Chimeric Antigen Receptor Cell Therapy: Current Status and Its Potential in Aging and Alzheimer’s Disease
Abstract
1. Introduction
2. Inflammation in Aging
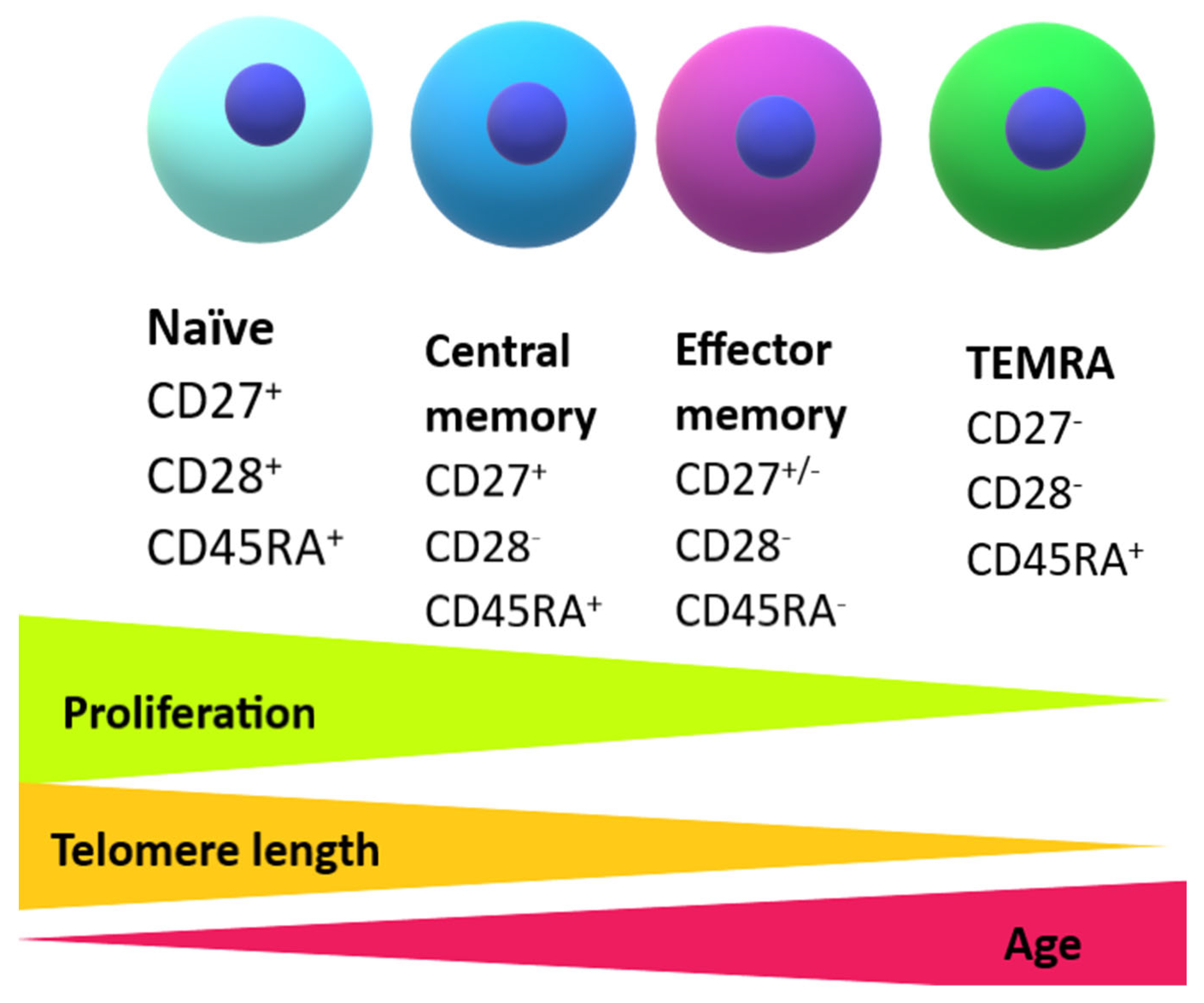
2.1. The Development of Senescent Immune Cells
2.2. Altered Function of Senescent T Cells
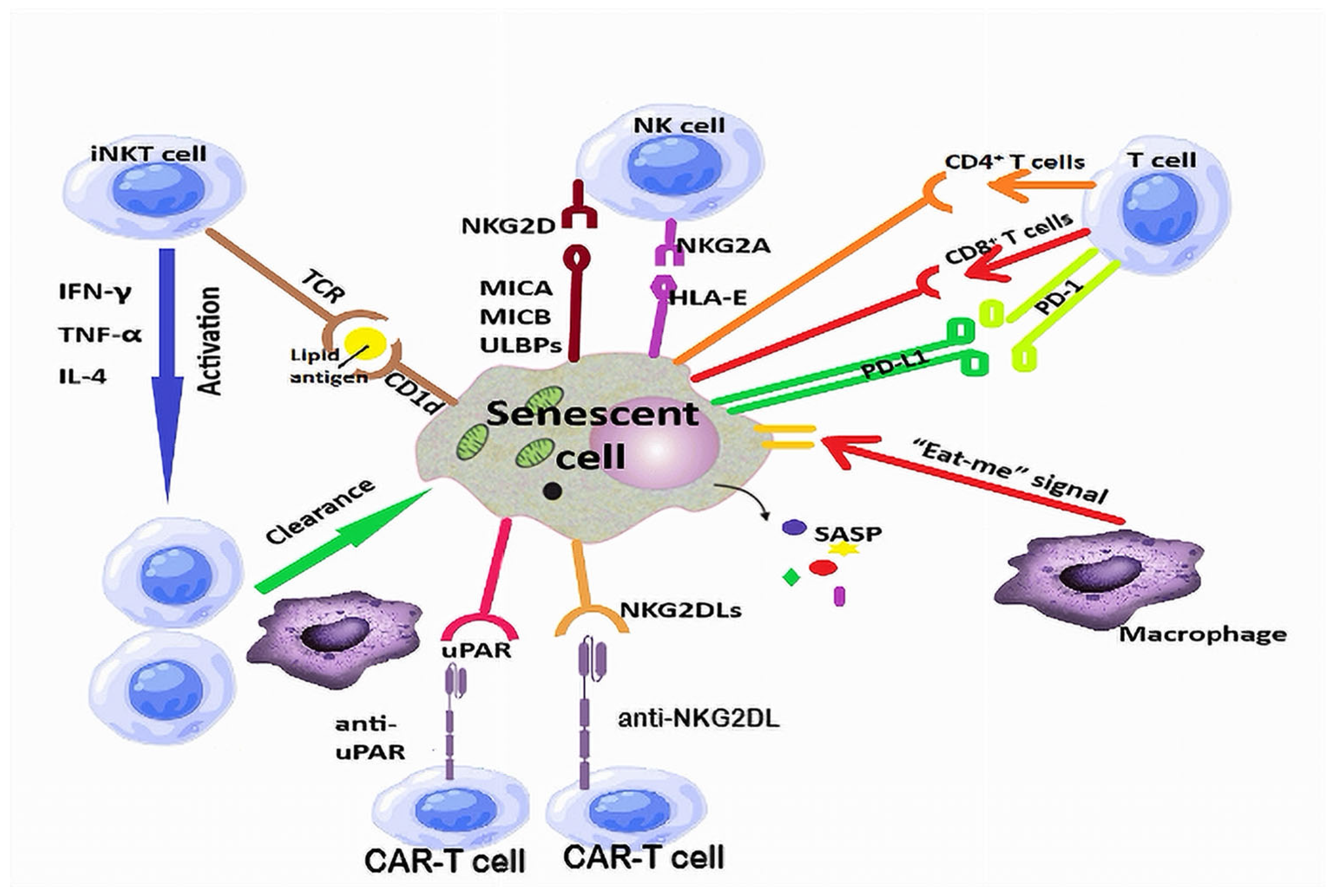
2.3. Immune Surveillance of Senescent Cells
3. Neuroinflammation in Alzheimer’s Disease
3.1. Genome-Wide Association Studies Support the Contribution of Neuroinflammation in the Development of Alzheimer’s Disease
3.2. The Innate Immune System in Alzheimer’s Disease
3.3. The Adaptive Immune System in Alzheimer’s Disease
3.3.1. B Cell Responses Against Amyloid Beta and Tau
3.3.2. T Cells in Alzheimer’s Disease
- -
- Brain antigens transported via the CSF can access the nasal mucosa via the cribriform plate, from where they are further drained by the lymphatic circulation into the deep cervical lymph nodes [119].
- -
- Antigens in the CSF can also be drained directly by meningeal lymphatic vessels into the deep cervical lymph nodes [119].
- -
- CNS antigens from the CSF can be captured by meningeal dendritic cells, which also reach the deep cervical lymph nodes through the meningeal lymphatics and contribute to priming of the immune cells [119].
4. CAR Cell Therapy
4.1. The CAR-T Cell Construct
- The first generation of CAR-T cells had an extracellular scFv domain linked to an intracellular CD3 signaling domain. These T cells had a low survival rate and could not produce a long-lasting immunological response.
- The second generation of CAR molecules was developed by adding a co-stimulatory domain (CD28 or 4-1BB) to the CD3 signaling domain, leading to increased resistance to exhaustion of the engineered cells [133].
- Third-generation CAR constructs used 2 co-stimulatory domains, which increased efficacy, proliferation rate, and durability of the transferred cells [134].
- The fourth generation is also known as TRUCK cells (T Redirected Universal Cytokine Killer Cells) and is engineered to also produce cytokines (IL-2, IL-15, IL-18), enzymes, and other biochemical substances that enhance the anti-tumor effect and the persistence of CAR-T cells in the tumor microenvironment [135].
- Fifth-generation CARs include additional elements that enable engineered cells to recognize multiple targets even in an environment with low antigen density [136].
4.2. Current Applications of CAR-T Cell Therapy
4.3. Side Effects of CAR-T Cell Therapy
4.4. Other CAR Cell Constructs
5. The Potential of CAR Cell Therapy Against Senescent Cells
- (a)
- Elimination of senescent cells (senolysis)
- (b)
- Neutralization of the SASP (with senomorphics)
- (c)
- Immune-based clearance of senescent cells
5.1. CAR-T Cell Therapy Against Senescent Cells
5.2. CAR-NK Cell Therapy Against Senescent Cells
5.3. CAR-M Cell Therapy Against Senescent Cells
5.4. CAR-iNKT Cell Therapy Against Senescent Cells
6. The Potential of Cell Therapy in Alzheimer’s Disease
6.1. Aβ-Specific Tregs in Alzheimer’s Disease
6.2. Chimeric Antigen Receptor Macrophages in Alzheimer’s Disease
7. Limitations, Challenges, and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | amyloid beta |
| ABCA | ATP-binding cassette subfamily A |
| AD | Alzheimer’s disease |
| AI | artificial intelligence |
| Akt | protein kinase B |
| ALL | acute lymphoblastic leukemia |
| ARIA | amyloid-related imaging abnormalities |
| B-ALL | B-cell acute lymphoblastic leukemia |
| BBB | blood–brain barrier |
| BBIR | biotin-binding immune receptor |
| BCR | B cell receptor |
| BTK | Bruton’s kinase |
| CAR | chimeric antigen receptor |
| CAR-Ms | chimeric antigen receptor macrophages |
| CAR-NKs | chimeric antigen receptor natural killer cells |
| CAR-T | CAR-T cells |
| CAR-Tregs | chimeric antigen receptor regulatory T cells |
| Cas9 | caspase-9 |
| CD | cluster of differentiation |
| CLL | chronic lymphocytic leukemia |
| CNS | central nervous system |
| CR1 | complement component (3b/4b) receptor 1 |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| CRS | cytokine release syndrome |
| CSF | cerebrospinal fluid |
| DAP12 | DNAX activation protein of 12 kDa |
| DLBCL | diffuse large B-cell lymphoma |
| DNA | deoxyribonucleic acid |
| EM | effector memory (T cells) |
| EMA | European Medicines Agency |
| EphA1 | ephrin type-A receptor 1 |
| FDA | Food and Drug Administration |
| FL | follicular lymphoma |
| FoxP3 | forkhead box P3 |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GWAS | genome-wide association study |
| HAMA | human anti-mouse IgG antibody |
| HLA | human leukocyte antigen |
| ICANS | immune cell-associated neurological syndrome |
| IFN | interferon |
| IL | interleukin |
| iNKT | invariant natural killer T cells |
| iPSCs | induced pluripotent stem cells |
| KLRG | killer cell lectin-like receptor subfamily g |
| LBCL | large B-cell lymphoma |
| mAb | monoclonal antibody |
| MAPK | mitogen activated protein kinase |
| MCL | mantle cell lymphoma |
| MCP-1 | monocyte chemoattractant protein-1 |
| M-CSF | macrophage colony stimulating factor |
| MEK | mitogen-activated protein kinase |
| MHC | major histocompatibility complex |
| MICA | MHC class I chain-related protein A |
| MICB | MHC class I chain-related protein B |
| mRNA | messenger ribonucleic acid |
| mTOR | mammalian target of rapamycin |
| NFAT | nuclear factor of the activated T cell |
| NF-κB | nuclear factor- κB |
| NK | natural killer T cells |
| NKG2A | member A of the G2 receptor family of natural killer cells |
| NKG2DL | natural killer group 2 member D ligand |
| NRF1 | nuclear respiratory factor 1 |
| PD-1 | programmed death-1 |
| PD-L1 | corresponding ligand for the PD-1 receptor |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PI3K | phosphatidylinositol-3-kinase |
| PILRA | paired immunoglobulin-like type 2 receptor alpha |
| PMBCL | primary mediastinal large B-cell lymphoma |
| RUNX3 | Runt-related transcription factor 2 |
| SASP | senescence-associated secretory phenotype |
| scFv | single-chain variable fragment |
| SIGLECs | sialic acid-binding Ig-superfamily of lectins |
| SIRPα | signal-regulated protein α |
| SLL | small lymphocytic lymphoma |
| sMAC | sestrin-activated MAPKinase activation complex |
| SNP | single-nucleotide polymorphism |
| S1P | sphingosine 1-phosphate |
| S1PR1 | sphingosine 1-phosphate receptor-1 |
| STAT | signal transducer and activator of transcription |
| TCF1 | T-cell factor 1 |
| TCR | T-cell receptor |
| TEMRA | terminally effector memory re-expressing CD45RA |
| TFAM | mitochondrial transcription factor protein A |
| TGF-β | transforming growth factor-β |
| Th | T helper cell |
| ThPOK | T-helper-inducing POZ/Krueppel-like factor |
| TN | naïve T cells |
| TNF-α | tumor necrosis factor alpha |
| Treg | regulatory T cell |
| TREM2 | triggering receptor expressed on myeloid cells 2 |
| TRIB2 | Tribbles homolog 2 |
| TRUCK | T redirected universal cytokine killer cell |
| UCAR | universal CAR-T cell |
| ULBP2 | UL16 binding protein 2 |
| uPAR | urokinase plasminogen activator receptor |
| USA | United States of America |
| WHO | World Health Organization |
References
- Jin, R.; Chan, A.K.Y.; Wu, J.; Lee, T.M.C. Relationships between Inflammation and Age-Related Neurocognitive Changes. Int. J. Mol. Sci. 2022, 23, 12573. [Google Scholar] [CrossRef] [PubMed]
- Peplow, P.V. Reprogramming T cells as an emerging treatment to slow human age-related decline in health. Front. Med. Technol. 2024, 6, 1384648. [Google Scholar] [CrossRef]
- Yu, P.-J.; Zhou, M.; Liu, Y.; Du, J. Senescent T cells in Age-Related Diseases. Aging Dis. 2024, 16, 321–344. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Jurcau, M.C.; Jurcau, A.; Cristian, A.; Hogea, V.O.; Diaconu, R.G.; Nunkoo, V.S. Inflammaging and Brain Aging. Int. J. Mol. Sci. 2024, 25, 10535. [Google Scholar] [CrossRef]
- Meyer, J.L.; Buczak-Stec, E.; König, H.H.; Hajek, A. Fear of Dementia among Middle-Aged and Older Adults in Germany. Dement. Geriatr. Cogn. Dis. Extra 2024, 14, 96–105. [Google Scholar] [CrossRef] [PubMed]
- The Good Care Group. Available online: https://www.thegoodcaregroup.com/news/dementia-overtakes-cancer-uks-most-feared-illness/ (accessed on 20 June 2025).
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Silva, M.V.F.; Loures, C.d.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.d.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef]
- Asher, S.; Priefer, R. Alzheimer’s disease failed clinical trials. Life Sci. 2022, 306, 120861. [Google Scholar] [CrossRef]
- Riesland, M.; Orr, M.E. Translating the Biology of Aging into New Therapeutics for Alzheimer’s Disease: Senolytics. J. Prev. Alzheimers Dis. 2023, 10, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Walford, R.L. The Immunologic Theory of Aging. Gerontologist 1964, 4, 195–197. [Google Scholar] [CrossRef]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huo, T.; Lu, M.; Zhao, Y.; Zhang, J.; He, W.; Chen, H. Recent Advances in Aging and Immunosenescence: Mechanisms and Therapeutic Strategies. Cells 2025, 14, 499. [Google Scholar] [CrossRef]
- Andronie-Cioara, F.L.; Ardelean, A.I.; Nistor-Cseppento, C.D.; Jurcau, A.; Jurcau, M.C.; Pascalau, N.; Marcu, F. Molecular Mechanisms of Neuroinflammation in Aging and Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2023, 24, 1869. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Watling, M.; Imbimbo, C.; Nisticò, R. Plasma ATN(I) classification and precision pharmacology in Alzheimer’s disease. Alzheimers Dement. 2023, 19, 4729–4734. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.B.; Xiao, Q.; Yan, P.; Pan, Q.; Pandey, G.; Grathwohl, S.; Gonzales, E.; Xu, I.; Cho, Y.; Haecker, H.; et al. Chimeric antigen receptor macrophages target and resorb amyloid plaques. JCI Insight 2024, 9, e175015. [Google Scholar] [CrossRef] [PubMed]
- Yeapuri, P.; Machhi, J.; Lu, Y.; Abdelmoaty, M.M.; Kadry, R.; Patel, M.; Bhattarai, S.; Lu, E.; Namminga, K.L.; Olson, K.E.; et al. Amyloid-β specific regulatory T cells attenuate Alzheimer’s disease pathobiology in APP/PS1 mice. Mol. Neurodegener. 2023, 18, 97. [Google Scholar] [CrossRef]
- Akbar, A.N.; Gilroy, D.W. Aging immunity may exacerbate COVID-19. Science 2020, 369, 256–257. [Google Scholar] [CrossRef]
- Pereira, B.; Xu, X.N.; Akbar, A.N. Targeting Inflammation and Immunosenescence to Improve Vaccine Responses in the Elderly. Front. Immunol. 2020, 11, 583019. [Google Scholar] [CrossRef]
- Okuyama, H.; Weyand, C.M.; Goronzy, J.J. Generation and durability of immune memory in older adults. J. Allergy Clin. Immunol. 2023, 152, 601–603. [Google Scholar] [CrossRef]
- MacLeod, M.K.; Kappler, J.W.; Marrack, P. Memory CD4 T cells: Generation, reactivation and re-assignment. Immunology 2010, 130, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Sumida, T.S.; Cheru, N.T.; Hafler, D.A. The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases. Nat. Rev. Immunol. 2024, 24, 503–517. [Google Scholar] [CrossRef]
- Zong, Y.; Deng, K.; Chong, W.P. Regulation of Treg cells by cytokine signaling and co-stimulatory molecules. Front. Immunol. 2024, 15, 1387975. [Google Scholar] [CrossRef]
- Paiva, R.A.; Ramos, C.V.; Leiria, G.; Martins, V.C. IL-7 receptor drives early T lineage progenitor expansion. J. Immunol. 2022, 209, 1942–1949. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Bae, H.; Lee, K.; Lee, S.; Ma, J.; Jo, K.; Kim, I.; Jee, B.; Kang, M.; et al. IL-15 promotes self-renewal of progenitor exhausted CD8 T cells during persistent antigenic stimulation. Front. Immunol. 2023, 14, 1117092. [Google Scholar] [CrossRef]
- Thomas, A.L.; Godarova, A.; Wayman, J.A.; Miraldi, E.R.; Hildeman, D.A.; Chougnet, C.A. Accumulation of immune-suppressive CD4 + T cells in aging—Tempering inflammaging at the expense of immunity. Semin. Immunol. 2023, 70, 101836. [Google Scholar] [CrossRef]
- Mix, M.R.; Harty, J.T. Keeping T Cell Memories in Mind. Trends Immunol. 2022, 43, 1018–1031. [Google Scholar] [CrossRef]
- Hallisey, V.M.; Schwab, S.R. Get me out of here: Sphingosine 1-phosphate signaling and T cell exit from tissues during an immune response. Immunol. Rev. 2023, 317, 8–19. [Google Scholar] [CrossRef]
- Xu, W.; Bergsbaken, T.; Edelblum, K.L. The multifunctional nature of CD103 (αEβ7 integrin) signaling in tissue-resident lymphocytes. Am. J. Physiol. Cell Physiol. 2022, 323, C1161–C1167. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.V.; Ruef, N.; Wissmann, S. Organ-Specific Surveillance and Long-Term Residency Strategies Adapted by Tissue-Resident Memory CD8+ T Cells. Front. Immunol. 2021, 12, 626019. [Google Scholar] [CrossRef] [PubMed]
- Laphanuwat, P.; Gomes, D.C.O.; Akbar, A.N. Senescent T cells: Beneficial and detrimental roles. Immunol. Rev. 2023, 316, 160–175. [Google Scholar] [CrossRef]
- Henson, S.M.; Riddell, N.E.; Akbar, A.N. Properties of end-stage human T cells defined by CD45RA re-expression. Curr. Opin. Immunol. 2012, 24, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Lanna, A.; Henson, S.M.; Akbar, A.N. The regulation of T cell senescence and metabolism by p38 MAPKinase signaling. Innov. Aging 2017, 1, 1254. [Google Scholar] [CrossRef][Green Version]
- Xia, T.; Zhou, Y.; An, J.; Cui, Z.; Zhong, X.; Cui, T.; Lv, B.; Zhao, X.; Gao, X. Benefit delayed immunosenescence by regulating CD4+ T cells: A promising therapeutic target for aging-related diseases. Aging Cell 2024, 23, e14317. [Google Scholar] [CrossRef]
- Richmond, L.; Keeshan, K. Pseudokinases: A tribble-edged sword. FEBS J. 2020, 287, 4170–4182. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Sturmlechner, I.; Zhang, H.; Jin, J.; Hu, B.; Jadhav, R.R.; Fang, F.; Weyand, C.M.; Goronzy, J.J. TRIB2 safeguards naive T cell homeostasis during aging. Cell Rep. 2023, 42, 112195. [Google Scholar] [CrossRef]
- Zhang, H.; Jadhav, R.R.; Cao, W.; Goronzy, I.N.; Zhao, T.V.; Jin, J.; Ohtsuki, S.; Hu, Z.; Morales, J.; Greenleaf, W.J.; et al. Aging-associated HELIOS deficiency in naïve CD4+ T cells alters chromatin remodeling and promotes effector cell responses. Nat. Immunol. 2023, 24, 96–109. [Google Scholar] [CrossRef]
- Han, S.; Georgiev, P.; Ringel, A.E.; Sharpe, A.H.; Haigis, M.C. Age-associated remodeling of T cell immunity and metabolism. Cell Metab. 2023, 35, 36–55. [Google Scholar] [CrossRef]
- Desdín-Micó, G.; Soto-Heredero, G.; Aranda, J.F.; Oller, J.; Carrasco, E.; Gabandé-Rodríguez, E.; Blanco, E.M.; Alfranca, A.; Cussó, L.; Desco, M.; et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 2020, 368, 1371–1376. [Google Scholar] [CrossRef]
- Sun, X.; Nguyen, T.; Achour, A.; Ko, A.; Cifello, J.; Ling, C.; Sharma, J.; Hiroi, T.; Zhang, Y.; Chia, C.W.; et al. Longitudinal analysis reveals age-related changes in the T cell receptor repertoire of human T cell subsets. J. Clin. Investig. 2022, 132, e158122. [Google Scholar] [CrossRef]
- Callender, L.A.; Carroll, E.C.; Bober, E.A.; Akbar, A.N.; Solito, E.; Henson, S.M. Mitochondrial mass governs the extent of human T cell senescence. Aging Cell 2020, 19, e13067. [Google Scholar] [CrossRef]
- Hu, B.; Jadhav, R.R.; Gustafson, C.E.; Le Saux, S.; Ye, Z.; Li, X.; Tian, L.; Weyand, C.M.; Goronzy, J.J. Distinct Age-Related Epigenetic Signatures in CD4 and CD8 T Cells. Front. Immunol. 2020, 11, 585168, Erratum in Front. Immunol. 2022, 13, 911132. [Google Scholar] [CrossRef]
- Franco, F.; Jaccard, A.; Romero, P.; Yu, Y.R.; Ho, P.C. Metabolic and epigenetic regulation of T-cell exhaustion. Nat. Metab. 2020, 2, 1001–1012. [Google Scholar] [CrossRef]
- Chen, D.; Tang, T.X.; Deng, H.; Yang, X.P.; Tang, Z.H. Interleukin-7 Biology and Its Effects on Immune Cells: Mediator of Generation, Differentiation, Survival, and Homeostasis. Front. Immunol. 2021, 12, 747324. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 2021, 22, 687–698. [Google Scholar] [CrossRef]
- Lanna, A.; Vaz, B.; D’Ambra, C.; Valvo, S.; Vuotto, C.; Chiurchiù, V.; Devine, O.; Sanchez, M.; Borsellino, G.; Akbar, A.N.; et al. An intercellular transfer of telomeres rescues T cells from senescence and promotes long-term immunological memory. Nat Cell Biol. 2022, 24, 1461–1474. [Google Scholar] [CrossRef]
- Slaets, H.; Veeningen, N.; de Keizer, P.L.J.; Hellings, N.; Hendrix, S. Are immunosenescent T cells really senescent? Aging Cell 2024, 23, e14300. [Google Scholar] [CrossRef]
- Lanna, A.; Gomes, D.C.; Muller-Durovic, B.; McDonnell, T.; Escors, D.; Gilroy, D.W.; Lee, J.H.; Karin, M.; Akbar, A.N. A sestrin-dependent Erk-Jnk-p38 MAPK activation complex inhibits immunity during aging. Nat Immunol. 2017, 18, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kang, I.; Kim, Y.; Ku, K.B.; Park, J.H.; Kim, H.J.; Kim, C.W.; La, J.; Jung, H.E.; Kim, H.C.; et al. Regulation of c-SMAC formation and AKT-mTOR signaling by the TSG101-IFT20 axis in CD4+ T cells. Cell. Mol. Immunol. 2023, 20, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.I.; De Maeyer, R.P.H.; Covre, L.P.; Nehar-Belaid, D.; Lanna, A.; Ward, S.; Marches, R.; Chambers, E.S.; Gomes, D.C.O.; Riddell, N.E.; et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. 2020, 21, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, T.; Suzuki, J.; Matsuoka, Y.; Kikugawa, T.; Saika, T.; Yamashita, M. Senescent CD8+ T cells acquire NK cell-like innate functions to promote antitumor immunity. Cancer Sci. 2023, 114, 2810–2820. [Google Scholar] [CrossRef]
- Wang, T.-W.; Nakanishi, M. Immune surveillance of senescence: Potential application to age-related diseases. Trends Cell Biol. 2025, 35, 248–257. [Google Scholar] [CrossRef]
- Chen, H.A.; Ho, Y.J.; Mezzadra, R.; Adrover, J.M.; Smolkin, R.; Zhu, C.; Woess, K.; Bernstein, N.; Schmitt, G.; Fong, L.; et al. Senescence Rewires Microenvironment Sensing to Facilitate Antitumor Immunity. Cancer Discov. 2023, 13, 432–453. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, E.; Ranzino, L.; Tolotto, V.; Dalla, E.; Burelli, M.; Gualandi, N.; Brancolini, C. Transcription of endogenous retroviruses in senescent cells contributes to the accumulation of double-stranded RNAs that trigger an anti-viral response that reinforces senescence. Cell Death Dis. 2024, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.R.; Zong, J.B.; Liu, Y.X.; Aili, T.; Qiu, M.; Wu, J.H.; Hu, B. Endogenous Retroviruses Unveiled: A Comprehensive Review of Inflammatory Signaling/Senescence-Related Pathways and Therapeutic Strategies. Aging Dis. 2024, 16, 738–756. [Google Scholar]
- Giannoula, Y.; Kroemer, G.; Pietrocola, F. Cellular senescence and the host immune system in aging and age-related disorders. Biomed. J. 2023, 46, 100581. [Google Scholar] [CrossRef]
- Kang, T.W.; Yevsa, T.; Woller, N.; Hoenicke, L.; Wuestefeld, T.; Dauch, D.; Hohmeyer, A.; Gereke, M.; Rudalska, R.; Potapova, A.; et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011, 479, 547–551. [Google Scholar] [CrossRef]
- Schloesser, D.; Lindenthal, L.; Sauer, J.; Chung, K.J.; Chavakis, T.; Griesser, E.; Baskaran, P.; Maier-Habelsberger, U.; Fundel-Clemens, K.; Schlotthauer, I.; et al. Senescent cells suppress macrophage-mediated corpse removal via upregulation of the CD47-QPCT/L axis. J. Cell Biol. 2023, 222, e202207097. [Google Scholar] [CrossRef] [PubMed]
- Muneekaew, S.; Sasithong, P.; Chupradit, K.; Saiprayong, K.; Nuchphongsai, T.; Wattanapanitch, M. Enhancing macrophage phagocytosis of cancers by disrupting the SIRPα/CD47 signaling axis and targeting MUC1 antigen. FEBS J. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.I.; Devine, O.P.; Vukmanovic-Stejic, M.; Chambers, E.S.; Subramanian, P.; Patel, N.; Virasami, A.; Sebire, N.J.; Kinsler, V.; Valdovinos, A.; et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat. Commun. 2019, 10, 2387. [Google Scholar] [CrossRef]
- Li, D.; Brackenridge, S.; Walters, L.C.; Swanson, O.; Harlos, K.; Rozbesky, D.; Cain, D.W.; Wiehe, K.; Scearce, R.M.; Barr, M.; et al. Mouse and human antibodies bind HLA-E-leader peptide complexes and enhance NK cell cytotoxicity. Commun Biol. 2022, 5, 271. [Google Scholar] [CrossRef]
- Onorati, A.; Havas, A.P.; Lin, B.; Rajagopal, J.; Sen, P.; Adams, P.D.; Dou, Z. Upregulation of PD-L1 in Senescence and Aging. Mol. Cell. Biol. 2022, 42, e0017122. [Google Scholar] [CrossRef]
- Wang, T.W.; Johmura, Y.; Suzuki, N.; Omori, S.; Migita, T.; Yamaguchi, K.; Hatakeyama, S.; Yamazaki, S.; Shimizu, E.; Imoto, S.; et al. Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature 2022, 611, 358–364. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Ippati, S.; Watling, M.; Balducci, C. A critical appraisal of tau-targeting therapies for primary and secondary tauopathies. Alzheimers Dement. 2022, 18, 1008–1037. [Google Scholar] [CrossRef]
- Venkataraman, A.V.; Mansur, A.; Rizzo, G.; Bishop, C.; Lewis, Y.; Kocagoncu, E.; Lingford-Hughes, A.; Huiban, M.; Passchier, J.; Rowe, J.B.; et al. Widespread cell stress and mitochondrial dysfunction occur in patients with early Alzheimer’s disease. Sci. Transl. Med. 2022, 14, eabk1051. [Google Scholar] [CrossRef]
- Tzioras, M.; McGeachan, R.I.; Durrant, C.S.; Spires-Jones, T.L. Synaptic degeneration in Alzheimer disease. Nat. Rev. Neurol. 2023, 19, 19–38. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2024. Alzheimers Dement. 2024, 10, e12465. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in neuroinflammation and neurodegeneration: From understanding to therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef]
- Harkins, A.L.; Kopec, A.L.; Keeler, A.M. Regulatory T Cell Therapeutics for Neuroinflammatory Disorders. Crit. Rev. Immunol. 2022, 42, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Schiera, G.; Di Liegro, C.M.; Schirò, G.; Sorbello, G.; Di Liegro, I. Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood–Brain Barrier. Cells 2024, 13, 150. [Google Scholar] [CrossRef]
- Groh, J.; Knöpper, K.; Arampatzi, P.; Yuan, X.; Lößlein, L.; Saliba, A.E.; Kastenmüller, W.; Martini, R. Accumulation of cytotoxic T cells in the aged CNS leads to axon degeneration and contributes to cognitive and motor decline. Nat. Aging 2021, 1, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Liston, A.; Dooley, J.; Yshii, L. Brain-resident regulatory T cells and their role in health and disease. Immunol. Lett. 2022, 248, 26–30. [Google Scholar] [CrossRef]
- Hemmers, S.; Schizas, M.; Rudensky, A.Y. T reg cell-intrinsic requirements for ST2 signaling in health and neuroinflammation. J. Exp. Med. 2020, 218, e20201234. [Google Scholar] [CrossRef]
- Xue, D.; Guo, X.; Liu, J.; Li, Y.; Liu, L.; Liao, G.; Zhang, M.; Cao, J.; Liu, Y.; Lou, J.; et al. Tryptophan-rich diet and its effects on Htr7+ Tregs in alleviating neuroinflammation and cognitive impairment induced by lipopolysaccharide. J. Neuroinflammation 2024, 21, 241. [Google Scholar] [CrossRef]
- Shi, L.; Sun, Z.; Su, W.; Xu, F.; Xie, D.; Zhang, Q.; Dai, X.; Iyer, K.; Hitchens, T.K.; Foley, L.M.; et al. Treg cell-derived osteopontin promotes microglia-mediated white matter repair after ischemic stroke. Immunity 2021, 54, 1527–1542.e8. [Google Scholar] [CrossRef]
- Bertram, L.; Lange, C.; Mullin, K.; Parkinson, M.; Hsiao, M.; Hogan, M.F.; Schjeide, B.M.M.; Hooli, B.; Divito, J.; Ionita, I.; et al. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am. J. Hum. Genet. 2008, 83, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Rikos, D.; Siokas, V.; Mentis, A.A.; Aloizou, A.M.; Liampas, I.; Tsouris, Z.; Peristeri, E.; Stamati, P.; Hadjigeorgiou, G.M.; Dardiotis, E. TREM2 R47H variant and risk for Alzheimer’s disease: Assessment in a Greek population and updated meta-analysis. Int. J. Neurosci. 2022, 134, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Cosker, K.; Mallach, A.; Limaye, J.; Piers, T.M.; Staddon, J.; Neame, S.J.; Hardy, J.; Pocock, J.M. Microglial signalling pathway deficits associated with the patient derived R47H TREM2 variants linked to AD indicate inability to activate inflammasome. Sci. Rep. 2021, 11, 13316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiang, X.; Li, Y.; Bu, G.; Chen, X.F. TREM2 and sTREM2 in Alzheimer’s disease: From mechanisms to therapies. Mol. Neurodegener. 2025, 20, 43. [Google Scholar] [CrossRef]
- Yang, Z.; Sheng, J.; Zhang, Q.; Chou, J.T.C.; Xin, Y.; Wang, L. Coupling relationship between glucose and oxygen metabolisms to serve as an imaging biomarker for Alzheimer’s disease. Sci. Rep. 2025, 15, 16838. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Yao, Q.Y.; Ruan, S.S.; Hu, J.W.; Long, W.J.; Dai, W.Z.; Ma, T.; Zhu, X.C. Explore the role of CR1 genetic variants in late-onset Alzheimer’s disease susceptibility. Psychiatr. Genet. 2021, 31, 216–229. [Google Scholar] [CrossRef]
- Milinkeviciute, G.; Green, K.N. Clusterin/apolipoprotein J, its isoforms and Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1167886. [Google Scholar] [CrossRef] [PubMed]
- Lopatko Lindman, K.; Jonsson, C.; Weidung, B.; Olsson, J.; Pandey, J.P.; Prokopenko, D.; Tanzi, R.E.; Hallmans, G.; Eriksson, S.; Elgh, F.; et al. PILRA polymorphism modifies the effect of APOE4 and GM17 on Alzheimer’s disease risk. Sci. Rep. 2022, 12, 13264. [Google Scholar] [CrossRef] [PubMed]
- Owens, H.A.; Thorburn, L.E.; Walsby, E.; Moon, O.R.; Rizkallah, P.; Sherwani, S.; Tinsley, C.L.; Rogers, L.; Cerutti, C.; Ridley, A.J.; et al. Alzheimer’s disease-associated P460L variant of EphA1 dysregulates receptor activity and blood-brain barrier function. Alzheimers Dement. 2024, 20, 2016–2033. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.D.; Yin, R.; Li, J.Q.; Cao, X.P.; Yu, J.T.; Tan, L.; Alzheimer’s Disease Neuroimaging Initiative. Common Variants in ABI3 Influence Cerebrospinal Fluid Total Tau Levels and Cognitive Decline in Progressive Mild Cognitive Impairment Patients. J. Alzheimers Dis. 2019, 70, 17–23. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, M.; Wu, Y.; Jiang, D.; Wu, T.; Zhao, Y.; Wu, D.; Cui, J.; Li, G. Regulation of the Late Onset Alzheimer’s Disease Associated HLA-DQA1/DRB1 Expression. Am. J. Alzheimers Dis. Other Demen. 2022, 37, 15333175221085066. [Google Scholar] [CrossRef]
- Dos Santos, L.R.; Pimassoni, L.H.S.; Sena, G.G.S.; Camporez, D.; Belcavello, L.; Trancozo, M.; Morelato, R.L.; Errera, F.I.V.; Bueno, M.R.P.; de Paula, F. Validating GWAS Variants from Microglial Genes Implicated in Alzheimer’s Disease. J. Mol. Neurosci. 2017, 62, 215–221. [Google Scholar] [CrossRef]
- Wang, L.; Jiao, Y.; Zhao, A.; Xu, X.; Ye, G.; Zhang, Y.; Wang, Y.; Deng, Y.; Xu, W.; Liu, J. Analysis of Genetic Association Between ABCA7 Polymorphism and Alzheimer’s Disease Risk in the Southern Chinese Population. Front. Aging Neurosci. 2022, 14, 819499. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef]
- Wiens, K.R.; Wasti, N.; Ulloa, O.O.; Klegeris, A. Diversity of Microglia-Derived Molecules with Neurotrophic Properties That Support Neurons in the Central Nervous System and Other Tissues. Molecules 2024, 29, 5525. [Google Scholar] [CrossRef] [PubMed]
- Malvaso, A.; Gatti, A.; Negro, G.; Calatozzolo, C.; Medici, V.; Poloni, T.E. Microglial Senescence and Activation in Healthy Aging and Alzheimer’s Disease: Systematic Review and Neuropathological Scoring. Cells 2023, 12, 2824. [Google Scholar] [CrossRef]
- Wen, L.; Bi, D.; Shen, Y. Complement-mediated synapse loss in Alzheimer’s disease: Mechanisms and involvement of risk factors. Trends Neurosci. 2024, 47, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Ayyubova, G. Dysfunctional microglia and tau pathology in Alzheimer’s disease. Rev. Neurosci. 2022, 34, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef]
- Chen, Y.; Colonna, M. Microglia in Alzheimer’s disease at single-cell level. Are there common patterns in humans and mice? J. Exp. Med. 2021, 218, e20202717. [Google Scholar] [CrossRef]
- Chen, Y.; Colonna, M. Spontaneous and induced adaptive immune responses in Alzheimer’s disease: New insights into old observations. Curr. Opin. Immunol. 2022, 77, 102233. [Google Scholar] [CrossRef]
- Degn, S.E.; Tolar, P. Towards a unifying model for B-cell receptor triggering. Nat. Rev. Immunol. 2025, 25, 77–91. [Google Scholar] [CrossRef]
- Jurcău, M.C.; Andronie-Cioara, F.L.; Jurcău, A.; Marcu, F.; Ţiț, D.M.; Pașcalău, N.; Nistor-Cseppentö, D.C. The Link between Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation in the Pathophysiology of Alzheimer’s Disease: Therapeutic Implications and Future Perspectives. Antioxidants 2022, 11, 2167. [Google Scholar] [CrossRef]
- Balestri, W.; Sharma, R.; da Silva, V.A.; Bobotis, B.C.; Curle, A.J.; Kothakota, V.; Kalantarnia, F.; Hangad, M.V.; Hoorfar, M.; Jones, J.L.; et al. Modeling the neuroimmune system in Alzheimer’s and Parkinson’s disease. J. Neuroinflammation 2024, 21, 32. [Google Scholar] [CrossRef]
- Späni, C.; Suter, T.; Derungs, R.; Ferretti, M.T.; Welt, T.; Wirth, F.; Gericke, C.; Nitsch, R.M.; Kulic, L. Reduced β-amyloid pathology in an APP transgenic mouse model of Alzheimer’s disease lacking functional B and T cells. Acta Neuropathol. Commun. 2015, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Söllvander, S.; Ekholm-Pettersson, F.; Brundin, R.M.; Westman, G.; Kilander, L.; Paulie, S.; Lannfelt, L.; Sehlin, D. Increased Number of Plasma B Cells Producing Autoantibodies Against Aβ42 Protofibrils in Alzheimer’s Disease. J. Alzheimers Dis. 2015, 48, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Stein, P.; Cavazzoni, P. Approval of Aducanumab for Alzheimer Disease-The FDA’s Perspective. JAMA Intern. Med. 2021, 181, 1276–1278. [Google Scholar] [CrossRef] [PubMed]
- Pascual, G.; Wadia, J.S.; Zhu, X.; Keogh, E.; Kükrer, B.; van Ameijde, J.; Inganäs, H.; Siregar, B.; Perdok, G.; Diefenbach, O.; et al. Immunological memory to hyperphosphorylated tau in asymptomatic individuals. Acta Neuropathol. 2017, 133, 767–783. [Google Scholar] [CrossRef]
- Brioschi, S.; Wang, W.L.; Peng, V.; Wang, M.; Shchukina, I.; Greenberg, Z.J.; Bando, J.K.; Jaeger, N.; Czepielewski, R.S.; Swain, A.; et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science 2021, 373, eabf9277. [Google Scholar] [CrossRef]
- Rickenbach, C.; Gericke, C. Specificity of Adaptive Immune Responses in Central Nervous System Health, Aging and Diseases. Front. Neurosci. 2022, 15, 806260. [Google Scholar] [CrossRef]
- Itagaki, S.; McGeer, P.L.; Akiyama, H. Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer’s disease brain tissue. Neurosci. Lett. 1988, 91, 259–264. [Google Scholar] [CrossRef]
- Togo, T.; Akiyama, H.; Iseki, E.; Kondo, H.; Ikeda, K.; Kato, M.; Oda, T.; Tsuchiya, K.; Kosaka, K. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 2002, 124, 83–92. [Google Scholar] [CrossRef]
- Unger, M.S.; Li, E.; Scharnagl, L.; Poupardin, R.; Altendorfer, B.; Mrowetz, H.; Hutter-Paier, B.; Weiger, T.M.; Heneka, M.T.; Attems, J.; et al. CD8+ T cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain Behav. Immun. 2020, 89, 67–86. [Google Scholar] [CrossRef]
- Gate, D.; Saligrama, N.; Leventhal, O.; Yang, A.C.; Unger, M.S.; Middeldorp, J.; Chen, K.; Lehallier, B.; Channappa, D.; De Los Santos, M.B.; et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 2020, 577, 399–404. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Liang, X.; Zhao, M.; Yu, X.; Fu, H.; Yang, W. CD4+ T-Cell Senescence in Neurodegenerative Disease: Pathogenesis and Potential Therapeutic Targets. Cells 2024, 13, 749. [Google Scholar] [CrossRef]
- Knopp, R.C.; Erickson, M.A.; Rhea, E.M.; Reed, M.J.; Banks, W.A. Cellular senescence and the blood-brain barrier: Implications for aging and age-related diseases. Exp. Biol. Med. 2023, 248, 399–411. [Google Scholar] [CrossRef]
- Mittal, K.; Eremenko, E.; Berner, O.; Elyahu, Y.; Strominger, I.; Apelblat, D.; Nemirovsky, A.; Spiegel, I.; Monsonego, A. CD4 T Cells Induce A Subset of MHCII-Expressing Microglia that Attenuates Alzheimer Pathology. iScience 2019, 16, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Sirkis, D.W.; Warly Solsberg, C.; Johnson, T.P.; Bonham, L.W.; Oddi, A.P.; Geier, E.G.; Miller, B.L.; Rabinovici, G.D.; Yokoyama, J.S. Expansion of highly interferon-responsive T cells in early-onset Alzheimer’s disease. Alzheimers Dement. 2024, 20, 5062–5070. [Google Scholar] [CrossRef]
- Machhi, J.; Yeapuri, P.; Lu, Y.; Foster, E.; Chikhale, R.; Herskovitz, J.; Namminga, K.L.; Olson, K.E.; Abdelmoaty, M.M.; Gao, J.; et al. CD4+ effector T cells accelerate Alzheimer’s disease in mice. J. Neuroinflammation 2021, 18, 272. [Google Scholar] [CrossRef]
- Laurent, C.; Dorothée, G.; Hunot, S.; Martin, E.; Monnet, Y.; Duchamp, M.; Dong, Y.; Légeron, F.P.; Leboucher, A.; Burnouf, S.; et al. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain 2017, 140, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Van Olst, L.; Kamermans, A.; Halters, S.; van der Pol, S.M.A.; Rodriguez, E.; Verberk, I.M.W.; Verberk, S.G.S.; Wessels, D.W.R.; Rodriguez-Mogeda, C.; Verhoeff, J.; et al. Adaptive immune changes associate with clinical progression of Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, Z.; Herz, J.; Kipnis, J. Meningeal Lymphatics: From Anatomy to Central Nervous System Immune Surveillance. J. Immunol. 2020, 204, 286–293. [Google Scholar] [CrossRef]
- Faridar, A.; Thome, A.D.; Zhao, W.; Thonhoff, J.R.; Beers, D.R.; Pascual, B.; Masdeu, J.C.; Appel, S.H. Restoring regulatory T-cell dysfunction in Alzheimer’s disease through ex vivo expansion. Brain Commun. 2020, 2, fcaa112. [Google Scholar] [CrossRef]
- Abbott, V.; Housden, B.E.; Houldsworth, A. Could immunotherapy and regulatory T cells be used therapeutically to slow the progression of Alzheimer’s disease? Brain Commun. 2025, 7, fcaf092. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Park, S.Y.; Baek, H.; Lee, C.; Chung, G.; Liu, X.; Lee, J.H.; Kim, B.; Kwon, M.; Choi, H.; et al. Adoptive therapy with amyloid-β specific regulatory T cells alleviates Alzheimer’s disease. Theranostics 2022, 12, 7668–7680. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Yang, J.; Yang, H.; Cho, I.; Kim, J.Y.; Bae, H. Therapeutic Effects of Aβ-Specific Regulatory T Cells in Alzheimer’s Disease: A Study in 5xFAD Mice. Int. J. Mol. Sci. 2024, 25, 783. [Google Scholar] [CrossRef]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Han, W.; Zhang, Y. Tisagenlecleucel, an approved anti-CD19 chimeric antigen receptor T-cell therapy for the treatment of leukemia. Drugs Today 2017, 53, 597–608. [Google Scholar] [CrossRef]
- Goyco Vera, D.; Waghela, H.; Nuh, M.; Pan, J.; Lulla, P. Approved CAR-T therapies have reproducible efficacy and safety in clinical practice. Hum. Vaccin. Immunother. 2024, 20, 2378543. [Google Scholar] [CrossRef]
- Gaimari, A.; De Lucia, A.; Nicolini, F.; Mazzotti, L.; Maltoni, R.; Rughi, G.; Zurlo, M.; Marchesini, M.; Juan, M.; Parras, D.; et al. Significant Advancements and Evolutions in Chimeric Antigen Receptor Design. Int. J. Mol. Sci. 2024, 25, 12201. [Google Scholar] [CrossRef]
- Savoldo, B.; Ramos, C.A.; Liu, E.; Mims, M.P.; Keating, M.J.; Carrum, G.; Kamble, R.T.; Bollard, C.M.; Gee, A.P.; Mei, Z.; et al. CD28 Costimulation Improves Expansion and Persistence of Chimeric Antigen Receptor-Modified T Cells in Lymphoma Patients. J. Clin. Investig. 2011, 121, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Rallis, K.S.; Hillyar, C.R.T.; Sideris, M.; Davies, J.K. T-cell-based Immunotherapies for Haematological Cancers, Part B: A SWOT Analysis of Adoptive Cell Therapies. Anticancer Res. 2021, 41, 1143–1156. [Google Scholar] [CrossRef]
- Hadiloo, K.; Taremi, S.; Safa, S.H.; Amidifar, S.; Esmaeilzadeh, A. The new era of immunological treatment, last updated and future consideration of CAR T cell-based drugs. Pharmacol. Res. 2024, 203, 107158. [Google Scholar] [CrossRef] [PubMed]
- Battram, A.M.; Bachiller, M.; Lopez, V.; Fernández de Larrea, C.; Urbano-Ispizua, A.; Martín-Antonio, B. IL-15 Enhances the Persistence and Function of BCMA-Targeting CAR-T Cells Compared to IL-2 or IL-15/IL-7 by Limiting CAR-T Cell Dysfunction and Differentiation. Cancers 2021, 13, 3534. [Google Scholar] [CrossRef]
- Lickefett, B.; Chu, L.; Ortiz-Maldonado, V.; Warmuth, L.; Barba, P.; Doglio, M.; Henderson, D.; Hudecek, M.; Kremer, A.; Markman, J.; et al. Lymphodepletion—An essential but undervalued part of the chimeric antigen receptor T-cell therapy cycle. Front. Immunol. 2023, 14, 1303935. [Google Scholar] [CrossRef]
- Arjomandnejad, M.; Kopec, A.L.; Keeler, A.M. CAR-T Regulatory (CAR-Treg) Cells: Engineering and Applications. Biomedicines 2022, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, S.; Elahi, R.; Khosh, E.; Esmaeilzadeh, A. Programmable and multi-targeted CARs: A new breakthrough in cancer CAR-T cell therapy. Clin. Transl. Oncol. 2021, 23, 1003–1019. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, J.; Huang, B.; Zhang, Z.; Wang, S.; Tang, F.; Teng, M.; Li, Y. Special Chimeric Antigen Receptor (CAR) Modifications of T Cells: A Review. Front. Oncol. 2022, 12, 832765. [Google Scholar] [CrossRef]
- Patel, K.K.; Tariveranmoshabad, M.; Kadu, S.; Shobaki, N.; June, C. From concept to cure: The evolution of CAR-T cell therapy. Mol. Ther. 2025, 33, 2123–2140. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, A.; Tommasi, A.; Saha, K. Advances in manufacturing chimeric antigen receptor immune cell therapies. Semin. Immunopathol. 2024, 46, 12. [Google Scholar] [CrossRef]
- Cho, J.H.; Collins, J.J.; Wong, W.W. Universal Chimeric Antigen Receptors for Multiplexed and Logical Control of T Cell Responses. Cell 2018, 173, 1426–1438.e11. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, Y.; Liu, D.; Jiao, X.; Liu, C.; Wang, Y.; Zhang, Z.; Jia, K.; Gong, J.; Yang, Z.; et al. The landscape of cancer research and cancer care in China. Nat. Med. 2023, 29, 3022–3032. [Google Scholar] [CrossRef]
- Qin, C.; Tian, D.S.; Zhou, L.Q.; Shang, K.; Huang, L.; Dong, M.H.; You, Y.F.; Xiao, J.; Xiong, Y.; Wang, W.; et al. Anti-BCMA CAR T-cell therapy CT103A in relapsed or refractory AQP4-IgG seropositive neuromyelitis optica spectrum disorders: Phase 1 trial interim results. Signal Transduct. Target Ther. 2023, 8, 5. [Google Scholar] [CrossRef]
- Jain, H.; Karulkar, A.; Kalra, D.; Ravikumar, S.; Shah, S.; Firfiray, A.; Pendhari, J.; Manivasagam, S.; Vaibhaw, A.; Saroha, A.; et al. Real world data of novel talicabtagene autoleucel (humanized CD19 CAR-T) from India; Ensuring equitable access with excellent safety and efficacy profile. Blood 2024, 144, 3755. [Google Scholar] [CrossRef]
- Nie, T. Talicabtagene Autoleucel: First Approval. Mol. Diagn. Ther. 2024, 28, 495–499. [Google Scholar] [CrossRef]
- Hu, D.; Yang, R.; Wang, G.; Li, H.; Fan, X.; Liang, G. Emerging Strategies to Overcome Current CAR-T Therapy Dilemmas—Exosomes Derived from CAR-T Cells. Int. J. Nanomed. 2024, 19, 2773–2791. [Google Scholar] [CrossRef] [PubMed]
- Hayden, P.J.; Roddie, C.; Bader, P.; Basak, G.W.; Bonig, H.; Bonini, C.; Chabannon, C.; Ciceri, F.; Corbacioglu, S.; Ellard, R.; et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann. Oncol. 2022, 33, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y.; et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020, 368, eaaz7548. [Google Scholar] [CrossRef]
- Hay, K.A.; Hanafi, L.A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; López, J.A.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef]
- Pensato, U.; Muccioli, L.; Cani, I.; Janigro, D.; Zinzani, P.L.; Guarino, M.; Cortelli, P.; Bisulli, F. Brain dysfunction in COVID-19 and CAR-T therapy: Cytokine storm-associated encephalopathy. Ann. Clin. Transl. Neurol. 2021, 8, 968–979. [Google Scholar] [CrossRef]
- Genoud, V.; Migliorini, D. Novel pathophysiological insights into CAR-T cell associated neurotoxicity. Front. Neurol. 2023, 14, 1108297. [Google Scholar] [CrossRef]
- Gust, J.; Finney, O.C.; Li, D.; Brakke, H.M.; Hicks, R.M.; Futrell, R.B.; Gamble, D.N.; Rawlings-Rhea, S.D.; Khalatbari, H.K.; Ishak, G.E.; et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann. Neurol. 2019, 86, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Sidana, S.; Dueck, A.C.; Thanarajasingam, G.; Griffin, J.M.; Thompson, C.; Durani, U.; Burtis, M.; Warsame, R.; Paludo, J.; Gertz, M.A.; et al. Longitudinal Patient Reported Outcomes with CAR-T Cell Therapy Versus Autologous and Allogeneic Stem Cell Transplant. Transplant. Cell Ther. 2022, 28, 473–482. [Google Scholar] [CrossRef]
- Barata, A.; Hoogland, A.I.; Kommalapati, A.; Logue, J.; Welniak, T.; Hyland, K.A.; Eisel, S.L.; Small, B.J.; Jayani, R.V.; Booth-Jones, M.; et al. Change in Patients’ Perceived Cognition Following Chimeric Antigen Receptor T-Cell Therapy for Lymphoma. Transplant. Cell Ther. 2022, 28, 401.e1–401.e7. [Google Scholar] [CrossRef]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef]
- Clinical Trials.gov. Available online: https://clinicaltrials.gov/ (accessed on 9 August 2025).
- Su, S.; Lei, A.; Wang, X.; Lu, H.; Wang, S.; Yang, Y.; Li, N.; Zhang, Y.; Zhang, J. Induced CAR-Macrophages as a Novel Therapeutic Cell Type for Cancer Immune Cell Therapies. Cells 2022, 11, 1652. [Google Scholar] [CrossRef]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Depil, S.; Duchateau, P.; Grupp, S.A.; Mufti, G.; Poirot, L. ‘Off-the-shelf’ allogeneic CAR T cells: Development and challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, A.; Accardi, G.; Aiello, A.; Caruso, C.; Galimberti, D.; Candore, G. Senotherapeutics to Counteract Senescent Cells Are Prominent Topics in the Context of Anti-Ageing Strategies. Int. J. Mol. Sci. 2024, 25, 1792. [Google Scholar] [CrossRef] [PubMed]
- Herdy, J.R.; Traxler, L.; Agarwal, R.K.; Karbacher, L.; Schlachetzki, J.C.M.; Boehnke, L.; Zangwill, D.; Galasko, D.; Glass, C.K.; Mertens, J.; et al. Increased post-mitotic senescence in aged human neurons is a pathological feature of Alzheimer’s disease. Cell Stem Cell. 2022, 29, 1637–1652.e6. [Google Scholar] [CrossRef]
- Rachmian, N.; Krizhanovsky, V. Senescent cells in the brain and where to find them. FEBS J. 2023, 290, 1256–1266. [Google Scholar] [CrossRef]
- Carver, C.; Schafer, M. Spatially resolved mapping of cell-specific senescent profiles in aging mouse brain. Innov. Aging 2022, 6, 590–591. [Google Scholar] [CrossRef]
- Moreno-Blas, D.; Gorostieta-Salas, E.; Pommer-Alba, A.; Muciño-Hernández, G.; Gerónimo-Olvera, C.; Maciel-Barón, L.A.; Konigsberg, M.; Massieu, L.; Castro-Obregón, S. Cortical neurons develop a senescence-like phenotype promoted by dysfunctional autophagy. Aging 2019, 11, 6175–6198. [Google Scholar] [CrossRef]
- Matsudaira, T.; Nakano, S.; Konishi, Y.; Kawamoto, S.; Uemura, K.; Kondo, T.; Sakurai, K.; Ozawa, T.; Hikida, T.; Komine, O.; et al. Cellular senescence in white matter microglia is induced during ageing in mice and exacerbates the neuroinflammatory phenotype. Commun. Biol. 2023, 6, 665. [Google Scholar] [CrossRef]
- Jin, W.N.; Shi, K.; He, W.; Sun, J.H.; Van Kaer, L.; Shi, F.D.; Liu, Q. Neuroblast senescence in the aged brain augments natural killer cell cytotoxicity leading to impaired neurogenesis and cognition. Nat. Neurosci. 2021, 24, 61–73. [Google Scholar] [CrossRef]
- Dehkordi, S.K.; Walker, J.; Sah, E.; Bennett, E.; Atrian, F.; Frost, B.; Woost, B.; Bennett, R.E.; Orr, T.C.; Zhou, Y.; et al. Profiling senescent cells in human brains reveals neurons with CDKN2D/p19 and tau neuropathology. Nat. Aging 2021, 1, 1107–1116. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Su, J.; Ren, S.; Wang, X.; Zhang, Y.; Yuan, Z.; He, X.; Wu, X.; Li, M.; et al. Rejuvenation Strategy for Inducing and Enhancing Autoimmune Response to Eliminate Senescent cells. Aging Dis. 2025, 16, 2273–2292. [Google Scholar] [CrossRef]
- Shin, E.; Bak, S.H.; Park, T.; Kim, J.W.; Yoon, S.R.; Jung, H.; Noh, J.Y. Understanding NK cell biology for harnessing NK cell therapies: Targeting cancer and beyond. Front. Immunol. 2023, 14, 1192907. [Google Scholar] [CrossRef] [PubMed]
- Brauning, A.; Rae, M.; Zhu, G.; Fulton, E.; Admasu, T.D.; Stolzing, A.; Sharma, A. Aging of the Immune System: Focus on Natural Killer Cells Phenotype and Functions. Cells 2022, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Etxebeste-Mitxeltorena, M.; Del Rincón-Loza, I.; Martín-Antonio, B. Tumor Secretome to Adoptive Cellular Immunotherapy: Reduce Me Before I Make You My Partner. Front. Immunol. 2021, 12, 717850. [Google Scholar] [CrossRef]
- Sansoni, P.; Cossarizza, A.; Brianti, V.; Fagnoni, F.; Snelli, G.; Monti, D.; Marcato, A.; Passeri, G.; Ortolani, C.; Forti, E. Lymphocyte subsets and natural killer activity in healthy old people and centenarians. Blood 1993, 82, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, E.; Gómez de Las Heras, M.M.; Gabandé-Rodríguez, E.; Desdín-Micó, G.; Aranda, J.F.; Mittelbrunn, M. The role of T cells in age-related diseases. Nat. Rev. Immunol. 2022, 22, 97–111. [Google Scholar]
- Sagiv, A.; Krizhanovsky, V. Immunosurveillance of senescent cells: The bright side of the senescence program. Biogerontology 2013, 14, 617–628. [Google Scholar] [CrossRef]
- Kale, A.; Sharma, A.; Stolzing, A.; Desprez, P.Y.; Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef]
- Arora, S.; Thompson, P.J.; Wang, Y.; Bhattacharyya, A.; Apostolopoulou, H.; Hatano, R.; Naikawadi, R.P.; Shah, A.; Wolters, P.J.; Koliwad, S.; et al. Invariant Natural Killer T cells coordinate removal of senescent cells. Med 2021, 2, 938–950. [Google Scholar] [CrossRef]
- Kurioka, A.; Klenerman, P. Aging unconventionally: γδ T cells, iNKT cells, and MAIT cells in aging. Semin. Immunol. 2023, 69, 101816. [Google Scholar] [CrossRef]
- Qin, Y.; Bao, X.; Zheng, M. CD8+ T-cell immunity orchestrated by iNKT cells. Front. Immunol. 2023, 13, 1109347. [Google Scholar] [CrossRef] [PubMed]
- Nunkoo, V.S.; Cristian, A.; Jurcau, A.; Diaconu, R.G.; Jurcau, M.C. The Quest for Eternal Youth: Hallmarks of Aging and Rejuvenating Therapeutic Strategies. Biomedicines 2024, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Ya, J.; Bayraktutan, U. Senolytics and Senomorphics Targeting p38MAPK/NF-κB Pathway Protect Endothelial Cells from Oxidative Stress-Mediated Premature Senescence. Cells 2024, 13, 1292. [Google Scholar] [CrossRef]
- Deng, Y.; Kumar, A.; Xie, K.; Schaaf, K.; Scifo, E.; Morsy, S.; Li, T.; Ehninger, A.; Bano, D.; Ehninger, D. Targeting senescent cells with NKG2D-CAR T cells. Cell Death Discov. 2024, 10, 217. [Google Scholar] [CrossRef]
- Escriche-Navarro, B.; Garrido, E.; Escudero, A.; Montoya-Méndez, I.; Sancenón, F.; García-Fernández, A.; Martínez-Máñez, R. Targeting the senescent surfaceome through DPP4 antibody-functionalized nanoparticles. An application to cancer therapy. Biomaterials 2025, 324, 123461. [Google Scholar] [CrossRef]
- Han, J.; Zheng, J.; Li, Q.; Hong, H.; Yao, J.; Wang, J.; Zhao, R.C. An Antibody-directed and Immune Response Modifier-augmented Photothermal Therapy Strategy Relieves Aging via Rapid Immune Clearance of Senescent Cells. Aging Dis. 2024, 15, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Suda, M.; Shimizu, I.; Katsuumi, G.; Yoshida, Y.; Hayashi, Y.; Ikegami, R.; Matsumoto, N.; Yoshida, Y.; Mikawa, R.; Katayama, A.; et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat. Aging 2021, 1, 1117–1126. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Miao, Q.; Chen, Y. The therapeutic potential of PD-1/PD-L1 pathway on immune-related diseases: Based on the innate and adaptive immune components. Biomed. Pharmacother. 2023, 167, 115569. [Google Scholar] [CrossRef]
- Rivera, N.; Boada, A.; Bielsa, M.I.; Fernández-Figueras, M.T.; Carcereny, E.; Moran, M.T.; Ferrándiz, C. Hair Repigmentation During Immunotherapy Treatment with an Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Agent for Lung Cancer. JAMA Dermatol. 2017, 153, 1162–1165. [Google Scholar] [CrossRef]
- Rettko, N.J.; Campisi, J.; Wells, J.A. Engineering Antibodies Targeting p16 MHC-Peptide Complexes. ACS Chem. Biol. 2022, 17, 545–555. [Google Scholar] [CrossRef]
- Poblocka, M.; Bassey, A.L.; Smith, V.M.; Falcicchio, M.; Manso, A.S.; Althubiti, M.; Sheng, X.; Kyle, A.; Barber, R.; Frigerio, M.; et al. Targeted clearance of senescent cells using an antibody-drug conjugate against a specific membrane marker. Sci. Rep. 2021, 11, 20358. [Google Scholar] [CrossRef]
- Hoare, M.; Ito, Y.; Kang, T.W.; Weekes, M.P.; Matheson, N.J.; Patten, D.A.; Shetty, S.; Parry, A.J.; Menon, S.; Salama, R.; et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell Biol. 2016, 18, 979–992. [Google Scholar] [CrossRef]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132, Erratum in Nature 2024, 627, E9. [Google Scholar] [CrossRef]
- Cui, H.; Xie, N.; Banerjee, S.; Dey, T.; Liu, R.M.; Antony, V.B.; Sanders, Y.Y.; Adams, T.S.; Gomez, J.L.; Thannickal, V.J.; et al. CD38 Mediates Lung Fibrosis by Promoting Alveolar Epithelial Cell Aging. Am. J. Respir. Crit. Care Med. 2022, 206, 459–475. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, X.; Chou, C.H.; Hsueh, M.F.; Attarian, D.; Li, Y.J.; Kraus, V.B. Association of Dipeptidylpeptidase 4 (CD26) With Chondrocyte Senescence and Radiographic Progression in Knee Osteoarthritis. Arthritis Rheumatol. 2023, 75, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mercado, E.; Prieto-Chávez, J.L.; Arriaga-Pizano, L.A.; Hernández-Gutierrez, S.; Mendlovic, F.; Königsberg, M.; López-Díazguerrero, N.E. Increased CD47 and MHC Class I Inhibitory Signals Expression in Senescent CD1 Primary Mouse Lung Fibroblasts. Int. J. Mol. Sci. 2021, 22, 10215. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Ke-Hong, C.; Fei, X.; Huan-Zi, D.; Jie, Y.; Li-Ming, W.; Xiao-Yue, W.; Jian-Guo, Z.; Ya-Ni, H. Decoy receptor 2 mediation of the senescent phenotype of tubular cells by interacting with peroxiredoxin 1 presents a novel mechanism of renal fibrosis in diabetic nephropathy. Kidney Int. 2020, 98, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Riegger, J.; Brenner, R.E. Increase of cell surface vimentin is associated with vimentin network disruption and subsequent stress-induced premature senescence in human chondrocytes. eLife 2025, 12, e91453, Erratum in elife 2025, 14, e108668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, P.; Chen, J.; Chen, Z.; Liu, Z.; Feng, G.; Sha, F.; Li, Z.; Xu, Z.; Huang, Y.; et al. CD44 connects autophagy decline and ageing in the vascular endothelium. Nat. Commun. 2023, 14, 5524. [Google Scholar] [CrossRef]
- Yang, D.; Sun, B.; Li, S.; Wei, W.; Liu, X.; Cui, X.; Zhang, X.; Liu, N.; Yan, L.; Deng, Y.; et al. NKG2D-CAR T cells eliminate senescent cells in aged mice and nonhuman primates. Sci. Transl. Med. 2023, 15, eadd1951. [Google Scholar] [CrossRef]
- Childs, B.G.; Glusevic, M.; Bajer, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of aging. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Amor, C.; Fernández-Maestre, I.; Chowdhury, S.; Ho, Y.J.; Nadella, S.; Graham, C.; Carrasco, S.E.; Nnuji-John, E.; Feucht, J.; Hinterleitner, C.; et al. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat. Aging 2024, 4, 336–349. [Google Scholar] [CrossRef]
- Baumeister, S.H.; Murad, J.; Werner, L.; Daley, H.; Trebeden-Negre, H.; Gicobi, J.K.; Schmucker, A.; Reder, J.; Sentman, C.L.; Gilham, D.E.; et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol. Res. 2019, 7, 100–112. [Google Scholar] [CrossRef]
- Hong, M.; Clubb, J.D.; Chen, Y.Y. Engineering CAR-T Cells for Next-Generation Cancer Therapy. Cancer Cell. 2020, 38, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Chen, Y.T. A Fresh Approach to Targeting Aging Cells: CAR-T Cells Enhance Senolytic Specificity. Cell Stem Cell 2020, 27, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK cells: A promising cellular immunotherapy for cancer. eBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Leivas, A.; Valeri, A.; Córdoba, L.; García-Ortiz, A.; Ortiz, A.; Sánchez-Vega, L.; Graña-Castro, O.; Fernández, L.; Carreño-Tarragona, G.; Pérez, M.; et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J. 2021, 11, 146. [Google Scholar] [CrossRef]
- Kilgour, M.K.; Bastin, D.J.; Lee, S.H.; Ardolino, M.; McComb, S.; Visram, A. Advancements in CAR-NK therapy: Lessons to be learned from CAR-T therapy. Front. Immunol. 2023, 14, 1166038. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Yang, P.; Yu, F.; Li, Z.; Yao, Z.; Martinez, J.; Li, M.; Xu, H. Combining adoptive NK cell infusion with a dopamine-releasing peptide reduces senescent cells in aged mice. Cell Death Dis. 2022, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 2012, 12, 623–635. [Google Scholar] [CrossRef]
- Zimmer, J.; Hentges, M.B.; Chekenya, M.; Boucraut, J. NK Cells in Central Nervous System. J. Immunol. 2013, 190, 5355–5362. [Google Scholar] [CrossRef]
- Lindsay, H.G.; Hendrix, C.J.; Gonzalez Murcia, J.D.; Haynie, C.; Weber, K.S. The Role of Atypical Chemokine Receptors in Neuroinflammation and Neurodegenerative Disorders. Int. J. Mol. Sci. 2023, 24, 16493. [Google Scholar] [CrossRef]
- Ning, Z.; Liu, Y.; Guo, D.; Lin, W.J.; Tang, Y. Natural killer cells in the central nervous system. Cell Commun. Signal. 2023, 21, 341. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Ahmed, M.; Osaid, Z.; Hamoudi, R.; Harati, R. Insights into Exosome Transport through the Blood–Brain Barrier and the Potential Therapeutical Applications in Brain Diseases. Pharmaceuticals 2023, 16, 571. [Google Scholar] [CrossRef] [PubMed]
- Thangudu, S.; Cheng, F.-Y.; Su, C.-H. Advancements in the Blood–Brain Barrier Penetrating Nanoplatforms for Brain Related Disease Diagnostics and Therapeutic Applications. Polymers 2020, 12, 3055. [Google Scholar] [CrossRef]
- Maalej, K.M.; Merhi, M.; Inchakalody, V.P.; Mestiri, S.; Alam, M.; Maccalli, C.; Cherif, H.; Uddin, S.; Steinhoff, M.; Marincola, F.M.; et al. CAR-cell therapy in the era of solid tumor treatment: Current challenges and emerging therapeutic advances. Mol. Cancer 2023, 22, 20. [Google Scholar] [CrossRef]
- Kronenberg, M.; Ascui, G. The α glycolipid rules the NKT cell TCR. J. Exp. Med. 2025, 222, e20242099. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.G.; Ponnusamy, K.; Ren, H.; Taylor, G.P.; Cook, L.B.M.; Karadimitris, A. CAR-iNKT cells targeting clonal TCRVβ chains as a precise strategy to treat T cell lymphoma. Front. Immunol. 2023, 14, 1118681. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, J. Global trends and frontiers in iNKT cells: A bibliometric and visualized analysis. Front. Immunol. 2025, 16, 1618254. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Hamlin, D.; Ryall, C.; Turner, C.; Faull, R.L.M.; Murray, H.C.; Curtis, M.A. Characterization of neurofibrillary tangle immunophenotype signatures to classify tangle maturity in Alzheimer’s disease. Alzheimers Dement. 2024, 20, 4803–4817. [Google Scholar] [CrossRef]
- Cummings, J. Anti-Amyloid Monoclonal Antibodies are Transformative Treatments that Redefine Alzheimer’s Disease Therapeutics. Drugs 2023, 83, 569–576. [Google Scholar] [CrossRef]
- Hampel, H.; Elhage, A.; Cho, M.; Apostolova, L.G.; Nicoll, J.A.R.; Atri, A. Amyloid-related imaging abnormalities (ARIA): Radiological, biological and clinical characteristics. Brain 2023, 146, 4414–4424. [Google Scholar] [CrossRef]
- Cogswell, P.M.; Barakos, J.A.; Barkhof, F.; Benzinger, T.S.; Jack, C.R., Jr.; Poussaint, T.Y.; Raji, C.A.; Ramanan, V.K.; Whitlow, C.T. Amyloid-Related Imaging Abnormalities with Emerging Alzheimer Disease Therapeutics: Detection and Reporting Recommendations for Clinical Practice. AJNR Am. J. Neuroradiol. 2022, 43, E19–E35. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Nunkoo, V.S.; Jurcau, A.; Les, M.; Cristian, A.; Militaru, M.; Marge, C.; Iovanovici, D.C.; Jurcau, M.C. Circulating Biomarkers for the Early Diagnosis of Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 7268. [Google Scholar] [CrossRef]
- Dansokho, C.; Ait Ahmed, D.; Aid, S.; Toly-Ndour, C.; Chaigneau, T.; Calle, V.; Cagnard, N.; Holzenberger, M.; Piaggio, E.; Aucouturier, P.; et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 2016, 139, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Saetzler, V.; Riet, T.; Schienke, A.; Henschel, P.; Freitag, K.; Haake, A.; Heppner, F.L.; Buitrago-Molina, L.E.; Noyan, F.; Jaeckel, E.; et al. Development of Beta-Amyloid-Specific CAR-Tregs for the Treatment of Alzheimer’s Disease. Cells 2023, 12, 2115. [Google Scholar] [CrossRef] [PubMed]
- Siebrand, C.J.; Bergo, N.J.; Lee, S.; Andersen, J.K.; Walton, C.C. Chimeric antigen receptors discriminate between tau and distinct amyloid-beta species. J. Transl. Med. 2025, 23, 605. [Google Scholar] [CrossRef]
- Xu, Z.; Rao, Y.; Huang, Y.; Zhou, T.; Feng, R.; Xiong, S.; Yuan, T.F.; Qin, S.; Lu, Y.; Zhou, X.; et al. Efficient Strategies for Microglia Replacement in the Central Nervous System. Cell Rep. 2020, 32, 108041. [Google Scholar] [CrossRef] [PubMed]
- Simard, A.R.; Soulet, D.; Gowing, G.; Julien, J.P.; Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 2006, 49, 489–502. [Google Scholar] [CrossRef]
- d’Errico, P.; Ziegler-Waldkirch, S.; Aires, V.; Hoffmann, P.; Mezö, C.; Erny, D.; Monasor, L.S.; Liebscher, S.; Ravi, V.M.; Joseph, K.; et al. Microglia contribute to the propagation of Aβ into unaffected brain tissue. Nat. Neurosci. 2022, 25, 20–25. [Google Scholar] [CrossRef]
- Yan, P.; Kim, K.W.; Xiao, Q.; Ma, X.; Czerniewski, L.R.; Liu, H.; Rawnsley, D.R.; Yan, Y.; Randolph, G.J.; Epelman, S.; et al. Peripheral monocyte-derived cells counter amyloid plaque pathogenesis in a mouse model of Alzheimer’s disease. J. Clin. Investig. 2022, 132, e152565. [Google Scholar] [CrossRef]
- Melenhorst, J.J.; Chen, G.M.; Wang, M.; Porter, D.L.; Chen, C.; Collins, M.A.; Gao, P.; Bandyopadhyay, S.; Sun, H.; Zhao, Z.; et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 2022, 602, 503–509, Erratum in Nature 2022, 612, E22. [Google Scholar] [CrossRef]
- DiNofia, A.M.; Grupp, S.A. Will allogeneic CAR T cells for CD19+ malignancies take autologous CAR T cells ‘off the shelf’? Nat. Rev. Clin. Oncol. 2021, 18, 195–196. [Google Scholar] [CrossRef]
- Bishop, E.L.; Gudgeon, N.; Dimeloe, S. Control of T Cell Metabolism by Cytokines and Hormones. Front. Immunol. 2021, 12, 653605. [Google Scholar] [CrossRef]
- Fu, T.E.; Zhou, Z. Senescent cells as a target for anti-aging interventions: From senolytics to immune therapies. J. Transl. Int. Med. 2025, 13, 33–47. [Google Scholar] [CrossRef]
- Haghikia, A.; Schett, G.; Mougiakakos, D. B cell-targeting chimeric antigen receptor T cells as an emerging therapy in neuroimmunological diseases. Lancet Neurol. 2024, 23, 615–624. [Google Scholar] [CrossRef]
- Brown, C.E.; Aguilar, B.; Starr, R.; Yang, X.; Chang, W.C.; Weng, L.; Chang, B.; Sarkissian, A.; Brito, A.; Sanchez, J.F.; et al. Optimization of IL13Rα2-Targeted Chimeric Antigen Receptor T Cells for Improved Anti-tumor Efficacy against Glioblastoma. Mol. Ther. 2018, 26, 31–44. [Google Scholar] [CrossRef]
- Priceman, S.J.; Tilakawardane, D.; Jeang, B.; Aguilar, B.; Murad, J.P.; Park, A.K.; Chang, W.C.; Ostberg, J.R.; Neman, J.; Jandial, R.; et al. Regional Delivery of Chimeric Antigen Receptor-Engineered T Cells Effectively Targets HER2+ Breast Cancer Metastasis to the Brain. Clin. Cancer Res. 2018, 24, 95–105. [Google Scholar] [CrossRef]
- Qu, Z.; Luo, J.; Li, Z.; Yang, R.; Zhao, J.; Chen, X.; Yu, S.; Shu, H. Advancements in strategies for overcoming the blood-brain barrier to deliver brain-targeted drugs. Front. Aging Neurosci. 2024, 16, 1353003. [Google Scholar] [CrossRef]
- Arcangeli, S.; Bove, C.; Mezzanotte, C.; Camisa, B.; Falcone, L.; Manfredi, F.; Bezzecchi, E.; El Khoury, R.; Norata, R.; Sanvito, F.; et al. CAR T cell manufacturing from naive/stem memory T lymphocytes enhances antitumor responses while curtailing cytokine release syndrome. J. Clin. Investig. 2022, 132, e150807. [Google Scholar] [CrossRef]
- Zurko, J.C.; Chaney, K.; Xu, H.; Schneider, D.; Hari, P.; Johnson, B.; Shah, N.H. Manufacturing bispecific LV20.19 CAR T-cells with IL-17 & IL-15 for a shorter duration improves CAR T-cell immunophenotype while maintaining target cell dose. Blood 2021, 138, 3883–3885. [Google Scholar]
- Lynn, R.C.; Weber, E.W.; Sotillo, E.; Gennert, D.; Xu, P.; Good, Z.; Anbunathan, H.; Lattin, J.; Jones, R.; Tieu, V.; et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature 2019, 576, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhu, X.; Xiao, Y. Advances in CAR-T therapy for central nervous system tumors. Biomark. Res. 2024, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Lontos, K.; Wang, Y.; Joshi, S.K.; Frisch, A.T.; Watson, M.J.; Kumar, A.; Menk, A.V.; Wang, Y.; Cumberland, R.; Lohmueller, J.; et al. Metabolic reprogramming via an engineered PGC-1α improves human chimeric antigen receptor T-cell therapy against solid tumors. J. Immunother. Cancer 2023, 11, e006522. [Google Scholar] [CrossRef]
- Boretti, A. The transformative potential of AI-driven CRISPR-Cas9 genome editing to enhance CAR T-cell therapy. Comput. Biol. Med. 2024, 182, 109137. [Google Scholar] [CrossRef]
- Lu, L.; Yu, X.; Cai, Y.; Sun, M.; Yang, H. Application of CRISPR/Cas9 in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 803894. [Google Scholar] [CrossRef]
- Lau, N.C.H.; Yam, J.P.W. From Exosome Biogenesis to Absorption: Ket Takeaways for cancer research. Cancers 2023, 15, 1992. [Google Scholar] [CrossRef] [PubMed]
- Nistor-Cseppentö, D.C.; Jurcău, M.C.; Jurcău, A.; Andronie-Cioară, F.L.; Marcu, F. Stem Cell- and Cell-Based Therapies for Ischemic Stroke. Bioengineering 2022, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.; Dhar, R.; Mukherjee, S.; Nag, S.; Gorai, S.; Mukerjee, N.; Mukherjee, D.; Vatsa, R.; Chandrakanth Jadhav, M.; Ghosh, A.; et al. Exosome-Based Smart Drug Delivery Tool for Cancer Theranostics. ACS Biomater. Sci. Eng. 2023, 9, 577–594. [Google Scholar] [CrossRef]
- Kim, H.Y.; Min, H.K.; Song, H.W.; Yoo, A.; Lee, S.; Kim, K.P.; Park, J.O.; Choi, Y.H.; Choi, E. Delivery of human natural killer cell-derived exosomes for liver cancer therapy: An in vivo study in subcutaneous and orthotopic animal models. Drug Deliv. 2022, 29, 2897–2911. [Google Scholar] [CrossRef]
- Andronie-Cioară, F.L.; Jurcău, A.; Jurcău, M.C.; Nistor-Cseppentö, D.C.; Simion, A. Cholesterol Management in Neurology: Time for Revised Strategies? J. Pers. Med. 2022, 12, 1981. [Google Scholar] [CrossRef]
- Asami, T.; Endo, K.; Matsui, R.; Sawa, T.; Tanaka, Y.; Saiki, T.; Tanba, N.; Haga, H.; Tanaka, S. Long-term caloric restriction ameliorates T cell immunosenescence in mice. Mech. Ageing Dev. 2022, 206, 111710. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, R.; Kumar, U.; Kumar, S.; Luthra, K.; Dada, R. Yoga maintains Th17/Treg cell homeostasis and reduces the rate of T cell aging in rheumatoid arthritis: A randomized controlled trial. Sci. Rep. 2023, 13, 14924. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A. The Role of Natural Antioxidants in the Prevention of Dementia—Where Do We Stand and Future Perspectives. Nutrients 2021, 13, 282. [Google Scholar] [CrossRef]
- Wei, T.T.; Feng, Y.K.; Cao, J.H.; Li, J.H.; Yuan, S.L.; Ding, Y.; Chai, Y.R. Dosage effects of resveratrol on thymus involution in D-galactose-treated mice. J. Food Biochem. 2021, 45, e13709. [Google Scholar] [CrossRef] [PubMed]
- Ghamar Talepoor, A.; Khosropanah, S.; Doroudchi, M. Partial recovery of senescence in circulating follicular helper T cells after Dasatinib treatment. Int. Immunopharmacol. 2021, 94, 107465. [Google Scholar] [CrossRef] [PubMed]

| Gene | Gene Product | Role in Neuroinflammation | Polymorphisms | Effect | References |
|---|---|---|---|---|---|
| TREM2 | TREM2 (triggering receptor expressed on myeloid cells 2) | Microglial phagocytosis | R47H variant | Increases neuritic dystrophy caused by Aβ deposition and reduces microglial function in Caucasians | [78,79] |
| R63H variant | Increases the risk for AD in individuals of European ancestry | [80] | |||
| H157Y variant | Increases the shedding of the soluble form of the receptor and decreases cell surface expression of the receptor; increases the risk for AD mainly in Chinese populations | [80] | |||
| CD33 | A transmembrane receptor on the myeloid lineage cells that belongs to the SIGLECs (sialic acid-binding Ig-superfamily of lectins) | Inhibits microglial amyloid clearance | SNP rs3865444 | Protective effect by promoting Aβ42 clearance by microglial cells | [81] |
| CR1 | Complement component (3b/4b) receptor 1 | Complements activation, synapse pruning | SNP rs6656401 | Increases the susceptibility for late-onset AD | [82] |
| CLU | Clusterin, an astrocytic protein with anti-amyloidogenic functions | Complements regulation, immune recruitment | rs11136000 | Increases the risk for AD mainly in Caucasians | [83] |
| PILRA | PILRA (paired immunoglobulin-like type 2 receptor alpha), a microglial surface inhibitory receptor | Microglial inhibitory signaling | G78R (rs1859788) | Protects carriers from the risk of AD | [84] |
| EPHA1 | EPHA1 (ephrin type-A receptor 1) | Recruitment of immune cells | P460L variant | Increases the risk for late-onset AD | [85] |
| ABI3 | ABI3 (ABI interactor family member 3) | Microglial cytoskeletal regulator; impacts motility and microglial response to Aβ plaques | SNPs rs5978930 and rs16947151 | Increase the risk of AD, associated with faster cognitive decline | [86] |
| HLA-DRB1 | Beta chain of the human leukocyte antigen class II molecule | Involved in antigen presentation to CD4+ T cells and microglial activation | SNP rs9271192 | Associated with late-onset AD | [87] |
| MS4A6A | Membrane spanning 4-domains A6A | Involved in the immune clearance of Aβ | rs610932 | Associated with late-onset AD | [88] |
| ABCA7 | ATP-binding cassette sub-family A member 7 | Regulates the communication between microglia and astrocytes through the NLRP3 inflammasome and the release of proinflammatory cytokines | rs3764650 | Increases the risk for AD | [89] |
| Surface Marker | Cell Type | Aging Model and Detection Pathway | Reference |
|---|---|---|---|
| B2M | Bladder cancer cells | Senescence—induced by doxorubicin | [186] |
| NOTCH1 | Fibroblasts | Senescence was induced by mitogen-activated extracellular signal-regulated kinase (MEK) and DNA damage | [187] |
| PD-L1 | fibroblasts | Senescence was induced by DNA damage and Nutlin-3 | [64] |
| uPAR | Liver fibrosis cells and lung adenocarcinoma cells | Senescence induced by replication induction, MEK and CDK4/6 inhibitors, liver fibrosis induced by CCl4 | [188] |
| GPNMB | Vascular endothelial cells | Senescence was induced by feeding a high-fat diet | [182] |
| CD38 | Alveolar epithelial cells | Lung fibrosis was induced in mice by intratracheal bleomycin | [189] |
| CD26 (DPP4) | Human cartilage | Chondrocytes were acquired from individuals undergoing total knee replacement; research evaluated co-expression of DPP4 with established senescence markers by flow cytometry, and expression levels of anabolic and catabolic genes, senescence related genes and SASP phenotypes in DPP4+ and DPP4− cells | [190] |
| CD47 | Fibroblasts | Premature senescence was induced with hydrogen peroxide | [191] |
| DcR2 | Renal tubular epithelial cells | Renal fibrosis was induced with streptozotocin in mice with diabetic nephropathy | [192] |
| Oxidized vimentin | Human chondrocytes | Doxorubicin-based in vitro model of stress-induced premature senescence | [193] |
| CD44 | Vascular endothelial cells | Replicative senescence induced by serial passages | [194] |
| MICA, MICB, ULBP2 (NKG2D ligands) | Lung fibroblasts | Senescence was induced by DNA damage, replication deletion, and triggering oncogenes | [195] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurcau, M.C.; Iovanovici, C.D.; Jurcau, A.; Militaru, M.; Udrea, R.B.; Comanescu, A.; Nunkoo, V.S. Chimeric Antigen Receptor Cell Therapy: Current Status and Its Potential in Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 9009. https://doi.org/10.3390/ijms26189009
Jurcau MC, Iovanovici CD, Jurcau A, Militaru M, Udrea RB, Comanescu A, Nunkoo VS. Chimeric Antigen Receptor Cell Therapy: Current Status and Its Potential in Aging and Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(18):9009. https://doi.org/10.3390/ijms26189009
Chicago/Turabian StyleJurcau, Maria Carolina, Carina Diana Iovanovici, Anamaria Jurcau, Marius Militaru, Radu Bogdan Udrea, Alexandra Comanescu, and Vharoon Sharma Nunkoo. 2025. "Chimeric Antigen Receptor Cell Therapy: Current Status and Its Potential in Aging and Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 18: 9009. https://doi.org/10.3390/ijms26189009
APA StyleJurcau, M. C., Iovanovici, C. D., Jurcau, A., Militaru, M., Udrea, R. B., Comanescu, A., & Nunkoo, V. S. (2025). Chimeric Antigen Receptor Cell Therapy: Current Status and Its Potential in Aging and Alzheimer’s Disease. International Journal of Molecular Sciences, 26(18), 9009. https://doi.org/10.3390/ijms26189009






