New Derivatives of 2-(Cyclohexylamino)thiazol-4(5H)-one as Strong Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1: Synthesis, Antiproliferative and Redox-Modulating Activity
Abstract
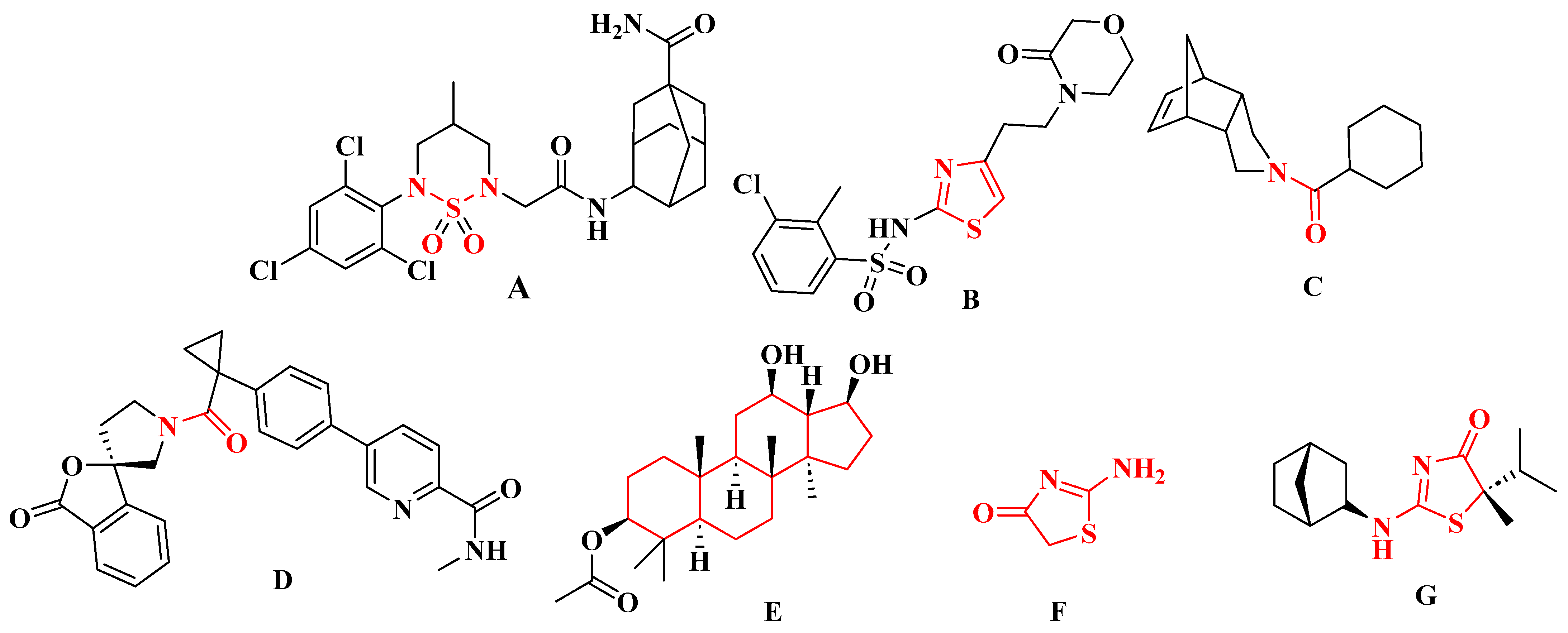
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. 11β-HSD Inhibitory Activity
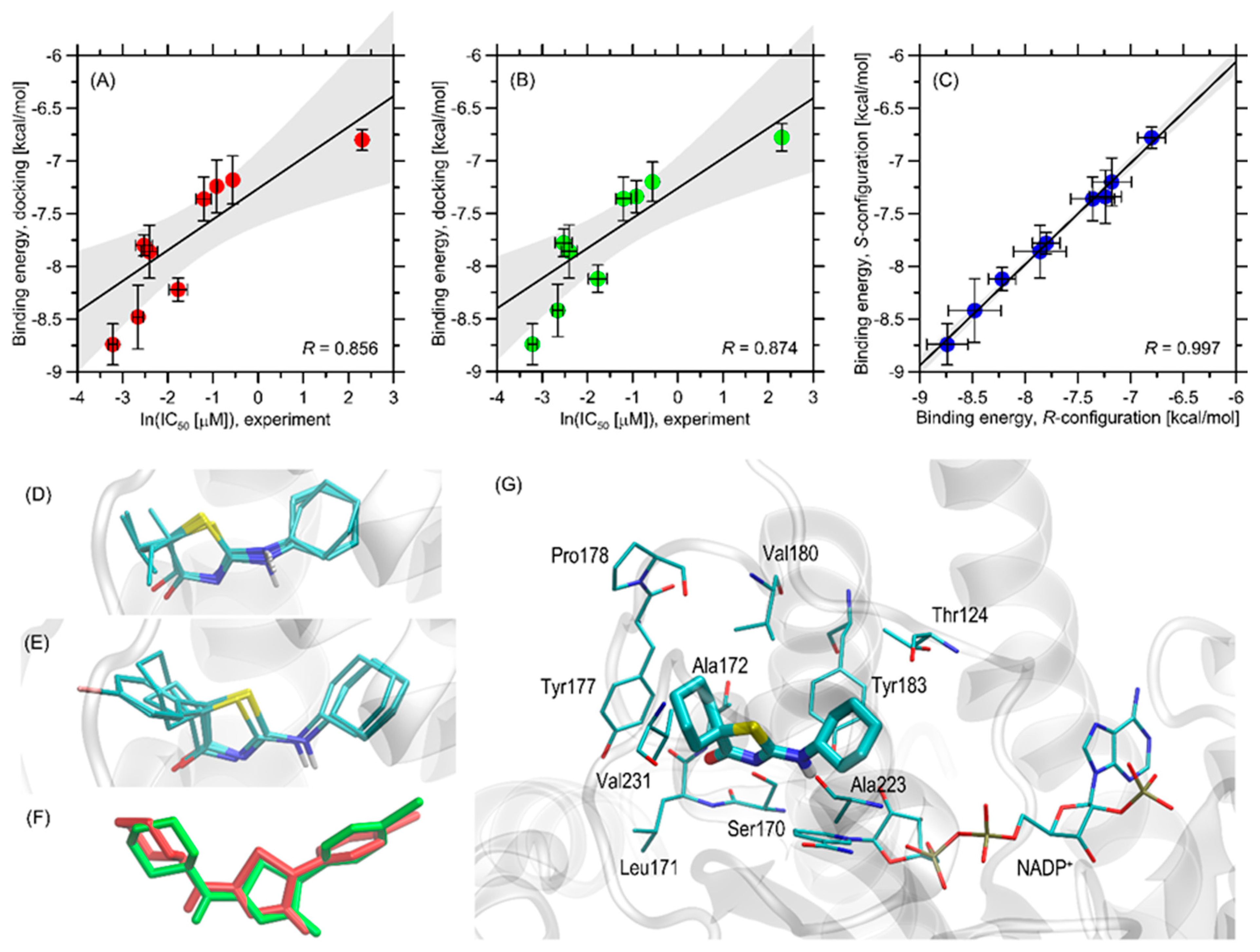
2.3. Results of the Docking Studies
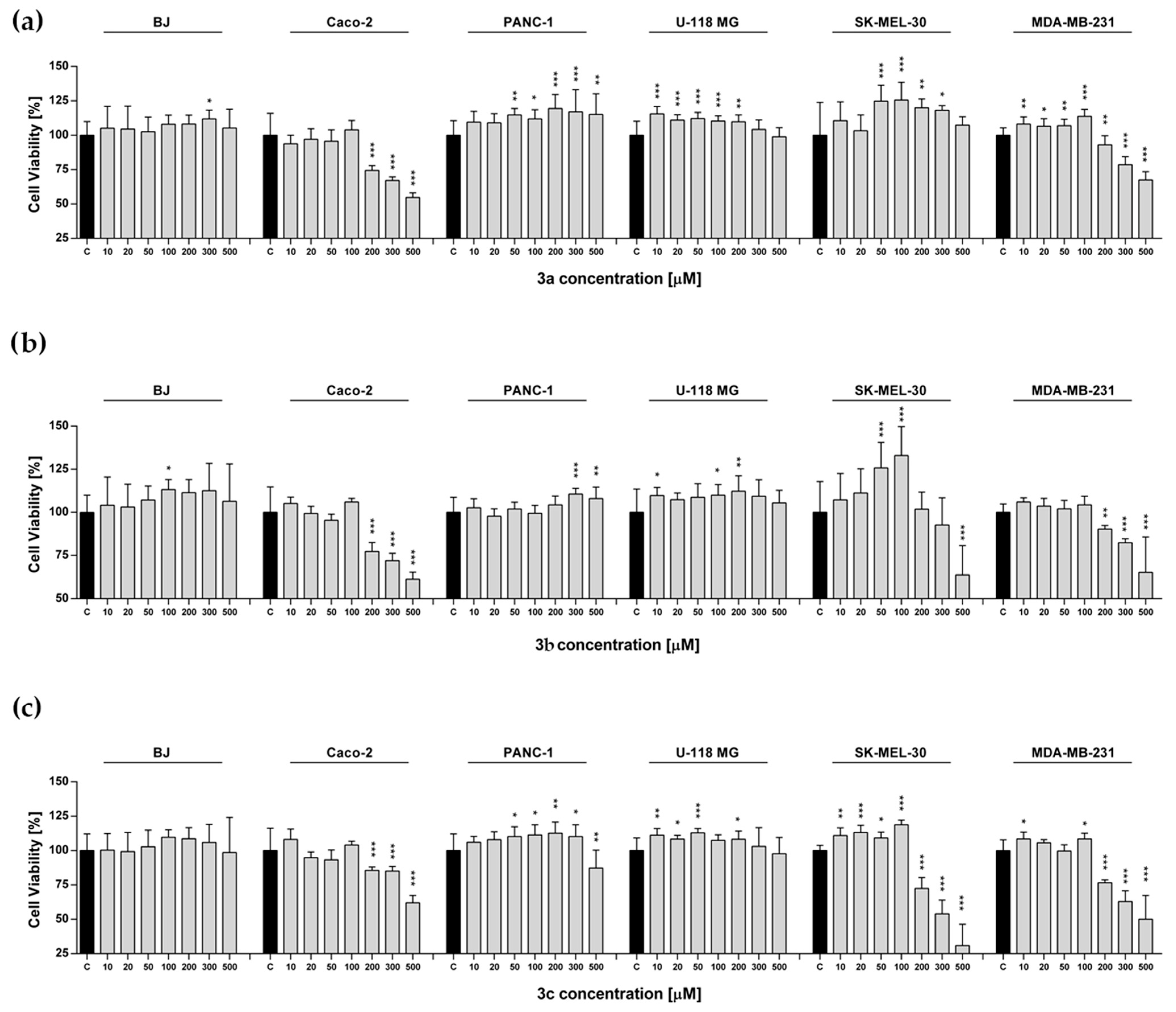
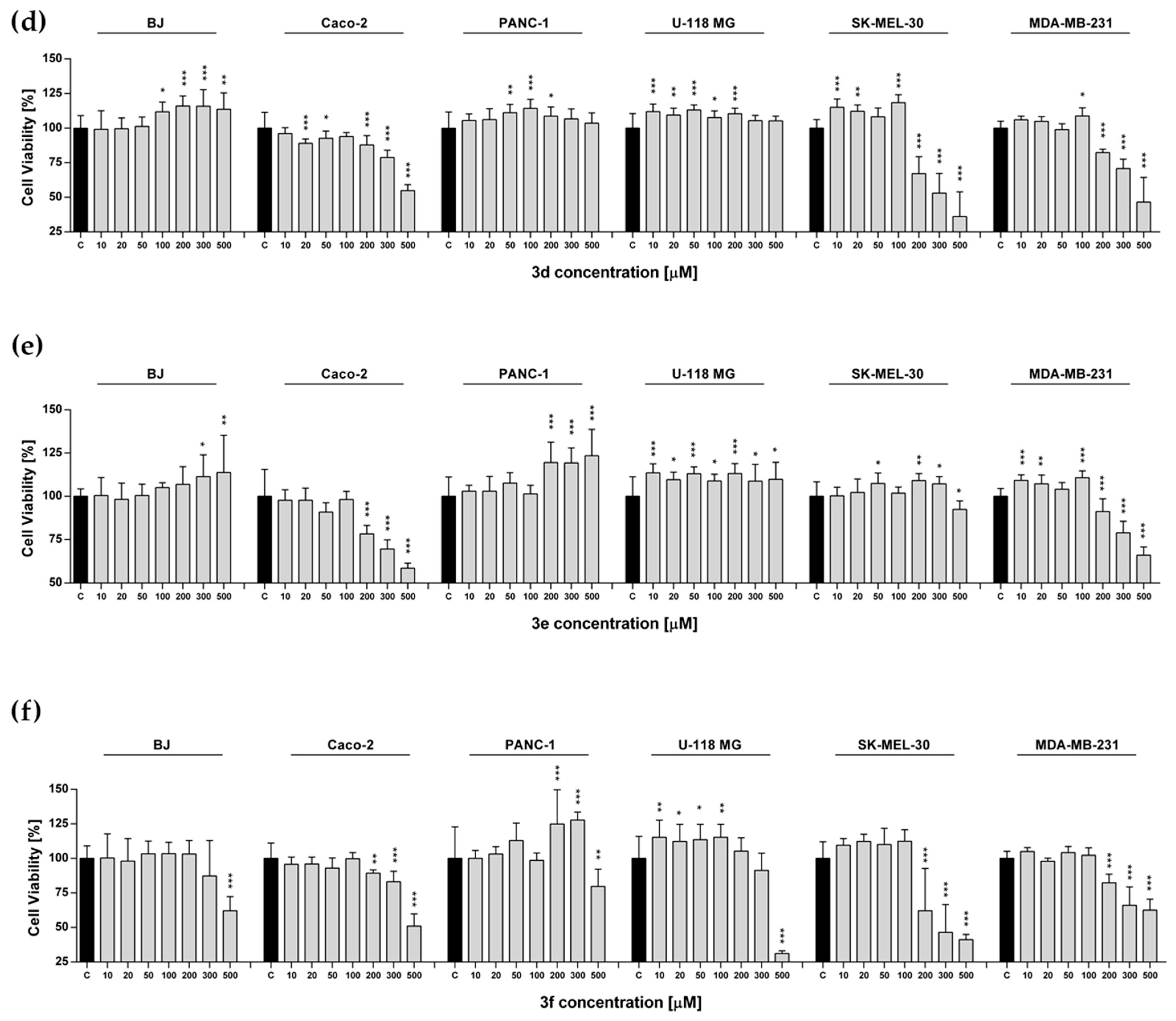
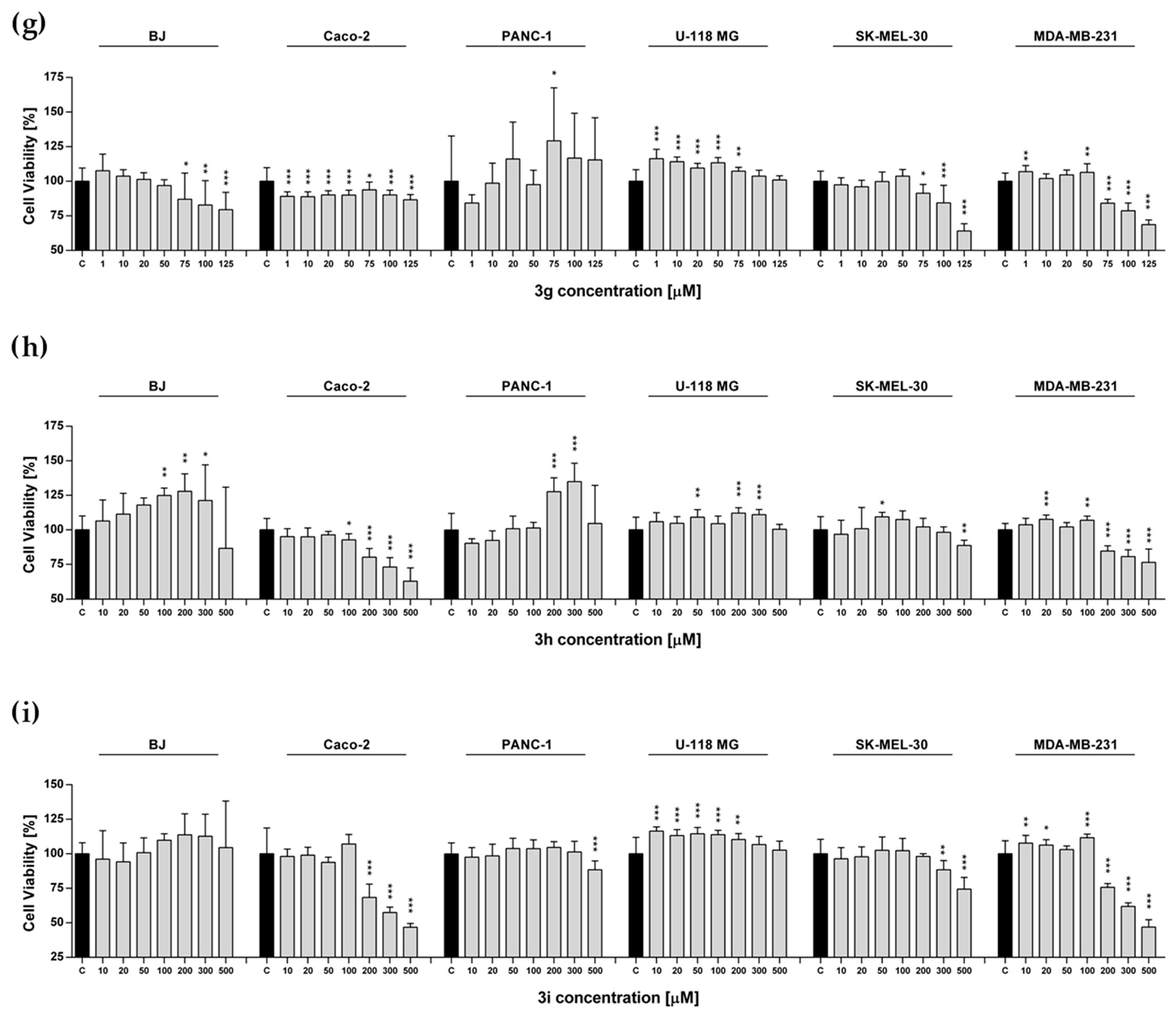
2.4. Metabolic Activity
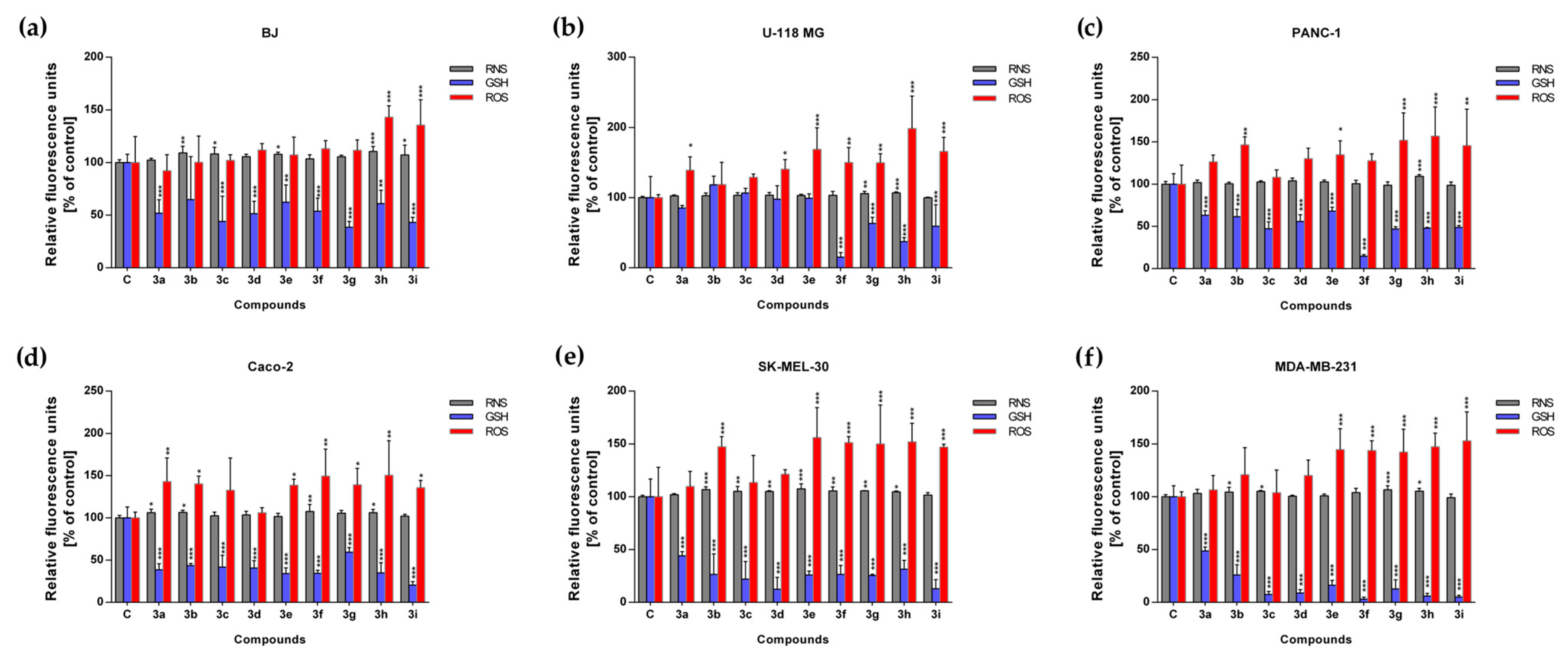
2.5. Evaluation of Intracellular Redox Homeostasis
2.6. Predicting Medicinal Chemistry Structural Alerts
3. Materials and Methods
3.1. Chemistry
3.2. Synthesis Procedures
3.2.1. Method A
3.2.2. Method B
3.2.3. Method C
3.3. 11β-HSD Inhibition Assays
3.4. Molecular Docking
3.5. Cell Culture
3.6. MTS Assay
3.7. Analysis of Intracellular Redox Homeostasis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seckl, J.R.; Walker, B.R. Minireview: 11beta-hydroxysteroid dehydrogenase type 1—A tissue-specific amplifier of glucocorticoid action. Endocrinology 2001, 142, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.; Holmes, M.; Seckl, J. 11β-hydroxysteroid dehydrogenases: Intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef]
- Kupczyk, D.; Studzińska, R.; Kołodziejska, R.; Baumgart, S.; Modrzejewska, M.; Woźniak, A. 11β-Hydroxysteroid dehydrogenase type 1 as a potential treatment target in cardiovascular diseases. J. Clin. Med. 2022, 11, 6190. [Google Scholar] [CrossRef]
- Scott, J.S.; Goldberg, F.W.; Turnbull, A.V. Medicinal chemistry of inhibitors of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). J. Med. Chem. 2014, 57, 4466–4486. [Google Scholar] [CrossRef]
- Dekker, M.J.; Tiemeier, H.; Luijendijk, H.J.; Kuningas, M.; Hofman, A.; de Jong, F.H.; Stewart, P.M.; Koper, J.W.; Lamberts, S.W. The effect of common genetic variation in 11β-hydroxysteroid dehydrogenase type 1 on hypothalamic-pituitary-adrenal axis activity and incident depression. J. Clin. Endocrinol. Metab. 2012, 97, E233–E237. [Google Scholar] [CrossRef]
- Slattery, D.A.; Uzunov, D.P.; Cryan, J.F. 11-β hydroxysteroid type 1 knockout mice display an antidepressant-like phenotype in the forced swim test. Acta Neuropsychiatr. 2016, 28, 55–60. [Google Scholar] [CrossRef]
- Li, H.; Hu, S.; Wu, R.; Zhou, H.; Zhang, K.; Li, K.; Lin, W.; Shi, Q.; Chen, H.; Lv, S. 11β-Hydroxysteroid dehydrogenase type 1 facilitates osteoporosis by turning on osteoclastogenesis through hippo signaling. Int. J. Biol. Sci. 2023, 19, 3628–3639. [Google Scholar] [CrossRef] [PubMed]
- Schwab, D.; Sturm, C.; Portron, A.; Fuerst-Recktenwald, S.; Hainzl, S.; Jordan, P.; Stewart, W.C.; Tepedino, M.E.; DuBiner, H. Oral administration of the 11β-hydroxysteroid-dehydrogenase type 1 inhibitor RO5093151 to patients with glaucoma: An adaptive, randomised, placebo-controlled clinical study. BMJ Open Ophthalmol. 2017, 1, e000063. [Google Scholar] [CrossRef][Green Version]
- Taves, M.D.; Otsuka, S.; Taylor, M.A.; Donahue, K.M.; Meyer, T.J.; Cam, M.C.; Ashwell, J.D. Tumors produce glucocorticoids by metabolite recycling, not synthesis, and activate Tregs to promote growth. J. Clin. Investig. 2023, 133, e164599. [Google Scholar] [CrossRef] [PubMed]
- Swatler, J.; Ju, Y.J.; Anderson, A.C.; Lugli, E. Tumors recycle glucocorticoids to drive Treg-mediated immunosuppression. J. Clin. Investig. 2023, 133, e173141. [Google Scholar] [CrossRef]
- Poinot, H.; Dupuychaffray, E.; Arnoux, G.; Alvarez, M.; Tachet, J.; Ezzar, O.; Moore, J.; Bejuy, O.; Olesti, E.; Visconti, G.; et al. Activation of endogenous glucocorticoids by HSD11B1 inhibits the antitumor immune response in renal cancer. Oncoimmunology 2023, 13, 2286820. [Google Scholar] [CrossRef]
- Saito, R.; Miki, Y.; Abe, T.; Miyauchi, E.; Abe, J.; Nanamiya, R.; Inoue, C.; Sato, I.; Sasano, H. 11β hydroxysteroid dehydrogenase 1: A new marker for predicting response to immune-checkpoint blockade therapy in non-small-cell lung carcinoma. Br. J. Cancer 2020, 123, 61–71. [Google Scholar] [CrossRef]
- Melo, L.M.N.; Herrera-Rios, D.; Hinze, D.; Löffek, S.; Oezel, I.; Turiello, R.; Klein, J.; Leonardelli, S.; Westedt, I.V.; Al-Matary, Y.; et al. Glucocorticoid activation by HSD11B1 limits T cell-driven interferon signaling and response to PD-1 blockade in melanoma. J. Immunother. Cancer 2023, 11, e004150. [Google Scholar] [CrossRef]
- Lee, J.H.; Bok, J.H.; Park, S.B.; Pagire, H.S.; Na, Y.J.; Rim, E.; Jung, W.H.; Song, J.S.; Kang, N.S.; Seo, H.W.; et al. Optimization of cyclic sulfamide derivatives as 11β-hydroxysteroid dehydrogenase 1 inhibitors for the potential treatment of ischemic brain injury. Bioorg. Med. Chem. Lett. 2020, 30, 126787. [Google Scholar] [CrossRef]
- Joharapurkar, A.; Dhanesha, N.; Shah, G.; Kharul, R.; Jain, M. 11β-hydroxysteroid dehydrogenase type 1: Potential therapeutic target for metabolic syndrome. Pharmacol. Rep. 2012, 64, 1055–1085. [Google Scholar] [CrossRef] [PubMed]
- Leiva, R.; Grinan-Ferre, C.; Seira, C.; Valverde, E.; McBride, A.; Binnie, M.; Pérez, B.; Luque, F.J.; Pallàs, M.; Bidon-Chanal, A.; et al. Design, synthesis and in vivo study of novel pyrrolidine-based 11β-HSD1 inhibitors for age-related cognitive dysfunction. Eur. J. Med. Chem. 2017, 139, 412–428. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, M.; He, C.; Zhuo, J.; Burns, D.M.; Qian, D.Q.; Lin, Q.; Li, Y.L.; Chen, L.; Shi, E.; et al. Discovery of 1′-(1-phenylcyclopropane-carbonyl)-3H-spiro[isobenzofuran-1,3′-pyrrolidin]-3-one as a novel steroid mimetic scaffold for the potent and tissue-specific inhibition of 11β-HSD1 using a scaffold-hopping approach. Bioorg. Med. Chem. Lett. 2022, 69, 128782. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.D.; Bao, Y.; Shen, Y.; Su, J.; Leng, Y.; Zhao, Q.S. Synthesis of selective 11β-HSD1 inhibitors based on dammarane scaffold. Eur. J. Med. Chem. 2017, 135, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Kimura, R.E.; Zhang, X.; Nam, J.; Amore, B.M.; Hickman, D.; Greg Slatter, J.; Emery, M.G. Intestinal and hepatic first-pass extraction of the 11β-HSD1 inhibitor AMG 221 in rats with chronic vascular catheters. Xenobiotica 2014, 44, 264–269. [Google Scholar] [CrossRef]
- Baumgart, S.; Kupczyk, D.; Archała, A.; Koszła, O.; Sołek, P.; Płaziński, W.; Płazińska, A.; Studzińska, R. Synthesis of novel 2-(cyclopentylamino)thiazol-4(5H)-one derivatives with potential anticancer, antioxidant, and 11β-HSD inhibitory activities. Int. J. Mol. Sci. 2023, 24, 7252. [Google Scholar] [CrossRef]
- Studzińska, R.; Kupczyk, D.; Płaziński, W.; Baumgart, S.; Bilski, R.; Paprocka, R.; Kołodziejska, R. Novel 2-(adamantan-1-yloamino)thiazol-4(5H)-one derivatives and their inhibitory activity towards 11β-HSD1—Synthesis molecular docking and in vitro studies. Int. J. Mol. Sci. 2021, 22, 8609. [Google Scholar] [CrossRef] [PubMed]
- Kupczyk, D.; Studzińska, R.; Baumgart, S.; Bilski, R.; Kosmalski, T.; Kołodziejska, R.; Woźniak, A. A novel N-tert-butyl derivatives of pseudothiohydantoin as potential target in anti-cancer therapy. Molecules 2021, 26, 2612. [Google Scholar] [CrossRef] [PubMed]
- Mądra-Gackowska, K.; Baumgart, S.; Jędrzejewski, M.; Studzińska, R.; Szeleszczuk, Ł.; Gackowski, M. Computational QSAR study of novel 2-aminothiazol-4(5H)-one derivatives as 11β-HSD1 inhibitors. J. Comput. Aided. Mol. Des. 2025, 39, 67. [Google Scholar] [CrossRef] [PubMed]
- Studzińska, R.; Kołodziejska, R.; Płaziński, W.; Kupczyk, D.; Kosmalski, T.; Jasieniecka, K.; Modzelewska-Banachiewicz, B. Synthesis of the N-methyl derivatives of 2-aminothiazol-4(5H)-one and their interactions with 11βHSD1-molecular modeling and in vitro studies. Chem. Biodivers. 2019, 16, e1900065. [Google Scholar] [CrossRef]
- Kupczyk, D.; Studzińska, R.; Bilski, R.; Baumgart, S.; Kołodziejska, R.; Woźniak, A. Synthesis of novel 2-(isopropylamino)thia-zol-4(5H)-one derivatives and their inhibitory activity of 11β-HSD1 and 11β-HSD2 in aspect of carcinogenesis prevention. Molecules 2020, 25, 4233. [Google Scholar] [CrossRef]
- Studzińska, R.; Kołodziejska, R.; Kupczyk, D.; Płaziński, W.; Kosmalski, T. A novel derivatives of thiazol-4(5H)-one and their activity in the inhibition of 11β-hydroxysteroid dehydrogenase type 1. Bioorg. Chem. 2018, 79, 115–121. [Google Scholar] [CrossRef]
- Wan, Y.; Long, J.; Gao, H.; Tang, Z. 2-Aminothiazole: A privileged scaffold for the discovery of anti-cancer agents. Eur. J. Med. Chem. 2010, 210, 112953. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef]
- Rai, M.; Singh, A.V.; Paudel, N.; Kanase, A.; Falletta, E.; Kerkar, P.; Heyda, J.; Barghash, R.F.; Pratap Singh, S.; Soos, M. Herbal concoction Unveiled: A computational analysis of phytochemicals’ pharmacokinetic and toxicological profiles using novel approach methodologies (NAMs). Curr. Res. Toxicol. 2023, 5, 100118. [Google Scholar] [CrossRef]
- SwissADME. Available online: http://swissadme.ch (accessed on 24 October 2024).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R.J. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A.; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
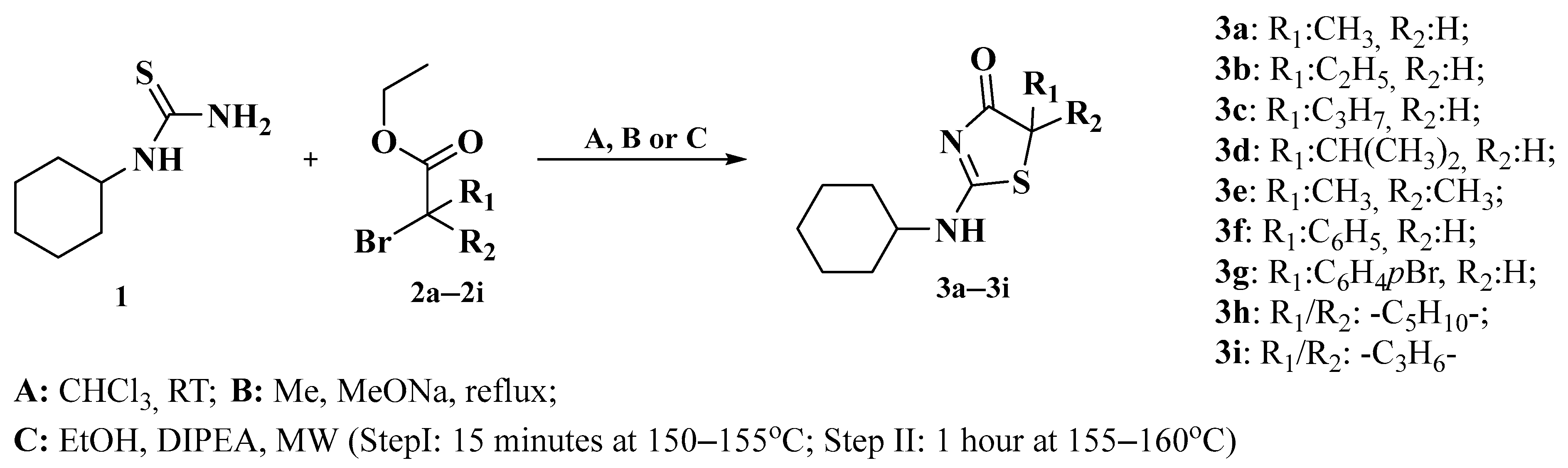
| Compound | R1 | R2 | Synthesis Method | Isolated Yield (%) | Melting Point (°C) |
|---|---|---|---|---|---|
| 3a | H | CH3 | A | 54.79 1 | 164.2–165.3 |
| 3b | H | C2H5 | A | 80.06 1 | 152.1–153.0 |
| 3c | H | C3H7 | A | 82.75 1 | 147.5–146.8 |
| 3d | H | CH(CH3)2 | B | 14.55 | 160–162 |
| 3e | CH3 | CH3 | B | 11.78 | 218.5–221.3 |
| 3f | H | C6H5 | C | 74.68 | 208–210 |
| 3g | H | C6H4p-Br | C | 28.30 | 255 (dec.) |
| 3h | -(CH2)5- | C | 1.40 | 244.2–245.4 | |
| 3i | -(CH2)3- | C | 34.62 | 196.8–197.3 | |
| Compound | % of 11β-HSD1 Inhibition | IC50 11β-HSD1 | % of 11β-HSD2 Inhibition |
|---|---|---|---|
| 3a | 27.48 ± 2.12 | >10 | 42.03 ± 2.50 |
| 3b | 68.47 ± 4.28 | 0.57 ± 0.06 | 38.34 ± 9.96 |
| 3c | 85.72 ± 4.43 | 0.4 ± 0.005 | 47.10 ± 0.82 |
| 3d | 90.38 ± 2.74 | 0.08 ± 0.015 | 47.01 ± 1.67 |
| 3e | 82.56 ± 1.79 | 0.3 ± 0.05 | 38.59 ± 5.56 |
| 3f | 83.84 ± 4.53 | 0.17 ± 0.035 | 39.96 ± 8.34 |
| 3g | 90.15 ± 1.59 | 0.07 ± 0.008 | 47.20 ± 1.17 |
| 3h | 93.99 ± 2.35 | 0.04 ± 0.004 | 45.65 ± 5.28 |
| 3i | 87.35 ± 0.99 | 0.09 ± 0.016 | 50.97 ± 0.73 |
| Control | 90.42 ± 1.86 | 0.08 ± 0.006 | 55.22 ± 0.13 a 46.82 ± 3.75 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumgart, S.; Kupczyk, D.; Płazińska, A.; Koszła, O.; Sołek, P.; Archała, A.; Płaziński, W.; Studzińska, R. New Derivatives of 2-(Cyclohexylamino)thiazol-4(5H)-one as Strong Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1: Synthesis, Antiproliferative and Redox-Modulating Activity. Int. J. Mol. Sci. 2025, 26, 8972. https://doi.org/10.3390/ijms26188972
Baumgart S, Kupczyk D, Płazińska A, Koszła O, Sołek P, Archała A, Płaziński W, Studzińska R. New Derivatives of 2-(Cyclohexylamino)thiazol-4(5H)-one as Strong Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1: Synthesis, Antiproliferative and Redox-Modulating Activity. International Journal of Molecular Sciences. 2025; 26(18):8972. https://doi.org/10.3390/ijms26188972
Chicago/Turabian StyleBaumgart, Szymon, Daria Kupczyk, Anita Płazińska, Oliwia Koszła, Przemysław Sołek, Aneta Archała, Wojciech Płaziński, and Renata Studzińska. 2025. "New Derivatives of 2-(Cyclohexylamino)thiazol-4(5H)-one as Strong Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1: Synthesis, Antiproliferative and Redox-Modulating Activity" International Journal of Molecular Sciences 26, no. 18: 8972. https://doi.org/10.3390/ijms26188972
APA StyleBaumgart, S., Kupczyk, D., Płazińska, A., Koszła, O., Sołek, P., Archała, A., Płaziński, W., & Studzińska, R. (2025). New Derivatives of 2-(Cyclohexylamino)thiazol-4(5H)-one as Strong Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1: Synthesis, Antiproliferative and Redox-Modulating Activity. International Journal of Molecular Sciences, 26(18), 8972. https://doi.org/10.3390/ijms26188972



_Kim.png)



