In Silico Study About Substituent Effects, Electronic Properties, and the Biological Potential of 1,3-Butadiene Analogues
Abstract
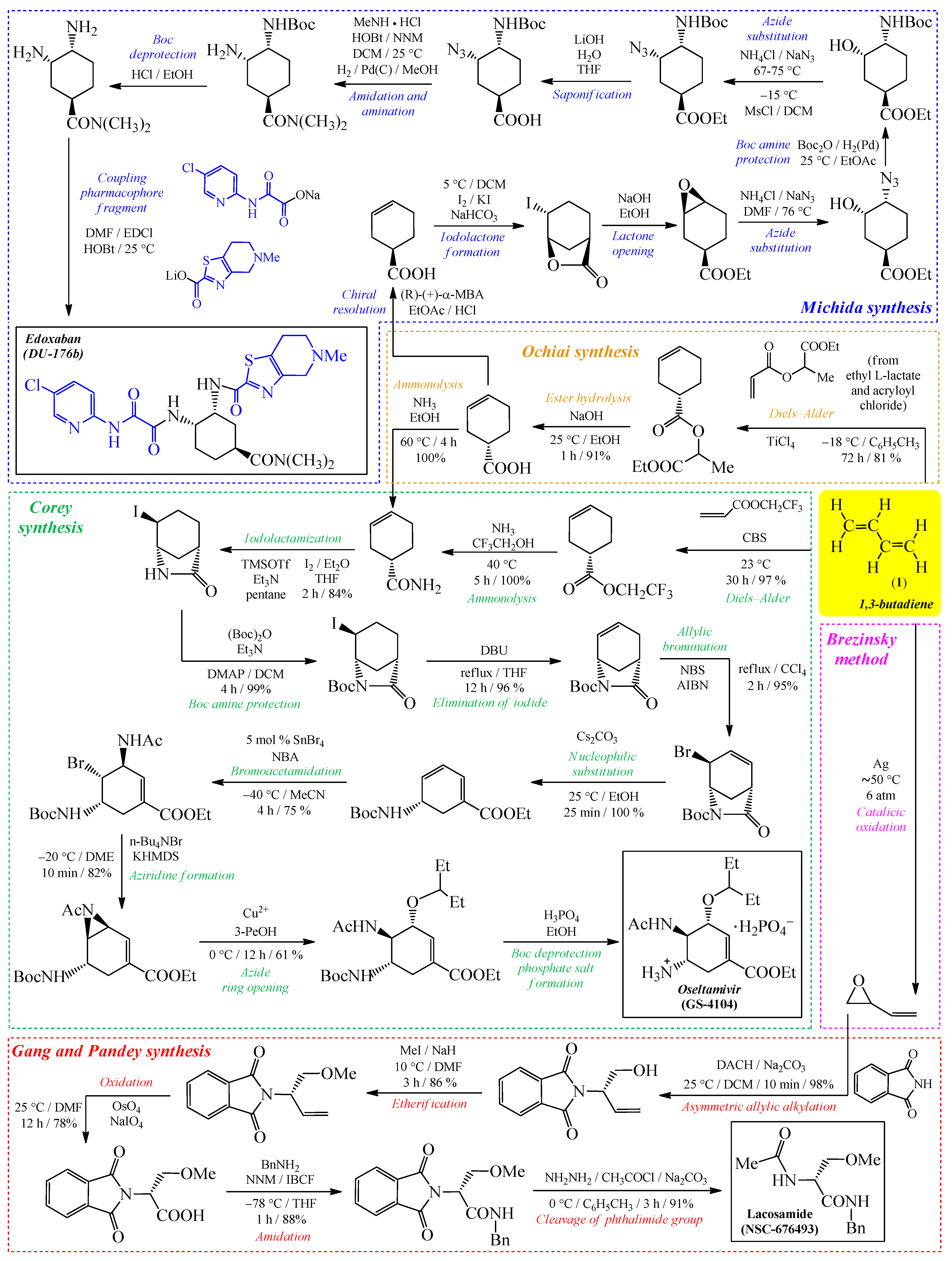
1. Introduction
2. Results and Discussion
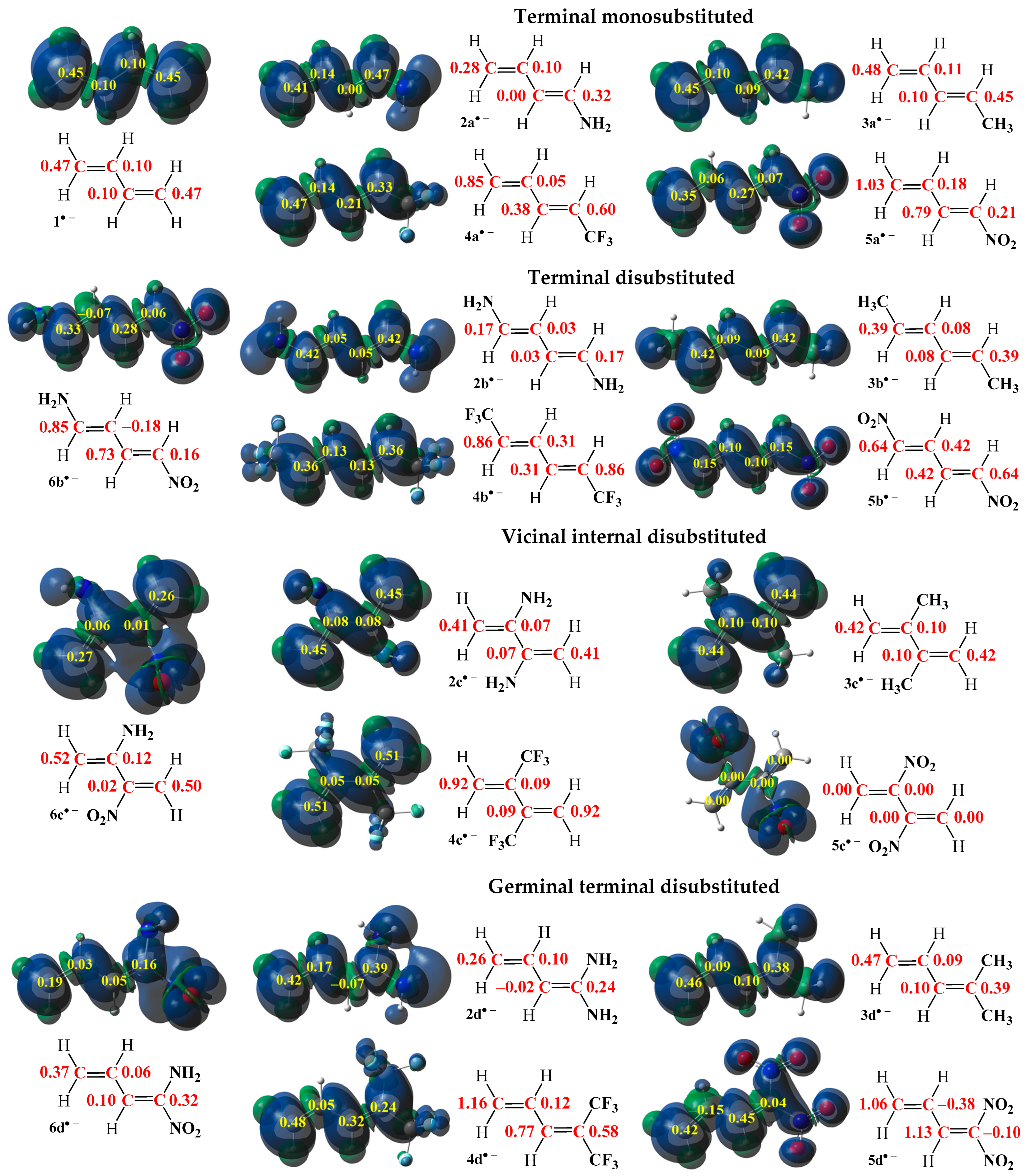
2.1. Study of Electron Density Distribution Based on MEDT
2.1.1. Analysis of the Electronic Structure Based on ELF, NPA, and MEP

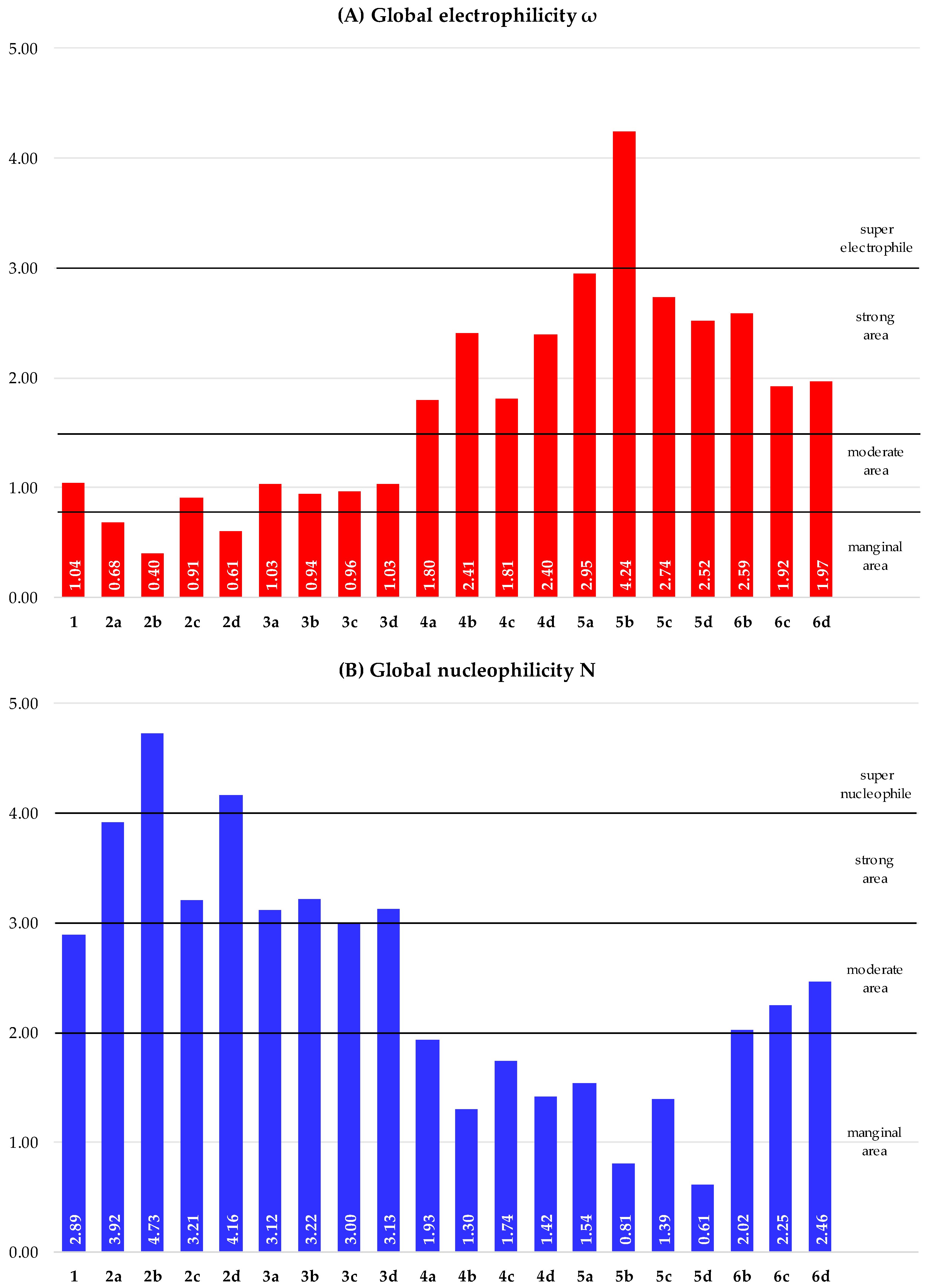
2.1.2. Analysis of the CDFT Reactivity Indices

2.2. Biological Potential Based on ADME and PASS Studies
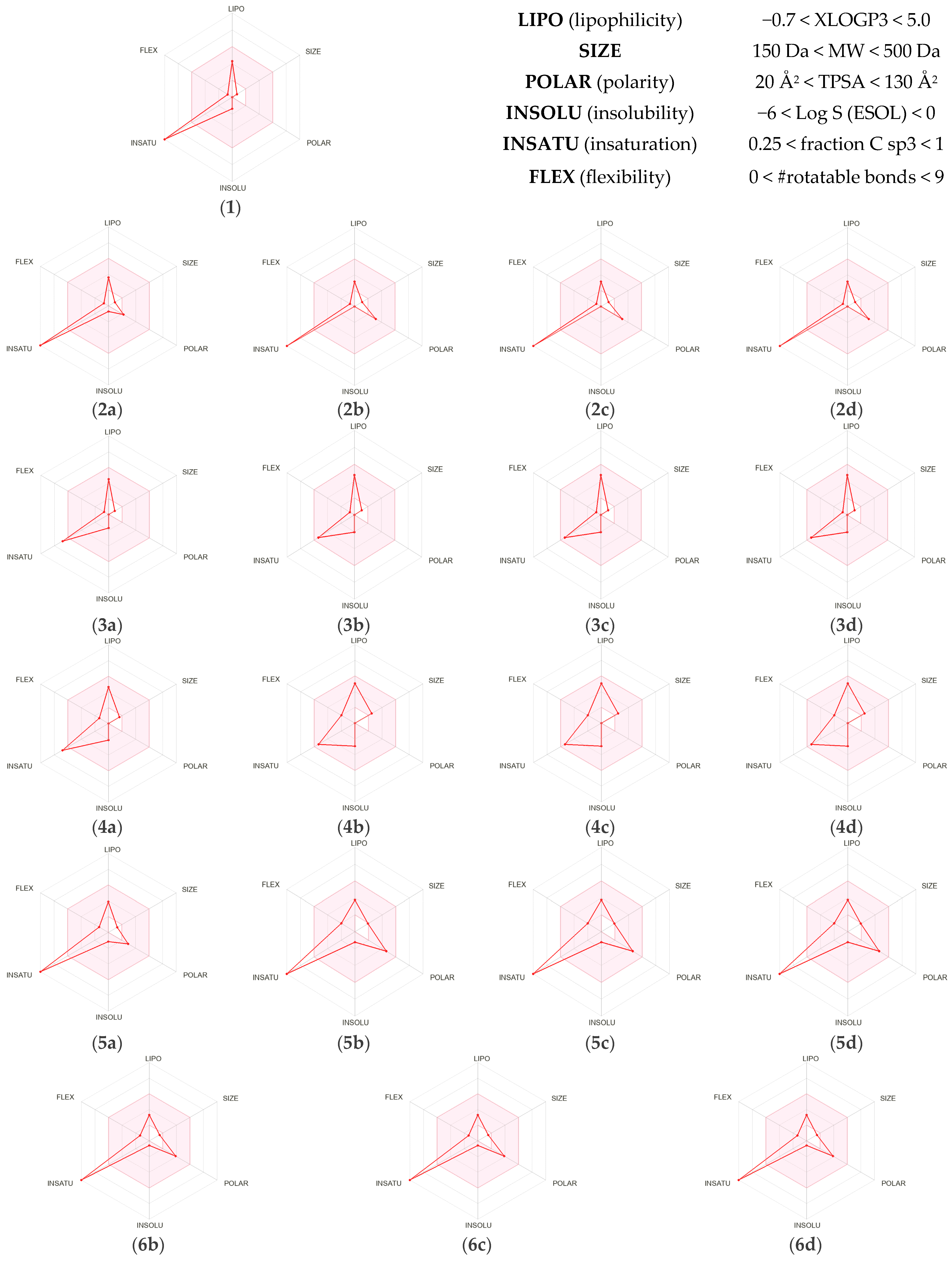
2.2.1. Analysis of Pharmacokinetic Properties and Drug-likeness Based on ADME
2.2.2. Assessment of Biological Potential Based on PASS
3. Materials and Methods
4. Conclusions and Future Prospective
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiberg, K.B.; Rablen, P.R.; Baraban, J.H. Butadiene and heterodienes revisited. J. Org. Chem. 2018, 83, 8473–8482. [Google Scholar] [CrossRef]
- Soengas, R.G.; Rodríguez-Solla, H. Modern Synthetic Methods for the Stereoselective Construction of 1,3-Dienes. Molecules 2021, 26, 249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, K.; Wang, G.; Zhang, S.; Zhu, X.; Li, C.; Shan, H. Factors influencing 1,3-butadiene formation for light alkane dehydrogenation. J. Taiwan Inst. Chem. Eng. 2020, 113, 187–197. [Google Scholar] [CrossRef]
- White, W.C. Butadiene production process overview. Chem.-Biol. Interact. 2007, 166, 10–14. [Google Scholar] [CrossRef]
- Madeira, L.M.; Portela, M.F. Catalytic oxidative dehydrogenation of n-butane. Catal. Rev. 2002, 44, 247–286. [Google Scholar] [CrossRef]
- Mukherjee, A.; Samanta, C.; Bordoloi, A. On-Purpose Production of Light Olefins Through Oxidative Dehydrogenation: An Overview of Recent Developments. ChemCatChem 2025, 16, e202401187. [Google Scholar] [CrossRef]
- Wu, J.; Liang, Y.; Li, G.; Wan, C. Carbon Nanotubes Modified by BiMo Metal Oxides for Oxidative Dehydrogenation of 1-Butene to 1,3-Butadiene without Steam. Chemistry 2022, 4, 370–379. [Google Scholar] [CrossRef]
- Moon, J.; Gbadago, D.Q.; Hwang, S. 3-D Multi-Tubular Reactor Model Development for the Oxidative Dehydrogenation of Butene to 1,3-Butadiene. Chemengineering 2020, 4, 46. [Google Scholar] [CrossRef]
- Production Capacity of Butadiene Worldwide from 2024 to 2028. Available online: https://www.statista.com/statistics/1067436/global-butadiene-production-capacity/ (accessed on 25 June 2025).
- 1,3 Butadiene Market Size, Share & Trends Analysis Report by Application. Available online: https://www.grandviewresearch.com/industry-analysis/butadiene-market# (accessed on 25 August 2025).
- Sadhu, S.; Bhowmick, A.K. Preparation and properties of nanocomposites based on acrylonitrile–butadiene rubber, styrene–butadiene rubber, and polybutadiene rubber. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1573–1585. [Google Scholar] [CrossRef]
- Kumar, A.; Mohanty, S.; Gupta, V.R. Butadiene Rubber: Synthesis, Microstructure, and Role of Catalysts. Rubber Chem. Technol. 2021, 94, 393–409. [Google Scholar] [CrossRef]
- Pomalaza, G.; Arango Ponton, P.; Capron, M.; Dumeignil, F. Ethanol-to-butadiene: The reaction and its catalysts. Catal. Sci. Technol. 2020, 10, 4860–4911. [Google Scholar] [CrossRef]
- Makshina, E.V.; Dusselier, M.; Janssens, W.; Degreve, J.; Jacobs, P.A.; Sels, B.F. Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem. Soc. Rev. 2014, 43, 7917–7953. [Google Scholar] [CrossRef]
- Loukili, M.; Rivilla, I.; Cossio, F.P.; Cammi, R.; Chen, B. Cycloaddition of Butadiene with Perfluoroethylene: Prediction of a Periselectivity Switch under Pressure. J. Org. Chem. 2024, 89, 17768–17772. [Google Scholar] [CrossRef]
- Stephenson, L.M.; Smith, D.E.; Current, S.P. Endo preference in the Diels-Alder cycloaddition of butadiene and maleic anhydride. J. Org. Chem. 1982, 47, 4170–4171. [Google Scholar] [CrossRef]
- Kaberdin, R.V.; Potkin, V.I.; Zapol’skii, V.A. Synthesis and reactions of mixed halogenbuta-1,3-dienes. Russ. Chem. Rev. 1999, 68, 765–779. [Google Scholar] [CrossRef]
- Fałowska, A.; Grzybowski, S.; Kapuściński, D.; Sambora, K.; Łapczuk, A. Modeling of the General Trends of Reactivity and Regioselectivity in Cyclopentadiene–Nitroalkene Diels–Alder Reactions. Molecules 2025, 30, 2467. [Google Scholar] [CrossRef]
- Petrillo, G.; Benzi, A.; Bianchi, L.; Maccagno, M.; Pagano, A.; Tavani, C.; Spinelli, D. Recent advances in the use of conjugated nitro or dinitro-1,3-butadienes as building-blocks for the synthesis of heterocycles. Tetrahedron Lett. 2020, 61, 152297–152309. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, Y.; Gong, D.; He, X.; Shi, J.; Zhang, Q.; Tu, G. Novel swivel-cruciform 5, 5′-bibenzothiadiazole based small molecule donors for efficient organic solar cells. Org. Electron. 2020, 77, 105521. [Google Scholar] [CrossRef]
- Magano, J. Synthetic approaches to the neuraminidase inhibitors zanamivir (Relenza) and oseltamivir phosphate (Tamiflu) for the treatment of influenza. Chem. Rev. 2009, 109, 4398–4438. [Google Scholar] [CrossRef]
- Yeung, Y.Y.; Hong, S.; Corey, E.J. A short enantioselective pathway for the synthesis of the anti-influenza neuramidase inhibitor oseltamivir from 1,3-butadiene and acrylic acid. J. Am. Chem. Soc. 2006, 128, 6310–6311. [Google Scholar] [CrossRef]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming Challenges in Small-Molecule Drug Bioavailability: A Review of Key Factors and Approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef]
- Ochiai, H.; Hayashi, W.; Nishiyama, A.; Fujita, R.; Kubota, S.; Sasagawa, M.; Nishi, T. Asymmetric Synthesis of Optically Active 3-Cyclohexene-1-carboxylic Acid Utilizing Lactic Ester as a Chiral Auxiliary in the Diastereoselective Diels–Alder Reaction. Org. Process Res. Dev. 2022, 26, 1002–1009. [Google Scholar] [CrossRef]
- Michida, M.; Ishikawa, H.; Kaneda, T.; Tatekabe, S.; Nakamura, Y. Development of an Efficient Manufacturing Process for a Key Intermediate in the Synthesis of Edoxaban. Org. Process Res. Dev. 2019, 23, 524–534. [Google Scholar] [CrossRef]
- Brezinsky, K.; Burke, E.J.; Glassman, I. The high temperature oxidation of butadiene. Symp. (Int.) Combust. 1985, 20, 613–622. [Google Scholar] [CrossRef]
- Garg, Y.; Pandey, S.K. An enantioselective approach to functionalized amino acids: Total synthesis of antiepileptic drug (R)-lacosamide. J. Org. Chem. 2015, 80, 4201–4203. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Moya, J.; Zurita, D.A.; Cadena-Cruz, J.E.; Alcívar-León, C.D. Diaza-1,3-butadienes as Useful Intermediate in Heterocycles Synthesis. Molecules 2022, 27, 6708. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, L.; Ma, C.; Jiang, Y.; Yu, B. Functionalization of 1,3-Butadiene Derivatives under Photo/Electrocatalysis. Eur. J. Org. Chem. 2024, 27, 202400403. [Google Scholar] [CrossRef]
- Wu, C.; Liu, B.; Lin, F.; Wang, M.; Cui, D. cis -1,4-Selective Copolymerization of Ethylene and Butadiene: A Compromise between Two Mechanisms. Angew. Chem. Int. Ed. 2017, 56, 6975–6979. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, C.; Zhou, T.; Zhang, A. Cross-linking process of cis-polybutadiene rubber with peroxides studied by two-dimensional infrared correlation spectroscopy: A detailed tracking. RSC Adv. 2015, 5, 10231–10242. [Google Scholar] [CrossRef]
- Huang, X.; Huang, J.; Wu, J.; Yu, X.; Gao, Q.; Luo, Y.; Hu, H. Fabrication and properties of polybutadiene rubber-interpenetrating cross-linking poly(propylene carbonate) network as gel polymer electrolytes for lithium-ion battery. RSC Adv. 2015, 5, 52978–52984. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, G.H.; Nam, G.M.; Kang, D.G.; Seo, K.H. Oil resistance and low-temperature characteristics of plasticized nitrile butadiene rubber compounds. J. Appl. Polym. Sci. 2019, 136, 47851. [Google Scholar] [CrossRef]
- Akhlaghi, S.; Pourrahimi, A.M.; Hedenqvist, M.S.; Sjöstedt, C.; Bellander, M.; Gedde, U.W. Degradation of carbon-black-filled acrylonitrile butadiene rubber in alternative fuels: Transesterified and hydrotreated vegetable oils. Polym. Degrad. Stab. 2016, 123, 69–79. [Google Scholar] [CrossRef]
- Zapol’skii, V.A.; Fischer, R.; Namyslo, J.C.; Kaufmann, D.E. Chemistry of polyhalogenated nitrobutadienes, 8: Nitropolychlorobutadienes—Precursors for insecticidal neonicotinoids. Bioorganic Med. Chem. 2009, 17, 4206–4215. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, M.; Kula, K. Nitro-functionalized Analogues of 1,3-Butadiene: An Overview of Characteristic, Synthesis, Chemical Transformations and Biological Activity. Curr. Chem. Lett. 2024, 13, 15–30. [Google Scholar] [CrossRef]
- De Crescentini, L.; Attanasi, O.A.; Favi, G.; Filippone, P.; Mantellini, F.; Perrulli, F.R. 1,2-Diaza-1,3-dienes as a Multifaceted Synthon for the Synthesis of Heterocycles: A Fifteen-Years Update. Eur. J. Org. Chem. 2024, 27, 202400716. [Google Scholar] [CrossRef]
- Sadowski, M.; Synkiewicz-Musialska, B.; Kula, K. (1E,3E)-1,4-Dinitro-1,3-butadiene—Synthesis, Spectral Characteristics and Computational Study Based on MEDT, ADME and PASS Simulation. Molecules 2024, 29, 542. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, M.; Kula, K. Unexpected Course of Reaction Between (1E,3E)-1,4-Dinitro-1,3-butadiene and N-Methyl Azomethine Ylide—A Comprehensive Experimental and Quantum-Chemical Study. Molecules 2024, 29, 5066. [Google Scholar] [CrossRef]
- Kula, K.; Jasiński, R. Synthesis of bis(het)aryl systems via domino reaction involving (2E,4E)-2,5-dinitrohexa-2,4-diene: DFT mechanistic considerations. Chem. Heterocycl. Compd. 2024, 60, 600–610. [Google Scholar] [CrossRef]
- Raczyńska, E.D.; Gal, J.-F.; Maria, P.-C.; Saeidian, H. Push–Pull Effect on the Gas-Phase Basicity of Nitriles: Transmission of the Resonance Effects by Methylenecyclopropene and Cyclopropenimine π-Systems Substituted by Two Identical Strong Electron Donors. Symmetry 2021, 13, 1554. [Google Scholar] [CrossRef]
- Guo, H.; He, J.; Guo, Y.; Chang, Y.; Ju, H.; Li, Y. Electron Push-Pull Effect of Benzotrithiophene-Based Covalent Organic Frameworks on the Photocatalytic Degradation of Pharmaceuticals and Personal Care Products. Molecules 2025, 30, 336. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Ballini, R.; Araújo, N.; Gil, M.V.; Román, E.; Serrano, J.A. Conjugated Nitrodienes. Synthesis and Reactivity. Chem. Rev. 2013, 113, 3493–3515. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E. Drug-Like Properties: Concepts, Structure Design and Methods from ADME to Toxicity Optimization; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Woliński, P.; Zawadzińska-Wrochniak, K.; Dresler, E.; Jasiński, R. On the Question of the Course of the Hetero Diels–Alder Reactions Between N-(2,2,2-Trichloroethylidene)Carboxamides and Dicyclohexylcarbodiimide: A New Case of the Stepwise Zwitterionic Cycloaddition Process. Molecules 2025, 30, 2692. [Google Scholar] [CrossRef]
- Zawadzińska-Wrochniak, K.; Kula, K.; Ríos-Gutiérrez, M.; Gostyński, B.; Krawczyk, T.; Jasiński, R. A Comprehensive Study of the Synthesis, Spectral Characteristics, Quantum–Chemical Molecular Electron Density Theory, and In Silico Future Perspective of Novel CBr3-Functionalyzed Nitro-2-Isoxazolines Obtained via (3 + 2) Cycloaddition of (E)-3,3,3-Tribromo-1-Nitroprop-1-ene. Molecules 2025, 30, 2149. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A Simple Measure of Electron Localization in Atomic and Molecular Systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int. Ed. Engl. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Łapczuk, A.; Ríos-Gutiérrez, M. Mechanistic Aspects of [3+2] Cycloaddition Reaction of Trifluoroacetonitrile with Diarylnitrilimines in Light of Molecular Electron Density Theory Quantum Chemical Study. Molecules 2025, 30, 85. [Google Scholar] [CrossRef] [PubMed]
- Ryachi, K.; Mohammad-Salim, H.; Al-Sadoon, M.K.; Zeroual, A.; de Julián-Ortiz, J.V.; Idrissi, M.E.; Tounsi, A. Quantum study of the [3 + 2] cycloaddition of nitrile oxide and carvone oxime: Insights into toxicity, pharmacokinetics, and mechanism. Chem. Heterocycl. Compd. 2024, 60, 646–654. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Polo, V.; Andres, J.; Berski, S.; Domingo, L.R.; Silvi, B. Understanding reaction mechanisms in organic chemistry from catastrophe theory applied to the electron localization function topology. J. Phys. Chem. A 2008, 112, 7128–7136. [Google Scholar] [CrossRef]
- Sadowski, M.; Dresler, E.; Zawadzińska, K.; Wróblewska, A.; Jasiński, R. Syn-Propanethial S-Oxide as an Available Natural Building Block for the Preparation of Nitro-Functionalized, Sulfur-Containing Five-Membered Heterocycles: An MEDT Study. Molecules 2024, 29, 4892. [Google Scholar] [CrossRef]
- Kula, K.; Łapczuk, A.; Sadowski, M.; Kras, J.; Zawadzińska, K.; Demchuk, O.M.; Gaurav, G.K.; Wróblewska, A.; Jasiński, R. On the Question of the Formation of Nitro-Functionalized 2,4-Pyrazole Analogs on the Basis of Nitrylimine Molecular Systems and 3,3,3-Trichloro-1-Nitroprop-1-Ene. Molecules 2022, 27, 8409. [Google Scholar] [CrossRef]
- Sadowski, M.; Dresler, E.; Wróblewska, A.; Jasiński, R. A New Insight into the Molecular Mechanism of the Reaction between 2-Methoxyfuran and Ethyl (Z)-3-phenyl-2-nitroprop-2-enoate: An Molecular Electron Density Theory (MEDT) Computational Study. Molecules 2024, 29, 4876. [Google Scholar] [CrossRef]
- Messaadia, S.; Nacereddine, A.K.; Djerourou, A. Exploring the factors controlling the mechanism and the high stereoselectivity of the polar [3 + 2] cycloaddition reaction of the N,N’-cyclic azomethine imine with 3-nitro-2-phenyl-2H-chromene. A Molecular Electron Density Theory study. Chem. Heterocycl. Compd. 2023, 59, 128–137. [Google Scholar] [CrossRef]
- Rattananakin, P.; Pittman, C.U.; Collier, W.E.; Saebo, S. Ab initio studies of push–pull systems. Struct. Chem. 2007, 18, 399–407. [Google Scholar] [CrossRef]
- Stojiljković, I.N.; Rančić, M.P.; Marinković, A.D.; Cvijetić, I.N.; Milčić, M.K. Assessing the potential of para-donor and para-acceptor substituted 5-benzylidenebarbituric acid derivatives as push-pull electronic systems: Experimental and quantum chemical study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 253, 119576. [Google Scholar] [CrossRef] [PubMed]
- Kacka-Zych, A.; Jasinski, R. Understanding the molecular mechanism of the stereoselective conversion of N–trialkylsilyloxy nitronates into bicyclic isoxazoline derivatives. New J. Chem. 2021, 45, 9491–9500. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Jasiński, R. Unexpected molecular mechanism of trimethylsilyl bromide elimination from 2-(trimethylsilyloxy)-3-bromo-3-methyl-isoxazolidines. Theor. Chem. Acc. 2019, 138, 81–86. [Google Scholar] [CrossRef]
- Wróblewska, A.; Sadowski, M.; Jasiński, R. Selectivity and molecular mechanism of the Au(III)-catalyzed [3 + 2] cycloaddition reaction between (Z)-C,N-diphenylnitrone and nitroethene in the light of the molecular electron density theory computational study. Chem. Heterocycl. Compd. 2024, 60, 639–645. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Kula, K. Application of β-phosphorylated nitroethenes in [3 + 2] cycloaddition reactions involving benzonitrile N-oxide in the light of DFT computational study. Organics 2021, 2, 26–37. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Jasiński, R. Molecular mechanism of Hetero Diels-Alder reactions between (E)-1,1,1-trifluoro-3-nitrobut-2-enes and enamine systems in the light of Molecular Electron Density Theory. J. Mol. Graph. Model. 2020, 101, 107714. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Wróblewska, A.; Wielgus, E.; Dolot, R.; Jasiński, R. Fully Selective Synthesis of Spirocyclic-1,2-oxazine N-Oxides via Non-Catalysed Hetero Diels-Alder Reactions with the Participation of Cyanofunctionalysed Conjugated Nitroalkenes. Molecules 2023, 28, 4586. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Mirosław, B.; Wielgus, E.; Olszewska, A.; Jasiński, R. Green, one-pot synthesis of 1,2-oxazine-type herbicides via non-catalyzed Hetero Diels-Alder reactions comprising (2E)-3-aryl-2-nitroprop-2-enenitriles. J. Clean. Prod. 2022, 356, 131878. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Leboeuf, M.; Koster, A.M.; Jug, K. Topological analysis of the molecular electrostatic potential. J. Chem. Phys. 1999, 111, 4893–4905. [Google Scholar] [CrossRef]
- Sadowski, M.; Dresler, E.; Jasiński, R. The Puzzle of the Regioselectivity and Molecular Mechanism of the (3+2) Cycloaddition Reaction Between E-2-(Trimethylsilyl)-1-Nitroethene and Arylonitrile N-Oxides: Molecular Electron Density Theory (MEDT) Quantumchemical Study. Molecules 2025, 30, 974. [Google Scholar] [CrossRef]
- Jasiński, R.; Mirosław, B.; Demchuk, O.M.; Babyuk, D.; Łapczuk-Krygier, A. In the search for experimental and quantumchemical evidence for zwitterionic nature of (2E)-3-[4-(dimethylamino)phenyl]-2-nitroprop-2-enenitrile—An extreme example of donor-p-acceptor push-pull molecule. J. Mol. Struct. 2016, 1108, 689–697. [Google Scholar] [CrossRef]
- Kaya, S.; Putz, M.V. Atoms-In-Molecules’ Faces of Chemical Hardness by Conceptual Density Functional Theory. Molecules 2022, 27, 8825. [Google Scholar] [CrossRef]
- Geerlings, P. From Density Functional Theory to Conceptual Density Functional Theory and Biosystems. Pharmaceuticals 2022, 15, 1112. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Parr, R.G.; Gadre, S.R.; Bartolotti, L.J. Local Density Functional Theory of Atoms and Molecules. Proc. Natl. Acad. Sci. USA 1979, 76, 2522–2526. [Google Scholar] [CrossRef]
- Sadowski, M.; Utnicka, J.; Wójtowicz, A.; Kula, K. The Global and Local Reactivity of C,N-diarylnitryle Imines in [3+2] Cycloaddition Processes with Trans-β-nitrostyrene according to Molecular Electron Density Theory: A computational study. Curr. Chem. Lett. 2023, 12, 421–430. [Google Scholar] [CrossRef]
- Flores-Holguín, N.; Frau, J.; Glossman-Mitnik, D. Virtual Screening of Marine Natural Compounds by Means of Chemoinformatics and CDFT-Based Computational Peptidology. Mar. Drugs 2020, 18, 478. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P. A quantum chemical topological analysis of the C–C bond formation in organic reactions involving cationic species. Phys. Chem. Chem. Phys. 2014, 16, 14108–14115. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Bębenek, E.; Sokal, A.; Książek, M.; Chrobak, E. Crystal Structure and Spectroscopic Analysis of 3-Diethoxyphosphoryl-28-[1-(1-deoxy-β-D-glucopyranosyl)-1H-1,2,3-triazol-4-yl]carbonylbetulin. Crystals 2023, 13, 1488. [Google Scholar] [CrossRef]
- Benmerabet, A.; Bouhadiba, A.; Belhocine, Y.; Rahali, S.; Sbei, N.; Seydou, M.; Boucheriha, I.; Omeiri, I.; Assaba, I.M. DFT Investigation on the Complexation of β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin as Recognition Hosts with Trichloroethylene. Atoms 2023, 11, 153. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. A Useful Classification of Organic Reactions Based on the Flux of the Electron Density. Sci. Radices 2023, 2, 1–24. [Google Scholar] [CrossRef]
- Sustmann, R. Orbital energy control of cycloaddition reactivity. Pure Appl. Chem. 1974, 40, 569–593. [Google Scholar] [CrossRef]
- Parr, R.G.; von Szentpaly, L.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P. The Nucleophilicity N Index in Organic Chemistry. Org. Biomol. Chem. 2011, 9, 7168–7175. [Google Scholar] [CrossRef]
- Domingo, L.R. 1999–2024, a quarter century of the Parr’s electrophilicity ω index. Sci. Radices 2024, 3, 157–186. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Electrophilicity w and Nucleophilicity N Scales for Cationic and Anionic Species. Sci. Radices 2025, 4, 1–17. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Saz Sousa, A.; Domingo, L.R. Electrophilicity and nucleophilicity scales at different DFT computational levels. J. Phys. Org. Chem. 2023, 36, 4503. [Google Scholar] [CrossRef]
- Aurell, M.J.; Domingo, L.R.; Pérez, P.; Contreras, R. A Theoretical Study on the Regioselectivity Of 1,3-Dipolar Cycloadditions Using DFT-based Reactivity Indexes. Tetrahedron 2004, 60, 11503–11509. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the Local Reactivity in Polar Organic Reactions through Electrophilic and Nucleophilic Parr Functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Chamorro, E.; Pérez, P.; Domingo, L.R. On the nature of Parr functions to predict the most reactive sites along organic polar reactions. Chem. Phys. Lett. 2013, 582, 141–143. [Google Scholar] [CrossRef]
- Kula, K.; Zawadzińska, K. Local Nucleophile-Electrophile Interactions in [3 + 2] Cycloaddition Reactions between Benzonitrile N-Oxide and Selected Conjugated Nitroalkenes in the Light of MEDT Computational Study. Curr. Chem. Lett. 2021, 10, 9–16. [Google Scholar] [CrossRef]
- Duescher, R.J.; Elfarra, A.A. Human liver microsomes are efficient catalysts of 1,3-butadiene oxidation: Evidence for major roles by cytochromes P450 2A6 and 2E1. Arch. Biochem. Biophys. 1994, 311, 342–349. [Google Scholar] [CrossRef]
- Tates, A.D.; Van Dam, F.J.; De Zwart, F.A.; Van Teylingen, C.M.M.; Natarajan, A.T. Development of a cloning assay with high cloning efficiency to detect induction of 6-thioguanine-resistant lymphocytes in spleen of adult mice following in vivo inhalation exposure to 1,3-butadiene. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1994, 309, 299–306. [Google Scholar] [CrossRef]
- Marty, M.S.; Erraguntla, N.; North, C.; Barranco, W.T.; Kirman, C.R.; Cagen, S.; Rushton, E.K.; Koehler, M.W.; Budinsky, R. A reproductive and developmental toxicity screening study of 1,3-butadiene in Sprague-Dawley rats. Regul. Toxicol. Pharmacol. 2021, 127, 105066. [Google Scholar] [CrossRef]
- Recio, L.; Pluta, L.J.; Meyer, K.G. The in vivo mutagenicity and mutational spectrum at the lacI transgene recovered from the spleens of B6C3F1 lacI transgenic mice following a 4-week inhalation exposure to 1,3-butadiene. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 401, 99–110. [Google Scholar] [CrossRef]
- Lambert, I.B.; Singer, T.M.; Boucher, S.E.; Douglas, G.R. Detailed review of transgenic rodent mutation assays. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 590, 1–280. [Google Scholar] [CrossRef]
- Aydinli, S.G.; Ozdamar, Y.; Sayil, C.; Gulsah Deniz, N.; Stasevych, M.; Zvarych, V.; Komarovska-Porokhnyavets, O.; Novikov, V. Synthesis, characterization and investigation of antibacterial and antifungal activities of novel 1,3-butadiene compounds. Synth. Commun. 2020, 50, 3234–3244. [Google Scholar] [CrossRef]
- Abdassalam, A.F.; Deniz, N.G.; Sayil, C.; Aydinli, S.G.; Lubenets, V.; Komarovska-Porokhnyavets, O.; Zvarych, V.; Stasevych, M. A novel series of (E)-S and (E)-N, S-polyhalonitrobutadiene analogues: Design and evaluation of antibacterial and antifungicidial activity. J. Chem. Sci. 2021, 133, 132. [Google Scholar] [CrossRef]
- Durden, J.A.; Heywood, D.L.; Sousa, A.A.; Spurr, H.W. Synthesis and Microbial Toxicity of Dinitrobutadienes and Related Compounds. J. Agric. Food Chem. 1970, 18, 50–56. [Google Scholar] [CrossRef]
- Sinicropi, M.S.; Tavani, C.; Rosano, C.; Ceramella, J.; Iacopetta, D.; Barbarossa, A.; Bianchi, L.; Benzi, A.; Maccagno, M.; Ponassi, M.; et al. A Nitrocarbazole as a New Microtubule-Targeting Agent in Breast Cancer Treatment. Appl. Sci. 2021, 11, 9139. [Google Scholar] [CrossRef]
- Zapol’skii, V.A.; Bilitewski, U.; Kupiec, S.R.; Ramming, I.; Kaufmann, D.E. Polyhalonitrobutadienes as Versatile Building Blocks for the Biotargeted Synthesis of Substituted N-Heterocyclic Compounds. Molecules 2020, 25, 2863. [Google Scholar] [CrossRef]
- Aydinli, S.G.; Bulut, E.; Deniz, N.G.; Sayil, C.; Komarovska-Porokhnyavets, O.; Lubenets, V.; Zvarych, V.; Stasevych, M.; Nesterkina, M.; Kravchenko, I. New ketene dithioacetals generated from 2-nitroperchlorobutadiene and investigation of their antibacterial, antifungal, anticonvulsant and antidepressant activities. Chem. Biodivers. 2022, 19, e202100931. [Google Scholar] [CrossRef]
- Bürgi, M.; Zapol’Skii, V.A.; Hinkelmann, B.; Köster, M.; Kaufmann, D.E.; Sasse, F.; Hauser, H.; Etcheverrigaray, M.; Kratje, R.; Bollati-Fogolín, M.; et al. Screening and characterization of molecules that modulate the biological activity of IFNs-I. J. Biotechnol. 2016, 233, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Zapol’skii, V.A.; Bürgi, M.; Oggero, M.; Bollati Fogolín, M.; Kaufmann, D.E. Chemistry of polyhalogenated nitrobutadienes, 19: Synthesis of new types of compounds modulating the biological activity of Type I Interferons (IFN-I). Arkivoc 2023, 7, 202311989. [Google Scholar] [CrossRef]
- SwissADME. Swiss Institute of Bioinformatics. Available online: http://www.swissadme.ch/ (accessed on 1 July 2025).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Way2Drug, PASS Online. Available online: http://www.way2drug.com/passonline/ (accessed on 1 July 2025).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Dulsat, J.; López-Nieto, B.; Estrada-Tejedor, R.; Borrell, J.I. Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments. Molecules 2023, 28, 776. [Google Scholar] [CrossRef]
- Sumontri, S.; Eiamart, W.; Tadtong, S.; Samee, W. Utilizing ADMET Analysis and Molecular Docking to Elucidate the Neuroprotective Mechanisms of a Cannabis-Containing Herbal Remedy (Suk-Saiyasna) in Inhibiting Acetylcholinesterase. Int. J. Mol. Sci. 2025, 26, 3189. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Mauri, A.; Bertola, M. Alvascience: A New Software Suite for the QSAR Workflow Applied to the Blood–Brain Barrier Permeability. Int. J. Mol. Sci. 2022, 23, 12882. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, I.; Hirono, S.; Nakagome, I.; Hirano, H. Comparison of Reliability of Log P Values for Drugs Calculated by Several Methods. Chem. Pharm. Bull. 1994, 42, 976–978. [Google Scholar] [CrossRef]
- Wildman, S.A.; Crippen, G.M. Prediction of Physicochemical Parameters by Atomic Contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Morak-Młodawska, B.; Jeleń, M.; Martula, E.; Korlacki, R. Study of Lipophilicity and ADME Properties of 1,9-Diazaphenothiazines with Anticancer Action. Int. J. Mol. Sci. 2023, 24, 6970. [Google Scholar] [CrossRef]
- Redka, M.; Baumgart, S.; Kupczyk, D.; Kosmalski, T.; Studzińska, R. Lipophilic Studies and In Silico ADME Profiling of Biologically Active 2-Aminothiazol-4(5H)-one Derivatives. Int. J. Mol. Sci. 2023, 24, 12230. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, X.; Hyun, C.-G. Isolation, Characterization, Genome Annotation, and Evaluation of Tyrosinase Inhibitory Activity in Secondary Metabolites of Paenibacillus sp. JNUCC32: A Comprehensive Analysis through Molecular Docking and Molecular Dynamics Simulation. Int. J. Mol. Sci. 2024, 25, 2213. [Google Scholar] [CrossRef]
- Alturki, M.S.; Alkhodier, R.A.; Gomaa, M.S.; Hussein, D.A.; Tawfeeq, N.; Al Khzem, A.H.; Pottoo, F.H.; Albugami, S.A.; Aldawsari, M.F.; Rants’o, T.A. Structure-Based Discovery of Orthosteric Non-Peptide GLP-1R Agonists via Integrated Virtual Screening and Molecular Dynamics. Int. J. Mol. Sci. 2025, 26, 6131. [Google Scholar] [CrossRef] [PubMed]
- Porras, M.; Hernández, D.; Boto, A. Short Synthesis of Structurally Diverse N-Acylhomoserine Lactone Analogs and Discovery of Novel Quorum Quenchers Against Gram-Negative Pathogens. Int. J. Mol. Sci. 2025, 26, 1775. [Google Scholar] [CrossRef]
- Kralj, S.; Jukič, M.; Bren, U. Comparative Analyses of Medicinal Chemistry and Cheminformatics Filters with Accessible Implementation in Konstanz Information Miner (KNIME). Int. J. Mol. Sci. 2022, 23, 5727. [Google Scholar] [CrossRef]
- Lopez-Mercado, S.; Enríquez, C.; Valderrama, J.A.; Pino-Rios, R.; Ruiz-Vásquez, L.; Ruiz Mesia, L.; Vargas-Arana, G.; Buc Calderon, P.; Benites, J. Exploring the Antibacterial and Antiparasitic Activity of Phenylaminonaphthoquinones—Green Synthesis, Biological Evaluation and Computational Study. Int. J. Mol. Sci. 2024, 25, 10670. [Google Scholar] [CrossRef]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. In Silico Prediction of Aqueous Solubility Using Simple QSPR Models: The Importance of Phenol and Phenol-like Moieties. J. Chem. Inf. Model. 2012, 52, 2950–2957. [Google Scholar] [CrossRef]
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Mannhold, R.; Poda, G.I.; Ostermann, C.; Tetko, I.V. Calculation of molecular lipophilicity: State-of-the-art and comparison of log P methods on more than 96,000 compounds. J. Pharm. Sci. 2009, 98, 861–893. [Google Scholar] [CrossRef]
- He, Z.; Yang, D.; Fan, X.; Zhang, M.; Li, Y.; Gu, X.; Yang, M. The Roles and Mechanisms of lncRNAs in Liver Fibrosis. Int. J. Mol. Sci. 2020, 21, 1482. [Google Scholar] [CrossRef]
- Vučkovski, M.; Filipović, A.; Jadranin, M.; Korićanac, L.; Žakula, J.; Bondžić, B.P.; Bondžić, A.M. Enhanced Bioactivity of Quercetin–Tetrahydroisoquinoline Derivatives: Effect on Lipophilicity, Enzymes Inhibition, Antioxidant Potential, and Cytotoxicity. Int. J. Mol. Sci. 2024, 25, 13076. [Google Scholar] [CrossRef] [PubMed]
- Nicze, M.; Borówka, M.; Dec, A.; Niemiec, A.; Bułdak, Ł.; Okopień, B. The Current and Promising Oral Delivery Methods for Protein- and Peptide-Based Drugs. Int. J. Mol. Sci. 2024, 25, 815. [Google Scholar] [CrossRef]
- Sergazy, S.; Berikkhanova, K.; Gulyayev, A.; Shulgau, Z.; Maikenova, A.; Bilal, R.; Terzic, M.; Zhumadilov, Z.; Aljofan, M. Cell-Based Drug Delivery Systems: Innovative Drug Transporters for Targeted Therapy. Int. J. Mol. Sci. 2025, 26, 8143. [Google Scholar] [CrossRef]
- Abbas, A.M.; Nasrallah, H.H.; Aboelmagd, A.; Kishk, S.M.; Boyd, W.C.; Kalil, H.; Orabi, A.S. Design, Synthesis, Anti-Inflammatory Activity, DFT Modeling and Docking Study of New Ibuprofen Derivatives. Int. J. Mol. Sci. 2024, 25, 3558. [Google Scholar] [CrossRef]
- Muller, T.; Demizieux, L.; Troy-Fioramonti, S.; Buch, C.; Leemput, J.; Belloir, C.; Pais de Barros, J.-P.; Jourdan, T.; Passilly-Degrace, P.; Fioramonti, X.; et al. Chemical Synthesis, Pharmacokinetic Properties and Biological Effects of JM-00266, a Putative Non-Brain Penetrant Cannabinoid Receptor 1 Inverse Agonist. Int. J. Mol. Sci. 2022, 23, 2923. [Google Scholar] [CrossRef]
- Cozzini, P.; Kellogg, G.E.; Spyrakis, F.; Abraham, D.J.; Costantino, G.; Emerson, A.; Fanelli, F.; Gohlke, H.; Kuhn, L.A.; Morris, G.M. Target flexibility: An emerging consideration in drug discovery and design. J. Med. Chem. 2008, 51, 6237–6255. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Li, Y.; Wang, R.; Lai, L. Computation of Octanol−Water Partition Coefficients by Guiding an Additive Model with Knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.u.; Muhammad, I.; Abbas, S.Q.; Hassan, M.; Majid, M.; Jin, H.-Z.; Bungau, S. Stress Driven Discovery of Natural Products from Actinobacteria with Anti-Oxidant and Cytotoxic Activities Including Docking and ADMET Properties. Int. J. Mol. Sci. 2021, 22, 11432. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, D.A.; Rudik, A.V.; Dmitriev, A.V.; Poroikov, V.V. Computer-Aided Estimation of Biological Activity Profiles of Drug-Like Compounds Taking into Account Their Metabolism in Human Body. Int. J. Mol. Sci. 2020, 21, 7492. [Google Scholar] [CrossRef]
- Bistrović, A.; Harej, A.; Grbčić, P.; Sedić, M.; Kraljević Pavelić, S.; Cetina, M.; Raić-Malić, S. Synthesis and Anti-Proliferative Effects of Mono- and Bis-Purinomimetics Targeting Kinases. Int. J. Mol. Sci. 2017, 18, 2292. [Google Scholar] [CrossRef]
- Lagunin, A.A.; Rudik, A.V.; Pogodin, P.V.; Savosina, P.I.; Tarasova, O.A.; Dmitriev, A.V.; Ivanov, S.M.; Biziukova, N.Y.; Druzhilovskiy, D.S.; Filimonov, D.A.; et al. CLC-Pred 2.0: A Freely Available Web Application for In Silico Prediction of Human Cell Line Cytotoxicity and Molecular Mechanisms of Action for Druglike Compounds. Int. J. Mol. Sci. 2023, 24, 1689. [Google Scholar] [CrossRef]
- Stepanchikova, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. Prediction of biological activity spectra for substances: Evaluation on the diverse sets of drug-like structures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar] [CrossRef]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. Pass: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef]
- Takahashi, I.; Seto, S.; Ojima, N.; Ogura, K. Purification and characterization of dimethylallyl pyrophosphate: Aspulvinone dimethylallyltransferase from Aspergillus terreus. Biochemistry 1978, 17, 2696–2702. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B. GAUSSIAN 09, Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Tirado-Rives, J.; Jorgensen, W.L. Performance of B3LYP Density Functional Methods for a Large Set of Organic Molecules. J. Chem. Theory Comput. 2008, 4, 297–306. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A Complete Basis Set Model Chemistry. The Total Energies of Closed-Shell Atoms and Hydrides of the First-Row Atoms. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Dresler, E.; Allnajar, R.; Jasiński, R. Sterical index: A novel, simple tool for the interpretation of organic reaction mechanisms. Sci. Rad. 2023, 2, 69–74. [Google Scholar] [CrossRef]
- Kula, K.; Sadowski, M. Regio- and stereoselectivity of [3+2] cycloaddition reactions between (Z)-C-(9-anthryl)-N-methylnitrone and analogues of trans- -nitrostyrene in the light of MEDT computational study. Chem. Heterocycl. Compd. 2023, 59, 138–144. [Google Scholar] [CrossRef]
- Abbasi, M.; Farnia, S.M.F.; Tahghighi, A. The possibility of applying some heteroatom-decorated g-C3N4 heterocyclic nanosheets for delivering 5-aminosalicylic acid anti-inflammatory agent. Chem. Heterocycl. Comp. 2024, 60, 655–662. [Google Scholar] [CrossRef]
- Ameur, S.; Barhoumi, A.; Abdallaoui, H.E.A.; Syed, A.; Belghiti, M.E.; Elgorban, A.M.; Wong, L.S.; Wang, S.; El Idrissi, M.; Zeroual, A.; et al. Molecular docking, exploring diverse selectivities and mechanistic insights in the cycloaddition reaction between 3-benzoylpyrrolo-[1,2-a]quinoxaline-1,2,4(5H)-triones and butyl vinyl ether. Chem. Heterocycl. Compd. 2024, 60, 584–591. [Google Scholar] [CrossRef]
- Fryźlewicz, A.; Olszewska, A.; Zawadzińska, K.; Woliński, P.; Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. On the Mechanism of the Synthesis of Nitrofunctionalised Δ2-Pyrazolines via [3+2] Cycloaddition Reactions between α-EWG-Activated Nitroethenes and Nitrylimine TAC Systems. Organics 2022, 3, 59–76. [Google Scholar] [CrossRef]
- Aitouna, A.O.; Syed, A.; Alfagham, A.T.; Mazoir, N.; de Julián-Ortiz, J.V.; Elgorban, A.M.; El idrissi, M.; Wong, L.S.; Zeroual, A. Investigating the chemical reactivity and molecular docking of 2-diazo-3,3,3-trifluoro-1-nitropropane with phenyl methacrylate using computational methods. Chem. Heterocycl. Compd. 2024, 60, 592–599. [Google Scholar] [CrossRef]
- Mtiraou, H.; Ghabi, A.; Al-Ghulikah, H.; Habib, M.A.; Hajji, M. Structural and chemical reactivity insights of a benzimidazolidinone-based N-heterocycle: A multiapproach quantum-chemical study. Chem. Heterocycl. Compd. 2024, 60, 611–616. [Google Scholar] [CrossRef]
- Demchuk, O.M.; Jasiński, R.; Strzelecka, D.; Dziuba, K.; Kula, K.; Chrzanowski, J.; Krasowska, D. A clean and simple method for deprotection of phosphines from borane complexes. Pure Appl. Chem. 2018, 90, 49–62. [Google Scholar] [CrossRef]
- Antol, I.; Štrbac, P.; Murata, Y.; Margetić, D. Theoretical Study of the Mechanism of the Formation of Azomethine Ylide from Isatine and Sarcosine and Its Reactivity in 1,3-Dipolar Cycloaddition Reaction with 7-Oxabenzonorbornadiene. Int. J. Mol. Sci. 2024, 25, 6524. [Google Scholar] [CrossRef]
- Chiacchio, M.A.; Legnani, L. Density Functional Theory Calculations: A Useful Tool to Investigate Mechanisms of 1,3-Dipolar Cycloaddition Reactions. Int. J. Mol. Sci. 2024, 25, 1298. [Google Scholar] [CrossRef]
- Jasiński, R.; Żmigrodzka, M.; Dresler, E.; Kula, K. A full regio- and stereoselective synthesis of 4-nitroisoxazolidines via stepwise [3 + 2] cycloaddition reactions between (Z)-C-(9-anthryl)-N-arylnitrones and (E)-3,3,3-trichloro-1-nitroprop-1-ene: Comprehensive experimental and theoretical study. J. Heterocycl. Chem. 2017, 54, 3314–3320. [Google Scholar] [CrossRef]
- Ochterski, J.W. Vibrational Analysis in Gaussian. 2020. Available online: https://gaussian.com/vib/ (accessed on 18 May 2025).
- Reveles, J.U.; Köster, A.M. Geometry optimization in density functional methods. J. Comput. Chem. 2004, 25, 1109–1116. [Google Scholar] [CrossRef]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computational tools for the electron localization function topological analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.0; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Yavari, I.; Malekafzali, A.; Eivazzadeh-Keihan, R.; Skoulika, S.; Alivaisi, R. A one-pot synthesis of trichloromethylated pyrimidines from trichloroacetimidamides and acetylenic esters. Tetrahedron Lett. 2016, 57, 1733–1735. [Google Scholar] [CrossRef]
- Furuya, T.; Kamlet, A.S.; Ritter, T. Catalysis for fluorination and trifluoromethylation. Nature 2011, 473, 470–477. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Łapczuk-Krygier, A.; Rykała, K.; Biernasiuk, A.; Wnorowski, A.; Popiolek, Ł.; Maziarka, A.; Hordyjewska, A.; Jasiński, R. Novel Synthesis Scheme and In Vitro Antimicrobial Evaluation of a Panel of (E)-2-aryl-1-cyano-1-nitroethenes. J. Enzym. Inhib. Med. Chem. 2016, 31, 900–907. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Kula, K.; Wnorowski, A.; Biernasiuk, A.; Popiolek, Ł.; Miodowski, D.; Demchuk, O.M.; Jasiński, R. Novel Functionalized β-nitrostyrenes: Promising Candidates for New Antibacterial Drugs. Saudi Pharm. J. 2019, 27, 593–601. [Google Scholar] [CrossRef]
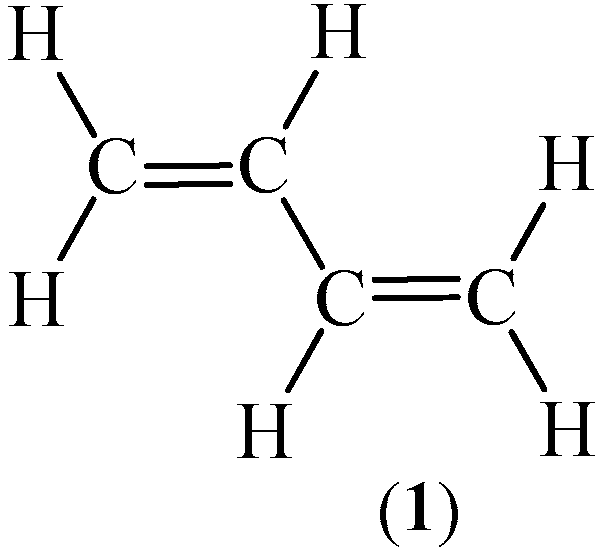
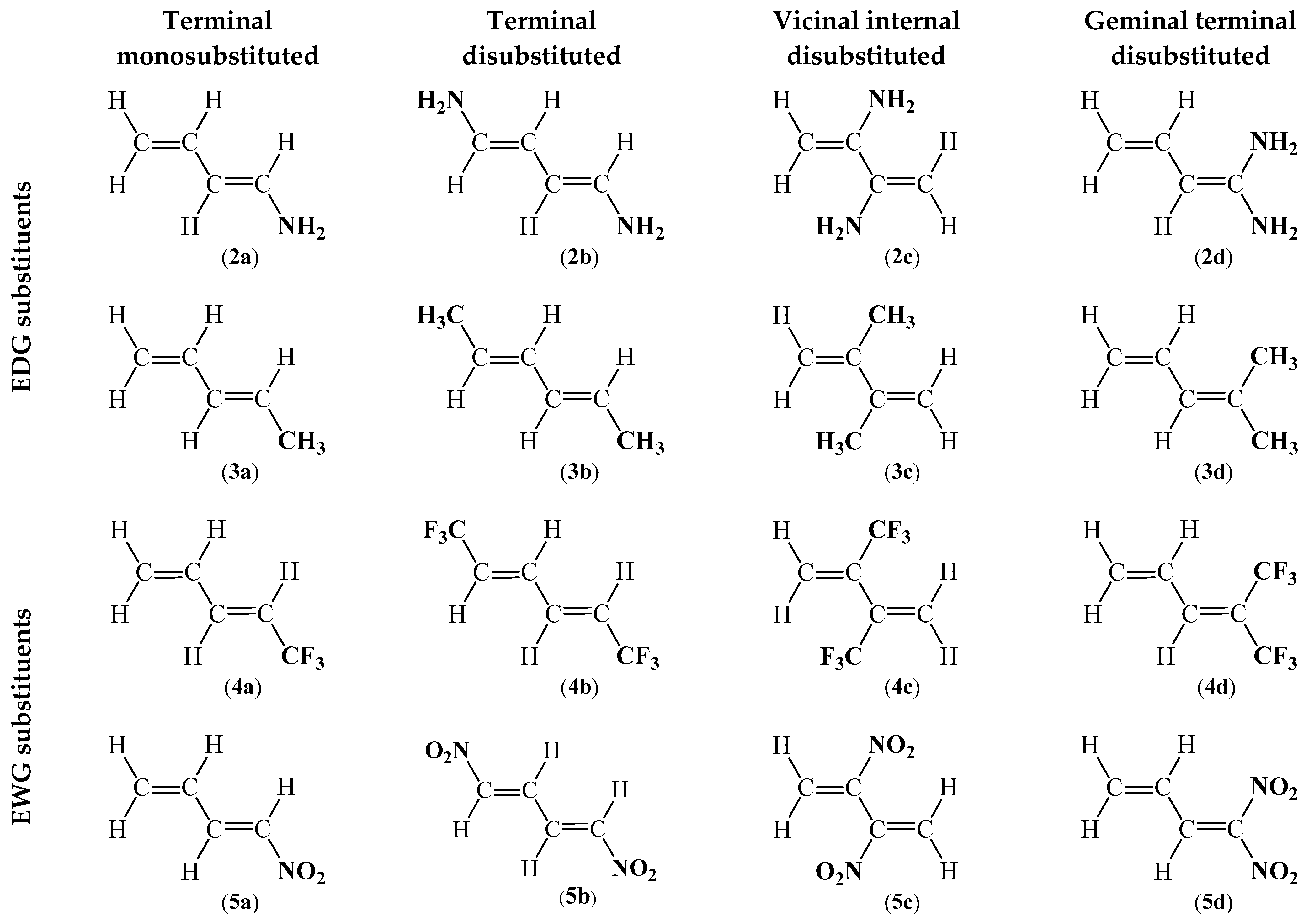
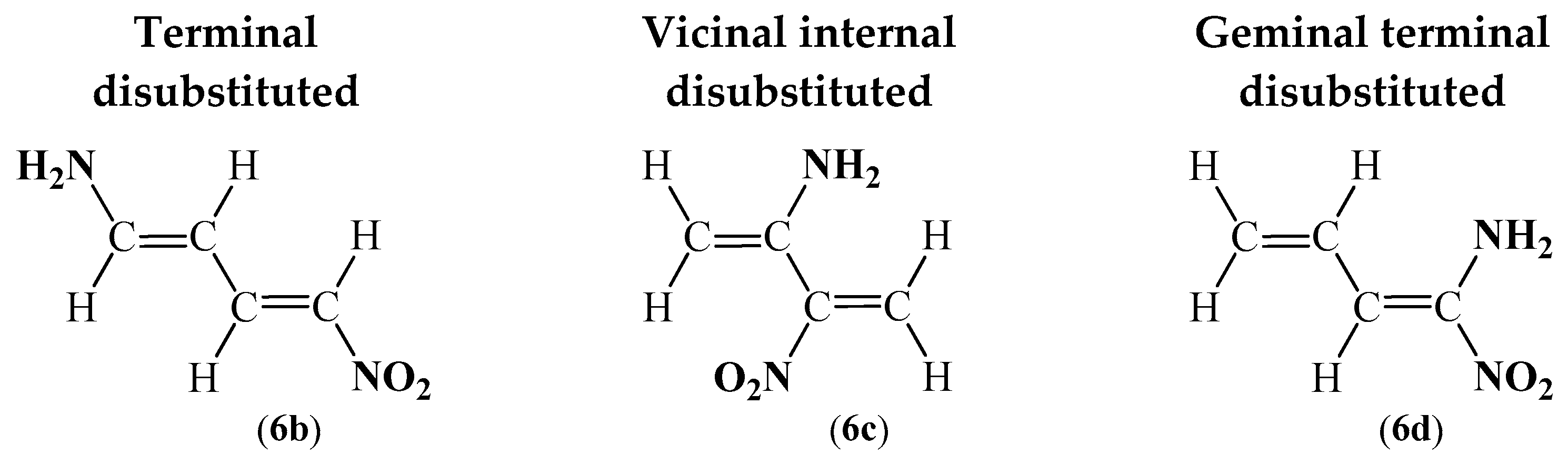


| Valence Basin Populations, N [e] | |||||||
|---|---|---|---|---|---|---|---|
| V (C1,C2) | V’ (C1,C2) | Vt (C1,C2) | V (C3,C4) | V’ (C3,C4) | Vt (C3,C4) | V (C2,C3) | |
| 1 | 1.72 | 1.72 | 3.44 | 1.72 | 1.72 | 3.44 | 2.18 |
| 2a | 1.77 | 1.81 | 3.58 | 1.74 | 1.77 | 3.51 | 2.23 |
| 2b | 1.83 | 1.83 | 3.66 | 1.83 | 1.83 | 3.66 | 2.20 |
| 2c | 1.80 | 1.80 | 3.60 | 1.80 | 1.80 | 3.60 | 2.29 |
| 2d | 1.85 | 1.88 | 3.73 | 1.75 | 1.76 | 3.51 | 2.23 |
| 3a | 1.70 | 1.79 | 3.49 | 1.69 | 1.78 | 3.47 | 2.21 |
| 3b | 1.75 | 1.75 | 3.50 | 1.75 | 1.75 | 3.50 | 2.20 |
| 3c | 1.75 | 1.75 | 3.50 | 1.75 | 1.75 | 3.50 | 2.22 |
| 3d | 1.72 | 1.82 | 3.54 | 1.71 | 1.74 | 3.45 | 2.21 |
| 4a | 1.74 | 1.74 | 3.48 | 1.70 | 1.70 | 3.40 | 2.21 |
| 4b | 1.68 | 1.78 | 3.46 | 1.68 | 1.78 | 3.46 | 2.20 |
| 4c | 1.75 | 1.75 | 3.50 | 1.75 | 1.75 | 3.50 | 2.20 |
| 4d | 1.78 | 1.80 | 3.58 | 1.66 | 1.70 | 3.36 | 2.23 |
| 5a | 1.76 | 1.76 | 3.52 | 1.68 | 1.68 | 3.36 | 2.22 |
| 5b | 1.73 | 1.73 | 3.46 | 1.73 | 1.73 | 3.46 | 2.23 |
| 5c | 1.74 | 1.84 | 3.58 | 1.74 | 1.84 | 3.58 | 2.30 |
| 5d | 1.89 | 1.89 | 3.78 | 1.63 | 1.63 | 3.26 | 2.27 |
| 6b | 1.73 | 1.73 | 3.46 | 1.76 | 1.76 | 3.52 | 2.24 |
| 6c | 1.78 | 1.78 | 3.56 | 1.81 | 1.81 | 3.62 | 2.29 |
| 6d | 1.89 | 1.90 | 3.79 | 1.66 | 1.75 | 3.41 | 2.20 |
| Main Reactivity Descriptors [eV] | |||
|---|---|---|---|
| μ | η | S | |
| 1 | −3.42 | 5.62 | 0.18 |
| 2a | −2.65 | 5.11 | 0.20 |
| 2b | −1.96 | 4.86 | 0.21 |
| 2c | −3.17 | 5.50 | 0.18 |
| 2d | −2.47 | 4.99 | 0.20 |
| 3a | −3.33 | 5.35 | 0.19 |
| 3b | −3.19 | 5.42 | 0.18 |
| 3c | −3.30 | 5.64 | 0.18 |
| 3d | −3.32 | 5.34 | 0.19 |
| 4a | −4.45 | 5.49 | 0.18 |
| 4b | −5.11 | 5.43 | 0.18 |
| 4c | −4.54 | 5.69 | 0.18 |
| 4d | −5.05 | 5.30 | 0.19 |
| 5a | −5.25 | 4.67 | 0.21 |
| 5b | −6.11 | 4.40 | 0.23 |
| 5c | −5.23 | 5.00 | 0.20 |
| 5d | −5.51 | 6.01 | 0.17 |
| 6b | −4.84 | 4.52 | 0.22 |
| 6c | −4.38 | 4.99 | 0.20 |
| 6d | −4.30 | 4.70 | 0.21 |
| 1 | 2a | 2b | 2c | 2d | 3a | 3b | 3c | 3d | 4a | 4b | 4c | 4d | 5a | 5b | 5c | 5d | 6b | 6c | 6d | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physchem. properties | MW [g/mol] | 54.09 | 69.11 | 84.12 | 84.12 | 84.12 | 68.12 | 82.14 | 82.14 | 82.14 | 122.09 | 190.09 | 190.09 | 190.09 | 99.09 | 144.09 | 144.09 | 144.09 | 114.10 | 114.10 | 114.10 |
| #heavy atoms | 4 | 5 | 6 | 6 | 6 | 5 | 6 | 6 | 6 | 8 | 12 | 12 | 12 | 7 | 10 | 10 | 10 | 8 | 8 | 8 | |
| #arom. heavy atoms | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| #rotatable bonds | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 2 | 2 | 2 | |
| #H-bond acceptors | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 6 | 6 | 6 | 2 | 4 | 4 | 4 | 2 | 2 | 2 | |
| #H-bond donors | 0 | 1 | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | |
| Molar refractivity | 20.39 | 23.10 | 25.81 | 25.81 | 25.81 | 25.20 | 30.01 | 30.01 | 30.01 | 25.39 | 30.39 | 30.39 | 30.39 | 28.50 | 36.60 | 36.60 | 36.60 | 31.21 | 31.21 | 31.21 | |
| TPSA [Å2] | 0.00 | 26.02 | 52.04 | 52.04 | 52.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 45.82 | 91.64 | 91.64 | 91.64 | 71.84 | 71.84 | 71.84 | |
| Lipophilicity | Log Po/w (iLOGP) | 1.68 | 1.29 | 0.93 | 0.94 | 1.11 | 1.91 | 2.14 | 2.03 | 2.13 | 1.83 | 2.13 | 2.02 | 2.05 | 1.21 | −2.36 | 0.65 | 0.34 | 0.73 | 1.52 | 0.92 |
| Log Po/w (XLOGP3) | 1.99 | 0.82 | −0.21 | 0.07 | 0.34 | 2.40 | 2.80 | 3.09 | 2.70 | 2.60 | 3.35 | 4.13 | 3.74 | 1.33 | 0.81 | 1.09 | 1.36 | 0.30 | 2.09 | 0.85 | |
| Log Po/w (WLOGP) | 1.36 | 0.64 | −0.07 | −0.07 | −0.07 | 1.75 | 2.14 | 2.14 | 2.14 | 3.55 | 5.74 | 5.74 | 5.74 | 1.49 | 1.61 | 1.61 | 1.61 | 0.77 | 1.88 | 0.77 | |
| Log Po/w (MLOGP) | 1.56 | 0.49 | −0.43 | −0.43 | −0.03 | 1.97 | 2.35 | 2.35 | 2.35 | 2.53 | 3.36 | 3.36 | 3.36 | −0.22 | −0.78 | −1.59 | −0.78 | −1.07 | 0.19 | −0.25 | |
| Log Po/w (SILICOS-IT) | 0.88 | 0.01 | −0.86 | −0.82 | −0.84 | 1.15 | 1.42 | 1.45 | 1.44 | 2.07 | 3.16 | 3.20 | 3.18 | −0.73 | −2.34 | −2.31 | −2.33 | −1.60 | −0.51 | −1.58 | |
| Consensus Log Po/w | 1.49 | 0.65 | −0.13 | −0.06 | 0.10 | 1.84 | 2.17 | 2.21 | 2.15 | 2.52 | 3.55 | 3.69 | 3.61 | 0.62 | −0.61 | −0.11 | 0.04 | −0.17 | 1.03 | 0.14 | |
| Water solubility | Log S (ESOL) | −1.36 | −0.72 | −0.16 | −0.34 | −0.51 | −1.71 | −2.05 | −2.23 | −1.98 | −2.10 | −2.93 | −3.42 | −3.18 | −1.16 | −1.05 | −1.22 | −1.39 | −0.60 | −1.73 | −0.95 |
| solubility [mg/mL] | 2.34 | 13.2 | 57.8 | 38.5 | 26.0 | 1.33 | 0.737 | 0.484 | 0.854 | 0.963 | 0.223 | 0.072 | 0.127 | 6.85 | 13.0 | 8.64 | 5.84 | 28.4 | 2.13 | 12.8 | |
| Log S (Ali) | −1.62 | −0.95 | −0.43 | −0.72 | −1.00 | −2.04 | −2.46 | −2.76 | −2.35 | −2.25 | −3.03 | −3.84 | −3.43 | −1.89 | −2.32 | −2.61 | −2.89 | −1.37 | −2.68 | −1.94 | |
| solubility [mg/mL] | 1.31 | 7.18 | 31.5 | 16.2 | 8.47 | 0.619 | 0.287 | 0.144 | 0.364 | 0.688 | 0.178 | 0.028 | 0.070 | 1.27 | 0.696 | 0.356 | 0.187 | 4.85 | 0.235 | 1.30 | |
| Log S (SILICOS-IT) | −0.58 | 0.09 | 0.77 | 0.06 | 0.41 | −0.65 | −0.72 | −1.43 | −1.08 | −1.26 | −1.83 | −2.55 | −2.19 | −0.20 | 0.24 | −0.47 | −0.11 | 0.50 | −0.96 | 0.14 | |
| solubility [mg/mL] | 14.3 | 85.0 | 293 | 95.7 | 217 | 15.1 | 15.7 | 3.04 | 6.90 | 6.77 | 2.78 | 0.538 | 1.22 | 63.2 | 253 | 49.0 | 112 | 358 | 12.4 | 158 | |
| Pharmacokinetics | CYP1A2 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| CYP2C9 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| IG absorption | High | High | High | High | High | High | High | High | High | High | High | High | High | High | High | High | High | High | High | High | |
| BBB permeant | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| Druglikeness | Lipinski et al. (Pfizer) [107] | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ghose et al. (Amgen) [108] | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | |
| Veber et al. (GSK) [109] | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Egan et al. (Pharmacia) [110] | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Muegge et al. (Bayer) [111] | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| 1 | 2a | 2b | 2c | 2d | 3a | 3b | 3c | 3d | 4a | 4b | 4c | 4d | 5a | 5b | 5c | 5d | Sum. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aspulvinone dimethylallyltransferase inhibitor | 0.940 | 0.913 | 0.916 | 0.917 | 0.965 | 0.936 | 0.942 | 0.913 | 8 | |||||||||
| Antieczematic | 0.918 | 1 | ||||||||||||||||
| Fatty-acyl-CoA synthase inhibitor | 0.917 | 0.935 | 0.919 | 0.903 | 0.914 | 5 | ||||||||||||
| Beta-adrenergic receptor kinase inhibitor | 0.902 | 0.911 | 2 | |||||||||||||||
| G-protein-coupled receptor kinase inhibitor | 0.902 | 0.911 | 2 | |||||||||||||||
| Arachidonate-CoA ligase inhibitor | 0.917 | 1 | ||||||||||||||||
| Phobic disorder treatment | 0.908 | 0.904 | 2 | |||||||||||||||
| NADPH peroxidase inhibitor | 0.900 | 0.903 | 2 | |||||||||||||||
| Integrin alphaVbeta3 antagonist | 0.909 | 1 | ||||||||||||||||
| Apoptosis agonist | 0.909 | 1 | ||||||||||||||||
| Mucomembranous protector | 0.908 | 1 | ||||||||||||||||
| Ubiquinol-cytochrome-c reductase inhibitor | 0.934 | 1 | ||||||||||||||||
| Phosphatidylcholine-retinol O-acyltransferase inhibitor | 0.910 | 0.928 | 2 | |||||||||||||||
| Cl--transporting ATPase inhibitor | 0.918 | 1 | ||||||||||||||||
| Cardiotonic | 0.902 | 1 | ||||||||||||||||
| Antineoplastic (breast cancer) | 0.938 | 0.938 | 0.936 | 0.934 | 4 | |||||||||||||
| Antineoplastic (lung cancer) | 0.929 | 0.929 | 0.928 | 3 | ||||||||||||||
| Epidermal growth factor antagonist | 0.980 | 1 | ||||||||||||||||
| Growth factor antagonist | 0.946 | 1 | ||||||||||||||||
| Epidermal growth factor receptor kinase inhibitor | 0.936 | 1 | ||||||||||||||||
| Antineoplastic (colorectal cancer) | 0.921 | 1 | ||||||||||||||||
| Vitamin D-like | 0.919 | 0.966 | 2 | |||||||||||||||
| Atherosclerosis treatment | 0.927 | 0.949 | 2 | |||||||||||||||
| Saccharopepsin inhibitor | 0.912 | 1 | ||||||||||||||||
| Acrocylindropepsin inhibitor | 0.912 | 1 | ||||||||||||||||
| Chymosin inhibitor | 0.912 | 1 | ||||||||||||||||
| Sum. | 5 | 0 | 3 | 4 | 0 | 2 | 4 | 6 | 6 | 2 | 7 | 2 | 2 | 0 | 3 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kula, K.; Kuś, E. In Silico Study About Substituent Effects, Electronic Properties, and the Biological Potential of 1,3-Butadiene Analogues. Int. J. Mol. Sci. 2025, 26, 8983. https://doi.org/10.3390/ijms26188983
Kula K, Kuś E. In Silico Study About Substituent Effects, Electronic Properties, and the Biological Potential of 1,3-Butadiene Analogues. International Journal of Molecular Sciences. 2025; 26(18):8983. https://doi.org/10.3390/ijms26188983
Chicago/Turabian StyleKula, Karolina, and Emilia Kuś. 2025. "In Silico Study About Substituent Effects, Electronic Properties, and the Biological Potential of 1,3-Butadiene Analogues" International Journal of Molecular Sciences 26, no. 18: 8983. https://doi.org/10.3390/ijms26188983
APA StyleKula, K., & Kuś, E. (2025). In Silico Study About Substituent Effects, Electronic Properties, and the Biological Potential of 1,3-Butadiene Analogues. International Journal of Molecular Sciences, 26(18), 8983. https://doi.org/10.3390/ijms26188983







