Ampelopsis japonica Extract Exhibited Significant Uric Acid-Lowering Effect by Downregulating URAT1/GLUT9 and Alleviates Inflammation Through TLR4/NF-κB Pathway
Abstract
1. Introduction
2. Results
2.1. Main Active Ingredient Analysis of AJE
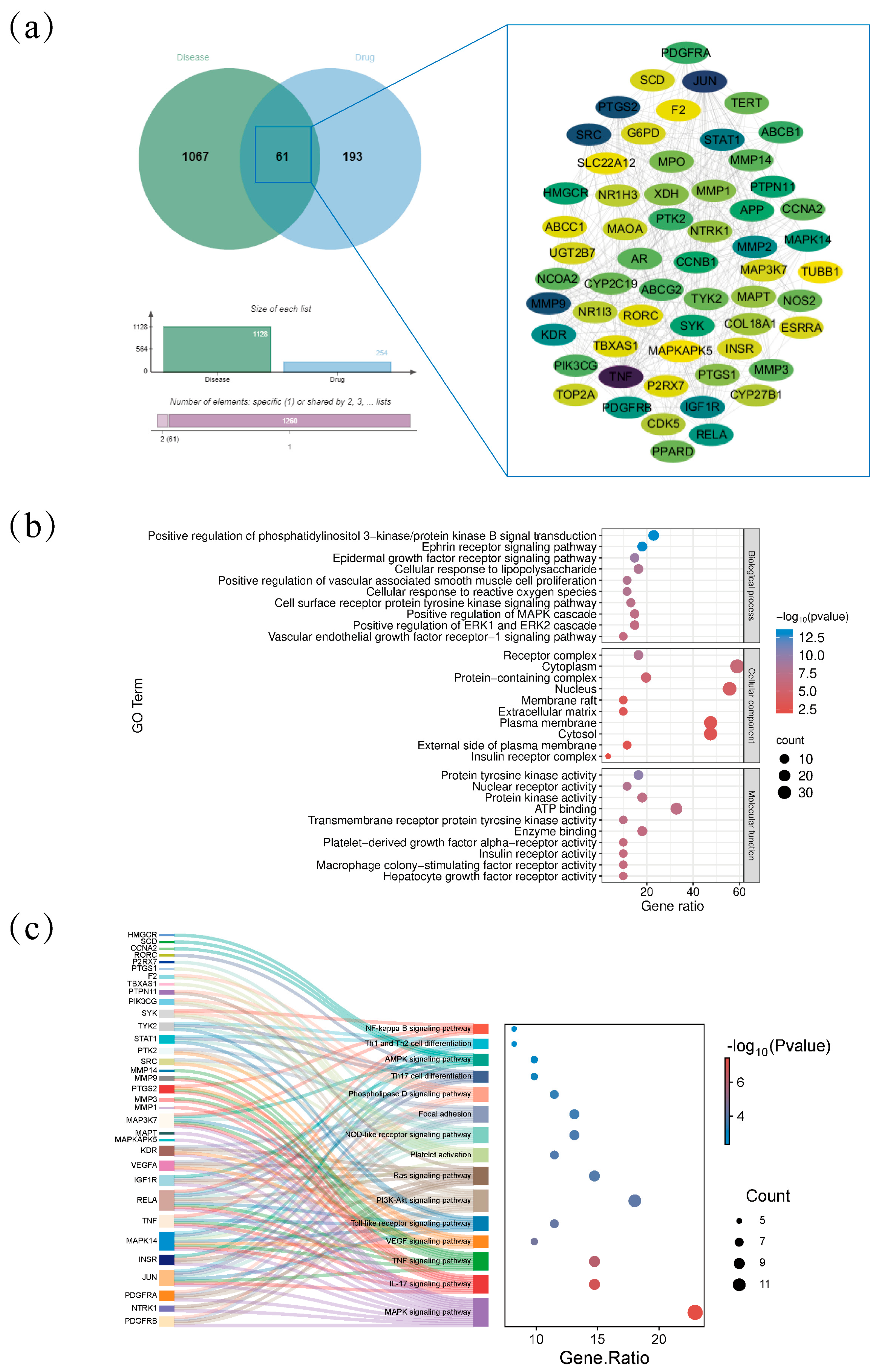
2.2. Intersection of the Targets Between AJE and Hyperuricemia
2.3. Effects of AJE on Serum UA, CRE, BUN, and Body Weight in Hyperuricemia Mice
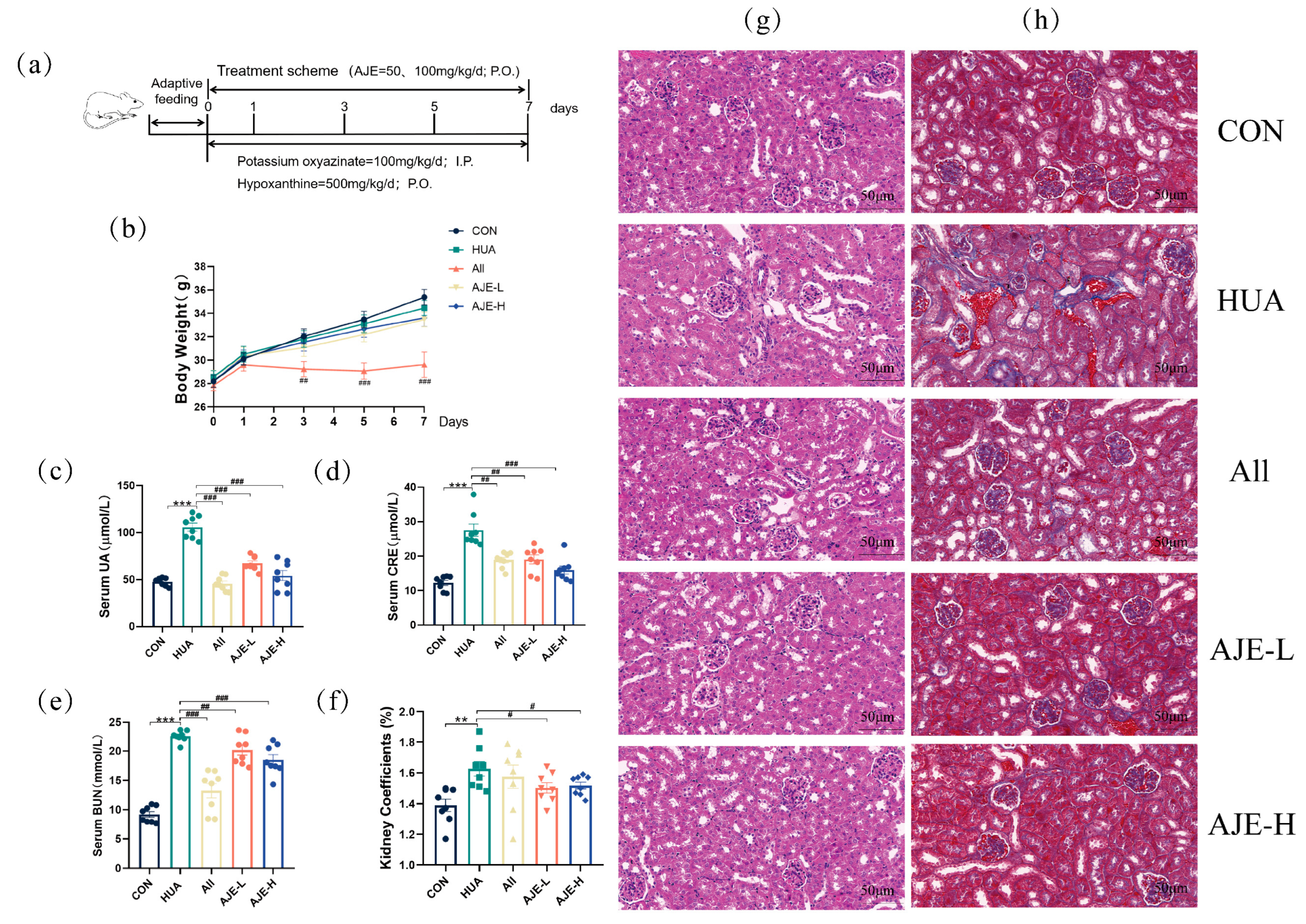
2.4. Effects of AJE on Kidney Injury in Hyperuricemia Mice
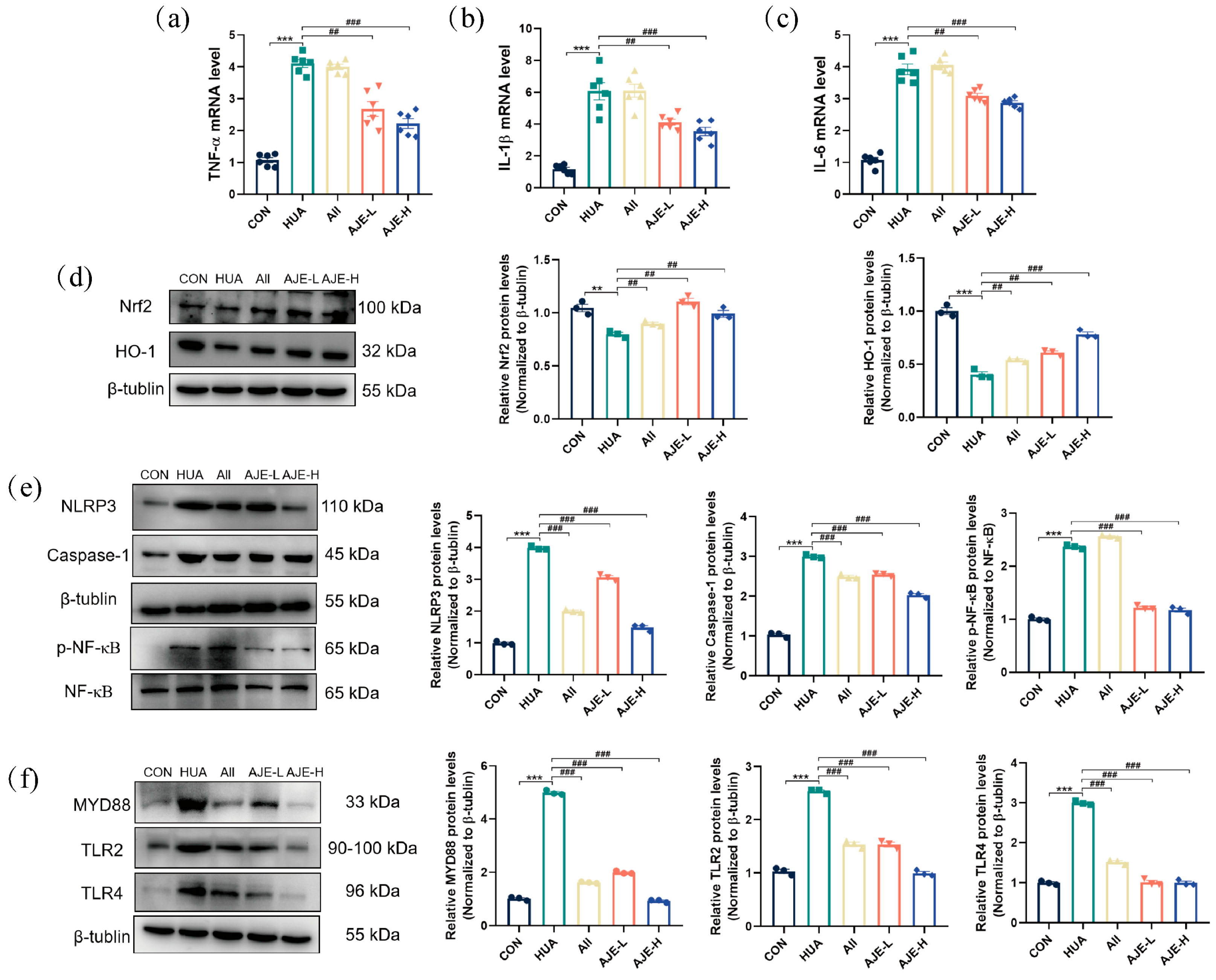
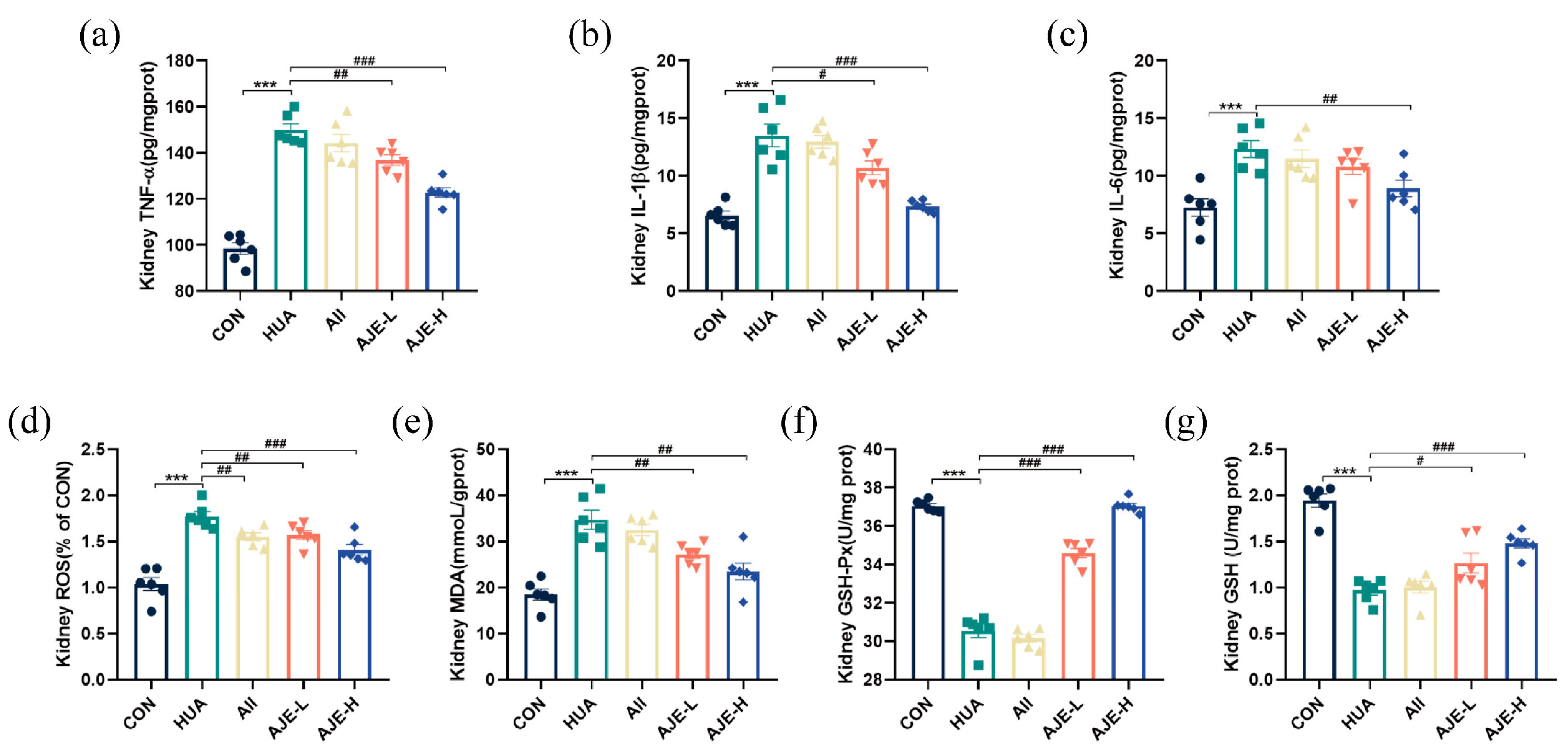
2.5. AJE Ameliorates HUA-Induced Renal Inflammation
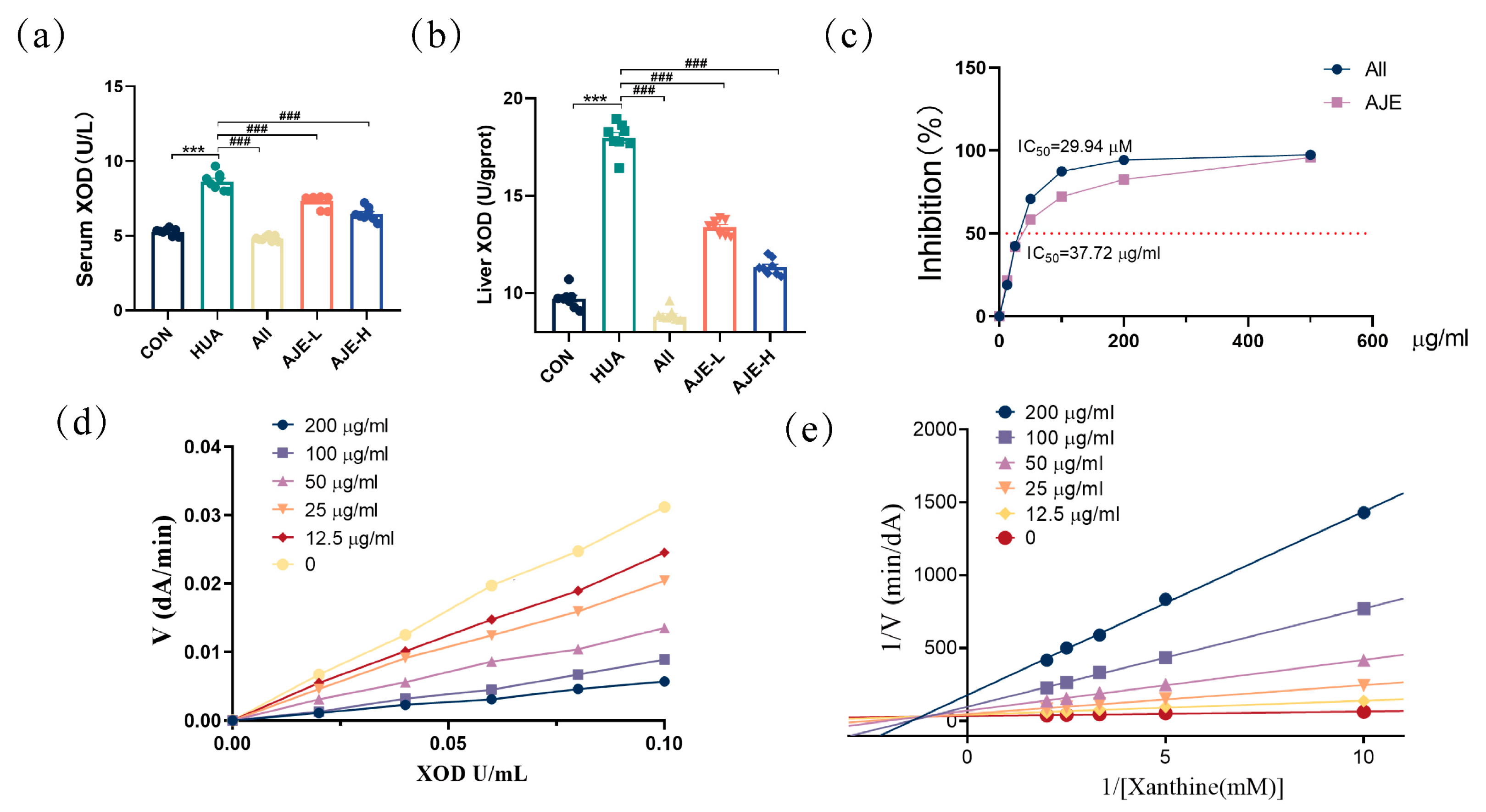
2.6. XOD Inhibitory Activity of AJE
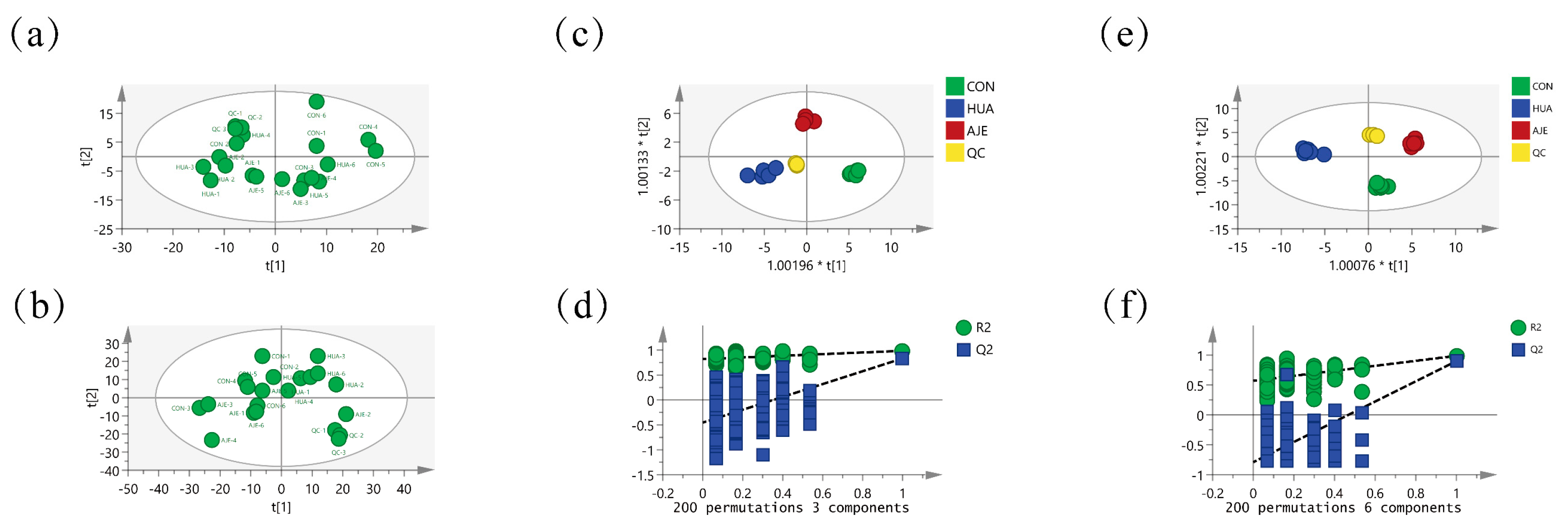
2.7. Serum Principal Component Analysis
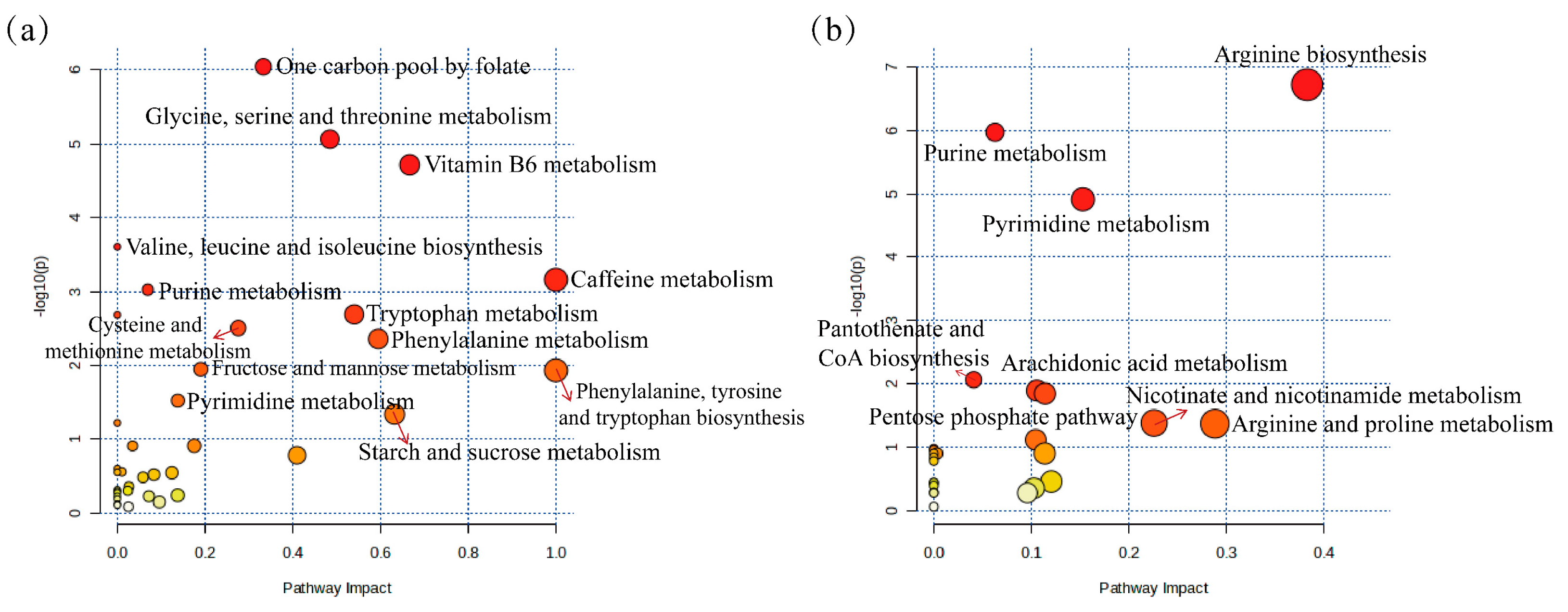
2.8. Screening and Identification of Potential Biomarkers
| No. | Metabolites | Formula | VIP | Trend | |
|---|---|---|---|---|---|
| CON vs. HUA | HUA vs. AJE | ||||
| 1 | Thymine | C5H6N2O2 | 2.11 | ↓ | ↑ |
| 2 | Deoxycytidine | C9H13N3O4 | 1.79 | ↓ | ↑ |
| 3 | Cytidine | C9H13N3O5 | 1.56 | ↓ | ↑ |
| 4 | Xanthosine | C10H12N4O6 | 2.11 | ↓ | ↑ |
| 5 | Adenine | C5H5N5 | 1.95 | ↓ | ↑ |
| 6 | Xanthine | C5H4N4O2 | 1.38 | ↓ | ↑ |
| 7 | Guanine | C5H5N5O | 1.59 | ↓ | ↑ |
| 8 | Hypoxanthine | C5H4N4O | 1.71 | ↓ | ↑ |
| 9 | Inosine | C10H12N4O5 | 2.27 | ↓ | ↑ |
| 10 | Adenosine | C10H13N5O4 | 2.22 | ↑ | ↓ |
| 11 | Urea | CH4N2O | 2.37 | ↑ | ↓ |
| 12 | Uracil | C4H4N2O2 | 2.44 | ↑ | ↓ |
| 13 | Uric acid | C5H4N4O3 | 2.44 | ↑ | ↓ |
| 14 | Leukotriene B4 | C20H32O4 | 2.18 | ↑ | ↓ |
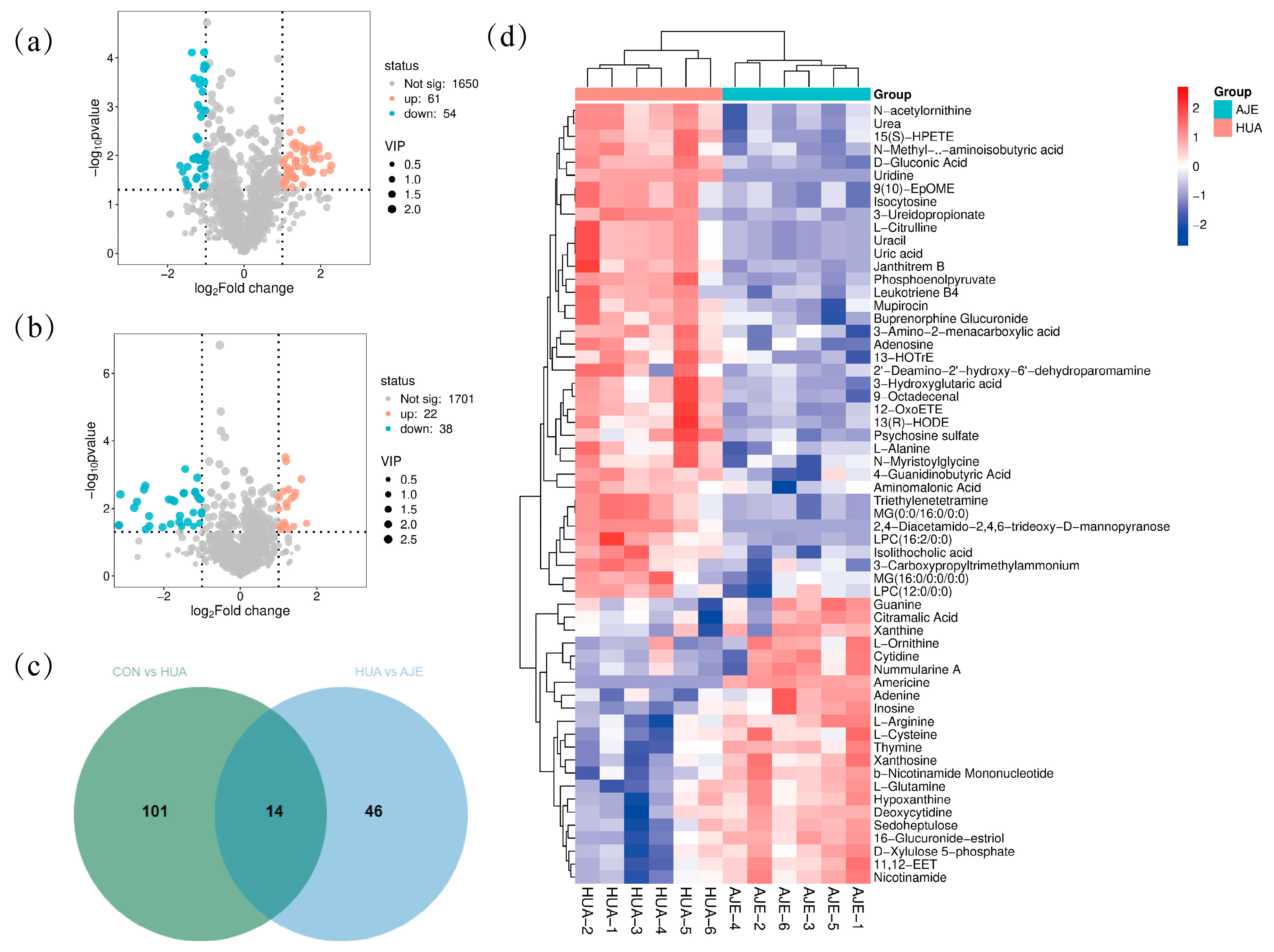
2.9. Metabolic Pathway Analysis
2.10. The Effect of AJE on the TLR4/NF-κB Pathway in the Renal Tissue of Hyperuricemia Mice
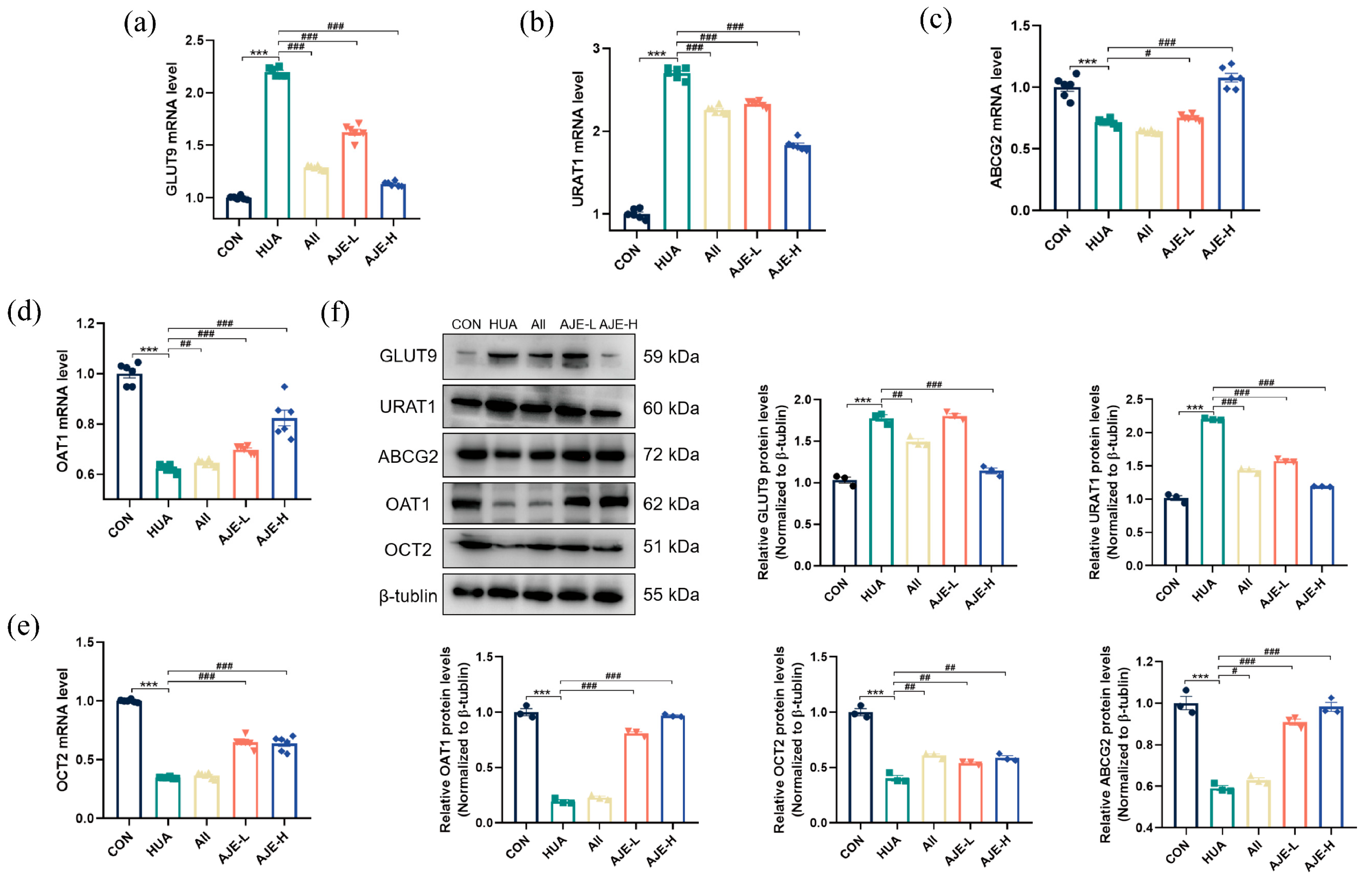
2.11. AJE Up-Regulated ABCG2 and Down-Regulated GLUT9 and URAT1
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Network Pharmacology Analysis of AJE
4.3. Animals and Prevention
4.4. Sample Collection
4.5. Histological Examination
4.6. Determination of Serum and Organ Tissues Biochemical Indicators
4.7. Determination of XOD Inhibitory Activity
4.8. Metabolomics Analysis
4.8.1. Serum Sample Preparation
4.8.2. UPLC-QTOF/MS Analysis
4.8.3. Data Processing and Multivariate Statistical Analysis
4.9. RT-qPCR Analysis
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJ | Ampelopsis japonica (Thunb.) Makino |
| AJE | Ampelopsis japonica extract |
| ABCG2 | ATP-binding cassette subfamily G member 2 |
| BUN | Blood urea nitrogen |
| Cre | Creatinine |
| GLUT9 | Glucose transporter 9 |
| HUA | Hyperuricemia |
| HX | Hypoxanthine |
| NF-κB | Nuclear factor-κB |
| OAT1 | Organic anion transporter protein 1 |
| OPLS-DA | Orthogonal partial least-squares discriminant analysis |
| PCA | Principal component analysis |
| PO | Potassium oxonate |
| TLR4 | Toll-like receptor 4 |
| UA | Uric acid |
| URAT1 | Urate transporter 1 |
| UPLC-QTOF/MS | Ultra-performance liquid chromatography-quadruple time-of-flight/mass spectrometry |
| XOD | Xanthine oxidase |
| VIP | Variable importance projection |
References
- Lian, Y.; Fu, G.; Liang, X.; He, X.; Xu, J.; Fan, H.; Wan, Y. Combination of Artemisia selengensis Turcz Leaves Polysaccharides and Dicaffeoylquinic Acids Could Be a Potential Inhibitor for Hyperuricemia. Int. J. Biol. Macromol. 2024, 271, 132687. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shen, J.; Chen, X.; Dawuti, T.; Xiao, H. Evaluating Renal Injury Characteristics in Different Rat Models of Hyperuricemia and Elucidating Pathological Molecular Mechanisms via Serum Metabolomics. Front. Pharmacol. 2024, 15, 1433991. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, X.-X.; Zhao, S.-L.; Ishii, Y.; Yu, B.-Y.; Li, R.-S. Supplements Extracted from Lophatherum gracile Brongn. Ameliorates Hyperuricemia by Regulating Nucleotide Metabolic Enzymes and Urate Transporters. Food Med. Homol. 2025. [Google Scholar] [CrossRef]
- Sun, L.; Ni, C.; Zhao, J.; Wang, G.; Chen, W. Probiotics, Bioactive Compounds and Dietary Patterns for the Effective Management of Hyperuricemia: A Review. Crit. Rev. Food Sci. Nutr. 2024, 64, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, S.; Li, K.; Wu, G.; Zheng, X.; Zheng, J.; Lu, X.; Zhang, L.; Li, J.; Su, Z.; et al. Coptisine Protects against Hyperuricemic Nephropathy through Alleviating Inflammation, Oxidative Stress and Mitochondrial Apoptosis via PI3K/Akt Signaling Pathway. Biomed. Pharmacother. 2022, 156, 113941. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, L.; Hu, S.; Gan, R.; Zeng, L. The Chemistry, Processing, and Preclinical Anti-Hyperuricemia Potential of Tea: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 7065–7090. [Google Scholar] [CrossRef]
- Terkeltaub, R. Emerging Urate-Lowering Drugs and Pharmacologic Treatment Strategies for Gout: A Narrative Review. Drugs 2023, 83, 1501–1521. [Google Scholar] [CrossRef]
- Zeng, L.; Deng, Y.; Zhou, X.; Ji, S.; Peng, B.; Lu, H.; He, Q.; Bi, J.; Kwan, H.Y.; Zhou, L.; et al. Simiao Pills Alleviates Renal Injury Associated with Hyperuricemia: A Multi-Omics Analysis. J. Ethnopharmacol. 2024, 333, 118492. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, S.; Chen, L.; Lu, J.; Zhao, F.; Long, T.; Wen, J.; Huang, J.; Mao, Y.; Qi, Z.; et al. In Vivo Anti-Hyperuricemia and Anti-Gouty Arthritis Effects of the Ethanol Extract from Amomumvillosum Lour. Biomed. Pharmacother. 2023, 161, 114532. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Z.; Chen, H.; Zhang, S.; Bao, Y.; Xie, Z.; Ke, J.; Ye, W.; Liang, J.; Chen, J.; et al. Salinomycin, a Potent Inhibitor of XOD and URAT1, Ameliorates Hyperuricemic Nephropathy by Activating NRF2, Modulating the Gut Microbiota, and Promoting SCFA Production. Chem.-Biol. Interact. 2024, 403, 111220. [Google Scholar] [CrossRef]
- Du, J.; Wang, N.; Yu, D.; He, P.; Gao, Y.; Tu, Y.; Li, Y. Data Mining-Guided Alleviation of Hyperuricemia by Paeonia Veitchii Lynch through Inhibition of Xanthine Oxidase and Regulation of Renal Urate Transporters. Phytomedicine 2024, 124, 155305. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, Z.; Zhu, M.; Sun, Y.; Wang, Y.; Li, B.; Wang, Q.; Kuang, H. Research Progress of Treating Hyperuricemia in Rats and Mice with Traditional Chinese Medicine. Front. Pharmacol. 2024, 15, 1428558. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Chen, X.-L.; Zhou, K.; Wang, L.-L.; Zhong, Y.-Z.; Peng, J.; Ge, B.-S.; Ho, C.-T.; Lu, C.-Y. Fucoidan Dose-Dependently Alleviated Hyperuricemia and Modulated Gut Microbiota in Mice. Food Med. Homol. 2025. [Google Scholar] [CrossRef]
- Cong, B. Perspectives in Food & Medicine Homology. Food Med. Homol. 2024, 1, 9420018. [Google Scholar] [CrossRef]
- Liang, J.-H.; Lin, H.-R.; Yang, C.-S.; Liaw, C.-C.; Wang, I.-C.; Chen, J.-J. Bioactive Components from Ampelopsis Japonica with Antioxidant, Anti-α-Glucosidase, and Antiacetylcholinesterase Activities. Antioxidants 2022, 11, 1228. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, H.; Yang, B.; Kim, S.; Jeong, H.; Kim, H. Anti-Inflammatory Effects of Ampelopsis Japonica Root on Contact Dermatitis in Mice. Chin. J. Integr. Med. 2022, 28, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ma, N.; Li, X.; Kang, M.; Guo, M.; Song, L. Application of GC/MS-Based Metabonomic Profiling in Studying the Therapeutic Effects of Aconitum Carmichaeli with Ampelopsis Japonica Extract on Collagen-Induced Arthritis in Rats. Molecules 2019, 24, 1934. [Google Scholar] [CrossRef] [PubMed]
- Nutmakul, T. A Review on Benefits of Quercetin in Hyperuricemia and Gouty Arthritis. Saudi Pharm. J. 2022, 30, 918–926. [Google Scholar] [CrossRef]
- Rabbaa, S.; Bouchab, H.; Laaziouez, Y.; Limami, Y.; Nasser, B.; Andreoletti, P.; Cherkaoui-Malki, M.; El Kebbaj, R. Argan Oil: A Natural Bioactive Lipid Modulating Oxidative Stress and Inflammation. Antioxidants 2025, 14, 515. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Yong, T.; Diao, X.; Chen, S.; Chen, D.; Xiao, C.; Zuo, D.; Xie, Y.; Zhou, X.; Hu, H. Hypouricaemic and Nephroprotective Effects of Poria cocos in Hyperuricemic Mice by up-Regulating ATP-Binding Cassette Super-Family G Member 2. Pharm. Biol. 2021, 59, 273–284. [Google Scholar] [CrossRef]
- Dhouibi, R.; Affes, H.; Salem, M.B.; Moalla, D.; Marekchi, R.; Charfi, S.; Hammami, S.; Sahnoun, Z.; Jamoussi, K.; Zeghal, K.M.; et al. Creation of an Adequate Animal Model of Hyperuricemia (Acute and Chronic Hyperuricemia); Study of Its Reversibility and Its Maintenance. Life Sci. 2021, 268, 118998. [Google Scholar] [CrossRef]
- Nho, K.J.; Chun, J.M.; Kim, D.-S.; Kim, H.K. Ampelopsis Japonica Ethanol Extract Suppresses Migration and Invasion in Human MDA-MB-231 Breast Cancer Cells. Mol. Med. Rep. 2015, 11, 3722–3728. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Shim, J.S.; Kim, H.G.; Lee, H.; Oh, M.S. Ampelopsis Radix Protects Dopaminergic Neurons against 1-Methyl-4-Phenylpyridinium/1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Toxicity in Parkinson’s Disease Models In Vitro and In Vivo. Evid.-Based Complement. Altern. Med. 2013, 2013, 346438. [Google Scholar] [CrossRef]
- Bekyarova, G.Y.; Vankova, D.G.; Madjova, V.H.; Bekyarov, N.A.; Salim, A.S.; Ivanova, D.G.; Stoeva, S.M.; Gerova, D.I.; Kiselova-Kaneva, Y.D. Association between Nfr2, HO-1, NF-kB Expression, Plasma ADMA, and Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 17067. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Li, W.; Feng, J.; Chen, Y.; Chen, R.; Hu, L.; Wei, J. Fasudil Attenuates Oxidative Stress-Induced Partial Epithelial-Mesenchymal Transition of Tubular Epithelial Cells in Hyperuricemic Nephropathy via Activating Nrf2. Eur. J. Pharmacol. 2024, 975, 176640. [Google Scholar] [CrossRef]
- Wen, S.; Arakawa, H.; Tamai, I. Uric Acid in Health and Disease: From Physiological Functions to Pathogenic Mechanisms. Pharmacol. Ther. 2024, 256, 108615. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yin, J.; Xu, T.; Dai, X.; Liu, T.; Zhang, Y.; Wang, S.; Liu, Y.; Shi, H.; Zhang, Y.; et al. Fuling-Zexie Formula Attenuates Hyperuricemia-Induced Nephropathy and Inhibits JAK2/STAT3 Signaling and NLRP3 Inflammasome Activation in Mice. J. Ethnopharmacol. 2024, 319, 117262. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, B.; Wang, X.; Duan, J.; Li, J.; Wang, J.; Xia, N.; Zhang, S.; Wang, J.; Guo, D.; et al. Spike Lavender Essential Oil Attenuates Hyperuricemia and Induced Renal Injury by Modulating the TLR4/NF-κB/NLRP3 Signalling Pathway. Arab. J. Chem. 2024, 17, 105897. [Google Scholar] [CrossRef]
- Majumder, S.; Pushpakumar, S.B.; Almarshood, H.; Ouseph, R.; Gondim, D.D.; Jala, V.R.; Sen, U. Toll-like Receptor 4 Mutation Mitigates Gut Microbiota-Mediated Hypertensive Kidney Injury. Pharmacol. Res. 2024, 206, 107303. [Google Scholar] [CrossRef]
- Chen, Y.; Pei, C.; Chen, Y.; Xiao, X.; Zhang, X.; Cai, K.; Deng, S.; Liang, R.; Xie, Z.; Li, P.; et al. Kidney Tea Ameliorates Hyperuricemia in Mice via Altering Gut Microbiota and Restoring Metabolic Profile. Chem.-Biol. Interact. 2023, 376, 110449. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, Y.; Wang, W.; Zhang, X.; Gao, L.; Qiu, X. Mechanism Study on the Preventive Effect of ELITEA Compound Tea on Hyperuricemia in Rats Based on Serum Untargeted Metabolomics. Metabolites 2025, 15, 336. [Google Scholar] [CrossRef]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Qin, N.; Qin, M.; Shi, W.; Kong, L.; Wang, L.; Xu, G.; Guo, Y.; Zhang, J.; Ma, Q. Investigation of Pathogenesis of Hyperuricemia Based on Untargeted and Targeted Metabolomics. Sci. Rep. 2022, 12, 13980. [Google Scholar] [CrossRef]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Zhou, A.; Chen, J.; Dong, X.; Bai, P.; Zhu, Q.; Meng, H.-M.; Li, Z. Bimetallic CuRu Nanozymes for Colorimetric and Smartphone-Assisted Rapid Visual Hypoxanthine Biosensing in Serum Samples. Microchim. Acta 2025, 192, 159. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, R.; Dong, Y.; Su, J.; Yu, J.; Yan, M.; Chen, S.; Lv, G. The Impact of Chrysanthemi Indici Flos-Enriched Flavonoid Part on the Model of Hyperuricemia Based on Inhibiting Synthesis and Promoting Excretion of Uric Acid. J. Ethnopharmacol. 2024, 333, 118488. [Google Scholar] [CrossRef]
- Li, L.; Zhao, K.; Luo, J.; Tian, J.; Zheng, F.; Lin, X.; Xie, Z.; Jiang, H.; Li, Y.; Zhao, Z.; et al. Piperine Improves Hyperuricemic Nephropathy by Inhibiting URAT1/GLUT9 and the AKT-mTOR Pathway. J. Agric. Food Chem. 2024, 72, 6565–6574. [Google Scholar] [CrossRef]
- Wang, L.; Tao, Y.; Wang, X.; Gan, Y.; Zeng, Y.; Li, S.; Zhu, Q. Aqueous Extract of Phellinus Igniarius Ameliorates Hyperuricemia and Renal Injury in Adenine/Potassium Oxonate-Treated Mice. Biomed. Pharmacother. 2024, 177, 116859. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-X.; Liu, L.-D.; Zhu, J.-Y.; Zhu, S.-S.; Ye, B.-Q.; Yang, J.-L.; Huang, J.-Y.; Huang, Z.-H.; You, Y.; Li, W.-K.; et al. Alistipes Indistinctus-Derived Hippuric Acid Promotes Intestinal Urate Excretion to Alleviate Hyperuricemia. Cell Host Microbe 2024, 32, 366–381.e9. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xiao, H.; Zou, X.; Pan, L.; Song, Q.; Hou, L.; Zeng, Y.; Han, Y.; Zhou, Z. Mechanisms of Levan in Ameliorating Hyperuricemia: Insight into Levan on Serum Metabolites, Gut Microbiota, and Function in Hyperuricemia Rats. Carbohydr. Polym. 2025, 347, 122665. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Liu, F.; Zhao, L.-Y.; Li, H.-X.; Jumai, A.; Xu, Z.-F.; Qiu, S.-X. Xanthine Oxidase Inhibitory Constituents from the Roots of Ampelopsis japonica. Nat. Prod. Res. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Liang, D.; Chen, S.; Xiao, C.; Gao, X.; Wu, Q.; Xie, Y.; Huang, L.; Hu, H.; Li, X.; et al. Caffeic Acid Phenethyl Ester Alleviated Hypouricemia in Hyperuricemic Mice through Inhibiting XOD and Up-Regulating OAT3. Phytomedicine 2022, 103, 154256. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Xiang, H.; Hu, X.; Chen, S.; Wu, Y.; Xu, J.; Yang, X. Novel Potential XOD Inhibitory Peptides Derived from Trachinotus Ovatus: Isolation, Identification and Structure-Function Analysis. Food Biosci. 2022, 47, 101639. [Google Scholar] [CrossRef]
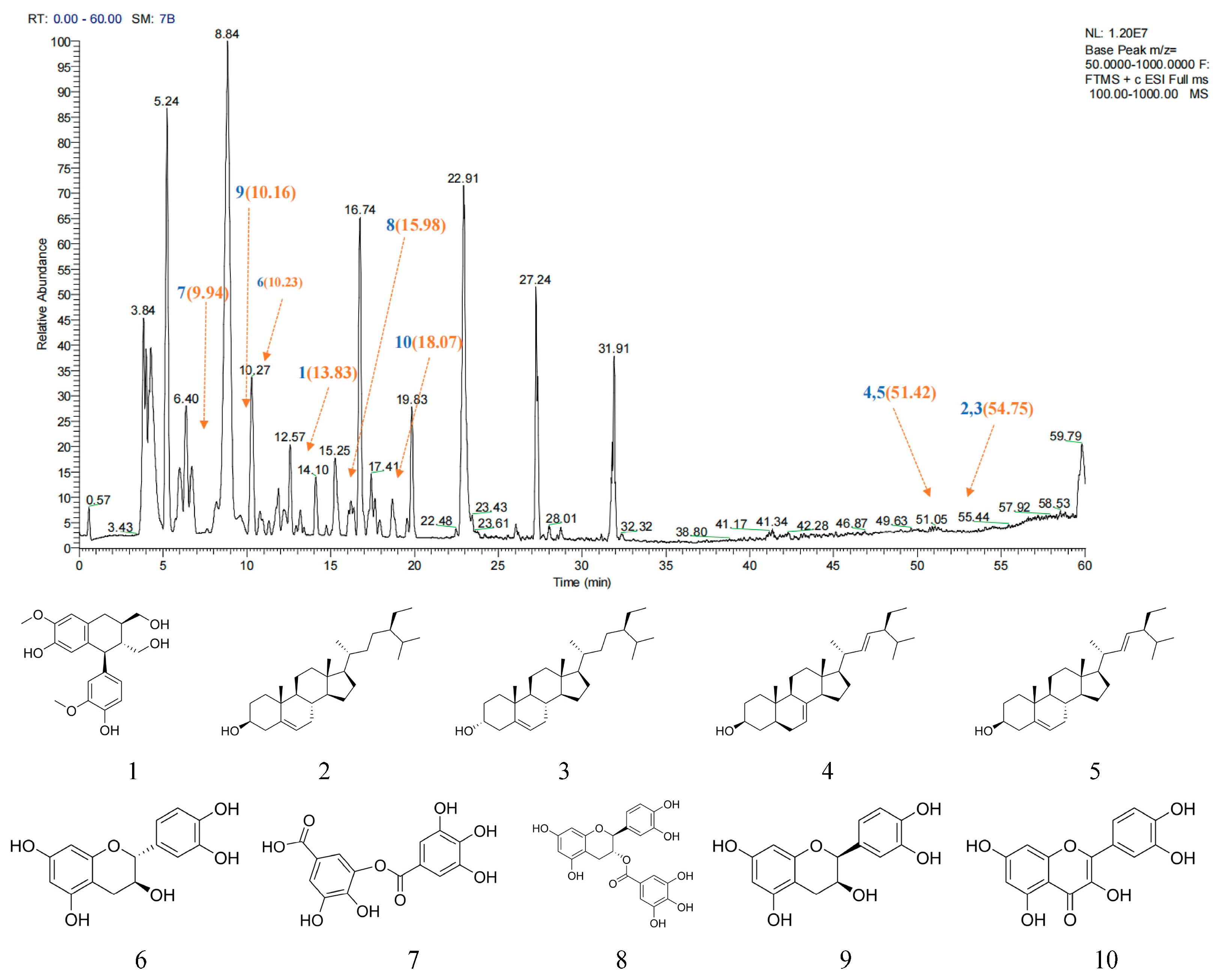
| No. | Molecule | MW | AlogP | Hdon | Hack | OB (%) | DL |
|---|---|---|---|---|---|---|---|
| AJE-01 | (2R,3R,4S)-4-(4-hydroxy-3-methoxy-phenyl)-7-methoxy-2,3-dimethylo | 360.44 | 2.25 | 4 | 6 | 66.51 | 0.39 |
| AJE-02 | Beta-sitosterol | 414.79 | 8.08 | 1 | 1 | 36.91 | 0.75 |
| AJE-03 | Sitosterol | 414.79 | 8.08 | 1 | 1 | 36.91 | 0.75 |
| AJE-04 | Spinasterol | 412.77 | 7.64 | 1 | 1 | 42.98 | 0.76 |
| AJE-05 | Stigmasterol | 412.77 | 7.64 | 1 | 1 | 43.83 | 0.76 |
| AJE-06 | (+)-Catechin | 290.29 | 1.92 | 5 | 6 | 54.83 | 0.24 |
| AJE-07 | Digallate | 322.24 | 1.53 | 6 | 9 | 61.85 | 0.26 |
| AJE-08 | (−)-Catechin gallate | 442.40 | 3.16 | 7 | 10 | 53.57 | 0.75 |
| AJE-09 | ent-Epicatechin | 290.29 | 1.92 | 5 | 6 | 48.96 | 0.24 |
| AJE-10 | Quercetin | 302.25 | 1.5 | 5 | 7 | 46.43 | 0.28 |
| Description | GenBank | Primer Name | Primer Sequences (5′-3′) | Product Size (bp) | Tm (°C) |
|---|---|---|---|---|---|
| Actb | NM_007393.5 | M-Actb-F | GATATCGCTGCGCTGGTCG | 132 | 61.37 |
| M-Actb-R | CATTCCCACCATCACACCCT | 59.67 | |||
| IL-1β | NM_008361.4 | M-IL-1β-F | AATGCCACCTTTTGACAGTGA | 139 | 58.33 |
| M-IL-1β-R | GATGTGCTGCTGCGAGATTT | 59.27 | |||
| TNF-α | NM_001278601.1 | M-TNF-α-F | GATCGGTCCCCAAAGGGATG | 92 | 60.18 |
| M-TNF-α-R | CCACTTGGTGGTTTGTGAGTG | 59.59 | |||
| URAT1 | NM_009203.3 | M-URAT1-F | TCATCACGGTAGGCAAGCTG | 100 | 60.11 |
| M-URAT1-R | ACCTCCATGGTCAACGTGTC | 59.97 | |||
| GLUT9 | NM_001012363.2 | M-GLUT9-F | TGCATTGGCGTGTTTTCTGG | 193 | 59.97 |
| M-GLUT9-R | GGAAGGCTTTCATGGCTCCT | 60.03 | |||
| ABCG2 | NM_001355477.2 | M-ABCG2-F | GATGCCGCTGGAATGCAAAA | 95 | 60.11 |
| M-ABCG2-R | ACTACGAACAGCTCCACAGC | 60.04 | |||
| OAT1 | NM_008766.3 | M-OAT1-F | CGTCGGAAGGTGCTGATCTT | 121 | 60.11 |
| M-OAT1-R | TAGCCAAAGACATGCCCGAG | 60.11 | |||
| OAT3 | NM_001164634.2 | M-OAT3-F | TGGCTACAGTTGTCCGTGTC | 133 | 59.97 |
| M-OAT3-R | TAGCCACACGTTGGAGTGTC | 59.97 | |||
| IL-6 | NM_001314054.1 | M-IL-6-F | CACTTCACAAGTCGGAGGCT | 355 | 59.97 |
| M-IL-6-R | GCCACTCCTTCTGTGACTCC | 60.04 | |||
| OCT2 | NM_001355767.1 | M-OCT2-F | CAGAATTTGTTGGGCTGGGC | 147 | 60.04 |
| M-OCT2-R | AGAAGTTGGGCAGAGTCACG | 59.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Li, B.-L.; Liu, M.; Chen, S.; Wu, Y.; Jumai, A.; Zhao, L.; Qiu, S.-X. Ampelopsis japonica Extract Exhibited Significant Uric Acid-Lowering Effect by Downregulating URAT1/GLUT9 and Alleviates Inflammation Through TLR4/NF-κB Pathway. Int. J. Mol. Sci. 2025, 26, 8999. https://doi.org/10.3390/ijms26188999
Liu F, Li B-L, Liu M, Chen S, Wu Y, Jumai A, Zhao L, Qiu S-X. Ampelopsis japonica Extract Exhibited Significant Uric Acid-Lowering Effect by Downregulating URAT1/GLUT9 and Alleviates Inflammation Through TLR4/NF-κB Pathway. International Journal of Molecular Sciences. 2025; 26(18):8999. https://doi.org/10.3390/ijms26188999
Chicago/Turabian StyleLiu, Fen, Bai-Lin Li, Meilan Liu, Shaohua Chen, Yaodan Wu, Aikebaier Jumai, Liyun Zhao, and Sheng-Xiang Qiu. 2025. "Ampelopsis japonica Extract Exhibited Significant Uric Acid-Lowering Effect by Downregulating URAT1/GLUT9 and Alleviates Inflammation Through TLR4/NF-κB Pathway" International Journal of Molecular Sciences 26, no. 18: 8999. https://doi.org/10.3390/ijms26188999
APA StyleLiu, F., Li, B.-L., Liu, M., Chen, S., Wu, Y., Jumai, A., Zhao, L., & Qiu, S.-X. (2025). Ampelopsis japonica Extract Exhibited Significant Uric Acid-Lowering Effect by Downregulating URAT1/GLUT9 and Alleviates Inflammation Through TLR4/NF-κB Pathway. International Journal of Molecular Sciences, 26(18), 8999. https://doi.org/10.3390/ijms26188999





