2-Azidobenzaldehyde-Enabled Construction of Quinazoline Derivatives: A Review
Abstract
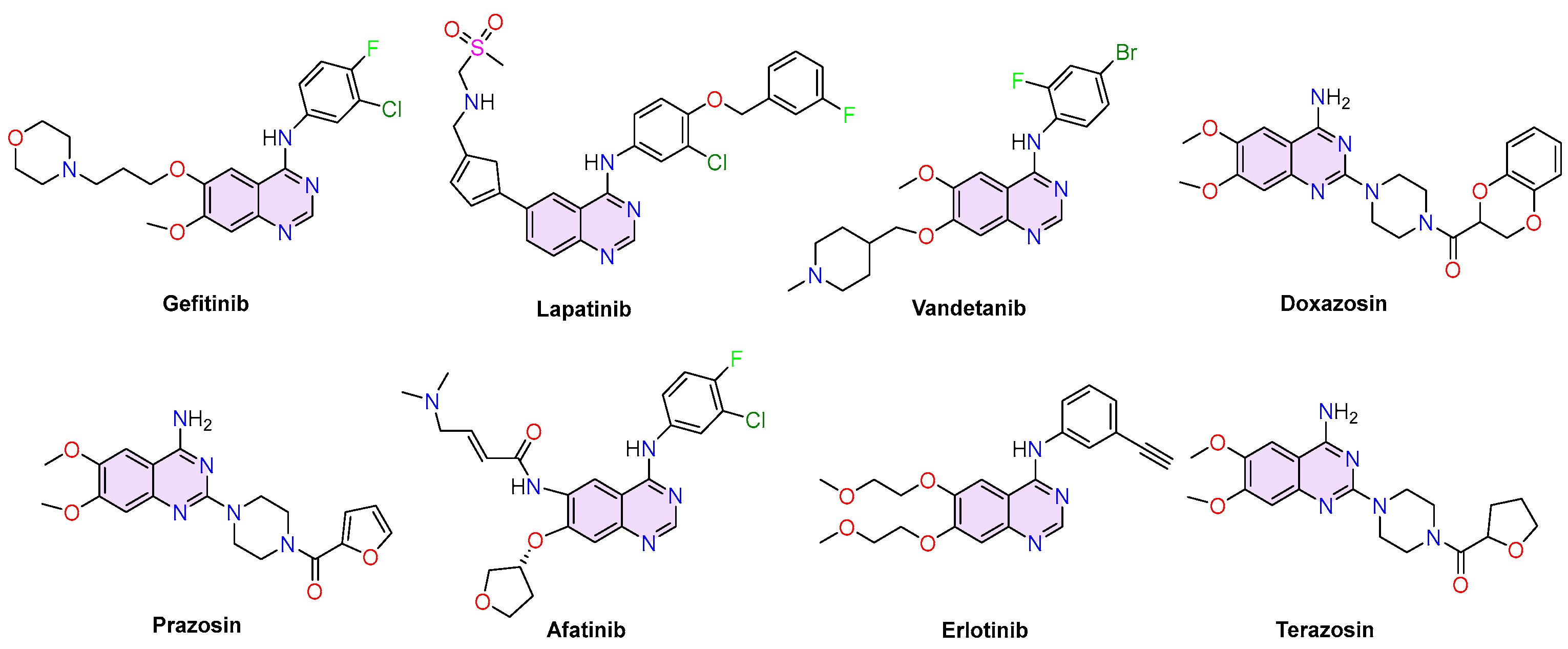
1. Introduction
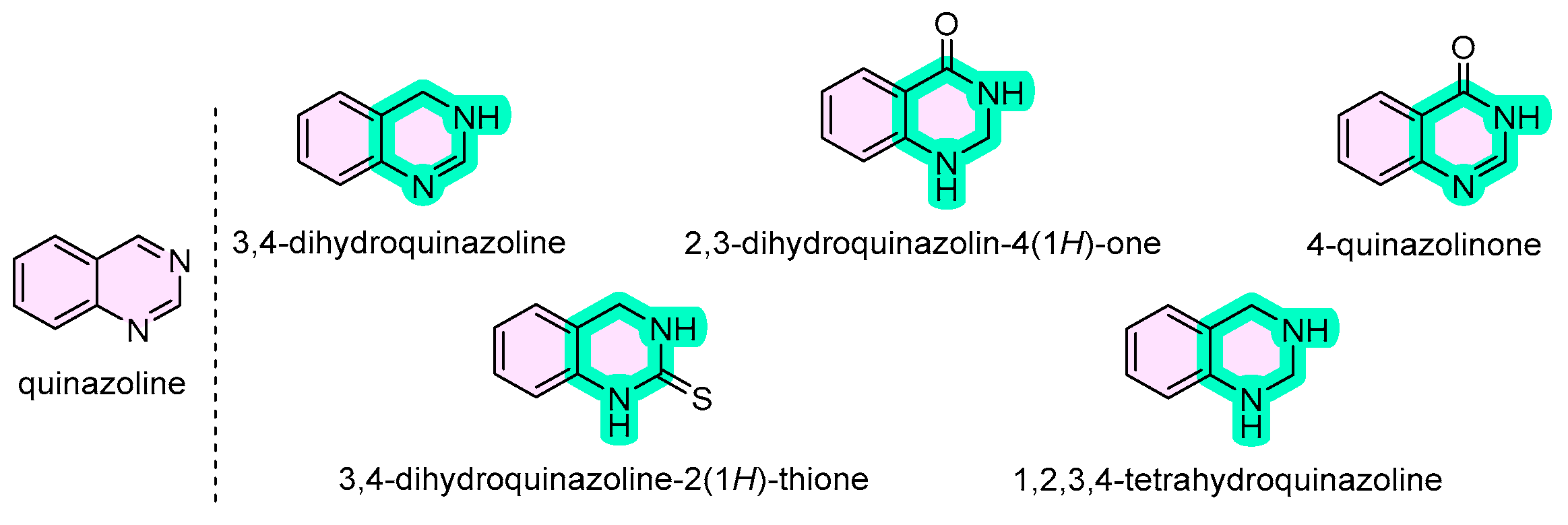
2. Synthesis of Quinazoline Derivatives
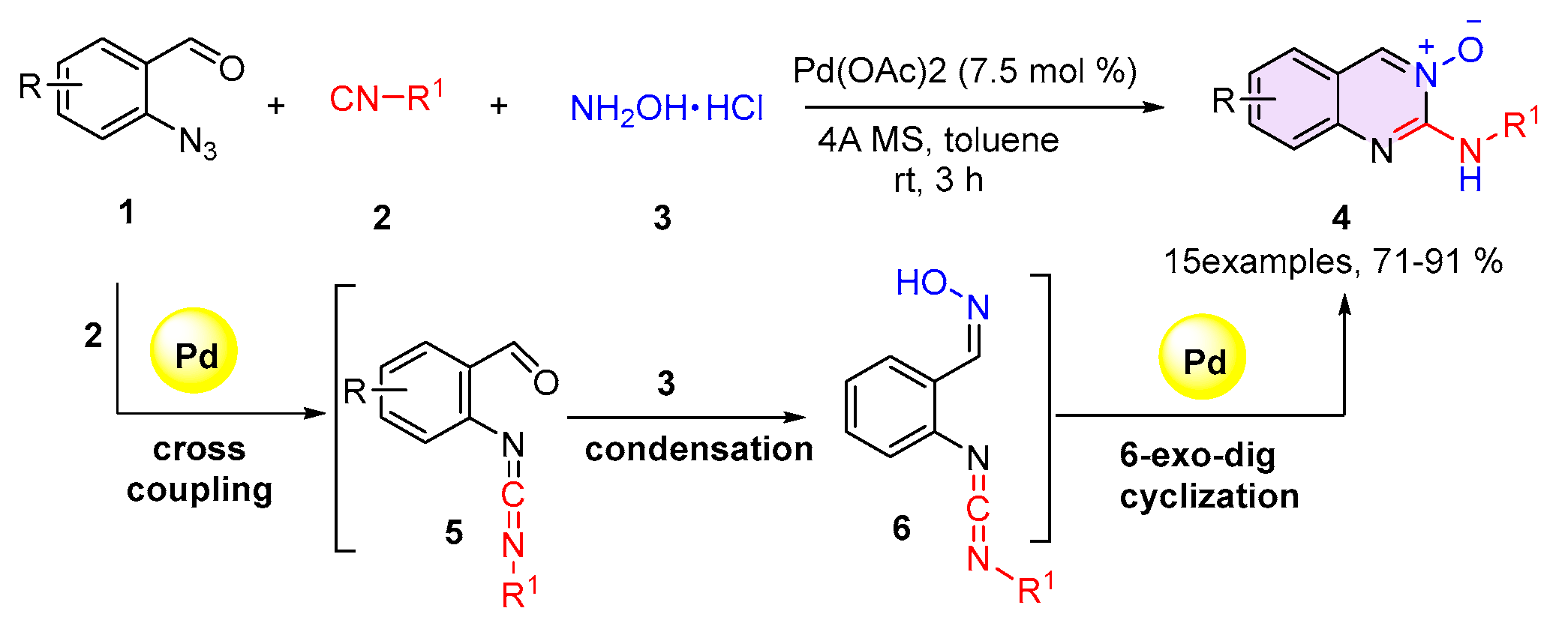
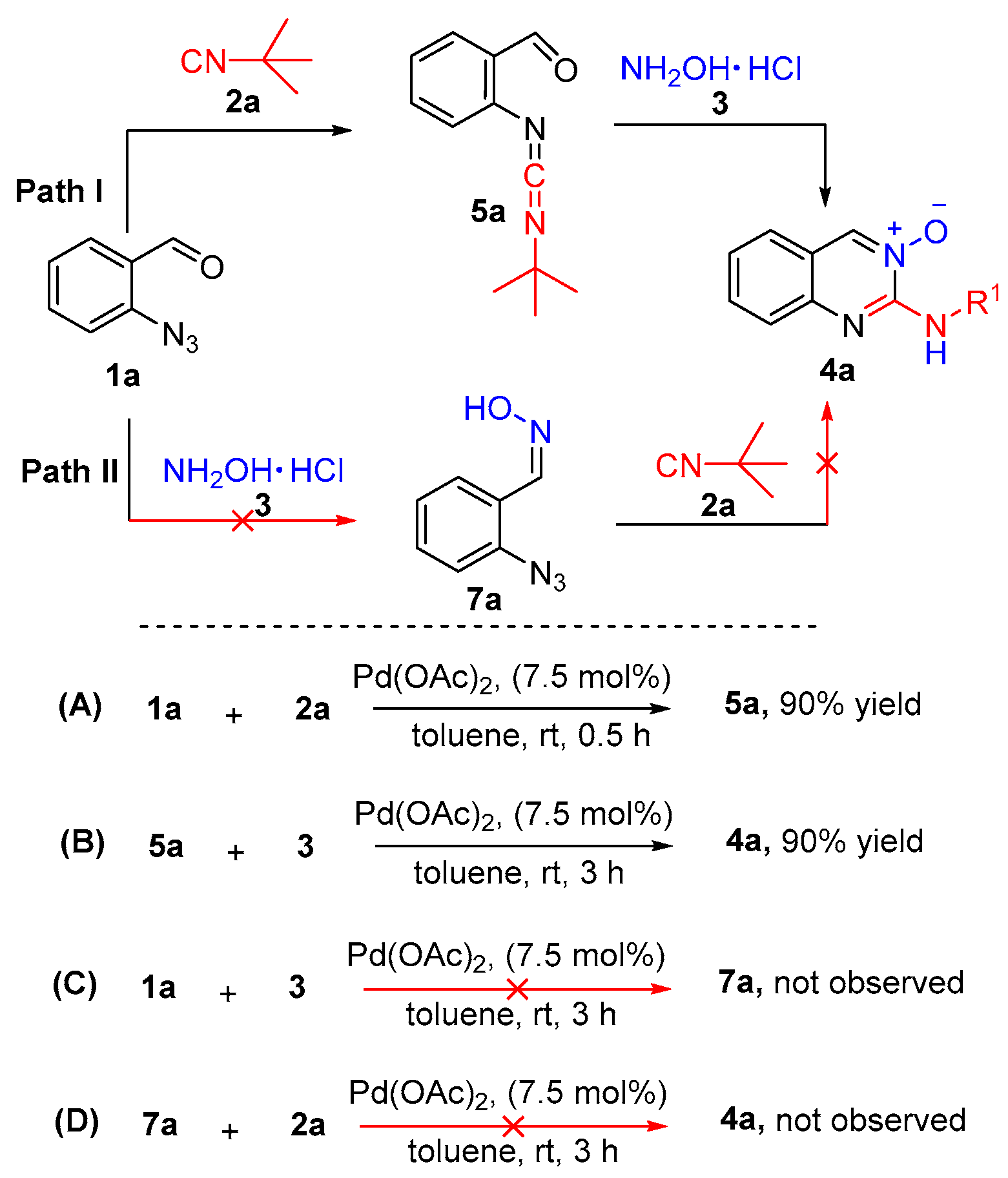
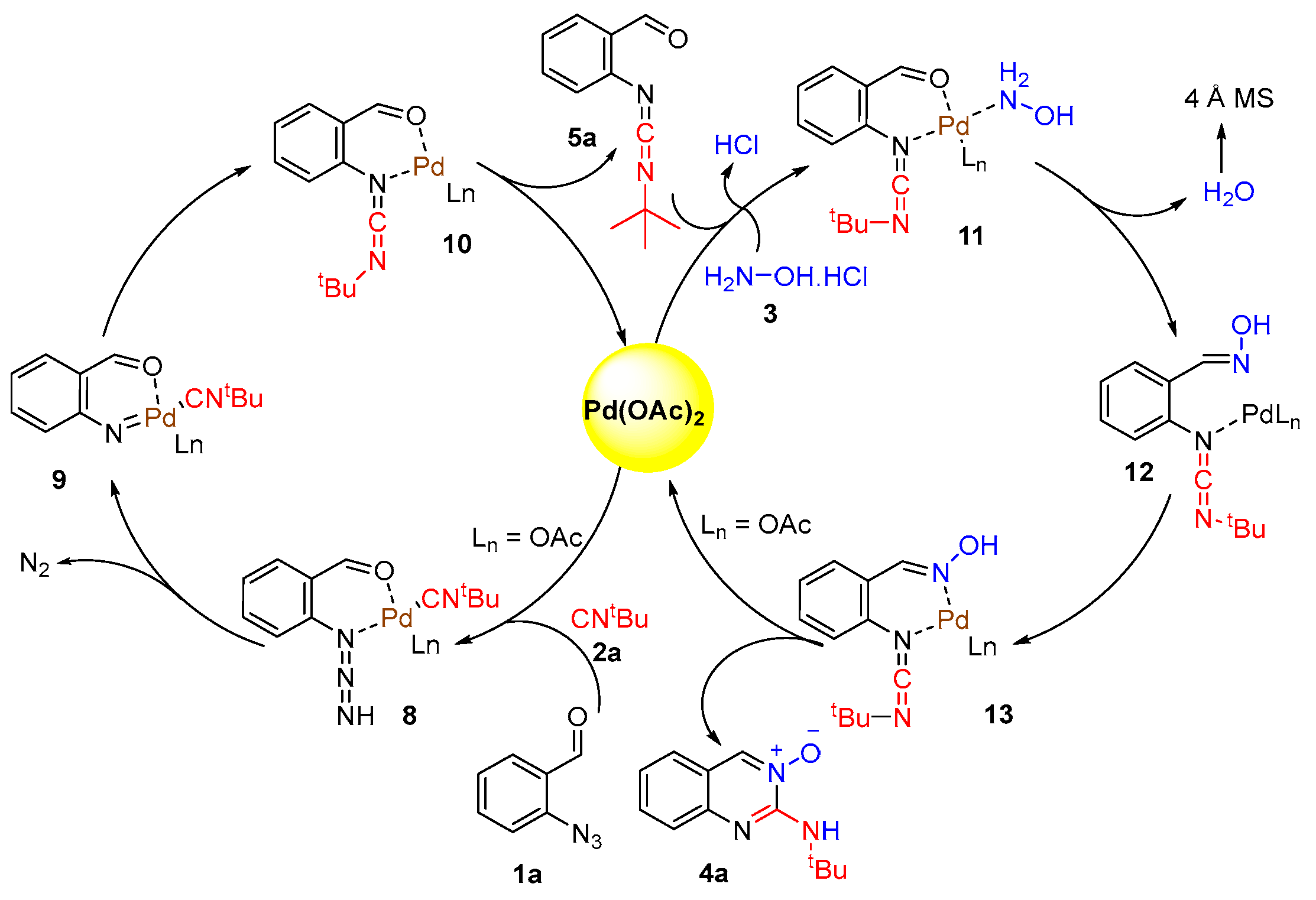
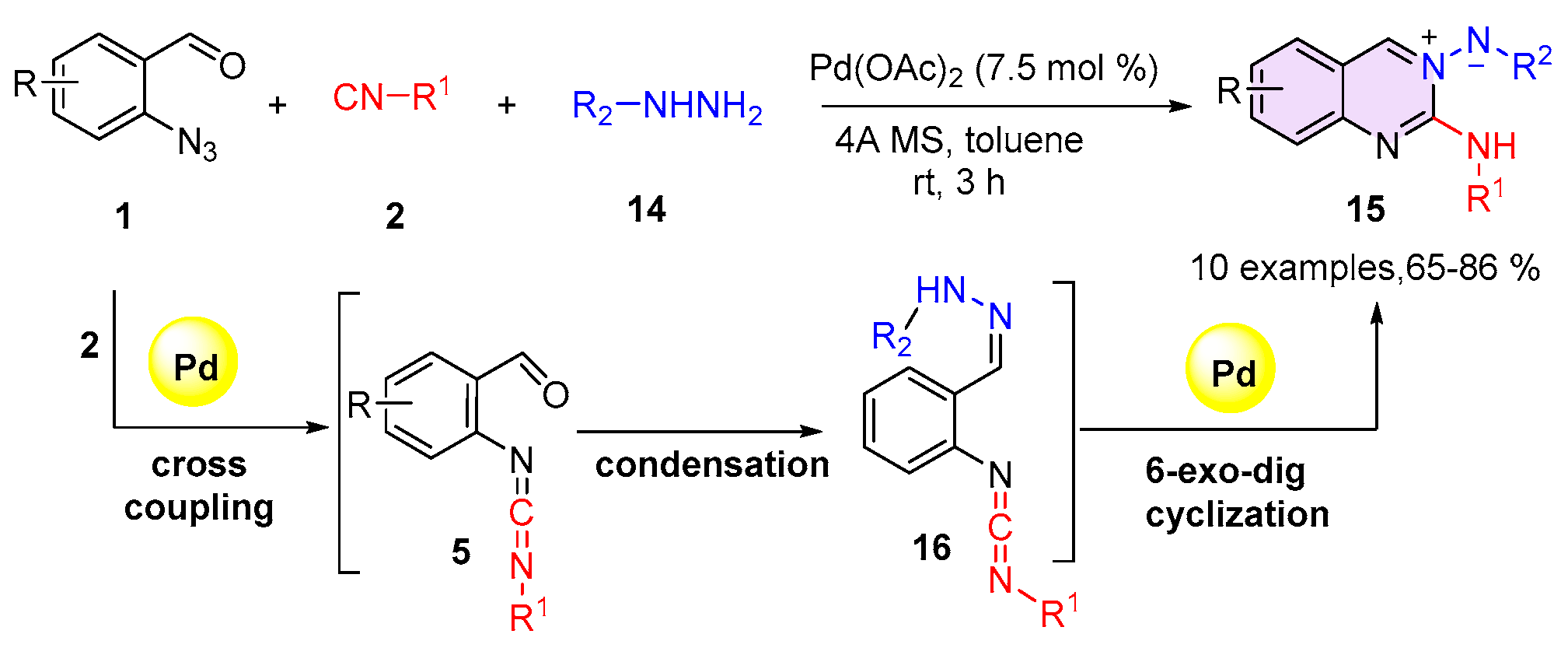
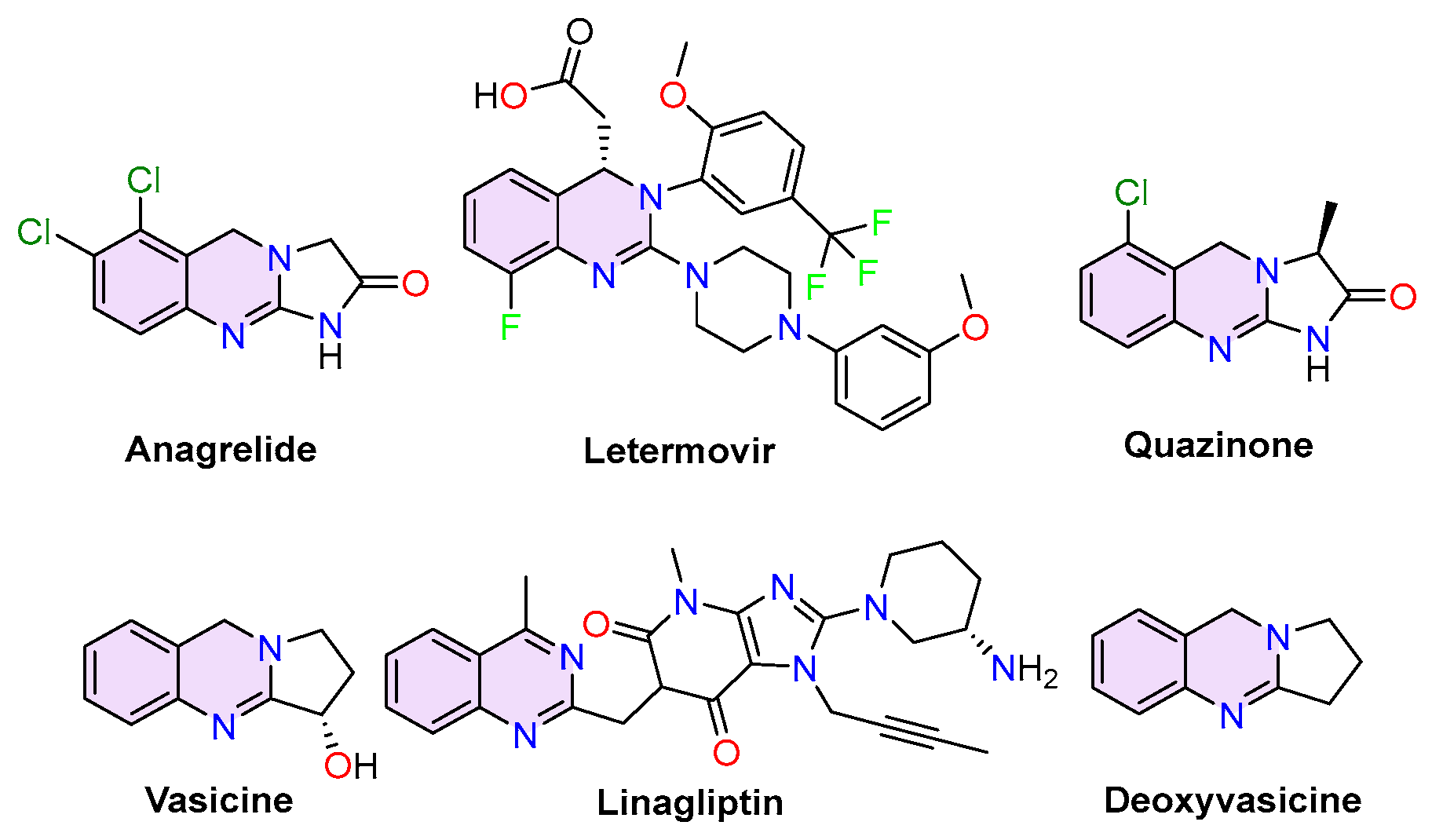
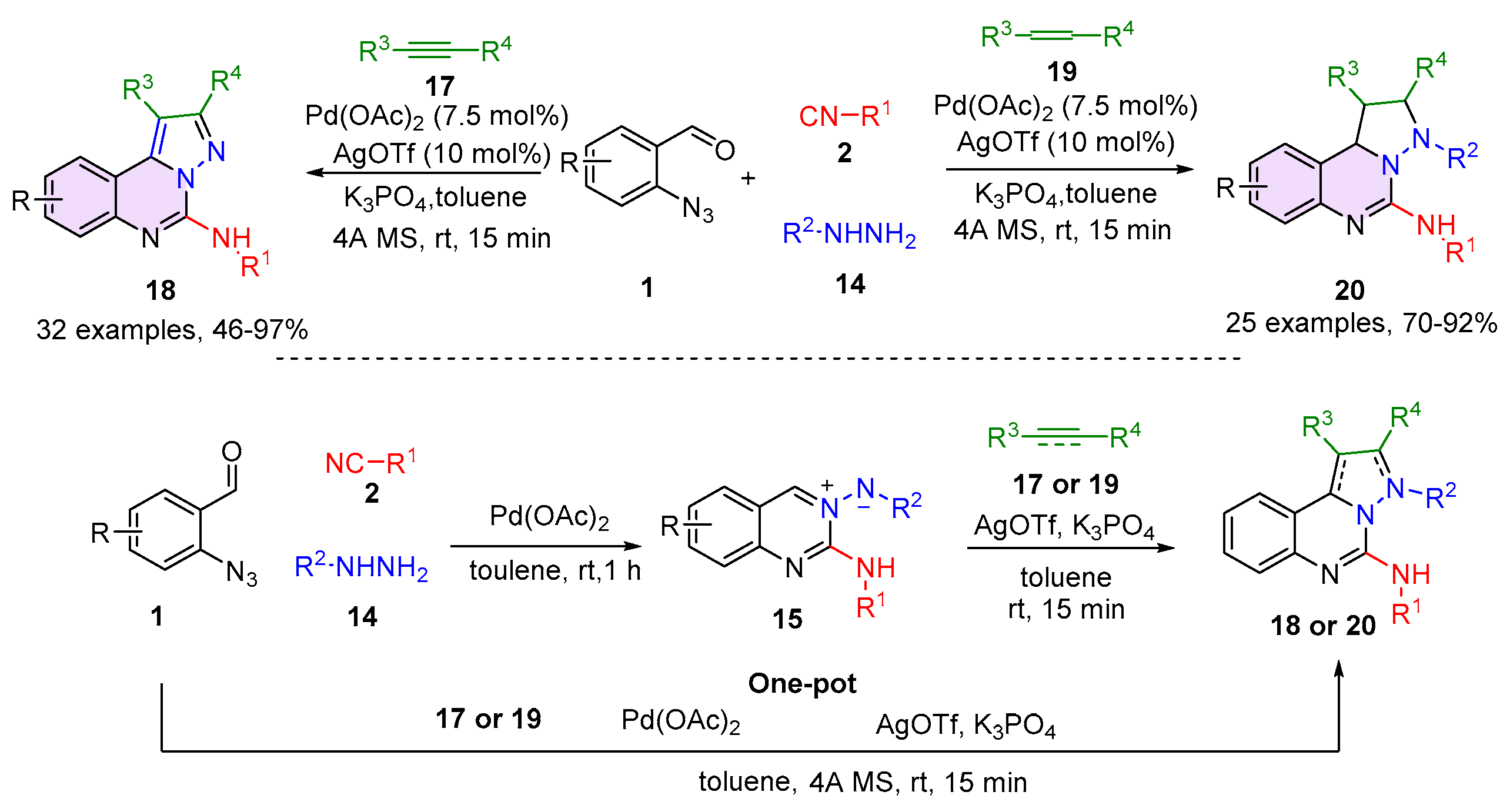
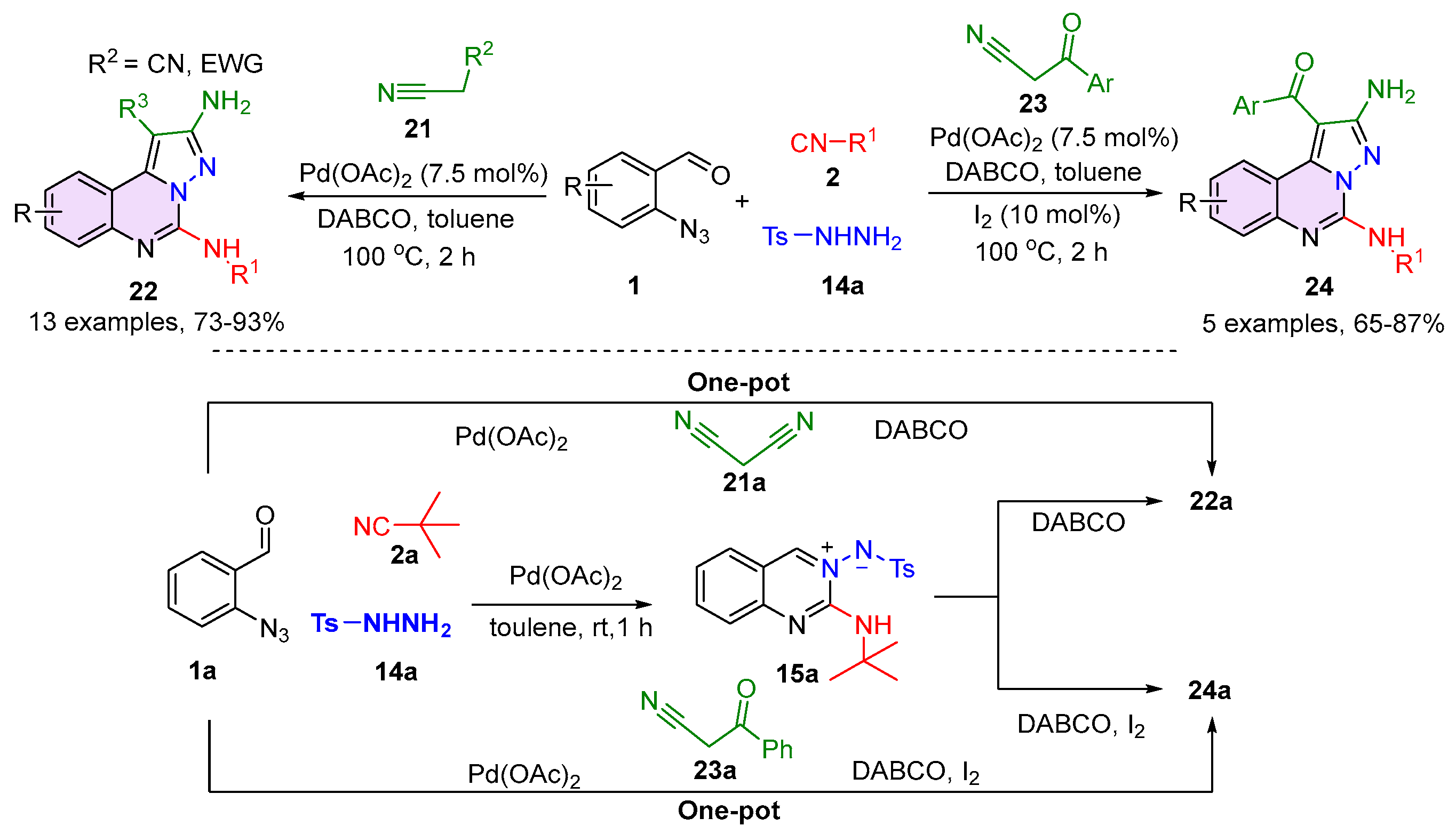
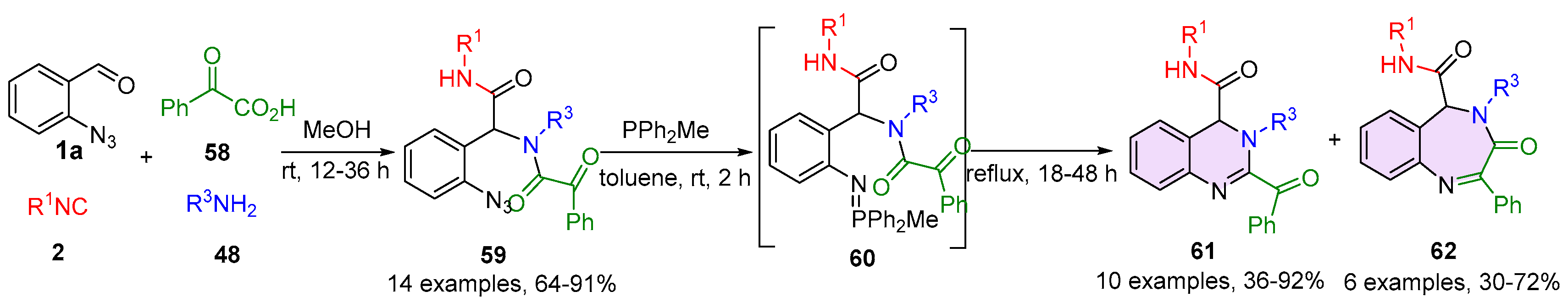
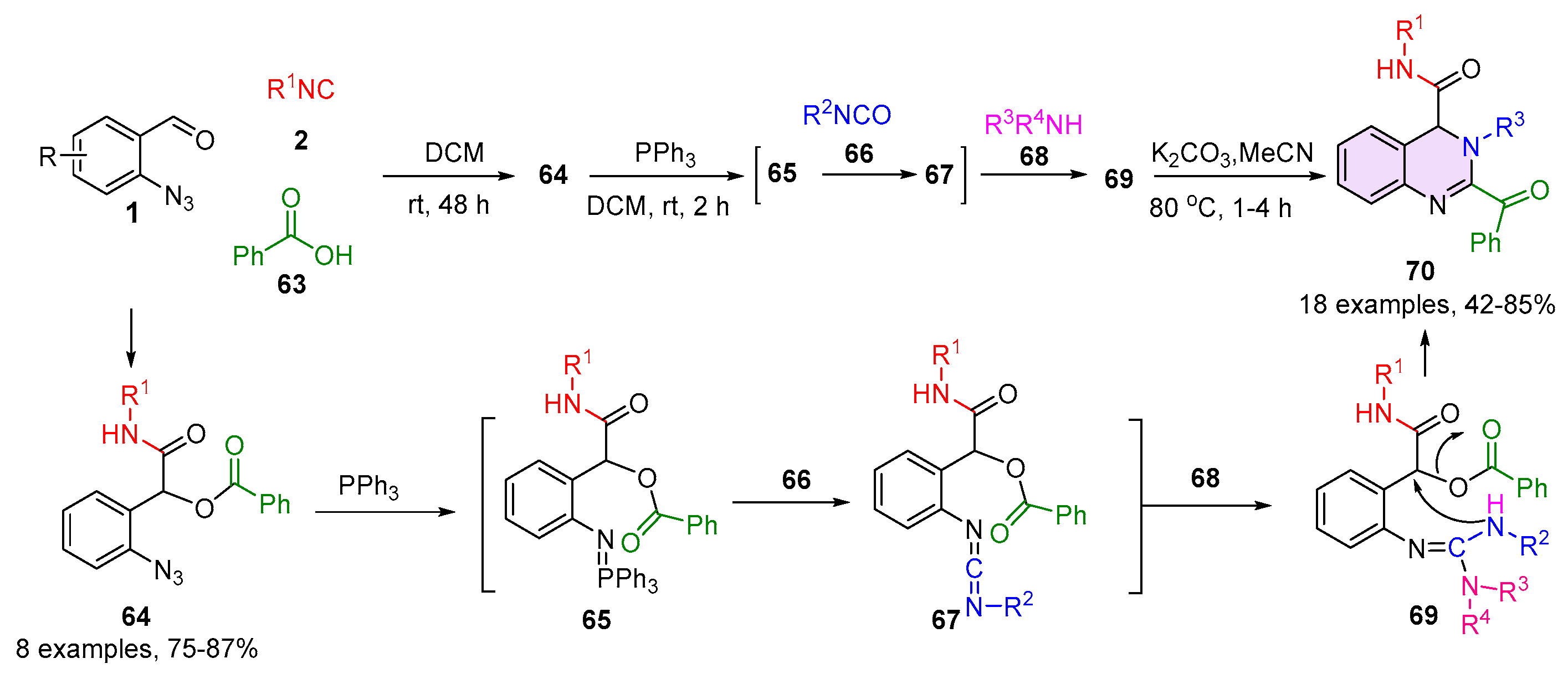
2.1. Quinazolines
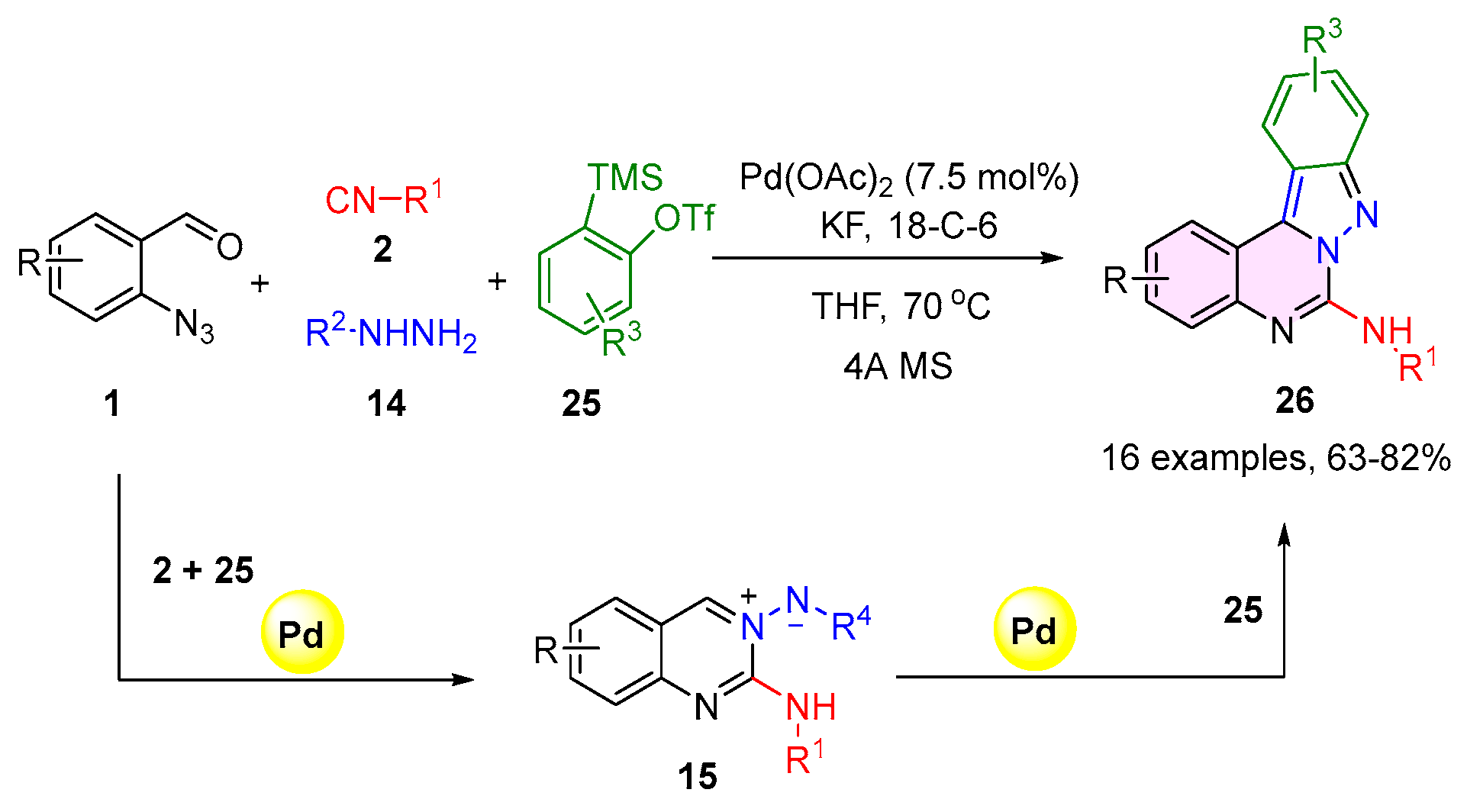
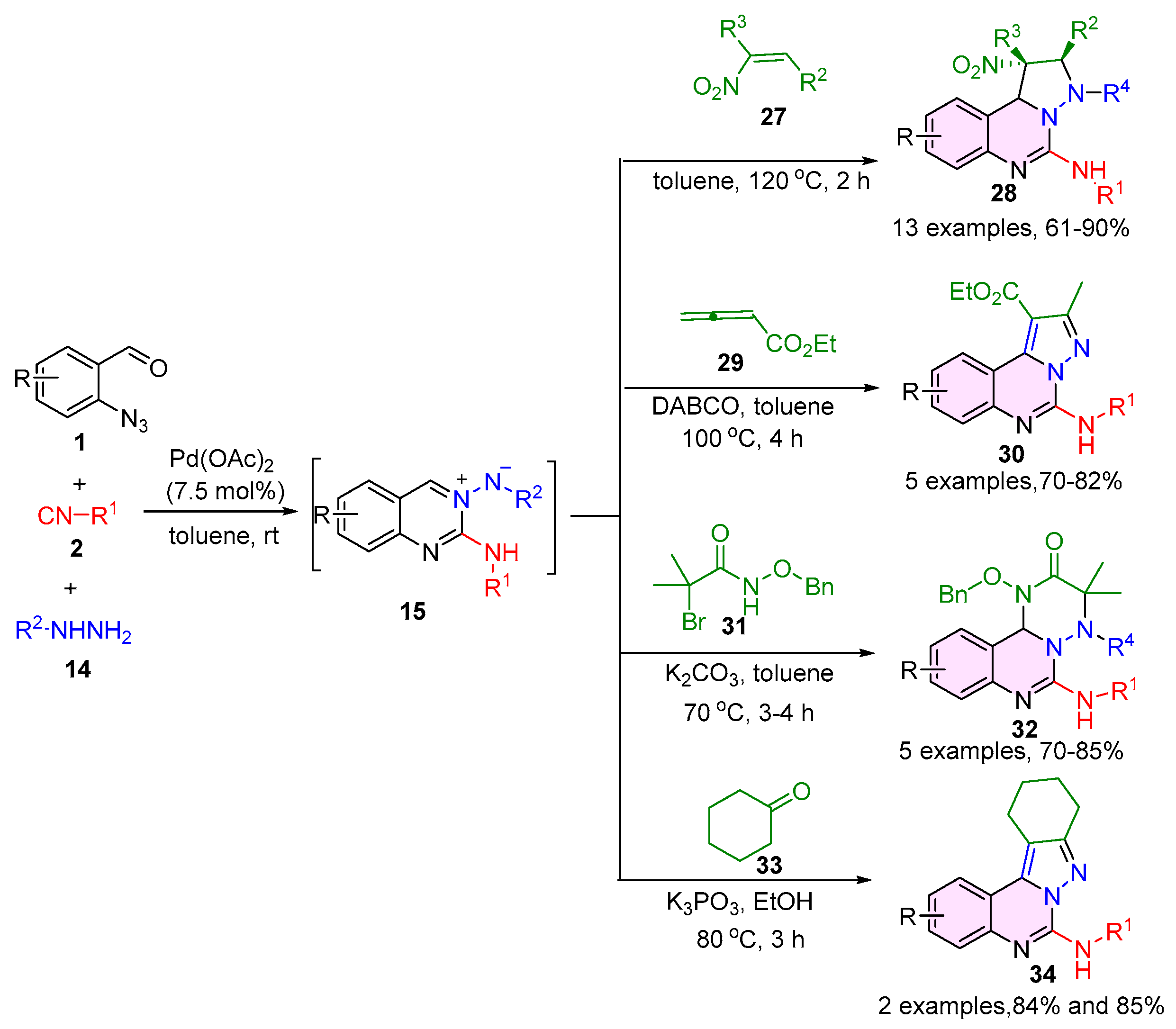
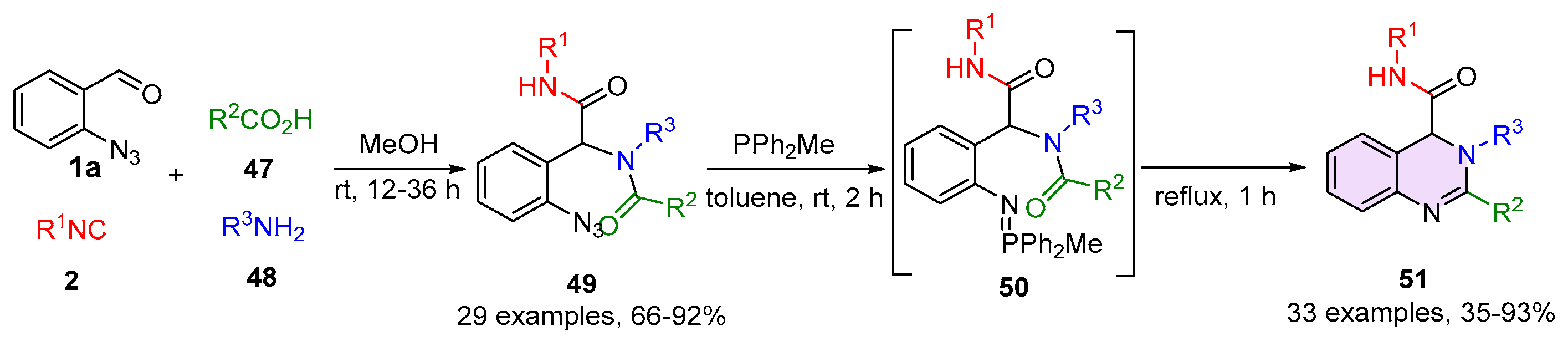
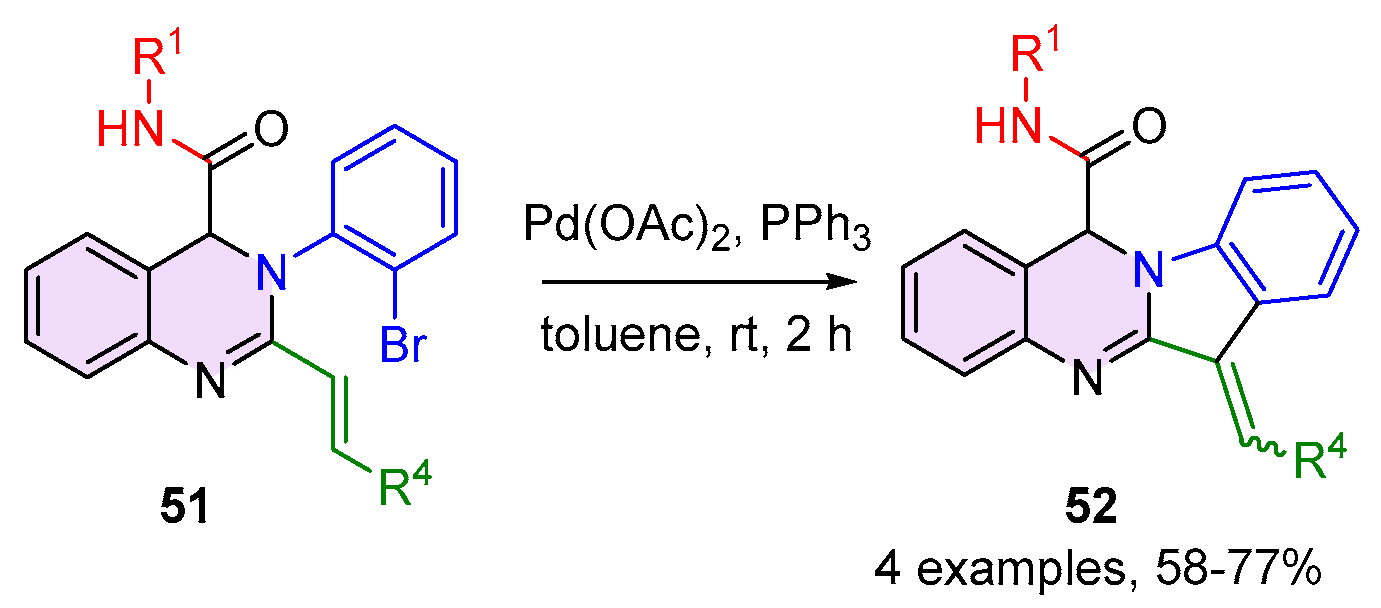
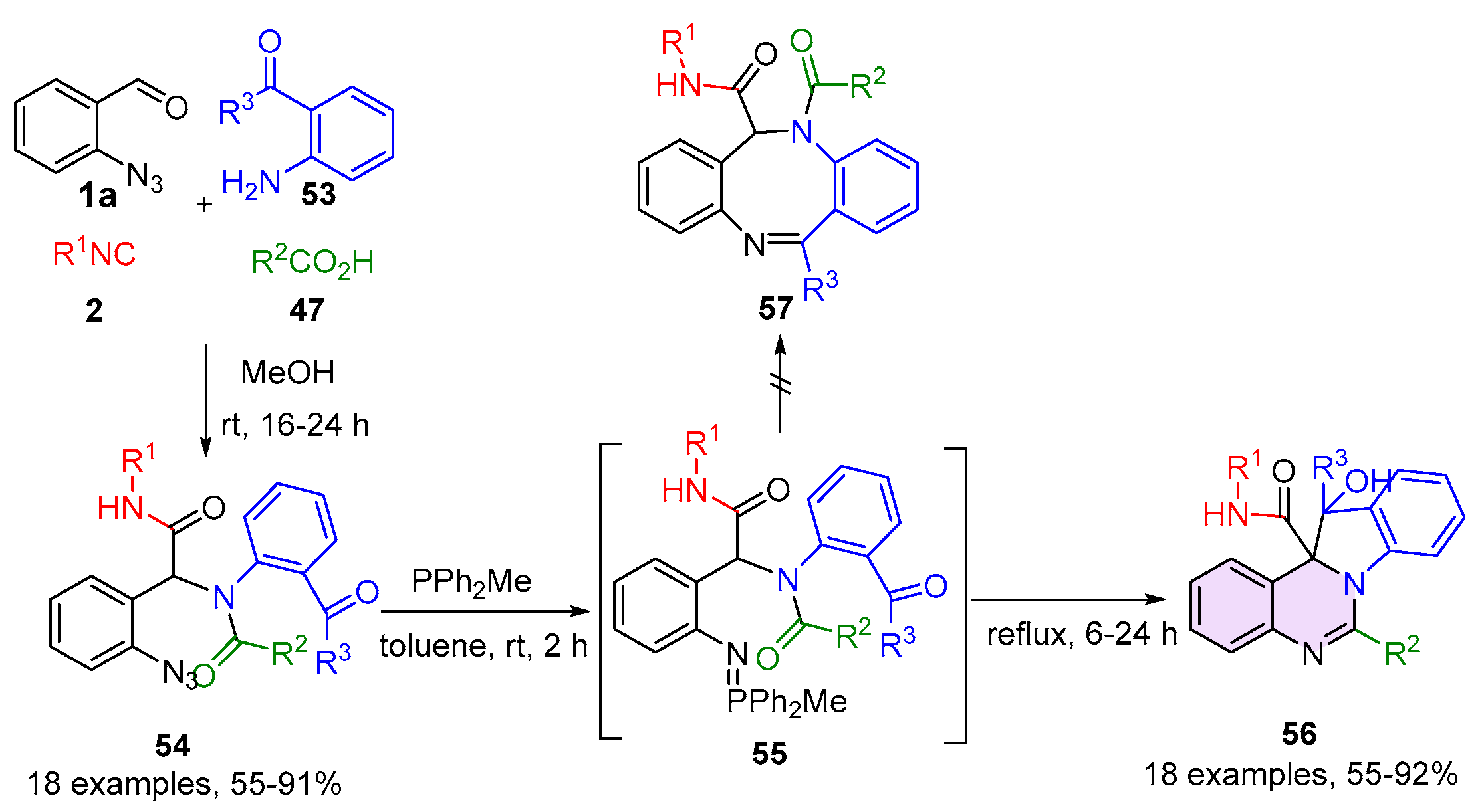
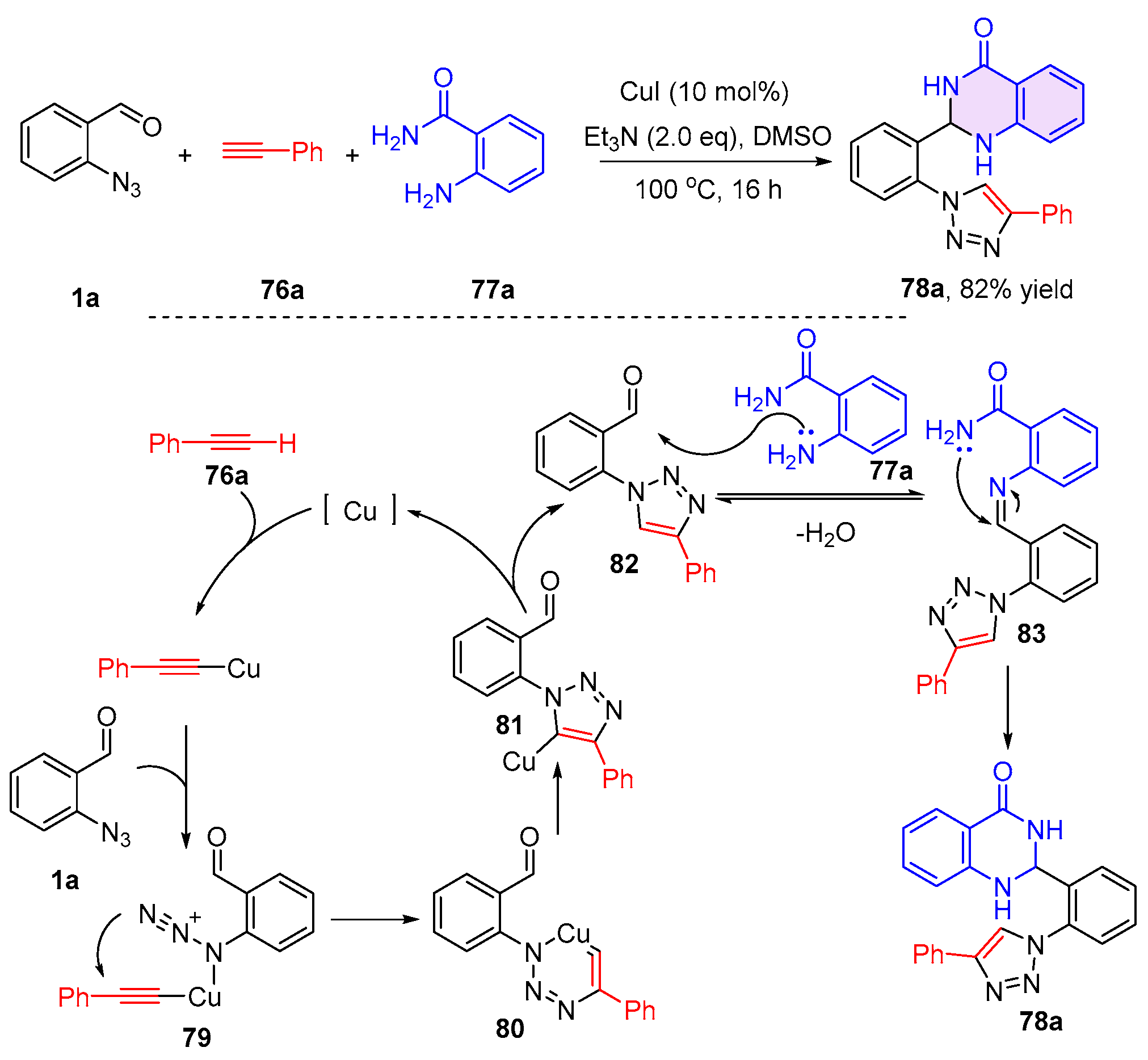
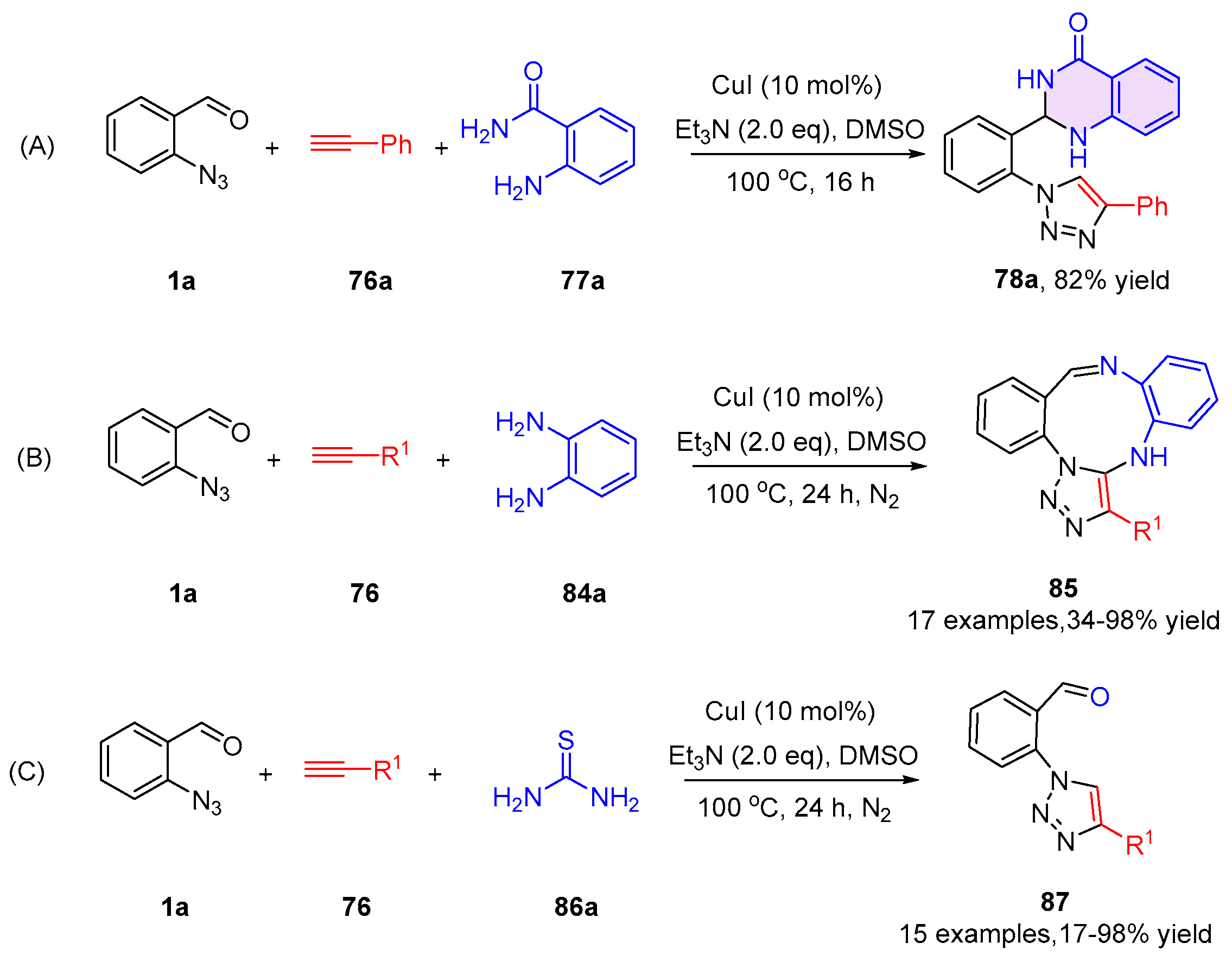
2.2. 3,4-Dihydroquinazolines
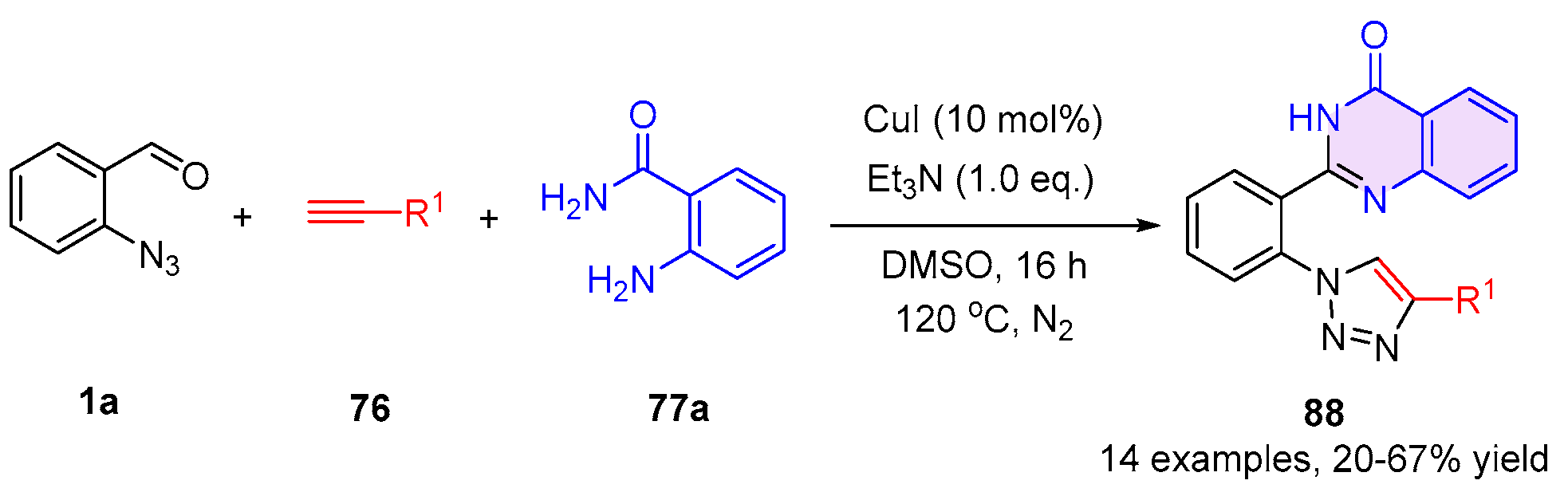
2.3. 2,3-Dihydroquinazolin-4(1H)-One and 4-Quinazolinone
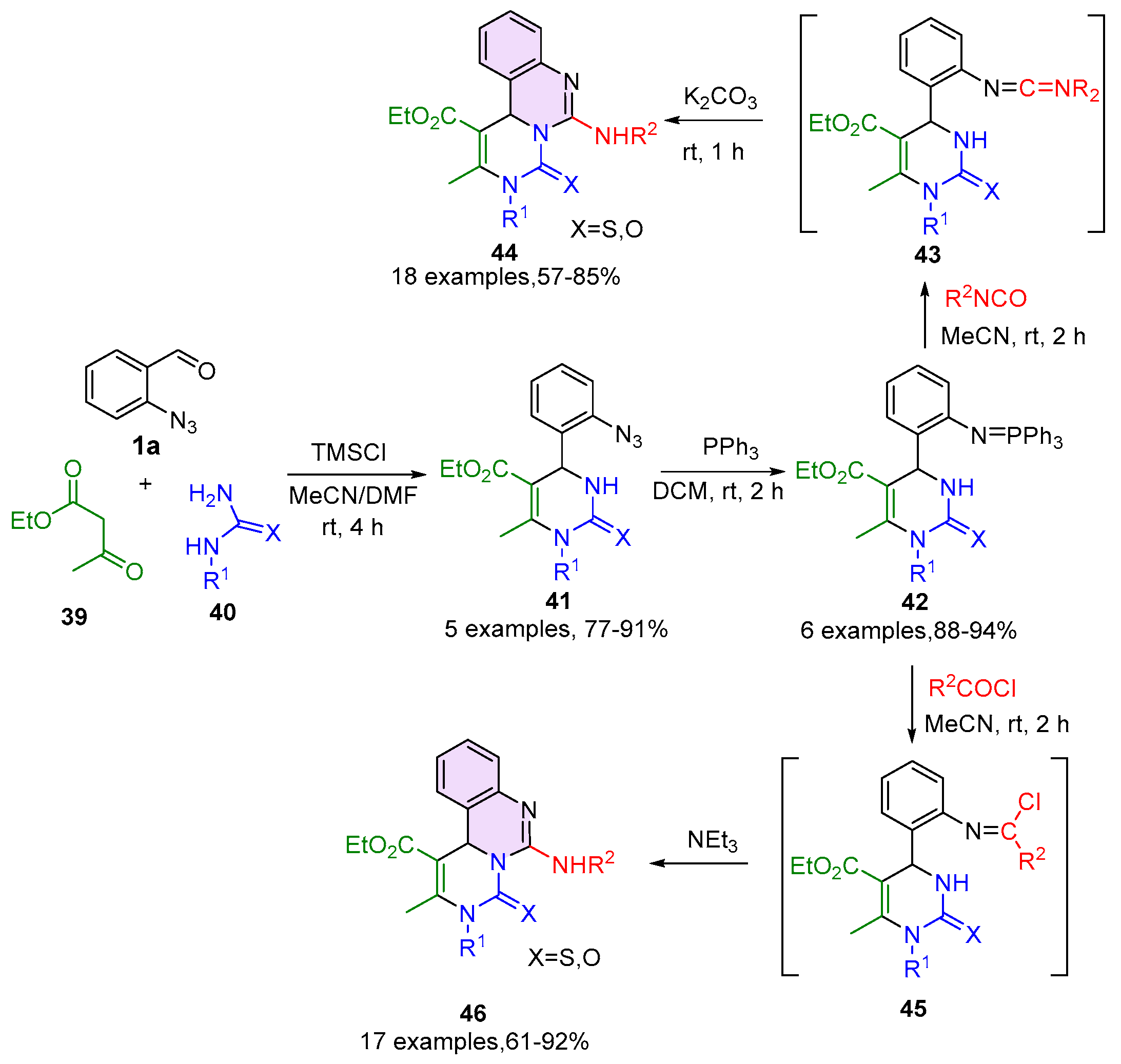
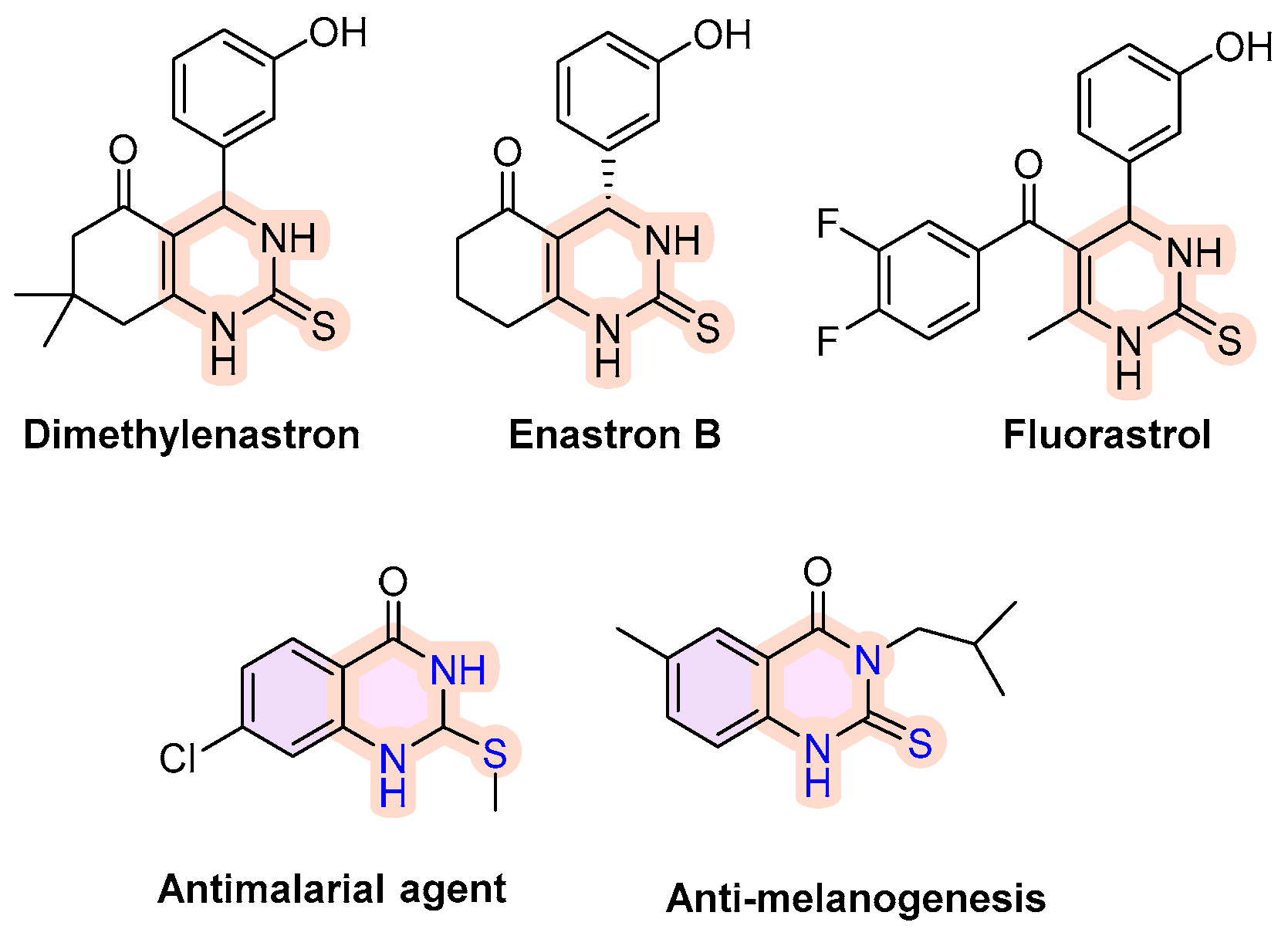
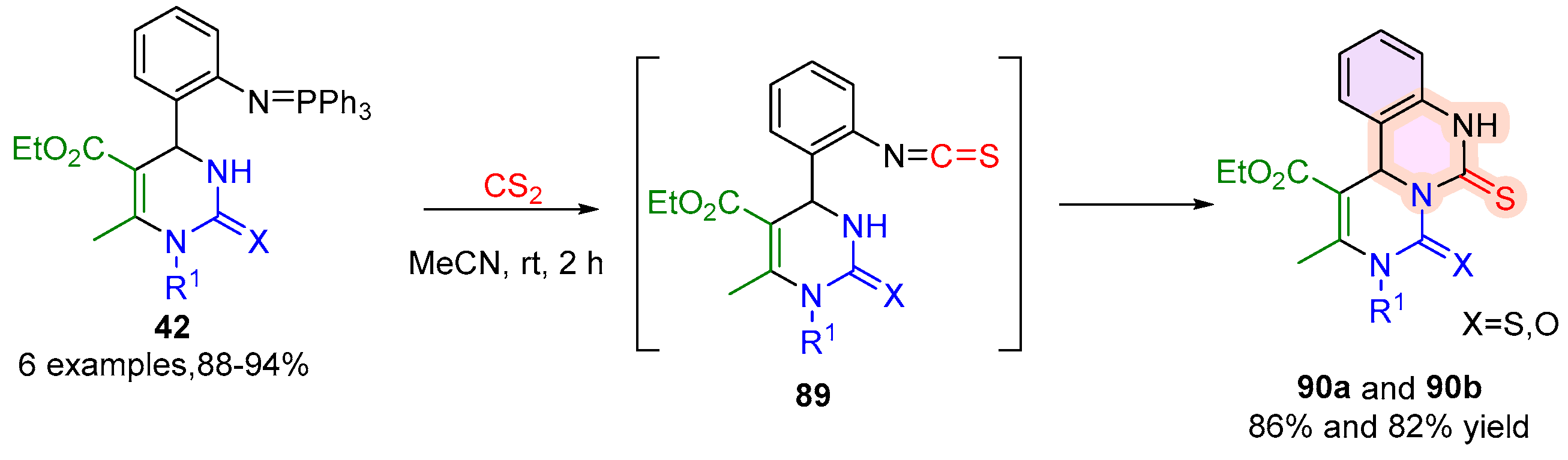
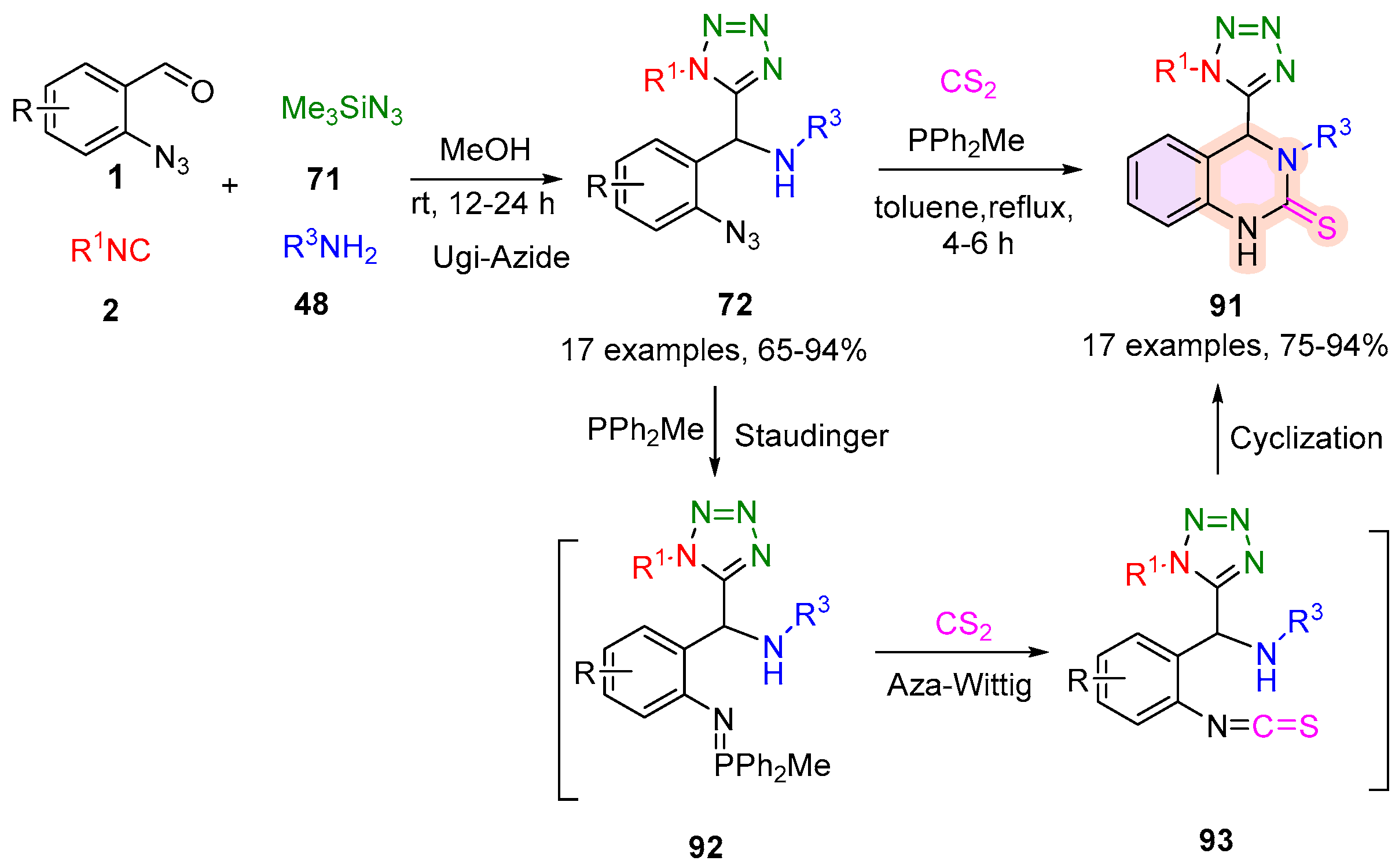
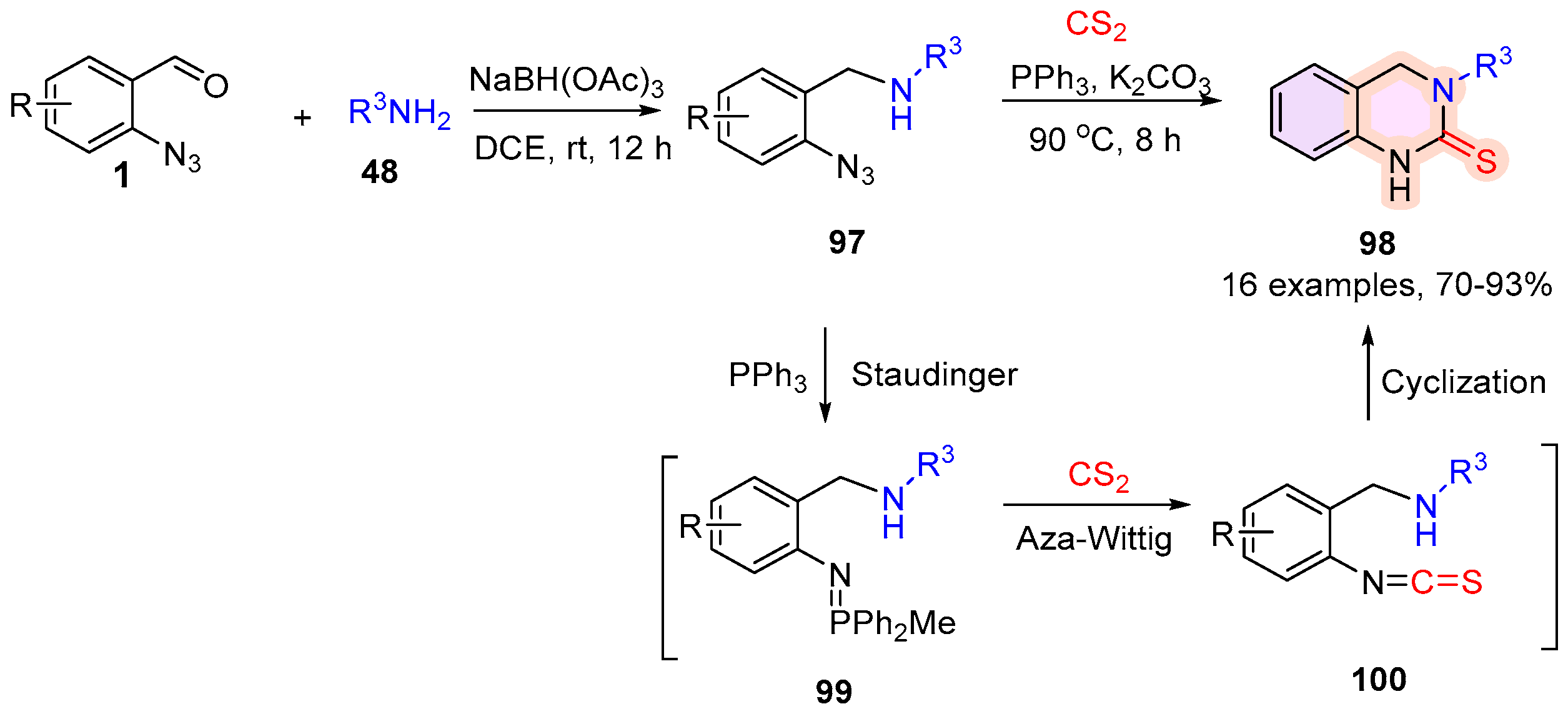
2.4. 3,4-Dihydroquinazoline-2(1H)-Thione
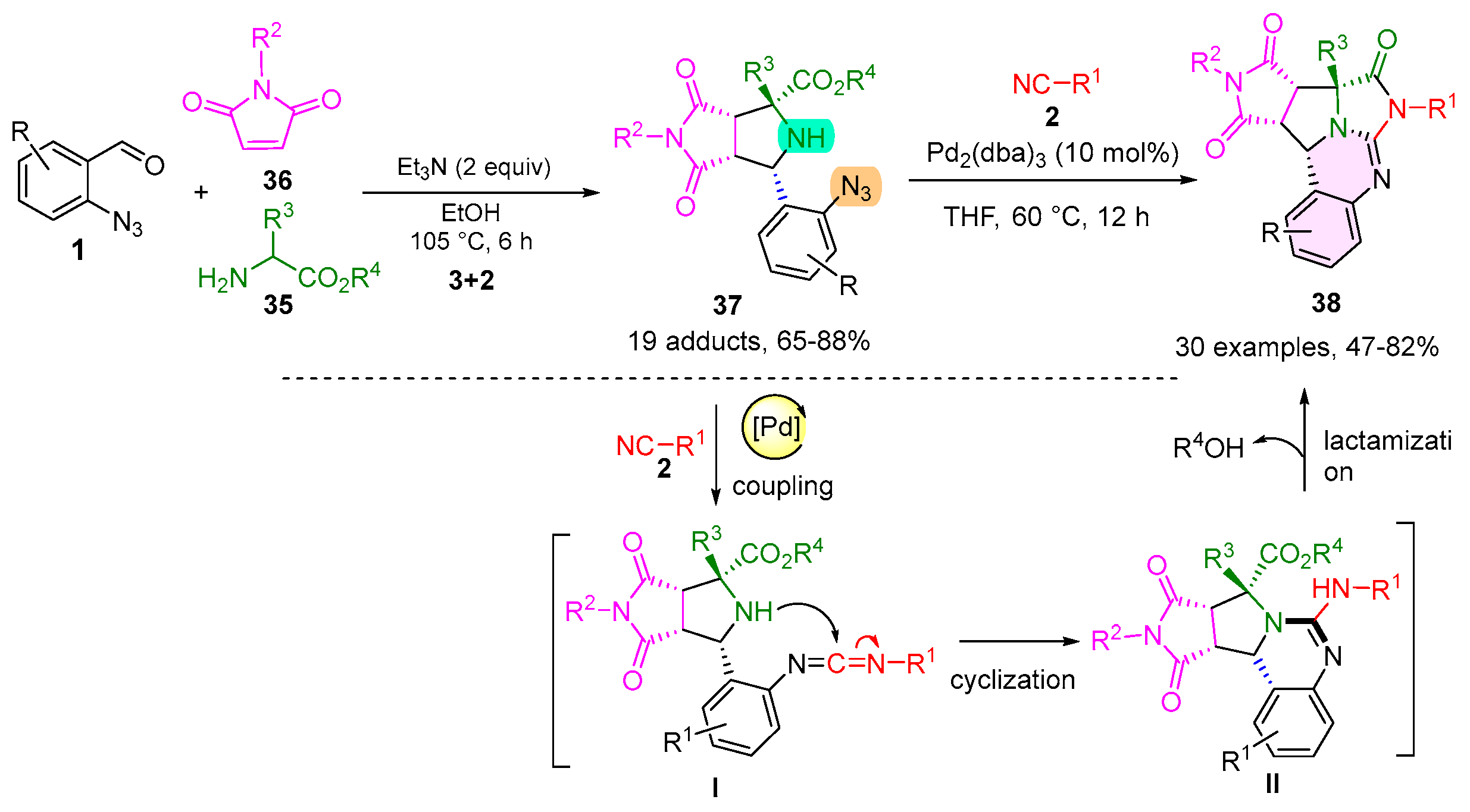
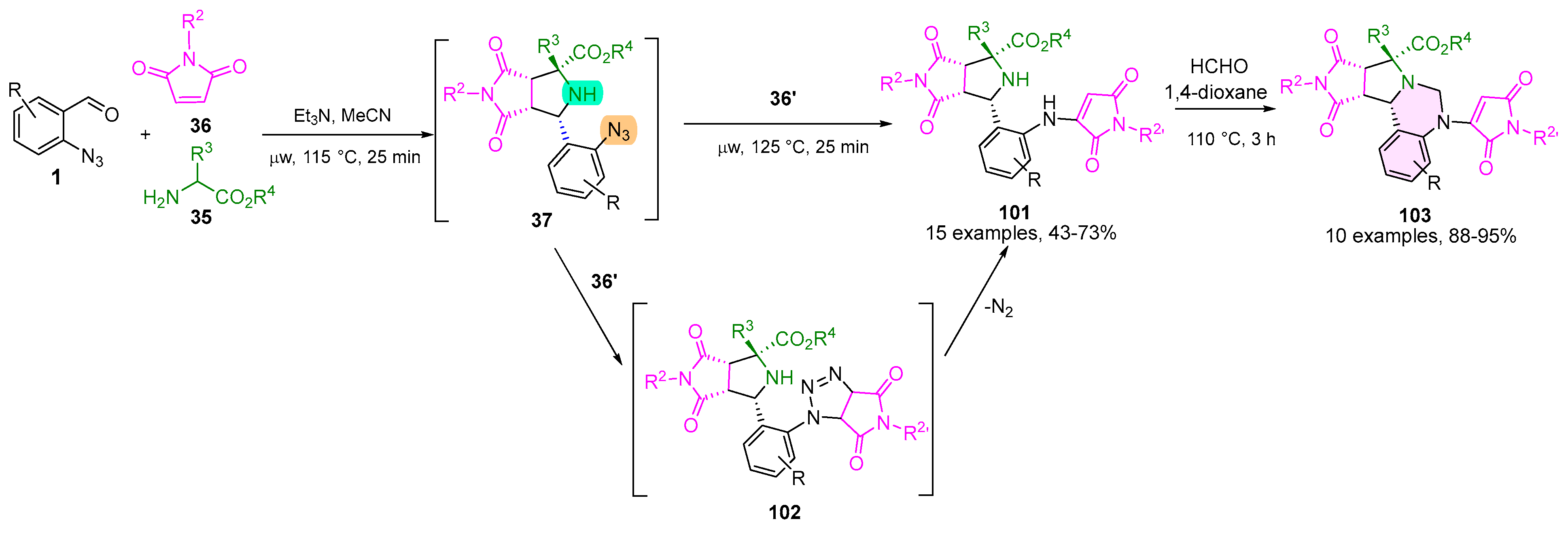
2.5. 1,2,3,4-Tetrahydroquinazoline
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, D.; Gao, F. Quinazoline Derivatives: Synthesis and Bioactivities. Chem. Cent. J. 2013, 7, 95. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.A.-H.; El-Ganzoury, E.M.; Zeid, I.F.; Zayed, E.M.; El-Sayed, W.A. Quinazolines Linked to Sugar Derivatives as Nucleoside Analogs, Synthesis and Biological Aspects. Egypt. J. Chem. 2024, 67, 209–223. [Google Scholar] [CrossRef]
- Ajani, O.O. Undeniable Pharmacological Potentials of Quinazoline Motifs in Therapeutic Medicine. Am. J. Drug Discov. Dev. 2017, 7, 1–24. [Google Scholar] [CrossRef]
- Bala, I.A.; Asiri, A.M.; El-Shishtawy, R.M. Quinazoline Derivatives and Hybrids: Recent Structures with Potent Bioactivity. Med. Chem. Res. 2024, 33, 2372–2419. [Google Scholar] [CrossRef]
- Szumilak, M.; Lichota, A.; Olczak, A.; Szczesio, M.; Stańczak, A. Molecular Insight into Quinazoline Derivatives with Cytotoxic Activity. J. Mol. Struct. 2019, 1194, 28–34. [Google Scholar] [CrossRef]
- Singh, M.; Chandraker, V.; Karthikeyan, C.; Moorthy, N.S.H.N. Anti-Colorectal Cancer Activity of Quinazoline Derivatives: A Comprehensive Review. Lett. Drug Des. Discov. 2024, 21, 1287–1301. [Google Scholar] [CrossRef]
- Marzaro, G.; Guiotto, A.; Chilin, A. Quinazoline Derivatives as Potential Anticancer Agents: A Patent Review (2007–2010). Expert Opin. Ther. Pat. 2012, 22, 223–252. [Google Scholar] [CrossRef] [PubMed]
- Haghighijoo, Z.; Zamani, L.; Moosavi, F.; Emami, S. Therapeutic Potential of Quinazoline Derivatives for Alzheimer’s Disease: A Comprehensive Review. Eur. J. Med. Chem. 2021, 227, 113949. [Google Scholar] [CrossRef]
- Jin, H.; Dan, H.-G.; Rao, G.-W. Research Progress in Quinazoline Derivatives as Multi-Target Tyrosine Kinase Inhibitors. Heterocycl. Commun. 2018, 24, 1–10. [Google Scholar] [CrossRef]
- Medina-Franco, J.L.; López-López, E.; Martínez-Fernández, L.P. 7-Aminoalkoxy-Quinazolines from Epigenetic Focused Libraries Are Potent and Selective Inhibitors of DNA Methyltransferase 1. Molecules 2022, 27, 2892. [Google Scholar] [CrossRef]
- Sundriyal, S.; Malmquist, N.A.; Caron, J.; Blundell, S.; Liu, F.; Chen, X.; Srimongkolpithak, N.; Jin, J.; Charman, S.A.; Scherf, A.; et al. Development of Diaminoquinazoline Histone Lysine Methyltransferase Inhibitors as Potent Blood-Stage Antimalarial Compounds. ChemMedChem 2014, 9, 2360–2373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, P.; Hu, D.; Jin, L.; Yang, S. Research Progress on Quinazoline Derivatives with Epidermal Growth Factor Receptor Inhibiting Bioactivity. Chin. J. Org. Chem. 2012, 32, 444. [Google Scholar] [CrossRef]
- Chilin, A.; Conconi, M.T.; Marzaro, G.; Guiotto, A.; Urbani, L.; Tonus, F.; Parnigotto, P. Exploring Epidermal Growth Factor Receptor (EGFR) Inhibitor Features: The Role of Fused Dioxygenated Rings on the Quinazoline Scaffold. J. Med. Chem. 2010, 53, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi-Baboli, M. In Silico Evaluation, Molecular Docking and QSAR Analysis of Quinazoline-Based EGFR-T790M Inhibitors. Mol. Divers. 2015, 20, 729–739. [Google Scholar] [CrossRef]
- Li, D.-D.; Lv, P.-C.; Zhang, H.; Zhang, H.-J.; Hou, Y.-P.; Liu, K.; Ye, Y.-H.; Zhu, H.-L. The Combination of 4-Anilinoquinazoline and Cinnamic Acid: A Novel Mode of Binding to the Epidermal Growth Factor Receptor Tyrosine Kinase. Bioorg. Med. Chem. 2011, 19, 5012–5022. [Google Scholar] [CrossRef]
- Chen, X.M.; Wei, H.; Yin, L.; Li, X.S. A Convenient Synthesis of Quinazoline Derivatives via Cascade Imino-Diels-Alder and Oxidation Reaction. Chin. Chem. Lett. 2010, 21, 782–786. [Google Scholar] [CrossRef]
- Baitiche, M.; Mahamoud, A.; Benachour, D.; Merbah, M.; Barbe, J. Synthesis of new quinazoline derivatives. Heterocycl. Commun. 2004, 10, 269–272. [Google Scholar] [CrossRef]
- Devi, M.; Kumari, A.; Yadav, A.; Kumar, A.; Dwivedi, J.; Kaur, N. Synthesis of Quinazoline Derivatives. Curr. Org. Chem. 2025, 29, 1107–1127. [Google Scholar] [CrossRef]
- Mahdavi, M.; Lotfi, V.; Saeedi, M.; Kianmehr, E.; Shafiee, A. Synthesis of Novel Fused Quinazolinone Derivatives. Mol. Divers. 2016, 20, 677–685. [Google Scholar] [CrossRef]
- Nandwana, N.K.; Patel, O.P.S.; Mehra, M.K.; Kumar, A.; Salvino, J.M. Recent Advances in Metal-Catalyzed Approaches for the Synthesis of Quinazoline Derivatives. Molecules 2024, 29, 2353. [Google Scholar] [CrossRef] [PubMed]
- Besson, T.; Chosson, E. Microwave-Assisted Synthesis of Bioactive Quinazolines and Quinazolinones. Comb. Chem. High Throughput Screen. 2007, 10, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Gondi, S.R.; Bera, A.K.; Westover, K.D. Green Synthesis of Substituted Anilines and Quinazolines from Isatoic Anhydride-8-Amide. Sci. Rep. 2019, 9, 14258. [Google Scholar] [CrossRef]
- Kushwaha, P.; Bhardwaj, A. Rashi One Pot Synthesis and Mechanistic Insights of Quinazoline and Related Molecules. Tetrahedron 2025, 179, 134635. [Google Scholar] [CrossRef]
- Ghotekar, B.K.; Jachak, M.N.; Toche, R.B. New One-step Synthesis of Pyrazolo [1,5-a]Pyrimidine and Pyrazolo [1,5-a]Quinazoline Derivatives via Multicomponent Reactions. J. Heterocycl. Chem. 2009, 46, 708–713. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Foroughifar, N.; Mosleh, T.; Hamta, A. Synthesis of New Pyrimidine, Quinazoline and Diazatricyclo Derivatives by Multicomponent Reaction and Evaluation of Their Biological Activity. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 728–734. [Google Scholar] [CrossRef]
- Nandi, S.; Jamatia, R.; Sarkar, R.; Sarkar, F.K.; Alam, S.; Pal, A.K. One-Pot Multicomponent Reaction: A Highly Versatile Strategy for the Construction of Valuable Nitrogen-Containing Heterocycles. ChemistrySelect 2022, 7, e202201901. [Google Scholar] [CrossRef]
- Xiang, J.; Wang, M.; Sun, Y.; Wu, A. Multicomponent Reactions. In Synthesis of Bioactive Heterocycles; CRC Press: Boca Raton, FL, USA, 2017; pp. 201–228. [Google Scholar] [CrossRef]
- Park, B.; Nam, J.H.; Kim, J.H.; Kim, H.J.; Onnis, V.; Balboni, G.; Lee, K.-T.; Park, J.H.; Catto, M.; Carotti, A.; et al. 3,4-Dihydroquinazoline Derivatives Inhibit the Activities of Cholinesterase Enzymes. Bioorg. Med. Chem. Lett. 2017, 27, 1179–1185. [Google Scholar] [CrossRef]
- Kang, H.B.; Rim, H.-K.; Park, J.Y.; Choi, H.W.; Choi, D.L.; Seo, J.-H.; Chung, K.-S.; Huh, G.; Kim, J.; Choo, D.J.; et al. In Vivo Evaluation of Oral Anti-Tumoral Effect of 3,4-Dihydroquinazoline Derivative on Solid Tumor. Bioorg. Med. Chem. Lett. 2011, 22, 1198–1201. [Google Scholar] [CrossRef]
- Singh, M.; Raghav, N. 2,3-Dihydroquinazolin-4(1H)-One Derivatives as Potential Non-Peptidyl Inhibitors of Cathepsins B and H. Bioorg. Chem. 2014, 59, 12–22. [Google Scholar] [CrossRef]
- O’Brien, N.S.; Gilbert, J.; McCluskey, A.; Sakoff, J.A. 2,3-Dihydroquinazolin-4(1H)-Ones and Quinazolin-4(3H)-Ones as Broad-Spectrum Cytotoxic Agents and Their Impact on Tubulin Polymerisation. RSC Med. Chem. 2024, 15, 1686–1708. [Google Scholar] [CrossRef] [PubMed]
- Dahabiyeh, L.A.; Hourani, W.; Darwish, W.; Hudaib, F.; Abu-Irmaileh, B.; Deb, P.K.; Venugopala, K.N.; Mohanlall, V.; Abu-Dahab, R.; Semreen, M.H.; et al. Molecular and Metabolic Alterations of 2,3-Dihydroquinazolin-4(1H)-One Derivatives in Prostate Cancer Cell Lines. Sci. Rep. 2022, 12, 21599. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Jiang, L.; Wan, S.; Zhang, L.; Yu, R.; Jiang, T. 2-Pyridinyl-4(3H)-Quinazolinone: A Scaffold for Anti-influenza A Virus Compounds. Chem. Biol. Drug Des. 2015, 86, 1221–1225. [Google Scholar] [CrossRef]
- Gatadi, S.; Lakshmi, T.V.; Nanduri, S. 4(3H)-Quinazolinone Derivatives: Promising Antibacterial Drug Leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef]
- Chen, K.; Wang, S.; Fu, S.; Kim, J.; Park, P.; Liu, R.; Lei, K. 4(3H)-Quinazolinone: A Natural Scaffold for Drug and Agrochemical Discovery. Int. J. Mol. Sci. 2025, 26, 2473. [Google Scholar] [CrossRef]
- Chen, X.-W.; Rao, L.; Chen, J.-L.; Zou, Y. Unexpected Assembly Machinery for 4(3H)-Quinazolinone Scaffold Synthesis. Nat. Commun. 2022, 13, 6522. [Google Scholar] [CrossRef]
- Thanigaimalai, P.; Sharma, V.K.; Lee, K.-C.; Yun, C.-Y.; Kim, Y.; Jung, S.-H. Refinement of the Pharmacophore of 3,4-Dihydroquinazoline-2(1H)-Thiones for Their Anti-Melanogenesis Activity. Bioorg. Med. Chem. Lett. 2010, 20, 4771–4773. [Google Scholar] [CrossRef]
- Thanigaimalai, P.; Lee, K.-C.; Bang, S.-C.; Lee, J.-H.; Yun, C.-Y.; Roh, E.; Hwang, B.-Y.; Kim, Y.; Jung, S.-H. Evaluation of 3,4-Dihydroquinazoline-2(1H)-Thiones as Inhibitors of Alpha-MSH-Induced Melanin Production in Melanoma B16 Cells. Bioorg. Med. Chem. 2009, 18, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Correa, W.H.; Papadopoulos, S.; Radnidge, P.; Roberts, B.A.; Scott, J.L. Direct, Efficient, Solvent-Free Synthesis of 2-Aryl-1,2,3,4-Tetrahydroquinazolines. Green Chem. 2002, 4, 245–251. [Google Scholar] [CrossRef]
- Marchenko, A.; Koidan, G.; Hurieva, A.N.; Shishkina, S.; Rusanov, E.; Kostyuk, A. Ring Enlargement of N-Phosphanyl-1,2,3,4-Tetrahydroquinazolines. J. Org. Chem. 2020, 85, 14467–14472. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Qiu, W.; Zhang, W. 2-Azidobenzaldehyde-Based [4+2] Annulation for the Synthesis of Quinoline Derivatives. Molecules 2024, 29, 1241. [Google Scholar] [CrossRef] [PubMed]
- Alagarsamy, V.; Chitra, K.; Saravanan, G.; Solomon, V.R.; Sulthana, M.T.; Narendhar, B. An Overview of Quinazolines: Pharmacological Significance and Recent Developments. Eur. J. Med. Chem. 2018, 151, 628–685. [Google Scholar] [CrossRef] [PubMed]
- Pathare, R.S.; Maurya, A.K.; Kumari, A.; Agnihotri, V.K.; Verma, V.P.; Sawant, D.M. Synthesis of Quinazoline-3-Oxides via a Pd(Ii) Catalyzed Azide–Isocyanide Coupling/Cyclocondensation Reaction. Org. Biomol. Chem. 2018, 17, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Renaut*, P.P.; Durand, P.; Ratel, P. 3,9-Dihydro-2H-[1,2,4]-Oxadiazolo [3,2-b]Quinazolin-2-Ones: First Synthesis of the Parent Heterocycle, 7- and 9-Substituted Derivatives. Synthesis 2000, 2000, 2009–2012. [Google Scholar] [CrossRef]
- Heaney, F.; Lawless, E. 2-Vinyl Quinazoline 3-oxides; Preparation from Acid Induced Cyclocondensation of 2-acylaminoaryloximes. J. Heterocycl. Chem. 2007, 44, 569–574. [Google Scholar] [CrossRef]
- Madabhushi, S.; Mallu, K.K.R.; Jillella, R.; Kurva, S.; Singh, R. One-Step Method for Synthesis of 2,4-Disubstituted Quinazoline 3-Oxides by Reaction of a 2-Aminoaryl Ketone with a Hydroxamic Acid Using Zn(OTf)2 as the Catalyst. Tetrahedron Lett. 2014, 55, 1979–1982. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Hu, Z.; Dong, J.; Zhang, X.; Xu, X. Silver-Catalyzed Isocyanide Insertion into N−H Bond of Ammonia: [5+1] Annulation to Quinazoline Derivatives. Adv. Synth. Catal. 2018, 360, 1938–1942. [Google Scholar] [CrossRef]
- Qiu, G.; Ding, Q.; Wu, J. Recent Advances in Isocyanide Insertion Chemistry. Chem. Soc. Rev. 2013, 42, 5257–5269. [Google Scholar] [CrossRef]
- Zhao, L.-M.; Wang, Y.-J. Quinazoline-Derived Azomethine Imines as Substrates To Access Polycyclic Compounds. J. Org. Chem. 2024, 89, 15393–15403. [Google Scholar] [CrossRef]
- Zhou, L.; Zeng, Y.; Gao, X.; Wang, Q.; Wang, C.; Wang, B.; Wang, W.; Wu, Y.; Zheng, B.; Guo, H. A Chiral Squaramide-Catalyzed Asymmetric Dearomative Tandem Annulation Reaction through a Kinetic Resolution of MBH Alcohols: Highly Enantioselective Synthesis of Three-Dimensional Heterocyclic Compounds. Chem. Commun. 2019, 55, 10464–10467. [Google Scholar] [CrossRef]
- Zhou, L.; Yuan, C.; Zeng, Y.; Wang, Q.; Wang, C.; Liu, M.; Wang, W.; Wu, Y.; Zheng, B.; Guo, H. Direct Activation of Unmodified Morita–Baylis–Hillman Alcohols through Phosphine Catalysis for Rapid Construction of Three-Dimensional Heterocyclic Compounds. Org. Lett. 2019, 21, 4882–4886. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, R.; Dong, Y.; Yu, S.; Wu, Y.; Xiao, Y.; Guo, H. 4-Vinylbenzodioxinones as a New Type of Precursor for Palladium-Catalyzed (4+3) Cycloaddition of Azomethine Imines. Chem. Commun. 2024, 60, 1436–1439. [Google Scholar] [CrossRef]
- Ansari, A.J.; Pathare, R.S.; Kumawat, A.; Maurya, A.K.; Verma, S.; Agnihotri, V.K.; Joshi, R.; Metre, R.K.; Sharon, A.; Pardasani, R.T.; et al. A Diversity-Oriented Synthesis of Polyheterocycles via the Cyclocondensation of Azomethine Imine. N. J. Chem. 2019, 43, 13721–13724. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, B.H.; Park, S.J.; Kang, S.B.; Rhim, H.; Park, J.-Y.; Lee, J.-H.; Jeong, S.-W.; Lee, J.Y. 3,4-Dihydroquinazoline Derivatives as Novel Selective T-Type Ca2+ Channel Blockers. Bioorg. Med. Chem. Lett. 2004, 14, 3379–3384. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Liao, W.-J.; Lin, X.-Y.; Liang, C.-F. A Facile Access to N-Sulfonylthioimidates and Their Use for the Transformation to 3,4-Dihydroquinazolines. Org. Biomol. Chem. 2020, 18, 8881–8885. [Google Scholar] [CrossRef]
- Chen, T.; Huang, T.; Ye, M.; Shen, J. Acid-Catalyzed, Metal- and Oxidant-Free C=C Bond Cleavage of Enaminones: One-Pot Synthesis of 3,4-Dihydroquinazolines. Molecules 2025, 30, 350. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, J.; Zhang, X.-Y.; Jia, G.-K.; Cao, Z.; Tang, Z.; Yu, X.; Xu, X.; He, W.-M. Palladium-Catalyzed Selective Synthesis of 3,4-Dihydroquinazolines from Electron-Rich Arylamines, Electron-Poor Arylamines and Glyoxalates. Org. Biomol. Chem. 2018, 16, 5050–5054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Barker, J.; Lou, B.; Saneii, H. Solid-Phase Synthesis of 3,4-Dihydroquinazoline Derivatives. Tetrahedron Lett. 2001, 42, 8405–8408. [Google Scholar] [CrossRef]
- Magyar, C.L.; Wall, T.J.; Davies, S.B.; Campbell, M.V.; Barna, H.A.; Smith, S.R.; Savich, C.J.; Mosey, R.A. Triflic Anhydride Mediated Synthesis of 3,4-Dihydroquinazolines: A Three-Component One-Pot Tandem Procedure. Org. Biomol. Chem. 2019, 17, 7995–8000. [Google Scholar] [CrossRef]
- Díaz, J.E.; Ranieri, S.; Gruber, N.; Orelli, L.R. Syntheses of 3,4- and 1,4-Dihydroquinazolines from 2-Aminobenzylamine. Beilstein J. Org. Chem. 2017, 13, 1470–1477. [Google Scholar] [CrossRef]
- Xiong, J.; Wei, X.; Wan, Y.-C.; Ding, M.-W. One-Pot and Regioselective Synthesis of Polysubstituted 3,4-Dihydroquinazolines and 4,5-Dihydro-3H-1,4-Benzodiazepin-3-Ones by Sequential Ugi/Staudinger/Aza-Wittig Reaction. Tetrahedron 2019, 75, 1072–1078. [Google Scholar] [CrossRef]
- Xiong, J.; Wei, X.; Yan, Y.-M.; Ding, M.-W. One-Pot and Regioselective Synthesis of 3,4-Dihydroquinazolines by Sequential Ugi/Staudinger/Aza-Wittig Reaction Starting from Functionalized Isocyanides. Tetrahedron 2017, 73, 5720–5724. [Google Scholar] [CrossRef]
- Xiong, J.; He, H.-T.; Yang, H.-Y.; Zeng, Z.-G.; Zhong, C.-R.; Shi, H.; Ouyang, M.-L.; Tao, Y.-Y.; Pang, Y.-L.; Zhang, Y.-H.; et al. Synthesis of 4-Tetrazolyl-Substituted 3,4-Dihydroquinazoline Derivatives with Anticancer Activity via a One-Pot Sequential Ugi-Azide/Palladium-Catalyzed Azide-Isocyanide Cross-Coupling/Cyclization Reaction. J. Org. Chem. 2022, 87, 9488–9496. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, L.; Ding, M.-W. New Efficient Synthesis of 2,3,4-Trisubstituted 3,4-Dihydroquinazolines by a Ugi 4CC/Staudinger/Aza-Wittig Sequence. Tetrahedron 2011, 67, 3714–3723. [Google Scholar] [CrossRef]
- Collins, I.; Jones, A.M. Diversity-Oriented Synthetic Strategies Applied to Cancer Chemical Biology and Drug Discovery. Molecules 2014, 19, 17221–17255. [Google Scholar] [CrossRef]
- Schreiber, S.L. Target-Oriented and Diversity-Oriented Organic Synthesis in Drug Discovery. Science 2000, 287, 1964–1969. [Google Scholar] [CrossRef]
- Sawant, D.M.; Sharma, S.; Pathare, R.S.; Joshi, G.; Kalra, S.; Sukanya, S.; Maurya, A.K.; Metre, R.K.; Agnihotri, V.K.; Khan, S.; et al. Relay Tricyclic Pd(II)/Ag(I) Catalysis: Design of a Four-Component Reaction Driven by Nitrene-Transfer on Isocyanide Yields Inhibitors of EGFR. Chem. Commun. 2018, 54, 11530–11533. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.J.; Joshi, G.; Yadav, U.P.; Maurya, A.K.; Agnihotri, V.K.; Kalra, S.; Kumar, R.; Singh, S.; Sawant, D.M. Exploration of Pd-Catalysed Four-Component Tandem Reaction for One-Pot Assembly of Pyrazolo [1,5-c]Quinazolines as Potential EGFR Inhibitors. Bioorg. Chem. 2019, 93, 103314. [Google Scholar] [CrossRef]
- Ansari, A.J.; Joshi, G.; Sharma, P.; Maurya, A.K.; Metre, R.K.; Agnihotri, V.K.; Chandaluri, C.G.; Kumar, R.; Singh, S.; Sawant, D.M. Pd-Catalyzed Four-Component Sequential Reaction Delivers a Modular Fluorophore Platform for Cell Imaging. J. Org. Chem. 2019, 84, 3817–3825. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, C.; Li, P.; Ai, W.; Chen, H.; Wang, C.; Larock, R.C.; Shi, F. Synthesis of Pyrido [1,2-b]Indazoles via Aryne [3 + 2] Cycloaddition with N-Tosylpyridinium Imides. J. Org. Chem. 2011, 76, 6837–6843. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, X.; Awad, J.M.; Xie, G.; Qiu, W.; Zhang, W. One-Pot Synthesis of Tetrahydro-Pyrrolobenzodiazepines and Tetrahydro-Pyrrolobenzodiazepinones through Sequential 1,3-Dipolar Cycloaddition/ N-Alkylation (N-Acylation)/Staudinger/Aza-Wittig Reactions. Green Chem. 2019, 21, 4489–4494. [Google Scholar] [CrossRef]
- Zhang, X.; Zhi, S.; Wang, W.; Liu, S.; Jasinski, J.P.; Zhang, W. A Pot-Economical and Diastereoselective Synthesis Involving Catalyst-Free Click Reaction for Fused-Triazolobenzodiazepines. Green Chem. 2016, 18, 2642–2646. [Google Scholar] [CrossRef]
- Ma, X.; Gao, Z.; Niu, J.; Zhang, S.; Luo, L.; Yan, S.; Zhang, Q.; Zhang, W. Cascade Pd-Catalyzed Azide-Isocyanide Cross Coupling/Cyclization/Lactamization Reactions for the Synthesis of Tricyclic Guanidine-Containing Polyheterocycles. J. Org. Chem. 2025, 90, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Liu, S.; He, P.; Ding, M.-W. New Efficient Synthesis of Pyrimido [1,6-c]Quinazolin-4-Ones by a Biginelli 3CC/Staudinger/Aza-Wittig Sequence. Tetrahedron 2010, 66, 8151–8159. [Google Scholar] [CrossRef]
- He, P.; Nie, Y.-B.; Wu, J.; Ding, M.-W. Unexpected Synthesis of Indolo [1,2-c]Quinazolines by a Sequential Ugi 4CC–Staudinger–Aza-Wittig–Nucleophilic Addition Reaction. Org. Biomol. Chem. 2010, 9, 1429–1436. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, H.; Ding, M. Regioselective Synthesis of 2-Acylquinazolines and 3H-1,4-Benzodiazepin-3-ones by a Ugi 4CC/Staudinger/Aza-Wittig Sequence. J. Heterocycl. Chem. 2015, 52, 330–335. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, M.-L.; Liu, M.; Ding, M.-W. New Efficient Synthesis of Polysubstituted 3,4-Dihydroquinazolines and 4H-3,1-Benzothiazines through a Passerini/Staudinger/Aza-Wittig/Addition/Nucleophilic Substitution Sequence. Beilstein J. Org. Chem. 2022, 18, 286–292. [Google Scholar] [CrossRef]
- Deng, Z.; Li, J.; Zhu, P.; Wang, J.; Kong, Y.; Hu, Y.; Cai, J.; Dong, C. Quinazolinones as Potential Anticancer Agents: Synthesis and Action Mechanisms. Biomolecules 2025, 15, 210. [Google Scholar] [CrossRef]
- Wahan, S.K.; Sharma, S.; Chawla, P.A. Synthesis of Quinazolinone and Quinazoline Derivatives Using Green Chemistry Approach. Phys. Sci. Rev. 2022, 8, 3079–3094. [Google Scholar] [CrossRef]
- Amrutkar, R.D.; Amrutkar, S.V.; Ranawat, M.S. Quinazolin-4-One: A Varsatile Molecule. Curr. Bioact. Compd. 2020, 16, 370–382. [Google Scholar] [CrossRef]
- Chen, J.; Wu, D.; He, F.; Liu, M.; Wu, H.; Ding, J.; Su, W. Gallium(III) Triflate-Catalyzed One-Pot Selective Synthesis of 2,3-Dihydroquinazolin-4(1H)-Ones and Quinazolin-4(3H)-Ones. Tetrahedron Lett. 2008, 49, 3814–3818. [Google Scholar] [CrossRef]
- Tashrifi, Z.; Mohammadi-Khanaposhtani, M.; Biglar, M.; Larijani, B.; Mahdavi, M. Isatoic Anhydride: A Fascinating and Basic Molecule for the Synthesis of Substituted Quinazolinones and Benzo Di/Triazepines. Curr. Org. Chem. 2019, 23, 1090–1130. [Google Scholar] [CrossRef]
- Sacramento, M.; Piúma, L.P.A.; Nascimento, J.E.R.; Cargnelutti, R.; Jacob, R.G.; Lenardão, E.J.; Alves, D. Selective Synthesis of 2-(1,2,3-Triazoyl) Quinazolinones through Copper-Catalyzed Multicomponent Reaction. Catalysts 2021, 11, 1170. [Google Scholar] [CrossRef]
- Peringer, F.; Nascimento, J.E.R.d.; Abib, P.B.; Barcellos, T.; der Eycken, E.V.V.; Perin, G.; Jacob, R.G.; Alves, D. Copper-Catalyzed Multicomponent Reactions: Synthesis of Fused 1,2,3-Triazolo-1,3,6-triazonines. Eur. J. Org. Chem. 2017, 2017, 2579–2586. [Google Scholar] [CrossRef]
- Costa, G.P.; Baldinotti, R.S.M.; Fronza, M.G.; Nascimento, J.E.R.; Dias, Í.F.C.; Sonego, M.S.; Seixas, F.K.; Collares, T.; Perin, G.; Jacob, R.G.; et al. Synthesis, Molecular Docking, and Preliminary Evaluation of 2-(1,2,3-Triazoyl)Benzaldehydes As Multifunctional Agents for the Treatment of Alzheimer’s Disease. ChemMedChem 2020, 15, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.Y.; El-Bayouki, K.A.M.; Basyouni, W.M. Utilization of Isatoic Anhydride in the Syntheses of Various Types of Quinazoline and Quinazolinone Derivatives. Synth. Commun. 2016, 46, 993–1035. [Google Scholar] [CrossRef]
- Gomaa, H.A.M. A Comprehensive Review of Recent Advances in the Biological Activities of Quinazolines. Chem. Biol. Drug Des. 2022, 100, 639–655. [Google Scholar] [CrossRef]
- Faisal, M.; Saeed, A. Chemical Insights Into the Synthetic Chemistry of Quinazolines: Recent Advances. Front. Chem. 2021, 8, 594717. [Google Scholar] [CrossRef]
- Das, R.; Mehta, D.K.; Dhanawat, M. Bestowal of Quinazoline Scaffold in Anticancer Drug Discovery. Anti-Cancer Agents Med. Chem. 2020, 21, 1350–1368. [Google Scholar] [CrossRef]
- Azizi, N.; Edrisi, M. Practical Approach to 2-Thioxo-2,3-Dihydroquinazolin-4(1H)-One via Dithiocarbamate–Anthranilic Acid Reaction. Chin. Chem. Lett. 2017, 28, 109–112. [Google Scholar] [CrossRef]
- Ivachtchenko, A.V.; Kovalenko, S.M.; Drushlyak, O.G. Synthesis of Substituted 4-Oxo-2-Thioxo-1,2,3,4-Tetrahydroquinazolines and 4-Oxo-3,4-Dihydroquinazoline-2-Thioles. J. Comb. Chem. 2003, 5, 775–788. [Google Scholar] [CrossRef]
- Xiong, J.; Min, Q.; Yao, G.; Zhang, J.-A.; Yu, H.-F.; Ding, M.-W. New Facile Synthesis of 3,4-Dihydroquinazoline-2(1H)-Thiones by a Sequential Ugi-Azide/Staudinger/Aza-Wittig/Cyclization Reaction. Synlett 2019, 30, 1053–1056. [Google Scholar] [CrossRef]
- Morja, M.I.; Viradiya, R.H.; Chikhalia, K.H. Multicomponent Approaches Involving Carbon Disulfide. Asian J. Org. Chem. 2023, 12, e202300034. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Ma, X.; Zhang, W. One-Pot Synthesis of Dihydroquinazolinethione-Based Polycyclic System. Tetrahedron Lett. 2018, 59, 3845–3847. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Zhou, H.; Su, X.; Zhang, X.; Zhang, W. One-Pot, Two-Step Synthesis of 3,4-Dihydroquinazoline-2(1H)-Thiones from o-Azidobenzenealdehydes, Aryl Amines and Carbon Disulfide. Tetrahedron Lett. 2021, 81, 153361. [Google Scholar] [CrossRef]
- Zhang, X.; Pham, K.; Liu, S.; Legris, M.; Muthengi, A.; Jasinski, J.P.; Zhang, W. Stereoselective Synthesis of Fused TetrahydroQuinazolines through One-pot Double [3 + 2] Dipolar Cycloadditions Followed by [5 + 1] Annulation. Beilstein J. Org. Chem. 2016, 12, 2204–2210. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, W.; Zhan, D.; Ma, X.; Zhang, X. 2-Azidobenzaldehyde-Enabled Construction of Quinazoline Derivatives: A Review. Int. J. Mol. Sci. 2025, 26, 8955. https://doi.org/10.3390/ijms26188955
Qiu W, Zhan D, Ma X, Zhang X. 2-Azidobenzaldehyde-Enabled Construction of Quinazoline Derivatives: A Review. International Journal of Molecular Sciences. 2025; 26(18):8955. https://doi.org/10.3390/ijms26188955
Chicago/Turabian StyleQiu, Weiqi, Desheng Zhan, Xiaoming Ma, and Xiaofeng Zhang. 2025. "2-Azidobenzaldehyde-Enabled Construction of Quinazoline Derivatives: A Review" International Journal of Molecular Sciences 26, no. 18: 8955. https://doi.org/10.3390/ijms26188955
APA StyleQiu, W., Zhan, D., Ma, X., & Zhang, X. (2025). 2-Azidobenzaldehyde-Enabled Construction of Quinazoline Derivatives: A Review. International Journal of Molecular Sciences, 26(18), 8955. https://doi.org/10.3390/ijms26188955





