1. Introduction
Antithrombin (AT), a serpin-class protein, is a key inhibitor of procoagulant proteases and a central component of the natural anticoagulant system that preserves hemostatic balance (
Figure S1). Heparin polysaccharide chains act as an essential cofactor that enhances AT’s inhibitory activity against multiple coagulation proteases [
1]. Owing to the potent multi-target anticoagulant activity of the AT–heparin complex, heparin remains the most widely used anticoagulant drug. Long heparin chains simultaneously bind AT and target proteases, facilitating inhibition by bridging the transient Michaelis complex formed between the two proteins (
Figure 1). Within these chains, a specific pentasaccharide sequence (H5) allosterically activates AT, enhancing its reactivity toward factors IXa and Xa (FIXa, FXa) (
Figure S2). Notably, the heparin-binding site (HBS) and the reactive center loop (RCL)—which interacts with the protease—reside at opposite ends of the AT structure (
Figure 2). Although the AT structural changes underlying the allosteric transition from the native activity-silent (R-repressed) to the heparin-bound activated (AH) conformation have been elucidated, the specific residues comprising the allosteric communication network (ACN) that links the HBS and RCL, as well as the operational mechanism of this network, remained incompletely defined. A detailed understanding of this allosteric mechanism at the residue level would enable the rational design of fully activated heparin-independent AT-based anticoagulants with broad specificity. Such therapeutics could potentially replace heparin and mitigate the risk of heparin-induced thrombocytopenia (HIT).
The structures of the AH–FXa and AH-FIXa Michaelis complexes revealed that only the RCL P1-R393 residue, which binds into the S1 pocket of the enzyme’s active site, and the adjacent exosite pocket, which is bound by an Arg residue located in a loop by the enzyme’s active site, engage with the proteases [
2,
3]. This minimal-contact interface suggests that AT has evolved to minimize its intrinsic reactivity toward proteases by limiting protein–protein interactions [
4,
5,
6]. During the allosteric transition from the silenced (R) to the activated (AH) form, both the exosite pocket and the P1-R393 residue become accessible for reaction with FIXa or FXa [
7,
8,
9,
10].
The HBS and the RCL/exosite domains are structurally connected through the protein core, which undergoes conformational rearrangements during the R-to-AH transition [
11]. In particular, the helix D (hD) and βsA domains undergo structural shifts upon heparin binding, which are transmitted to the RCL/exosite domain. This highlights the central role of the protein core in repositioning the reactive domain to enable protease recognition and inhibition [
12].
With the help of comparative structural analysis, several residues at the interdomain interfaces were identified as undergoing significant positional changes between the R and AH states. The analysis of single and multiple mutations in these residues by protease inhibition reaction kinetics and thermodynamics, protein thermal stability assays, and molecular dynamic simulations (MDS) revealed that these residues are part of an ACN that functions as a molecular lock, silencing the R form and preventing activation in the absence of heparin. The disruption of this ACN by mutagenesis released the locked conformation, producing constitutively activated native AT variants (A*) that react independently of heparin. Additional mutagenesis studies at residues connecting the RCL with the body of the protein further support a mechanism in which structural signals from the core are relayed to the reactive site, enabling its activation.
3. Discussion
The present studies on the allosteric activation of AT build upon previous structural and kinetic analyses demonstrating that the AT inhibitory reactivity toward FXa requires the allosteric, heparin-dependent activation of the RCL/exosite domain [
2,
7]. These past studies also established that the HBS and the RCL/exosite domains are structurally linked through core domains that undergo rearrangements upon heparin binding, resulting in significant conformational differences between the native, activity-silent (R) and heparin-bound, activated (AH) forms [
11]. In the present study, through computational structural analysis, we identified an evolutionarily conserved network of residues extending from the HBS to the RCL/exosite domain, consistent with the architecture of an ACN that mediates the transmission of conformational changes. Our findings align with a previous report that mapped global structural shifts and highlighted several of the same residues [
19]. The mutational analysis of residues in this network showed significant increments in the reactivity of native AT, supporting a model in which this network functions to maintain R in an activity-silent conformation. Together, these results support a mechanistic model in which the R form is stabilized through a locking system that is allosterically disengaged upon heparin binding, thereby enabling the transition toward the reactive AH state.
AT functions as a two-state conformational switch (
Figure S9), in which the native A form binds heparin with significantly higher affinity than the R form. In the heparin-bound AH state, the RCL/exosite domain adopts an exposed, reactive configuration exhibiting a second-order association rate constant value of ~1 × 10
6 M
−1s
−1 toward FXa. In contrast, in the R state, the RCL P1-R393 residue and the exosite are inaccessible for protease interaction, thereby suppressing reactivity [
10]. The reactivity of the R form with FXa has been estimated at approximately 0.5–1 × 10
3 M
−1s
−1, based on the kinetic analyses of AT variants with their RCL hinge residues P14–S380 or P13–E381 immobilized [
12,
20]. These values support a model in which the basal reactivity of AT in the native equilibrium mixture (R ⇄ A), measured at ~4 × 10
3 M
−1s
−1, results from a population distribution heavily skewed toward the R state—approximately 99.5% R and 0.5% A. This extremely biased equilibrium reflects a stabilization energy that is remarkably high compared to that of most other proteins, underscoring the tightly controlled nature of AT’s allosteric regulation.
Achieving the native equilibrium bias (approximately 99.5% R) likely depends on strong cooperative interactions among the HBS, hD, βsA, and RCL/exosite domains. Our previous scanning mutagenesis study targeting the RCL P14–S380 residue demonstrated that changes in native reactivity correlate with shifts in protein melting temperature [
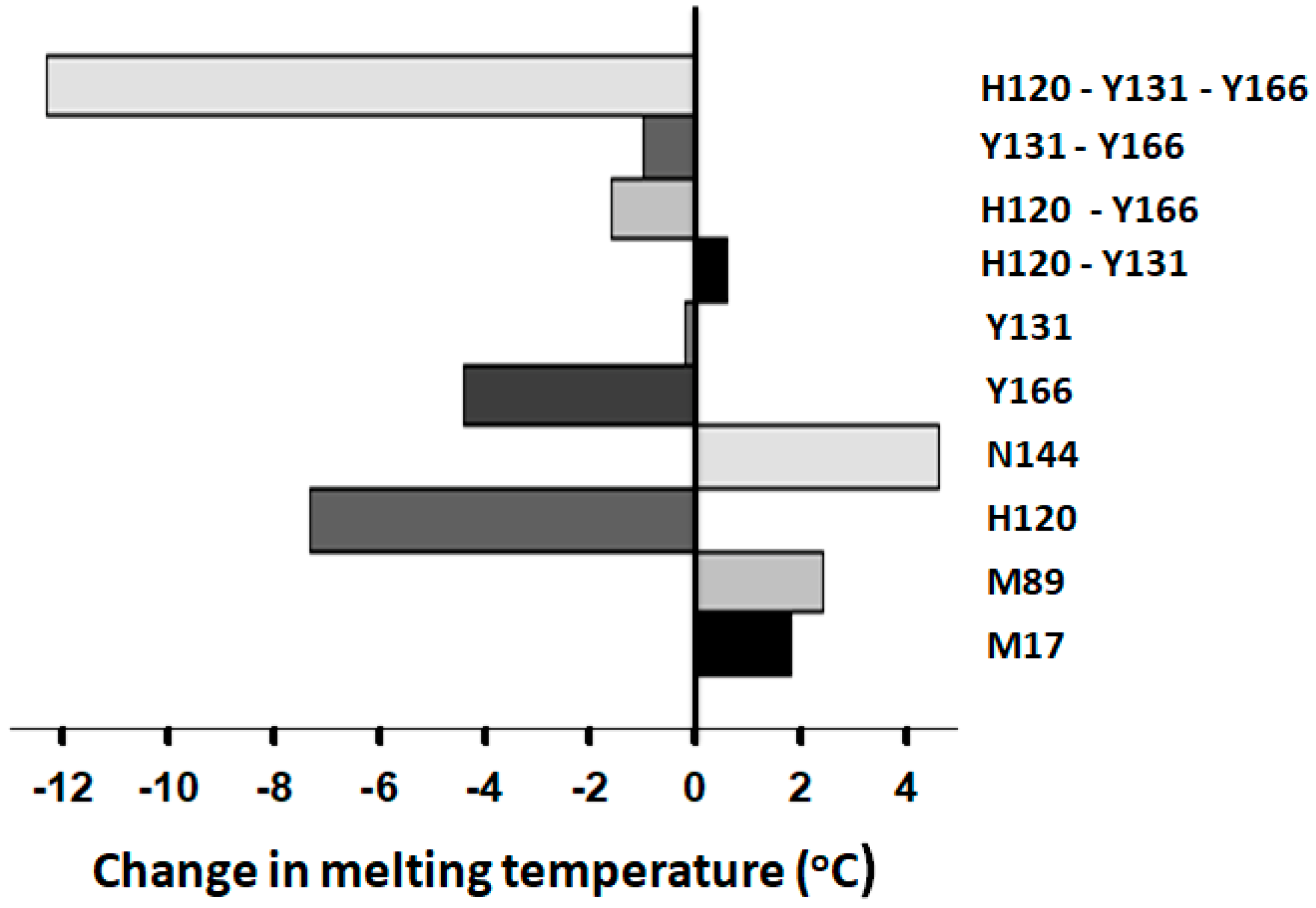
16]. Specifically, shifts toward the R form resulted in increased thermal stability, while shifts favoring the A form were associated with decreased melting temperatures. This inverse relationship indicates that the R state is stabilized by interactions that are weakened or lost in the A conformation.
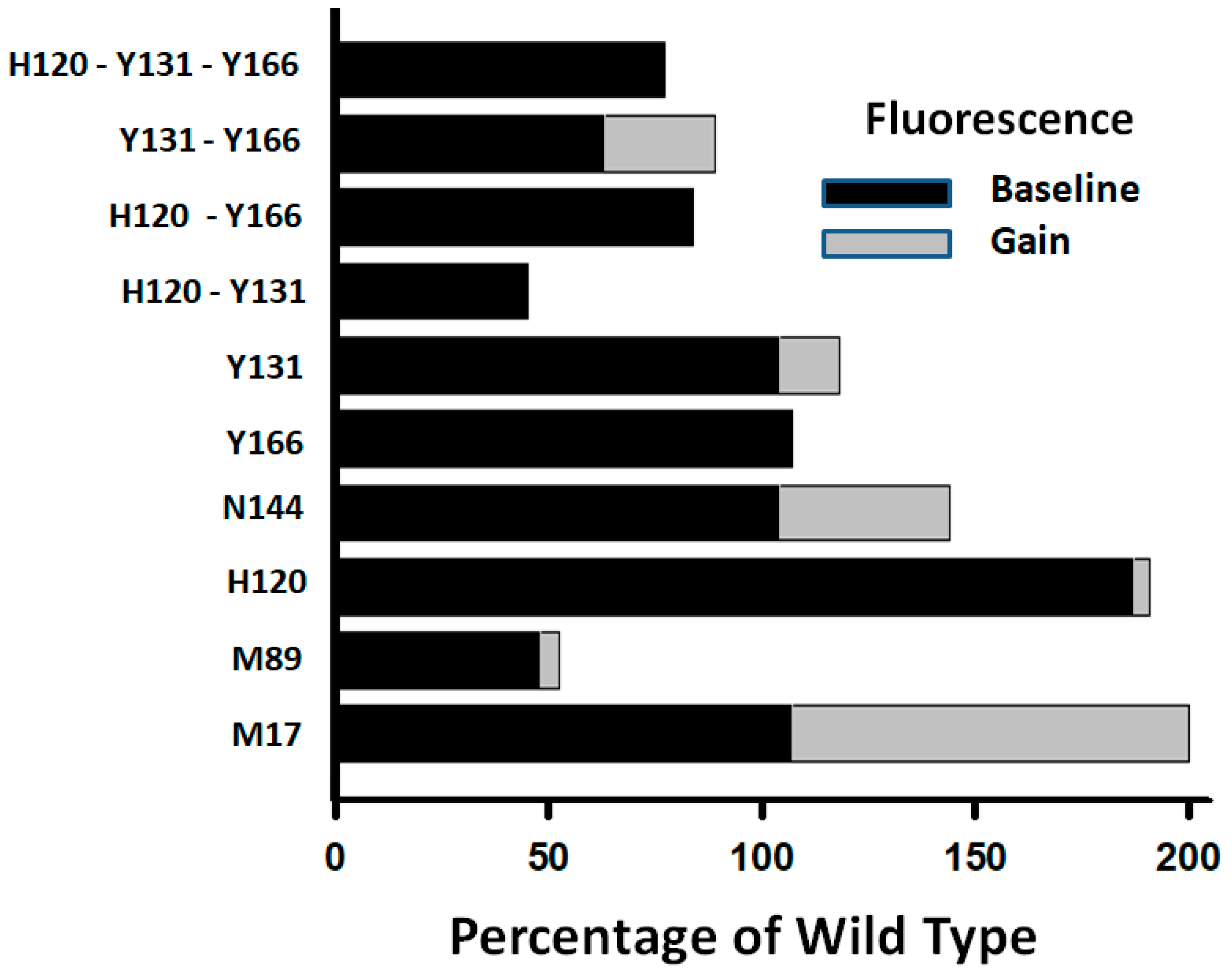
The results of the present study are consistent with this interpretation. Mutations of the ACN residues H120, Y131, and Y166 significantly increased native AT reactivity (A* forms) toward FXa and were accompanied by substantial reductions in melting temperature, indicating a destabilization of the R form. Additionally, proteins with mutations in these ACN residues bound stronger to immobilized heparin during purification than the WT protein, suggesting a shift in conformation toward the activated A state. These mutants also exhibited enhanced reactivity toward FXa in the presence of H5, confirming that they remained responsive to heparin activation. However, unlike the WT protein, the mutants failed to show the typical ~40% increase in tryptophan fluorescence upon heparin binding, a signal we routinely use to quantify binding affinity. This loss of the gain in fluorescence further supports the idea that these mutants exist in conformations resembling that of the AH form.
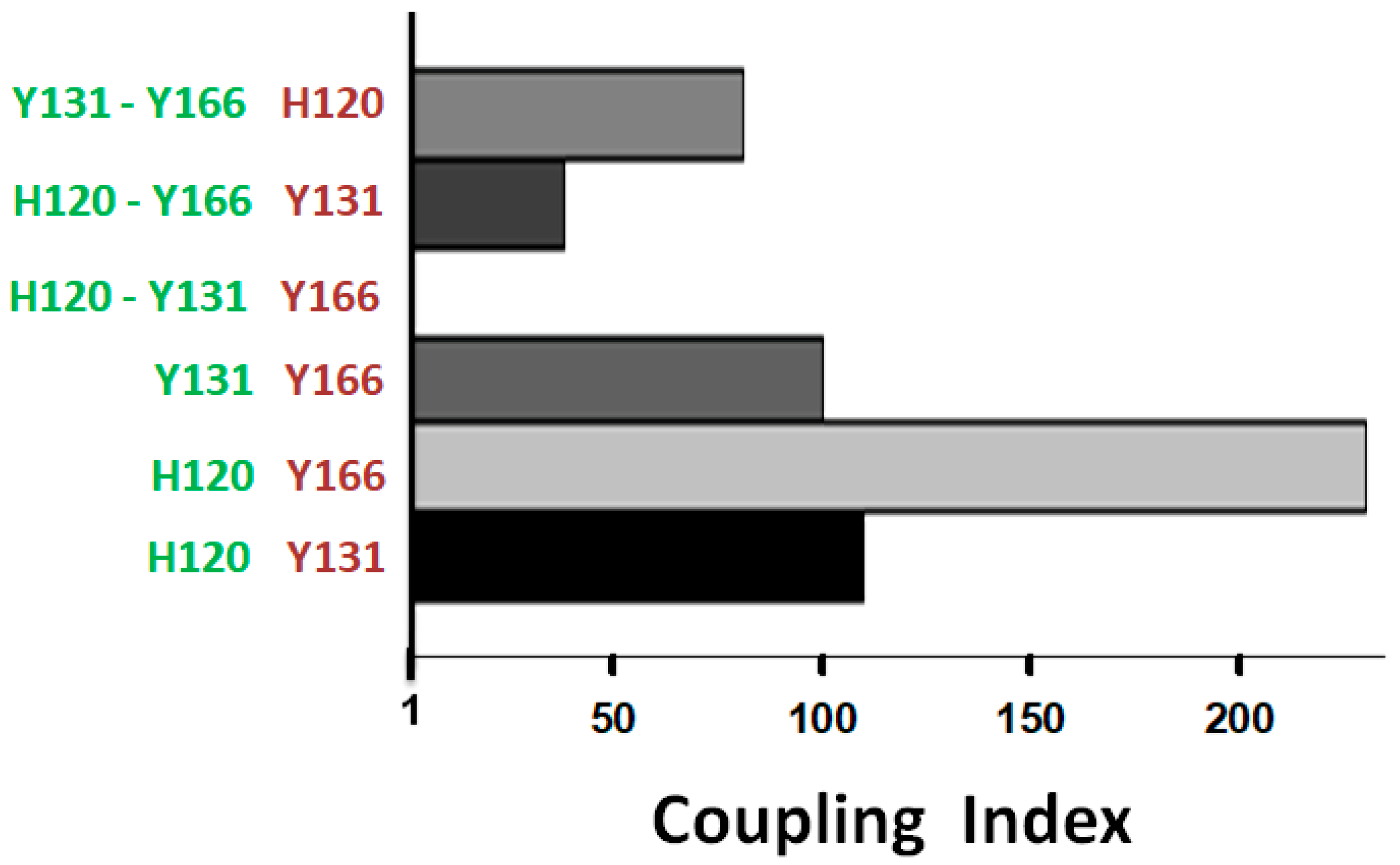
Moreover, the effects of the combined mutations on reactivity were found to be strongly thermodynamically coupled, suggesting cooperative interactions among H120, Y131, and Y166 in regulating the allosteric mechanism. Together, these findings support our hypothesis that high-energy interactions involving the ACN residues act collectively to stabilize the structurally repressed, and activity-silent R conformation.
The HBS and RCL/exosite domains are connected via the hD and βsA domains, which adopt distinct conformations in the R and AH forms, as suggested by their crystal structures. These four domains appear to operate in a modular fashion, both structurally and functionally, as it is indicated by measurements of heparin-binding affinity with AT variants whose structural linkages between the HBS and RCL/exosite domains were disrupted [
21,
22]. Additionally, our prior studies have shown that mutations in the RCL hinge P14–S380 residue can shift the RH–AH equilibrium by decoupling local activating conformational changes and stabilizing so-called intermediate RH forms [
16]. One other crystal structure of heparin-bound AT further supports this modular model, as the structure revealed only a partial set of conformational changes typically associated with the fully activated AH form [
23]. Collectively, these findings suggest that in the native R state, each domain is held in a biased equilibrium, stabilized by strong interdomain interactions. Upon allosteric activation, these interdomain constraints are relaxed, allowing each domain to sample a broader and less restricted conformational space, consistent with a more flexible AH form.
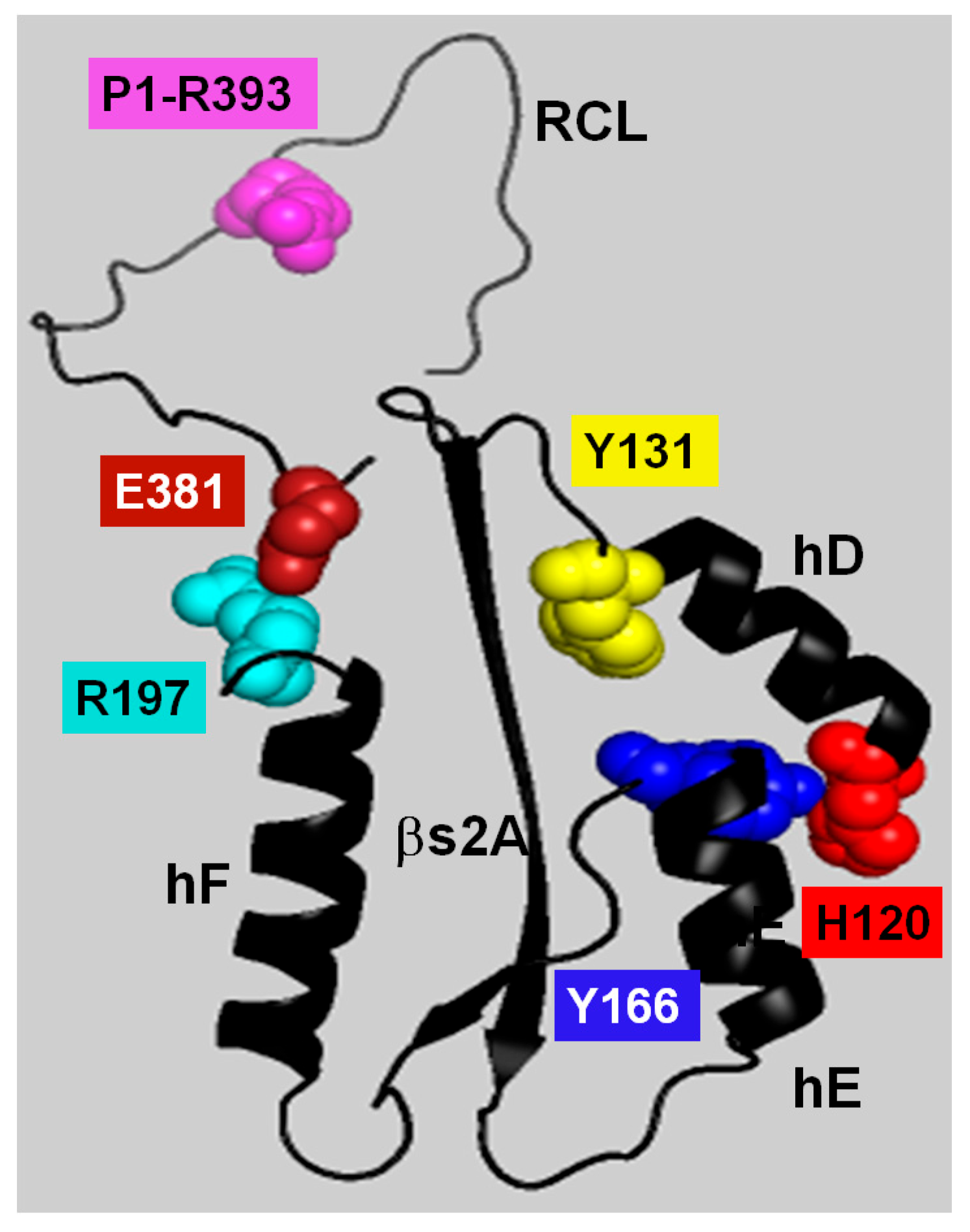
Based on the spatial positioning and displacement of the ACN residues in the R-to-AH conformational transition, along with the effects of their mutation on the AT reactivity and stability, we developed a mechanistic model that explains the role of these residues in stabilizing the R form and mediating activation upon heparin binding. This model proposes a three-step, or “slingshot”, allosteric mechanism in which the first step is the energy stored in an elastic β-sheet A where strands 3A and 5A are stretched apart. The second step is the locking or holding phase where the system is in its tensioned R state and stabilized by the residues H120, Y131, and Y166 that prevent the premature release of the stored energy. The third step is the release of the stored energy triggered by the binding of heparin that disengages the latch and propells an activating conformational shift transmitted to the RCL/exosite domain.
The latching role of the locking mechanism seems to be played by the H120-Y166, and βs2A-hD-Y131 interactions. In previous studies, we forced the elongation of hD by introducing helix stabilizing mutations, thereby demonstrating that this extension alone—along with removal of Y131—was sufficient to induce significant activation [
24,
25]. At the bottom of hD, H120 strenghtens the non-extended conformation of the helix by forming a stabilizing interaction with Y166, and this interaction in turn helps maintain a rigid hD-βs2A structure. The binding of heparin release the lock by forcing H120 away from Y166, therefore extenings hD and loosening the hD-βs2A interaction. Simultaneously, Y166 inserts between N144 and M89 into a secure position. The single H120V mutation showed the central role this residue plays in the locking mechanism by triggering the largest activating effect.
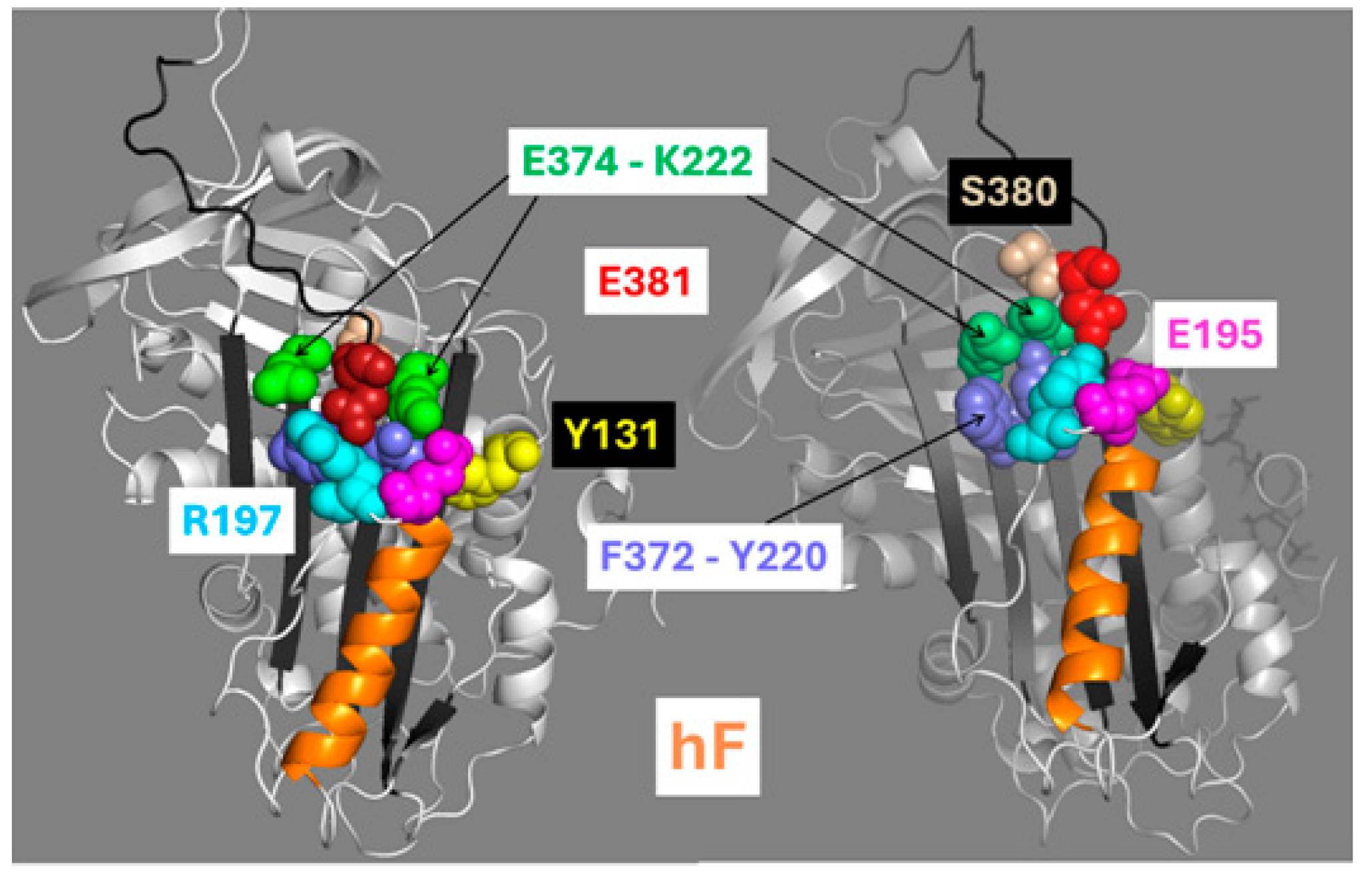
Our past models of the mechanism for the transmission of structural changes to the RCL were influenced by two prominent conformational changes in the R-to-AH transition revealed by crystallography: (1) the closing of the gap between β-strands 3A and 5A, and (2) the expulsion of the RCL hinge region—specifically residues P13-E381 and P14-S380—from this gap. These structural rearrangements should therefore expose the P1 residue and bring residues Y220 and K222 in βs3A into closer proximity with F372 and E374 in βs5A to form the stabilizing pairs Y220–F372 (hydrophobic) and K222–E374 (electrostatic). However, the present MDS studies suggest that the allosteric transmission of conformational changes may not require the expulsion of the RCL hinge from its insertion between β-strands 3A and 5A. However, it is important to point out that the length of the MDSs probably does not cover the whole R-to-A* structural transitions, and that adding heparin to the FXa inhibition reactions with the ACN mutants still further enhanced reactivity.
A distinctive feature of the RCL hinge is the salt bridge between the RCL P13-E381 and R197 residues. As reported by the crystal structures, this salt bridge is preserved after activation. The disruption of this bridge in the present study confirmed its critical role in mediating the allosteric communication necessary for activation. It also showed that the position of E381 is stabilized by the insertion of the RCL P14-S380 residue into a pocket between β-strands 3A and 5A. The opposing forces between R197 and S380 seem to stabilize the E381 position around a critical threshold. Our previous mutagenesis studies have shown that mutations that reinforce S380 insertion—such as those that increase side chain bulk—further stabilize the RCL hinge and reduce AT reactivity by hindering the movement of E381 [
16]. In contrast, mutations like S380G increased reactivity by weakening the insertion of the P14 residue, thereby loosening the anchoring of E381 and facilitating its movement. The disruption of the E381–R197 salt bridge seems to have altered this stability threshold. For example, the E381G mutation eliminated the salt bridge and led to reduced reactivity, as was also observed in a previous study with the E381A mutation [
26]. Without an anchored E381 to hold the RCL in place, S380 adopts a more deeply inserted position, thus reinforcing RCL stability and reducing reactivity. Another example is the electrostatic charge-switch mutation between E195 and R197 that appears to have redirected the E381 salt bridge from R197 to R195. Since R195 lies farther from the βs3A–βs5A gap, this shift would pull the RCL away and decrease S380 insertion, therefore, partially activating the protein. Notably, the combination of the S380G and charge-switch mutations resulted in a synergistic increase in reactivity that exceeded by far the additive effects of each mutation alone, suggesting that S380 and E381 act in a cooperative manner to regulate RCL positioning. In contrast, combining the S380G mutation with Y131L did not yield cooperative effects, indicating that Y131 operates independently from the RCL hinge. Overall, these findings underscore the importance of a precise tuning of the interplay between S380 insertion and the E381–R197 salt bridge that is essential in setting the correct response threshold for proper RCL movement and regulation of the P1-R393 residue.
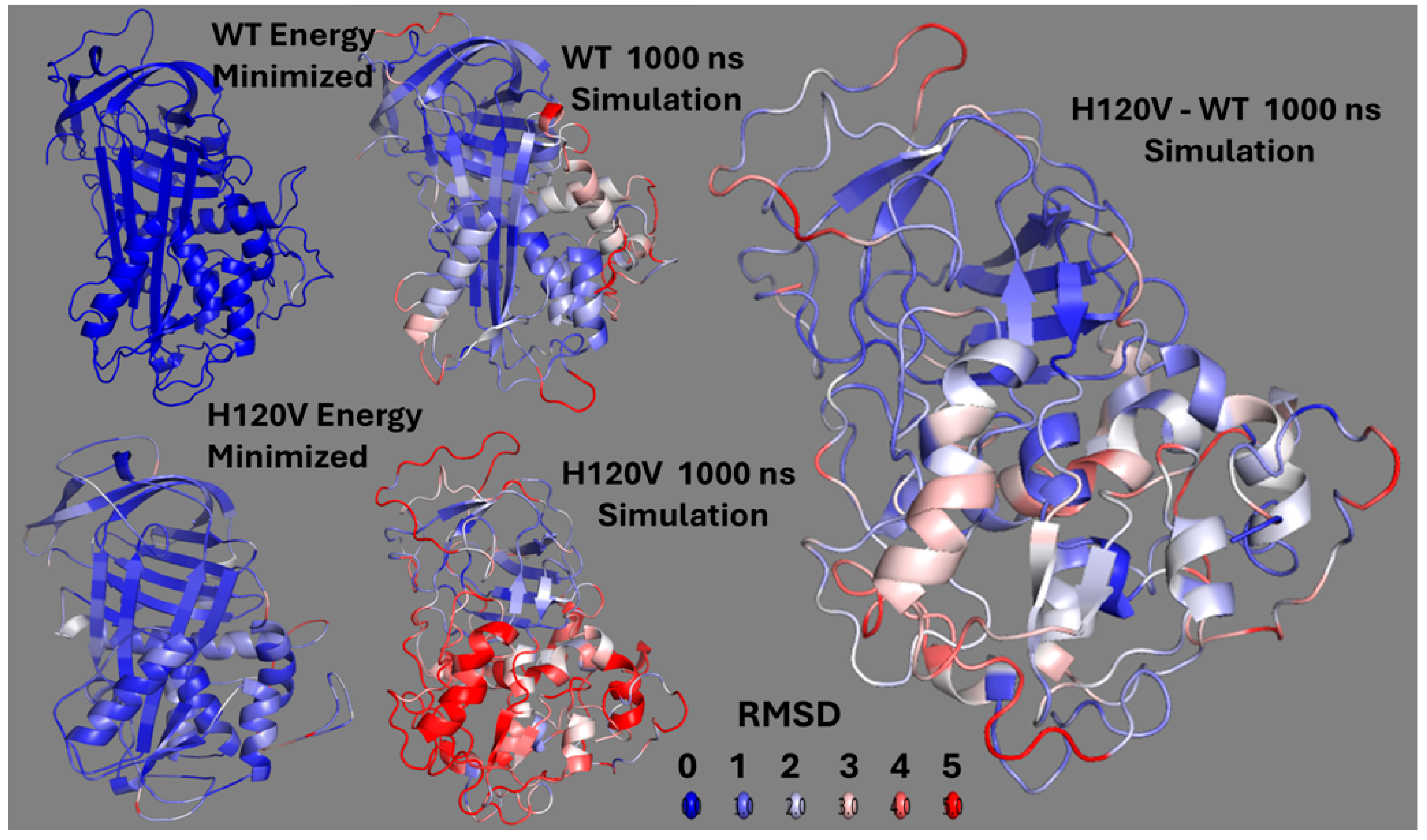
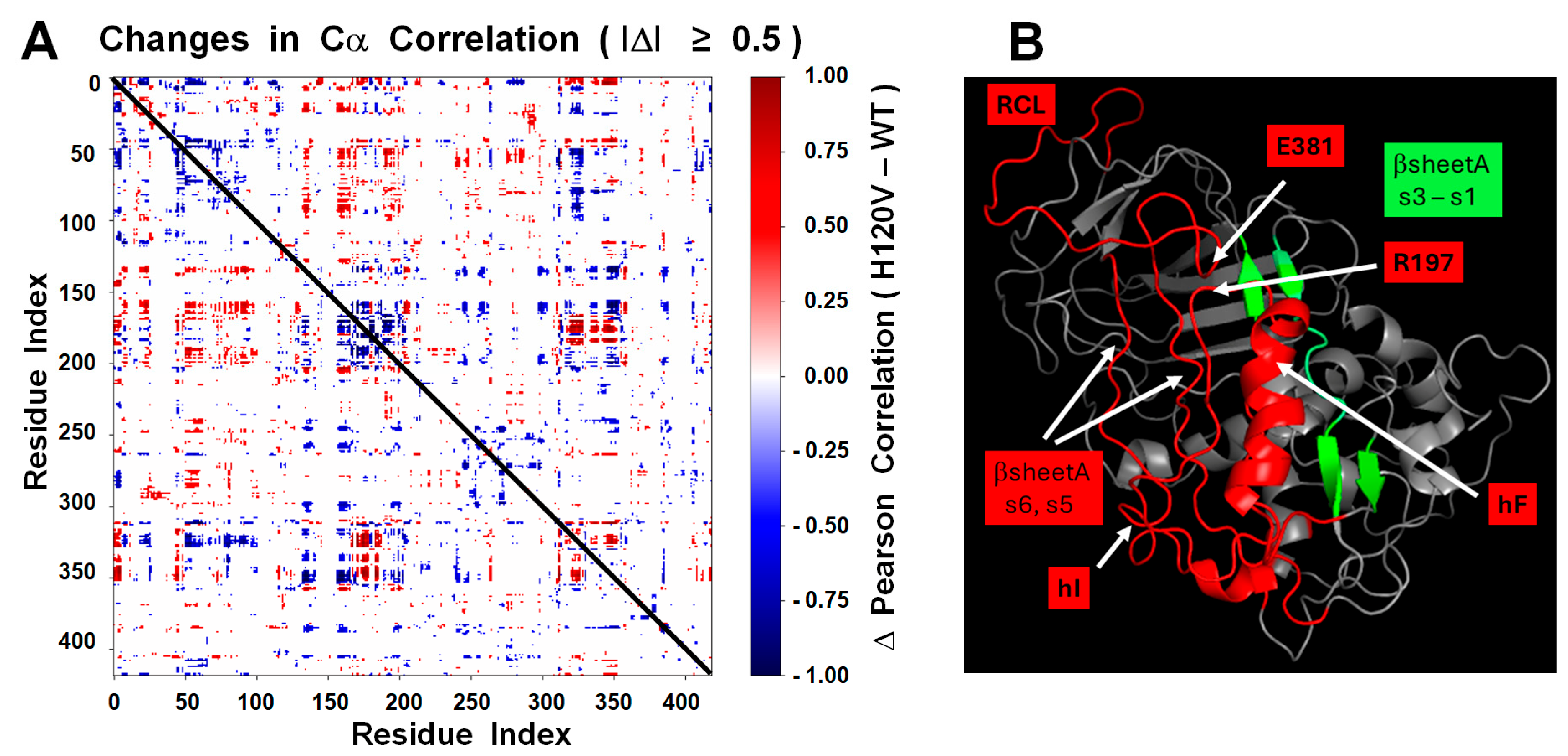
The solid trajectory obtained by MDS of the H120V structure provided a good glimpse of the structural changes that allosterically link the HBS and RCL and that should lead to AT activation. In the WT simulation, the protein remained stable without proceeding into a trajectory. On the contrary, the H120V mutation unleashed a dramatic structural change consistent with the protein being in a tense and unstable state. Noticeably, high levels of flexibility were immeditely attained at both the HBS and RCL/exosite domains, suggestive of allosteric communication between the two protein domains. Changes in correlation among residues identified helix F as the most flexible secondary structure followed by the RCL, and strands 5 and 6 of βsA. The connection between hF and RCL through the E381-R197 bridge suggests that this is a critical route of allosteric communication between the HBS and RCL (
Figure 12).
In conclusion, our findings delineate a comprehensive mechanistic framework for the allosteric activation of AT, revealing a strong conformational locking system that stabilizes the R state and is disengaged upon heparin binding. Central to this mechanism is an evolutionarily conserved ACN, in which residues H120, Y131, and Y166 operate cooperatively to stabilize structural constraints linking the HBS to the RCL. The three-step “slingshot” model proposed here accounts for both the structural energy storage in the R state and its triggered release upon heparin binding, ultimately promoting RCL displacement and AT activation. Mutagenesis and biophysical analyses, together with MDSs, provided strong evidence for the dynamic coupling between the HBS and RCL regions, with helix F acting as conduit for allosteric transmission through the modulation of the finely tuned position of the RCL by the S380 insertion and the E381–R197 salt bridge. These insights clarify long-standing questions about the AT’s activation mechanism and highlight critical residues and interactions that may be targeted in the therapeutic modulation of its anticoagulant function.
4. Materials and Methods
4.1. Computational Analysis of Protein Structures
The structures of the heparin-free and H5-bound AT forms were aligned using the software RCSB PDB Viewer 4.10 [
27]. The crystal structures of the R (PDB code 1T1F) and AH (PDB code 1EO3) AT forms were overlayed by their backbone atoms down to an RMSD of 1.48 Ȧ. The distance of the most distal carbon atom for each residue side chain between the two structures was measured. The atomic distances are listed in
Tables S1–S3.
4.2. Protein Production
Recombinant AT variants were constructed on an N135Q background to block glycosylation as in our previous studies [
9,
12]. Site-directed mutagenesis was carried out using the Stratagene QuikChange Site-Directed Mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA) and the appropriate primers. Mutations were confirmed by DNA sequencing. Variants were produced in baculovirus-infected insect cells (sf9) using the expression system from Invitrogen (Thermo Fisher Scientific, Carlsbad, CA, USA), as described by the manufacturer. AT protein variants were purified from culture supernatants by immobilizing the protein onto heparin-Sepharose followed by elution with a sodium chloride gradient. Most proteins mutated in the ACN residues bound heparin with higher-than-normal affinity and eluted at sodium chloride concentrations higher than 2 M producing preps of over 95% purity. Otherwise, preps were run over ion exchange chromatography using a mono Q column (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA, USA). Those fractions containing pure antithrombin were finally concentrated and desalted by ultrafiltration, and concentrations were determined in 10% SDS-PAGE by comparison to calibrated concentrations of bovine serum albumin after staining with Coomassie Blue. Finally, proteins were stored in 100 mM Hepes buffer pH 7.4, 100 mM NaCl at −80 °C in aliquots.
4.3. Baseline Protein Fluorescence Measurement
Tryptophane protein fluorescence was determined in 500–800 μL of 100 mM Hepes buffer pH 7.4, 100 mM NaCl at 25 °C by adding repeated volumes of the AT solution (2.5 μM) into a quartz cuvette. Fluorescence readings (wavelengths: excitation = 280 nm, emission = 345 nm) were recorded using a PTI Spectrofluorometer (Photon Technology International, Horiba Scientific, Piscataway, NJ, USA) and adjusted for buffer fluorescence and volume changes. The linear dependence of fluorescence increments on adjusted protein concentrations was taken as a measure of the baseline protein fluorescence.
4.4. Protein Thermal Denaturation
Thermal denaturation of AT variants was monitored by the temperature-induced changes in tryptophan fluorescence measured at the same conditions as described above for baseline protein fluorescence determinations. A temperature-controlled SLM 8000 (SLM Instruments, Inc. Urbana, IL, USA) spectrofluorometer, upgraded with a temperature control (Quantum Northwest, Olis, Inc., Liberty Lake, WA, USA), was used for temperature scanning in the range from 20 to 85 °C at intervals of 2 to 5 degrees (
Figure S5). AT concentrations used were from 400 nM to 2 μM depending on the baseline fluorescence. The data were fitted to the Van’t Hoff equation to determine the melting temperature (T
m).
4.5. Protease Inhibition Reactions
Assays of protease activity reactions with chromogenic substrates were measured as described in our previous studies [
9]. Reactions were conducted at 25 °C in 0.02 M sodium phosphate buffer pH 7.4, 0.1 M NaCl, 0.1 mM EDTA, 0.1% polyethylene glycol 8000, 0.1 M S-2238 (BioMedica Diagnostics, Windsor, NS, Canada) for thrombin, and 0.1 M Hepes buffer pH 7.4, 0.1 M NaCl, 5 mM CaCl2, 0.1 mM EDTA, 0.1% polyethylene glycol 8000, 0.1 M Spectrozyme-FXa (Sekisui Diagnostics, Burlington, MA, USA) for FXa. Reactions were run in 1 mL cuvettes and the linear increase in absorbance at 405 nm was monitored using a temperature-controlled DU-640 multicell Spectrophotometer (Beckman-Coulter, Brea, CA, USA).
Second order association rate constant values for the inhibition of 3–5 nM FXa (Enzyme Research Laboratories, South Bend, IN, USA) or thrombin (U.S. Biochemical Corp, Cleveland, OH, USA) by AT variants were determined in the absence or presence of heparin pentasaccharide (Fondaparinux, Sanofi-Aventis, Toulouse, France). Reaction rates were measured under pseudo-first-order conditions by using at least 10-fold molar excess of inhibitor over protease as in previous studies [
9]. Inhibition reactions (100 μL) were conducted in 1 mL cuvettes for the assigned time and terminated by adding 900 μL of protease substrate solution for measuring the residual enzymatic activity. Residual enzyme activity values at time points that span the full inhibition reaction time courses for the exponential loss of enzyme activity were fit by a single exponential function with a zero-activity end point to obtain the pseudo-first order rate value. This value was then divided by the AT concentration (0.1–1.0 μM) to obtain the second order association rate constant value. For reactions containing heparin, the pentasaccharide was present at levels that saturated AT. Rate values were corrected by factoring the stoichiometry of inhibition of the protease by the AT variants (
Tables S4–S6).
The stoichiometries for the reactions of the AT variants with thrombin and FXa were determined from reactions in which increasing concentrations of the inhibitor were added to a fixed concentration (100 nM) of protease (25–100 μL). Reactions were carried out both in the absence and in the presence of heparin pentasaccharide, which was fixed at an equimolar concentration with the inhibitor. After incubating for times sufficient to complete the reaction (95%), 5 μL of the inhibition reaction mixture were added to 1 mL of substrate (100 μM S-2238 for thrombin or 100 μM Spectrozyme-FXa for FXa), and the residual enzymatic activity was measured from the linear increase in absorbance. The decrease in protease activity with increasing molar ratio of inhibitor/protease was fit by linear regression to obtain the stoichiometry from the abscissa intercept.
4.6. Molecular Dynamic Simulations of Antithrombin Structures
Simulations were conducted on a virtual machine (VM) provisioned through Google Cloud Platform (GCP) using a G2-standard instance type, designed for GPU-accelerated workloads. The VM was configured with the following specifications: Instance Type: G2-standard; GPU: 1× NVIDIA L4 Tensor Core GPU; CPU: 8 vCPUs; Memory: 48 GB RAM; Operating System: Ubuntu 22.04 LTS; Storage: 200 GB persistent SSD; Zone: us-central1b.
Energy minimization of the antithrombin structure was performed using the program OpenMM 8.0. The starting structure was refined using the Amber14 force field (amber14-all.xml) with the TIP3P explicit water model (amber14/tip3p.xml). The system was constructed with periodic boundary conditions and Particle Mesh Ewald (PME) electrostatics, using a nonbonded cutoff of 1.0 nm and constraints applied to hydrogen bonds (HBonds). The structure was solvated by enclosing it in a cubic water box using Modeller, with a padding distance of 10 Å from the protein surface to the box edge and then neutralized by adding the required number of counterions (Na+ or Cl−) to achieve overall charge neutrality.
The simulation used a Langevin integrator at 310 K with a friction coefficient of 1 ps
−1 and a time step of 2 fs. The potential energy of the system was minimized using steepest descent followed by the L-BFGS algorithm, limited to 10,000 iterations. The pre- and post-minimization structures were compared to calculate RMSD values using PyMOL software. The scripts to perform minimizations using OpenMM 8.0 in the VM and structural alignments using PyMOL in a local computer are available in the
Supplementary Materials.
Molecular dynamics simulations were performed using the OpenMM engine on the energy-minimized structures (AT_minimized.pdb). The Amber14 force field with TIP3P water model (amber14-all.xml, amber14/tip3p.xml) was used to parameterize the system. Long-range electrostatic interactions were handled using the PME method with a 1.0 nm nonbonded cutoff. Hydrogen-containing bond lengths were constrained using the HBonds setting, allowing a 2 femtosecond (fs) integration time step. The system was coupled to a Langevin thermostat at 310 K (1/ps friction coefficient) and a Monte Carlo barostat to maintain 1 atm pressure. Velocities were initialized according to the Maxwell-Boltzmann distribution at 310 K. The simulation was run for a total of 1000 nanoseconds, corresponding to 500 million steps. Snapshots of the atomic coordinates were saved into separate PDB files enabling structural analysis at discrete time points throughout the trajectory. The script is available in the
Supplementary Materials.
4.7. Principal Component Analysis of Antithrombin Simulations
Principal Component Analysis (PCA) was performed to compare the large-scale conformational dynamics of WT and mutant antithrombin variants over the course of 1000 ns MDS. Simulations were conducted at 2 fs timesteps, and coordinates were saved every 20 ps; therefore, each trajectory contained 50,000 frames.
Analysis focused on the protein backbone by extracting only Cα atoms from the simulation trajectories using MDTraj 1.9.9. Frames were aligned to the initial structure to remove global translational and rotational motion. The Cartesian coordinates (x, y, z) of each Cα atom were flattened per frame to form a two-dimensional matrix of shape (frames × atoms × 3), which served as input for PCA.
Dimensionality reduction was performed using the PCA implementation from scikit-learn 1.4.2 (Python 3.10). The first 20 principal components (PCs) were computed for each trajectory, and their associated variance ratios were used to assess the distribution of collective motions. Time-resolved PCA was also performed on the final 15% of each trajectory to evaluate convergence and dynamic stability.
All analyses were performed on a Google Cloud Platform virtual machine equipped with an NVIDIA A100 GPU, 24 virtual CPUs, and 96 GB RAM, running Ubuntu 22.04 LTS. Visualization of PCA results, including PC projections and explained variance trends, was performed using Matplotlib 3.8.4 and Seaborn 0.13.2. Script is available in the
Supplementary Materials.
4.8. RMSD Calculations
Root-mean-square deviation (RMSD) analyses were performed using PyMOL (version 2.5.10) to assess conformational changes in WT and mutant structures during MDS. Energy-minimized structures were aligned to their respective 1000 ns simulation endpoints using the align function in PyMOL 2.5.10 to ensure consistent backbone superposition. RMSD heatmaps were generated by running a custom Python script within PyMOL 2.5.10, which computes per-residue RMSD values between the energy-minimized and simulated structures. Visualizations were rendered using a red-white-blue color gradient representing increasing structural displacement (0–5 Å). For direct comparison between the WT and mutant simulations, all 1000 ns endpoint structures were aligned, and the atom wise RMSD values were calculated similarly to highlight mutation-induced structural divergence. Atom counts were verified to be equal across pairs to avoid alignment artifacts. Script is available in the
Supplementary Materials.
4.9. Pearson Correlation Analysis of Residue Dynamics
To quantify correlated motions between residues during MDS, Pearson correlation coefficients were computed based on the atomic displacements of Cα atoms. Trajectory processing and correlation analysis were conducted using MDTraj 1.9.9 and NumPy 1.26.4 within a Conda-based Python 3.10 environment. Frames from the final 15% of each trajectory (750–1000 ns) were extracted, aligned to the initial minimized structure to remove global rotation and translation, and reduced to only Cα atoms.
Cα atomic displacements were reshaped into 2D matrices (frames × atoms × 3 coordinates), then flattened and mean-centered. Pearson correlation coefficients were computed using NumPy’s corrcoef function, yielding a symmetric residue–residue matrix per system. Δ-correlation matrices were generated by direct subtraction of the WT matrix from the mutant matrix. The most shifted residue pairs were identified by ranking the largest positive and negative Δ-correlation values. Visualization was performed with Matplotlib 3.8.4 and Seaborn 0.13.2. The script is available in the
Supplementary Materials.