Population Genetic Structure of Invasive and Non-Invasive Streptococcus pneumoniae Isolates After Fifteen Years of Routine PCV10 Vaccination in Bulgaria
Abstract
1. Introduction
2. Results
2.1. Studied Population
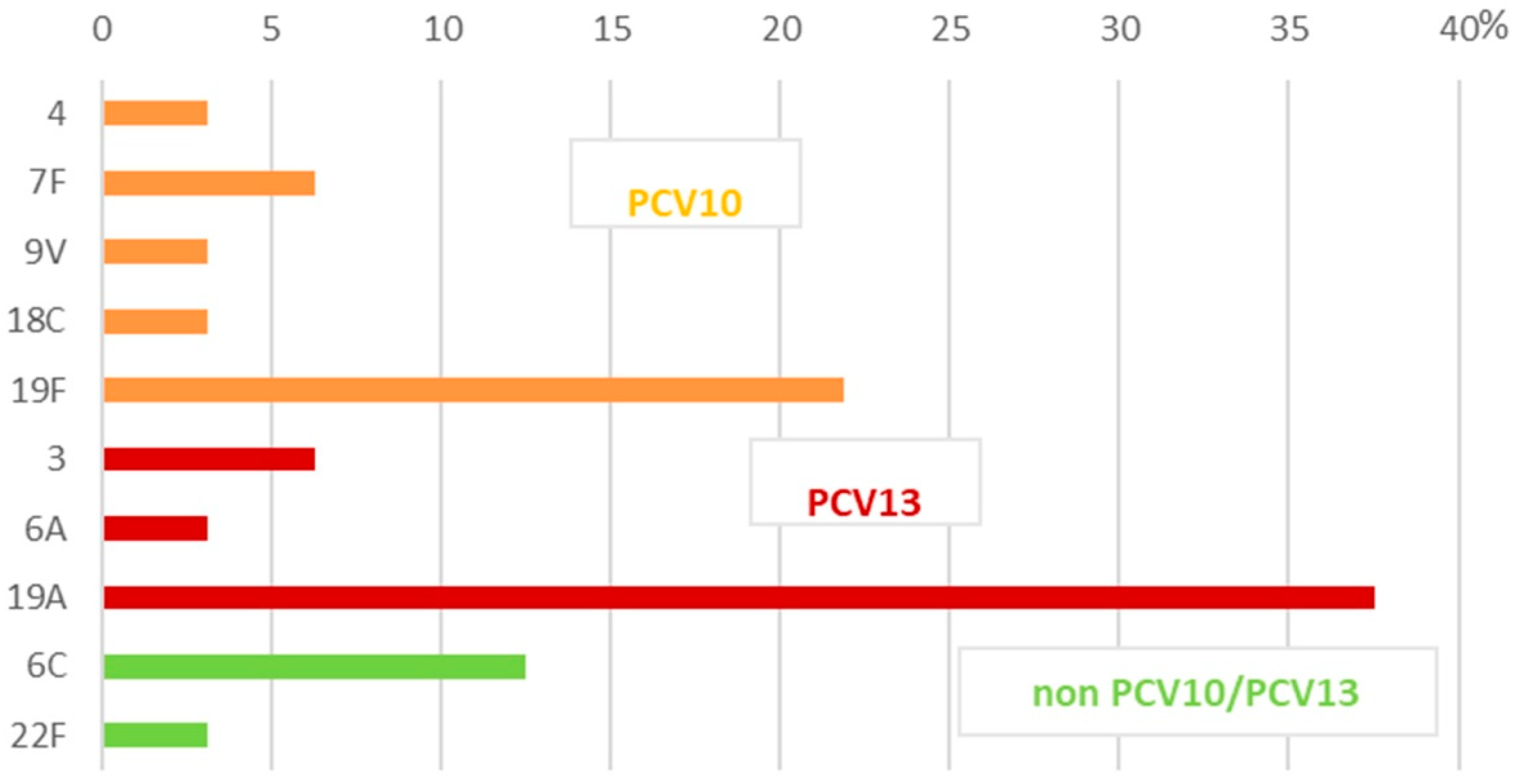
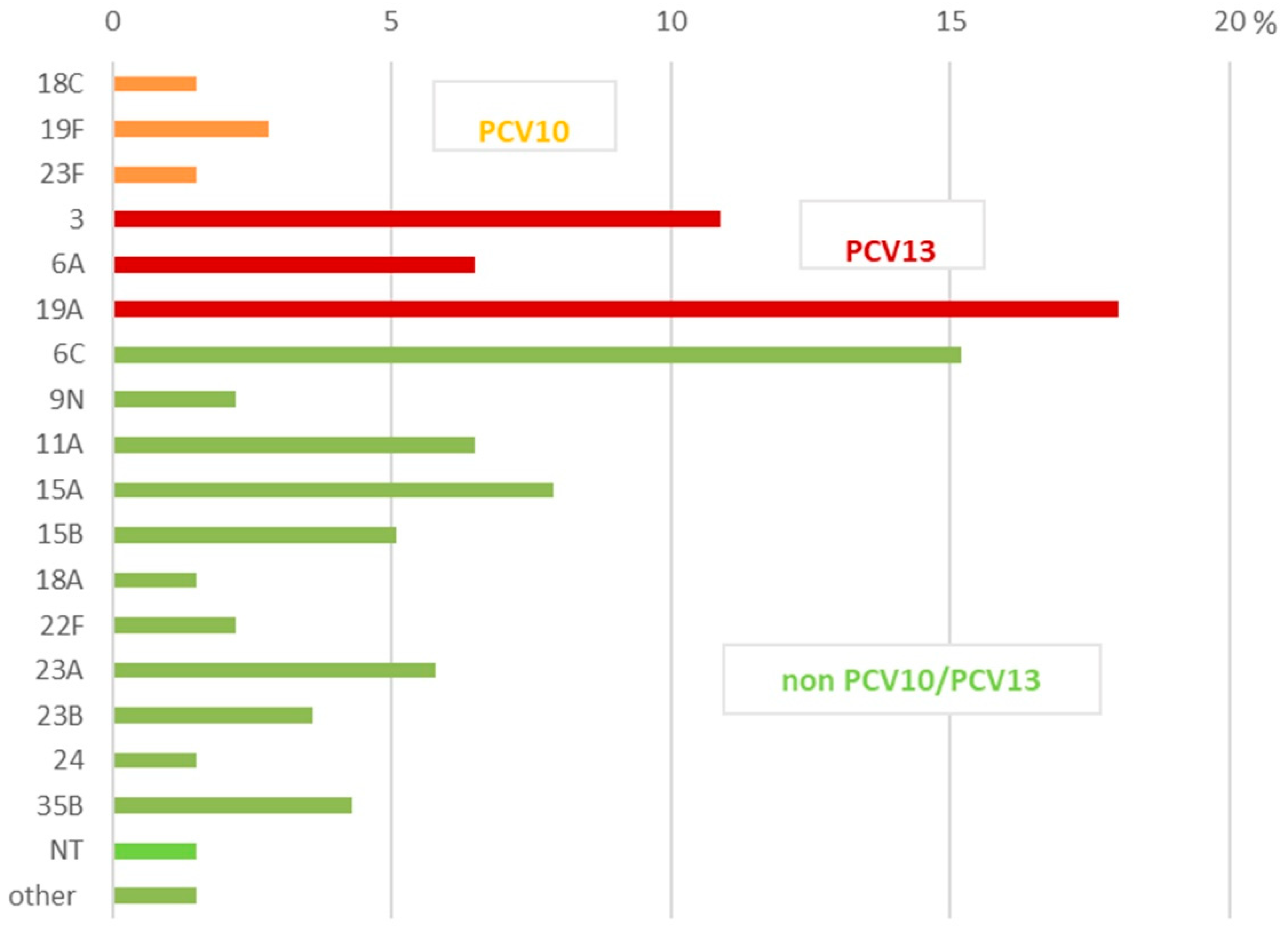
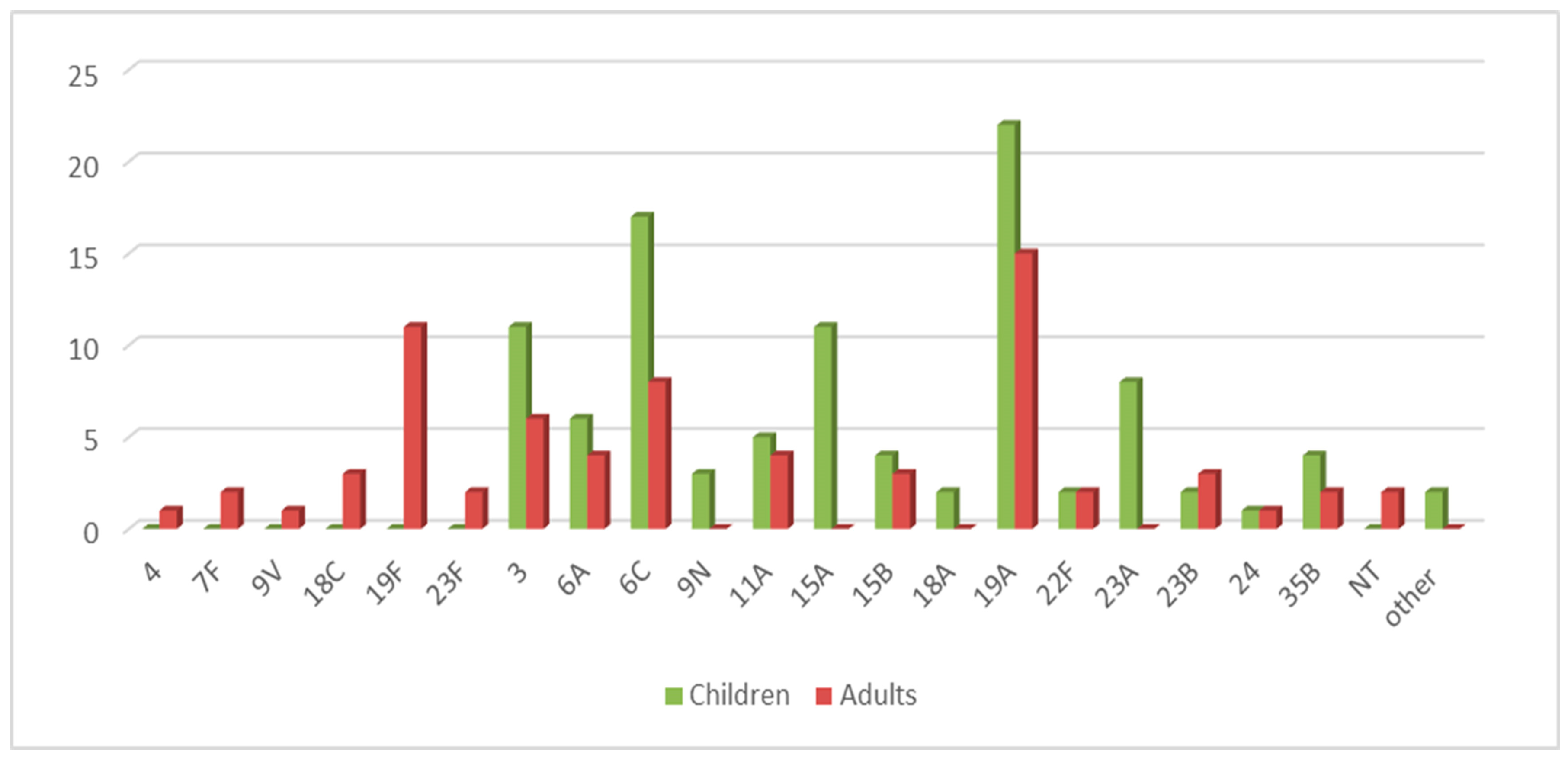
2.2. Serotyping
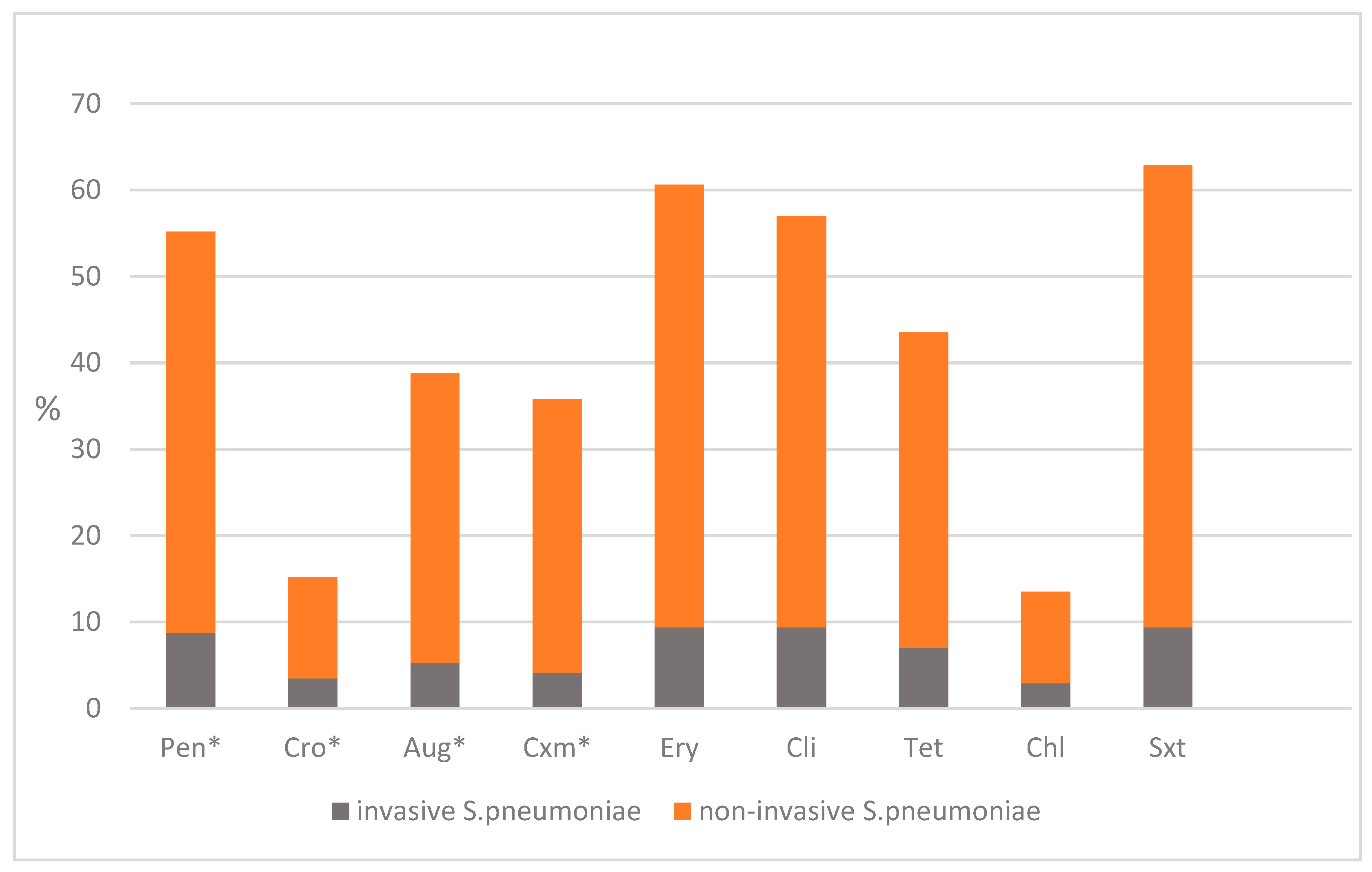
2.3. Antimicrobial Susceptibility
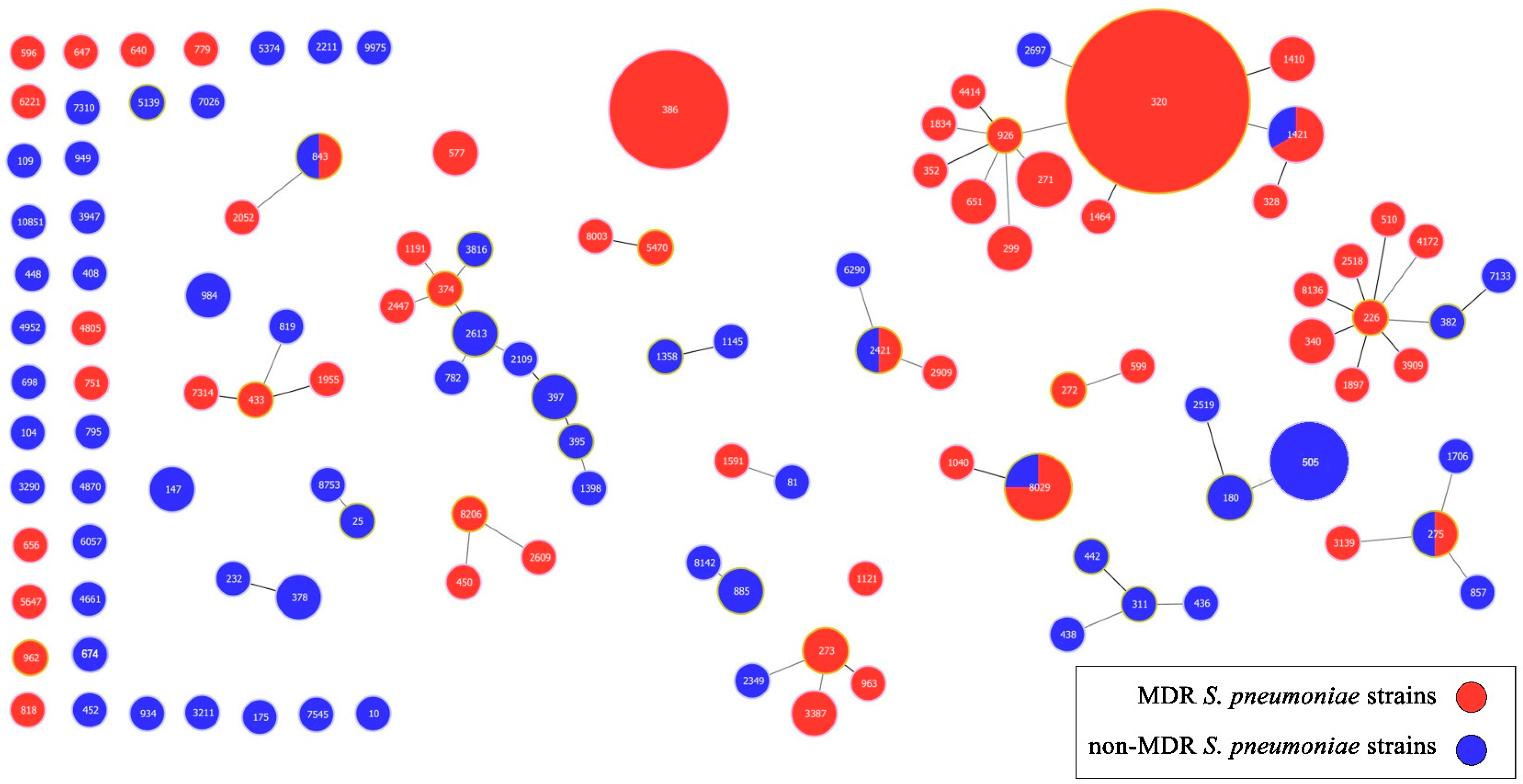
2.4. Multilocus Sequence Typing (MLST)
3. Discussion
4. Materials and Methods
4.1. Patients and Specimen Collection
4.2. Identification
4.3. Serotyping
4.4. Antimicrobial Susceptibility Testing
4.5. Multilocus Sequence Typing (MLST)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hiller, N.L.; Sá-Leão, R. Puzzling Over the Pneumococcal Pangenome. Front. Microbiol. 2018, 9, 2580. [Google Scholar] [CrossRef]
- Andam, C.P.; Hanage, W.P. Mechanisms of genome evolution of Streptococcus. Infect. Genet. Evol. 2015, 33, 334–342. [Google Scholar] [CrossRef]
- Brooks, L.R.K.; Mias, G.I. Streptococcus pneumoniae’s Virulence and Host Immunity: Aging, Diagnostics, and Prevention. Front. Immunol. 2018, 9, 1366. [Google Scholar] [CrossRef]
- Gil, E.; Noursadeghi, M.; Brown, J.S. Streptococcus pneumoniae interactions with the complement system. Front. Cell Infect. Microbiol. 2022, 12, 929483. [Google Scholar] [CrossRef]
- Almomani, E.; Maria van der Putten, I.; van Mastrigt, G.; Elabbasy, D.; Al-Lahham, A.; Paulus, A. Intersectoral costs of pneumococcal diseases: A systematic review of partial economic evaluation studies. Expert Rev. Pharmacoecon. Outcomes Res. 2025, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Koslap-Petraco, M. Pneumococcal vaccine. Nurs. Clin. N. Am. 2025, 60, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Loughran, A.J.; Orihuela, C.J.; Tuomanen, E.I. Streptococcus pneumoniae: Invasion and Inflammation. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Martens, P.; Worm, S.W.; Lundgren, B.; Konradsen, H.B.; Benfield, T. Serotype-specific mortality from invasive Streptococcus pneumoniae disease revisited. BMC Infect. Dis. 2004, 4, 21. [Google Scholar] [CrossRef]
- Cedrone, F.; Montagna, V.; Del Duca, L.; Camplone, L.; Mazzocca, R.; Carfagnini, F.; Fortunato, V.; Di Martino, G. The Burden of Streptococcus pneumoniae-Related Admissions and In-Hospital Mortality: A Retrospective Observational Study between the Years 2015 and 2022 from a Southern Italian Province. Vaccines 2023, 11, 1324. [Google Scholar] [CrossRef]
- Poolman, J.T. Pneumococcal vaccine development. Expert Rev. Vaccines 2004, 3, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Pletz, M.W.; Bahrs, C. Erratum zu: Pneumokokkenimpfstoffe [Pneumococcal vaccination]. Internist 2021, 62, 807–815, Erratum in Internist 2021, 62, 1256. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Romano, M.R.; Carboni, F.; Adamo, R.; Berti, F. Strengths and weaknesses of pneumococcal conjugate vaccines. Glycoconj J. 2023, 40, 135–148. [Google Scholar] [CrossRef]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal capsules and their types: Past, present, and future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef]
- Chang, B.; Nariai, A.; Sekizuka, T.; Akeda, Y.; Kuroda, M.; Oishi, K.; Ohnishi, M. Capsule Switching and Antimicrobial Resistance Acquired during Repeated Streptococcus pneumoniae Pneumonia Episodes. J. Clin. Microbiol. 2015, 53, 3318–3324. [Google Scholar] [CrossRef] [PubMed]
- Hyams, C.; Camberlein, E.; Cohen, J.M.; Bax, K.; Brown, J.S. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun. 2010, 78, 704–715. [Google Scholar] [CrossRef]
- Lysenko, E.S.; Lijek, R.S.; Brown, S.P.; Weiser, J.N. Within-host competition drives selection for the capsule virulence determinant of Streptococcus pneumoniae. Curr. Biol. 2010, 20, 1222–1226. [Google Scholar] [CrossRef][Green Version]
- Ganaie, F.A.; Saad, J.S.; Lo, S.W.; McGee, L.; van Tonder, A.J.; Hawkins, P.A.; Calix, J.J.; Bentley, S.D.; Nahm, M.H. Novel pneumococcal capsule type 33E results from the inactivation of glycosyltransferase WciE in vaccine type 33F. J. Biol. Chem. 2023, 299, 105085. [Google Scholar] [CrossRef]
- Ganaie, F.A.; Saad, J.S.; Lo, S.W.; McGee, L.; Bentley, S.D.; van Tonder, A.J.; Hawkins, P.; Keenan, J.D.; Calix, J.J.; Nahm, M.H. Discovery and Characterization of Pneumococcal Serogroup 36 Capsule Subtypes, Serotypes 36A and 36B. J. Clin. Microbiol. 2023, 61, e0002423. [Google Scholar] [CrossRef]
- Musher, D.M.; Anderson, R.; Feldman, C. The remarkable history of pneumococcal vaccination: An ongoing challenge. Pneumonia 2022, 14, 5. [Google Scholar] [CrossRef]
- Kobayashi, M.; Leidner, A.; Gierke, R.; Xing, W.; Accorsi, E.; Moro, P.; Kamboj, M.; Kuchel, G.A.; Schechter, R.; Loehr, J.; et al. Expanded Recommendations for Use of Pneumococcal Conjugate Vaccines Among Adults Aged ≥50 Years: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR Morb. Mortal Wkly. Rep. 2025, 74, 1–8. [Google Scholar] [CrossRef]
- Kobayashi, M.; Leidner, A.; Gierke, R.; Farrar, J.; Morgan, R.; Campos-Outcalt, D.; Schechter, R.; Poehling, K.; Long, S.; Loehr, J.; et al. Use of 21-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR Morb. Mortal Wkly. Rep. 2024, 73, 793–798. [Google Scholar] [CrossRef]
- Daniels, C.C.; Rogers, P.D.; Shelton, C.M. A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Recommendations and Future Protein Antigens. J. Pediatr. Pharmacol. Ther. 2016, 21, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, M.H.; Lynfield, R.; Schaffner, W.; Craig, A.S.; Hadler, J.; Reingold, A.; Thomas, A.R.; Harrison, L.H.; Bennett, N.M.; Farley, M.M.; et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 2006, 354, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, C.I.; Castañeda-Orjuela, C.; Brandileone, M.C.C.; Echániz-Aviles, G.; Almeida, S.C.G.; Carnalla-Barajas, M.N.; Regueira, M.; Fossati, S.; Alarcón, P.; Araya, P.; et al. The direct effect of pneumococcal conjugate vaccines on invasive pneumococcal disease in children in the Latin American and Caribbean region (SIREVA 2006-17): A multicentre, retrospective observational study. Lancet Infect. Dis. 2021, 21, 405–417. [Google Scholar] [CrossRef] [PubMed]
- von Gottberg, A.; Kleynhans, J.; de Gouveia, L.; Tempia, S.; Meiring, S.; Quan, V.; du Plessis, M.; von Mollendorf, C.; Crowther-Gibson, P.; Avenant, T.; et al. Long-term effect of pneumococcal conjugate vaccines on invasive pneumococcal disease incidence among people of all ages from national, active, laboratory-based surveillance in South Africa, 2005–2019: A cohort observational study. Lancet Glob. Health 2024, 12, e1470–e1484. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.Q.; Shi, W.; Yu, D.; Yao, K.H. Epidemiology of non-vaccine serotypes of Streptococcus pneumoniae before and after universal administration of pneumococcal conjugate vaccines. Hum. Vaccines Immunother. 2021, 17, 5628–5637. [Google Scholar] [CrossRef]
- Setchanova, L.P.; Alexandrova, A.; Mitov, I.; Nashev, D.; Kantardjiev, T.; Group for Microbiological Surveillance of Pneumococci. Serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates in Bulgaria before the introduction of pneumococcal conjugate vaccine. J. Chemother. 2012, 24, 12–17. [Google Scholar] [CrossRef]
- Alexandrova, A.; Pencheva, D.; Mitov, I.; Setchanova, L. Phenotypic and genotypic characteristics of non-invasive S. pneumoniae isolates recovered from PCV10-vaccinated children in Bulgaria. Indian J. Med. Microbiol. 2022, 40, 61–67. [Google Scholar] [CrossRef]
- Gergova, R.; Boyanov, V.; Muhtarova, A.; Alexandrova, A. A Review of the Impact of Streptococcal Infections and Antimicrobial Resistance on Human Health. Antibiotics 2024, 13, 360. [Google Scholar] [CrossRef]
- Liñares, J.; Ardanuy, C.; Pallares, R.; Fenoll, A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin. Microbiol. Infect. 2010, 16, 402–410. [Google Scholar] [CrossRef]
- Zahari, N.I.N.; Engku Abd Rahman, E.N.S.; Irekeola, A.A.; Ahmed, N.; Rabaan, A.A.; Alotaibi, J.; Alqahtani, S.A.; Halawi, M.Y.; Alamri, I.A.; Almogbel, M.S.; et al. A Review of the Resistance Mechanisms for β-Lactams, Macrolides and Fluoroquinolones among Streptococcus pneumoniae. Medicina 2023, 59, 1927. [Google Scholar] [CrossRef]
- Li, L.; Ma, J.; Yu, Z.; Li, M.; Zhang, W.; Sun, H. Epidemiological characteristics and antibiotic resistance mechanisms of Streptococcus pneumoniae: An updated review. Microbiol. Res. 2023, 266, 127221. [Google Scholar] [CrossRef]
- Maeda, H.; Morimoto, K. Global distribution and characteristics of pneumococcal serotypes in adults. Hum. Vaccines Immunother. 2025, 21, 2469424. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chiu, C.H.; Woo, P.C.; Muttalif, A.R.; Dhar, R.; Kit, L.C.; Morales, G.; Ozbilgili, E. Pneumococcal serotype prevalence and antibiotic resistance in children in South and Southeast Asia, 2012–2024. Hum. Vaccines Immunother. 2024, 20, 2417554. [Google Scholar] [CrossRef]
- Ugrekhelidze, D.; Anis, S.; Sępek, J.; Grys, M.; Zalewska, M.; Pieniążek, I. Pneumococcal disease in children in the Middle East and Northern Africa: A systematic literature review of clinical burden, serotype distribution, and vaccination programs. Hum. Vaccines Immunother. 2024, 20, 2421630. [Google Scholar] [CrossRef] [PubMed]
- Fenoll, A.; Ardanuy, C.; Liñares, J.; Cercenado, E.; Marco, F.; Fleites, A.; Rodríguez-Mayo, M.; López-Hontangas, J.L.; Palop, B.; Aller, A.I.; et al. Serotypes and genotypes of S. pneumoniae isolates from adult invasive disease in Spain: A 5-year prospective surveillance after pediatric PCV13 licensure. The ODIN study. Vaccine 2018, 36, 7993–8000. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Kleynhans, J.; de Gouveia, L.; Meiring, S.; Cohen, C.; Hathaway, L.J.; von Gottberg, A.; GERMS-SA. Streptococcus pneumoniae Serotypes Associated with Death, South Africa, 2012–2018. Emerg. Infect. Dis. 2022, 28, 166–179. [Google Scholar] [CrossRef]
- Koelman, D.L.H.; Brouwer, M.C.; van de Beek, D. Resurgence of pneumococcal meningitis in Europe and Northern America. Clin. Microbiol. Infect. 2020, 26, 199–204. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Jareño, M.; Roig-Sena, F.J.; Pérez-Pérez, E.; Gil-Brusola, A.; López-Hontangas, J.L.; Valentín-Gómez, E.; Pineda-Lucena, A.; Pemán, J. Study of pediatric invasive pneumococcal disease in the 13-pneumococcal conjugated vaccine era. Diagn. Microbiol. Infect. Dis. 2024, 110, 116532. [Google Scholar] [CrossRef]
- de Sevilla, M.F.; Alcaraz-Soler, C.; Soldevila, N.; Izquierdo, C.; Esteva, C.; Moraga-Llop, F.; González-Peris, S.; Ciruela, P.; Díaz-Conradi, A.; Pérez-Argüello, A.; et al. Clinical manifestations, serotype distribution, and incidence of pediatric invasive pneumococcal disease in Catalonia (Spain), 2018–2022. Eur. J. Pediatr. 2025, 184, 323. [Google Scholar] [CrossRef]
- Garrido Rodríguez, M.; Borrull Senra, A.M.; Hernández-Bou, S.; Artetxe Barroso, A.; Servitje Verdaguer, B.; Hurtado Mingo, Á.; Rivas García, A.; Collazo Vallduriola, I.; Gangoiti, I.; Velasco, R.; et al. Streptococcus pneumoniae remains leading cause of bacteremia in children despite 13-valent pneumococcal conjugate vaccine introduction. Acta Paediatr. 2025, 1–10. [Google Scholar] [CrossRef]
- Puzia, W.; Gawor, J.; Gromadka, R.; Żuchniewicz, K.; Wróbel-Pawelczyk, I.; Ronkiewicz, P.; Gołębiewska, A.; Hryniewicz, W.; Sadowy, E.; Skoczyńska, A. Highly Resistant Serotype 19A Streptococcus pneumoniae of the GPSC1/CC320 Clone from Invasive Infections in Poland Prior to Antipneumococcal Vaccination of Children. Infect. Dis. Ther. 2023, 12, 2017–2037. [Google Scholar] [CrossRef]
- Opavski, N.; Jovicevic, M.; Kabic, J.; Kekic, D.; Vasiljevic, Z.; Tosic, T.; Medic, D.; Laban, S.; Ranin, L.; Gajic, I. Serotype distribution, antimicrobial susceptibility and molecular epidemiology of invasive Streptococcus pneumoniae in the nine-year period in Serbia. Front. Microbiol. 2023, 14, 1244366. [Google Scholar] [CrossRef]
- Golden, A.R.; Lefebvre, B.; Deceuninck, G.; Brousseau, N.; De Wals, P.; Quach, C.; Demczuk, W.H.B.; Martin, I. Clonal diversity of Streptococcus pneumoniae serotype 19A collected from children < 5 years old in Québec, Canada, 2016–2021. Vaccine 2023, 41, 6612–6618. [Google Scholar] [CrossRef] [PubMed]
- Azarsa, M.; Mosadegh, M.; Ghahfarokhi, S.H.; Pourmand, M.R. Serotype Distribution and Multi Locus Sequence Type (MLST) of Erythromycin-Resistant Streptococcus Pneumoniae Isolates in Tehran, Iran. Rep. Biochem. Mol. Biol. 2023, 12, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, M.; Mereckiene, J.; Cotter, S.; Murchan, S.; Lo, S.W.; McGee, L.; Breiman, R.F.; Cunney, R.; Humphreys, H.; Bentley, S.D.; et al. Using genomics to examine the persistence of Streptococcus pneumoniae serotype 19A in Ireland and the emergence of a sub-clade associated with vaccine failures. Vaccine 2021, 39, 5064–5073. [Google Scholar] [CrossRef]
- Bennett, J.C.; Knoll, M.D.; Kagucia, E.W.; Quesada, M.Q.; Zeger, S.L.; Hetrich, M.K.; Yang, Y.; Herbert, C.; Ogyu, A.; Cohen, A.L.; et al. Global impact of ten-valent and 13-valent pneumococcal conjugate vaccines on invasive pneumococcal disease in all ages (the PSERENADE project): A global surveillance analysis. Lancet Infect Dis. 2024, 25, 457–470. [Google Scholar] [CrossRef]
- Mavroidi, A.; Paraskakis, I.; Pangalis, A.; Kirikou, E.; Charisiadou, A.; Athanasiou, T.; Tassios, P.T.; Tzouvelekis, L.S. Spread of the Streptococcus pneumoniae Taiwan19F-14 clone among children in Greece. Clin. Microbiol. Infect. 2007, 13, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Ip, M.; Ang, I.; Liyanapathirana, V.; Ma, H.; Lai, R. Genetic analyses of penicillin binding protein determinants in multidrug-resistant Streptococcus pneumoniae serogroup 19 CC320/271 clone with high-level resistance to third-generation cephalosporins. Antimicrob. Agents Chemother. 2015, 59, 4040–4045. [Google Scholar] [CrossRef]
- Fortuna, L.B.D.P.; Miranda, F.M.; Antunes, I.M.F.; Silva, A.B.; Cabral, A.S.; Dolores, Í.M.; Cardoso-Marques, N.T.; Teixeira, L.M.; Neves, F.P.G. Prevalence, capsular types, antimicrobial resistance and risk factors associated with pneumococcal carriage among children after long-term 10-valent pneumococcal conjugate vaccine use in Brazil. Vaccine 2023, 41, 3111–3118. [Google Scholar] [CrossRef] [PubMed]
- Hjálmarsdóttir, M.Á.; Haraldsson, G.; Quirk, S.J.; Haraldsson, Á.; Erlendsdóttir, H.; Kristinsson, K.G. Reduction of antimicrobial resistant pneumococci seven years after introduction of pneumococcal vaccine in Iceland. PLoS ONE 2020, 15, e0230332. [Google Scholar] [CrossRef]
- Krajcar, N.; Trkulja, V.; Butić, I.; Tešović, G. Pneumococcal CROcarriage Study Group. Impact of the 10-valent pneumococcal conjugate vaccine (PCV10) on pneumococcal carriage in healthy children and children with acute otitis media and pneumonia: Emergence of serotypes 3, 6C and 19A in Croatia. Vaccine 2025, 50, 126848. [Google Scholar] [CrossRef]
- Quesada, M.G.; Peterson, M.E.; Bennett, J.C.; Hayford, K.; Zeger, S.L.; Yang, Y.; Hetrich, M.K.; Feikin, D.R.; Cohen, A.L.; von Gottberg, A.; et al. Serotype distribution of remaining invasive pneumococcal disease after extensive use of ten-valent and 13-valent pneumococcal conjugate vaccines (the PSERENADE project): A global surveillance analysis. Lancet Infect Dis. 2024, 25, 445–456. [Google Scholar] [CrossRef]
- Kandasamy, R.; Voysey, M.; Collins, S.; Berbers, G.; Robinson, H.; Noel, I.; Hughes, H.; Ndimah, S.; Gould, K.; Fry, N.; et al. Persistent Circulation of Vaccine Serotypes and Serotype Replacement After 5 Years of Infant Immunization With 13-Valent Pneumococcal Conjugate Vaccine in the United Kingdom. J. Infect. Dis. 2020, 221, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Rafei, R.; Zaylaa, M.; Diab, M.; Kassem, I.I.; El Omari, K.; Halimeh, F.B.; El Moujaber, G.; Achour, A.; Ismail, B.; Mallat, H.; et al. Nasopharyngeal Carriage, Antimicrobial Resistance, and Serotype Distribution of Streptococcus pneumoniae in Children Under Five in Lebanon: Baseline Data Prior to PCV13 Introduction. Antibiotics 2025, 14, 168. [Google Scholar] [CrossRef]
- Bhatt, V.; Ponnampalavanar, S.S.S.; Chong, C.W.; Tang, L.Y.; Krishnasamy, K.; Goh, S.S.L.; The, C.S.J. Socio-demographic factors and public knowledge of antibiotic resistance. Healthcare 2023, 11, 2284. [Google Scholar] [CrossRef] [PubMed]
- Berical, A.C.; Harris, D.; Dela Cruz, C.S.; Possick, J.D. Pneumococcal vaccination strategies. An update and perspective. Ann. Am. Thorac. Soc. 2016, 13, 933–944. [Google Scholar] [CrossRef]
- Flem, E.; Mouawad, C.; Palmu, A.A.; Platt, H.; Johnson, K.D.; McIntosh, E.D.; Abadi, J.; Buchwald, U.K.; Feemster, K. Indirect protection in adults ≥18 years of age from pediatric pneumococcal vaccination: A review. Expert Rev. Vaccines 2024, 23, 997–1010. [Google Scholar] [CrossRef]
- Cillóniz, C.; Garcia-Vidal, C.; Ceccato, A.; Torres, A. Antimicrobial resistance among Streptococcus pneumoniae. In Antimicrobial Resistance in the 21st Century; Springer: Cham, Switzweland, 2018; Volume 7, pp. 13–38. [Google Scholar] [CrossRef]
- Abaye, G.; Fekadu, H.; Haji, K.; Alemu, D.; Anjulo, A.A.; Yadate, D.T. Prevalence and risk factors of pneumococcal nasopharyngeal carriage in healthy children attending kindergarten, in district of Arsi Zone, South East, Ethiopia. BMC Res. Notes 2019, 12, 253. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Belman, S.; Lefrancq, N.; Nzenze, S.; Downs, S.; du Plessis, M.; Lo, S.W.; Global Pneumococcal Sequencing Consortium; McGee, L.; Madhi, S.A.; von Gottberg, A.; et al. Geographical migration and fitness dynamics of Streptococcus pneumoniae. Nature 2024, 631, 386–392. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Dawood, H.N.; Ikram, D.; Al-Jabban, A. Pneumococcal disease: Global disease prevention strategies with a focus on the challenges in Iraq. Int. J. Gen. Med. 2023, 16, 2095–2110. [Google Scholar] [CrossRef]
- Reis, J.N.; Azevedo, J.; de Oliveira, A.M.L.; Menezes, A.P.O.; Pedrosa, M.; Dos Santos, M.S.; Ribeiro, L.C.; Freitas, H.F.; Gouveia, E.L.; Teles, M.B.; et al. Long-term surveillance of invasive pneumococcal disease: The impact of 10-valent pneumococcal conjugate vaccine in the metropolitan region of Salvador, Brazil. Vaccine 2024, 42, 591–597. [Google Scholar] [CrossRef]
- Ozisik, L. The new era of pneumococcal vaccination in adults: What is next? Vaccines 2025, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Greve, T.; Møller, J.K. Accuracy of using the lytA gene to distinguish Streptococcus pneumoniae from related species. J Med. Microbiol. 2012, 61, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Xiao, M.; Kong, F.; Oftadeh, S.; Zhou, F.; Liu, C.; Gilbert, G.L. Simple, accurate, serotype-specific PCR assay to differentiate Streptococcus pneumoniae serotypes 6A, 6B, and 6C. J. Clin. Microbiol. 2009, 47, 2470–2474. [Google Scholar] [CrossRef] [PubMed]
- da Gloria Carvalho, M.; Pimenta, F.C.; Jackson, D.; Roundtree, A.; Ahmad, Y.; Millar, E.V.; O’Brien, K.L.; Whitney, C.G.; Cohen, A.L.; Beall, B.W. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J. Clin. Microbiol. 2010, 48, 1611–1618. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 15.0; The European Committee on Antimicrobial Susceptibility Testing: Basel, Switzerland, 2025; Available online: http://www.eucast.org (accessed on 1 January 2025).
- Enright, M.C.; Spratt, B.G. Multilocus sequence typing. Trends Microbiol. 1999, 7, 482–487. [Google Scholar] [CrossRef]
- Gladstone, R.A.; Loa, S.W.; Lees, J.A.; Croucher, N.J.; van Tondera, A.J.; Jukka Corander, J.; Page, A.J.; Marttinen, P.; Bentley, L.J.; Ochoa, T.J.; et al. International genomic definition of pneumococcal lineages, to contextualise disease, antibiotic resistance and vaccine impact. EBioMedicine 2019, 43, 338–346. [Google Scholar] [CrossRef] [PubMed]
| Vaccine | Serotypes |
|---|---|
| *PCV10 | 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F |
| PCV13 | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F |
| PCV15 | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, 33F |
| PCV20 | 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, 33F |
| PCV24 | 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F |
| **PPV23 | 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F |
| IPD/NIPD 1 Isolates | Patient Specimens 2 | n 3 (%) of All Isolates | Serotype (n of Isolates) | *PCV10, PCV13, non-PCV10/PCV13 Serotypes |
|---|---|---|---|---|
| Invasive isolates n = 32 | CSF | 16 (9.4) | 4 (1), 7F (2), 19F (4) | PCV10 |
| 3 (2), 19A (7) | PCV13 | |||
| Blood | 12 (7.1) | 9V (1), 19F (2) | PCV10 | |
| 6A (1), 19A (4) | non-PCV10 | |||
| 6C (3) | PCV13 | |||
| 22F (1) | non-PCV10/PCV13 | |||
| Pleural fluids | 4 (2.3) | 18C (1), 19F (2) | PCV10 | |
| 19A (1) | PCV13 | |||
| Non-invasive isolates n = 138 | Nph samples | 45 (26.5) | 23F (1) | PCV10 |
| 3 (2), 6A (4), 19A (8) | PCV13 | |||
| 6C (4), 11A (6), 15A (8), 15B (4), 22F (2), 23A(1), 23B (3), 35B (2) | non-PCV10/PCV13 | |||
| Nose | 36 (21.2) | 19F(1) | PCV10 | |
| 3 (2), 6C (6), 19A (8) | PCV13 | |||
| 9N (1), 11A (3), 15A (3), 23A (5), 24 (2), 35B (4), Other (1) | non-PCV10/PCV13 | |||
| Sputum | 10 (5.8) | 18C (1), 19F(2) | PCV10 | |
| 6A(1) | non-PCV10 | |||
| 6C (1), 9N (1), 15B (1), 22F (1), 23B (2) | non-PCV10/PCV13 | |||
| BAL | 5 (2.9) | 23F (1) | PCV10 | |
| 3 (2), 19A (1) | PCV13 | |||
| NT (1) | non-PCV10/PCV13 | |||
| Eye fluids | 8 (4.7) | 18C (1) | PCV10 | |
| 6C (1), 19A (2) | PCV13 | |||
| 15B (2), NT (1), Other (1) | non-PCV10/PCV13 | |||
| Middle ear fluid | 29 (17.2) | 3 (9), 6A (3), 19A (5) | PCV13 | |
| 6C (8), 15C (2), 18A (2) | non-PCV10/PCV13 | |||
| Wound | 5 (2.9) | 6A (2), 19A (2) | non-PCV10 | |
| 9N (1) | non-PCV10 |
| Serotype | CC 1 | ST 2 (n) | GPSC 3 Type (ST) | PMEN 4 | |
|---|---|---|---|---|---|
| PCV10 serotypes | 4 | - | 7026 (1) | 70 (7026) | DLV of Denmark12F-34 |
| 7F | - | 6057 (1), 5374 (1) | 32 (6057) | - | |
| 9V | - | 4952 (1) | - | - | |
| 18C | - | 843 (1), 1358 (1), 9975 (1) | 22 (843) 11 (1358, 9975) | SLV of Greece21-30 (ST1358) | |
| 23F | - | 647 (1), 408 (1) | 13 (647) 3 (408) | - | |
| 19F | CC320 | 320 (2), 328 (1), 1421 (2), 1464 (1), 2697 (1), 4414 (1), 651 (1), 926 (1), 1834 (1) | 1 (320, 1421, 1464, 2697, 4414, 651, 926) | SLV of Taiwan19F-14 (STs 651, 926, 1834) DLV of Taiwan19F-14 (STs 320, 328, 1421, 1464, 2697) | |
| non-PCV10 serotypes | 19A | 320 (13), 271 (3), 299 (2), 1410 (2),352 (1), 651 (1), 1421 (1) | 1 (271,320, 651, 1421) | SLV of Taiwan19F-14 (299,352,651) DLV of Taiwan19F-14 (320, 271, 1410, 1421) | |
| 3 | CC505 | 505 (5), 180 (2), 2519 (1) | 12 (505, 180) | Netherlands3-31 (180) SLV of Netherlands3-31 (2519) DLV of Netherlands3-31 (505) | |
| 3 | CC378 | 378 (2), 232 (1) | 83 (378, 232) | - | |
| 3 | - | 782 (1), 1145 (1), 4661(1), 3290 (1), 4805 (1), 7545 (1) | 9 (782) 11 (1145) 43 (4661) 36 (3290) 51 (7545) | SLV of Sweden15A-25 (782) DLV of Greece21-30 (1145) | |
| 6A, 6C | CC273 | 273 (2), 147 (3), 885 (2), 2349 (1), 8142 (1) | 23 (273, 147) | Greece6B-22 (273) SLV of Greece6B-22 (2349) DLV of Greece6B-22 (147, 885, 8142) | |
| 6A | - | 5470 (1), 81 (1) | 13 (5470) 16 (81) | - | |
| 6A, 6C | CC386 | 386 (9) | 47 | DLV of Poland6B-20 (386) | |
| 6C | CC3387 | 3387 (2), 104 (1) | 23 (3387, 104) | SLV of Spain6B-2 (3387, 104) | |
| 6C | CC2421 | 2421 (2), 6290 (1), 2909 (1) | 37 (2421, 6290, 2909) | - | |
| 6C | - | 640 (1), 7310 (1), 8003 (1), 5647 (1), 751 (1), 3211 (1), 674 (1), 818 (1),1121 (1) | 23 (640, 1121) 13 (7310, 8003) 52 (5647) 37 (751, 3211) | DLV of Tennessee23F-4 (674) | |
| 9N | - | 4870 (1), 843 (1), 2211 (1) | 17 (4870) 22 (843) 26 (2211) | - | |
| 11A | - | 25 (1), 175 (1), 596(1), 630 (1), 934(1), 8753 (1), 596(1), 934 (1) | 18 (25, 8753) 55 (175) | SLV of England14-9 (25) CSR19A-11 (175) | |
| 11A | CC984 | 984 (2), 6221 (1) | 18 (984, 6221) | - | |
| 15A | CC2613 | 2613 (2),374 (1), 1191 (1) | 9 (2613, 374, 1191) | SLV of Sweden15A-25 (2613, 374, 1191) | |
| 15A | 5139 (1), 2052 (1), 962 (1), 10 (1) | 11 (5139) 22 (2052) | - | ||
| 15A,15B | CC397 | 397 (2), 395 (1) | 29 (395) | SLV of Portugal6A-41 (395) DLV of Portugal6A-41 (397) | |
| 15A, 15B | CC275 | 275 (2), 3139 (1), 857 (1), 1706 (1) | 48 (275) | - | |
| 15B | CC2447 | 2447 (1), 3816 (1) | 9 (2447,3816) | SLV of Sweden15A-25 (2447, 3816) | |
| 18A | - | 795 (1), 2109 (1) | - | - | |
| 19A | CC450 | 450 (1), 2609 (1), 8206 (1) | 4 (450) | SLV of Netherlands15B-37 (2609, 8206) DLV of Netherlands15B-37 (450) | |
| 19A | CC340 | 340 (2), 7133 (1), 4172 (1), 8136 (1), 2518 (1) | - | SLV of Hungary19A-6 (340, 7133) DLV of Hungary19A-6 (4172, 8136, 2518) | |
| 19A | CC226 | 226 (1), 382 (1), 510(1), 1897 (1), 3909 (1) | 738 (226) | SLV of Denmark14-32 (226, 382) DLV of Denmark14-32 (510, 1897, 3909) | |
| 22F | CC433 | 433(1), 1955 (1), 7314 (1) | 19 (433, 1955,7314) | - | |
| 22F | - | 698 (1) | 61 (698) | - | |
| 23A, 23B | CC8029 | 8029 (4),1040 (1) | - | - | |
| 23A | - | 442 (1), 656 (1), 577 (1) | 39 (656) | SLV of Netherlands14-35 (656, 577) | |
| 23A | CC311 | 311(1), 438 (1) | 7 (311, 438) | ||
| 23B | CC272 | 272 (1), 599 (1) | SLV of Poland23F-16 (272) | ||
| 23B | - | 1398 (1) | 29 (1398) | - | |
| 24 | - | 109(1), 819 (1) | - | - | |
| 35B | - | 452 (1), 577 (1), 779 (1), 963 (1), 1591(1), 3947 (1) | 75 (452) 715 (779) 16 (1591) | SLV of Netherlands8-33 (577) SLV of Spain23F-1 (1591) DLV of Spain6B-2 (963) | |
| NT 5 | - | 436(1), 448 (1) | 7 (436) 60 (448) | USANT-43 (448) | |
| Other 6 | - | 949 (1), 10851 (1) | 304 (10851) | DLV of Netherlands14-35 (949) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrova, A.S.; Boyanov, V.S.; Mihova, K.Y.; Hristova, P.M.; Hitkova, H.Y.; Marteva-Proevska, Y.; Gergova, R.T. Population Genetic Structure of Invasive and Non-Invasive Streptococcus pneumoniae Isolates After Fifteen Years of Routine PCV10 Vaccination in Bulgaria. Int. J. Mol. Sci. 2025, 26, 9028. https://doi.org/10.3390/ijms26189028
Alexandrova AS, Boyanov VS, Mihova KY, Hristova PM, Hitkova HY, Marteva-Proevska Y, Gergova RT. Population Genetic Structure of Invasive and Non-Invasive Streptococcus pneumoniae Isolates After Fifteen Years of Routine PCV10 Vaccination in Bulgaria. International Journal of Molecular Sciences. 2025; 26(18):9028. https://doi.org/10.3390/ijms26189028
Chicago/Turabian StyleAlexandrova, Alexandra S., Vasil S. Boyanov, Kalina Y. Mihova, Preslava M. Hristova, Hristina Y. Hitkova, Yuliya Marteva-Proevska, and Raina T. Gergova. 2025. "Population Genetic Structure of Invasive and Non-Invasive Streptococcus pneumoniae Isolates After Fifteen Years of Routine PCV10 Vaccination in Bulgaria" International Journal of Molecular Sciences 26, no. 18: 9028. https://doi.org/10.3390/ijms26189028
APA StyleAlexandrova, A. S., Boyanov, V. S., Mihova, K. Y., Hristova, P. M., Hitkova, H. Y., Marteva-Proevska, Y., & Gergova, R. T. (2025). Population Genetic Structure of Invasive and Non-Invasive Streptococcus pneumoniae Isolates After Fifteen Years of Routine PCV10 Vaccination in Bulgaria. International Journal of Molecular Sciences, 26(18), 9028. https://doi.org/10.3390/ijms26189028







