
- Chahrour, M.; Jung, S.Y.; Shaw, C.; Zhou, X.; Wong, S.T.C.; Qin, J.; Zoghbi, H.Y. MeCP2, a Key Contributor to Neurological Disease, Activates and Represses Transcription. Science 2008, 320, 1224–1229. [Google Scholar] [CrossRef]
- Clemens, A.W.; Wu, D.Y.; Moore, J.R.; Christian, D.L.; Zhao, G.; Gabel, H.W. MeCP2 Represses Enhancers through Chromosome Topology-Associated DNA Methylation. Mol. Cell 2020, 77, 279–293.e8. [Google Scholar] [CrossRef]
- Riedmann, C.; Fondufe-Mittendorf, Y.N. Comparative analysis of linker histone H1, MeCP2, and HMGD1 on nucleosome stability and target site accessibility. Sci. Rep. 2016, 6, 33186. [Google Scholar] [CrossRef] [PubMed]
- Gulmez Karaca, K.; Brito, D.V.C.; Oliveira, A.M.M. MeCP2: A Critical Regulator of Chromatin in Neurodevelopment and Adult Brain Function. Int. J. Mol. Sci. 2019, 20, 4577. [Google Scholar] [CrossRef]
- Ramocki, M.B.; Tavyev, Y.J.; Peters, S.U. The MECP2 duplication syndrome. Am. J. Med. Genet. A 2010, 152A, 1079–1088. [Google Scholar] [CrossRef]
- Ramocki, M.B.; Peters, S.U.; Tavyev, Y.J.; Zhang, F.; Carvalho, C.M.; Schaaf, C.P.; Richman, R.; Fang, P.; Glaze, D.G.; Lupski, J.R.; et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann. Neurol. 2009, 66, 771–782. [Google Scholar] [CrossRef]
- Guy, J.; Hendrich, B.; Holmes, M.; Martin, J.E.; Bird, A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001, 27, 322–326. [Google Scholar] [CrossRef]
- Zhou, Z.; Hong, E.J.; Cohen, S.; Zhao, W.-N.; Ho, H.-Y.H.; Schmidt, L.; Chen, W.G.; Lin, Y.; Savner, E.; Griffith, E.C.; et al. Brain-Specific Phosphorylation of MeCP2 Regulates Activity-Dependent Bdnf Transcription, Dendritic Growth, and Spine Maturation. Neuron 2006, 52, 255–269. [Google Scholar] [CrossRef]
- Peters, S.U.; Hundley, R.J.; Wilson, A.K.; Warren, Z.; Vehorn, A.; Carvalho, C.M.; Lupski, J.R.; Ramocki, M.B. The Behavioral Phenotype in MECP2 Duplication Syndrome: A Comparison With Idiopathic Autism. Autism Res. 2012, 6, 42–50. [Google Scholar] [CrossRef]
- Rezapour, M.; Walker, S.J.; Ornelles, D.A.; McNutt, P.M.; Atala, A.; Gurcan, M.N. Analysis of gene expression dynamics and differential expression in viral infections using generalized linear models and quasi-likelihood methods. Front. Microbiol. 2024, 15, 1342328. [Google Scholar] [CrossRef]
- Rezapour, M.; Walker, S.J.; Ornelles, D.A.; Niazi, M.K.K.; McNutt, P.M.; Atala, A.; Gurcan, M.N. A comparative analysis of RNA-Seq and NanoString technologies in deciphering viral infection response in upper airway lung organoids. Front. Genet. 2024, 15, 1327984. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.; Wesolowski, R.; Gurcan, M.N. Identifying Key Genes Involved in Axillary Lymph Node Metastasis in Breast Cancer Using Advanced RNA-Seq Analysis: A Methodological Approach with GLMQL and MAS. Int. J. Mol. Sci. 2024, 25, 7306. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.; Narayanan, A.; Gurcan, M.N. Machine Learning Analysis of RNA-Seq Data Identifies Key Gene Signatures and Pathways in Mpox Virus-Induced Gastrointestinal Complications Using Colon Organoid Models. Int. J. Mol. Sci. 2024, 25, 11142. [Google Scholar] [CrossRef]
- Rezapour, M.; Murphy, S.V.; Ornelles, D.A.; McNutt, P.M.; Atala, A. Tracing the evolutionary pathway of SARS-CoV-2 through RNA sequencing analysis. Sci. Rep. 2025, 15, 23961. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Benjamini, Y.; Heller, R.; Yekutieli, D. Selective inference in complex research. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 4255–4271. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- de Winter, J.C.F.; Gosling, S.D.; Potter, J. Comparing the Pearson and Spearman correlation coefficients across distributions and sample sizes: A tutorial using simulations and empirical data. Psychol. Methods 2016, 21, 273–290. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. WIREs Comp. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Kleinbaum, D.G.; Klein, M.; Pryor, E.R. Logistic Regression. In Logistic Regression: A Self-Learning Text, 3rd ed.; Gail, M., Krickeberg, K., Samet, J.M., Tsiatis, A., Wong, W., Eds.; Statistics for Biology and Health; Springer: New York, NY, USA, 2010. [Google Scholar]
- Christodoulou, E.; Ma, J.; Collins, G.S.; Steyerberg, E.W.; Verbakel, J.Y.; Van Calster, B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J. Clin. Epidemiol. 2019, 110, 12–22. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef]
- Filosa, S.; Pecorelli, A.; D’ESposito, M.; Valacchi, G.; Hajek, J. Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome. Free Radic. Biol. Med. 2015, 88, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Splawski, I.; Timothy, K.W.; Sharpe, L.M.; Decher, N.; Kumar, P.; Bloise, R.; Napolitano, C.; Schwartz, P.J.; Joseph, R.M.; Condouris, K.; et al. CaV1.2 Calcium Channel Dysfunction Causes a Multisystem Disorder Including Arrhythmia and Autism. Cell 2004, 119, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Pinggera, A.; Lieb, A.; Benedetti, B.; Lampert, M.; Monteleone, S.; Liedl, K.R.; Tuluc, P.; Striessnig, J. CACNA1D De Novo Mutations in Autism Spectrum Disorders Activate Cav1.3 L-Type Calcium Channels. Biol. Psychiatry 2015, 77, 816–822. [Google Scholar] [CrossRef]
- Rose, S.; Melnyk, S.; Pavliv, O.; Bai, S.; Nick, T.G.; Frye, R.E.; James, S.J. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl. Psychiatry 2012, 2, e134. [Google Scholar] [CrossRef]
- Kaur, I.; Behl, T.; Aleya, L.; Rahman, H.; Kumar, A.; Arora, S.; Akter, R. Role of metallic pollutants in neurodegeneration: Effects of aluminum, lead, mercury, and arsenic in mediating brain impairment events and autism spectrum disorder. Environ. Sci. Pollut. Res. 2021, e28, 8989–9001. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Westermark, B. Mechanism of Action and In Vivo Role of Platelet-Derived Growth Factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef]
- Sil, S.; Periyasamy, P.; Thangaraj, A.; Chivero, E.T.; Buch, S. PDGF/PDGFR axis in the neural systems. Mol. Asp. Med. 2018, 62, 63–74. [Google Scholar] [CrossRef]
- Coutelier, M.; Jacoupy, M.; Janer, A.; Renaud, F.; Auger, N.; Saripella, G.-V.; Ancien, F.; Pucci, F.; Rooman, M.; Gilis, D.; et al. NPTX1 mutations trigger endoplasmic reticulum stress and cause autosomal dominant cerebellar ataxia. Brain 2021, 145, 1519–1534. [Google Scholar] [CrossRef]
- O’bRien, R.J.; Xu, D.; Petralia, R.S.; Steward, O.; Huganir, R.L.; Worley, P. Synaptic Clustering of AMPA Receptors by the Extracellular Immediate-Early Gene Product Narp. Neuron 1999, 23, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.Á.; Vadászi, H.; Bulyáki, É.; Török, G.; Tóth, V.; Mátyás, D.; Kun, J.; Hunyadi-Gulyás, É.; Fedor, F.Z.; Csincsi, Á.; et al. Identification of Neuronal Pentraxins as Synaptic Binding Partners of C1q and the Involvement of NP1 in Synaptic Pruning in Adult Mice. Front. Immunol. 2021, 11, 599771. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, X.; Cai, Y.; Yu, H.; Cao, G.; Dai, E.; Kang, R.; Tang, D.; Hu, N.; Han, L. OSGIN1 promotes ferroptosis resistance by directly enhancing GCLM activity. Biochem. Biophys. Res. Commun. 2024, 740, 151015. [Google Scholar] [CrossRef]
- Yamashita, N.; Uchiyama, M.; Yamagata, R.; Hwang, G.-W. Methylmercury Induces Apoptosis in Mouse C17.2 Neural Stem Cells through the Induction of OSGIN1 Expression by NRF2. Int. J. Mol. Sci. 2024, 25, 3886. [Google Scholar] [CrossRef]
- De Felice, C.; Signorini, C.; Leoncini, S.; Pecorelli, A.; Durand, T.; Valacchi, G.; Ciccoli, L.; Hayek, J. The role of oxidative stress in Rett syndrome: An overview. Ann. N. Y. Acad. Sci. 2012, 1259, 121–135. [Google Scholar] [CrossRef]
- Vogelgesang, S.; Niebert, S.; Renner, U.; Möbius, W.; Hülsmann, S.; Manzke, T.; Niebert, M. Analysis of the Serotonergic System in a Mouse Model of Rett Syndrome Reveals Unusual Upregulation of Serotonin Receptor 5b. Front. Mol. Neurosci. 2017, 10, 61. [Google Scholar] [CrossRef]
- Mogilevsky, M.; Shimshon, O.; Kumar, S.; Mogilevsky, A.; Keshet, E.; Yavin, E.; Heyd, F.; Karni, R. Modulation of MKNK2 alternative splicing by splice-switching oligonucleotides as a novel approach for glioblastoma treatment. Nucleic Acids Res. 2018, 46, 11396–11404. [Google Scholar] [CrossRef] [PubMed]
- Maimon, A.; Mogilevsky, M.; Shilo, A.; Golan-Gerstl, R.; Obiedat, A.; Ben-Hur, V.; Lebenthal-Loinger, I.; Stein, I.; Reich, R.; Beenstock, J.; et al. Mnk2 Alternative Splicing Modulates the p38-MAPK Pathway and Impacts Ras-Induced Transformation. Cell Rep. 2014, 7, 501–513. [Google Scholar] [CrossRef]
- Scheper, M.; Sørensen, F.N.F.; Ruffolo, G.; Gaeta, A.; Lissner, L.J.; Anink, J.J.; Korshunova, I.; Jansen, F.E.; Riney, K.; van Hecke, W.; et al. Impaired GABAergic regulation and developmental immaturity in interneurons derived from the medial ganglionic eminence in the tuberous sclerosis complex. Acta Neuropathol. 2024, 147, 80. [Google Scholar] [CrossRef] [PubMed]
- Amorim, I.S.; Lach, G.; Gkogkas, C.G. The Role of the Eukaryotic Translation Initiation Factor 4E (eIF4E) in Neuropsychiatric Disorders. Front. Genet. 2018, 9, 561. [Google Scholar] [CrossRef]
- Counts, S.E.; Mufson, E.J. Regulator of Cell Cycle (RGCC) Expression during the Progression of Alzheimer’s Disease. Cell Transplant. 2017, 26, 693–702. [Google Scholar] [CrossRef]
- Xu, R.; Shang, C.; Zhao, J.; Han, Y.; Liu, J.; Chen, K.; Shi, W. Knockdown of response gene to complement 32 (RGC32) induces apoptosis and inhibits cell growth, migration, and invasion in human lung cancer cells. Mol. Cell. Biochem. 2014, 394, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, N.; Harrison, S.; Hwang, S.-H.; Chen, Z.; Karelina, M.; Deshpande, I.; Suomivuori, C.-M.; Palicharla, V.R.; Berry, S.P.; Tschaikner, P.; et al. GPR161 structure uncovers the redundant role of sterol-regulated ciliary cAMP signaling in the Hedgehog pathway. Nat. Struct. Mol. Biol. 2024, 31, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Tomar, S.; Sharma, D.; Mahindroo, N.; Udayabanu, M. Targeting sonic hedgehog signaling in neurological disorders. Neurosci. Biobehav. Rev. 2017, 74, 76–97. [Google Scholar] [CrossRef]
- Gulino, A.; Di Marcotullio, L.; Ferretti, E.; De Smaele, E.; Screpanti, I. Hedgehog signaling pathway in neural development and disease. Psychoneuroendocrinology 2007, 32, S52–S56. [Google Scholar] [CrossRef]
- Lyon, A.M.; Tesmer, J.J. Structural Insights into Phospholipase C-β Function. Mol. Pharmacol. 2013, 84, 488–500. [Google Scholar] [CrossRef]
- She, W.; Quairiaux, C.; Albright, M.J.; Wang, Y.; Sanchez, D.E.; Chang, P.; Welker, E.; Lu, H. Roles of mGluR5 in synaptic function and plasticity of the mouse thalamocortical pathway. Eur. J. Neurosci. 2009, 29, 1379–1396. [Google Scholar] [CrossRef]
- Calfa, G.; Li, W.; Rutherford, J.M.; Pozzo-Miller, L. Excitation/inhibition imbalance and impaired synaptic inhibition in hippocampal area CA3 of Mecp2 knockout mice. Hippocampus 2014, 25, 159–168. [Google Scholar] [CrossRef]
- Saresella, M.; Piancone, F.; Marventano, I.; Zoppis, M.; Hernis, A.; Zanette, M.; Trabattoni, D.; Chiappedi, M.; Ghezzo, A.; Canevini, M.P.; et al. Multiple inflammasome complexes are activated in autistic spectrum disorders. Brain Behav. Immun. 2016, 57, 125–133. [Google Scholar] [CrossRef]
- Mo, W.; Liu, J.; Zhang, Z.; Yu, H.; Yang, A.; Qu, F.; Hu, P.; Liu, Z.; Hu, F. A study of single nucleotide polymorphisms in CD157, AIM2 and JARID2 genes in Han Chinese children with autism spectrum disorder. Nord. J. Psychiatry 2017, 72, 179–183. [Google Scholar] [CrossRef]
- Smits, D.J.; Schot, R.; Popescu, C.A.; Dias, K.-R.; Ades, L.; Briere, L.C.; Sweetser, D.A.; Kushima, I.; Aleksic, B.; Khan, S.; et al. De novo MCM6 variants in neurodevelopmental disorders: A recognizable phenotype related to zinc binding residues. Hum. Genet. 2023, 142, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Simons Foundation Autism Research Initiative. MCM6 Gene—SFARI Gene. 2023. Available online: https://gene.sfari.org/database/human-gene/MCM6 (accessed on 2 July 2025).
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and Synaptic Function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.E.S.; Jehan, H.S.; Eman, G.B.; Sara, A.E.; Maha, S.Z.; Nora, N.E. Association of Cystathionine Beta Synthase Gene Polymorphism With Cognitive Disorders in Autistic Children. J. Innov. Pharm. Biol. Sci. 2017, 4, 20–24. [Google Scholar]
- Rossignol, D.A.; Frye, R.E. Mitochondrial dysfunction in autism spectrum disorders: A systematic review and meta-analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized Linear Models. J. R. Stat. Soc. Ser. A 1972, 135, 370–384. [Google Scholar] [CrossRef]
- Wedderburn, R.W.M. Quasi-Likelihood Functions, Generalized Linear Models, and the Gauss-Newton Method. Biometrika 1974, 61, 439. [Google Scholar] [CrossRef]
- Rezapour, M.; Walker, S.J.; Ornelles, D.A.; Niazi, M.K.K.; McNutt, P.M.; Atala, A.; Gurcan, M.N.; Choi, Y.H. Exploring the host response in infected lung organoids using NanoString technology: A statistical analysis of gene expression data. PLoS ONE 2024, 19, e0308849. [Google Scholar] [CrossRef] [PubMed]
- Rezapour, M.; Niazi, M.K.K.; Lu, H.; Narayanan, A.; Gurcan, M.N. Machine learning-based analysis of Ebola virus’ impact on gene expression in nonhuman primates. Front. Artif. Intell. 2024, 7, 1405332. [Google Scholar] [CrossRef] [PubMed]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezapour, M.; Bowser, J.; Richardson, C.; Gurcan, M.N. Transcriptional Consequences of MeCP2 Knockdown and Overexpression in Mouse Primary Cortical Neurons. Int. J. Mol. Sci. 2025, 26, 9032. https://doi.org/10.3390/ijms26189032
Rezapour M, Bowser J, Richardson C, Gurcan MN. Transcriptional Consequences of MeCP2 Knockdown and Overexpression in Mouse Primary Cortical Neurons. International Journal of Molecular Sciences. 2025; 26(18):9032. https://doi.org/10.3390/ijms26189032
Chicago/Turabian StyleRezapour, Mostafa, Joshua Bowser, Christine Richardson, and Metin Nafi Gurcan. 2025. "Transcriptional Consequences of MeCP2 Knockdown and Overexpression in Mouse Primary Cortical Neurons" International Journal of Molecular Sciences 26, no. 18: 9032. https://doi.org/10.3390/ijms26189032
APA StyleRezapour, M., Bowser, J., Richardson, C., & Gurcan, M. N. (2025). Transcriptional Consequences of MeCP2 Knockdown and Overexpression in Mouse Primary Cortical Neurons. International Journal of Molecular Sciences, 26(18), 9032. https://doi.org/10.3390/ijms26189032





