Neuroendocrine Regulation and Neural Circuitry of Parenthood: Integrating Neuropeptides, Brain Receptors, and Maternal Behavior
Abstract
1. Introduction
1.1. Motherhood and Parental Behaviors
1.2. Hormonal Influences on Maternal Behavior
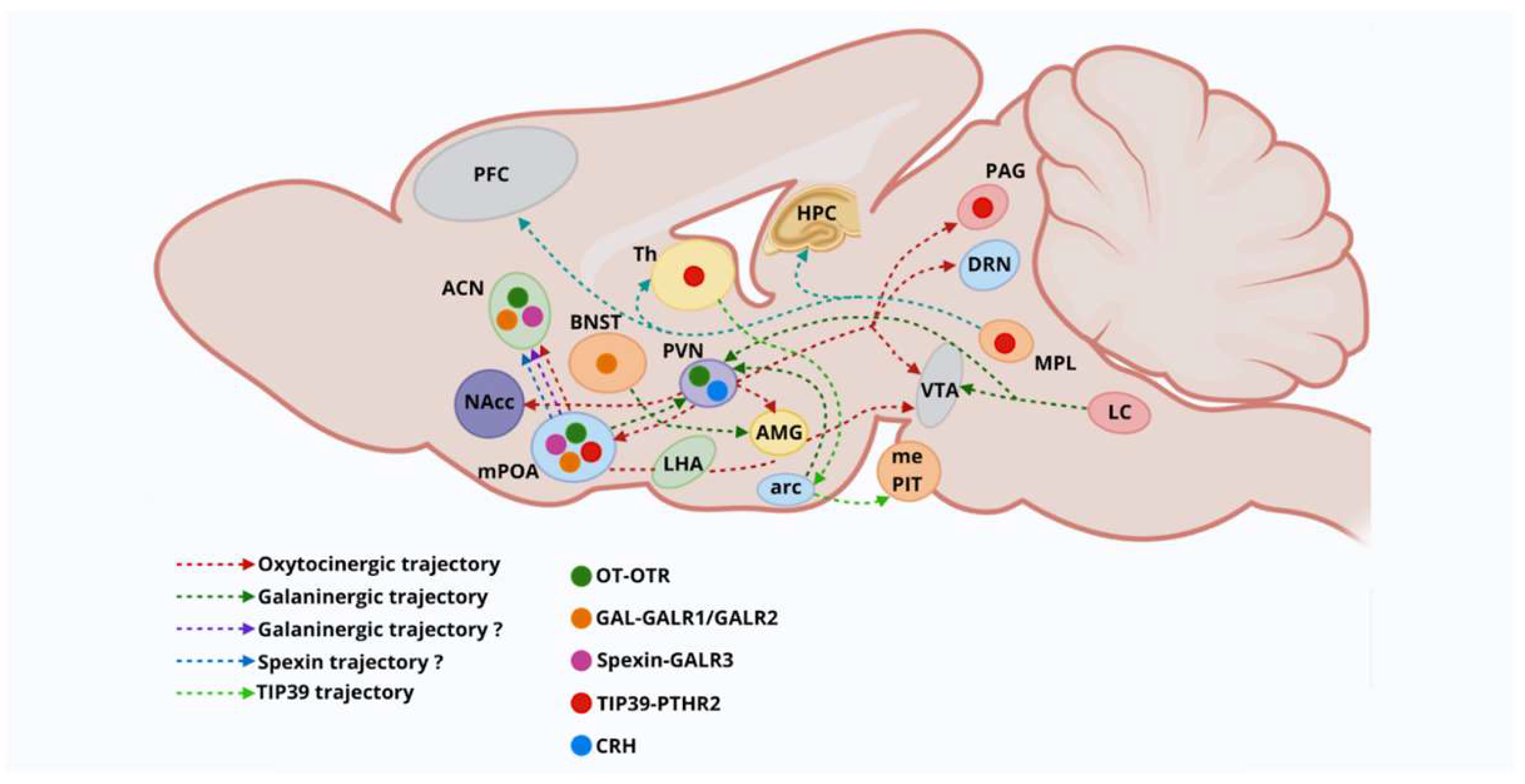
1.3. Neural Pathways Involved in Motherhood
1.4. Mesolimbic Dopamine Neural Pathways
1.5. Analyzing Hypothalamic Nuclei and Neural Pathways
1.6. Oxytocin Neurons: Magnocellular Neurosecretory Cells
1.7. Oxytocinergic Neural Pathways
1.8. Oxytocin Receptors
1.9. Oxytocin and Dopamine Heterodimeric Receptor Complexes
1.10. Oxytocin Neuromodulation
1.11. Brain Peptides Influencing Maternal Behavior
1.12. Urocortins
1.13. Endogenous Opioid Peptides
1.14. Opioid Receptors Modulating OT Neural Pathways
1.15. Galanin and Spexin Neural Peptides
1.16. Galanin, Serotonin, and Zinc Heterodimeric Receptor Complexes
1.17. Tuberoinfundibular Peptide 39
1.18. Prolactin Modulatory Bioactivities
1.19. Prolactin Cell-Signaling System
1.20. Prolactin Regulates Neural Functions
1.21. Prolactin Induces Maternal Behaviors
2. Stress Responses
2.1. CRH and HPA Axis Activity
2.2. Stress Effects on Maternal Behavior
2.3. Prolactin-Regulating Stress Responses
2.4. Oxytocin-Modulating Stress Responses
2.5. Galanin and Spexin Peptides: Pro- and Anti-Stress Responses
3. Psychiatric Disorders
3.1. Affective Disorders in Postpartum Period
3.2. Oxytocin in Mood Disorders
3.3. Neural Peptide Systems in Psychiatric Disorders
3.4. NPY and NPY Receptors in Mood-Related Disorders
3.5. PACAP-PAC1 Receptor in Psychiatric Disorders
3.6. Gal and Gal Receptors in Mood Disorders
4. Conclusions
5. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Broad, K.D.; Curley, J.P.; Keverne, E.B. Mother-infant bonding and the evolution of mammalian social relationships. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 2199–2214. [Google Scholar] [CrossRef]
- Kohl, J.; Autry, A.E.; Dulac, C. The neurobiology of parenting: A neural circuit perspective. Bioessays 2017, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hwong, A. Mothers and Others: The Evolutionary Origins of Mutual Understanding. Psychiatr. Serv. 2010, 61, 1050. [Google Scholar] [CrossRef]
- Georgescu, T.; Swart, J.M.; Grattan, D.R.; Brown, R.S.E. The Prolactin Family of Hormones as Regulators of Maternal Mood and Behavior. Front. Glob. Womens Health 2021, 2, 767467. [Google Scholar] [CrossRef] [PubMed]
- Dobolyi, A.; Cservenák, M.; Young, L.J. Thalamic integration of social stimuli regulating parental behavior and the oxytocin system. Front. Neuroendocr. 2018, 51, 102–115. [Google Scholar] [CrossRef]
- Goodson, J.L. The vertebrate social behavior network: Evolutionary themes and variations. Horm. Behav. 2005, 48, 11–22. [Google Scholar] [CrossRef]
- Royle, N.J.; Russell, A.F.; Wilson, A.J. The evolution of flexible parenting. Science 2014, 345, 776–781. [Google Scholar] [CrossRef]
- Rilling, J.K.; Young, L.J. The biology of mammalian parenting and its effect on offspring social development. Science 2014, 345, 771–776. [Google Scholar] [CrossRef]
- Schultz, W.; Tremblay, L.; Hollerman, J.R. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb. Cortex 2000, 10, 272–284. [Google Scholar] [CrossRef]
- Chiba, T.; Kayahara, T.; Nakano, K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 2001, 888, 83–101. [Google Scholar] [CrossRef]
- Pritschet, L.; Taylor, C.M.; Cossio, D.; Faskowitz, J.; Santander, T.; Handwerker, D.A.; Grotzinger, H.; Layher, E.; Chrastil, E.R.; Jacobs, E.G. Neuroanatomical changes observed over the course of a human pregnancy. Nat. Neurosci. 2024, 27, 2253–2260. [Google Scholar] [CrossRef]
- Rincón-Cortés, M.; Grace, A.A. Adaptations in reward-related behaviors and mesolimbic dopamine function during motherhood and the postpartum period. Front. Neuroendocr. 2020, 57, 100839. [Google Scholar] [CrossRef]
- Barba-Müller, E.; Craddock, S.; Carmona, S.; Hoekzema, E. Brain plasticity in pregnancy and the postpartum period: Links to maternal caregiving and mental health. Arch. Womens Ment. Health 2019, 22, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Hoekzema, E.; Barba-Müller, E.; Pozzobon, C.; Picado, M.; Lucco, F.; García-García, D.; Soliva, J.C.; Tobeña, A.; Desco, M.; Crone, E.A.; et al. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 2017, 20, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Lonstein, J.S.; Lévy, F.; Fleming, A.S. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Horm. Behav. 2015, 73, 156–185. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wong, S.; Mitchell, B.F. Effects of RU486 on estrogen, progesterone, oxytocin, and their receptors in the rat uterus during late gestation. Endocrinology 1997, 138, 2763–2768. [Google Scholar] [CrossRef]
- Rezapour, M.; Bäckström, T.; Ulmsten, U. Myometrial steroid concentration and oxytocin receptor density in parturient women at term. Steroids 1996, 61, 338–344. [Google Scholar] [CrossRef]
- Bayerl, D.S.; Bosch, O.J. Brain vasopressin signaling modulates aspects of maternal behavior in lactating rats. Genes Brain Behav. 2019, 18, e12517. [Google Scholar] [CrossRef]
- Bosch, O.J.; Neumann, I.D. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Horm. Behav. 2012, 61, 293–303. [Google Scholar] [CrossRef]
- McNeilly, A.S. Lactation and fertility. J. Mammary Gland. Biol. Neoplasia 1997, 2, 291–298. [Google Scholar] [CrossRef]
- Kim, P. Human Maternal Brain Plasticity: Adaptation to Parenting. New Dir. Child. Adolesc. Dev. 2016, 2016, 47–58. [Google Scholar] [CrossRef]
- Numan, M.; Rosenblatt, J.S.; Komisaruk, B.R. Medial preoptic area and onset of maternal behavior in the rat. J. Comp. Physiol. Psychol. 1977, 91, 146–164. [Google Scholar] [CrossRef] [PubMed]
- Bridges, R.S.; Robertson, M.C.; Shiu, R.P.C.; Friesen, H.G.; Stuer, A.M.; Mann, P.E. Endocrine communication between conceptus and mother: Placental lactogen stimulation of maternal behavior. Neuroendocrinology 1996, 64, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.S.; Luebke, C. Timidity prevents the virgin female rat from being a good mother: Emotionality differences between nulliparous and parturient females. Physiol. Behav. 1981, 27, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Vizi, E.S.; Fekete, A.; Karoly, R.; Mike, A. Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. Br. J. Pharmacol. 2010, 160, 785–809. [Google Scholar] [CrossRef]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, function, and regulation of secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef]
- Pratt, M.; Apter-Levi, Y.; Vakart, A.; Feldman, M.; Fishman, R.; Feldman, T.; Zagoory-Sharon, O.; Feldman, R. Maternal depression and child oxytocin response; moderation by maternal oxytocin and relational behavior. Depress. Anxiety 2015, 32, 635–646. [Google Scholar] [CrossRef]
- Seip, K.M.; Morrell, J.I. Exposure to pups influences the strength of maternal motivation in virgin female rats. Physiol. Behav. 2008, 95, 599–608. [Google Scholar] [CrossRef][Green Version]
- Wong, W.M.; Nagel, M.; Hernandez-Clavijo, A.; Pifferi, S.; Menini, A.; Spehr, M.; Meeks, J.P. Sensory Adaptation to Chemical Cues by Vomeronasal Sensory Neurons. eNeuro 2018, 5, ENEURO.0223-18.2018. [Google Scholar] [CrossRef]
- Fang, Y.-Y.; Yamaguchi, T.; Song, S.C.; Tritsch, N.X.; Lin, D. A Hypothalamic Midbrain Pathway Essential for Driving Maternal Behaviors. Neuron 2018, 98, 192–207.e10. [Google Scholar] [CrossRef]
- Numan, M.; Smith, H.G. Maternal behavior in rats: Evidence for the involvement of preoptic projections to the ventral tegmental area. Behav. Neurosci. 1984, 98, 712–727. [Google Scholar] [CrossRef]
- Li, C.; Chen, P.; Smith, M.S. Neural populations in the rat forebrain and brainstem activated by the suckling stimulus as demonstrated by cFos expression. Neuroscience 1999, 94, 117–129. [Google Scholar] [CrossRef]
- Del Cerro, M.C.R.; Perez Izquierdo, M.A.; Rosenblatt, J.S.; Johnson, B.M.; Pacheco, P.; Komisaruk, B.R. Brain 2-deoxyglucose level related to maternal behavior-inducing stimuli in the rat. Brain Res. 1995, 696, 213–220. [Google Scholar] [CrossRef]
- Febo, M.; Numan, M.; Ferris, C.F. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J. Neurosci. 2005, 25, 11637–11644. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R.; Harbaugh, C.R. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiol. Behav. 1989, 45, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Corodimas, K.P.; Rosenblatt, J.S.; Canfield, M.E.; Morrell, J.I. Neurons in the lateral subdivision of the habenular complex mediate the hormonal onset of maternal behavior in rats. Behav. Neurosci. 1993, 107, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Barofsky, A.L.; Taylor, J.; Tizabi, Y.; Kumar, R.; Jones-Quartey, K. Specific neurotoxin lesions of median raphe serotonergic neurons disrupt maternal behavior in the lactating rat. Endocrinology 1983, 113, 1884–1893. [Google Scholar] [CrossRef]
- Lonstein, J.S.; Simmons, D.A.; Swann, J.M.; Stern, J.M. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience 1998, 82, 267–281. [Google Scholar] [CrossRef]
- Lonstein, J.S.; Stern, J.M. Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal, and aggressive behaviors in rats. Brain Res. 1998, 804, 21–35. [Google Scholar] [CrossRef]
- Morgan, H.D.; Watchus, J.A.; Fleming, A.S. The effects of electrical stimulation of the medial preoptic area and the medial amygdala on maternal responsiveness in female rats. Ann. N. Y. Acad. Sci. 1997, 807, 602–605. [Google Scholar] [CrossRef]
- Cservenák, M.; Kis, V.; Keller, D.; Dimén, D.; Menyhárt, L.; Oláh, S.; Szabó, É.R.; Barna, J.; Renner, É.; Usdin, T.B.; et al. Maternally involved galanin neurons in the preoptic area of the rat. Brain Struct. Funct. 2017, 222, 781–798. [Google Scholar] [CrossRef]
- Kohl, J.; Babayan, B.M.; Rubinstein, N.D.; Autry, A.E.; Marin-Rodriguez, B.; Kapoor, V.; Miyamishi, K.; Zweifel, L.S.; Luo, L.; Uchida, N.; et al. Functional circuit architecture underlying parental behaviour. Nature 2018, 556, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.; Prigge, M.; Yizhar, O.; Kimchi, T. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 2015, 525, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.H.; Bains, J.S.; Ludwig, M.; Stern, J.E. Physiological regulation of magnocellular neurosecretory cell activity: Integration of intrinsic, local and afferent mechanisms. J. Neuroendocr. 2013, 25, 678–710. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.; Vila-Porcile, E.; Hökfelt, T.; Calas, A. Differential routing of coexisting neuropeptides in vasopressin neurons. Eur. J. Neurosci. 2003, 17, 579–589. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Sasaki, K.; Satomi, Y.; Shimbara, T.; Kageyama, H.; Mondal, M.S.; Toshinai, K.; Date, Y.; González, L.J.; Shioda, S.; et al. Peptidomic identification and biological validation of neuroendocrine regulatory peptide-1 and -2. J. Biol. Chem. 2007, 282, 26354–26360. [Google Scholar] [CrossRef]
- Fujihara, H.; Sasaki, K.; Mishiro-Sato, E.; Ohbuchi, T.; Dayanithi, G.; Yamasaki, M.; Ueta, Y.; Minamino, N. Molecular characterization and biological function of neuroendocrine regulatory peptide-3 in the rat. Endocrinology 2012, 153, 1377–1386. [Google Scholar] [CrossRef]
- Gillard, E.R.; León-Olea, M.; Mucio-RamírEz, S.; Coburn, C.G.; SánChez-Islas, E.; de Leon, A.; Mussenden, H.; Bauce, L.G.; Pittman, Q.J.; Currás-Collazo, M.C. A novel role for endogenous pituitary adenylate cyclase activating polypeptide in the magnocellular neuroendocrine system. Endocrinology 2006, 147, 791–803. [Google Scholar] [CrossRef]
- Froemke, R.C.; Young, L.J. Oxytocin, Neural Plasticity, and Social Behavior. Annu. Rev. Neurosci. 2021, 44, 359–381. [Google Scholar] [CrossRef]
- Son, S.; Manjila, S.B.; Newmaster, K.T.; Wu, Y.T.; Vanselow, D.J.; Ciarletta, M.; Anthony, T.E.; Cheng, K.C.; Kim, Y. Whole-Brain Wiring Diagram of Oxytocin System in Adult Mice. J. Neurosci. 2022, 42, 5021–5033. [Google Scholar] [CrossRef]
- Dulac, C.; O’Connell, L.A.; Wu, Z. Neural control of maternal and paternal behaviors. Science 2014, 345, 765–770. [Google Scholar] [CrossRef]
- Leng, G.; Meddle, S.L.; Douglas, A.J. Oxytocin and the maternal brain. Curr. Opin. Pharmacol. 2008, 8, 731–734. [Google Scholar] [CrossRef]
- Mitre, M.; Marlin, B.J.; Schiavo, J.K.; Morina, E.; Norden, S.E.; Hackett, T.A.; Aoki, C.J.; Chao, M.V.; Froemke, R.C. A Distributed Network for Social Cognition Enriched for Oxytocin Receptors. J. Neurosci. 2016, 36, 2517–2535. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Benusiglio, D.; Lefevre, A.; Hilfiger, L.; Althammer, F.; Bludau, A.; Hagiwara, D.; Baudon, A.; Darbon, P.; Schimmer, J.; et al. Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat. Neurosci. 2020, 23, 1125–1137. [Google Scholar] [CrossRef] [PubMed]
- Veening, J.G.; de Jong, T.; Barendregt, H.P. Oxytocin-messages via the cerebrospinal fluid: Behavioral effects; a review. Physiol. Behav. 2010, 101, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.V.; Young, L.J. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev. 2017, 76, 87–98. [Google Scholar] [CrossRef]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Salighedar, R.; Erfanparast, A.; Tamaddonfard, E.; Soltanalinejad, F. Medial prefrontal cortex oxytocin-opioid receptors interaction in spatial memory processing in rats. Physiol. Behav. 2019, 209, 112599. [Google Scholar] [CrossRef]
- Nisbett, K.E.; Vendruscolo, L.F.; Koob, G.F. Koob µ-Opioid receptor antagonism facilitates the anxiolytic-like effect of oxytocin in mice. Transl. Psychiatry 2024, 14, 125. [Google Scholar] [CrossRef]
- Amato, S.; Averna, M.; Guidolin, D.; Ceccoli, C.; Gatta, E.; Candiani, S.; Pedrazzi, M.; Capraro, M.; Maura, G.; Agnati, L.F.; et al. Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes. Int. J. Mol. Sci. 2023, 24, 4677. [Google Scholar] [CrossRef]
- Petersson, M.; Uvnäs-Moberg, K. Interactions of Oxytocin and Dopamine-Effects on Behavior in Health and Disease. Biomedicines 2024, 12, 2440. [Google Scholar] [CrossRef]
- de la Mora, M.P.; Pérez-Carrera, D.; Crespo-Ramírez, M.; Tarakanov, A.; Fuxe, K.; Borroto-Escuela, D.O. Signaling in dopamine D2 receptor-oxytocin receptor heterocomplexes and its relevance for the anxiolytic effects of dopamine and oxytocin interactions in the amygdala of the Rat. Biochim. Biophys. Acta-Mol. Basis Dis. 2016, 1862, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Z.X. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 2003, 121, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Romero-Fernandez, W.; Borroto-Escuela, D.O.; Agnati, L.F.; Fuxe, K. Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor–receptor interactions. Mol. Psychiatry 2013, 18, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Priest, M.F.; Nasenbeny, J.; Lu, T.; Kozorovitskiy, Y. Biased Oxytocinergic Modulation of Midbrain Dopamine Systems. Neuron 2017, 95, 368–384.e5. [Google Scholar] [CrossRef]
- Rivas, M.; Ferreira, A.; Torterolo, P.; Benedetto, L. Hypocretins, sleep, and maternal behavior. Front. Behav. Neurosci. 2023, 17, 1184885. [Google Scholar] [CrossRef]
- Kuebler, I.R.K.; Suárez, M.; Wakabayashi, K.T. Sex differences and sex-specific regulation of motivated behavior by Melanin-concentrating hormone: A short review. Biol. Sex Differ. 2024, 15, 33. [Google Scholar] [CrossRef]
- Alcantara, I.C.; Li, C.; Gao, C.; Rodriguez González, S.; Mickelsen, L.E.; Papas, B.N.; Goldschmidt, A.I.; Cohen, I.M.; Mazzone, C.M.; de Araujo Salgado, I.; et al. A hypothalamic circuit that modulates feeding and parenting behaviours. Nature 2025. epub ahead of print. [Google Scholar] [CrossRef]
- Kormos, V.; Gaszner, B. Role of neuropeptides in anxiety, stress, and depression: From animals to humans. Neuropeptides 2013, 47, 401–419. [Google Scholar] [CrossRef]
- Janssen, D.; Kozicz, T. Is it really a matter of simple dualism? Corticotropin- releasing factor receptors in body and mental health. Front. Endocrinol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Gaszner, B.; Jensen, K.O.; Farkas, J.; Reglodi, D.; Csernus, V.; Roubos, E.W.; Kozicz, T. Effects of maternal separation on dynamics of urocortin 1 and brain-derived neurotrophic factor in the rat non-preganglionic Edinger-Westphal nucleus. Int. J. Dev. Neurosci. 2009, 27, 439–451. [Google Scholar] [CrossRef]
- Kozicz, T.; Tilburg-Ouwens, D.; Faludi, G.; Palkovits, M.; Roubos, E. Gender-related urocortin 1 and brain-derived neurotrophic factor expression in the adult human midbrain of suicide victims with major depression. Neuroscience 2008, 152, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Sierra, S.; Lueptow, L.; Gupta, A.; Gouty, S.; Margolis, E.B.; Cox, B.M.; Devi, L.A. Biased signaling by endogenous opioid peptides. Proc. Natl. Acad. Sci. USA 2020, 117, 11820–11828. [Google Scholar] [CrossRef] [PubMed]
- Sumner, B.E.; Coombes, J.E.; Pumford, K.M.; Russell, J.A. Opioid receptor subtypes in the supraoptic nucleus and posterior pituitary gland of morphine-tolerant rats. Neuroscience 1990, 37, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.H.; Russell, J.A.; Leng, G. Opioid modulation of magnocellular neurosecretory cell activity. Neurosci. Res. 2000, 36, 97–120. [Google Scholar] [CrossRef]
- Douglas, A.J.; Neumann, I.; Meeren, H.K.; Leng, G.; Johnstone, L.E.; Munro, G.; Russell, J.A. Central endogenous opioid inhibition of supraoptic oxytocin neurons in pregnant rats. J. Neurosci. 1995, 15 Pt 1, 5049–5057. [Google Scholar] [CrossRef]
- Wigger, A.; Neumann, I.D. Endogenous opioid regulation of stress-induced oxytocin release within the hypothalamic paraventricular nucleus is reversed in late pregnancy: A microdialysis study. Neuroscience 2002, 112, 121–129. [Google Scholar] [CrossRef]
- Lang, R.; Gundlach, A.L.; Holmes, F.E.; Hobson, S.A.; Wynick, D.; Hökfelt, T.; Kofler, B. Physiology, signaling, and pharmacology of galanin peptides and receptors: Three decades of emerging diversity. Pharmacol. Rev. 2015, 67, 118–175. [Google Scholar] [CrossRef]
- Webling, K.E.; Runesson, J.; Bartfai, T.; Langel, U. Galanin receptors and ligands. Front. Endocrinol. 2012, 3, 146. [Google Scholar] [CrossRef]
- Mirabeau, O.; Perlas, E.; Severini, C.; Audero, E.; Gascuel, O.; Possenti, R.; Birney, E.; Rosenthal, N.; Gross, C. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. 2007, 17, 320–327. [Google Scholar] [CrossRef]
- Wan, B.; Wang, X.-R.; Zhou, Y.-B.; Zhang, X.; Huo, K.; Han, Z.-G. C12ORF39, a novel secreted protein with a typical amidation processing signal. Biosci. Rep. 2009, 30, 1–10. [Google Scholar] [CrossRef]
- Ma, A.; Bai, J.; He, M.; Wong, A.O.L. Spexin as a neuroendocrine signal with emerging functions. Gen. Comp. Endocrinol. 2018, 265, 90–96. [Google Scholar] [CrossRef]
- Mohd Zahir, I.; Ogawa, S.; Dominic, N.A.; Soga, T.; Parhar, I.S. Spexin and Galanin in Metabolic Functions and Social Behaviors with a Focus on Non-Mammalian Vertebrates. Front. Endocrinol. 2022, 13, 882772. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.K.H.; He, M.; Sze, K.H.; Huang, T.; Ko, W.K.W.; Bian, Z.X.; Wong, A.O.L. Mouse Spexin: (I) NMR Solution Structure, Docking Models for Receptor Binding, and Histological Expression at Tissue Level. Front. Endocrinol. 2021, 12, 681646. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Brunner, S.M.; Bianchini, R.; Ramspacher, A.; Emberger, M.; Sternberg, F.; Schlager, S.; Kofler, B. Galanin is a potent modulator of cytokine and chemokine expression in human macrophages. Sci. Rep. 2019, 9, 7237. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cabiale, Z.; Flores-Burgess, A.; Parrado, C.; Narváez, M.; Millón, C.; Puigcerver, A.; Coveñas, R.; Fuxe, K.; Narváez, J.A. Galanin receptor/Neuropeptide Y receptor interactions in the central nervous system. Curr. Protein Pept. Sci. 2014, 15, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Mirchandani-Duque, M.; Choucri, M.; Hernández-Mondragón, J.C.; Crespo-Ramírez, M.; Pérez-Olives, C.; Ferraro, L.; Franco, R.; Pérez de la Mora, M.; Fuxe, K.; Borroto-Escuela, D.O. Membrane Heteroreceptor Complexes as Second-Order Protein Modulators: A Novel Integrative Mechanism through Allosteric Receptor-Receptor Interactions. Membranes 2024, 14, 96. [Google Scholar] [CrossRef]
- Fuxe, K.; von Euler, G.; Agnati, L.F.; Ogren, S.O. Galanin selectively modulates 5-hydroxytryptamine 1A receptors in the rat ventral limbic cortex. Neurosci. Lett. 1988, 85, 163–167. [Google Scholar] [CrossRef]
- Bellido, I.; Diaz-Cabiale, Z.; Jiménez-Vasquez, P.A.; Andbjer, B.; Mathé, A.A.; Fuxe, K. Increased density of galanin binding sites in the dorsal raphe in a genetic rat model of depression. Neurosci. Lett. 2002, 317, 101–105. [Google Scholar] [CrossRef]
- Wirz, S.A.; Davis, C.N.; Lu, X.; Zal, T.; Bartfai, T. Homodimerization and internalization of galanin type 1 receptor in living CHO cells. Neuropeptides 2005, 39, 535–546. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Narvaez, M.; Marcellino, D.; Parrado, C.; Narvaez, J.A.; Tarakanov, A.O.; Agnati, L.F.; Díaz-Cabiale, Z.; Fuxe, K. Galanin receptor-1 modulates 5-hydroxtryptamine-1A signaling via heterodimerization. Biochem. Biophys. Res. Commun. 2010, 393, 767–772. [Google Scholar] [CrossRef]
- Tena-Campos, M.; Ramon, E.; Borroto-Escuela, D.O.; Fuxe, K.; Garriga, P. The zinc binding receptor GPR39 interacts with 5-HT1A and GalR1 to form dynamic heteroreceptor complexes with signaling diversity. Biochim. Biophys. Acta 2015, 1852, 2585–2592. [Google Scholar] [CrossRef] [PubMed]
- Tena-Campos, M.; Ramon, E.; Lupala, C.S.; Pérez, J.J.; Koch, K.W.; Garriga, P. Zinc Is Involved in Depression by Modulating G Protein-Coupled Receptor Heterodimerization. Mol. Neurobiol. 2016, 53, 2003–2015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fuxe, K.; Borroto-Escuela, D.O.; Romero-Fernandez, W.; Tarakanov, A.O.; Calvo, F.; Garriga, P.; Tena, M.; Narvaez, M.; Millón, C.; Parrado, C.; et al. On the existence and function of galanin receptor heteromers in the central nervous system. Front. Endocrinol. 2012, 3, 127. [Google Scholar] [CrossRef] [PubMed]
- Millón, C.; Flores-Burgess, A.; Narváez, M.; Borroto-Escuela, D.O.; Santín, L.; Gago, B.; Narváez, J.A.; Fuxe, K.; Díaz-Cabiale, Z. Galanin (1-15) enhances the antidepressant effects of the 5-HT1A receptor agonist 8-OH-DPAT: Involvement of the raphe-hippocampal 5-HT neuron system. Brain Struct. Funct. 2016, 221, 4491–4504. [Google Scholar] [CrossRef]
- Bridges, R.S. Neuroendocrine regulation of maternal behavior. Front. Neuroendocr. 2015, 36, 178–196. [Google Scholar] [CrossRef]
- Keller, D.; Tsuda, M.C.; Usdin, T.B.; Dobolyi, A. Behavioural actions of tuberoinfundibular peptide 39 (parathyroid hormone 2). J. Neuroendocrinol. 2022, 34, e13130. [Google Scholar] [CrossRef]
- Dobolyi, A.; Oláh, S.; Keller, D.; Kumari, R.; Fazekas, E.A.; Csikós, V.; Renner, É.; Cservenák, M. Secretion and Function of Pituitary Prolactin in Evolutionary Perspective. Front. Neurosci. 2020, 14, 621. [Google Scholar] [CrossRef]
- Costa-Brito, A.R.; Gonçalves, I.; Santos, C.R.A. The brain as a source and a target of prolactin in mammals. Neural Regen. Res. 2022, 17, 1695–1702. [Google Scholar] [CrossRef]
- Herman, A.; Bignon, C.; Daniel, N.; Grosclaude, J.; Gertler, A.; Djiane, J. Functional heterodimerization of prolactin and growth hormone receptors by ovine placental lactogen. J. Biol. Chem. 2000, 275, 6295–6301. [Google Scholar] [CrossRef]
- Vermani, B.; Mukherjee, S.; Kumar, G.; Patnaik, R. Prolactin attenuates global cerebral ischemic injury in rat model by conferring neuroprotection. Brain Inj. 2020, 34, 685–693. [Google Scholar] [CrossRef]
- Bernard, V.; Young, J.; Chanson, P.; Binart, N. New insights in prolactin: Pathological implications. Nat. Rev. Endocrinol. 2015, 11, 265–275. [Google Scholar] [CrossRef]
- Costa-Brito, A.R.; Quintela, T.; Gonçalves, I.; Duarte, A.C.; Costa, A.R.; Arosa, F.A.; Cavaco, J.E.; Lemos, M.C.; Santos, C.R.A. The choroid plexus is an alternative source of prolactin to the rat brain. Mol. Neurobiol. 2021, 58, 1846–1858. [Google Scholar] [CrossRef]
- Buhimschi, C.S. Endocrinology of lactation. Obstet. Gynecol. Clin. N. Am. 2004, 31, 963–979, xii. [Google Scholar] [CrossRef]
- Bole-Feysot, C.; Goffin, V.; Edery, M.; Binart, N.; Kelly, P.A. Prolactin (PRL) and its receptor: Actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 1998, 19, 225–268. [Google Scholar] [CrossRef]
- Finidori, J.; Kelly, P.A. Cytokine receptor signaling through two novel families of transducer molecules: Janus kinases, and signal transducers and activators of transcription. J. Endocrinol. 1995, 147, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.S.; Kraemer, G.W. Molecular and genetic bases of mammalian maternal behavior. Gend. Genome 2019, 3, 247028971982730. [Google Scholar] [CrossRef]
- Hennighausen, L.; Robinson, G.W. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008, 22, 711–721. [Google Scholar] [CrossRef]
- Liu, X.; Robinson, G.W.; Wagner, K.U.; Garrett, L.; Wynshaw-Boris, A.; Hennighausen, L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997, 11, 179–186. [Google Scholar] [CrossRef]
- Yip, S.H.; Eguchi, R.; Grattan, D.R.; Bunn, S.J. Prolactin signalling in the mouse hypothalamus is primarily mediated by signal transducer and activator of transcription factor 5b but not 5a. J. Neuroendocrinol. 2012, 24, 1484–1491. [Google Scholar] [CrossRef]
- de Dios, N.; Orrillo, S.; Irizarri, M.; Theas, M.S.; Boutillon, F.; Candolfi, M.; Seilicovich, A.; Goffin, V.; Pisera, D.; Ferraris, J. JAK2/STAT5 Pathway Mediates Prolactin-Induced Apoptosis of Lactotropes. Neuroendocrinology 2019, 108, 84–97. [Google Scholar] [CrossRef]
- Brown, R.S.; Kokay, I.C.; Phillipps, H.R.; Yip, S.H.; Gustafson, P.; Wyatt, A.; Larsen, C.M.; Knowles, P.; Ladyman, S.R.; LeTissier, P.; et al. Conditional deletion of the prolactin receptor reveals functional subpopulations of dopamine neurons in the arcuate nucleus of the hypothalamus. J. Neurosci. 2016, 36, 9173–9185. [Google Scholar] [CrossRef]
- Lyons, D.J.; Hellysaz, A.; Broberger, C. Prolactin regulates tuberoinfundibular dopamine neuron discharge pattern: Novel feedback control mechanisms in the lactotrophic axis. J. Neurosci. 2012, 32, 8074–8083. [Google Scholar] [CrossRef]
- Georgescu, T.; Ladyman, S.R.; Brown, R.S.E.; Grattan, D.R. Acute effects of prolactin on hypothalamic prolactin receptor expressing neurons in the mouse. J. Neuroendocr. 2020, 32, 12908. [Google Scholar] [CrossRef]
- Brown, R.S.E.; Piet, R.; Herbison, A.E.; Grattan, D.R. Differential actions of prolactin on electrical activity and intracellular signal transduction in hypothalamic neurons. Endocrinology 2012, 153, 2375–2384. [Google Scholar] [CrossRef]
- Romanò, N.; Yip, S.H.; Hodson, D.J.; Guillou, A.; Parnaudeau, S.; Kirk, S.; Tronche, F.; Bonnefont, X.; Le Tissier, P.; Bunn, S.J.; et al. Plasticity of Hypothalamic Dopamine Neurons during Lactation Results in Dissociation of Electrical Activity and Release. J. Neurosci. 2013, 33, 4424–4433. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.J.; Ruparel, S.B.; Henry, M.A.; Akopian, A.N. Prolactin regulates TRPV1, TRPA1, and TRPM8 in sensory neurons in a sex-dependent manner: Contribution of prolactin receptor to inflammatory pain. Am. J. Physiol. Endocrinol. Metab. 2013, 305, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.J.; Henry, M.A.; Akopian, A.N. Prolactin receptor in regulation of neuronal excitability and channels. Channels 2014, 8, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Buonfiglio, D.C.; Ramos-Lobo, A.M.; Silveira, M.A.; Furigo, I.C.; Hennighausen, L.; Frazão, R.; Donato, J., Jr. Neuronal STAT5 signaling is required for maintaining lactation but not for postpartum maternal behaviors in mice. Horm. Behav. 2015, 71, 60–68. [Google Scholar] [CrossRef]
- Augustine, R.A.; Ladyman, S.R.; Bouwer, G.T.; Alyousif, Y.; Sapsford, T.J.; Scott, V.; Kokay, I.C.; Grattan, D.R.; Brown, C.H. Prolactin regulation of oxytocin neuron activity in pregnancy and lactation. J. Physiol. 2017, 595, 3591–3605. [Google Scholar] [CrossRef]
- Lucas, B.K.; Ormandy, C.J.; Binart, N.; Bridges, R.S.; Kelly, P.A. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology 1998, 139, 4102–4107. [Google Scholar] [CrossRef]
- Bridges, R.S.; Numan, M.; Ronsheim, P.M.; Mann, P.E.; Lupini, C.E. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc. Natl. Acad. Sci. USA 1990, 87, 8003–8007. [Google Scholar] [CrossRef] [PubMed]
- Bridges, R.S.; Robertson, M.C.; Shiu, R.P.C.; Sturgis, J.D.; Henriquez, B.M.; Mann, P.E. Central lactogenic regulation of maternal behavior in rats: Steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen I. Endocrinology 1997, 138, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Zarrow, M.X.; Gandelman, R.; Denenberg, V.H. Lack of nest building and maternal behavior in the mouse following olfactory bulb removal. Horm. Behav. 1971, 2, 227–238. [Google Scholar] [CrossRef]
- Li, X.-Y.; Han, Y.; Zhang, W.; Wang, S.-R.; Wei, Y.-C.; Li, S.-S.; Lin, J.-K.; Yan, J.-J.; Chen, A.-X.; Zhang, X.; et al. AGRP neurons project to the medial preoptic area and modulate maternal nest-building. J. Neurosci. 2019, 39, 456–471. [Google Scholar] [CrossRef]
- Anderson, M.V.; Rutherford, M.D. Evidence of a nesting psychology during human pregnancy. Evol. Hum. Behav. 2013, 34, 390–397. [Google Scholar] [CrossRef]
- Aguilera, G.; Liu, Y. The molecular physiology of CRH neurons. Front. Neuroendocr. 2012, 33, 67–84. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Tasker, J.G.; Herman, J.P. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress 2011, 14, 398–406. [Google Scholar] [CrossRef]
- Familari, M.; Smith, A.I.; Smith, R.; Funder, J.W. Arginine vasopressin is a much more potent stimulus to ACTH release from ovine anterior pituitary cells than ovine corticotropin-releasing factor. 1. In vitro studies. Neuroendocrinology 1989, 50, 152–157. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Engeland, W.C. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology 2002, 76, 79–92. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Figueiredo, H.F.; Ostrander, M.M.; Choi, D.C.; Engeland, W.C.; Herman, J.P. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E965–E973. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Engeland, W.C.; Ehrhart-Bornstein, M.; Herman, J.P. Dissociation of ACTH and glucocorticoids. Trends Endocrinol. Metab. 2008, 19, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Packard, A.E.B.; Egan, A.E.; Ulrich-Lai, Y.M. HPA Axis Interactions with Behavioral Systems. Compr. Physiol. 2016, 6, 1897–1934. [Google Scholar] [CrossRef] [PubMed]
- Klampfl, S.M.; Neumann, I.D.; Bosch, O.J. Reduced brain corticotropin-releasing factor receptor activation is required for adequate maternal care and maternal aggression in lactating rats. Eur. J. Neurosci. 2013, 38, 2742–2750. [Google Scholar] [CrossRef]
- Altemus, M.; Deuster, P.A.; Galliven, E.; Carter, C.S.; Gold, P.W. Suppression of hypothalamic-pituitary-adrenal axis responses to stress in lactating women. J. Clin. Endocrinol. Metab. 1995, 80, 2954–2959. [Google Scholar] [CrossRef] [PubMed]
- Hillerer, K.M.; Reber, S.O.; Neumann, I.D.; Slattery, D.A. Exposure to chronic pregnancy stress reverses peripartum-associated adaptations: Implications for postpartum anxiety and mood disorders. Endocrinology 2011, 152, 3930–3940. [Google Scholar] [CrossRef]
- Walsh, K.; McCormack, C.A.; Webster, R.; Pinto, A.; Lee, S.; Feng, T.; Krakovsky, H.S.; O’Grady, S.M.; Tycko, B.; Champagne, F.A.; et al. Maternal prenatal stress phenotypes associate with fetal neurodevelopment and birth outcomes. Proc. Natl. Acad. Sci. USA 2019, 116, 23996–24005. [Google Scholar] [CrossRef]
- Jafari, Z.; Mehla, J.; Kolb, B.E.; Mohajerani, M.H. Prenatal noise stress impairs HPA axis and cognitive performance in mice. Sci. Rep. 2017, 7, 10560. [Google Scholar] [CrossRef]
- Possamai-Della, T.; Cararo, J.H.; Aguiar-Geraldo, J.M.; Peper-Nascimento, J.; Zugno, A.I.; Fries, G.R.; Quevedo, J.; Valvassori, S.S. Prenatal Stress Induces Long-Term Behavioral Sex-Dependent Changes in Rats Offspring: The Role of the HPA Axis and Epigenetics. Mol. Neurobiol. 2023, 60, 5013–5033. [Google Scholar] [CrossRef]
- Zietlow, A.L.; Nonnenmacher, N.; Reck, C.; Ditzen, B.; Müller, M. Emotional stress during pregnancy—Associations with maternal anxiety disorders, infant cortisol reactivity, and mother-child interaction at pre-school age. Front. Psychol. 2019, 10, 2179. [Google Scholar] [CrossRef]
- Shanks, N.; Windle, R.J.; Perks, P.; Wood, S.; Ingram, C.D.; Lightman, S.L. The hypothalamic-pituitary-adrenal axis response to endotoxin is attenuated during lactation. J. Neuroendocr. 1999, 11, 857–865. [Google Scholar] [CrossRef]
- De Guzman, R.M.; Rosinger, Z.J.; Parra, K.E.; Jacobskind, J.S.; Justice, N.J.; Zuloaga, D.G. Alterations in corticotropin-releasing factor receptor type 1 in the preoptic area and hypothalamus in mice during the postpartum period. Horm. Behav. 2021, 135, 105044. [Google Scholar] [CrossRef]
- Klampfl, S.M.; Schramm, M.M.; Gaßner, B.M.; Hübner, K.; Seasholtz, A.F.; Brunton, P.J.; Bosch, O.J. Maternal stress and the MPOA: Activation of CRF receptor 1 impairs maternal behavior and triggers local oxytocin release in lactating rats. Neuropharmacology 2018, 133, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Gammie, S.C.; Bethea, E.D.; Stevenson, S.A. Altered maternal profiles in corticotropin-releasing factor receptor 1 deficient mice. BMC Neurosci. 2007, 8, 17. [Google Scholar] [CrossRef]
- Gammie, S.C.; Seasholtz, A.F.; Stevenson, S.A. Deletion of corticotropin-releasing factor binding protein selectively impairs maternal, but not intermale aggression. Neuroscience 2008, 157, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Gelman, P.L.; Flores-Ramos, M.; López-Martínez, M.; Fuentes, C.C.; Grajeda, J.P. Hypothalamic-pituitary-adrenal axis function during perinatal depression. Neurosci. Bull. 2015, 31, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, P.; Bunn, S.J.; Grattan, D.R. The role of prolactin in the suppression of Crh mRNA expression during pregnancy and lactation in the mouse. J. Neuroendocr. 2017, 29, 12511. [Google Scholar] [CrossRef]
- Weber, R.F.A.; Calogero, A.E. Prolactin stimulates rat hypothalamic corticotropin-releasing hormone and pituitary adrenocorticotropin secretion in vitro. Neuroendocrinology 1991, 54, 248–253. [Google Scholar] [CrossRef]
- Torner, L.; Toschi, N.; Pohlinger, A.; Landgraf, R.; Neumann, I.D. Anxiolytic and anti-stress effects of brain prolactin: Improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J. Neurosci. 2001, 21, 3207–3214. [Google Scholar] [CrossRef]
- Donner, N.; Bredewold, R.; Maloumby, R.; Neumann, I.D. Chronic intracerebral prolactin attenuates neuronal stress circuitries in virgin rats. Eur. J. Neurosci. 2007, 25, 1804–1814. [Google Scholar] [CrossRef]
- Gustafson, P.; Kokay, I.; Sapsford, T.; Bunn, S.; Grattan, D. Prolactin regulation of the HPA axis is not mediated by a direct action upon CRH neurons: Evidence from the rat and mouse. Brain Struct. Funct. 2017, 222, 3191–3204. [Google Scholar] [CrossRef]
- Torner, L.; Toschi, N.; Nava, G.; Clapp, C.; Neumann, I.D. Increased hypothalamic expression of prolactin in lactation: Involvement in behavioural and neuroendocrine stress responses. Eur. J. Neurosci. 2002, 15, 1381–1389. [Google Scholar] [CrossRef]
- Dunkel Schetter, C.; Tanner, L. Anxiety, depression and stress in pregnancy: Implications for mothers, children, research, and practice. Curr. Opin. Psychiatry 2012, 25, 141–148. [Google Scholar] [CrossRef]
- Reck, C.; Tietz, A.; Müller, M.; Seibold, K.; Tronick, E. The impact of maternal anxiety disorder on mother-infant interaction in the postpartum period. PLoS ONE 2018, 13, e0194763. [Google Scholar] [CrossRef]
- Buijs, R.M.; Swaab, D.F.; Dogterom, J.; van Leeuwen, F.W. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978, 192, 423–435. [Google Scholar] [CrossRef]
- Menon, R.; Grund, T.; Zoicas, I.; Althammer, F.; Fiedler, D.; Biermeier, V.; Bosch, O.J.; Hiraoka, Y.; Nishimori, K.; Eliava, M.; et al. Oxytocin signaling in the lateral septum prevents social fear during lactation. Curr. Biol. 2018, 28, 1066–1078. [Google Scholar] [CrossRef]
- Kendrick, K.M.; Keverne, E.B.; Hinton, M.R.; Goode, J.A. Oxytocin, amino acid and monoamine release in the region of the medial preoptic area and bed nucleus of the stria terminalis of the sheep during parturition and suckling. Brain Res. 1992, 569, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Neumann, I.; Ludwig, M.; Engelmann, M.; Pittman, Q.J.; Landgraf, R. Simultaneous microdialysis in blood and brain: Oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology 1993, 58, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Smith, E.J.; Holmes, N.M.; Lingawi, N.W.; Panayi, M.C.; Westbrook, R.F. Oxytocin signaling in basolateral and central amygdala nuclei differentially regulates the acquisition, expression, and extinction of context-conditioned fear in rats. Learn. Mem. 2015, 22, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Young, L.J. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm. Behav. 2016, 77, 98–112. [Google Scholar] [CrossRef]
- Caughey, S.D.; Klampfl, S.M.; Bishop, V.R.; Pfoertsch, J.; Neumann, I.D.; Bosch, O.J.; Meddle, S.L. Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. J. Neuroendocr. 2011, 23, 1113–1124. [Google Scholar] [CrossRef]
- Meddle, S.L.; Bishop, V.R.; Gkoumassi, E.; van Leeuwen, F.W.; Douglas, A.J. Dynamic changes in oxytocin receptor expression and activation at parturition in the rat brain. Endocrinology 2007, 148, 5095–5104. [Google Scholar] [CrossRef]
- Zimmermann-Peruzatto, J.M.; Lazzari, V.M.; Agnes, G.; Becker, R.O.; de Moura, A.C.; Guedes, R.P.; Lucion, A.B.; Almeida, S.; Giovenardi, M. The impact of oxytocin gene knockout on sexual behavior and gene expression related to neuroendocrine systems in the brain of female mice. Cell Mol. Neurobiol. 2017, 37, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Jurek, B.; Slattery, D.A.; Maloumby, R.; Hillerer, K.; Koszinowski, S.; Neumann, I.D.; van den Burg, E.H. Differential contribution of hypothalamic MAPK activity to anxiety-like behaviour in virgin and lactating rats. PLoS ONE 2012, 7, e37060. [Google Scholar] [CrossRef] [PubMed]
- Slattery, D.A.; Neumann, I.D. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J. Physiol. 2008, 586, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.A.; Prange, A.J., Jr. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl. Acad. Sci. USA 1979, 76, 6661–6665. [Google Scholar] [CrossRef]
- Neumann, I.; Landgraf, R. Septal and hippocampal release of oxytocin, but not vasopressin, in the conscious lactating rat during suckling. J. Neuroendocr. 1989, 1, 305–308. [Google Scholar] [CrossRef]
- Tomizawa, K.; Iga, N.; Lu, Y.F.; Moriwaki, A.; Matsushita, M.; Li, S.T.; Miyamoto, O.; Itano, T.; Matsui, H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat. Neurosci. 2003, 6, 384–390. [Google Scholar] [CrossRef]
- Marlin, B.J.; Mitre, M.; D’amour, J.A.; Chao, M.V.; Froemke, R.C. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 2015, 520, 499–504. [Google Scholar] [CrossRef]
- Rich, M.E.; deCárdenas, E.J.; Lee, H.J.; Caldwell, H.K. Impairments in the initiation of maternal behavior in oxytocin receptor knockout mice. PLoS ONE 2014, 9, e98839. [Google Scholar] [CrossRef]
- Pedersen, C.A.; Vadlamudi, S.V.; Boccia, M.L.; Amico, J.A. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 2006, 5, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Sabihi, S.; Durosko, N.E.; Dong, S.M.; Leuner, B. Oxytocin in the prelimbic medial prefrontal cortex reduces anxiety-like behavior in female and male rats. Psychoneuroendocrinology 2014, 45, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Sabihi, S.; Dong, S.M.; Durosko, N.E.; Leuner, B. Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front. Behav. Neurosci. 2014, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Šípková, J.; Kramáriková, I.; Hynie, S.; Klenerová, V. The galanin and galanin receptor subtypes, its regulatory role in the biological and pathological functions. Physiol. Res. 2017, 66, 729–740. [Google Scholar] [CrossRef]
- Khoshbouei, H.; Cecchi, M.; Dove, S.; Javors, M.; Morilak, D.A. Behavioral reactivity to stress: Amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol. Biochem. Behav. 2002, 71, 407–417. [Google Scholar] [CrossRef]
- Swanson, C.J.; Blackburn, T.P.; Zhang, X.; Zheng, K.; Xu, Z.-Q.D.; Hökfelt, T.; Wolinsky, T.D.; Konkel, M.J.; Chen, H.; Zhong, H.; et al. Anxiolytic- and antidepressant-like profiles of the galanin-3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299. Proc. Natl. Acad. Sci. USA 2005, 102, 17489–17494. [Google Scholar] [CrossRef]
- Kuteeva, E.; Hökfelt, T.; Wardi, T.; Ogren, S.O. Galanin, galanin receptor subtypes and depression-like behaviour. Cell Mol. Life Sci. 2008, 65, 1854–1863. [Google Scholar] [CrossRef]
- Saar, I.; Lahe, J.; Langel, K.; Runesson, J.; Webling, K.; Järv, J.; Rytkönen, J.; Närvänen, A.; Bartfai, T.; Kurrikoff, K.; et al. Novel systemically active galanin receptor 2 ligands in depression-like behavior. J. Neurochem. 2013, 127, 114–123. [Google Scholar] [CrossRef]
- Le Maître, T.W.; Xia, S.; Le Maitre, E.; Dun, X.P.; Lu, J.; Theodorsson, E.; Ogren, S.O.; Hökfelt, T.; Xu, Z.Q. Galanin receptor 2 overexpressing mice display an antidepressive-like phenotype: Possible involvement of the subiculum. Neuroscience 2011, 190, 270–288. [Google Scholar] [CrossRef]
- Zhao, X.; Seese, R.R.; Yun, K.; Peng, T.; Wang, Z. The role of galanin system in modulating depression, anxiety, and addiction-like behaviors after chronic restraint stress. Neuroscience 2013, 246, 82–93. [Google Scholar] [CrossRef]
- Keszler, G.; Molnár, Z.; Rónai, Z.; Sasvári-Székely, M.; Székely, A.; Kótyuk, E. Association between anxiety and non-coding genetic variants of the galanin neuropeptide. PLoS ONE 2019, 14, e0226228. [Google Scholar] [CrossRef]
- Yun, S.; Reyes-Alcaraz, A.; Lee, Y.-N.; Yong, H.J.; Choi, J.; Ham, B.-J.; Sohn, J.-W.; Son, G.H.; Kim, H.; Kwon, S.-G.; et al. Spexin-Based Galanin Receptor Type 2 Agonist for Comorbid Mood Disorders and Abnormal Body Weight. Front. Neurosci. 2019, 13, 391. [Google Scholar] [CrossRef]
- Dennis, C.L.; Falah-Hassani, K.; Shiri, R. Prevalence of antenatal and postnatal anxiety: Systematic review and meta-analysis. Br. J. Psychiatry 2017, 210, 315–323. [Google Scholar] [CrossRef]
- O’Hara, M.W.; McCabe, J.E. Postpartum depression: Current status and future directions. Annu. Rev. Clin. Psychol. 2013, 9, 379–407. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Slattery, D.A.; Neumann, I.D. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology 2010, 58, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Williams, K.; Birkett, S.; Nicholson, H.; Glue, P.; Nutt, D.J. Neuroendocrine and clinical effects of electroconvulsive therapy and their relationship to treatment outcome. Psychol. Med. 1994, 24, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Cyranowski, J.M.; Hofkens, T.L.; Frank, E.; Seltman, H.; Cai, H.M.; Amico, J.A. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosom. Med. 2008, 70, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Bendix, M.; Uvnäs-Moberg, K.; Petersson, M.; Gustavsson, P.; Svanborg, P.; Åsberg, M.; Jokinen, J. Plasma oxytocin and personality traits in psychiatric outpatients. Psychoneuroendocrinology 2015, 57, 102–110. [Google Scholar] [CrossRef]
- Bell, C.J.; Nicholson, H.; Mulder, R.T.; Luty, S.E.; Joyce, P.R. Plasma oxytocin levels in depression and their correlation with the temperament dimension of reward dependence. J. Psychopharmacol. 2006, 20, 656–660. [Google Scholar] [CrossRef]
- Purba, J.S.; Hoogendijk, W.J.; Hofman, M.A.; Swaab, D.F. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch. Gen. Psychiatry 1996, 53, 137–143. [Google Scholar] [CrossRef]
- Meynen, G.; Unmehopa, U.A.; Hofman, M.A.; Swaab, D.F.; Hoogendijk, W.J. Hypothalamic oxytocin mRNA expression and melancholic depression. Mol. Psychiatry 2007, 12, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Kroll-Desrosiers, A.R.; Nephew, B.C.; Babb, J.A.; Guilarte-Walker, Y.; Moore Simas, T.A.; Deligiannidis, K.M. Association of peripartum synthetic oxytocin administration and depressive and anxiety disorders within the first postpartum year. Depress. Anxiety 2017, 34, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Riem, M.M.E.; Loheide-Niesmann, L.; Beijers, R.; Tendolkar, I.; Mulders, P.C. Boosting oxytocin in postpartum depression: Intranasal oxytocin enhances maternal positive affect and regard for the infant. Psychoneuroendocrinology 2025, 179, 107530. [Google Scholar] [CrossRef] [PubMed]
- Morales-Medina, J.C.; Dumont, Y.; Benoit, C.E.; Bastianetto, S.; Flores, G.; Fournier, A.; Quirion, R. Role of neuropeptide Y Y1 and Y2 receptors on behavioral despair in a rat model of depression with co-morbid anxiety. Neuropharmacology 2012, 62, 200–208. [Google Scholar] [CrossRef]
- Cohen, H.; Liu, T.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Mathé, A.A. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 2012, 37, 350–363. [Google Scholar] [CrossRef]
- Zambello, E.; Zanetti, L.; Hédou, G.F.; Angelici, O.; Arban, R.; Tasan, R.O.; Sperk, G.; Caberlotto, L. Neuropeptide Y-Y2 receptor knockout mice: Influence of genetic background on anxiety-related behaviors. Neuroscience 2011, 176, 420–430. [Google Scholar] [CrossRef]
- Walker, M.W.; Wolinsky, T.D.; Jubian, V.; Chandrasena, G.; Zhong, H.; Huang, X.; Miller, S.; Hegde, L.G.; Marsteller, D.A.; Marzabadi, M.R.; et al. The novel neuropeptide Y Y5 receptor antagonist Lu AA33810 [N-[[trans-4-[(4,5-dihydro [1]benzothiepino [5,4-d]thiazol-2-yl)amino]cyclohexyl]methyl]-methanesulfonamide] exerts anxiolytic- and antidepressant-like effects in rat models of stress sensitivity. J. Pharmacol. Exp. Ther. 2009, 328, 900–911. [Google Scholar] [CrossRef]
- Otto, C.; Martin, M.; Wolfer, D.P.; Lipp, H.P.; Maldonado, R.; Schütz, G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res. Mol. Brain Res. 2001, 92, 78–84. [Google Scholar] [CrossRef]
- Stroth, N.; Eiden, L.E. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience 2010, 165, 1025–1030. [Google Scholar] [CrossRef]
- Gaszner, B.; Kormos, V.; Kozicz, T.; Hashimoto, H.; Reglodi, D.; Helyes, Z. The behavioral phenotype of pituitary adenylate-cyclase activating polypeptide-deficient mice in anxiety and depression tests is accompanied by blunted c-Fos expression in the bed nucleus of the stria terminalis, central projecting Edinger-Westphal nucleus, ventral lateral septum, and dorsal raphe nucleus. Neuroscience 2012, 202, 283–299. [Google Scholar] [CrossRef]
- Hattori, T.; Baba, K.; Matsuzaki, S.; Honda, A.; Miyoshi, K.; Inoue, K.; Taniguchi, M.; Hashimoto, H.; Shintani, N.; Baba, A.; et al. A novel DISC1-interacting partner DISC1-Binding Zinc-finger protein: Implication in the modulation of DISC1-dependent neurite outgrowth. Mol. Psychiatry 2007, 12, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R.; Hashimoto, H.; Shintani, N.; Ohi, K.; Hori, H.; Saitoh, O.; Kosuga, A.; Tatsumi, M.; Iwata, N.; Ozaki, N.; et al. Possible association between the pituitary adenylate cyclase-activating polypeptide (PACAP) gene and major depressive disorder. Neurosci. Lett. 2010, 468, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Ressler, K.J.; Mercer, K.B.; Bradley, B.; Jovanovic, T.; Mahan, A.; Kerley, K.; Norrholm, S.D.; Kilaru, V.; Smith, A.K.; Myers, A.J.; et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 2011, 470, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Almli, L.M.; Mercer, K.B.; Kerley, K.; Feng, H.; Bradley, B.; Conneely, K.N.; Ressler, K.J. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013, 162B, 262–272. [Google Scholar] [CrossRef]
- Mitsukawa, K.; Lu, X.; Bartfai, T. Galanin, galanin receptors, and drug targets. Exp. Suppl. 2010, 102, 7–23. [Google Scholar] [CrossRef]
- Rao, S.; Yao, Y.; Zheng, C.; Ryan, J.; Mao, C.; Zhang, F.; Meyre, D.; Xu, Q. Common variants in CACNA1C and MDD susceptibility: A comprehensive meta-analysis. Am. J. Med. Genet. Part B 2016, 171, 896–903. [Google Scholar] [CrossRef]
- Unschuld, P.G.; Ising, M.; Roeske, D.; Erhardt, A.; Specht, M.; Kloiber, S.; Uhr, M.; Müller-Myhsok, B.; Holsboer, F.; Binder, E.B. Gender-specific association of galanin polymorphisms with HPA-axis dysregulation, symptom severity, and antidepressant treatment response. Neuropsychopharmacology 2010, 35, 1583–1592. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leff-Gelman, P.; Pellón-Díaz, G.; Camacho-Arroyo, I.; Palomera-Garfias, N.; Flores-Ramos, M. Neuroendocrine Regulation and Neural Circuitry of Parenthood: Integrating Neuropeptides, Brain Receptors, and Maternal Behavior. Int. J. Mol. Sci. 2025, 26, 9007. https://doi.org/10.3390/ijms26189007
Leff-Gelman P, Pellón-Díaz G, Camacho-Arroyo I, Palomera-Garfias N, Flores-Ramos M. Neuroendocrine Regulation and Neural Circuitry of Parenthood: Integrating Neuropeptides, Brain Receptors, and Maternal Behavior. International Journal of Molecular Sciences. 2025; 26(18):9007. https://doi.org/10.3390/ijms26189007
Chicago/Turabian StyleLeff-Gelman, Philippe, Gabriela Pellón-Díaz, Ignacio Camacho-Arroyo, Nadia Palomera-Garfias, and Mónica Flores-Ramos. 2025. "Neuroendocrine Regulation and Neural Circuitry of Parenthood: Integrating Neuropeptides, Brain Receptors, and Maternal Behavior" International Journal of Molecular Sciences 26, no. 18: 9007. https://doi.org/10.3390/ijms26189007
APA StyleLeff-Gelman, P., Pellón-Díaz, G., Camacho-Arroyo, I., Palomera-Garfias, N., & Flores-Ramos, M. (2025). Neuroendocrine Regulation and Neural Circuitry of Parenthood: Integrating Neuropeptides, Brain Receptors, and Maternal Behavior. International Journal of Molecular Sciences, 26(18), 9007. https://doi.org/10.3390/ijms26189007






