The Achilles Heel of Protein Biochemistry: Insolubility of Recombinant Proteins—A Case Study About Producing a Rice Enzyme
Abstract
1. Introduction
2. Results
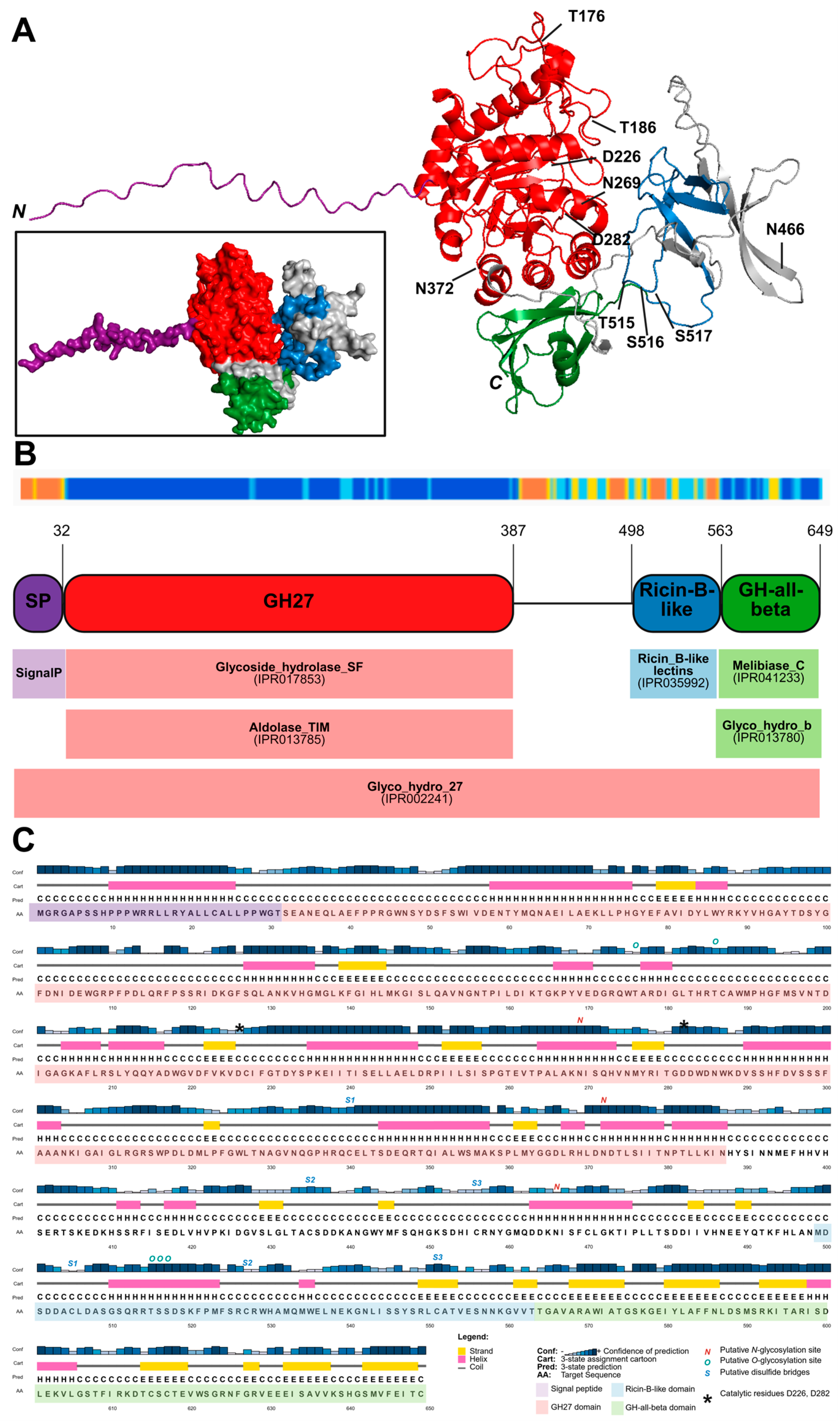
2.1. Characteristics of OsAPSE
2.2. Expression in E. coli Leads to Mostly Insoluble Proteins
2.2.1. Effect of Codon Optimization
2.2.2. Effect of Codon Harmonization and Mutational Variants
2.2.3. Utilization of E. coli Strains Capable of Synthesizing Disulfide Bridges
2.2.4. Usage of Solubility Tags
2.2.5. Exploration of Protein Refolding
2.2.6. Enzymatic Activity of Soluble GH27_OsAPSE and Refolded OsAPSE
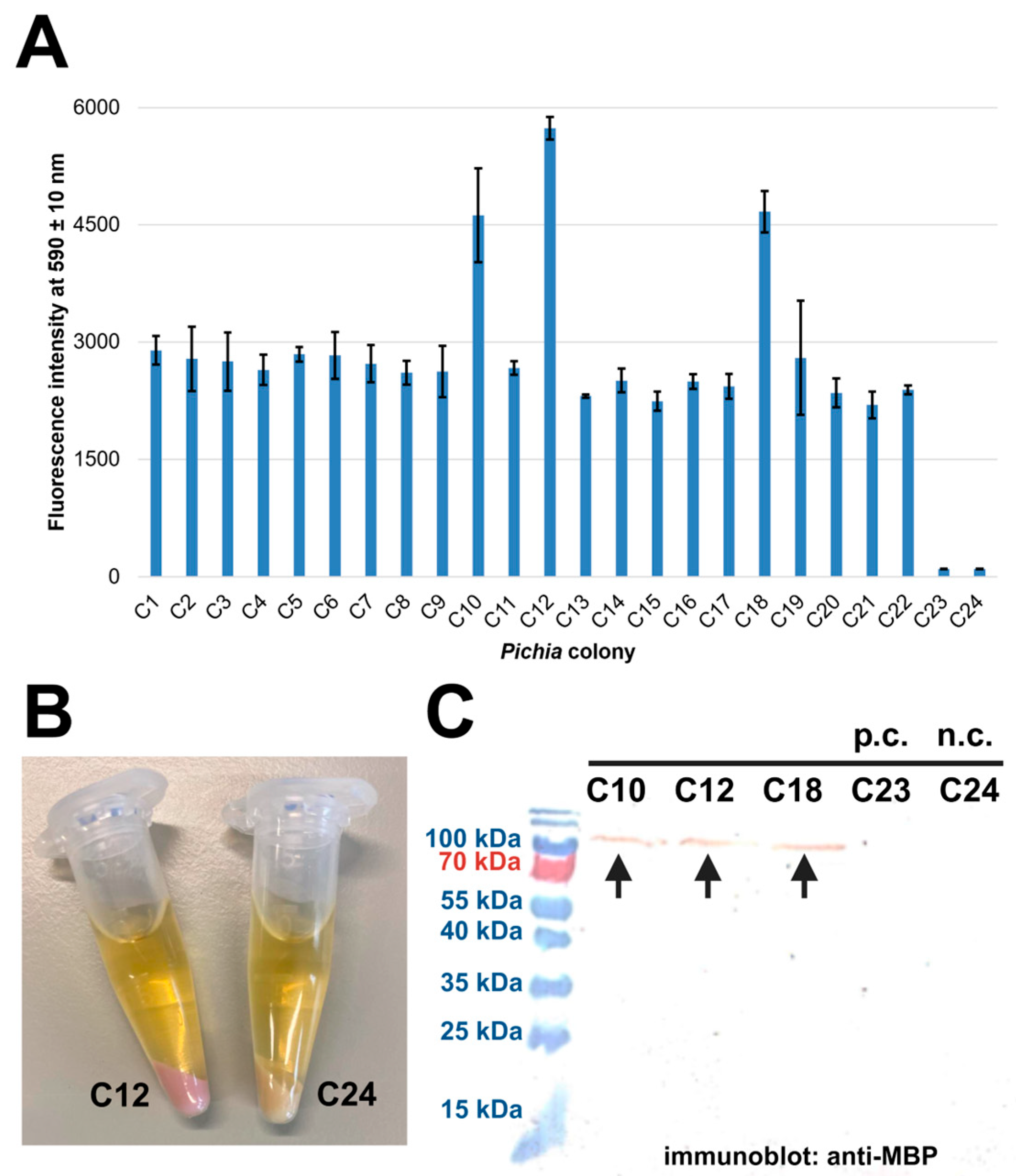
2.3. Expression in P. pastoris Yields Inactive Proteins of Interest
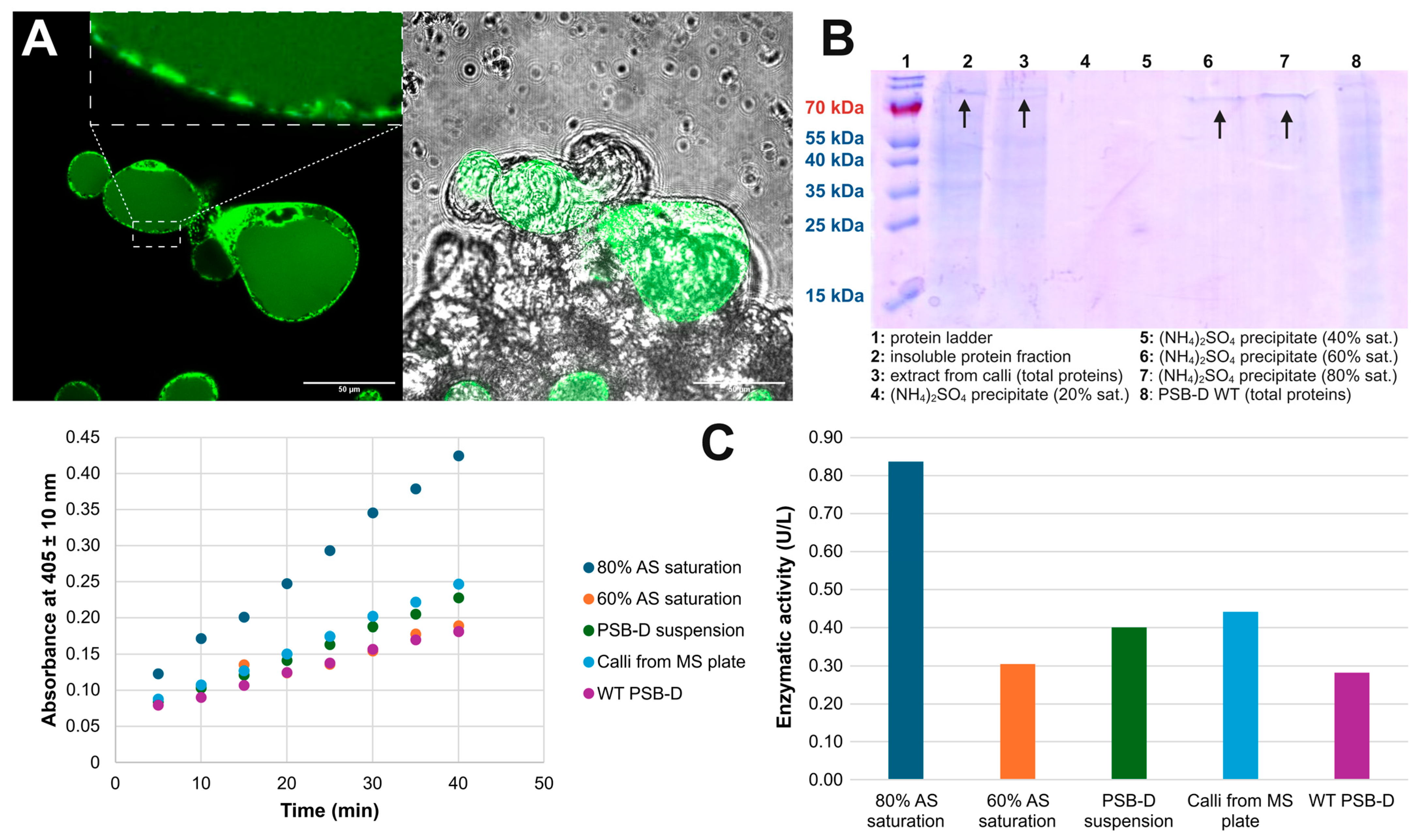
2.4. Expression in A. thaliana PSB-D Cell Cultures Results in Low Yields
3. Discussion
- First, it is recommended to optimize the operational production parameters such as the incubation temperature, shaking speed for aeration of the cultures, incubation time, concentration of inducer molecule for transcript expression, medium composition (f.i., presence of solubility enhancing additives, considering auto-induction medium), culture volume and cell lysis method [8,66]. In practice, it is advised to reduce the temperature during the induction phase because this reduces the protein biosynthesis rate and increases the chance of obtaining a soluble POI.
- Second, reconsideration of the expression construct may be advised. Researchers should take codon bias into account and optimize/harmonize the coding sequence. Several online tools make adjustments to the amino acid sequence, including deep learning and artificial intelligence, are available [39,40,67]. To circumvent issues with solubility, it might be considered to mutate hydrophobic residues to hydrophilic residues, thereby increasing the hydrophilicity of the POI and increasing the chance of obtaining soluble POI. This specific approach was successful for the production of Interleukin-2, after several point mutations [68,69]. However, the targeted residues should be chosen carefully, as catalytic pockets or ligand binding sites often involve hydrophobic residues, for instance in the case of carbohydrate-binding proteins. In addition, if non-optimized sequences are used in a prokaryotic system such as E. coli, the Rosetta® strain can be considered. This strain is engineered with additional transfer-RNAs for enhancing translation of eukaryotic proteins with ‘rare codons’ [70]. Next to codon bias, the addition of solubility tags may be considered. Widely used solubility tags include MBP, GST and TRX [71]. Successful protein production is, however, not guaranteed when employing solubility tags. Several parameters can exert an effect on the solubility of the new fusion protein [72,73], for instance the positioning (C- or N-terminal) of the solubility tag, the size of the tag, the number of tags … It should be taken into account that fusion with a large solubility tag may affect protein activity by sterically shielding active states and introducing the need for proteolytic removal of the solubility tag. If a TEV site is used, the simultaneous production of a TEV protease can be considered allowing in vivo proteolytic cleavage, thereby avoiding the need for purchasing expensive commercial enzymes and simplifying the downstream purification procedure. Finally, selection of a proper solubility tag and positioning towards the protein domain of interest often needs to be established and/or optimized empirically.
- Another option for prokaryotic protein production is to use modified host strains. Several modified hosts are available that may accommodate the researchers’ individual needs and are often equipped with additional chaperones. These chaperones are able to recognize unproperly folded proteins and prevent them from aggregation. Typical chaperons include the heat-shock proteins and have been engineered in strains to circumvent issues with protein aggregation [74], and may assist in proper protein folding [75]. The E. coli ArcticExpress® strain coproduces the Cpn10 and Cpn60 chaperonins from Oleispira antarctica, allowing protein production at lowered temperatures (4–10 °C), potentially accommodating a lower protein biosynthesis rate and therefore limiting the risk of protein aggregation and IB formation [76]. Another example is the E. coli SHuffle® strain, which is equipped with the disulfide bond isomerase chaperone, allowing formation of disulfide bridges in the cytosol [42], hereby increasing solubility of proteins that require disulfide bridges [77]. The engineered GlycoDelete strain of P. pastoris, allows recombinant protein production in the absence of hyper-glycosylation [25];
- Next to usage of engineered host strains, it can be considered to co-express molecular chaperones that are situated upstream of downstream from the native gene of interest. There is sufficient evidence that these chaperones, mostly heat-shock proteins, are co-expressed under native conditions to ensure proper POI folding [78].
- A frequently utilized approach is to produce the POI in IBs and perform subsequent protein unfolding and refolding [16]. Protein refolding is controversial since the refolding step does not always restore the native folding; it might trap the protein in a non-native state resulting in an inactive protein. Protein refolding protocols require extensive optimization and are highly empirical [79,80,81]. Nevertheless, the performance of refolding strategies has been demonstrated many times before [14,15];
- Changing the expression host may be considered, since the success of recombinant protein production is for a large part determined by the host used. A study producing 29 human proteins in E. coli and P. pastoris demonstrated that all of the POI were soluble when using P. pastoris, compared to only 31% when using E. coli [82]. Eventually, CFPS or phage/yeast display may be opted when traditional cell-based strategies are not successful [83]. CFPSs make use of cell lysates and contain all the necessary components for protein synthesis. Both prokaryotic CFPS (f.i., cell lysates of E. coli, archaeans) and eukaryotic CFPS (f.i., tobacco Bright Yellow-2 lysates, rabbit reticulocyte lysates) systems exist, but similar to conventional recombinant protein production, the CFPS should be chosen carefully, taking into account the same considerations as mentioned above. However, CFPS may be confronted with reduced yields [84,85]. Phage/yeast display has the advantage that the POI is produced by the host and presented at the cell surface, thereby removing the need for tedious or laborious optimization of protein production and purification. However, yeast/phage display may be confronted with similar issues as with traditional recombinant protein production, since the same constraints regarding non-native expression remain valid;
- A final option is considering to produce a homolog of the POI, as it was shown before that the success of recombinant protein production may vary between homologues [64]. It should be kept in mind that this research avenue is especially suitable when exploring, f.i., enzyme families or other cases where researchers are not bound to one particular POI.
4. Materials and Methods
4.1. Construct Design, Gene Cloning and Host Transformation
4.1.1. Gene Cloning for Expression in E. coli
4.1.2. Gene Cloning for Expression in P. pastoris
4.1.3. Gene Cloning for Expression in A. thaliana PSB-D Cell Cultures
4.2. Protein Production and Extraction
4.2.1. Escherichia coli
4.2.2. Pichia pastoris
4.2.3. Arabidopsis thaliana PSB-D Cell Cultures
4.3. Protein Analysis
4.3.1. Protein Concentration
4.3.2. SDS-PAGE and Western Blot
4.4. Downstream Analyses
4.4.1. Protein Refolding
4.4.2. Enzymatic Activity Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGAL | α-D-Galactopyranosidase |
| BSA | Bovine Serum Albumin |
| CAI | Codon Adaptation Index |
| CFPS | Cell-Free Production System |
| EGFP | Enhanced Green Fluorescent Protein |
| ER | Endoplasmic Reticulum |
| GST | Glutathione S-Transferase |
| IB | Inclusion Body |
| LB | Lysogeny Broth |
| MBP | Maltose-Binding Protein |
| MSMO | Murashige and Skoog medium with Minimal Organics |
| OD600 | Optical Density at 600 nm |
| PMSF | Phenyl Methyl Sulfonyl Fluoride |
| pNP-α-D-Galp | p-4-nitrophenol-α-D-Galactopyranoside |
| POI | Protein of Interest |
| PROSS | Protein Repair One-Stop Shop |
| PSB-D | Plant Systems Biology—Dark |
| PTM | Post-Translational Modification |
| RSCU | Relative Synonymous Codon Usage |
| RFP | Red Fluorescent Protein |
| RMSD | Root-Mean Square Deviation |
| TCA | Trichloroacetic acid |
| TEV | Tobacco Etch Virus |
| TGH | Tris-Glycerol-HEPES |
| TRX | Thioredoxin |
References
- Francis, D.M.; Page, R. Strategies to Optimize Protein Expression in E. coli. CP Protein Sci. 2010, 61, 5–24. [Google Scholar] [CrossRef]
- Balen, B.; Krsnik-Rasol, M. N-Glycosylation of Recombinant Therapeutic Glycoproteins in Plant Systems. Food Technol. Biotechnol. 2007, 45, 1–10. [Google Scholar]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A Highly Successful Expression System for Optimal Synthesis of Heterologous Proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, H.; Lv, B.; Li, C. Regulating Strategies for Producing Carbohydrate Active Enzymes by Filamentous Fungal Cell Factories. Front. Bioeng. Biotechnol. 2020, 8, 691. [Google Scholar] [CrossRef] [PubMed]
- Schütz, A.; Bernhard, F.; Berrow, N.; Buyel, J.F.; Ferreira-da-Silva, F.; Haustraete, J.; van den Heuvel, J.; Hoffmann, J.-E.; de Marco, A.; Peleg, Y.; et al. A Concise Guide to Choosing Suitable Gene Expression Systems for Recombinant Protein Production. STAR Protoc. 2023, 4, 102572. [Google Scholar] [CrossRef]
- Lee, J.M.; Hammarén, H.M.; Savitski, M.M.; Baek, S.H. Control of Protein Stability by Post-Translational Modifications. Nat. Commun. 2023, 14, 201. [Google Scholar] [CrossRef]
- Overton, T.W. Recombinant Protein Production in Bacterial Hosts. Drug Discov. Today 2014, 19, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Bhatwa, A.; Wang, W.; Hassan, Y.I.; Abraham, N.; Li, X.-Z.; Zhou, T. Challenges Associated with the Formation of Recombinant Protein Inclusion Bodies in Escherichia coli and Strategies to Address Them for Industrial Applications. Front. Bioeng. Biotechnol. 2021, 9, 630551. [Google Scholar] [CrossRef]
- Ferrer-Miralles, N.; Saccardo, P.; Corchero, J.L.; Garcia-Fruitós, E. Recombinant Protein Production and Purification of Insoluble Proteins. In Insoluble Proteins; Garcia Fruitós, E., Arís Giralt, A., Eds.; Methods in Molecular Biology; Springer US: New York, NY, USA, 2022; Volume 2406, pp. 1–31. ISBN 978-1-07-161858-5. [Google Scholar]
- Kramer, R.M.; Shende, V.R.; Motl, N.; Pace, C.N.; Scholtz, J.M. Toward a Molecular Understanding of Protein Solubility: Increased Negative Surface Charge Correlates with Increased Solubility. Biophys. J. 2012, 102, 1907–1915. [Google Scholar] [CrossRef]
- Muntau, A.C.; Leandro, J.; Staudigl, M.; Mayer, F.; Gersting, S.W. Innovative Strategies to Treat Protein Misfolding in Inborn Errors of Metabolism: Pharmacological Chaperones and Proteostasis Regulators. J. Inherit. Metab. Dis. 2014, 37, 505–523. [Google Scholar] [CrossRef]
- Onuchic, J.N.; Luthey-Schulten, Z.; Wolynes, P.G. Theory of Protein Folding: The Energy Landscape Perspective. Annu. Rev. Phys. Chem. 1997, 48, 545–600. [Google Scholar] [CrossRef]
- Finkelstein, A.V.; Bogatyreva, N.S.; Ivankov, D.N.; Garbuzynskiy, S.O. Protein Folding Problem: Enigma, Paradox, Solution. Biophys. Rev. 2022, 14, 1255–1272. [Google Scholar] [CrossRef]
- García-Fruitós, E.; González-Montalbán, N.; Morell, M.; Vera, A.; Ferraz, R.M.; Arís, A.; Ventura, S.; Villaverde, A. Aggregation as Bacterial Inclusion Bodies Does Not Imply Inactivation of Enzymes and Fluorescent Proteins. Microb. Cell Fact. 2005, 4, 27. [Google Scholar] [CrossRef]
- Flores, S.S.; Nolan, V.; Perillo, M.A.; Sánchez, J.M. Superactive β-Galactosidase Inclusion Bodies. Colloids Surf. B Biointerfaces 2019, 173, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Upadhyay, V.; Upadhyay, A.K.; Singh, S.M.; Panda, A.K. Protein Recovery from Inclusion Bodies of Escherichia coli Using Mild Solubilization Process. Microb. Cell Fact. 2015, 14, 41. [Google Scholar] [CrossRef]
- Vallejo, L.F.; Rinas, U. Strategies for the Recovery of Active Proteins through Refolding of Bacterial Inclusion Body Proteins. Microb. Cell Fact. 2004, 3, 11. [Google Scholar] [CrossRef]
- Habibi, N.; Mohd Hashim, S.Z.; Norouzi, A.; Samian, M.R. A Review of Machine Learning Methods to Predict the Solubility of Overexpressed Recombinant Proteins in Escherichia coli. BMC Bioinform. 2014, 15, 134. [Google Scholar] [CrossRef]
- Bhandari, B.K.; Gardner, P.P.; Lim, C.S. Solubility-Weighted Index: Fast and Accurate Prediction of Protein Solubility. Bioinformatics 2020, 36, 4691–4698. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-Y.; Majewska, N.I.; Wang, Q.; Paul, J.T.; Betenbaugh, M.J. SnapShot: N-Glycosylation Processing Pathways across Kingdoms. Cell 2017, 171, 258.e1. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.J.; Narimatsu, Y.; Schjoldager, K.T.; Tytgat, H.L.P.; Aebi, M.; Clausen, H.; Halim, A. SnapShot: O-Glycosylation Pathways across Kingdoms. Cell 2018, 172, 632.e2. [Google Scholar] [CrossRef]
- Wu, X.; Oh, M.-H.; Kim, H.S.; Schwartz, D.; Imai, B.S.; Yau, P.M.; Clouse, S.D.; Huber, S.C. Transphosphorylation of E. coli Proteins during Production of Recombinant Protein Kinases Provides a Robust System to Characterize Kinase Specificity. Front. Plant Sci. 2012, 3, 362. [Google Scholar] [CrossRef]
- Sørensen, H.P.; Mortensen, K.K. Soluble Expression of Recombinant Proteins in the Cytoplasm of Escherichia coli. Microb. Cell Fact. 2005, 4, 1. [Google Scholar] [CrossRef]
- Martínez-Alarcón, D.; Blanco-Labra, A.; García-Gasca, T. Expression of Lectins in Heterologous Systems. Int. J. Mol. Sci. 2018, 19, 616. [Google Scholar] [CrossRef] [PubMed]
- Meuris, L.; Santens, F.; Elson, G.; Festjens, N.; Boone, M.; Dos Santos, A.; Devos, S.; Rousseau, F.; Plets, E.; Houthuys, E.; et al. GlycoDelete Engineering of Mammalian Cells Simplifies N-Glycosylation of Recombinant Proteins. Nat. Biotechnol. 2014, 32, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Piron, R.; Santens, F.; De Paepe, A.; Depicker, A.; Callewaert, N. Using GlycoDelete to Produce Proteins Lacking Plant-Specific N-Glycan Modification in Seeds. Nat. Biotechnol. 2015, 33, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Van Holle, S.; De Schutter, K.; Eggermont, L.; Tsaneva, M.; Dang, L.; Van Damme, E.J.M. Comparative Study of Lectin Domains in Model Species: New Insights into Evolutionary Dynamics. Int. J. Mol. Sci. 2017, 18, 1136. [Google Scholar] [CrossRef]
- De Coninck, T.; Verbeke, I.; Rougé, P.; Desmet, T.; Van Damme, E.J.M. OsAPSE Modulates Non-Covalent Interactions between Arabinogalactan Protein O-Glycans and Pectin in Rice Cell Walls. Front. Plant Sci. 2025, 16, 1588802. [Google Scholar] [CrossRef]
- Imaizumi, C.; Tomatsu, H.; Kitazawa, K.; Yoshimi, Y.; Shibano, S.; Kikuchi, K.; Yamaguchi, M.; Kaneko, S.; Tsumuraya, Y.; Kotake, T. Heterologous Expression and Characterization of an Arabidopsis β-L-Arabinopyranosidase and α-D-Galactosidases Acting on β-L-Arabinopyranosyl Residues. J. Exp. Bot. 2017, 68, 4651–4661. [Google Scholar] [CrossRef]
- De Coninck, T.; Gippert, G.P.; Henrissat, B.; Desmet, T.; Van Damme, E.J.M. Investigating Diversity and Similarity between CBM13 Modules and Ricin-B Lectin Domains Using Sequence Similarity Networks. BMC Genom. 2024, 25, 643. [Google Scholar] [CrossRef]
- Fujimoto, Z. Structure and Function of Carbohydrate-Binding Module Families 13 and 42 of Glycoside Hydrolases, Comprising a β-Trefoil Fold. Biosci. Biotechnol. Biochem. 2013, 77, 1363–1371. [Google Scholar] [CrossRef]
- Taylor, M.E.; Drickamer, K. Convergent and Divergent Mechanisms of Sugar Recognition across Kingdoms. Curr. Opin. Struct. Biol. 2014, 28, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hazes, B. The (QxW) 3 Domain: A Flexible Lectin Scaffold. Protein Sci. 1996, 5, 1490–1501. [Google Scholar] [CrossRef]
- Steeves, R.M.; Denton, M.E.; Barnard, F.C.; Henry, A.; Lambert, J.M. Identification of Three Oligosaccharide Binding Sites in Ricin. Biochemistry 1999, 38, 11677–11685. [Google Scholar] [CrossRef]
- Boissinot, M.; Karnas, S.; Lepock, J.R.; Cabelli, D.E.; Tainer, J.A.; Getzoff, E.D.; Hallewell, R.A. Function of the Greek Key Connection Analysed Using Circular Permutants of Superoxide Dismutase. EMBO J. 1997, 16, 2171–2178. [Google Scholar] [CrossRef]
- Kemplen, K.R.; De Sancho, D.; Clarke, J. The Response of Greek Key Proteins to Changes in Connectivity Depends on the Nature of Their Secondary Structure. J. Mol. Biol. 2015, 427, 2159–2165. [Google Scholar] [CrossRef]
- Ranaghan, M.J.; Li, J.J.; Laprise, D.M.; Garvie, C.W. Assessing Optimal: Inequalities in Codon Optimization Algorithms. BMC Biol. 2021, 19, 36. [Google Scholar] [CrossRef]
- Sharp, P.M.; Li, W.-H. The Codon Adaptation Index-a Measure of Directional Synonymous Codon Usage Bias, and Its Potential Applications. Nucleic Acids Res. 1987, 15, 1281–1295. [Google Scholar] [CrossRef]
- Listov, D.; Goverde, C.A.; Correia, B.E.; Fleishman, S.J. Opportunities and Challenges in Design and Optimization of Protein Function. Nat. Rev. Mol. Cell. Biol. 2024, 25, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Goldenzweig, A.; Goldsmith, M.; Hill, S.E.; Gertman, O.; Laurino, P.; Ashani, Y.; Dym, O.; Unger, T.; Albeck, S.; Prilusky, J.; et al. Automated Structure- and Sequence-Based Design of Proteins for High Bacterial Expression and Stability. Mol. Cell 2016, 63, 337–346. [Google Scholar] [CrossRef]
- Morgan, A.A.; Rubenstein, E. Proline: The Distribution, Frequency, Positioning, and Common Functional Roles of Proline and Polyproline Sequences in the Human Proteome. PLoS ONE 2013, 8, e53785. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, J.; Emrich, C.A.; Jeans, C.; Faulkner, M.; Riggs, P.; Berkmen, M. SHuffle, a Novel Escherichia coli Protein Expression Strain Capable of Correctly Folding Disulfide Bonded Proteins in Its Cytoplasm. Microb. Cell Fact 2012, 11, 753, Erratum in Microb. Cell Fact 2016, 15, 124. [Google Scholar] [CrossRef]
- Sun, P.; Tropea, J.E.; Waugh, D.S. Enhancing the Solubility of Recombinant Proteins in Escherichia coli by Using Hexahistidine-Tagged Maltose-Binding Protein as a Fusion Partner. In Heterologous Gene Expression in E. coli; Evans, T.C., Xu, M.-Q., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 705, pp. 259–274. ISBN 978-1-61737-966-6. [Google Scholar]
- Reuten, R.; Nikodemus, D.; Oliveira, M.B.; Patel, T.R.; Brachvogel, B.; Breloy, I.; Stetefeld, J.; Koch, M. Maltose-Binding Protein (MBP), a Secretion-Enhancing Tag for Mammalian Protein Expression Systems. PLoS ONE 2016, 11, e0152386. [Google Scholar] [CrossRef] [PubMed]
- Kellermann, O.; Szmelcman, S. Active Transport of Maltose in Escherichia coli K12: Involvement of a “Periplasmic” Maltose Binding Protein. Eur. J. Biochem. 1974, 47, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Lénon, M.; Ke, N.; Ren, G.; Meuser, M.E.; Loll, P.J.; Riggs, P.; Berkmen, M. A Useful Epitope Tag Derived from Maltose Binding Protein. Protein Sci. 2021, 30, 1235–1246. [Google Scholar] [CrossRef]
- Costa, S.; Almeida, A.; Castro, A.; Domingues, L. Fusion Tags for Protein Solubility, Purification and Immunogenicity in Escherichia coli: The Novel Fh8 System. Front. Microbiol. 2014, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Rao, J.; Chen, B. Plant Protein Solubility: A Challenge or Insurmountable Obstacle. Adv. Colloid Interface Sci. 2024, 324, 103074. [Google Scholar] [CrossRef]
- Bhaskar, B.; Ramachandra, G.; Virupaksha, T.K. Alpha-Galactosidase of Germinating Seeds of Cassia Sericea Sw. J. Food Biochem. 1990, 14, 45–59. [Google Scholar] [CrossRef]
- Gao, Z.; Schaffer, A.A. A Novel Alkaline α-Galactosidase from Melon Fruit with a Substrate Preference for Raffinose. Plant Physiol. 1999, 119, 979–988. [Google Scholar] [CrossRef]
- Chien, S.-F.; Chen, S.-H.; Chien, M.-Y. Cloning, Expression, and Characterization of Rice α-Galactosidase. Plant Mol. Biol. Rep. 2008, 26, 213–224. [Google Scholar] [CrossRef]
- Peters, S.; Egert, A.; Stieger, B.; Keller, F. Functional Identification of Arabidopsis ATSIP2 (At3g57520) as an Alkaline -Galactosidase with a Substrate Specificity for Raffinose and an Apparent Sink-Specific Expression Pattern. Plant Cell Physiol. 2010, 51, 1815–1819. [Google Scholar] [CrossRef]
- Sakharayapatna Ranganatha, K.; Venugopal, A.; Chinthapalli, D.K.; Subramanyam, R.; Nadimpalli, S.K. Purification, Biochemical and Biophysical Characterization of an Acidic α-Galactosidase from the Seeds of Annona Squamosa (Custard Apple). Int. J. Biol. Macromol. 2021, 175, 558–571. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Dai, H.; Miao, M. Characteristics and Expression Patterns of Six α-Galactosidases in Cucumber (Cucumis sativus L.). PLoS ONE 2021, 16, e0244714. [Google Scholar] [CrossRef]
- Davis, M.O.; Hata, D.J.; Johnson, S.A.; Jones, D.E.; Harmata, M.A.; Evans, M.L.; Walker, J.C.; Smith, D.S. Cloning, sequence, and expression of a blood group B active recombinant alpha-D-galactosidase from pinto bean (Phaseolus vulgaris). Biochem. Mol. Biol. Int. 1997, 42, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Khrapunov, S.; Cheng, H.; Hegde, S.; Blanchard, J.; Brenowitz, M. Solution Structure and Refolding of the Mycobacterium Tuberculosis Pentapeptide Repeat Protein MfpA. J. Biol. Chem. 2008, 283, 36290–36299. [Google Scholar] [CrossRef] [PubMed]
- Michaux, C.; Pomroy, N.C.; Privé, G.G. Refolding SDS-Denatured Proteins by the Addition of Amphipathic Cosolvents. J. Mol. Biol. 2008, 375, 1477–1488. [Google Scholar] [CrossRef]
- Dulermo, R.; Legras, J.-L.; Brunel, F.; Devillers, H.; Sarilar, V.; Neuvéglise, C.; Nguyen, H.-V. Truncation of Gal4p Explains the Inactivation of the GAL/MEL Regulon in Both Saccharomyces bayanus and Some Saccharomyces cerevisiae Wine Strains. FEMS Yeast Res. 2016, 16, fow070. [Google Scholar] [CrossRef][Green Version]
- Mot, A.C.; Puscas, C.; Miclea, P.; Naumova-Letia, G.; Dorneanu, S.; Podar, D.; Dissmeyer, N.; Silaghi-Dumitrescu, R. Redox Control and Autoxidation of Class 1, 2 and 3 Phytoglobins from Arabidopsis Thaliana. Sci. Rep. 2018, 8, 13714. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, S.; Wang, J.; Song, M.; Xu, M.; Zhang, M.; Shen, Y.; Hou, J.; Bao, X. N-Hypermannose Glycosylation Disruption Enhances Recombinant Protein Production by Regulating Secretory Pathway and Cell Wall Integrity in Saccharomyces Cerevisiae. Sci. Rep. 2016, 6, 25654. [Google Scholar] [CrossRef]
- Ma, J.; Li, Q.; Tan, H.; Jiang, H.; Li, K.; Zhang, L.; Shi, Q.; Yin, H. Unique N-Glycosylation of a Recombinant Exo-Inulinase from Kluyveromyces cicerisporus and Its Effect on Enzymatic Activity and Thermostability. J. Biol. Eng. 2019, 13, 81. [Google Scholar] [CrossRef]
- Klesmith, J.R.; Bacik, J.-P.; Wrenbeck, E.E.; Michalczyk, R.; Whitehead, T.A. Trade-Offs between Enzyme Fitness and Solubility Illuminated by Deep Mutational Scanning. Proc. Natl. Acad. Sci. USA 2017, 114, 2265–2270. [Google Scholar] [CrossRef]
- Fox, J.D.; Kapust, R.B.; Waugh, D.S. Single Amino Acid Substitutions on the Surface of Escherichia coli Maltose-binding Protein Can Have a Profound Impact on the Solubility of Fusion Proteins. Protein Sci. 2001, 10, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Morel, N.; Massoulié, J. Comparative Expression of Homologous Proteins. J. Biol. Chem. 2000, 275, 7304–7312. [Google Scholar] [CrossRef]
- Tanurdzic, M.; Vaughn, M.W.; Jiang, H.; Lee, T.-J.; Slotkin, R.K.; Sosinski, B.; Thompson, W.F.; Doerge, R.W.; Martienssen, R.A. Epigenomic Consequences of Immortalized Plant Cell Suspension Culture. PLoS Biol. 2008, 6, e302. [Google Scholar] [CrossRef]
- Atroshenko, D.L.; Sergeev, E.P.; Golovina, D.I.; Pometun, A.A. Additivities for Soluble Recombinant Protein Expression in Cytoplasm of Escherichia coli. Fermentation 2024, 10, 120. [Google Scholar] [CrossRef]
- Mignon, C.; Mariano, N.; Stadthagen, G.; Lugari, A.; Lagoutte, P.; Donnat, S.; Chenavas, S.; Perot, C.; Sodoyer, R.; Werle, B. Codon Harmonization—Going beyond the Speed Limit for Protein Expression. FEBS Lett. 2018, 592, 1554–1564. [Google Scholar] [CrossRef]
- Rojas, G.; Carmenate, T.; Santo-Tomás, J.F.; Valiente, P.A.; Becker, M.; Pérez-Riverón, A.; Tundidor, Y.; Ortiz, Y.; Fernandez De Cossio-Diaz, J.; Graça, L.; et al. Directed Evolution of Super-Secreted Variants from Phage-Displayed Human Interleukin-2. Sci. Rep. 2019, 9, 800. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, X.-Y.; Sun, M.; Hu, Y.-H.; Ou-Yang, K.-Q.; Ai, S.-X.C.; Hua, Z.-C. Expression and Purification of a Mutant of Human Interleukin-2 in Pichia Pastoris. ABAB 2006, 133, 77–86. [Google Scholar] [CrossRef]
- Novy, R.; Drott, D.; Yaeger, K.; Mierendorf, R. inNovations . June 2001, pp. 1–3. Available online: https://wolfson.huji.ac.il/expression/rosetta.pdf (accessed on 16 April 2025).
- Bell, M.R.; Engleka, M.J.; Malik, A.; Strickler, J.E. To Fuse or Not to Fuse: What Is Your Purpose? Protein Sci. 2013, 22, 1466–1477. [Google Scholar] [CrossRef]
- Sommer, B.; Friehs, K.; Flaschel, E.; Reck, M.; Stahl, F.; Scheper, T. Extracellular Production and Affinity Purification of Recombinant Proteins with Escherichia coli Using the Versatility of the Maltose Binding Protein. J. Biotechnol. 2009, 140, 194–202. [Google Scholar] [CrossRef]
- Raran-Kurussi, S.; Keefe, K.; Waugh, D.S. Positional Effects of Fusion Partners on the Yield and Solubility of MBP Fusion Proteins. Protein Expr. Purif. 2015, 110, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Piette, F.; Struvay, C.; Feller, G. The Protein Folding Challenge in Psychrophiles: Facts and Current Issues. Environ. Microbiol. 2011, 13, 1924–1933. [Google Scholar] [CrossRef]
- Saibil, H. Chaperone Machines for Protein Folding, Unfolding and Disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef]
- Ferrer, M.; Chernikova, T.N.; Timmis, K.N.; Golyshin, P.N. Expression of a Temperature-Sensitive Esterase in a Novel Chaperone-Based Escherichia coli Strain. Appl. Environ. Microbiol. 2004, 70, 4499–4504. [Google Scholar] [CrossRef]
- Kauzmann, W.; Douglas, R.G. The Effect of Disulfide Bonding on the Solubility of Unfolded Serum Albumin in Salt Solutions. Arch. Biochem. Biophys. 1956, 65, 106–119. [Google Scholar] [CrossRef]
- An, L.; Gao, H.; Zhong, Y.; Liu, Y.; Cao, Y.; Yi, J.; Huang, X.; Wen, C.; Tong, R.; Pan, Z.; et al. Molecular Chaperones HSP40, HSP70, STIP1, and HSP90 Are Involved in Stabilization of Cx43. Cytotechnology 2023, 75, 207–217. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Gouaux, E. Overexpression of a Glutamate Receptor (GluR2) Ligand Binding Domain in Escherichia coli: Application of a Novel Protein Folding Screen. Proc. Natl. Acad. Sci. USA 1997, 94, 13431–13436. [Google Scholar] [CrossRef]
- Armstrong, N.; Lencastre, A.D.; Gouaux, E. A New Protein Folding Screen: Application to the Ligand Binding Domains of a Glutamate and Kainate Receptor and to Lysozyme and Carbonic Anhydrase. Protein Sci. 1999, 8, 1475–1483. [Google Scholar] [CrossRef]
- Vincentelli, R.; Canaan, S.; Campanacci, V.; Valencia, C.; Maurin, D.; Frassinetti, F.; Scappucini-Calvo, L.; Bourne, Y.; Cambillau, C.; Bignon, C. High-throughput Automated Refolding Screening of Inclusion Bodies. Protein Sci. 2004, 13, 2782–2792. [Google Scholar] [CrossRef] [PubMed]
- Lueking, A.; Holz, C.; Gotthold, C.; Lehrach, H.; Cahill, D. A System for Dual Protein Expression in Pichia Pastoris and Escherichia coli. Protein Expr. Purif. 2000, 20, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-L.; DaSilva, N.A.; Chen, W. Functional Display of Complex Cellulosomes on the Yeast Surface via Adaptive Assembly. ACS Synth. Biol. 2013, 2, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Harbers, M. Wheat Germ Systems for Cell-free Protein Expression. FEBS Lett. 2014, 588, 2762–2773. [Google Scholar] [CrossRef]
- Zemella, A.; Thoring, L.; Hoffmeister, C.; Kubick, S. Cell-Free Protein Synthesis: Pros and Cons of Prokaryotic and Eukaryotic Systems. ChemBioChem 2015, 16, 2420–2431. [Google Scholar] [CrossRef]
- Agarwal, S.; Agarwal, S.; Biancucci, M.; Satchell, K.J.F. Induced Autoprocessing of the Cytopathic Makes Caterpillars Floppy-like Effector Domain of the Vibrio vulnificus MARTX Toxin: A Novel Cysteine Peptidase in Toxin Autoprocessing. Cell. Microbiol. 2015, 17, 1494–1509. [Google Scholar] [CrossRef] [PubMed]
- Shindyalov, I.N.; Bourne, P.E. Protein Structure Alignment by Incremental Combinatorial Extension (CE) of the Optimal Path. Protein Eng. Des. Sel. 1998, 11, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Kufareva, I.; Abagyan, R. Methods of Protein Structure Comparison. In Homology Modeling; Orry, A.J.W., Abagyan, R., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 857, pp. 231–257. ISBN 978-1-61779-587-9. [Google Scholar]
- Lampropoulos, A.; Sutikovic, Z.; Wenzl, C.; Maegele, I.; Lohmann, J.U.; Forner, J. GreenGate—A Novel, Versatile, and Efficient Cloning System for Plant Transgenesis. PLoS ONE 2013, 8, e83043. [Google Scholar] [CrossRef] [PubMed]
- Gerstmans, H.; Grimon, D.; Gutiérrez, D.; Lood, C.; Rodríguez, A.; Van Noort, V.; Lammertyn, J.; Lavigne, R.; Briers, Y. A VersaTile-Driven Platform for Rapid Hit-to-Lead Development of Engineered Lysins. Sci. Adv. 2020, 6, eaaz1136. [Google Scholar] [CrossRef]
- Karimi, M.; Inzé, D.; Depicker, A. GATEWAYTM Vectors for Agrobacterium-Mediated Plant Transformation. Trends Plant Sci. 2002, 7, 193–195. [Google Scholar] [CrossRef]
- Van Leene, J.; Eeckhout, D.; Persiau, G.; Van De Slijke, E.; Geerinck, J.; Van Isterdael, G.; Witters, E.; De Jaeger, G. Isolation of Transcription Factor Complexes from Arabidopsis Cell Suspension Cultures by Tandem Affinity Purification. In Plant Transcription Factors; Yuan, L., Perry, S.E., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 754, pp. 195–218. ISBN 978-1-61779-153-6. [Google Scholar]
- Dubiel, M.; De Coninck, T.; Osterne, V.J.S.; Verbeke, I.; Van Damme, D.; Smagghe, G.; Van Damme, E.J.M. The ArathEULS3 Lectin Ends up in Stress Granules and Can Follow an Unconventional Route for Secretion. Int. J. Mol. Sci. 2020, 21, 1659. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beygmoradi, A.; Homaei, A.; Hemmati, R.; Fernandes, P. Recombinant Protein Expression: Challenges in Production and Folding Related Matters. Int. J. Biol. Macromol. 2023, 233, 123407. [Google Scholar] [CrossRef]
- Dechavanne, V.; Barrillat, N.; Borlat, F.; Hermant, A.; Magnenat, L.; Paquet, M.; Antonsson, B.; Chevalet, L. A High-Throughput Protein Refolding Screen in 96-Well Format Combined with Design of Experiments to Optimize the Refolding Conditions. Protein Expr. Purif. 2011, 75, 192–203. [Google Scholar] [CrossRef]
- Illergård, K.; Ardell, D.H.; Elofsson, A. Structure Is Three to Ten Times More Conserved than Sequence—A Study of Structural Response in Protein Cores. Proteins 2009, 77, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Hofstee, B.H.J. Non-Inverted versus Inverted Plots in Enzyme Kinetics. Nature 1959, 184, 1296–1298. [Google Scholar] [CrossRef] [PubMed]
- Hanes, C.S. Studies on Plant Amylases: The Effect of Starch Concentration upon the Velocity of Hydrolysis by the Amylase of Germinated Barley. Biochem. J. 1932, 26, 1406–1421. [Google Scholar] [CrossRef] [PubMed]
- Tungekar, A.A.; Castillo-Corujo, A.; Ruddock, L.W. So You Want to Express Your Protein in Escherichia coli? Essays Biochem. 2021, 65, 247–260. [Google Scholar] [CrossRef]
| EXP | Host Organism | Strain or Type | Expression Plasmid | CDS a | CDS Adjustments | Tags | Cloning Method | Lysis Method | Result | Refolding |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | E. coli | BL21 | pET-22b(+) | OsAPSE | Codon Opt. | N-pelB+C-His6 | Res. & Lig | LyB | Ins. | Yes |
| 2 | BL21-AI | Ins. | No | |||||||
| 3 | pLysS | Ins. | No | |||||||
| 4 | Rosetta | Ins. | No | |||||||
| 5 | ArcticExpress | Ins. | No | |||||||
| 6 | SHuffle | N.P. | No | |||||||
| 7 | GH27 | Ins. | No | |||||||
| 8 | E. coli | BL21 | pET-28a(+) | OsAPSE | Codon Opt. | C-His6 | Res. & Lig | LyB | Ins. | No |
| 9 | ArcticExpress | Ins. | No | |||||||
| 10 | E. coli | BL21 | pVTD13 | OsAPSE | Codon Opt. | N-GST+C-His6 | VersaTile cloning | LyB | N.P. | No |
| 11 | GH27 | N.P. | No | |||||||
| 12 | ricin-B | Ins. | No | |||||||
| 13 | GH-all-beta | Ins. | No | |||||||
| 14 | E. coli | BL21 Star | pET-32a(+) b | OsAPSE | Codon Opt. | N-TRX+C-His6 | Res. & Lig | Son. | Ins. | Yes |
| 15 | ArcticExpress | Ins. | No | |||||||
| 16 | Rosetta | Ins. | No | |||||||
| 17 | E. coli | BL21 | pET-21a(+) | OsAPSE | Harm. c | C-His6 | Res. & Lig | LyB | Ins. | No |
| 18 | Rosetta | N.P. | No | |||||||
| 19 | BL21 | Codon Opt. d | Ins. | No | ||||||
| 20 | SHuffle | N.P. | No | |||||||
| 21 | BL21 | PROSS e | Ins. | No | ||||||
| 22 | SHuffle | N.P. | No | |||||||
| 23 | E. coli | BL21 | pDEST | OsAPSE | Codon Opt. | N-MBP+TEV site+C-FLAG3 or C-HA3 or C-His6 | GG cloning | LyB | Ins. | No |
| 24 | ricin-B | Ins. | No | |||||||
| 25 | GH-β | Ins. | No | |||||||
| 26 | GH27 | LyB + Son. | Soluble | No | ||||||
| 27 | N/C-MBP2+C-His6 | LyB | Ins. | No | ||||||
| 28 | N-GST+C-His6 | Ins. | No | |||||||
| 29 | P. pastoris | X-33 | pPICZαA | OsAPSE | Codon Opt. | N-α-factor+C-His6 | Res. & Lig | Beads + LyB | N.P. | No |
| 30 | GlycoDelete | N.P. | No | |||||||
| 31 | KM71H | N.P. | No | |||||||
| 32 | P. pastoris | X-33 | Modified pPICZαA | GH27 | Codon Opt. | N-MBP+C-RFP | GG cloning | Beads + LyB | Soluble | No |
| 33 | N/C-MBP2+C-His6 | N.P. | No | |||||||
| 34 | A. thaliana | PSB-D cell culture | pK7WG2D | OsAPSE | Codon Opt. | Reporter EGFP+C-His6 | GW cloning | Cryo + LyB | Soluble | No |
| 35 | GH27 | N.P. | No | |||||||
| 36 | ricin-B | N.P. | No | |||||||
| 37 | GH-β | N.P. | No | |||||||
| 38 | BY-2 CFPS | ALiCE | pALiCE02 | GH27 | Codon Opt. | C-His6 | Res. & Lig | None | Soluble f | No |
| WT | Variant 1 | Variant 2 | Variant 3 | Variant 4 | Variant 5 | Variant 6 | Variant 7 | Variant 8 | Variant 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sequence identity compared to OsAPSE | 100 | 98.7 | 97.8 | 97.7 | 96.5 | 95.7 | 95.5 | 94.6 | 93.5 | 92.8 |
| Number of mutated amino acid residues | 0 | 8 | 14 | 15 | 23 | 28 | 29 | 35 | 42 | 47 |
| RMSD (Å) compared to OsAPSE | 0 | 0.0684 | 0.0825 | 0.0881 | 0.0908 | 0.1017 | 0.1030 | 0.0963 | 0.1081 | 0.1113 |
| POI produced recombinantly? | Yes | Yes | No | Yes | No | Yes | No | Yes | No | No |
| Soluble POI? | No | No | No | No | No | No | No | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Coninck, T.; Vanhaeren, H.; Van Damme, E.J.M. The Achilles Heel of Protein Biochemistry: Insolubility of Recombinant Proteins—A Case Study About Producing a Rice Enzyme. Int. J. Mol. Sci. 2025, 26, 8974. https://doi.org/10.3390/ijms26188974
De Coninck T, Vanhaeren H, Van Damme EJM. The Achilles Heel of Protein Biochemistry: Insolubility of Recombinant Proteins—A Case Study About Producing a Rice Enzyme. International Journal of Molecular Sciences. 2025; 26(18):8974. https://doi.org/10.3390/ijms26188974
Chicago/Turabian StyleDe Coninck, Tibo, Hannes Vanhaeren, and Els J. M. Van Damme. 2025. "The Achilles Heel of Protein Biochemistry: Insolubility of Recombinant Proteins—A Case Study About Producing a Rice Enzyme" International Journal of Molecular Sciences 26, no. 18: 8974. https://doi.org/10.3390/ijms26188974
APA StyleDe Coninck, T., Vanhaeren, H., & Van Damme, E. J. M. (2025). The Achilles Heel of Protein Biochemistry: Insolubility of Recombinant Proteins—A Case Study About Producing a Rice Enzyme. International Journal of Molecular Sciences, 26(18), 8974. https://doi.org/10.3390/ijms26188974








