Insights into the Metabolic Adaptations of a Carbapenem-Resistant Klebsiella pneumoniae Strain on Exposure to Sublethal Concentrations of Ertapenem
Abstract
1. Introduction
2. Results
2.1. Sample Overview
2.2. Functional Categorization of Significantly Different Expressed Proteins
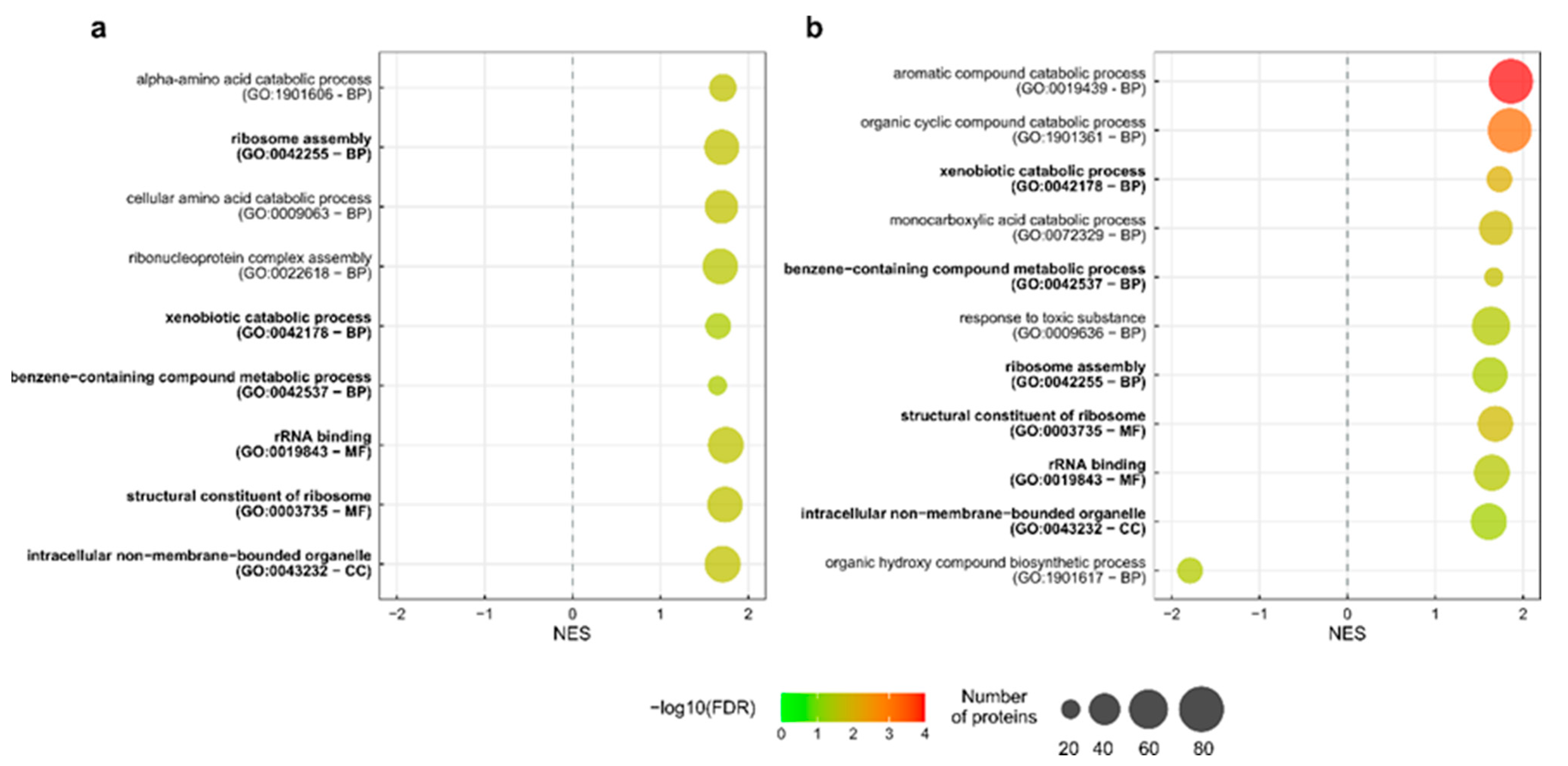
2.3. GO Terms and Pathway Enrichment Analysis
3. Discussion
3.1. Functional Categories
3.2. Enriched Metabolic Pathways and GO Terms
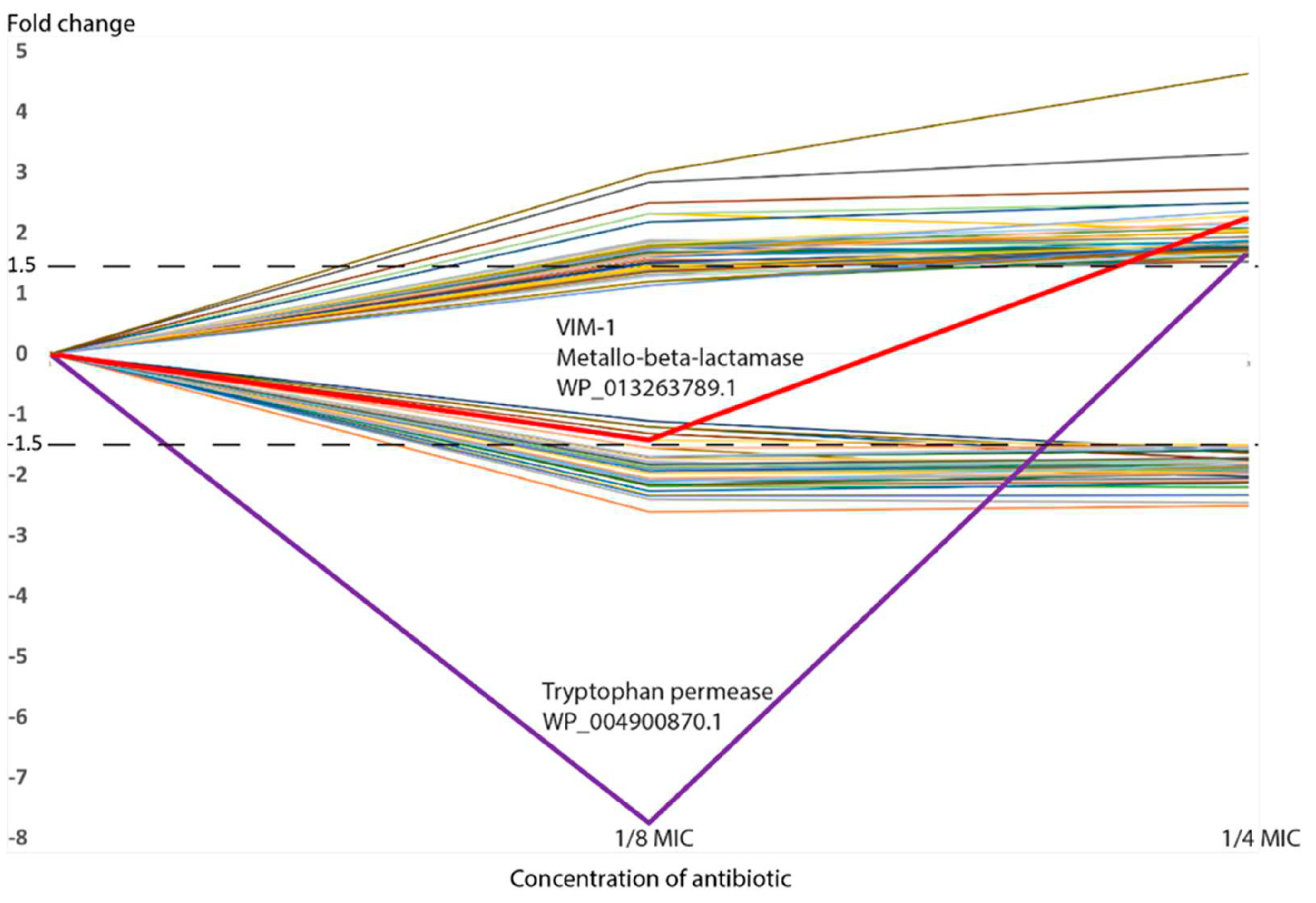
3.3. Beta-Lactamases and Other Antibiotic Resistance Genes
3.4. Adjustment of the Influx–Efflux Capacity
3.5. Penicillin-Binding Proteins, Cell Wall, and Peptidoglycan Metabolism
3.6. Regulome
4. Materials and Methods
4.1. Strain
4.2. Quantitative Proteomic Analysis
4.3. Proteomic Data Analysis
4.4. Enrichment Analyses and Protein Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cella, E.; Giovanetti, M.; Benedetti, F.; Scarpa, F.; Johnston, C.; Borsetti, A.; Ceccarelli, G.; Azarian, T.; Zella, D.; Ciccozzi, M. Joining Forces against Antibiotic Resistance: The One Health Solution. Pathogens 2023, 12, 1074. [Google Scholar] [CrossRef]
- Husna, A.; Rahman, M.M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, M.T.; Alam, J.; Ashour, H.M. Extended-spectrum β-lactamases (ESBL): Challenges and opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef]
- Lee, C.R.; Lee, J.H.; Park, K.S.; Kim, Y.B.; Jeong, B.C.; Lee, S.H. Global Dissemination of Carbapenemase-Producing Klebsiella pneumoniae: Epidemiology, Genetic Context, Treatment Options, and Detection Methods. Front. Microbiol. 2016, 7, 895. [Google Scholar] [CrossRef]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. 2024. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 1 May 2024).
- World Health Organization. Disease Outbreak News; Antimicrobial Resistance, Hypervirulent Klebsiella pneumoniae, Global situation. 2024. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON527 (accessed on 1 September 2024).
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Reyes, J.; Aguilar, A.C.; Caicedo, A. Carbapenem-Resistant Klebsiella pneumoniae: Microbiology Key Points for Clinical Practice. Int. J. Gen. Med. 2019, 12, 437–446. [Google Scholar] [CrossRef]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- vanDuin, D.; Arias, C.A.; Komarow, L.; Chen, L.; Hanson, B.M.; Weston, G.; Cober, E.; Garner, O.B.; Jacob, J.T.; Satlin, M.J.; et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): A prospective cohort study. Lancet Infect. Dis. 2020, 20, 731–741, Correction in Lancet Infect. Dis. 2020, 20, E116. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, A.A.; Bergen, P.J.; Rao, G.G.; Nation, R.L.; Landersdorfer, C.B. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int. J. Antimicrob. Agents 2020, 55, 105833. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, X.; Ma, X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, L.; Liu, C.; Huang, X.; Zheng, R.; Lu, Y.; Xia, W.; Ni, F.; Mei, Y.; Liu, G. Characterization of Carbapenem-Resistant Klebsiella pneumoniae ST15 Clone Coproducing KPC-2, CTX-M-15 and SHV-28 Spread in an Intensive Care Unit of a Tertiary Hospital. Infect. Drug Resist. 2021, 14, 767–773. [Google Scholar] [CrossRef]
- Xu, A.; Zheng, B.; Xu, Y.C.; Huang, Z.G.; Zhong, N.S.; Zhuo, C. National epidemiology of carbapenem-resistant and extensively drug-resistant Gram-negative bacteria isolated from blood samples in China in 2013. Clin. Microbiol. Infect. 2016, 1, S1–S8. [Google Scholar] [CrossRef]
- Guh, A.Y.; Limbago, B.M.; Kallen, A.J. Epidemiology and prevention of carbapenem-resistant Enterobacteriaceae in the United States. Expert. Rev. Anti. Infect. Ther. 2014, 12, 565–580. [Google Scholar] [CrossRef]
- David, S.; Reuter, S.; Harris, S.R.; Glasner, C.; Feltwell, T.; Argimon, S.; Abudahab, K.; Goater, R.; Giani, T.; Errico, G.; et al. Epidemic of carbapenem-resistant Klebsiella pneumoniae in Europe is driven by nosocomial spread. Nat. Microbiol. 2019, 4, 1919–1929. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655, Erratum in Lancet 2022, 400, 1102. [Google Scholar] [CrossRef] [PubMed]
- Kizny Gordon, A.E.; Mathers, A.J.; Cheong, E.Y.L.; Gottlieb, T.; Kotay, S.; Walker, A.S.; Peto, T.E.A.; Crook, D.W.; Stoesser, N. The Hospital Water Environment as a Reservoir for Carbapenem-Resistant Organisms Causing Hospital-Acquired Infections-A Systematic Review of the Literature. Clin. Infect. Dis. 2017, 64, 1435–1444, Erratum in Clin. Infect. Dis. 2017, 65, 1431–1433. [Google Scholar] [CrossRef]
- Leitner, E.; Zarfel, G.; Luxner, J.; Herzog, K.; Pekard-Amenitsch, S.; Hoenigl, M.; Valentin, T.; Feierl, G.; Grisold, A.J.; Högenauer, C.; et al. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob. Agents Chemother. 2015, 59, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.; Thom, K.A.; Masnick, M.; Johnson, J.K.; Harris, A.D.; Morgan, D.J. Frequency of Klebsiella pneumoniae carbapenemase (KPC)-producing and non-KPC-producing Klebsiella species contamination of healthcare workers and the environment. Infect. Control Hosp. Epidemiol. 2014, 35, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.Z.R.; de Lima, E.M.; Martins Aires, C.A.; Pereira, P.S.; Yim, J.; Silva, F.H.; Rodrigues, C.A.S.; Oliveira, T.R.T.E.; da Silva, P.P.; Eller, C.M.; et al. Outbreak report of polymyxin-carbapenem-resistant Klebsiella pneumoniae causing untreatable infections evidenced by synergy tests and bacterial genomes. Sci. Rep. 2023, 13, 6238. [Google Scholar] [CrossRef]
- Li, Y.; Ni, M. Regulation of biofilm formation in Klebsiella pneumoniae. Front. Microbiol. 2023, 14, 1238482. [Google Scholar] [CrossRef]
- Karimi, K.; Zarei, O.; Sedighi, P.; Taheri, M.; Doosti-Irani, A.; Shokoohizadeh, L. Investigation of Antibiotic Resistance and Biofilm Formation in Clinical Isolates of Klebsiella pneumoniae. Int. J. Microbiol. 2021, 2021, 5573388. [Google Scholar] [CrossRef]
- Nunez, C.; Kostoulias, X.; Peleg, A.; Short, F.; Qu, Y. A comprehensive comparison of biofilm formation and capsule production for bacterial survival on hospital surfaces. Biofilm 2023, 5, 100105. [Google Scholar] [CrossRef] [PubMed]
- Noel, D.J.; Keevil, C.W.; Wilks, S.A. Development of disinfectant tolerance in Klebsiella pneumoniae. J. Hosp. Infect. 2025, 155, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kumar, S.; Zhang, L. Mechanisms of Antibiotic Resistance and Developments in Therapeutic Strategies to Combat Klebsiella pneumoniae Infection. Infect. Drug Resist. 2024, 17, 1107–1119. [Google Scholar] [CrossRef]
- Aurilio, C.; Sansone, P.; Barbarisi, M.; Pota, V.; Giaccari, L.G.; Coppolino, F.; Barbarisi, A.; Passavanti, M.B.; Pace, M.C. Mechanisms of Action of Carbapenem Resistance. Antibiotics 2022, 11, 421. [Google Scholar] [CrossRef]
- Colquhoun, J.M.; Farokhyfar, M.; Hutcheson, A.R.; Anderson, A.; Bethel, C.R.; Bonomo, R.A.; Clarke, A.J.; Rather, P.N. OXA-23 β-Lactamase Overexpression in Acinetobacter baumannii Drives Physiological Changes Resulting in New Genetic Vulnerabilities. mBio. 2021, 12, e03137-21. [Google Scholar] [CrossRef]
- Bansal, O.P. A review on antibiotics in the environment, resistance in microbes, impact on human health and treatment and future strategies for tackling the global problem. World J. Adv. Res. Rev. 2023, 20, 959–971. [Google Scholar] [CrossRef]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef]
- Wang, F.; Fu, Y.-H.; Sheng, H.-J.; Topp, E.; Jiang, X.; Zhu, Y.-G.; Tiedje, J.M. Antibiotic resistance in the soil ecosystem: A One Health perspective. Curr. Opin. Environ. Sci. Health 2021, 20, 100230. [Google Scholar] [CrossRef]
- Amábile-Cuevas, C.F. Antibiotic resistance from, and to the environment. AIMS Environ. Sci. 2021, 8, 18–35. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, fux053. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.; Yang, X.; Ji, X.; Zou, H.; Ottoson, J.; Nilsson, L.E.; Berglund, B.; et al. Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: Its potential for resistance development and ecological and human risk. Environ. Int. 2018, 114, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of Resistant Bacteria at Very Low Antibiotic Concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.R.; Lam, N.B.; Jackson, J.L.; Dorenkott, S.M.; Ticer, T.; Maldosevic, E.; Velez, A.; Camden, M.R.; Ellis, T.N. Progressive Sub-MIC Exposure of Klebsiella pneumoniae 43816 to Cephalothin Induces the Evolution of Beta-Lactam Resistance without Acquisition of Beta-Lactamase Genes. Antibiotics 2023, 12, 887. [Google Scholar] [CrossRef]
- Baquero, F.; Martínez, J.L.; Lanza, V.F.; Rodríguez-Beltrán, J.; Galán, J.C.; San Millán, A.; Cantón, R.; Coque, T.M. Evolutionary Pathways and Trajectories in Antibiotic Resistance. Clin. Microbiol. Rev. 2021, 34, e00050-19. [Google Scholar] [CrossRef]
- Hodille, E.; Rose, W.; Diep, B.A.; Goutelle, S.; Lina, G.; Dumitrescu, O. The Role of Antibiotics in Modulating Virulence in Staphylococcus aureus. Clin. Microbiol. Rev. 2017, 30, 887–917. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, J.P.; de Macêdo Farias, L.; Ferreira, J.F.; Bruna-Romero, O.; da Glória de Souza, D.; de Carvalho, M.A.; dos Santos, K.V. Sub-Inhibitory Concentration of Piperacillin-Tazobactam May be Related to Virulence Properties of Filamentous Escherichia coli. Curr. Microbiol. 2016, 72, 19–28. [Google Scholar] [CrossRef]
- Shen, L.; Shi, Y.; Zhang, D.; Wei, J.; Surette, M.G.; Duan, K. Modulation of secreted virulence factor genes by subinhibitory concentrations of antibiotics in Pseudomonas aeruginosa. J. Microbiol. 2008, 46, 441–447. [Google Scholar] [CrossRef]
- Johnning, A.; Karami, N.; Tång Hallbäck, E.; Müller, V.; Nyberg, L.; Buongermino Pereira, M.; Stewart, C.; Ambjörnsson, T.; Westerlund, F.; Adlerberth, I.; et al. The resistomes of six carbapenem-resistant pathogens—A critical genotype-phenotype analysis. Microb. Genom. 2018, 4, e000233. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Song, J.; Wang, H.; Ye, N.; Wang, R.W. Tryptophan transport gene inactivation promotes the development of antibiotic resistance in Escherichia coli. FEMS Microbiol. Lett. 2024, 371, fnae057. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Alvarez, R.V.; Karamycheva, S.; Makarova, K.S.; Wolf, Y.I.; Landsman, D.; Koonin, E.V. COG database update 2024. Nucleic Acids Res. 2025, 53, D356–D363. [Google Scholar] [CrossRef]
- Woegerbauer, M.; Kuffner, M.; Domingues, S.; Nielsen, K.M. Involvement of aph(3′)-IIa in the formation of mosaic aminoglycoside resistance genes in natural environments. Front. Microbiol. 2015, 6, 442. [Google Scholar] [CrossRef]
- Kim, H.J.; Li, Y.; Zimmermann, M.; Lee, Y.; Lim, H.W.; Leong Tan, A.S.; Choi, I.; Ko, Y.; Lee, S.; Seo, J.J.; et al. Pharmacological perturbation of thiamine metabolism sensitizes Pseudomonas aeruginosa to multiple antibacterial agents. Cell Chem. Biol. 2022, 29, 1317–1324.e5. [Google Scholar] [CrossRef]
- Dalbanjan, N.P.; Kadapure, A.J.; Kumar, P.S.K. A Comprehensive review on latent role of stress proteins in antibiotic resistance. Microbe 2024, 4, 100151. [Google Scholar] [CrossRef]
- Jiao, M.; He, W.; Ouyang, Z.; Shi, Q.; Wen, Y. Progress in structural and functional study of the bacterial phenylacetic acid catabolic pathway, its role in pathogenicity and antibiotic resistance. Front. Microbiol. 2022, 13, 964019. [Google Scholar] [CrossRef] [PubMed]
- Caveney, N.A.; Caballero, G.; Voedts, H.; Niciforovic, A.; Worrall, L.J.; Vuckovic, M.; Fonvielle, M.; Hugonnet, J.E.; Arthur, M.; Strynadka, N.C.J. Structural insight into YcbB-mediated beta-lactam resistance in Escherichia coli. Nat. Commun. 2019, 10, 1849. [Google Scholar] [CrossRef]
- Van Laar, T.A.; Chen, T.; You, T.; Leung, K.P. Sublethal concentrations of carbapenems alter cell morphology and genomic expression of Klebsiella pneumoniae biofilms. Antimicrob. Agents Chemother. 2015, 59, 1707–1717. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, Y.; Ma, T.; Wang, J.; Li, S.; Wang, J.; Han, L.; Hou, X.; Ma, X.; Jiang, S.; et al. Transcriptomic and phenotype analysis revealed the role of rpoS in stress resistance and virulence of a novel ST3355 ESBL-producing hypervirulent Klebsiella pneumoniae isolate. Front. Cell Infect. Microbiol. 2023, 13, 1259472. [Google Scholar] [CrossRef] [PubMed]
- Luxton, T.N.; King, N.; Wälti, C.; Jeuken, L.J.C.; Sandoe, J.A.T. A Systematic Review of the Effect of Therapeutic Drug Monitoring on Patient Health Outcomes during Treatment with Carbapenems. Antibiotics 2022, 11, 1311. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, G.; Chao, X.; Xie, L.; Wang, H. The Characteristic of Virulence, Biofilm and Antibiotic Resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 2020, 17, 6278. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Sharma, D.; Faheem, M.; Bisht, D.; Khan, A.U. Proteomic analysis of a carbapenem-resistant Klebsiella pneumoniae strain in response to meropenem stress. J. Glob. Antimicrob. Resist. 2017, 8, 172–178. [Google Scholar] [CrossRef]
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Jenney, A.; Connor, T.R.; Hsu, L.Y.; Severin, J.; et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. USA 2015, 112, E3574–E3581. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.H.P.; Alorabi, M.; Hamilton, F.; Takebayashi, Y.; Mounsey, O.; Heesom, K.J.; Williams, P.B.; Williams, O.M.; Albur, M.; MacGowan, A.P.; et al. Trade-Offs between Antibacterial Resistance and Fitness Cost in the Production of Metallo-β-Lactamases by Enteric Bacteria Manifest as Sporadic Emergence of Carbapenem Resistance in a Clinical Setting. Antimicrob. Agents Chemother. 2021, 65, e0241220. [Google Scholar] [CrossRef] [PubMed]
- Jaén-Luchoro, D.; Karlsson, R.; Busquets, A.; Piñeiro-Iglesias, B.; Karami, N.; Marathe, N.P.; Moore, E.R.B. Knockout of Targeted Plasmid-Borne β-Lactamase Genes in an Extended-Spectrum-β-Lactamase-Producing Escherichia coli Strain: Impact on Resistance and Proteomic Profile. Microbiol. Spectr. 2023, 11, e03867-22. [Google Scholar] [CrossRef]
- Wang, P.; Li, R.Q.; Wang, L.; Yang, W.T.; Zou, Q.H.; Xiao, D. Proteomic Analyses of Acinetobacter baumannii Clinical Isolates to Identify Drug Resistant Mechanism. Front. Cell Infect. Microbiol. 2021, 11, 625430. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Kumar, R.; Khan, A.; Khan, A.U. Interaction of LysM BON family protein domain with carbapenems: A putative mechanism of carbapenem resistance. Int. J. Biol. Macromol. 2020, 160, 212–223. [Google Scholar] [CrossRef]
- Snell, A.P.; Manias, D.A.; Elbehery, R.R.; Dunny, G.M.; Willett, J.L.E. Arginine impacts aggregation, biofilm formation, and antibiotic susceptibility in Enterococcus faecalis. FEMS Microbes 2024, 5, xtae030. [Google Scholar] [CrossRef]
- Maurice, C.F.; Haiser, H.J.; Turnbaugh, P.J. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef]
- Yuan, P.B.; Ling, J.H.; Zhu, J.H.; Peng, C.; Chen, E.Z.; Zhong, Y.X.; Liu, W.T.; Wang, L.J.; Yang, L.; Chen, D.Q. Proteomics profiling of ertapenem challenged major porin deficient carbapenem-resistant Klebsiella pneumoniae. J. Proteomics. 2022, 268, 104715. [Google Scholar] [CrossRef]
- Hao, L.; Yang, X.; Chen, H.; Mo, Z.; Li, Y.; Wei, S.; Zhao, Z. Molecular Characteristics and Quantitative Proteomic Analysis of Klebsiella pneumoniae Strains with Carbapenem and Colistin Resistance. Antibiotics 2022, 11, 1341. [Google Scholar] [CrossRef]
- Salvà-Serra, F.; Jaén-Luchoro, D.; Marathe, N.P.; Adlerberth, I.; Moore, E.R.B.; Karlsson, R. Responses of carbapenemase-producing and non-producing carbapenem-resistant Pseudomonas aeruginosa strains to meropenem revealed by quantitative tandem mass spectrometry proteomics. Front. Microbiol. 2023, 13, 1089140. [Google Scholar] [CrossRef]
- Chen, X.; Tian, J.; Luo, C.; Wang, X.; Li, X.; Wang, M. Cell Membrane Remodeling Mediates Polymyxin B Resistance in Klebsiella pneumoniae: An Integrated Proteomics and Metabolomics Study. Front. Microbiol. 2022, 13, 810403. [Google Scholar] [CrossRef]
- Sugawara, E.; Kojima, S.; Nikaido, H. Klebsiella pneumoniae Major Porins OmpK35 and OmpK36 Allow More Efficient Diffusion of β-Lactams than Their Escherichia coli Homologs OmpF and OmpC. J. Bacteriol. 2016, 198, 3200–3208. [Google Scholar] [CrossRef]
- Tsai, Y.K.; Fung, C.P.; Lin, J.C.; Chen, J.H.; Chang, F.Y.; Chen, T.L.; Siu, L.K. Klebsiella pneumoniae outer membrane porins OmpK35 and OmpK36 play roles in both antimicrobial resistance and virulence. Antimicrob Agents Chemother. 2011, 55, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Gauba, A.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 2009, 1794, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Rocker, A.; Lacey, J.A.; Belousoff, M.J.; Wilksch, J.J.; Strugnell, R.A.; Davies, M.R.; Lithgow, T. Global Trends in Proteome Remodeling of the Outer Membrane Modulate Antimicrobial Permeability in Klebsiella pneumoniae. mBio 2020, 11, e00603–e00620. [Google Scholar] [CrossRef]
- Nicolas-Chanoine, M.-H.; Mayer, N.; Guyot, K.; Dumont, E.; Pagès, J.-M. Interplay Between Membrane Permeability and Enzymatic Barrier Leads to Antibiotic-Dependent Resistance in Klebsiella Pneumoniae. Front. Microbiol. 2018, 9, 1422. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.M.C.; Wick, R.R.; Watts, S.C.; Cerdeira, L.T.; Wyres, K.L.; Holt, K.E. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat. Commun. 2021, 12, 4188. [Google Scholar] [CrossRef]
- Wong, J.L.C.; Romano, M.; Kerry, L.E.; Kwong, H.S.; Low, W.W.; Brett, S.J.; Clements, A.; Beis, K.; Frankel, G. OmpK36-mediated Carbapenem resistance attenuates ST258 Klebsiella pneumoniae in vivo. Nat. Commun. 2019, 10, 3957. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Wang, Y.; Wen, X.; Peng, H.; Peng, R.; Shi, Q.; Xie, X.; Li, L. Outer Membrane Porins Contribute to Antimicrobial Resistance in Gram-Negative Bacteria. Microorganisms 2023, 11, 1690. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Deng, H.; Song, J.; Zhang, L.; Zhao, R.; Guo, Z.; Zhang, X.; Zhang, H.; Tian, T.; Ji, Y.; et al. Phage resistance mutation triggered by OmpC deficiency in Klebsiella pneumoniae induced limited fitness costs. Microb. Pathog. 2022, 167, 105556. [Google Scholar] [CrossRef] [PubMed]
- Sibinelli-Sousa, S.; Hespanhol, J.T.; Bayer-Santos, E. Targeting the Achilles’ Heel of Bacteria: Different Mechanisms to Break Down the Peptidoglycan Cell Wall during Bacterial Warfare. J. Bacteriol. 2021, 203, e00478-20. [Google Scholar] [CrossRef]
- Garde, S.; Chodisetti, P.K.; Reddy, M. Peptidoglycan: Structure, Synthesis, and Regulation. EcoSal Plus 2021, 9, eESP-0010-2020. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cai, Y.; Liu, Y.; An, H.; Deng, K.; Ashraf, M.A.; Zou, L.; Wang, J. Breaking down the cell wall: Still an attractive antibacterial strategy. Front. Microbiol. 2022, 13, 952633. [Google Scholar] [CrossRef] [PubMed]
- Kádár, B.; Kocsis, B.; Tóth, Á.; Kristóf, K.; Felső, P.; Kocsis, B.; Böddi, K.; Szabó, D. Colistin resistance associated with outer membrane protein change in Klebsiella pneumoniae and Enterobacter asburiae. Acta Microbiol. Immunol. Hung. 2017, 64, 217–227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hugonnet, J.E.; Mengin-Lecreulx, D.; Monton, A.; den Blaauwen, T.; Carbonnelle, E.; Veckerlé, C.; Brun, Y.V.; van Nieuwenhze, M.; Bouchier, C.; Tu, K.; et al. Factors essential for L,D-transpeptidase-mediated peptidoglycan cross-linking and β-lactam resistance in Escherichia coli. Elife 2016, 5, e19469. [Google Scholar] [CrossRef] [PubMed]
- Magnet, S.; Dubost, L.; Marie, A.; Arthur, M.; Gutmann, L. Identification of the L,D-transpeptidases for peptidoglycan cross-linking in Escherichia coli. J. Bacteriol. 2008, 190, 4782–4785. [Google Scholar] [CrossRef]
- Magnet, S.; Bellais, S.; Dubost, L.; Fourgeaud, M.; Mainardi, J.L.; Petit-Frère, S.; Marie, A.; Mengin-Lecreulx, D.; Arthur, M.; Gutmann, L. Identification of the L,D-transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. J. Bacteriol. 2007, 189, 3927–3931. [Google Scholar] [CrossRef]
- Braun, V.; Rehn, K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur. J. Biochem. 1969, 10, 426–438. [Google Scholar] [CrossRef]
- Chopra, S.; Ramkissoon, K.; Anderson, D.C. A systematic quantitative proteomic examination of multidrug resistance in Acinetobacter baumannii. J. Proteomics. 2013, 84, 17–39. [Google Scholar] [CrossRef]
- Jiménez-Castellanos, J.C.; Wan Nur Ismah, W.A.K.; Takebayashi, Y.; Findlay, J.; Schneiders, T.; Heesom, K.J.; Avison, M.B. Envelope proteome changes driven by RamA overproduction in Klebsiella pneumoniae that enhance acquired β-lactam resistance. J. Antimicrob. Chemother. 2018, 73, 88–94. [Google Scholar] [CrossRef]
- De Majumdar, S.; Yu, J.; Fookes, M.; McAteer, S.P.; Llobet, E.; Finn, S.; Spence, S.; Monaghan, A.; Kissenpfennig, A.; Ingram, R.J.; et al. Elucidation of the RamA Regulon in Klebsiella pneumoniae Reveals a Role in LPS Regulation. PLoS Pathog. 2015, 11, e1004627. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.D.; Ellermeier, C.D. Extra cytoplasmic function σ factor activation. Curr. Opin. Microbiol. 2012, 15, 182–188. [Google Scholar] [CrossRef][Green Version]
- Dong, T.; Schellhorn, H.E. Role of RpoS in Virulence of Pathogens. Infect. Immun. 2010, 78, 887–897. [Google Scholar] [CrossRef]
- Hengge-Aronis, R. Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 2002, 66, 373–395. [Google Scholar] [CrossRef] [PubMed]
- Mahren, S.; Braun, V. The FecI extracytoplasmic-function sigma factor of Escherichia coli interacts with the beta’ subunit of RNA polymerase. J. Bacteriol. 2003, 185, 1796–1802. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Guidance Document on Broth Microdilution Testing, Version 12.0. 2024. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/MIC_testing/Reading_guide_BMD_v_5.0_2024.pdf (accessed on 20 March 2024).
- ISO 20776-1:2019; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents Against Rapid. ISO: Geneva, Switzerland, 2019.
- European Committee on Antimicrobial Susceptibility Testing. EUCAST Clinical Breakpoint Table. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 7 June 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: http://www.R-project.org (accessed on 1 May 2024).
- Wickham, H.; Sievert, C. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Volume 10, ISBN 978-3-319-24277-4. [Google Scholar]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 1 May 2024).
- Bonnot, T.; Gillard, M.; Nagel, D. A simple protocol for informative visualization of enriched gene ontology terms. Bio-Protocol 2019, 9, e3429. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
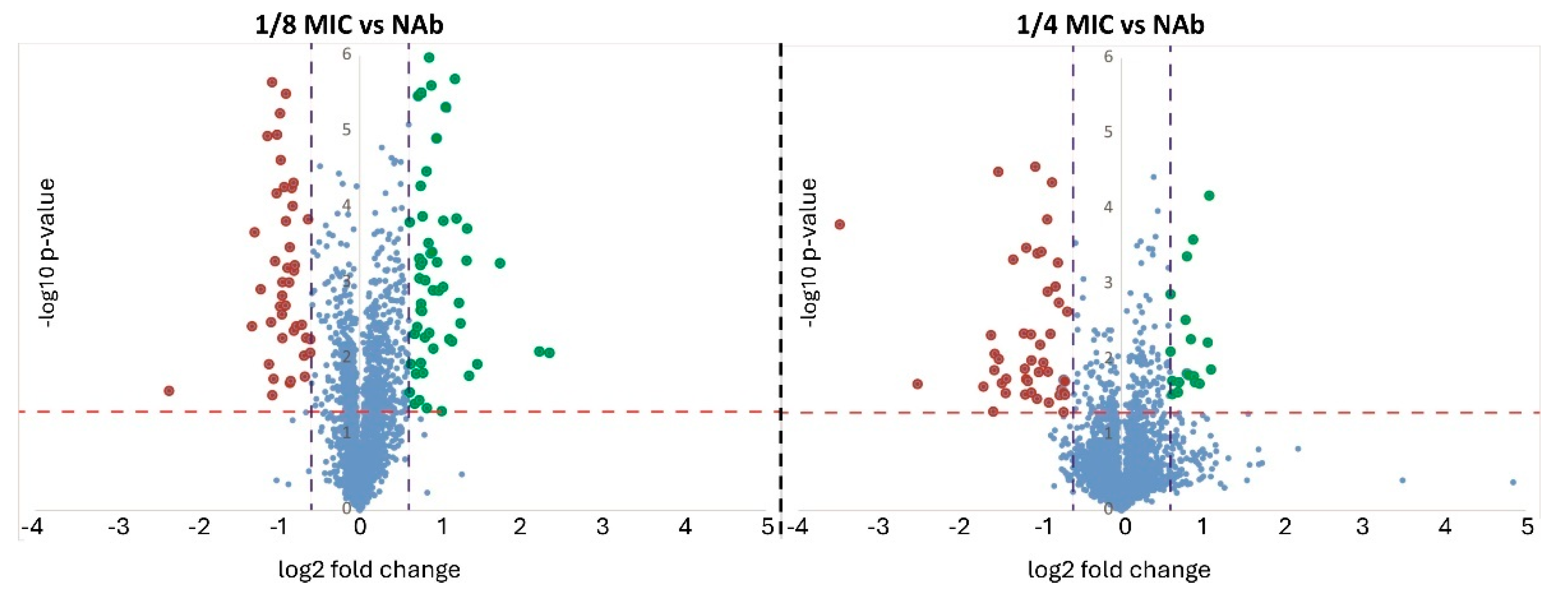


| Comparison | Proteins with Higher Abundance | Protein with Lower Abundance | Total |
|---|---|---|---|
| 1/8 MIC vs. NAb | 33 | 28 | 61 |
| 1/4 MIC vs. NAb | 48 | 39 | 87 |
| 1/8 MIC vs. NAb | 1/4 MIC vs. NAb | ||||
|---|---|---|---|---|---|
| Accession No. | Description of the Protein | FC | p-Value | FC | p-Value |
| WP_004143727.1 | Stationary phase-induced ribosome-associated protein | 2.99 | 0.00 | 4.63 | 0.00 |
| WP_004224493.1 | phenylacetate-CoA oxygenase subunit PaaI | 2.83 | 0.01 | 3.31 | 0.00 |
| WP_004224492.1 | 1,2-phenylacetyl-CoA epoxidase subunit B | 2.49 | 0.00 | 2.73 | 0.00 |
| WP_023302002.1 | phenylacetate-CoA oxygenase/reductase subunit PaaK | 2.18 | 0.01 | 2.50 | 0.00 |
| WP_002921438.1 | C4-dicarboxylate transporter | 2.31 | 0.00 | 2.49 | 0.00 |
| WP_002910896.1 | YcgN family cysteine cluster protein | 1.72 | 0.06 | 2.36 | 0.02 |
| WP_023280306.1 | L,D-transpeptidase | 1.82 | 0.00 | 2.28 | 0.00 |
| WP_013263789.1 | subclass B1 metallo-beta-lactamase VIM-1 | −1.42 | 0.00 | 2.25 | 0.00 |
| WP_002917960.1 | galactarate dehydratase | 1.61 | 0.04 | 2.19 | 0.00 |
| WP_004148220.1 | 1,2-phenylacetyl-CoA epoxidase subunit A | 1.84 | 0.05 | 2.15 | 0.00 |
| WP_019705815.1 | LysM peptidoglycan-binding domain-containing protein | 1.80 | 0.00 | 2.08 | 0.00 |
| WP_004143718.1 | hypothetical protein | 2.32 | 0.21 | 2.04 | 0.00 |
| WP_000124850.1 | 50S ribosomal protein L20 | 1.77 | 0.06 | 2.01 | 0.03 |
| WP_002909082.1 | lipoprotein | 1.74 | 0.01 | 1.93 | 0.00 |
| WP_110244503.1 | carbon starvation-induced protein CsiD | 1.81 | 0.00 | 1.92 | 0.00 |
| WP_002907763.1 | alkene reductase | 1.60 | 0.03 | 1.87 | 0.01 |
| WP_002915259.1 | L-serine ammonia-lyase | 1.78 | 0.00 | 1.85 | 0.00 |
| WP_023302381.1 | 2′,3′-cyclic-nucleotide 2′-phosphodiesterase | 1.13 | 0.10 | 1.84 | 0.00 |
| WP_023301830.1 | ABC transporter substrate-binding protein | 1.45 | 0.00 | 1.83 | 0.00 |
| WP_002907918.1 | membrane protein | 1.88 | 0.03 | 1.81 | 0.02 |
| WP_002915106.1 | RNA polymerase sigma factor RpoS | 1.87 | 0.00 | 1.81 | 0.00 |
| WP_004185056.1 | ethanolamine utilization acetate kinase EutQ | 1.86 | 0.00 | 1.80 | 0.00 |
| WP_002916849.1 | DUF1190 family protein | 1.68 | 0.02 | 1.77 | 0.00 |
| WP_002914189.1 | multidrug efflux RND transporter periplasmic adaptor subunit OqxA | 1.52 | 0.00 | 1.75 | 0.00 |
| WP_002907759.1 | superoxide dismutase SodC2 | 1.20 | 0.15 | 1.74 | 0.02 |
| WP_002889376.1 | ribonuclease HII | 1.45 | 0.03 | 1.74 | 0.05 |
| WP_001144069.1 | 30S ribosomal protein S21 | 1.36 | 0.15 | 1.71 | 0.05 |
| WP_004151997.1 | ethanolamine utilization microcompartment protein EutK | 1.45 | 0.01 | 1.71 | 0.00 |
| WP_004152003.1 | aldehyde dehydrogenase EutE | 1.68 | 0.00 | 1.70 | 0.00 |
| WP_002923306.1 | 6-phospho-alpha-glucosidase | 1.75 | 0.01 | 1.70 | 0.00 |
| WP_004157740.1 | iron uptake system protein EfeO | 1.41 | 0.01 | 1.69 | 0.00 |
| WP_023301626.1 | phosphate acetyltransferase | 1.64 | 0.00 | 1.69 | 0.00 |
| WP_004152644.1 | single-stranded DNA-binding protein | 1.67 | 0.02 | 1.68 | 0.02 |
| WP_002912948.1 | hypothetical protein | 1.39 | 0.00 | 1.68 | 0.00 |
| WP_004900870.1 | tryptophan permease | −7.75 | 0.00 | 1.68 | 0.00 |
| WP_023302039.1 | peptide ABC transporter substrate-binding protein | 1.43 | 0.00 | 1.68 | 0.00 |
| WP_020802835.1 | DUF523 domain-containing protein | 1.46 | 0.24 | 1.66 | 0.00 |
| WP_002898195.1 | L,D-transpeptidase | 1.29 | 0.04 | 1.66 | 0.02 |
| WP_023301761.1 | U32 family peptidase | 1.75 | 0.02 | 1.66 | 0.00 |
| WP_004174759.1 | amino acid ABC transporter substrate-binding protein | 1.28 | 0.01 | 1.65 | 0.00 |
| WP_002914339.1 | multidrug export protein EmrA | 1.19 | 0.02 | 1.63 | 0.00 |
| WP_004184243.1 | ABC transporter ATP-binding protein | 1.49 | 0.00 | 1.61 | 0.01 |
| WP_023301892.1 | polyphosphate kinase 2 | 1.42 | 0.04 | 1.59 | 0.02 |
| WP_004144787.1 | nucleoside permease | 1.30 | 0.07 | 1.53 | 0.01 |
| WP_004174905.1 | ethanolamine utilization microcompartment protein EutL | 1.56 | 0.00 | 1.53 | 0.00 |
| WP_002885659.1 | RNA-binding protein Hfq | 1.44 | 0.05 | 1.52 | 0.00 |
| WP_004174538.1 | N-acetylmuramoyl-L-alanine amidase | 1.35 | 0.00 | 1.52 | 0.00 |
| WP_002907788.1 | cyclopropane fatty acyl phospholipid synthase | 1.45 | 0.00 | 1.52 | 0.00 |
| Accession No. | Description of the Protein | FC 1/8 MIC /NAb | p-Value 1/8 MIC /NAb | FC 1/4 MIC /NAb | p-Value 1/4 MIC /NAb |
|---|---|---|---|---|---|
| WP_004118241.1 | Fe(3+)-dicitrate ABC transporter substrate-binding protein FecB | −2.61 | 0.02 | −2.51 | 0.01 |
| WP_001287521.1 | transcription termination/antitermination protein NusG | −2.40 | 0.01 | −2.46 | 0.00 |
| WP_004152286.1 | LacI family DNA-binding transcriptional regulator | −2.33 | 0.01 | −2.34 | 0.00 |
| WP_001293886.1 | DUF86 domain-containing protein | −2.35 | 0.00 | −2.33 | 0.00 |
| WP_004152117.1 | Hsp20/alpha crystallin family protein | −2.19 | 0.01 | −2.20 | 0.00 |
| WP_004152102.1 | hypothetical protein | −2.27 | 0,.00 | −2.13 | 0.00 |
| WP_004152116.1 | heat shock survival AAA family ATPase ClpK | −2.17 | 0.00 | −2.12 | 0.00 |
| WP_003159185.1 | recombinase family protein | −2.11 | 0.01 | −2.06 | 0.00 |
| WP_004183942.1 | Heat shock protein | −1.56 | 0.03 | −2.04 | 0.,00 |
| WP_004145074.1 | heat shock chaperone IbpB | −1.80 | 0.04 | −2.03 | 0.00 |
| WP_032488579.1 | AAC(6′)-Ib family aminoglycoside 6′-N-acetyltransferase | −2.17 | 0.07 | −1.98 | 0.00 |
| WP_009483782.1 | diguanylate cyclase | −2.09 | 0.01 | −1.98 | 0.00 |
| WP_003032875.1 | Cu(+)/Ag(+) sensor histidine kinase | −2.06 | 0.00 | −1.97 | 0.,00 |
| WP_110244509.1 | choline transporter | −1.93 | 0.00 | −1.95 | 0.00 |
| WP_000018326.1 | aminoglycoside O-phosphotransferase APH(3′)-Ia | −1.97 | 0.00 | −1.94 | 0.00 |
| WP_004152099.1 | arsenical pump-driving ATPase | −1.78 | 0.00 | −1.94 | 0.01 |
| WP_004098955.1 | copper-translocating P-type ATPase | −1.91 | 0.03 | −1.91 | 0.00 |
| WP_002210514.1 | NAD(P)-dependent oxidoreductase | −1.83 | 0.00 | −1.89 | 0.00 |
| WP_004152280.1 | TonB-dependent siderophore receptor | −1.89 | 0.00 | −1.88 | 0.00 |
| WP_004151523.1 | heat shock protein IbpA | −1.70 | 0.03 | −1.88 | 0.00 |
| WP_004152291.1 | ATPase AAA | −1.88 | 0.00 | −1.86 | 0.00 |
| WP_004152097.1 | arsenate reductase | −1.94 | 0.01 | −1.83 | 0.00 |
| WP_000777555.1 | trimethoprim-resistant dihydrofolate reductase DfrA1 | −1.90 | 0.00 | −1.82 | 0.00 |
| WP_004152282.1 | iron-dicitrate ABC transporter ATP-binding subunit | −2.13 | 0.01 | −1.82 | 0.01 |
| WP_004152290.1 | ATPase | −1.73 | 0.02 | −1.81 | 0.01 |
| WP_000523813.1 | chromosome-partitioning protein ParA | −1.80 | 0.00 | −1.79 | 0.00 |
| WP_000259031.1 | sulfonamide-resistant dihydropteroate synthase Sul1 | −1.71 | 0.00 | −1.78 | 0.00 |
| WP_001206317.1 | ANT(3′′)-Ia family aminoglycoside nucleotidyltransferase AadA1 | −1.91 | 0.00 | −1.76 | 0.00 |
| WP_002907469.1 | collagenase-like protease | −1.86 | 0.00 | −1.76 | 0.00 |
| WP_023302347.1 | phosphomethylpyrimidine synthase ThiC | −1.21 | 0.01 | −1.76 | 0.00 |
| WP_004146300.1 | thiamine phosphate synthase | −1.32 | 0.00 | −1.74 | 0.00 |
| WP_004152079.1 | efflux RND transporter periplasmic adaptor subunit | −1.83 | 0.03 | −1.72 | 0.00 |
| WP_004177871.1 | thiazole biosynthesis adenylyltransferase ThiF | −1.21 | 0.08 | −1.64 | 0.00 |
| WP_004152308.1 | thiazole synthase | −1.11 | 0.11 | −1.61 | 0.00 |
| WP_004152062.1 | ParB/RepB/Spo0J family plasmid partition protein | −1.70 | 0.01 | −1.60 | 0.01 |
| WP_004152103.1 | hypothetical protein | −1.70 | 0.10 | −1.56 | 0.00 |
| WP_001188930.1 | DNA-binding response regulator | −1.56 | 0.04 | −1.53 | 0.01 |
| WP_004152101.1 | transcriptional regulator | −1.69 | 0.11 | −1.51 | 0.04 |
| WP_004152720.1 | type II toxin–antitoxin system RelE/ParE family toxin | −1.43 | 0.05 | −1.51 | 0.00 |
| Gene | Name | Conferring Resistance Against (Class-Subclass) |
|---|---|---|
| sul1 | Sulfonamide-resistant dihydropteroate synthase Sul1 | Sulfonamide |
| aac(6′)-Ib | AAC(6′)-Ib family aminoglycoside 6′-N-acetyltransferase gene family | Aminoglycosides-Gentamicin |
| aph(3′)-Ic | Aminoglycoside O-phosphotransferase APH(3′′)-Ic | Aminoglycosides-Streptomycin |
| dfrA1, ant(3″)-Ia | Trimethoprim-resistant dihydrofolate reductase DfrA1 gene family | Aminoglycosides-Spectinomycin/Streptomycin |
| blaVIM-1 | Subclass B1 metallo-beta-lactamase VIM-1 | Beta-lactamases-Carbapenem |
| blaSHV-200 | Class A beta-lactamase SHV-200 | Beta-lactamases-Penicililins, cephalosporins |
| blaKPC-2 | Carbapenem-hydrolyzing class A beta-lactamase KPC-2 | Beta-lactamases-Carbapenem |
| blaTEM-1 | Broad-spectrum class A beta-lactamase TEM-1 | Beta-lactamases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaén-Luchoro, D.; Salvà-Serra, F.; Piñeiro-Iglesias, B.; Marathe, N.; Moore, E.R.B.; Karlsson, R. Insights into the Metabolic Adaptations of a Carbapenem-Resistant Klebsiella pneumoniae Strain on Exposure to Sublethal Concentrations of Ertapenem. Int. J. Mol. Sci. 2025, 26, 8988. https://doi.org/10.3390/ijms26188988
Jaén-Luchoro D, Salvà-Serra F, Piñeiro-Iglesias B, Marathe N, Moore ERB, Karlsson R. Insights into the Metabolic Adaptations of a Carbapenem-Resistant Klebsiella pneumoniae Strain on Exposure to Sublethal Concentrations of Ertapenem. International Journal of Molecular Sciences. 2025; 26(18):8988. https://doi.org/10.3390/ijms26188988
Chicago/Turabian StyleJaén-Luchoro, Daniel, Francisco Salvà-Serra, Beatriz Piñeiro-Iglesias, Nachiket Marathe, Edward R. B. Moore, and Roger Karlsson. 2025. "Insights into the Metabolic Adaptations of a Carbapenem-Resistant Klebsiella pneumoniae Strain on Exposure to Sublethal Concentrations of Ertapenem" International Journal of Molecular Sciences 26, no. 18: 8988. https://doi.org/10.3390/ijms26188988
APA StyleJaén-Luchoro, D., Salvà-Serra, F., Piñeiro-Iglesias, B., Marathe, N., Moore, E. R. B., & Karlsson, R. (2025). Insights into the Metabolic Adaptations of a Carbapenem-Resistant Klebsiella pneumoniae Strain on Exposure to Sublethal Concentrations of Ertapenem. International Journal of Molecular Sciences, 26(18), 8988. https://doi.org/10.3390/ijms26188988







